Abstract
Aims
Stress Urinary Incontinence (SUI) affects women both acutely and chronically after vaginal delivery. Current SUI treatments assume the neuromuscular continence mechanism, comprised of the pudendal nerve (PN) and external urethral sphincter (EUS), is either intact or irreparable. This study investigated the ability of neurotrophin therapy to facilitate recovery of the neuromuscular continence mechanism.
Methods
Virgin, Sprague Dawley rats received simulated childbirth injury or sham injury and treatment with continuous infusion of brain derived neurotrophic factor (BDNF) or saline placebo to the site of PN injury. Continence was assessed by leak point pressure (LPP) and EUS electromyography (EMG) 14 and 21 days after injury. Structural recovery was assessed histologically. Molecular assessment of the muscular and neuroregenerative response was determined via measurement of EUS BDNF and PN βII-tubulin expression respectively, 4, 8, and 12 days after injury.
Results
Following injury, LPP was significantly reduced with saline compared to either BDNF treatment or sham injury. Similarly, compared to sham injury, resting EUS EMG amplitude and firing rate, as well as amplitude during LPP were significantly reduced with saline but not BDNF treatment. Histology confirmed improved EUS recovery with BDNF treatment. EUS BDNF and PN βII-tubulin expression demonstrated that BDNF treatment improved the neurogenerative response and may facilitate sphincteric recovery.
Conclusions
Continuous targeted neurotrophin therapy accelerates continence recovery after simulated childbirth injury likely through stimulating neuroregeneration and facilitating EUS recovery and re-innervation. Neurotrophins or other therapies targeting neuromuscular regeneration may be useful for treating SUI related to failure of the neuromuscular continence mechanism.
Keywords: Urinary Incontinence, Stress, Vaginal Delivery, Childbirth Injury, Pudendal Nerve, Neurotrophin
Introduction
Approximately 25%–46% of women suffer from urinary incontinence, with stress urinary incontinence (SUI) 250% more prevalent amongst women who experienced vaginal childbirth; a process associated with damage to pelvic floor tissues(1,2). Numerous surgical procedures and symptom management techniques for SUI exist, but none target its underlying neuromuscular pathophysiology(3,4). Current therapies assume the neuromuscular continence mechanism is either intact (i.e. pelvic floor physiotherapy or pharmacotherapy) or irreparable (i.e. urethral slings or bulking injections).
Animal models of vaginal birth demonstrate that skeletal muscle of the external urethral sphincter (EUS) is injured during simulated parturition(5). Injury to the pudendal nerve (PN), which innervates the EUS, has also been observed clinically and in animal models(6,7). Thus, childbirth inflicts a double insult to the neuromuscular urinary continence mechanism: damage to the muscles responsible for preventing leakage and interruption of the nerve supply controlling them. PN damage likely contributes appreciably to the pathogenesis of SUI, not only via directly impairing muscular function but also through limiting EUS recovery because of persistent denervation(8).
Following peripheral nerve injury, upregulation of brain-derived neurotrophic factor (BDNF) occurs in innervated target organs and axons distal to injury sites(9). BDNF is required for neuroregeneration, and local administration to nerve injury sites reduces damage-induced motoneuron death(10). In contrast, BDNF is inhibitory to and thus reduced during myogenic myoblast differentiation and both neuromuscular junction (NMJ) formation and restoration(11,12). The concurrent injury of EUS muscle and the PN during childbirth results in EUS BDNF downregulation that should facilitate sphincter muscle and NMJ recovery but likely impairs the PN neuroregenerative response since isolated PN crush without EUS injury causes BDNF upregulation, which is required for neuroregeneration(13,14).
EUS electromyography (EMG) and PN electroneurography (ENG), as well as urinary leak point pressure (LPP), show slowed recovery when the EUS and PN are simultaneously injured compared to either injury in isolation(13,14). A more severe loss of NMJ integrity at the EUS has also been demonstrated in this combined injury model(15). Therefore, exogenous BDNF may overcome the muscle injury-induced BDNF downregulation in the EUS and improve both PN recovery and EUS re-innervation, leading to improved continence following childbirth. This study aimed to determine if local continuous administration of BDNF improves the regenerative response of the PN as well as both structural and functional recovery of the EUS following simulated childbirth injury.
Materials and Methods
All experiments were pre-approved by the local Institutional Animal Care and Use Committee. Female, virgin, Sprague-Dawley rats (200–225g) were separated into 3 groups. The first (N=32) received vaginal distention (VD) and PN crush followed immediately by 4–21 days of BDNF treatment. The second (N=31) received identical injuries but was treated with a saline (containing albumin) placebo. The third (N=30) underwent sham PN crush and received no treatment. Childbirth injuries were done as previously described, using intraperitoneal ketamine (100mg/kg) and xylene (10mg/kg) or inhaled isofluroane anesthesia for functional and molecular analyses, respectively(14). Briefly, VD was created using a de-tipped Foley catheter filled with 3ml water and secured in the vagina with a 3-0 vicryl suture through the labia majora for 4 hours. Bilateral PNC was performed subsequently by dorsally isolating the PN in the ischiorectal fossa and crushing it with a Castro-Viejo needle holder twice, consecutively for 30 seconds each. PN isolation alone was performed for sham injury.
