Abstract
Objective
Clinical experience suggests that the majority of schwannomas arise within sensory ganglia, suggesting that intraganglionic glial cells represent a potential cell of origin for schwannomas. To support this clinical impression, we reviewed magnetic resonance imaging (MRI) studies performed over a 5 year period at our institution to determine the relationship of cranial and spinal nerve schwannomas with the ganglia of the associated nerves.
Study design
Retrospective cohort study
Setting
Tertiary referral center
Patients
Patients undergoing imaging study at our institution over a 5 year period.
Intervention(s)
Radiographical images at our institution were reviewed as well as published studies to determine the anatomic location of schwannomas.
Main outcome measure(s)
Anatomical location of schwannomas
Results
A total of 372 patients were found over the 5-year study period, 31 of those were diagnosed with neurofibromatosis type 2 (NF2). Vestibular schwannomas comprised the greatest number of schwannomas, followed by spinal schwannomas. In NF2 patients, spinal schwannomas were the most common tumor, followed by vestibular schwannomas. In NF2 patients and those with sporadic schwannomas, the overwhelming majority of tumors arose in nerves with a sensory component and were associated with sensory ganglia of the nerves (562/607, 92.6%). Very few tumors arose from pure motor nerves. This is supported by review of published articles on anatomic location of schwannomas.
Conclusions
Schwannomas are strongly associated anatomically with ganglia of sensory nerves. These findings raise the possibility that intraganglionic glial cells give rise to the majority of schwannomas.
Keywords: glia, Schwann cell, schwannoma, acoustic neuroma, satellite cells, dorsal root ganglion, ganglia, cell of origin
Introduction
Schwannomas are benign tumors of peripheral nerves that comprise about 7% of intracranial tumors(1) and 20% of spinal tumors(2). The vast majority of intracranial schwannomas arise from the vestibular nerves.
Neurofibromatosis type 2 (NF2) is characterized by multiple schwannomas along with other central nervous system tumors, with the most identifiable feature being bilateral vestibular schwannomas. Sporadic schwannomas and schwannomas in patients with NF2 are indistinguishable histopathologically and both result from inactivating mutations of the NF2 (merlin) tumor suppressor gene. (3,4) Patients with schwannomatosis develop multiple schwannomas but typically lack vestibular schwannomas. Mutation in the chromatin remodeling gene SMARCB1 accounts for 50% of familial forms of schwannomatosis and about 10% of the sporadic form. (5)
Investigation of schwannoma tumorigenesis has mainly focused on Schwann cells (SCs), despite the fact that the peripheral nervous system contains a variety of different glial cells in various stages of differentiation (Fig. 1). (6,7) Peripheral glia cells originate from neural crest cells and have different morphologic and molecular phenotypes. Several studies confirm that schwannoma cells express markers of immature SCs.(8) Yet the specific cells that give rise to schwannomas remain elusive.
Figure 1.
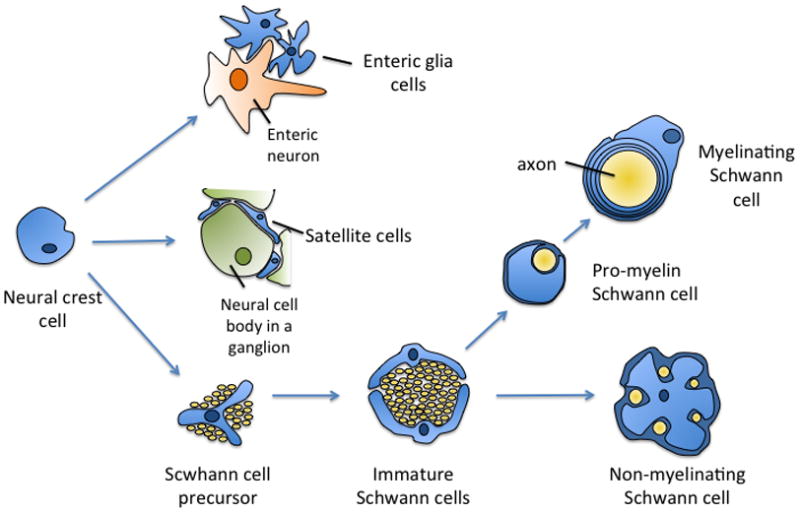
Schematic overview of development of peripheral glia from neural crest cells. Adapted in part from Jessen et al. (6) and Woodhoo et al. (7)
Different peripheral glialcells associate with different regions of neurons (e.g. cell body vs. axon) restricting their localization to discrete anatomic regions. (6) We surmise that the anatomical location of schwannomas provides insight into their pathogenesis including possible candidates for the cell(s) of origin that give rise to schwannomas. Clinical experience, especially in regards to vestibular, facial and lower cranial nerve schwannomas, suggests that the majority of the tumors arise within sensory ganglia. To support this clinical impression, we reviewed magnetic resonance imaging (MRI) studies performed over a 5 year period at our institution to determine the relationship of cranial and spinal nerve schwannomas with the ganglia of the associated nerves. We also reviewed prior publications to likewise determine the relationship of schwannomas with cranial or peripheral nerve ganglia. We report that the vast majority of schwannomas are associated with sensory ganglia and discuss the potential implications of this finding for the cell of origin and treatment strategies of schwannomas.
