Abstract
Success in a dynamically changing world requires both rapid shifts of attention to the location of important objects and the detection of changes in motivational contingencies that may alter future behavior. Here we addressed the relationship between these two processes by measuring the blood-oxygenation-level-dependent (BOLD) signal during a visual search task in which the location and the color of a salient cue respectively indicated where a rewarded target would appear and the monetary gain (large or small) associated with its detection. While cues that either shifted or maintained attention were presented every 4 to 8 seconds, the reward magnitude indicated by the cue changed roughly every 30 seconds, allowing us to distinguish a change in expected reward magnitude from a maintained state of expected reward magnitude. Posterior cingulate cortex was modulated by cues signaling an increase in expected reward magnitude, but not by cues for shifting versus maintaining spatial attention. Dorsal fronto-parietal regions in precuneus and FEF also showed increased BOLD activity for changes in expected reward magnitude from low to high, but in addition showed large independent modulations for shifting versus maintaining attention. In particular, the differential activation for shifting versus maintaining attention was not affected by expected reward magnitude. These results indicate that BOLD activations for shifts of attention and increases in expected reward magnitude are largely separate. Finally, visual cortex showed sustained spatially selective signals that were significantly enhanced when greater reward magnitude was expected, but this reward-related modulation was not observed in spatially selective regions of dorsal fronto-parietal cortex.
Keywords: shifts of spatial attention, changes in expected reward magnitude, maintenance of attention at a location
1.Introduction
Reward and spatial attention are closely intertwined during natural behavior, as animals and people frequently shift their attention to the location of potentially rewarding stimuli. Therefore, it is important to understand how the neural activity related to shifts of attention is affected by reward-related variables. Many studies have shown that in humans, dorsal fronto-parietal regions such as frontal eye field (FEF), intraparietal sulcus (IPS) and precuneus play a primary role in controlling spatial attention (Corbetta et al., 2008; Shulman et al., 2009; Kelley et al., 2008; Serences and Yantis, 2007). Mesulam has proposed that the posterior cingulate is an interface between this dorsal attention network and limbic areas traditionally associated with reward and motivation (Mesulam, 1981). In support, Mesulam and colleagues have reported that the correlation between neural activity in the posterior cingulate (PC) and the behavioral benefit of anticipatory shifts of attention is enhanced by motivational incentives (Small et al., 2005; Mohanty et al., 2008). However, these studies have left unclear whether the posterior cingulate is modulated specifically by shifts of attention. A second line of research involving single-unit studies in monkeys has led to a more general proposal that the posterior cingulate encodes environmental variables, such as reward outcomes or changes in reward contingencies, that affect the expected value of stimuli or actions and prompt changes in subsequent behavior (McCoy and Platt, 2005; Hayden et al., 2008; Pearson et al., 2011). According to this view, activity in the posterior cingulate is not necessarily tied to shifts of attention.
Conversely, it is unclear how different components of the dorsal attention network are affected by reward-related variables. In recent fMRI studies a clear functional-anatomical segregation has been found between signals for shifting attention from one location to another and signals for maintaining attention at contralateral versus ipsilateral locations (Shulman et al., 2009; Kelley et al., 2008; Greenberg et al., 2010). How these specific attentional signals within the dorsal network are modulated by reward-related variables, however, has not been studied. To address these issues we manipulated two kinds of reward-related signals: signals associated with a change in expected reward magnitude (e.g. a transition from low to high expected reward magnitude), and signals associated with a maintained state of expected reward magnitude (e.g. high expected reward magnitude). We also manipulated whether these reward-related signals occurred in conjunction with shifts of attention between locations or with maintenance of attention at a location. Specifically, subjects searched for a rewarded target object in one of two rapid-serial-visual-presentation (RSVP) streams presented left and right of fixation. The location of an occasional salient cue indicated which stream would contain a target. A cue might appear in the currently attended stream, indicating that attention should be maintained, or it might appear in the opposite stream, evoking a shift of attention. The color of the cue indicated whether the detection of subsequent targets in the cued stream would be rewarded with small or large monetary gains. Because the cue color only changed roughly every 30 seconds, activations due to a change in reward contingency (e.g. a shift from low to high expected reward magnitude) could be distinguished from activations reflecting a tonic state of high or low reward expectation.
2.Material and Methods
2.1. Participants
Twenty-seven right-hand volunteers with normal or corrected-to-normal vision and no history of psychiatric disorders gave written consent to participate in the study, in agreement with the guidelines of the human studies committee of Washington University School of Medicine. Data from twenty-six subjects were included in the fMRI study, while the data from one subject was excluded because of excessive eye movements.
