Abstract
Purpose
To investigate the value of gadoxetic acid-enhanced 3-dimensional T1-weighted MR cholangiography (T1w-MRC) in comparison to 3-dimensional T2-weighted MR cholangiopancreaticography (T2w-MRCP) in patients with primary sclerosing cholangitis (PSC).
Subjects and Methods
Thirty-four MR exams in 29 patients (46.0±16.1 years; 19 men, 10 women) scanned within a 14-month-period were retrospectively included. Two abdominal radiologists independently evaluated image quality regarding image contrast, image quality degradation due to artifacts, and visualization quality of ducts. The order of biliary tree branches that were visualized and reader preference towards each method were recorded. Helpfulness of T1w-MRC was scored in consensus. Confirmatory endoscopic retrograde cholangiopancreaticography (ERCP) performed within 3 months of the MR examination was available in 8 patients.
Results
Image quality of T1w-MRC and T2w-MRCP was graded good to excellent in all cases. There were advantages for both T1w-MRC (functional information, less degradation due to artifacts) and T2w-MRCP (higher order of visualized branches, better branch depiction). Both readers showed preference for T2w-MRCP; however, both readers found gadoxetic acid–enhanced T1w-MRC helpful in the majority of cases.
Conclusion
Gadoxetic acid-enhanced T1w-MRC is complementary to, but should not replace, T2w-MRCP. T1w-MRC is a useful adjunct to T2w-MRCP for morphologic evaluation and provides additional diagnostic information.
Keywords: Primary sclerosing cholangitis, magnetic resonance cholangiography, T1-weighted MR cholangiography, T2-weighted MR cholangiopancreaticography, gadoxetic acid, hepatobiliary contrast enhancement
INTRODUCTION
Primary sclerosing cholangitis (PSC), first described by Hoffman in 1867 (1), is an inflammatory biliary disorder responsible for significant morbidity, predominantly in middle-aged men (2). The exact etiology of PSC is unknown; however, the presence of low serum T-cells, increased auto-immune antibodies, and a well-established association with inflammatory bowel disease strongly suggest an autoimmune etiology (3). PSC is characterized by inflammation and fibrosis of the intrahepatic and/or extrahepatic bile ducts. The slow but relentlessly progressive nature of this disease leads to biliary cirrhosis, hepatic failure, paroxysmal bacterial cholangitis, biliary stone formation, and the dreaded complication of cholangiocarcinoma. Although PSC is a far less common cause of cirrhosis than viral hepatitis, alcohol abuse, or fatty liver disease, it is the fourth leading indication for liver transplantation in adults in the United States (2), which is currently the only definitive cure.
Patients with PSC often present with symptoms associated with cholestasis, including fatigue, pruritus, and steatorrhea. While these findings are non-specific, MR imaging can help establish the diagnosis by depicting the characteristic “beaded” appearance of the bile ducts with irregular, alternating areas of focal narrowing, dilations, and elongations. Further, MRI can help identify common complications such as the presence of intraductal stones, the development of dominant strictures and screen for the development of cholangiocarcinoma through the combined use of T2-weighted MR cholangiopancreatography (T2w-MRCP) and dynamic contrast enhanced T1w-MRI (4). Thus, MRI can help avoid endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC), both of which are invasive and involve exposure to ionizing radiation.
T2w-MRCP is well established as the noninvasive morphological imaging standard of the biliary system, and is routinely used to diagnose and evaluate PSC (5). However, with the recent FDA approval of a new hepatobiliary agent, gadoxetic acid (Eovist®, Gd-EOB-DTPA, Bayer Pharmaceuticals, Wayne, NJ), new opportunities for both anatomical and functional evaluation of the bile ducts are available (6,7). During dynamic phase imaging, gadoxetic acid behaves similarly to conventional extracellular gadolinium based contrast agents (GBCA). While extracellular GBCAs are cleared from the body primarily by renal filtration, approximately 50% of gadoxetic acid is taken up rapidly by functioning hepatocytes and excreted into bile, with peak biliary enhancement at approximately 20 minutes (8–10). Significant concentrations of gadolinium are excreted into the bile, facilitating T1 weighted (T1w) MR cholangiography (T1w-MRC). It is important to note that there is no explicit FDA-approval for biliary imaging using gadoxetic acid in the U.S, and therefore the use of gadoxetic acid to T1w-MRC is an off-label application. Unlike T2w-MRCP, gadoxetic-enhanced T1w-MRC therefore contains both morphological and functional information that allow, for example, determination of the patency of choledochoenteric anastomoses or the presence of bile leaks.
Although the general diagnostic value of gadoxetic acid has been described during phase II-III trials for focal liver lesions (8,9,11,12), there have been a limited number of reports on gadoxetic acid-enhanced T1w-MRC (10,13–16) and no reports on specific biliary diseases such as PSC. Therefore, it was the purpose of this study to investigate the value of high-resolution 3D T1w-MRC using gadoxetic acid in patients with PSC in comparison to 3D T2w-MRCP. In cases with ERCP performed within 3 months of the MR study, ERCP was used for confirmation.
MATERIALS AND METHODS
Patients
After obtaining approval from the local institutional review board, a HIPAA compliant retrospective analysis of patient examinations was performed. The necessity of retrospective consent was waived. Patients who underwent abdominal MRI between 8/1/2008 and 10/1/2009 and met the following criteria were included in this study:
Known or suspected diagnosis of PSC
The use of gadoxetic acid;
-
Availability of the following pulse sequences, all acquired on the same day:
3D T2w-MRCP
high resolution 3D T1w imaging performed 20–30 minutes after contrast injection.