Materials and methods
Accrual of institutional schwannoma data
The institutional review board of the University of Iowa approved the protocol for this study (ID# 201012711, PI: M. Hansen). This is a retrospective study of patients diagnosed with schwannoma and had radiological imaging in the time period from January 1. 2004 to December 31. 2008. Patients were accrued through a radiological database where reports were searched for the name schwannoma or neuroma. A chart review was performed on all patients to verify the diagnosis by either a re-review of images or if there had been a resection with a pathology report.
We also identified patients that were considered to have NF2 according to clinical criteria. (3,9) The tumors were divided into NF2 associated schwannomas, schwannomatosis associated schwannomas and sporadic schwannomas.
All radiological imaging and operating reports were reviewed and information on location of tumor and whether it was associated with a ganglion of a nerve were recorded. If the relationship of the tumor with the ganglion was not clear in the imaging or operative reports, the images were reviewed (GT, JK, JM); over 70% of images were reviewed. To be considered associated with a ganglion, the normal anatomic location of respective ganglia of cranial and spinal nerves was assessed and if the tumor was in the area of the ganglion, it was considered associated with the ganglion of the nerve.
Literature review
The literature was reviewed regarding anatomic locations of schwannomas. The PubMed database (January 1980 – Feb 2011) was searched with the keywords “schwannoma” or “neuroma”. Associated with the search were names of nerves such as: “spinal”, “peripheral”, “facial”, “glossopharyngeal”, “vagal”, “vestibular”, etc. Studies that were identified with this search were reviewed regarding the content and especially regarding the reporting of the anatomic location of the tumors. Only studies reporting anatomic locations of schwannomas were included. Studies were excluded if there was no information on anatomic location or if schwannomas were mixed with other tumor types or if tumor type was not discernable. Anatomic location of the tumors was noted from the papers and recorded (Table 3).
Table 3.
Relationship of schwannomas* in the literature with sensory nerve ganglia
| Schwannoma location | Tumors at ganglion/total tumors (percent) |
|---|---|
|
| |
| Facial nerve (10–17) | 345/608 (56.7) |
|
| |
| Vestibular nerve (18–22) | 3663/3684 (99.4) |
|
| |
| Cochlear nerve (23–29) | 90/90 (100) |
|
| |
| Neck | |
| X (30–32) | 21/21 (100) |
| CSC (31–35) | 22/22 (100) |
|
| |
| Cranial nerves III/IV/VI (36,37) | −/48 (0) |
|
| |
| Lower cranial nerves | |
| X (30,38) | 10/10 (100) |
| IX (38–40) | 50/50 (100) |
|
| |
| Trigeminal (41–46) | 144/197 (73.1) |
|
| |
| Spinal (47–52) | 693/699 (99.1) |
|
| |
| Peripheral (53–60) | −/495 (0) |
In most studies no differentiation was made between sporadic, neurofibromatosis type 2 and schwannomatosis patients; all are included in these numbers.
Results
Institutional schwannomas
Over the 5-year study period images from a total of 31 patients with NF2 were found. Sporadic schwannomas were much more common with 341 patients identified. No patients with definite diagnosis of schwannomatosis were identified. The results from the review of the sporadic tumors and NF2-associated tumors are presented in Tables 1 and 2, respectively. Vestibular schwannomas comprised the greatest number of schwannomas, followed by spinal schwannomas. In NF2 patients, spinal schwannomas were the most common tumor in our series followed by vestibular schwannomas. In both NF2 patients and those with sporadic schwannomas, the overwhelming majority of tumors, including smaller and larger tumors, arise in nerves with a sensory component and are associated with the sensory ganglia of the nerves (562/607, 92.6%). There was no significant difference in the percent of sporadic or NF2-associated schwannomas that were associated with sensory ganglia (92.2% vs 93.1%, respectively, p=0.657, chi square). Very few tumors arose from pure motor nerves such as cranial nerve III, IV, and VI (2.1% of sporadic and 2.8% of NF2-associated). Figures 2 to 4 illustrate typical imaging findings of schwannomas arising from the spinal and the auditory and vestibular nerves.
Table 1.
Institutional review of sporadic schwannomas and their relationship with ganglia
| Schwannoma location | Number of tumors | Number of tumors with tumor within ganglia (percent) |
|---|---|---|
| Facial nerve | 12 | 10 (83.3) |
| Vestibular nerve | 244 | 244 (100) |
| Cochlear nerve | 3 | 3 (100) |
| Neck | 12 | 11 (91.7) |
| Cranial nerves III/IV/VI | 7 | − (0) |
| Lower cranial nerves | 3 | 3 (100) |
| Trigeminal | 9 | 9 (100) |
| Spinal | 30 | 26 (86.7) |
| Peripheral | 12 | − (0) |
| Total | 332 | 306 (92.2) |
Table 2.