2.2. Paradigm and Stimuli
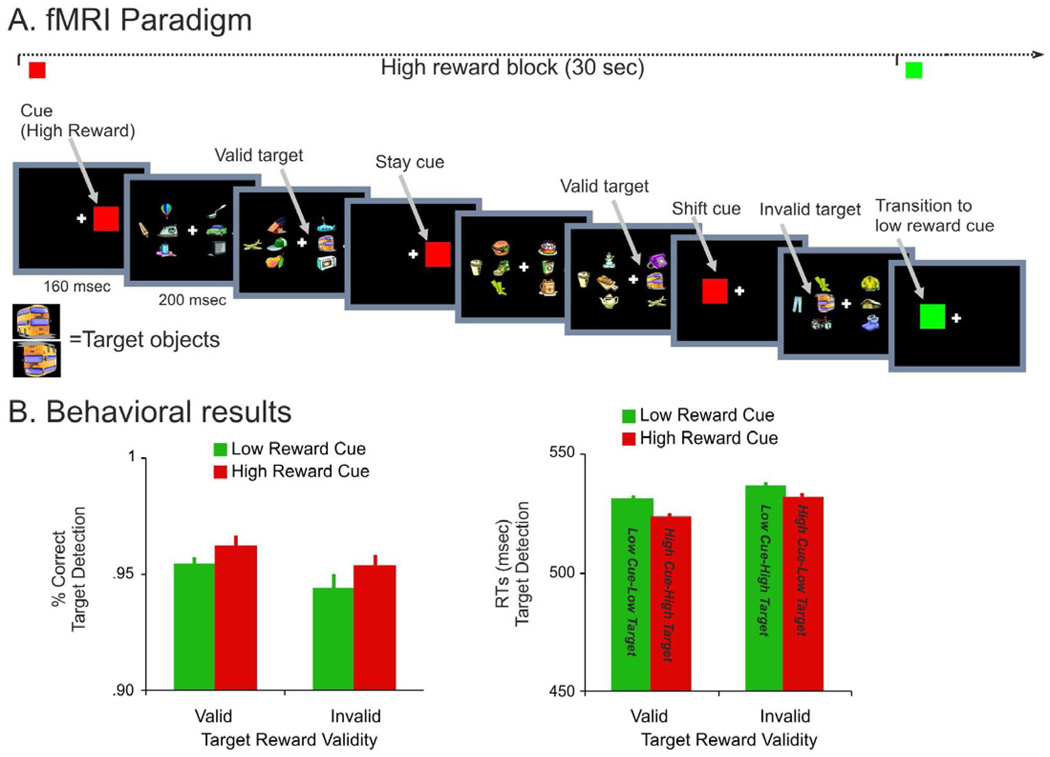
Subjects performed a RSVP (rapid serial visual presentation) visual search task involving search for a predefined target object in two visual streams of objects, one left and one right of fixation (Fig. 1A). Each task-relevant stream was surrounded by three task-irrelevant, distracting streams, ensuring that attention needed to be focally directed to the relevant stream. At regular intervals, a highly salient cue stimulus (a filled colored square) was presented in one of the two streams location, with no other objects presented in the other stream locations. The location (left, right) and the color of the cue (green, red) indicated, respectively, which of the two streams would contain subsequent targets and the reward value associated with detection of those targets. In half of the participants, the red and green cues were associated with a reward of 1 and 20 cents, respectively, while the association was reversed for the other half. The location of the cue always correctly predicted the location of the target (spatial cue validity = 100%) but only correctly predicted the reward associated with a detected target with probability .75 (reward cue validity = 75%). In order to manipulate the reward validity of the cue, two different orientations (upright and upside down) of the target object were presented. One orientation indicated that the expected reward would be received (e.g. high reward target following a high reward cue), while the other orientation indicated that the unexpected reward would be received (e.g. low reward target following a high reward cue). The effect of reward validity on the BOLD response is not discussed in the current paper since it focuses on the relationship between signals for reward expectation and spatial attention rather than on signals associated with the detection of rewarded targets such as the ‘reward-prediction’ errors produced by unexpected rewards (Sutton and Barto, 1981; Schultz, 1998, 2004).
Figure 1. fMRI paradigm and behavioral results.
A. Subjects searched for a target object (a bus with an upright or upside down orientation of 200 msec duration) in two visual streams of objects, one left and one right. The location of a cue stimulus (a filled color square stimulus of 160 msec duration) indicated where to attend/where the target object would appear, while its color indicated whether detection of any subsequent targets would be rewarded with a large or small monetary gain.