Treatment was provided using bilateral, dorsally placed, subcutaneous miniature-osmotic pumps with vinyl catheters (V/3A, Durect) to the PN injury site and secured to nearby paraspinal musculature with 5-0 prolene suture. Following an overnight incubation in 37°C physiologic saline, pumps provided a constant 0.5μl/hr efflux for 1 (Alzet Mini-Osmotic Pump, Model 1007D, Durect) or 2 (Model 2002) weeks. The albumin solution consisted of 0.25g albumin (Albumin from Rat Serum, A6414, Sigma-Aldrich) dissolved in 100ml sterile saline. This both acted as placebo treatment and served to stabilize the BDNF solution, which was created by adding 1mg BDNF (Recombinant Human BDNF, CYT-207, ProSpec) to 6ml of solution. Overall, this provided the injury site a local, targeted BDNF dose of 2μg/day.
Continuous EUS EMG was recorded during LPP 2 and 3 weeks after injury and treatment initiation, as described previously(13). Specifically, via pubic symphysectomy, the EUS was revealed and caudal extension of the incision exposed the bladder. A plane was then developed laterally to expose the right PN in the ischiorectal fossa and catheter placement and patency were confirmed. Next, polyethylene tubing was placed transurethrally into the bladder and platinum bipolar parallel rod electrodes rested ventrally on the EUS for EMG recording. Direct bladder compression was performed using a cotton-tipped applicator that was rapidly removed when leakage at the urethral meatus occurred. One second segments of electrophysiological and bladder pressure recordings from baseline and at LPP were analyzed using previously described techniques(13). Mean EUS EMG amplitude and firing rate, as well as mean bladder pressures were calculated for each animal from a minimum of 3 separate LPP maneuvers.
Spinal cord and urethra were procured from 5 animals in each of the 3 injury and treatment groups 4, 8, and 12 days after injury and treatment initiation. Following ketamine-xylazine anesthesia, sternotomy and intracardiac perfusion of heprinated, phosphate-buffered saline was performed. After an L2-S3 laminectomy, the spinal cord was frozen in-situ with liquid nitrogen and sharply transected at L3 and S2(16). The isolated segment was placed into a pre-cooled cryotube and stored in liquid nitrogen until embedded (Tissue-Tek OCT Compound, 4583OCT, Sakura Finetek) for cryostat sectioning. The anterior vaginal wall, urethra, and bladder neck were then removed en bloc after symphysectomy, embedded, flash frozen, and stored at −80°C. Cryostat sectioning of both the spinal cord (12μm) at L6 containing Onuf’s Nucleus (ON) and urogenital tissues at the EUS was performed. Sections were collected on alternating standard glass slides and laser microdissection (LMD) slides (Frame Slides PET-Membrane, 11505151, Leica Microsystems).
Thionin staining and ethanol-xylene fixation identified PN cell bodies that were collected via isolating the ON region using LMD. Striated EUS muscle was collected similarly. Collected cells were lysed and ribonucleic acid (RNA) was isolated (RNAqueous-Micro, Applied Biosystems) for reverse transcription (High Capacity cDNA Reverse Transcription Kit, Applied Biosystems) into complimentary deoxyribonucleic acid (cDNA) using pre-packaged kits. Polymerase chain reaction (PCR) pre-amplification (TaqMan PreAmp Master Mix, Applied Biosystems) specific for βII-tubulin (TaqMan Gene Expression Assay, Rn01435557_g1, Applied Biosystems) in PN samples or BDNF (TaqMan Gene Expression Assay, Rn02531967_s1, Applied Biosystems) in EUS samples was performed. Using standard reagents (TaqMan Gene Expression Master Mix, Applied Biosystems) quantitative-PCR (Q-PCR) normalized to 18S ribosomal RNA (TaqMan Gene Expression Assay, Rn03928990_g1, Applied Biosystems) was conducted to assess βII-tubulin expression in ON and BDNF in the EUS relative to that in the sham-injured group 12 days after injury.