During this period, 35 MR exams in 30 patients (average age 46.0 ± 16.1 years, range 18–81 years; body weight 80.5 ± 13.9 kg, range 56.7 – 115.2 kg; 19 men, 10 women) fulfilled these criteria and were included in the analysis. One study was excluded during the analysis due to significant artifacts and non-diagnostic image quality of both T2w-MRCP and T1w-MRC, related to pneumobilia caused by sphincterotomy and stent placement two days prior to the MR scan. Therefore, 34 MR scans in 29 patients were used for the analysis.
Confirmatory endoscopic retrograde cholangiopancreaticography (ERCP) performed within 3 months of the MR examination was available in 8 patients. In 21 patients, the diagnosis PSC was already established at the time of MR scanning. In three patients with ulcerative colitis and elevated liver function test MR imaging and ERCP confirmed the diagnosis of PSC. In the remaining five patients, PSC was not confirmed by either MR or ERCP. Fifteen patients had had no previous abdominal surgery; two patients had previously undergone orthotopic liver transplantation; six patients had a previous choledochojejunoatomy; six participants previously underwent a cholecystectomy.
Endoscopic retrograde cholangiopancreatography (ERCP) results in patients performed within a 3-month period of the MR examination was used for confirmation of MR imaging results, if available.
MR Imaging
All scans were performed on a clinical 1.5T scanner (Signa HDx, GE Healthcare, Waukesha, WI) using an eight-channel phased-array torso or cardiac coil.
Prior to contrast injection, 3D T2w-MRCP imaging was performed during free breathing with respiratory triggering. Imaging parameters were adapted to each individual’s anatomy to cover the biliary system: coronal oblique slab, FOV = 30 – 32cm, matrix = 288 x 288, TR = 3529–8571 ms, TEeff = 750–772 ms, echo train length (ETL) = 144, 129–131 slices, bandwidth = ± 50 kHz, slice thickness =1.8 mm. The true spatial resolution was 1.0 – 1.1x 1.0 – 1.1 x 1.8 mm3, interpolated to 0.6 x 0.6 x 0.9 mm3 through zero-filling. Fat and vessel suppression was enhanced using a T2 preparation (“T2 Prep”) magnetization preparation pulse (17,18).
Dynamic phase liver imaging was performed following clinical routine after the injection of 0.05 mmol/kg gadoxetic acid at a rate of 2.0 mL/s, followed by a 20 mL saline chaser. It should be noted that 0.05 mmol/kg gadoxetic acid is twice the recommended package insert dose (0.025mmol/kg) approved for detection and characterization of focal liver lesion. As we discuss below, dosing at 0.05mmol/kg is the local standard of care at some institutions, including ours, in order to achieve acceptable enhancement during the dynamic phase.
Approximately 20 minutes after contrast administration, high resolution T1w-MRC was performed in 32/34 exams using an investigational version of a navigator-gated high-resolution 3D spoiled gradient recalled echo (SPGR) sequence with intermittent spectrally selective partial inversion recovery fat suppression (LAVA) (19,20). Respiratory motion artifacts were avoided through use of a cylindrical navigator to monitor the position of the diaphragm and to acquire data only during end-expiration. Specific imaging parameters used for the breath hold T1w navigated acquisition included: axial slab excitation, FOV = 340–400 mm R/L x 272–320 mm A/P, acquisition matrix = 288x256, TR/TE = 5.5 – 5.9/2.5 – 2.7 ms, inversion time of spectrally selective fat-saturation pulse (TI) = 8 ms, flip angle = 40° (optimized for liver-to-biliary contrast (20–22)), bandwidth = ± 42 kHz. This resulted in a true spatial resolution of 1.2 – 1.4 mm R/L x 1.1 – 1.3 mm A/P x 1.8 mm S/I, interpolated to 0.5 – 0.7 x 0.5 – 0.6 x 0.9 mm3 through zero filling. Total scan time was 4–6 minutes depending on the respiratory pattern and rate. In 2/34 exams, a breath-held sequence with similar in plane resolution was used applying parallel imaging with an autocalibrated image-based approach (ARC (23)). The two breath hold exams were acquired with 66 and 86 slices of 3.0 and 2.4 mm slice thickness, respectively.
Image Quality Evaluation
Two fellowship-trained abdominal radiologists with expertise in MR (11 and 25 years of experience) independently reviewed the images. Helpfulness was judged in consensus using criteria listed below. Images were reviewed on a clinical PACS (McKesson, Richmond, British Columbia, Canada). Readers were able to reformat both T2w-MRCP and T1w-MRC images into multiplanar reformats (MPR) and maximum intensity projections (MIP) at user-defined thickness. Image quality was assessed for both T2w-MRCP and T1w-MRC acquisitions based on original images and MPR reformats.
The following objective criteria were independently evaluated:
-
Visual contrast
was determined by visually assessing the contrast between biliary structures and surrounding fatty and hepatic tissue using a Likert scale with 0 = non-diagnostic, 1 = diagnostic, moderate image quality, 2 = good image quality, and 3 = excellent image quality.