The cue stimulus could occur on the same side (stay cue) or the opposite side (shift cue) of the subject’s current focus of attention. Targets always occurred in the attended location but were presented with 75% probability in the expected orientation (valid reward i.e. reward that conformed to that indicated by the color of the cue) and with 25% probability in the unattended orientation (invalid reward).
B. Accuracy and reaction time results for detection of high and low reward targets as a function of reward validity. Error bars represent within-subjects standard error of the mean (s.e.m) calculated according to Cousineau, 2005.
A cue stimulus either occurred in the same location as the previous cue stimulus, indicating that attention should be maintained on the same stream (stay cue), or occurred in the opposite location, indicating that attention should be shifted to the opposite stream (shift cue). Cues occurred on average every 4.12, 6.18 or 8.24 sec within a temporal window of 400 msec centered on those time points. While shift and stay cues were selected randomly, the reward value of the cue, indicated by its color, changed roughly every 30 seconds, therefore producing blocks in which reward expectation was fixed. Modulations due to changes of reward contingencies were studied by distinguishing the cue that appeared at the beginning of a block (transition cue) from the cues that appeared within a block (stream cues).
Although the cue indicated both the location and reward value of subsequent targets, it never predicted when a target would occur. Targets occurred on average every 8 sec. Each non-cue display frame in the RSVP sequence was shown for 200 msec, resulting in a relatively high level of target identification (90% accuracy).
Target and distracter objects contained in the eight RSVP streams were 3 by 3 degrees (deg) drawings of colored inanimate objects. The cue stimulus was a 3 by 3 deg filled square of red or green color. Both target and cue stimuli were only presented in the more central, 5 deg eccentric RSVP stream and never in the distracter streams located one above, one below and one more eccentric to the target stream.
The experimental design, which was a modified version of a paradigm previously used to investigate the relationship between signals for shifting of attention and the frequency of shifting attention (Shulman et al., 2009), controlled for several important factors. First, it eliminated time-locked activations to the cues that were related to the temporal prediction of target onset (Coull and Nobre, 1998; Coull et al., 2000), ensuring that the cues provided information only about reward magnitude and the location of attention/reward. Second, by independently manipulating shifts of attention (shift versus stay cues), and changes in expected reward magnitude (transition versus stream cues), the effects of shifts of spatial attention were studied independently of changes in reward contingencies. Third, the design allowed a well controlled test of whether expected reward magnitude affected signals specifically associated with shifts of attention, since perceptually identical cue stimuli instructed subjects either to shift or to maintain attention under different levels of reward expectation in a factorial design.
2.3. Procedure
Before participating in the fMRI experiment subjects were familiarized with the task in a behavioral training session in which they were initially shown the target object (the bus in Fig. 1A) and then underwent a staircase procedure in which performance was monitored during a series of runs in which the duration of each target and distracter frame in the RSVP sequence was progressively reduced up to a frame duration of 200 msec. When the desired accuracy threshold was satisfied (target was detected with 90% accuracy), subjects were informed about the different reward values associated with the color of the cue (in half of the subjects red and green indicated a 20 cent reward and 1 cent reward for a detected target, respectively, while the color-reward association was reversed for the remaining half). Subjects were also told that the target would always occur at the cued location but that it could violate the reward expectation (high, low) indicated by the cue (75% valid reward) and were instructed about the reward significance of the upright and upside down targets. As mentioned above (see paradigm and stimuli section), violation of reward expectation associated with the cue stimulus was manipulated by presenting two opposite orientations (upright and upside down) of the same target object. For half of the subjects, the upright object was the most frequent target and had a reward value that conformed to that indicated by the color of the cue, while the upside down object was less frequent and had a reward value opposite to the one indicated by the cue. The opposite association was given to the other half of the subjects.
The eye movement records from the training session were used to screen out subjects who were not able to maintain fixation. Only subjects that did not exceed a threshold of about 3–4 eye movements per 3 minutes of recording were subsequently selected for the actual experiment.
The experiment started with the appearance of the fixation cross followed by the onset of a cue stimulus that directed attention to one of the two stream locations and the onset of the eight RSVP streams. The duration of each display frame containing the 8 objects of the RSVP streams was 200 msec. The duration of the cue frame was 160 msec, with no inter-stimulus interval (ISI) between cue and display frames or between consecutive display frames. Targets had a fixed, independent probability of being displayed within each 1 sec interval and occurred on average every 8 sec. The hazard function was flat, with the following exceptions. A minimum inter-target interval of 1 sec was instituted to allow detection responses to be separately collected on each target presentation. Cue and target onsets were independent except that a target could not occur simultaneously with a cue or in the 200 msec display frame preceding the onset of a cue. Each subject performed 16 scans/session where each scan was of 206.4 sec duration. In addition to their regular fixed participation fee, subjects could earn up to a maximum amount of 37 dollars based on their performance.