Glass slides were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 minutes and then washed 3 times with PBS. Tissues were blocked using standard universal blocking buffer for 30 minutes and then exposed to primary antibodies at 4°C overnight, targeting BDNF (1:50 Rabbit Polyclonal BDNF, SC546, Santa Cruz Biotechnology) in the EUS and βII-tubulin (1:50 Mouse Monoclonal βII-tubulin, SC47751, Santa Cruz Biotechnology) along with neurofilament (1:50 Rabbit Polyclonal Neurofilament, N4142, Sigma-Aldrich) in PN sections. Following 3 PBS washes, slides were exposed to secondary antibodies (1:400 AlexaFluro 488 Donkey anti-Mouse, A21202, or 1:400 AlexaFluro 488 Donkey anti-Rabbit, A21206, Invitrogen), with an additional antibody (1:1,200 DyLight 594 Goat Anti-Rabbit, 111-515-144, Thermo Scientific) used for PN sections. After 3 more PBS washes, slides underwent anti-fade (ProLong Gold, P36930, Invitrogen) treatment. Additional glass slides were fixed in Bouin’s solution and stained with Masson’s trichrome. Images were acquired with standard ultraviolet immunofluorescence and light microscopy.
Statistical evaluations (SigmaStat, Version 10.0, Systat Software) of normally distributed functional outcomes were performed using a standard One-Way ANOVA followed by Student-Newman-Keuls pairwise comparisons. A Kruskal-Wallis One-Way ANOVA on Ranks followed by Dunn’s pairwise comparisons were used for non-normally distributed data. Molecular expression data were analyzed using a Two-Way ANOVA followed by Holm-Sidak multiple pairwise comparisons. For all analyses, p<0.05 indicated a statistically significant difference between groups. Data is presented as mean ± standard error of the mean. Images were assessed qualitatively by a blinded observer.
Results
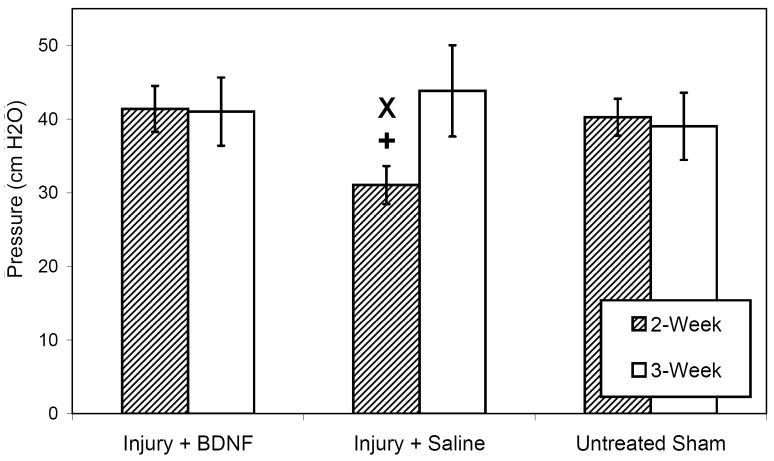
No adverse effects appeared to be associated with BDNF treatment; however, a number of rats across all experimental groups received overly-potent ketamine, which was subsequently recalled, and were appropriately excluded from analyses and euthanized due to auto-amputation of forelimb digits and corneal ulcerations. Animals treated with saline demonstrated significantly decreased mean LPP 2 weeks after injury compared to both untreated sham-injured (p=0.032) and BDNF-treated (p=0.033) animals (Fig 1). No significant differences in mean LPP were noted 3 weeks after injury. Baseline bladder pressures did not significantly differ at any time.
Figure 1.
Leak point pressure in all injury-treatment groups between 2 and 3 weeks as indicated in the legend. Results shown are the means and standard errors of data from 6 to 11 animals. Each symbol represents statistical significance within the same time point as follows: x indicates a statistically significant difference from the sham injury group, while + designates a statistically significant difference from the BDNF treatment cohort.
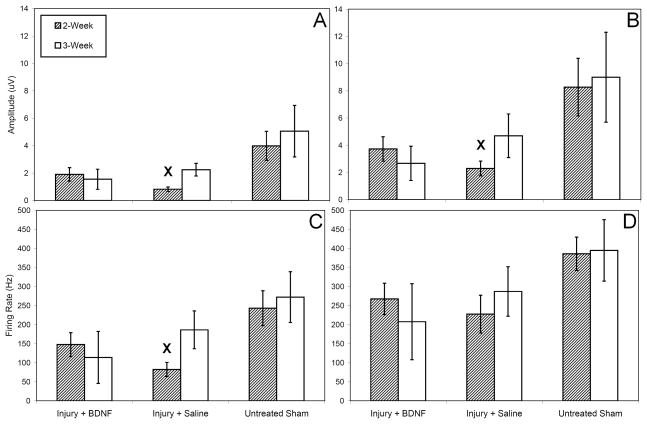
EMG firing patterns were qualitatively similar to those presented by Jiang et al. with regard to trends of the electrophysiologic activity and intermittent bursting during spontaneous voiding(13,17). Saline treatment resulted in significantly lower median resting EUS EMG amplitude 2 weeks after injury compared to sham injury (p<0.05) while BDNF treatment did not (Fig 2A). Similarly, 2 weeks after injury median EUS EMG amplitude at LPP was significantly reduced with saline treatment compared to sham injury (p<0.05) but not with BDNF treatment (Fig 2B). Three weeks after injury, no significant differences existed in mean EUS EMG amplitude at rest or during LPP. Saline treatment resulted in significantly reduced mean resting EUS EMG firing rate 2 weeks after injury compared to sham injury (p=0.006) while BDNF treatment did not (Fig 2C). No significant differences in mean EUS EMG firing rate at LPP existed 2 weeks after injury (Fig 2D). Three weeks after injury, no significant differences existed in mean EUS EMG firing rate at rest or during LPP.