-
Image degradation due to artifacts
was also scored with a Likert scale with 0 = non-diagnostic, 1 = diagnostic, moderate image quality, 2 = good image quality, and 3 = excellent image quality.
-
Visualized biliary duct branch order
was recorded as the highest order of the biliary tree branching that was well visualized.
-
Biliary duct depiction quality
was assessed for the quality of visualization of the common duct, left and right hepatic ducts and intrahepatic bile ducts following a Likert scale: 0 = non-visualized, 1 = visualized, moderate quality, 2 = good visualization, and 3 = excellent visualization
-
Helpfulness of T1w-MRC
was evaluated as 0 = not helpful or inferior, 1 = equal or confirmatory, 2 = added diagnostic value/information in comparison to T2w-MRCP. Additional information was considered as one of the following criteria: Marked improvement of diagnostic confidence towards PSC; additional morphological information regarding the intra- or extrahepatic bile ducts, lack of biliary excretion, or additional findings. Exclusion or identification of cholangiocarcinoma was not taken into account.
-
Overall Preference
of gadoxetic acid enhanced T1w-MRC or T2w-MRCP was noted as 0 = T2w-MRCP preferred, 1 = T1w-MRC preferred, 2 = no preference.
In the eight patients that also had ERCP available for comparison, both T2w-MRCP and T1w-MRC were scored in a consensus reading of both radiologists:
-
Diagnostic Concordance
with ERCP where 1 = MRI overdiagnosing disease severity, 0 = MRI concordant with ERCP, or −1 = MRI underdiagnosing disease severity.
Statistical Analysis
Patient data are reported as mean ± standard deviation (range). A Wilcoxon signed rank test was used to compare grading differences between groups (i.e., differences between readers or techniques). After dichotomization (scores 0 and 1 being non-diagnostic or of moderate quality vs. scores 2 and 3 confirming good to excellent quality), Fisher’s exact test was used to test for differences between techniques. For these analyses, ratings from both radiologists were considered as being independent observations and were subsequently pooled, hence doubling the numbers of compared pairs.
RESULTS
Image quality
Figure 1 shows an example of excellent image quality available with both T1w-MRC and T2w-MRCP in a patient with suspected PSC. ERCP images are shown for confirmation of morphology. In Figure 2, results from a patient with inferior image quality are presented. Nevertheless, both T1w-MRC and T2w-MRCP enable the reading radiologist to establish the diagnosis of PSC. While Figure 3 reveals an apparent different image quality of the MIP reconstructions, image grading on non-reformatted image quality and branch order depiction was rated equal by both readers and judged confirmatory (helpfulness scale 1). Tables 1 and 2 summarize the image quality ratings from both readers.
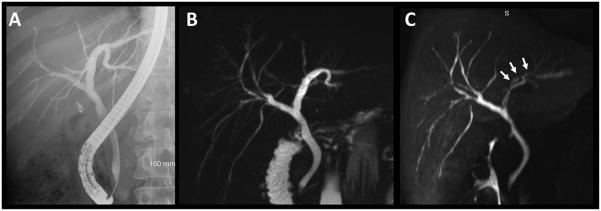
Figure 1.
33-year old man with ulcerative colitis and suspected primary sclerosing cholangitis. Comparison of ERCP (A), T2w MRCP MIP (B), and gadoxetic-enhanced T1w MRC MIP (C) underlines the similarity of results obtainable with all three methods. Note the decreased signal intensity of the left hepatic duct in the T1w MRC image (C, white arrows). This pseudo-stenosis is created due to layering of the contrast agent with the patient being in supine position. This finding underlines a) the necessity to read the axial images as opposed to MIP reformats alone and b) the complementary nature of T1w MRC and T2w MRCP.
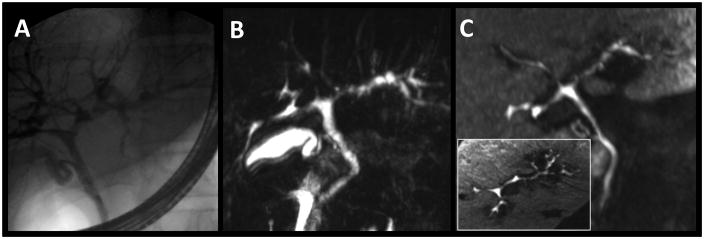
Figure 2.
26-year-old man with ulcerative colitis, primary sclerosing cholangitis (PSC), and elevated liver function tests. ERCP (A), T2w MRCP MIP (B), and gadoxetic acid-enhanced T1w MRC MIP (C, inset: axial MIP) depict the extent of the disease with the beaded appearance of multiple stenoses and segmental dilatations. Both T2w MRCP and T2w MRC were graded with matching good image quality (scale level “2”) and some deterioration due to artifacts (scale level “2”).
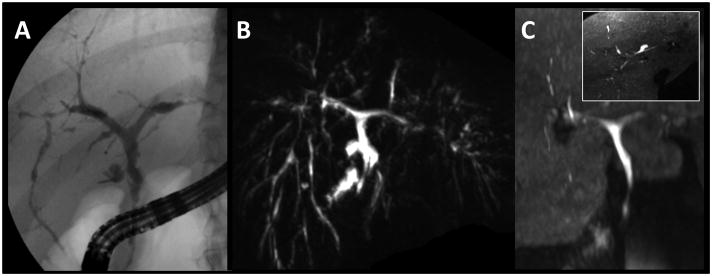
Figure 3.