2.4. Eye movement recording
Eye position was monitored and recorded in all subjects during both the behavioral training and the fMRI experiment via an infrared eye-tracking system (ISCAN ETL-220 during scanning and ASL 504 during behavioral measurements).
2.5. fMRI methods
Image acquisition and apparatus
Magnetic resonance imaging (MRI) scans were collected on a Siemens Allegra 3T scanner, using a gradient-echo echoplanar imaging sequence to measure BOLD contrast over the entire brain [Thirty-two contiguous 4 mm thick axial slides, 4 × 4 mm in-plane resolution, echo time (TE), 25 msec; flip angle, 90°, repetition time (TR), 2.06 sec]. Anatomical images were acquired using a magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence (TR, 1810 msec; TE, 3.93 msec; flip angle 12°; inversion time, 1200 msec, voxel size, 1 × 1 × 1.25 mm). Visual stimuli were presented with a Power Macintosh G4 computer running Matlab software with the psychophysics toolbox (Brainard, 1997; Pelli, 1997), projected to the head of the scanner via a liquid crystal display projector (sharp LCD, C20X) and viewed through a mirror attached to the head coil.
Preprocessing and statistical analyses
Differences in the acquisition time of each slice in a MR frame were compensated by sinc interpolation so that all slices were aligned to the start of the frame. Functional data were realigned within and across scans to correct for head movement using six-parameter rigid body realignment. A whole brain normalization factor was uniformly applied to all frames within a scan in order to equate signal intensity across scans. Images were resampled into 3 mm isotropic voxels and transformed into a standardized atlas space (Talairach and Tournoux, 1988). Movement correction and atlas transformation was accomplished in one resampling step to minimize blur and noise.
The hemodynamic responses time-locked to the presentation of cue and target stimuli were estimated without any shape assumption (Ollinger et al., 2001a; Ollinger et al., 2001b), on a voxel by voxel basis, according to the general linear model (GLM). Evoked responses to cue stimuli were analyzed as a function of 1) cue onset position, i.e. whether cues were presented at the beginning (transition cues) or within (stream cues) a constant reward block, 2) expected reward magnitude, i.e. whether cues signaled a low or a high expected reward magnitude of the target, 3) cue type, i.e. whether cues occurred in the opposite (shift cues) or in the same location (stay cues) as the previous cue stimulus and 4) cue location, whether cues were presented in the left or right RSVP stream (also classified as contralateral or ipsilateral to the region under examination).
Two different models were used. The first model included sixteen cue regressors (factorial combination of stay/shift, left/right, high/low reward, transition/stream cue) and eight target regressors (factorial combination of valid/invalid, left/right, high/low reward) for a total of 240 task-related regressors, in addition to baseline and linear trend regressors in each scan. The second model was identical to the first with the exception that cue regressors were collapsed across transition and stream cues for a total of 160 task-related regressors. Each cue and target regressor consisted of 10 time points regressors extending out to 18.5 sec. The time courses of the evoked response to these different types of cues and target stimuli were then analyzed at the whole brain level using voxelwise within-subject ANOVAs or within regions of interest (ROIs), identified from voxelwise maps (regional analyses). Before conducting the voxelwise ANOVAs, the time course data were spatially smoothed by a Gaussian filter with a full-width-at-half-maximum of 6 mm. Voxelwise ANOVAs were corrected for non-independence of time points by adjusting the degrees of freedom and for multiple comparisons using joint z-score/cluster size thresholds (Forman et al., 1995) corresponding to z = 3.0 and a cluster size of 13 face-contiguous voxels. The z-score/cluster size thresholds were determined using volume-based monte-carlo simulations. Regional ANOVAs were conducted on regions-of-interest (ROIs) that were automatically created from the voxel-level maps using a peak-finding routine (Kerr et al., 2004). For each subject and ROI, the BOLD time courses for a condition were averaged over all voxels in the ROI, and the regional time course for each condition was entered into an ANOVA, which corrected for non-independence of time points by adjusting the degrees of freedom. A significance threshold of p < .05, uncorrected for the number of tested ROIs, was adopted for regional analyses.