Figure 2.
External urethral sphincter electrophysiological data in all injury-treatment groups between 2 and 3 weeks as indicated in the legend: amplitude at baseline (A) and during leak point pressure (B) and firing rate at baseline (C) and during leak point pressure (D). Within the same time point, x designates statistical significance of a difference from the sham injury group. Results shown are the means and standard errors of data from 6 to 11 animals.
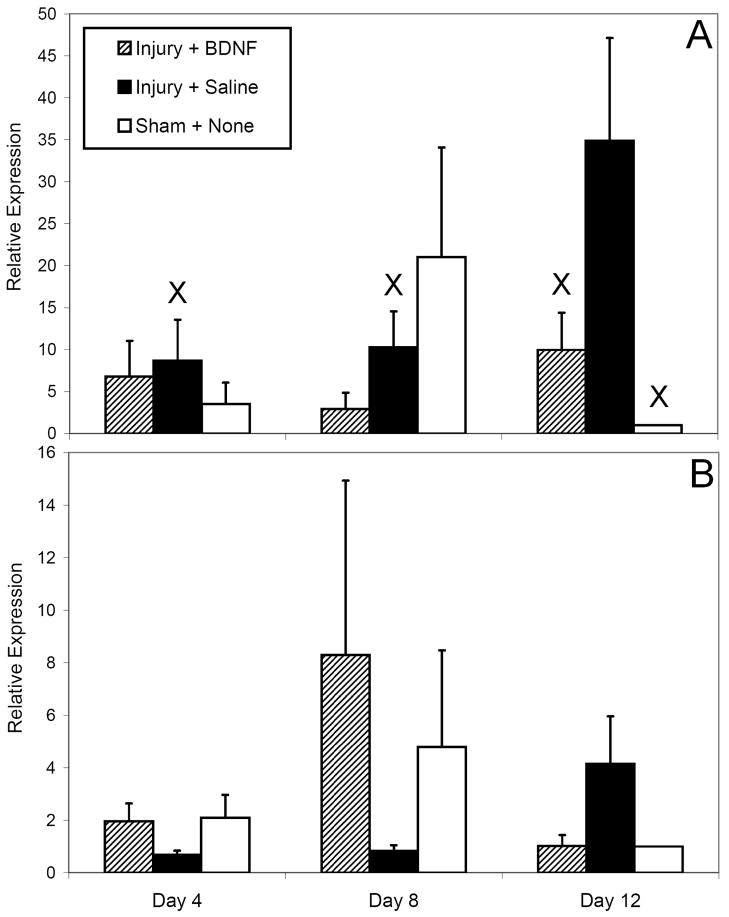
No significant differences in EUS BDNF expression were noted between groups at 4 or 8 days after injury (Fig 3A). Significantly higher EUS BDNF expression occurred 12 days after injury with saline treatment compared to BDNF treatment (p=0.007) or sham injury (p = 0.001); however, the latter was the basis of relative PCR comparisons and may be artifactual. Significantly less BDNF expression occurred with saline treatment 4 (p=0.005) and 8 (p=0.011) days after injury compared to 12 days after injury. Levels of EUS BDNF expression with PN BDNF treatment appeared relatively stable, while in sham-injured animals a transient increase in BDNF occurred, although these observations were not statistically significant.
Figure 3.
Relative gene expression levels, as assessed by quantitative PCR in all injury-treatment groups 4, 8, and 12 days after injury and treatment initiation as indicated in the legend: external urethral sphincter BDNF expression (A) and pudendal nerve βII-tubulin expression (B). Results shown are the means and standard errors of data from 3 to 5 animals. Irrespective of time point, x designates statistical significance of a difference from the sham injury group 12 days after injury.
No significant differences in PN βII-tubulin expression were detected (Fig 3B). However, saline treatment appeared to reduce βII-tubulin expression 4 and 8 days after injury compared to either BDNF treatment or sham injury, although non-significantly. A transient increase in PN βII-tubulin expression 8 days after injury appeared to occur with BDNF treatment compared to sham injury, but was not statistically significant.