Institutional review of NF2-associated schwannomas and their relationship with ganglia
| Schwannoma location | Number of tumors | Number of tumors with tumor within ganglia (percent) |
|---|---|---|
| Facial nerve | 10 | 10 (100) |
| Vestibular nerve | 58 | 58 (100) |
| Cochlear nerve | 5 | 5 (100) |
| Neck | 3 | 1 (33) |
| Cranial nerves III/IV/VI | 10 | − (0) |
| Lower cranial nerves | 16 | 16 (100) |
| Trigeminal | 17 | 17 (100) |
| Spinal | 149 | 149 (100) |
| Peripheral/soft tissue | 7 | − (0) |
| Total | 275 | 256 (93.1) |
Figure 2.
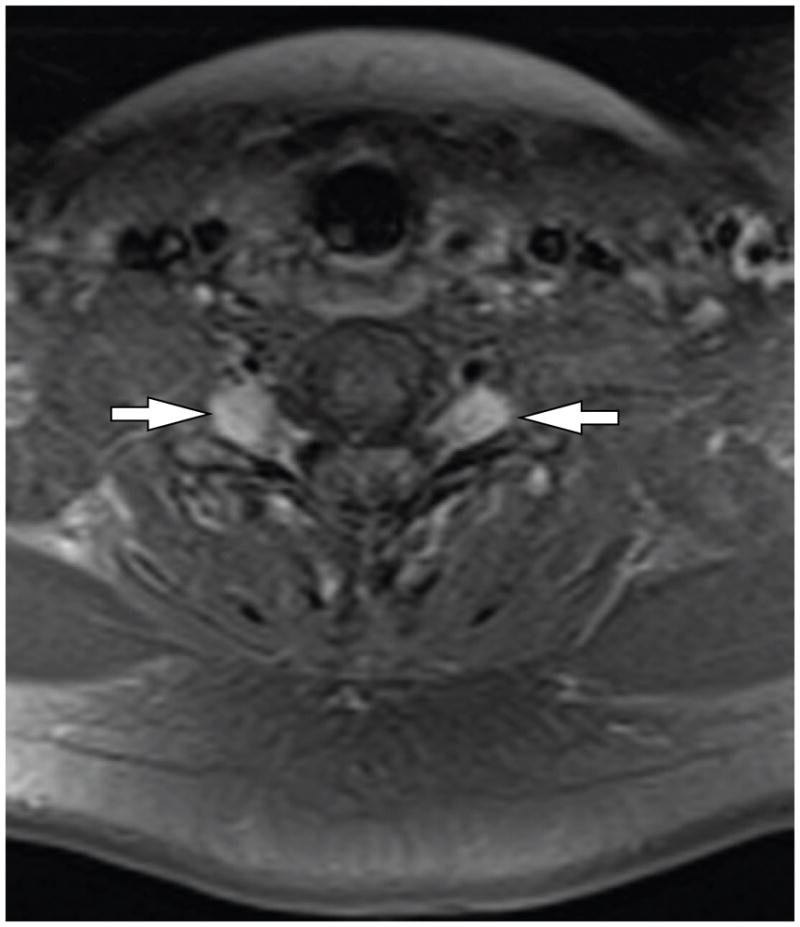
MRI image of bilateral spinal schwannomas in a patient with NF2. Axial T1-weighted MRI following gadolinium enhancement demonstrates bilateral schwannoma arising in association with dorsal root ganglia with tumor extending through neural foramina. Arrows indicate lesions.
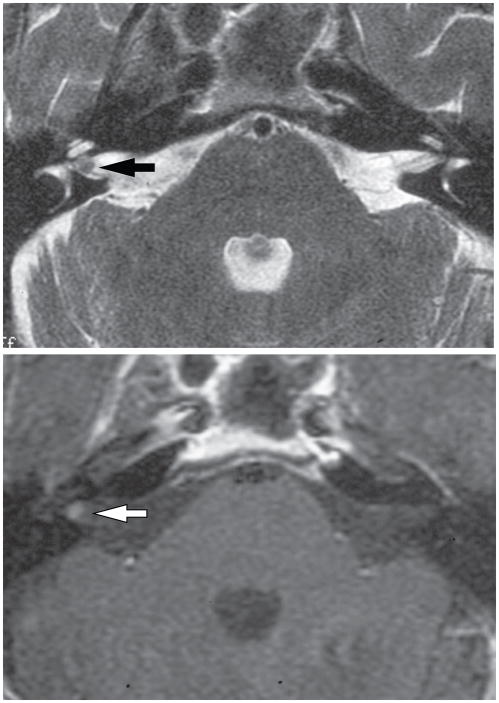
Figure 4.
MRI images of a small vestibular schwannoma. A. T2 weighted CISS sequence showing intracanalicular tumor lateral in the internal auditory canal. B. T1-weighted MRI following gadolinium enhancement demonstrates enhancement of schwannoma. Arrows indicate lesion.