To analyze the relationship between signals for reward expectation and spatial attention, we first identified regions of interest that responded to a certain type of modulation using voxelwise ANOVAs and then tested whether these regions were modulated by another type of effect using regional ANOVAs. Because the statistical term in the ANOVA used to select regions was independent of the term(s) reported from the ANOVA, this approach is unbiased. For example, regions that were modulated by a change in expected reward magnitude in a specific direction (e.g. from low to high), which was a main focus of this work, were first identified at the whole brain level by a voxelwise test for the interaction of cue onset position by expected reward magnitude by time. These regions were then tested for attention-related modulations such as shifts of attention (cue type by time) or cue location (cue location by time) via regional ANOVAs. Since both regional and voxelwise ANOVAs indicated the level of significance of an effect but not its direction, statistical significance was coupled with inspection of regional time courses of activation.
Finally, we note that a significant interaction between cue onset position, expected reward magnitude, and time identified regions whose BOLD time course was modulated by a change in expected reward magnitude in a specific direction, i.e. a greater BOLD signal change for going from low to high expected reward than from high to low expected reward, or the reverse pattern. Importantly, regions that show this interaction are not simply responding to effects of novelty (e.g. the change in stimulus color) or to any change in expected reward magnitude. These latter effects instead are reflected in the interaction of cue onset position by time, since they are equally present for a change in expected reward magnitude from low to high and from high to low. Moreover, regions that show the cue onset position by expected reward magnitude by time interaction are not simply responding more strongly to high than low reward expectation, which instead is reflected in the interaction of expected reward magnitude by time.
For display purposes, volumes were mapped to surface-based representations using the PALS atlas and CARET software (Van Essen, 2005).
3. Results
3.1. fMRI Behavior
Subjects detected the target with a mean accuracy of 95.5% with no significant difference between conditions. An ANOVA conducted on reaction time (RTs), however, indicated main effects of target reward validity (reward invalid versus reward valid targets: F1,25=11.2 p=.002) and reward expectation (high versus low expected reward magnitude: F1,25=8.5 p=.007) with no interaction between the two factors (F1,25=1.2 p=.3)(Fig. 1B). Although the RTs difference between reward conditions was small, these results indicate that subjects were significantly faster to detect targets that occurred during a period of high versus low reward expectation, independently of whether the targets conformed to the expected reward magnitude (reward validity). The independent effect of target reward validity could reflect the fact that valid targets occurred in the expected orientation, i.e. an effect of stimulus expectation, or that they signaled the expected reward. Notably, we did not observe better performance when an unexpected target (reward invalid) signaled a reward higher than expected (e.g. expect 1 cent target, instead a 20 cent target is actually presented), as compared to when an unexpected target signaled a reward lower than expected (e.g. expect 20 cent target, instead a 1 cent target is actually presented). Therefore, performance was more influenced by the reward that was expected for an upcoming target than by the reward actually provided by that target. Since we were interested in the relation between reward expectation and spatial attention, the main focus of the paper is on cue- and not on target-related activity and the principal aim of presenting the behavioral data is to show that reward expectation affected performance.
3.2. Imaging
3.2.1. Changes of reward expectation but not shifts of attention modulate posterior cingulate cortex
Our overall goal was to determine how representations for expected reward magnitude and changes in expected reward magnitude are distributed in the brain, and their relation to signals for shifting versus maintaining attention. As part of this aim, we first identified cortical regions that were more modulated by a change in expected reward magnitude, relative to the current expectation, in one direction than another (i.e. a larger BOLD signal when expected reward goes from low to high than from high to low, or the reverse pattern). Specifically, a voxelwise ANOVA tested for the interaction between cue onset position (transition, stream), expected reward magnitude (high, low) and time (10 time points). By identifying regions in which the BOLD signal depended on the direction of change, we eliminated the effects of two factors that were present for changes in expected reward magnitude in either direction and were not specific for reward. In particular, time-locked activations to transition cues could reflect activity for rare or novel events (Spencer et al., 1999; Downar et al., 2000; Stevens et al., 2000) or dishabituation of a color-specific mechanism.