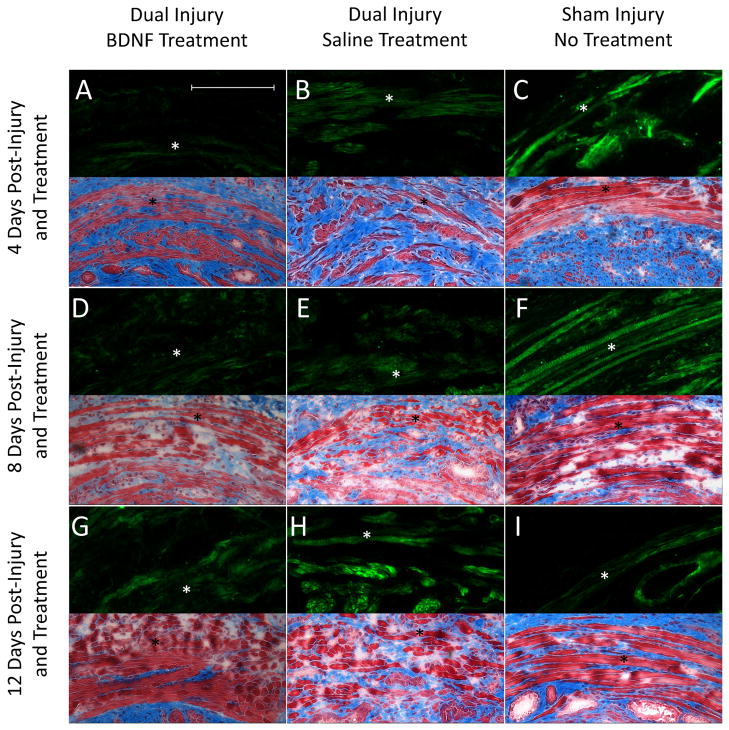
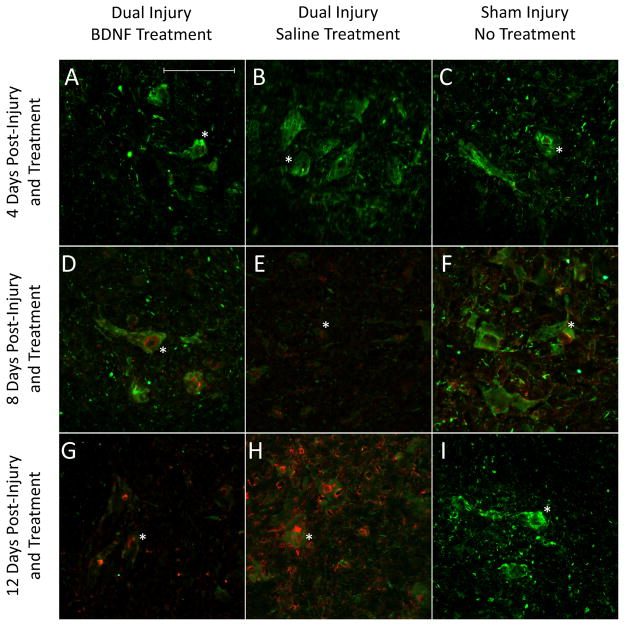
Sham-injured animals demonstrated normal appearing EUS histology (Fig 4). Less EUS atrophy, disruption, and fibrosis 4 and 8 days after injury were observed with BDNF compared to saline treatment. The EUS in BDNF-treated animals more closely resembled those of sham-injured animals 12 days after injury compared to those from of saline-treated animals. Immunofluorescence demonstrated a consistent pattern 4 and 8 days after injury with sham-injured rats expressing more EUS BDNF than injured animals treated with saline, which expressed more EUS BDNF than injured animals treated with BDNF (Fig 4). Injured animals treated with saline expressed the most EUS BDNF 12 days after injury, while sham-injured animals expressed the least. Immunofluorescence demonstrated more PN βII-tubulin 4 days after injury with BDNF treatment than either saline treatment or sham injury (Fig 5). BDNF-treated animals demonstrated the most βII-tubulin 8 days after injury, while saline-treated animals expressed the least. BDNF-treated animals expressed less βII-tubulin than saline-treated animals 12 day after injury, but more than to sham-injured animals (Fig 5).
Figure 4.
Representative immunofluorescence (upper half) and histology images (lower half) of the external urethral sphincter, indicated by the star, from all injury-treatment groups 4, 8, and 12 days after injury and treatment initiation. Immunofluorescence highlights BDNF (green) protein, while Masson’s trichrome histology illustrates muscle (red) and fibrosis (blue) within the sphincter. The scale bar represents approximately 1,000μm for all images.
Figure 5.