Literature review
Table 3 presents the results from the literature search. These results are likely biased by the tendency to report rare tumors in the literature. Nevertheless, the data show that reports of central schwannomas are much more common than peripheral schwannomas and that schwannomas arise much more commonly in nerves with a sensory component compared to purely motor nerves such as CN III, IV, VI, XI, and XII. Schwannomas associated with these motor nerves are mainly described in isolated case reports given their rarity rather than as large case series.
Discussion
Although schwannomas can potentially arise anywhere along a nerve, experience has lead clinicians to recognize that the location of these tumors is anything but random. Most of these tumors occur in sensory nerves near the sensory ganglia. The data in this study support this clinical impression, that the vast majority of schwannomas of cranial nerves are associated with a ganglion of a sensory nerve. These finding are derived from a radiographic association study and do not offer definitive proof that the majority of schwannomas originate in sensory ganglia. Since many of the tumors were not resected, the diagnosis of schwannoma was made exclusively based on MRI findings, although the diagnosis was universally confirmed to be schwannoma in those specimens for which histological records were available for review. Schwannomas rarely arise from a pure motor nerve or along the myelinated segments of mixed peripheral nerves. Our data also demonstrate that there is no difference in the pattern between sporadic schwannomas and schwannomas associated with NF2. A review of published articles confirms that these findings are comparable across institutions.
Cranial nerve schwannomas
Vestibular schwannoma is the most common intracranial schwannoma. Scarpa’s ganglion, the sensory ganglion of the vestibular nerves, lies in the lateral aspect of the internal auditory canal. (61) Small vestibular schwannomas are found exclusively in the internal auditory canal whereas larger tumors have an intracanalicular component with variable extension into the cerebellopontine angle. Review of the literature with respect to anatomic location of vestibular schwannomas as is seen in table 3 shows that over 99% of tumors have an intracanalicular component. Our review of both sporadic and NF2-associated vestibular schwannomas revealed that all tumors have a component within the internal auditory canal. Histological analysis of small vestibular schwannomas confirms these radiographic findings that vestibular schwannomas arise from cells within or near Scarpa’s ganglion rather than the central glial-Schwann cell junction as was a commonly held belief. (62,63)
Cochlear nerve schwannomas seem to arise almost exclusively within the cochlea, from the spiral ganglion but can also grow into the vestibule and into the fundus of the IAC and beyond. Khurana et al. (64) reported on a very interesting case of cochlear schwannoma illustrating the evolution of a cochlear schwannoma. When the tumor was initially recognized it was confined to the cochlea. Over the course of 12 years the tumor extended into the labyrinth, subsequently into the IAC, and finally developed a large CPA component. Furthermore all of the cochlear schwannomas that we identified radiographically in our institution and in the literature arose within the cochlea where the spiral ganglion lies.
In the past, facial nerve schwannomas have been described as being able to arise randomly along the facial nerve. (65) We found that 20 of 22 facial nerve schwannomas (both sporadic and NF2 related) involved the geniculate ganglion. Older studies show lower percentage of tumors arising from the geniculate but newer studies based on more sensitive MRI techniques confirm that facial nerve schwannomas involve the geniculate in up to 97% of cases. (16) The data here further confirm a predilection for the geniculate ganglion and they often involve multiple segments of the nerve both distal and proximal to the geniculate ganglion.
Trigeminal schwannomas demonstrate a clear predilection for the Gasserian ganglion. Both the results of this study (virtually all tumors) and a literature search show that the large majority of tumors involve the Gasserian ganglion. We did not encounter a trigeminal schwannoma arising exclusively in the extracranial portion of the nerve without involvement of the ganglion.
The lower cranial nerves include cranial nerves IX–XI, which exit the skull through the jugular foramen. The glossopharyngeal nerve has two ganglia that typically lie in the jugular foramen, the vagal nerve has two ganglia, the superior which lies in the jugular foramen and the inferior which lies high in the neck. Almost all of the vagal and glossopharyngeal schwannomas both in our study and in the literature occur in the jugular foramen or high in the neck associated with the ganglia. Song et al. (38) hypothesized that the growth pattern of cranial nerve IX and X schwannomas in the jugular foramen depends on which of the ganglia give rise to the tumor. The findings in this study demonstrating that the vast majority of CN IX and X schwannomas are associated with these ganglia are consistent with this hypothesis. Few cranial nerve XI schwannomas have been described, and we did not identify any in our study. Similarly, few cranial nerve XII schwannomas have been reported.
Spinal nerve and autonomic nervous system schwannomas
The sensory neurons of spinal nerves reside in the dorsal root ganglia in the posterior (dorsal) paraspinal region. Consistent with the pattern of schwannoma involvement in cranial nerves, nearly all spinal nerve schwannomas in our institutional and literature reviews involve the dorsal root ganglia, often creating a dumbbell tumor with intraspinal and extraspinal components. These results further support the notion that schwannomas most often arise from glial cells within the sensory ganglia.