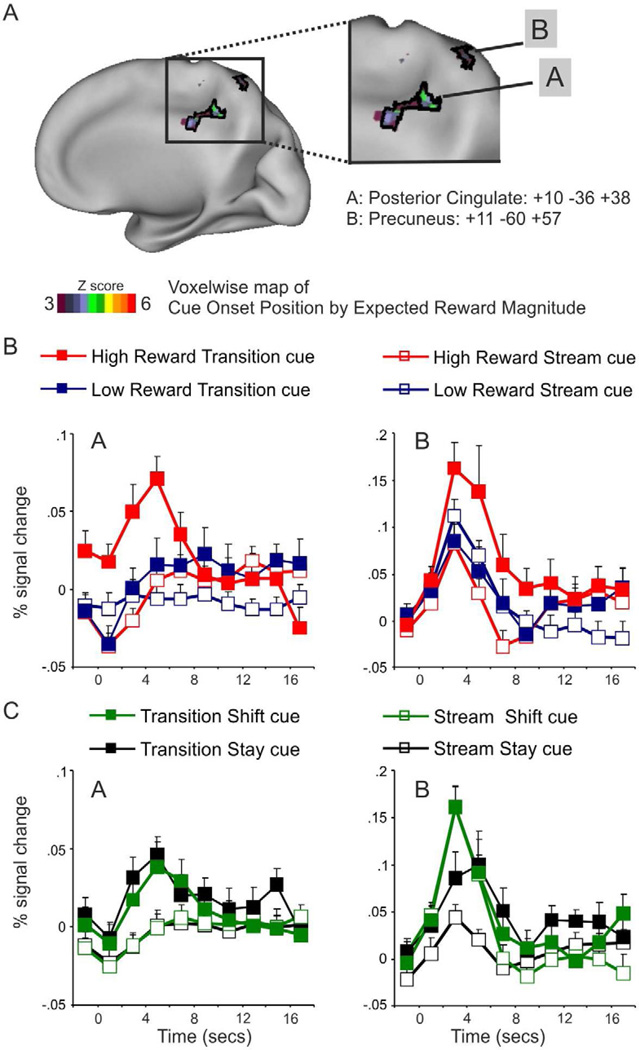
Over the whole brain, a significant interaction of cue onset position by expected reward magnitude by time was observed in two regions of the medial parietal cortex (Fig. 2A): a precuneus region whose location closely matched the shift-related activation in recent fMRI studies of spatial attention (Shulman et al., 2009), and a posterior cingulate region whose location closely matched the region identified in fMRI studies on the effect of incentive on the correlation between BOLD activity and performance (Small et al., 2005; Mohanty et al., 2008). The time course of activity in these two regions as a function of cue onset position and expected reward magnitude indicated that both posterior cingulate and precuneus regions showed a selective increase of activity following transition cues that indicated the beginning of a high reward period (Fig. 2B).
Figure 2. Effects of changes in expected reward magnitude: Voxelwise analysis.
A. Voxels that showed a significant interaction of cue onset position (transition, stream) by expected reward magnitude (high, low) by time (voxelwise ANOVA map corrected for multiple comparison) are superimposed over a medial representation of the right hemisphere of the PALS atlas. Two regions in right precuneus and posterior cingulate cortex with the greatest activation peak magnitude are outlined in black.
B–C. The time course of the BOLD signal in posterior cingulate and precuneus ROIs, defined from the voxelwise interaction map, are plotted as a function of expected reward magnitude and cue onset position (top graph), or cue type (stay, shift) and cue onset position (bottom graph). Error bars represent within-subjects s.e.m calculated according to Cousineau, 2005.
We next investigated whether the precuneus and posterior cingulate regions that were identified by the voxelwise ANOVAs of cue onset position by expected reward magnitude by time were additionally modulated by attention-related signals associated with shifts of spatial attention or with cue presentation in a contralateral or ipsilateral location. To this aim, we compared the BOLD responses of these two regions for shift versus stay cues and left versus right cues in a regional ANOVA with cue onset position (transition, stream), cue type (stay, shift), cue location (left, right), and time as factors. The results of these ANOVAs indicated that, while the precuneus was both significantly modulated by cues indicating a shift of attention (cue type by time: F9,225=8.5 p < .0001) and by cues indicating maintenance of attention at a contralateral versus ipsilateral location (cue location by time: F9,225=3 p= .001), the posterior cingulate did not show any evidence of increased BOLD signals to cues that shifted rather than maintained attention (F9,225=.5 p=.8) or that were presented in a contralateral versus ipsilateral location (F9,225=.6 p=.7) (see time course of precuneus and posterior cingulate to shift versus stay cues in Fig. 2C). Importantly, although the precuneus region was both modulated by changes in expected reward magnitude and by shifts of attention, it did not show any significant interaction between cue onset position, expected reward magnitude, cue type and time in a regional ANOVA, thus indicating that the reward and attention shifting modulations were independent one from another (cue onset position by expected reward magnitude by cue type by time: p=.8).
Therefore, while activity in the precuneus was significantly modulated both by increases in expected reward magnitude and by shifts of attention, with these modulations additive to one another, activity in posterior cingulate was only significantly modulated by increases in expected reward magnitude.