Representative immunofluorescence images of pudendal nerve motor nuclei from all injury-treatment groups 4, 8, and 12 days after injury and treatment initiation. Immunofluorescence highlights neurofilament (green) comprising the cell bodies and βII-tubulin (red) protein within them. The scale bar represents approximately 1,000μm for all images.
Discussion
SUI was estimated to cost the US over $19.5 billion in 2000, with the burden expected to increase as the population ages(18). Since postpartum incontinence is associated with a 2.4-fold greater likelihood of chronic SUI development, effectively treating and preventing this condition could provide substantial benefits(2). Mechanical SUI etiologies, including urethral hypermobility and mild pelvic organ prolapse, are amenable to surgical correction(4). However, sphincteric deficiency, whether from direct injury to the sphincter or its innervation, is managed sub-optimally with surgery, bulking injections, and physiotherapy(3). Regenerative treatments, such as stem cells, may repair sphincteric injury(19). However, in the presence of PN injury, targeting the nerve for regeneration is likely more beneficial than improving bulk in a denervated sphincter. Therefore, this investigation aimed to improve PN recovery to facilitate restoration of EUS function after simulated childbirth injury.
Neurotrophins, including BDNF, are necessary for the maintenance of innervation, normal neuromuscular function, and nerve regrowth(20). They are produced by myelinating cells and innervated target organs and are upregulated after nerve injury(9). Neurotrophin treatment improved functional and anatomic recovery in peripheral nerve injury models(21,22). The same rodent model used in this study demonstrated EUS neurotrophin upregulation following isolated PN crush(14). Therefore, BDNF treatment likely could improve functional recovery of the neuromuscular continence mechanism after PN injury. To date, localized continuous neurotrophin supplementation has been most effective at promoting nerve regeneration and provided the rationale behind utilizing miniature osmotic pumps in this study(20).
Despite facilitating regeneration of peripheral nerves, BDNF is detrimental to muscular recovery and development since it impairs NMJ formation as well as myogenic myoblast differentiation and maturation(11,12). Childbirth injures both the PN and EUS, as well as their NMJ, which together comprise the neuromuscular continence mechanism(5,14,15). In this animal model, BDNF downregulation has been observed after EUS injury, supporting the hypothesis it may hinder muscular recovery(12,14). Therefore, it is imperative that BDNF treatment intended to improve PN neuroregeneration neither impairs EUS nor NMJ recovery, providing rationale for the targeted delivery in this study.
Previously, the same combined PNC-VD model demonstrated LPP recovery 3 weeks after injury, similar to saline-treated animals in this study(14). In comparison, LPP recovered 10 days after isolated VD and 14 days after isolated PN crush, suggesting that combined EUS and PN injuries are synergistic and slow recovery(14). Other work using a more severe PN crush with and without VD showed persistent LPP deficits and EUS EMG impairments 3 weeks after injury(13). The current study found that BDNF treatment accelerated recovery from 3 to 2 weeks, likely as a result of concurrent PN and EUS improvement, as measured by LPP and EUS EMG. Histological findings of less EUS atrophy and fibrosis with BDNF treatment compared to saline treatment supported the functional findings, with the latter resembling previously described EUS changes after untreated VD-PNC(14).
A 2μg/day dose of localized, continuous BDNF treatment, higher than that used in nerve tubes, was given to account for diffusion from the catheter site(23). Both PCR and immunofluorescence detected lower EUS BDNF levels 4 days after PNC-VD than after sham injury, similar to previously observed EUS BDNF patterns 1 day after injury(14). Low EUS BDNF expression levels persisted 8 and 12 days after injury with BDNF treatment, suggesting neurotrophin therapy may prevent EUS BDNF upregulation after PN injury, which would facilitate EUS muscle recovery and NMJ restoration.
The PN neuroregenerative response, indicated by expression of βII-tubulin, a cytoskeletal protein upregulated in axonal growth and repair, was observed previously to increase 2.49-fold 7 days after isolated PN crush injury(16). This is similar to the 2.29-fold upregulation observed 8 days after untreated sham injury, despite the lack of statistical significance, which likely stems from PN trauma sustained during the sham injury. The published βII-tubulin upregulation following PN crush was no longer significant 14 days after injury, which was similarly observed 12 days after sham injury in this study(16). Patterns of PN βII-tubulin mirrored those of EUS BDNF expression after sham injury, suggesting EUS BDNF levels and PN neuroregenerative response may be related.
Similar trends between EUS BDNF expression and PN neuroregenerative response were evident after injury treated with saline. Both EUS neurotrophin upregulation and PN neuroregenerative response were delayed after injury treated with saline, as evidenced by near-stable EUS BDNF and PN βII-tubulin expression 4 and 8 days after injury. Thus, unlike sham injury, which likely involves isolated PN trauma that produces EUS BDNF upregulation, a combined PN and EUS injury appears to impair stimulation of the neuroregenerative response by delaying EUS BDNF upregulation.