29 year-old female with known PSC and ulcerative colitis. A multitude of strictures is depicted by T2w MRCP MIP (B) and ERCP (A). Gadoxetic acid-enhanced T1w MRC MIP (C) depicts the central ducts equally well. The MIP display in (C, inset: axial MIP) reveal the detail seen in the stack of axial images which revealed multiple stenotic and dilated segments to the 3rd (reader 1) or 4th (reader 2) branch.
Table 1.
Overall image quality and comparison of T2w MRCP and gadoxetic acid – enhanced T1w MRC.
| Reader 1 | Reader 2 | |||
|---|---|---|---|---|
| T2w MRCP | T1w MRC | T2w MRCP | T1w MRC | |
|
| ||||
| Image quality assessed by: | ||||
| A. Visual contrast | ||||
| 3 | 28 | 27 | 27 | 32 |
| 2 | 6 | 5 | 7 | 2 |
| 1 | 0 | 1 | 0 | 0 |
|
| ||||
| 0 | 0 | 0 | 0 | 0 |
|
| ||||
| B. Artifacts | ||||
| 3 | 13 | 18 | 22 | 29 |
| 2 | 16 | 11 | 11 | 4 |
| 1 | 5 | 4 | 1 | 1 |
|
| ||||
| 0 | 0 | 0 | 0 | 0 |
|
| ||||
| C. Visualized branch order | ||||
| 5 | 0 | 0 | 6 | 3 |
| 4 | 3 | 0 | 8 | 9 |
| 3 | 23 | 18 | 11 | 10 |
| 2 | 7 | 9 | 8 | 5 |
| 1 | 1 | 3 | 1 | 2 |
|
| ||||
| 0 | 0 | 4 | 0 | 4 |
|
| ||||
| Overall preference | T2w 3D MRC = 17 T1w MRC = 11 none = 6 |
T2w 3D MRC = 20 T1w MRC = 14 none = 0 |
||
Table 2.
Visualization grading of biliary segments for both T1w MRC and T2w MRCP.
| Reader 1 | Reader 2 | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T2w MRCP | T1w MRC | T2w MRCP | T1w MRC | ||||||
| [n] | [%] | [n] | [%] | [n] | [%] | [n] | [%] | ||
|
| |||||||||
| CHD | p=n.s | ||||||||
| 3 | 20 | 58.8 | 20 | 60.6 | 24 | 70.6 | 26 | 76.5 | |
| 2 | 13 | 38.2 | 7 | 21.2 | 6 | 17.6 | 3 | 8.8 | |
| 1 | 1 | 2.9 | 2 | 6.1 | 2 | 5.9 | 1 | 2.9 | |
|
| |||||||||
| 0 | 0 | 0 | 4 | 12.1 | 2 | 5.9 | 4 | 11.8 | |
|
| |||||||||
| LHD | p=n.s | ||||||||
| 3 | 19 | 55.9 | 20 | 60.6 | 20 | 58.8 | 22 | 66.7 | |
| 2 | 9 | 26.5 | 6 | 18.2 | 5 | 14.7 | 4 | 12.1 | |
| 1 | 5 | 14.7 | 2 | 6.1 | 7 | 20.6 | 1 | 3.0 | |
|
| |||||||||
| 0 | 1 | 2.9 | 5 | 15.2 | 2 | 5.9 | 6 | 18.2 | |
|
| |||||||||
| RHD | p<0.03 | ||||||||
| 3 | 17 | 50.0 | 20 | 60.6 | 17 | 50.0 | 17 | 51.5 | |
| 2 | 11 | 32.4 | 6 | 18.2 | 5 | 14.7 | 6 | 18.2 | |
| 1 | 5 | 14.7 | 3 | 9.1 | 8 | 23.5 | 4 | 12.1 | |
|
| |||||||||
| 0 | 1 | 2.9 | 4 | 12.1 | 4 | 11.8 | 6 | 18.2 | |
|
| |||||||||
| IHD | p<0.01 | ||||||||
| 3 | 14 | 41.2 | 15 | 45.5 | 16 | 47.1 | 10 | 30.3 | |
| 2 | 10 | 29.4 | 10 | 30.3 | 7 | 20.6 | 10 | 30.3 | |
| 1 | 9 | 26.5 | 2 | 8.8 | 10 | 29.4 | 5 | 15.2 | |
|
| |||||||||
| 0 | 1 | 2.9 | 6 | 11.8 | 1 | 2.9 | 8 | 24.2 | |
CHD = common hepatic duct, LHD = left hepatic duct, RHD = right hepatic duct, and IHD = intrahepatic ducts. Given p-values compare image quality ratings of T1w-MRC versus T2w-MRCP based on pooled results of both readers and dichotomization in good to excellent quality (grades “3” and “2”) versus moderate to non-diagnostic quality (grades “1” and “0”) image grading.
Both readers rated image quality good or excellent in the majority of both T2w-MRCP and gadoxetic acid-enhanced T1w-MRC exams. With respect to visual contrast and imaging artifacts, all exams were considered to be of diagnostic image quality. No statistically significant differences were detected between the readers. There was a trend towards decreased artifacts on T1w-MRC as opposed to T2w-MRCP, and visualization of higher order biliary duct branches using T2w-MRCP. These findings contributed to a slight overall preference of T2w-MRCP over gadoxetic acid-enhanced T1w-MRC in only a few more than half of cases (reader 1: 17/34, reader 2: 20/34).