3.2.2. Identification of regions for shifting and maintaining attention
We identified regions that were modulated by shifting and maintaining attention using whole-brain analyses. A voxelwise ANOVA was conducted with cue type (stay, shift), cue location (left, right) and time as factors. The interaction of cue type by time isolated regions of the dorsal [superior parietal lobule (SPL), frontal eye field (FEF), precuneus)] and ventral attention networks [right temporo-parietal junction (TPJ), right inferior frontal gyrus (IFG), bilateral prefrontal cortex (PFC)] (Yantis et al., 2002; Kelley et al., 2008; Shulman et al., 2009; Corbetta et al., 2000; Arrington et al., 2000). Activity in these regions was transient and time-locked to the presentation of the cue stimulus and increased in magnitude for shifting versus maintaining attention (Supplementary Fig. 1A).
Regions modulated by maintaining attention to the contralateral versus ipsilateral visual target streams were identified by the interaction of cue location by time. This comparison isolated large swaths of retinotopic visual cortex, including areas V1–V2, VP-V4, V3A-V7, MT-LO, parietal regions within the medial IPS previously shown to be topographically mapped (Sereno et al., 2001; Schluppeck et al., 2005; Schluppeck et al., 2006; Silver et al., 2005; Hagler and Sereno, 2006; Swisher et al., 2007; Jack et al., 2007), and small topographic parts of FEF (Supplementary Fig. 1B). Consistent with the results from a previous study that used the current paradigm (Shulman et al., 2009), regions in dorsal fronto-parietal cortex and throughout visual cortex showed a sustained, spatially selective signal in which BOLD activity was increased in regions contralateral to the attended RSVP stream. The sustained nature of the BOLD activity indicated that it was not a sensory response to the cue but reflected either the maintenance of attention at the cued location or the modulation of the sensory-evoked activity from the objects in the RSVP stream.
3.2.3. Independent signals for shifts of attention and reward expectation in shift-related regions of dorsal fronto-parietal cortex
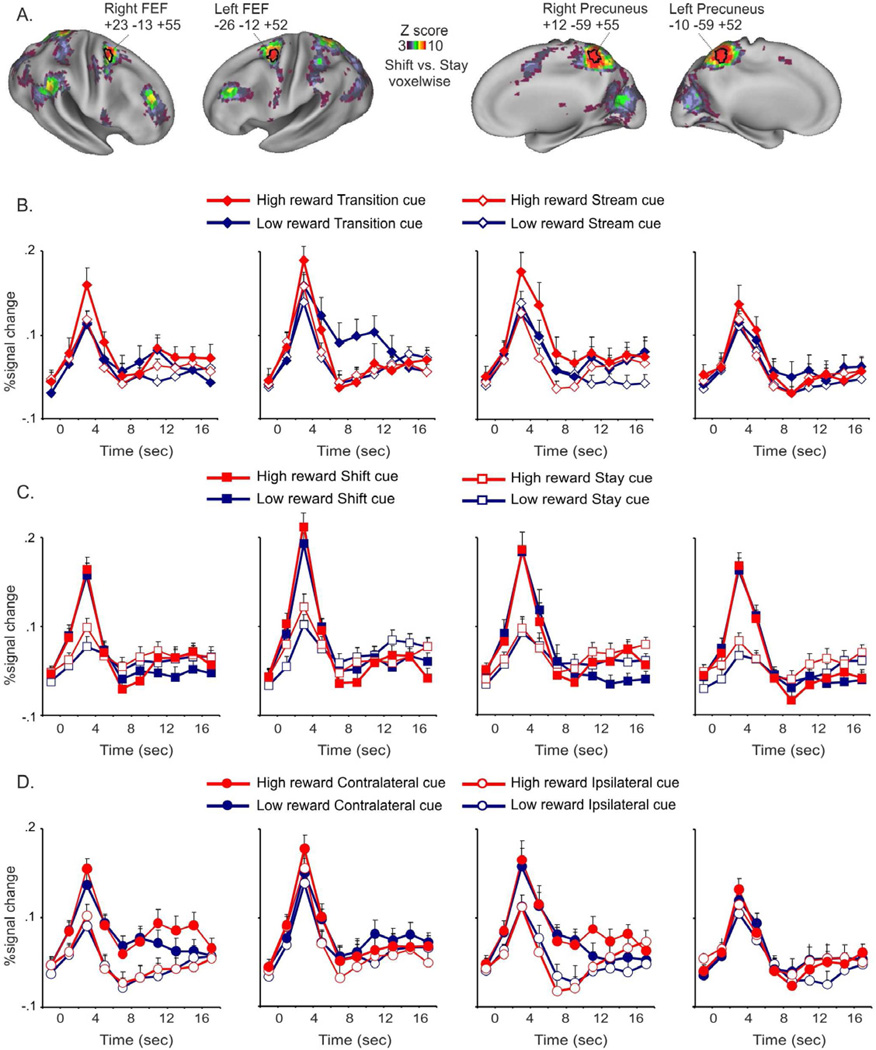
We next examined how reward-related signals modulated the activity of shift-related regions in dorsal-fronto parietal cortex, which were identified from the above voxelwise analyses. We first tested whether precuneus and FEF regions of dorsal fronto-parietal cortex (see black outlines in Fig. 3A) encoded signals for changes in expected reward magnitude in a specific direction, as assessed by the interaction between cue onset position (transition, stream), expected reward magnitude (high, low) and time. As shown in Figure 3B, transition cues indicating a change in expected reward magnitude from low to high produced the largest response in all four regions, similarly to what was observed in the posterior cingulate. Regional ANOVAs on the time courses, however, indicated that the interaction was only significant in left FEF and right precuneus (right precuneus: F9,225=3 p=.002, left FEF: F9,225=2.4 p=.012). The right precuneus ROI corresponded well to the precuneus region identified earlier (Fig. 2A) that showed significantly greater activations for high reward transition cues in a voxelwise analysis. The left FEF region also showed a significant main effect of expected reward magnitude (expected reward magnitude by time, F9,225= 1.9 p=.04). Therefore, a significant effect of an increase in expected reward magnitude was observed in the BOLD response of some shift-related regions of dorsal fronto-parietal cortex.