PN BDNF treatment appeared to overcome the lack of EUS BDNF upregulation associated with the combined PN and EUS insults produced by simulated childbirth injury. Treating the PN with BDNF was related to a 4.2-fold, but non-significantly higher neuroregenerative response 8 days after injury, which was not observed 12 days after injury. Furthermore, a decrease in EUS BDNF occurred concurrently with an increase in neuroregenerative response, suggesting another source of stimulus for PN recovery. Therefore, it is likely that BDNF treatment provided the drive for neuroregeneration.
A limitation of the current study is the use of a quadruped animal model of simulated delivery to investigate post-partum SUI. However, since no other animal undergoes as traumatic a delivery as humans, simulated parturition must be implemented for the preclinical testing of potential therapies. Additionally, the involvement of PN trauma in the sham injury model challenged analyses in the study. While the sham injury was intended to reproduce other physiologic effects of the surgical model, it was not intended to induce nerve trauma, which likely limited the significant differences detected in various analyses. Nonetheless, the study design and multi-modal analysis of BDNF treatment compared to saline treatment provided insight into the potential beneficial effects of neurotrophin therapy for maternal childbirth injuries.
With the growing interest in preventative medicine, postpartum treatments aimed at facilitating recovery of the neuromuscular continence system may help reduce the societal burdens of SUI. Degradable neurotrophin releasing beads have been shown to improve neuroregeneration and could feasibly be injected near the PN(24). PN electrical stimulation resulted in increased neurotrophin expression and PN neuroregenerative response in a rat model of childbirth injury(25). Adipose-derived stem cells have also been found to secrete BDNF and promote nerve healing(26). With that, the use of targeted gene therapy could serve as a means of selectively upregulating BDNF therapeutically. An example of this is demonstrated by reversal of erectile dysfunction in male Sprague-Dawley rats after cavernous nerve injury by viral vector-mediated delivery of neurturin, another neurotrophin(27). Therefore, treating the neuromuscular continence mechanism and optimizing recovery of both PN and EUS may be a feasible postpartum intervention.
Conclusions
Following simulated childbirth injury, localized and continuous PN treatment with BDNF accelerated recovery of the neuromuscular continence mechanism, as evidenced by functional, electrophysiological, anatomic, and molecular data. Since PN and EUS injury result in stimuli that oppose recovery of the other structure, overcoming this phenomenon therapeutically may provide a means of facilitating recovery from such complex neuromuscular injuries. Further investigation into means of increasing recovery of the neuromuscular continence mechanism is needed.
Acknowledgments
The authors thank Amy S. Nowacki, Ph.D. for statistical guidance. This work was supported in part by NIH RO1 HD38679, Cleveland Clinic, and Rehabilitation Research and Development Services of the Department of Veterans Affairs.
References
- 1.Gregory W, Nygaard I. Childbirth and Pelvic Floor Disorders. Clin Obstet Gynecol. 2004;47:394. doi: 10.1097/00003081-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Rortveit G, Kjersti A, Hannestad Y, et al. Urinary incontinence after vaginal delivery or Cesarean section. New Engl J Med. 2003;348:900. doi: 10.1056/NEJMoa021788. [DOI] [PubMed] [Google Scholar]
- 3.Latthe P, Foon R, Khan K. Nonsurgical Treatment of Stress Urinary Incontinence: Grading of Evidence in Systematic Reviews. BJOG. 2008;115:435. doi: 10.1111/j.1471-0528.2007.01629.x. [DOI] [PubMed] [Google Scholar]
- 4.Barber M. Surgical Treatment of Stress Urinary Incontinence. In: Bent A, Cundiff G, Swift S, editors. Ostergard’s Urogynecology and Pelvic Floor Dysfunction. 6. Vol. 2008 Philadelphia, PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 5.Phull HS, Pan HQ, Butler RS, et al. Vulnerability of continence structures to injury by simulated childbirth. Am J Physiol Renal Physiol. 2011;301:F641. doi: 10.1152/ajprenal.00120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith A, Hosker G, Warrell D. The Role of Pudendal Nerve Damage in the Aetiology of Genuine Stress Urinary Incontinence in Women. Br J Obstet Gynaecol. 1989;96:29. doi: 10.1111/j.1471-0528.1989.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 7.Allen R, Hosker G, Smith A, et al. Pelvic floor damage and childbirth: a neurophysiological study. Br J Obstet Gynaecol. 1990;97:770. doi: 10.1111/j.1471-0528.1990.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 8.Snooks S, Swash M, Mathers S, et al. Effect of Vaginal Delivery On the Pelvic Floor: A 5-Year Follow-Up. Br J Surg. 1990;77:1358. doi: 10.1002/bjs.1800771213. [DOI] [PubMed] [Google Scholar]