Table 2 presents the detailed results of duct visibility ratings performed on the common, main left, main right (CHD, LHD, and RHD, respectively), and the intrahepatic ducts (IHD). Overall, there was a high proportion of segments with diagnostic readings for both T2w-MRCP and gadoxetic acid-enhanced T1w-MRC (T2w-MRCP = 213 diagnostic vs. 52 non-diagnostic segments, T1w-MRC = 202 diagnostic vs. 63 non-diagnostic segments). Overall, differences for duct visibility between both techniques were not statistically significant (p = 0.15) despite a trend towards higher ratings by reader 1. There were, however, significant differences on a per-branch scale with readings for RHD and IHD differing significantly between both readers (RHD: p < 0.03; IHD: p < 0.01). No statistical difference was found for CHD and LHD, respectively (for both, p > 0.05).
Helpfulness of T1w-MRC
In the majority of cases (28/34 cases, 82.4%) both readers agreed that gadoxetic acid-enhanced T1w-MRC was helpful. Out of the 28 cases, 16 were judged as being equal or confirmatory (see Figures 1 and 2). In the remaining 12 cases, additional information was revealed regarding a markedly higher confidence regarding the diagnosis of PSC (n=5), and/or additional information regarding the intrahepatic ducts (n=4), and/or regarding the extrahepatic ducts (n=11). In one case, a bilioma was diagnosed as an additional finding. Nodular lesions at the dome of the gallbladder highly suspicious of a neoplasm were seen with both T1w-MRC and T2w-MRCP in one case which was denoted as being confirmatory.
Figures 4 and 5 show examples in which both readers agreed on the helpfulness of gadoxetic acid-enhanced T1w-MRC. Figure 4 shows findings in a 28 year-old woman with PSC. Although gadoxetic acid-enhanced T1w-MRC (C) does not provide a central cholangiogram at 20 min after injection, innumerable hyperintense foci of the liver may represent tiny peripheral bile ducts or biliary lakes. There is no excretion of the agent into the central biliary system at the time these images were acquired. Figure 5 depicts one potential gain in functional information provided by gadoxetic acid-enhanced T1w-MRC. This 65 year-old man presented with elevated liver function tests (LFT’s) after liver transplantation and choledochojejunostomy. Although the anatomy can be evaluated on both approaches, only gadoxetic acid-enhanced T1w-MRC proves the patency of the anastomosis by contrast agent passage.
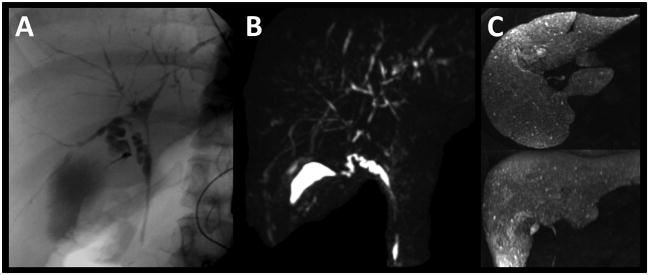
Figure 4.
28 year-old woman with PSC. Pruned intrahepatic biliary tree with multiple ductal irregularities. Clearly, ERCP (A) and T2w MRCP MIP (B) are able to depict the biliary tree. In contrast, gadoxetic acid-enhanced T1w MRC MIP (C) does not provide a central cholangiogram at 20 min after injection. Instead, innumerable hyperintense foci of the liver may represent tiny peripheral bile ducts or biliary lakes. Despite the lack of a central T1w MR cholangiogram, the study was graded “helpful (1)” during the evaluation since functional information on the excretion to the extrahepatic ducts is available.
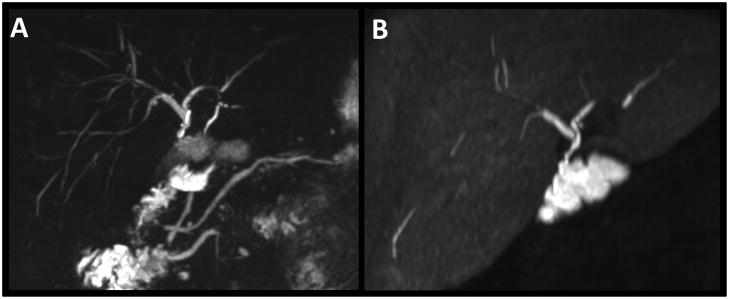
Figure 5.
65 year-old man status post liver transplantation due to PSC presenting with elevated LFT’s to rule out recurrent disease. Next to the depiction of the main biliary ducts with both techniques (A: T2w MRCP MIP, B: T1w MRC MIP), T1w MRC clearly proofs the patency of the choledochojejunostomy.
Both reviewers preferred T2w-MRCP over T1w-MRC using gadoxetic acid in this group of PSC patients. Although reader 1 preferred T2w-MRCP slightly more often than T1w-MRC, the fact that the readers showed no preference between methods in 6 cases strengthens the argument that both techniques are important.
Confirmation by ERCP
In eight patients, an ERCP was performed within three months before or after the MR exam, and was available for comparison to both T2w-MRCP and gadoxetic acid–enhanced T1w-MRC. In all eight cases, both readers confirmed equality of gadoxetic acid-enhanced T1w-MRC and T2w-MRCP in comparison to ERCP.