Most sympathetic chain schwannomas are associated with the superior most ganglion. In the literature only 22 sympathetic chain schwannomas that included data on their anatomic location were identified, which shows the rarity of these tumors.
Peripheral schwannomas
These tumors are worth mentioning because they appear to be the exception to the rule. In our material we found 12 schwannomas in the sporadic patient group and 7 in the neurofibromatosis type 2 patient group. These are far fewer than the central schwannomas. A review of the literature shows that these peripheral schwannomas can arise anywhere, in extremities(53), abdomen(56), or thyroid(54). Most of the reports are case reports of unusual tumors in these locations but these are well established, albeit rare, in the extremities and in the abdomen. Thus, not all schwannomas are associated with sensory ganglia suggesting that prototypical, peripheral nerve Schwann cells also give rise to schwannomas.
Implication for schwannoma cell of origin
The fact that schwannomas are almost always associated with sensory ganglia suggests the possibility that glia cells within sensory ganglia give rise to these tumors. Neural crest cells give rise to all glia cells in the peripheral nervous system including Schwann cells, satellite cells and enteric glia cells, thus these glial cells share many common histological features making it difficult to differentiate them histologically. The predominant glial cells in sensory and autonomic ganglia are the satellite cells. Satellite cells associate with the neural cell bodies in the ganglion as opposed to non-myelinating glia cells and Schwann cells, which associate with axons. The propensity of schwannomas to arise within or near sensory ganglia raises the possibility that intraganglionic glial cells, principally the satellite cells, represent a likely candidate for the cell of origin for many schwannnomas. Very few studies have characterized the cellular and molecular properties of these cells.
Implications for treatment
Management options of schwannomas include observation with serial imaging, treatment with microsurgical removal or stereotactic radiosurgery. For those tumors that require treatment, failure to address the associated ganglion may lead to a higher risk of recurrence or eventual tumor growth. For example, in microsurgical management of vestibular schwannomas failure to exposure and remove the schwannoma component within Scarpa’s ganglion is associated with a higher risk of recurrent growth. (66) It is not clear whether the radiation dose to the ganglion influences the response of schwannomas to radiosurgery.
Conclusions
In this review of our clinical material and the literature, we find that the majority of schwannomas associate with ganglia of sensory nerves. These results raise the possibility that intraganglionic glial cells including satellite cells, which represent the principal glia in sensory ganglia, give rise to a high percentage of schwannomas. Further investigation into the cellular and molecular traits of these intraganglionic glial cells is warranted in an effort to understand schwannoma pathogenesis. The association of schwannomas with sensory ganglia also carries implications for treatment strategies including the possibility that failure to adequately address the ganglia may increase the risk of subsequent growth.
Figure 3.
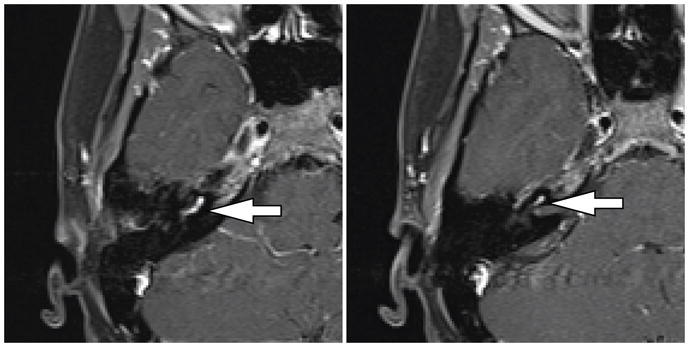
MRI images of cochlear schwannoma. A, B Axail T1-weighted fat-saturated MRI after administration of gadolinium demonstrating enhancement of the cochlear mass.