- 9.Omura T, Sano M, Omura K, et al. Different Expressions of BDNF, NT3, and NT4 in Muscle and Nerve After Various Types of Peripheral Nerve Injuries. JPNS. 2005;10:293. doi: 10.1111/j.1085-9489.2005.10307.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Luo X, Xian C, et al. Endogenous BDNF is Required for Myelination and Regeneration of Injured Sciatic Nerve in Rodents. Eur J Neurosci. 2000;12:4171. [PubMed] [Google Scholar]
- 11.Wells D, McKechnie B, Kelkar S, et al. Neurotrophins Regulate Agrin-Induced Postsynaptic Differentiation. Proc Natl Acad Sci. 1999;96:1112. doi: 10.1073/pnas.96.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mousavi K, Jasmin B. BDNF Is Expressed in Skeletal Muscle Satellite Cells and Inhibits Myogenic Differentiation. J Neurosci. 2006;26:5739. doi: 10.1523/JNEUROSCI.5398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang HH, Pan HQ, Gustilo-Ashby MA, et al. Dual simulated childbirth injuries result in slowed recovery of pudendal nerve and urethral function. Neurourol Urodyn. 2009;28:229. doi: 10.1002/nau.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan H, Kerns J, Lin D, et al. Dual Simulated Childbirth Injury Delays Anatomic Recovery. Am J Physiol Renal Physiol. 2009;296:277. doi: 10.1152/ajprenal.90602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong W, Lin D, Kinley B, et al. Urethral Neuromuscular Injury Following Simulated Childbirth Injury in a Rat Model. Neurourol Urodyn. 2009;28:597. [Google Scholar]
- 16.Sakamoto K, Smith G, Storer P, et al. Neuroregeneration and voiding behavior patterns after pudendal nerve crush in female rats. Neurourol Urodyn. 2000;19:311. doi: 10.1002/(sici)1520-6777(2000)19:3<311::aid-nau11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Jiang HH, Gustilo-Ashby AM, Salcedo LB, et al. Electrophysiological Function During Voiding after Simulated Childbirth Injuries. Exp Neurol. 2009;215:342. doi: 10.1016/j.expneurol.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu TW, Wagner TH, Bentkover JD, et al. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology. 2004;63:461. doi: 10.1016/j.urology.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 19.Carr LK, Steele D, Steele S, et al. 1-Year Follow-Up of Autologous Muscle-Derived Stem Cell Injection Pilot Study to Treat Stress Urinary Incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:881. doi: 10.1007/s00192-007-0553-z. [DOI] [PubMed] [Google Scholar]
- 20.Terenghi G. Peripheral Nerve Regeneration and Neurotrophic Factors. J Anat. 1999;194:1. doi: 10.1046/j.1469-7580.1999.19410001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon M, Porter R, Brown R, et al. Effect of NT-4 and BDNF deliver to damaged sciatic nerves on phenotypic recover of fast and slow muscle fibers. Eur J Neurosci. 2003;18:2460. doi: 10.1046/j.1460-9568.2003.02978.x. [DOI] [PubMed] [Google Scholar]
- 22.Utley D, Lewin S, Cheng E, et al. Brain-Derived Neurotrophic Factor and Collagen Tubilization Enhance Functional Recovery After Peripheral Nerve Transection and Repair. Arch Otolaryngol Head Neck Surg. 1996;122:407. doi: 10.1001/archotol.1996.01890160047009. [DOI] [PubMed] [Google Scholar]
- 23.Boyd J, Gordon T. A Dose-Dependent Facilitation and Inhibition of Peripheral Nerve Regeneration by Brain-Derived Neurotrophic Factor. Eur J Neurosci. 2002;15:613. doi: 10.1046/j.1460-9568.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- 24.Vogelin E, Baker J, Gates J, et al. Effects of Local Continuous Release of Brain Derived Neurotrophic Factor (BDNF) on Peripheral Nerve Regeneration in a Rat Model. Exp Neurol. 2006;199:348. doi: 10.1016/j.expneurol.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 25.Jiang HH, Gill BC, Salcedo LB, et al. Effects of electrical stimulation on the pudendal nerve on expression of neurotrophins in onuf’s nucleus following simulated childbirth injury. Neurourol Urodyn. 2010;29:254. [Google Scholar]
- 26.Lopatina T, Kalinina N, Karagyaur M, et al. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One. 2011;6:e17899. doi: 10.1371/journal.pone.0017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato R, Wolfe D, Coyle CH, et al. Herpes simplex virus vector-mediated delivery of neurturin rescues erectile dysfunction of cavernous nerve injury. Gene Ther. 2009;16:26. doi: 10.1038/gt.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]