DISCUSSION
To the best of our knowledge, this work is the first to provide a comparison of gadoxetic acid-enhanced T1w-MRC with T2w-MRCP in patients with primary sclerosing cholangitis. Findings from this study demonstrate that while there are advantages of T2w-MRCP over gadoxetic acid-enhanced T1w-MRC, both sequences offer good to excellent image quality in the majority of cases.
Each approach has its advantages. While gadoxetic acid-enhanced T1w-MRC is an effective tool for evaluating the morphology of the main biliary structures, it did not perform as well for peripheral, intrahepatic biliary ductal visualization as T2w-MRCP. In addition to superior depiction of higher order of biliary duct branches, T2w-MRCP was preferred over T1w-MRC for morphological evaluation by both readers. Even though morphological information can usually be collected using T2w-MRCP alone, T1w-MRC improved the reader confidence and both readers considered gadoxetic acid-enhanced hepatobiliary phase T1w-MRC to be a useful adjunct to conventional T2w-MRCP. However, it was clear that T1w-MRC should not replace T2w-MRCP, which is also less expensive since it does not require any contrast agent administration.
In addition, the excellent concordance between the three imaging modalities ERCP, T2w-MRCP, and gadoxetic acid–enhanced T1w-MRC was highly reassuring. Despite being limited by a small sample size, the utility of both non-invasive approaches was confirmed with the invasive gold standard, ERCP.
Aside from the anatomical information discussed above, gadoxetic acid-enhanced T1w-MRC offers functional information that is not available through T2w-MRCP alone. This fact was not incorporated into the helpfulness evaluation due to its uncertain diagnostic value at this point in time. In the future. T1w-MRC may prove even more helpful if this feature is shown to add diagnostic value. Several cases suggest that this may in fact be the case. The lack of biliary contrast enhancement was seen in five patients, as illustrated in Figure 4. Similarly, in two patients with a choledochojejunostomy, the patency of the anastomosis was proven by passage of contrast.
Although T2w-MRCP accurately depicts the ducts peripheral to a stricture, the lack of contrast agent in the ducts associated with liver parenchyma that is peripheral to the stricture may offer important information on the functional significance of a stricture. In our opinion, the presence of segmentally decreased uptake and excretion peripheral to a stricture may indicate that the stricture is functionally severe, resulting from local cholestasis peripheral to the stricture, and may possibly indicate the presence of a dominant stricture. Areas of functional parenchymal deficit associated with hypointensity of the liver and lack of biliary excretion could provide clinicians with useful adjunctive information, particularly if the finding is new in comparison to a prior study. The precise relationship between local cholestasis and stricture severity is not well understood and the ability of gadoxetic acid to grade such strictures is unknown. There are a few reports of decreased uptake of gadoxetic acid in the presence of liver failure, global cholestasis, or common duct stenosis, but no data relating the lack or extent of contrast excretion with the functional severity of local strictures (14). The exact interpretation of reduced uptake and excretion of gadoxetic acid excretion in the setting of local or global cholestasis is a topic of current investigation. At present there are insufficient data to recommend a grading scheme or practice guideline regarding the use of gadoxetic acid in the presence of severe cholestasis or strictures. However, the potential for more quantitative characterization of biliary disease and identification of dominant strictures is sufficiently compelling to warrant future studies.
The use of a free-breathing protocol for both the established T2w-MRCP and the navigator-gated gadoxetic acid–enhanced T1w-MRC has proven useful in routine clinical practice but is not available on all scanners and at all institutions. By removing the scan time limitations necessary for breath-held scans in these sick patients, higher spatial resolution images can be achieved. Especially for gadoxetic acid-enhanced T1w-MRC, imaging times on the order of 5 minutes for a single navigator-based sequence have become possible by making use of the relatively constant enhancement level of both the biliary system and the liver during the hepatobiliary phase. This pseudo-equilibrium phase enables the radiologist to use free-breathing sequences or repeated acquisitions, e.g., with different flip angles to optimize the contrast-to-noise-ratio (21,22). Most reports on the use of gadoxetic acid have confirmed that constant imaging conditions can be expected between 20–45 min after injection (8,9). A free-breathing protocol, however, has not yet been shown to be superior to a breath-held acquisition.
There are several limitations to this study. Although the sample size is relatively large relative to the prevalence of this disease, a comparison of different imaging methods would benefit from a prospective study with a larger numbers of patients. This is particularly true for validation with ERCP, which was limited to only eight patients. However, the comparison to ERCP was not the primary goal of this study. ERCP is rarely used for screening and diagnostic purposes, largely due to the success of MRI for non-invasive evaluation of biliary disease. Similarly, we have introduced some potential bias by allowing 5 patients to be included twice and by considering both examinations as being independent. This follows the clinical routine where follow-up examinations are scheduled 6–12 months later and could be influenced by patient-specific factors on image quality such as the ability to hold his/her breath.