Acknowledgments
Support: NIDCD R01 DC009801
References
- 1.Walker AE, Robins M, Weinfeld FD. Epidemiology of brain tumors: the national survey of intracranial neoplasms. Neurology. 1985;35:219–26. doi: 10.1212/wnl.35.2.219. [DOI] [PubMed] [Google Scholar]
- 2.Engelhard HH, Villano JL, Porter KR, et al. Clinical presentation, histology, and treatment in 430 patients with primary tumors of the spinal cord, spinal meninges, or cauda equina. J Neurosurg Spine. 2010;13:67–77. doi: 10.3171/2010.3.SPINE09430. [DOI] [PubMed] [Google Scholar]
- 3.Evans DG. Neurofibromatosis type 2 (NF2): a clinical and molecular review. Orphanet J Rare Dis. 2009;4:16. doi: 10.1186/1750-1172-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rouleau GA, Merel P, Lutchman M, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–21. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- 5.Hulsebos TJ, Plomp AS, Wolterman RA, et al. Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am J Hum Genet. 2007;80:805–10. doi: 10.1086/513207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–82. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 7.Woodhoo A, Sommer L. Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia. 2008;56:1481–90. doi: 10.1002/glia.20723. [DOI] [PubMed] [Google Scholar]
- 8.Hansen MR, Roehm PC, Chatterjee P, et al. Constitutive neuregulin-1/ErbB signaling contributes to human vestibular schwannoma proliferation. Glia. 2006;53:593–600. doi: 10.1002/glia.20316. [DOI] [PubMed] [Google Scholar]
- 9.Baser ME, Friedman JM, Wallace AJ, et al. Evaluation of clinical diagnostic criteria for neurofibromatosis 2. Neurology. 2002;59:1759–65. doi: 10.1212/01.wnl.0000035638.74084.f4. [DOI] [PubMed] [Google Scholar]
- 10.McMonagle B, Al-Sanosi A, Croxson G, et al. Facial schwannoma: results of a large case series and review. J Laryngol Otol. 2008;122:1139–50. doi: 10.1017/S0022215107000667. [DOI] [PubMed] [Google Scholar]
- 11.Saleh E, Achilli V, Naguib M, et al. Facial nerve neuromas: diagnosis and management. Am J Otol. 1995;16:521–6. [PubMed] [Google Scholar]
- 12.Falcioni M, Russo A, Taibah A, et al. Facial nerve tumors. Otol Neurotol. 2003;24:942–7. doi: 10.1097/00129492-200311000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Sherman JD, Dagnew E, Pensak ML, et al. Facial nerve neuromas: report of 10 cases and review of the literature. Neurosurgery. 2002;50:450–6. doi: 10.1097/00006123-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Salzman KL, Davidson HC, Harnsberger HR, et al. Dumbbell schwannomas of the internal auditory canal. Am J Neuroradiol. 2001;22:1368–76. [PMC free article] [PubMed] [Google Scholar]
- 15.Wiggins RH, 3rd, Harnsberger HR, Salzman KL, et al. The many faces of facial nerve schwannoma. Am J Neuroradiol. 2006;27:694–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson AL, Aviv RI, Chen JM, et al. Magnetic resonance imaging of facial nerve schwannoma. Laryngoscope. 2009;119:2428–36. doi: 10.1002/lary.20644. [DOI] [PubMed] [Google Scholar]
- 17.Perez R, Chen JM, Nedzelski JM. Intratemporal facial nerve schwannoma: a management dilemma. Otol Neurotol. 2005;26:121–6. doi: 10.1097/00129492-200501000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Strauss C, Bischoff B, Romstock J, et al. Hearing preservation in medial vestibular schwannomas. J Neurosurg. 2008;109:70–6. doi: 10.3171/JNS/2008/109/7/0070. [DOI] [PubMed] [Google Scholar]
- 19.Inamasu J, Shiobara R, Kagami H, et al. Medial (intra-cisternal) acoustic neuromas. Acta Otolaryngol. 2000;120:623–6. doi: 10.1080/000164800750000441. [DOI] [PubMed] [Google Scholar]
- 20.Tos M, Drozdziewicz D, Thomsen J. Medial acoustic neuromas. A new clinical entity. Arch Otolaryngol Head Neck Surg. 1992;118:127–33. doi: 10.1001/archotol.1992.01880020019009. [DOI] [PubMed] [Google Scholar]
- 21.Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery. 1997;40:1–9. doi: 10.1097/00006123-199701000-00001. discussion -10. [DOI] [PubMed] [Google Scholar]
- 22.Stangerup SE, Caye-Thomasen P, Tos M, et al. The natural history of vestibular schwannoma. Otol Neurotol. 2006;27:547–52. doi: 10.1097/01.mao.0000217356.73463.e7. [DOI] [PubMed] [Google Scholar]