A dose of 0.05 mmol/kg gadoxetic acid is the standard clinical dose at some institutions, including our own, and was used in all patients in this study. The package insert dose of gadoxetic acid is only 0.025mmol/kg, which is the FDA-approved dose for detection and characterization of focal liver lesions. Gadoxetic acid is not specifically approved for biliary imaging. This dose is based on the minimum effective dose for detection of liver lesions in the delayed phase, without regard to imaging characteristics of gadoxetic acid in the dynamic phase (11). Recent data demonstrate that 0.025 mmol/kg may be an insufficient dose for adequate arterial phase enhancement (22,24). This observation has also been confirmed at other institutions, including studies by Motosugi et al. and Lee et al. (16,25). Feuerlein et al. used a fixed dose of 10 mL per patient (resulting in an effective dose 0.02–0.06 mmol/kg), and observed improved enhancement using higher doses, although it did not reach statistical significance (26). In order to overcome this limitation, our institution routinely administers 0.05mmol/kg gadoxetic acid.
Clinical image interpretation is usually performed using thin slice and thin multi-planar reformatted (MPR) images. Maximum intensity projections (MIP’s), as shown in most of the figures in this work, are not typically used for image interpretation. However, MIP images allow a convenient summary in a single image and are useful for publication purposes. MIP image from gadoxetic acid – enhanced T1w-MRC suffer from the higher signal of the “background” liver, which is almost completely suppressed in T2w-MRCP. Therefore, the MIP images of T1w-MRC may not fully mirror the information contained in the MPR images (see also Figure 1).
In conclusion, this work demonstrates that high-resolution 0.05mmol/kg BW gadoxetic acid – enhanced T1w-MRC at using a navigator-gated, free breathing imaging sequence is a useful adjunct to T2w-MRCP imaging in patients with primary sclerosing cholangitis, but that it should not replace T2w-MRCP. Not only does gadoxetic acid–enhanced T1w-MRC provide potentially useful functional information, but it also provides an excellent “second-look” at morphological abnormalities in the bile ducts of patients with PSC. In routine clinical studies where dynamic contrast enhanced liver imaging is performed in conjunction with T2w-MRCP, the use of T1w-MRC to evaluate for the presence of PSC, its disease extent and severity can be achieved at minimal additional cost.
Acknowledgments
The authors gratefully acknowledge support from NIH (R01 DK083380, R01 DK088925 and RC1 EB010384), the Coulter Foundation, Bracco Diagnostics, and GE Healthcare.
Footnotes
Disclosures: S.K.N. and A.R.J. have no disclosures. F.K. has attended a symposium and received meals provided, honoraria, and travel reimbursement sponsored by Bracco Diagnostics. A.F. received an educational stipend from Bracco Diagnostics. The University of Wisconsin – Madison receives research support from Bracco Diagnostics and GE Healthcare. S.B.R. has a family member employed by GE Healthcare.
References
- 1.Chapman RW. Aetiology and natural history of primary sclerosing cholangitis--a decade of progress? Gut. 1991;32(12):1433–1435. doi: 10.1136/gut.32.12.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924–933. doi: 10.1056/NEJM199504063321406. [DOI] [PubMed] [Google Scholar]
- 3.Levy C, Lindor KD. Primary sclerosing cholangitis: epidemiology, natural history, and prognosis. Semin Liver Dis. 2006;26(1):22–30. doi: 10.1055/s-2006-933560. [DOI] [PubMed] [Google Scholar]
- 4.Vitellas KM, El-Dieb A, Vaswani KK, et al. MR cholangiopancreatography in patients with primary sclerosing cholangitis: interobserver variability and comparison with endoscopic retrograde cholangiopancreatography. AJR Am J Roentgenol. 2002;179(2):399–407. doi: 10.2214/ajr.179.2.1790399. [DOI] [PubMed] [Google Scholar]
- 5.Dave M, Elmunzer BJ, Dwamena BA, Higgins PD. Primary sclerosing cholangitis: meta-analysis of diagnostic performance of MR cholangiopancreatography. Radiology. 2010;256(2):387–396. doi: 10.1148/radiol.10091953. [DOI] [PubMed] [Google Scholar]
- 6.Clement O, Muhler A, Vexler V, Berthezene Y, Brasch RC. Gadolinium-ethoxybenzyl-DTPA, a new liver-specific magnetic resonance contrast agent. Kinetic and enhancement patterns in normal and cholestatic rats. Invest Radiol. 1992;27(8):612–619. [PubMed] [Google Scholar]
- 7.Schuhmann-Giampieri G, Schmitt-Willich H, Press WR, Negishi C, Weinmann HJ, Speck U. Preclinical evaluation of Gd-EOB-DTPA as a contrast agent in MR imaging of the hepatobiliary system. Radiology. 1992;183(1):59–64. doi: 10.1148/radiology.183.1.1549695. [DOI] [PubMed] [Google Scholar]