- 23.Grayeli AB, Fond C, Kalamarides M, et al. Diagnosis and management of intracochlear schwannomas. Otol Neurotol. 2007;28:951–7. doi: 10.1097/MAO.0b013e3181514485. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy RJ, Shelton C, Salzman KL, et al. Intralabyrinthine schwannomas: diagnosis, management, and a new classification system. Otol Neurotol. 2004;25:160–7. doi: 10.1097/00129492-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Falcioni M, Taibah A, Di Trapani G, et al. Inner ear extension of vestibular schwannomas. Laryngoscope. 2003;113:1605–8. doi: 10.1097/00005537-200309000-00037. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura T, Hosoi H. Progressive hearing loss in intracochlear schwannoma. Eur Arch Otorhinolaryngol. 2008;265:489–92. doi: 10.1007/s00405-007-0483-x. [DOI] [PubMed] [Google Scholar]
- 27.Miller ME, Moriarty JM, Linetsky M, et al. Intracochlear schwannoma presenting as diffuse cochlear enhancement: diagnostic challenges of a rare cause of deafness. Ir J Med Sci. 2010 doi: 10.1007/s11845-010-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soler YC, Fayad JN. Intracochlear schwannomas. Otol Neurotol. 2007;28:142. doi: 10.1097/01.mao.0000235963.22980.7a. [DOI] [PubMed] [Google Scholar]
- 29.Neff BA, Willcox TO, Jr, Sataloff RT. Intralabyrinthine schwannomas. Otol Neurotol. 2003;24:299–307. doi: 10.1097/00129492-200303000-00028. [DOI] [PubMed] [Google Scholar]
- 30.Green JD, Jr, Olsen KD, DeSanto LW, et al. Neoplasms of the vagus nerve. Laryngoscope. 1988;98:648–54. doi: 10.1288/00005537-198806000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Saito DM, Glastonbury CM, El-Sayed IH, et al. Parapharyngeal space schwannomas: preoperative imaging determination of the nerve of origin. Arch Otolaryngol Head Neck Surg. 2007;133:662–7. doi: 10.1001/archotol.133.7.662. [DOI] [PubMed] [Google Scholar]
- 32.Guerrissi JO. Solitary benign schwannomas in major nerve systems of the head and neck. J Craniofac Surg. 2009;20:957–61. doi: 10.1097/SCS.0b013e3181a14cbc. [DOI] [PubMed] [Google Scholar]
- 33.Lin CC, Wang CC, Liu SA, et al. Cervical sympathetic chain schwannoma. J Formos Med Assoc. 2007;106:956–60. doi: 10.1016/S0929-6646(08)60067-4. [DOI] [PubMed] [Google Scholar]
- 34.Anil G, Tan TY. Imaging characteristics of schwannoma of the cervical sympathetic chain: a review of 12 cases. Am J Neuroradiol. 2010;31:1408–12. doi: 10.3174/ajnr.A2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang CP, Hsiao JK, Ko JY. Splaying of the carotid bifurcation caused by a cervical sympathetic chain schwannoma. Ann Otol Rhinol Laryngol. 2004;113:696–9. doi: 10.1177/000348940411300904. [DOI] [PubMed] [Google Scholar]
- 36.Hatakeyama H, Saito K, Nagatani T, et al. Schwannoma in the crural cistern removed without permanent functional deficits--case report. Neurol Med Chir (Tokyo) 2003;43:95–9. doi: 10.2176/nmc.43.95. [DOI] [PubMed] [Google Scholar]
- 37.Vachata P, Sames M. Abducens nerve schwannoma mimicking intrinsic brainstem tumor. Acta neurochirurgica. 2009;151:1281–7. doi: 10.1007/s00701-009-0302-9. [DOI] [PubMed] [Google Scholar]
- 38.Song MH, Lee HY, Jeon JS, et al. Jugular foramen schwannoma: analysis on its origin and location. Otol Neurotol. 2008;29:387–91. doi: 10.1097/MAO.0b013e318164cb83. [DOI] [PubMed] [Google Scholar]
- 39.Vorasubin N, Sang UH, Mafee M, et al. Glossopharyngeal schwannomas: a 100 year review. Laryngoscope. 2009;119:26–35. doi: 10.1002/lary.20045. [DOI] [PubMed] [Google Scholar]
- 40.Sarma S, Sekhar LN, Schessel DA. Nonvestibular schwannomas of the brain: a 7- year experience. Neurosurgery. 2002;50:437–48. doi: 10.1097/00006123-200203000-00002. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 41.Fukaya R, Yoshida K, Ohira T, et al. Trigeminal schwannomas: experience with 57 cases and a review of the literature. Neurosurg Rev. 2010;34:159–71. doi: 10.1007/s10143-010-0289-y. [DOI] [PubMed] [Google Scholar]
- 42.Hasegawa T, Kida Y, Yoshimoto M, et al. Trigeminal schwannomas: results of gamma knife surgery in 37 cases. J Neurosurg. 2007;106:18–23. doi: 10.3171/jns.2007.106.1.18. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Yang Y, Xu S, et al. Trigeminal schwannomas: a report of 42 cases and review of the relevant surgical approaches. Clin Neurol Neurosurg. 2009;111:261–9. doi: 10.1016/j.clineuro.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Al-Mefty O, Ayoubi S, Gaber E. Trigeminal schwannomas: removal of dumbbell-shaped tumors through the expanded Meckel cave and outcomes of cranial nerve function. J Neurosurg. 2002;96:453–63. doi: 10.3171/jns.2002.96.3.0453. [DOI] [PubMed] [Google Scholar]
- 45.Ramina R, Mattei TA, Soria MG, et al. Surgical management of trigeminal schwannomas. Neurosurg Focus. 2008;25:E6. doi: 10.3171/FOC.2008.25.12.E6. discussion E. [DOI] [PubMed] [Google Scholar]