- 8.Bollow M, Taupitz M, Hamm B, Staks T, Wolf KJ, Weinmann HJ. Gadolinium-ethoxybenzyl-DTPA as a hepatobiliary contrast agent for use in MR cholangiography: results of an in vivo phase-I clinical evaluation. Eur Radiol. 1997;7(1):126–132. doi: 10.1007/s003300050125. [DOI] [PubMed] [Google Scholar]
- 9.Hamm B, Staks T, Muhler A, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995;195(3):785–792. doi: 10.1148/radiology.195.3.7754011. [DOI] [PubMed] [Google Scholar]
- 10.Ringe KI, Husarik DB, Gupta RT, Boll DT, Merkle EM. Hepatobiliary transit times of gadoxetate disodium (Primovist((R))) for protocol optimization of comprehensive MR imaging of the biliary system-What is normal? Eur J Radiol. 2011 Aug;79(2):201–5. doi: 10.1016/j.ejrad.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Reimer P, Rummeny EJ, Shamsi K, et al. Phase II clinical evaluation of Gd-EOB-DTPA: dose, safety aspects, and pulse sequence. Radiology. 1996;199(1):177–183. doi: 10.1148/radiology.199.1.8633143. [DOI] [PubMed] [Google Scholar]
- 12.Bluemke DA, Sahani D, Amendola M, et al. Efficacy and safety of MR imaging with liver-specific contrast agent: U.S. multicenter phase III study. Radiology. 2005;237(1):89–98. doi: 10.1148/radiol.2371031842. [DOI] [PubMed] [Google Scholar]
- 13.Dahlstrom N, Persson A, Albiin N, Smedby O, Brismar TB. Contrast-enhanced magnetic resonance cholangiography with Gd-BOPTA and Gd-EOB-DTPA in healthy subjects. Acta Radiol. 2007;48(4):362–368. doi: 10.1080/02841850701196922. [DOI] [PubMed] [Google Scholar]
- 14.Tschirch FT, Struwe A, Petrowsky H, Kakales I, Marincek B, Weishaupt D. Contrast-enhanced MR cholangiography with Gd-EOB-DTPA in patients with liver cirrhosis: visualization of the biliary ducts in comparison with patients with normal liver parenchyma. Eur Radiol. 2008;18(8):1577–1586. doi: 10.1007/s00330-008-0929-6. [DOI] [PubMed] [Google Scholar]
- 15.Lee NK, Kim S, Lee JW, et al. Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics. 2009;29(6):1707–1724. doi: 10.1148/rg.296095501. [DOI] [PubMed] [Google Scholar]
- 16.Lee MS, Lee JY, Kim SH, et al. Gadoxetic acid disodium-enhanced magnetic resonance imaging for biliary and vascular evaluations in preoperative living liver donors: Comparison with gadobenate dimeglumine-enhanced MRI. J Magn Reson Imaging. 2011;33(1):149–159. doi: 10.1002/jmri.22429. [DOI] [PubMed] [Google Scholar]
- 17.Brittain JH, Hu BS, Wright GA, Meyer CH, Macovski A, Nishimura DG. Coronary angiography with magnetization-prepared T2 contrast. Magn Reson Med. 1995;33(5):689–696. doi: 10.1002/mrm.1910330515. [DOI] [PubMed] [Google Scholar]
- 18.Busse RF, Brittain JH, Reeder SB, Kelcz F. Improved background suppression for 3D-MRCP using T2-Prep. Proc Int Soc Magn Reson Med. 2006;14:392. [Google Scholar]
- 19.Rofsky NM, Lee VS, Laub G, et al. Abdominal MR imaging with a volumetric interpolated breath-hold examination. Radiology. 1999;212(3):876–884. doi: 10.1148/radiology.212.3.r99se34876. [DOI] [PubMed] [Google Scholar]
- 20.Nagle SN, Busse RF, Brau AC, et al. High-Resolution Free-Breathing 3D T1 Weighted Hepatobiliary Imaging Optimized for Gd-EOB-DTPA. Proceedings of the Intl Soc Magn Reson Med. 2009;17:2076. [Google Scholar]
- 21.Bashir MR, Merkle EM. Improved liver lesion conspicuity by increasing the flip angle during hepatocyte phase MR imaging. Eur Radiol. 2010;21(2):291–294. doi: 10.1007/s00330-010-1917-1. [DOI] [PubMed] [Google Scholar]
- 22.Frydrychowicz A, Nagle SK, D’Souza SL, Vigen KK, Reeder SB. Optimized high-resolution contrast-enhanced hepatobiliary imaging at 3 tesla: A cross-over comparison of gadobenate dimeglumine and gadoxetic acid. Journal of magnetic resonance imaging : J Magn Reson Imaging. 2011 Jul 12; doi: 10.1002/jmri.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brau AC, Beatty PJ, Skare S, Bammer R. Comparison of reconstruction accuracy and efficiency among autocalibrating data-driven parallel imaging methods. Magn Reson Med. 2008;59(2):382–395. doi: 10.1002/mrm.21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brismar TB, Dahlstrom N, Edsborg N, Persson A, Smedby O, Albiin N. Liver vessel enhancement by Gd-BOPTA and Gd-EOB-DTPA: a comparison in healthy volunteers. Acta Radiol. 2009;50(7):709–715. doi: 10.1080/02841850903055603. [DOI] [PubMed] [Google Scholar]
- 25.Motosugi U, Ichikawa T, Sano K, et al. Double-Dose Gadoxetic Acid-Enhanced Magnetic Resonance Imaging in Patients With Chronic Liver Disease. Invest Radiol. 2010;46(2):141–145. doi: 10.1097/RLI.0b013e3181f9c487. [DOI] [PubMed] [Google Scholar]
- 26.Feuerlein S, Boll DT, Gupta RT, Ringe KI, Marin D, Merkle EM. Gadoxetate disodium-enhanced hepatic MRI: dose-dependent contrast dynamics of hepatic parenchyma and portal vein. AJR Am J Roentgenol. 2011;196(1):W18–24. doi: 10.2214/AJR.10.4387. [DOI] [PubMed] [Google Scholar]