- 46.Guthikonda B, Theodosopoulos PV, van Loveren H, et al. Evolution in the assessment and management of trigeminal schwannoma. Laryngoscope. 2008;118:195–203. doi: 10.1097/MLG.0b013e3181596091. [DOI] [PubMed] [Google Scholar]
- 47.Conti P, Pansini G, Mouchaty H, et al. Spinal neurinomas: retrospective analysis and long-term outcome of 179 consecutively operated cases and review of the literature. Surg Neurol. 2004;61:34–43. doi: 10.1016/s0090-3019(03)00537-8. discussion 4. [DOI] [PubMed] [Google Scholar]
- 48.Seppala MT, Haltia MJ, Sankila RJ, et al. Long-term outcome after removal of spinal schwannoma: a clinicopathological study of 187 cases. J Neurosurg. 1995;83:621–6. doi: 10.3171/jns.1995.83.4.0621. [DOI] [PubMed] [Google Scholar]
- 49.Jeon JH, Hwang HS, Jeong JH, et al. Spinal schwannoma; analysis of 40 cases. J Korean Neurosurg Soc. 2008;43:135–8. doi: 10.3340/jkns.2008.43.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jinnai T, Koyama T. Clinical characteristics of spinal nerve sheath tumors: analysis of 149 cases. Neurosurgery. 2005;56:510–5. doi: 10.1227/01.neu.0000153752.59565.bb. discussion -5. [DOI] [PubMed] [Google Scholar]
- 51.Hori T, Takakura K, Sano K. Spinal neurinomas--clinical analysis of 45 surgical cases. Neurol Med Chir (Tokyo) 1984;24:471–7. doi: 10.2176/nmc.24.471. [DOI] [PubMed] [Google Scholar]
- 52.Safavi-Abbasi S, Senoglu M, Theodore N, et al. Microsurgical management of spinal schwannomas: evaluation of 128 cases. Journal of neurosurgery Spine. 2008;9:40–7. doi: 10.3171/SPI/2008/9/7/040. [DOI] [PubMed] [Google Scholar]
- 53.Carvajal JA, Cuartas E, Qadir R, et al. Peripheral nerve sheath tumors of the foot and ankle. Foot Ankle Int. 2011;32:163–7. doi: 10.3113/FAI.2011.0163. [DOI] [PubMed] [Google Scholar]
- 54.Kandil E, Abdel Khalek M, Abdullah O, et al. Primary peripheral nerve sheath tumors of the thyroid gland. Thyroid. 2010;20:583–6. doi: 10.1089/thy.2009.0245. [DOI] [PubMed] [Google Scholar]
- 55.Levi AD, Ross AL, Cuartas E, et al. The surgical management of symptomatic peripheral nerve sheath tumors. Neurosurgery. 2010;66:833–40. doi: 10.1227/01.NEU.0000367636.91555.70. [DOI] [PubMed] [Google Scholar]
- 56.Agaimy A, Markl B, Kitz J, et al. Peripheral nerve sheath tumors of the gastrointestinal tract: a multicenter study of 58 patients including NF1-associated gastric schwannoma and unusual morphologic variants. Virchows Arch. 2010;456:411–22. doi: 10.1007/s00428-010-0886-8. [DOI] [PubMed] [Google Scholar]
- 57.Artico M, Cervoni L, Wierzbicki V, et al. Benign neural sheath tumours of major nerves: characteristics in 119 surgical cases. Acta neurochirurgica. 1997;139:1108–16. doi: 10.1007/BF01410969. [DOI] [PubMed] [Google Scholar]
- 58.Furniss D, Swan MC, Morritt DG, et al. A 10-year review of benign and malignant peripheral nerve sheath tumors in a single center: clinical and radiographic features can help to differentiate benign from malignant lesions. Plast Reconstr Surg. 2008;121:529–33. doi: 10.1097/01.prs.0000297636.93164.cb. [DOI] [PubMed] [Google Scholar]
- 59.Kang HJ, Shin SJ, Kang ES. Schwannomas of the upper extremity. J Hand Surg Br. 2000;25:604–7. doi: 10.1054/jhsb.2000.0472. [DOI] [PubMed] [Google Scholar]
- 60.Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246–55. doi: 10.3171/jns.2005.102.2.0246. [DOI] [PubMed] [Google Scholar]
- 61.Casselman J, Mermuys K, Delanote J, et al. MRI of the cranial nerves--more than meets the eye: technical considerations and advanced anatomy. Neuroimaging Clin N Am. 2008;18:197–231. doi: 10.1016/j.nic.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Sterkers JM, Perre J, Viala P, et al. The origin of acoustic neuromas. Acta Otolaryngol. 1987;103:427–31. [PubMed] [Google Scholar]
- 63.Roosli C, Linthicum FH, Jr, Cureoglu S, et al. What is the site of origin of cochleovestibular schwannomas? Audiology & neuro-otology. 2012;17:121–5. doi: 10.1159/000331394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khurana VG, Link MJ, Driscoll CL, et al. Evolution of a cochlear schwannoma on clinical and neuroimaging studies. Case report J Neurosurg. 2003;99:779–82. doi: 10.3171/jns.2003.99.4.0779. [DOI] [PubMed] [Google Scholar]
- 65.O’Donoghue GM, Brackmann DE, House JW, et al. Neuromas of the facial nerve. Am J Otol. 1989;10:49–54. [PubMed] [Google Scholar]
- 66.Linthicum FH, Jr, Saleh ES, Hitselberger WE, et al. Growth of postoperative remnants of unilateral vestibular nerve schwannoma: role of the vestibular ganglion. ORL J Otorhinolaryngol Relat Spec. 2002;64:138–42. doi: 10.1159/000057793. [DOI] [PubMed] [Google Scholar]