Figure 3. Effects of changes in expected reward magnitude in shift-related regions of dorsal fronto-parietal cortex.
A. Voxels that showed a significantly different activation following shift and stay cues (cue type by time ANOVA map, corrected for multiple comparisons) are superimposed over a lateral and medial representation of both hemispheres of the PALS atlas. Bilateral regions of FEF and Precuneus with the greatest activation peak are outlined in black.
The graphs show the time course of the BOLD signal following high and low reward cues as a function of cue onset position (B), cue type (C), cue location (D) in the FEF and Precuneus ROIs highlighted. Error bars represent within-subjects s.e.m calculated according to Cousineau, 2005.
We then tested whether signals for expected reward magnitude interacted with signals for shifting attention by conducting regional ANOVAs that tested for the interaction between cue onset position (transition, stream), expected reward magnitude (high, low), cue type (stay, shift) and time (10 time points). The interaction did not reach statistical significance in any of the four regions of dorsal fronto-parietal cortex (left precuneus, right precuneus: p= .8, right FEF: p=.9, left FEF: p=.4), indicating that the effect of expected reward magnitude was independent of whether attention was shifted or maintained on the current RSVP stream [see Fig. 3C for the time courses of activation to high and low reward cues (averaged across transition and stream cues) as a function of cue type]. In order to confirm the robustness of this result, we conducted ANOVAs separately on the transition and stream cues, reflecting effects of changes or maintenance of expected reward magnitude, respectively. No significant interaction was found between expected reward magnitude, cue type and time in any of the four regions (transition cues: left precuneus: p= .8, right precuneus: p=.5, right FEF: p=.6, left FEF: p=.9; stream cues: left precuneus: p= .3, right precuneus: p=.9, right FEF: p=.7, left FEF: p=.8). Although the absence of an interaction is a null result, the time courses in Figure 3C do not indicate even a trend for greater effects of reward during a shift of attention.
A second analysis investigated whether in these regions, signals for changes in expected reward magnitude or for maintained states of expected reward magnitude interacted with spatially-selective signals for maintaining attention at a location. Since both right FEF and right precuneus showed significantly stronger activity for contralateral than ipsilateral cues (cue location by time, right FEF: F9,225=3.9 p <0.001, right precuneus: F9,225=4.2 p <0.001), we tested whether the spatially-selective signals in these regions were affected by reward-related variables by testing the interaction between cue onset position, expected reward magnitude, cue location and time in regional ANOVAs. The results showed that the interaction did not reach significance or was marginally significant both in these regions and in regions of left precuneus and left FEF that showed the same non-significant trend of greater activity for contralateral versus ipsilateral cues (cue onset position by expected reward magnitude by cue location by time, right precuneus: p=.4, right FEF: p=.08, left precuneus: p=.6, left FEF: p=.1) [see Fig. 3D for the time courses of activation to high and low reward cues (averaged across transition and stream cues) as a function of cue location in the four regions of dorsal fronto-parietal cortex].
To summarize, dorsal fronto-parietal regions showed robust signals related to shifts of attention between locations. Some of these regions also showed more modest but significant BOLD modulations for cues indicating a change in expected reward magnitude from low to high, but this reward-related activity did not interact with the activity associated with shifting attention or with maintaining attention at a location.
3.2.4. No modulation by reward expectation in shift-related regions of ventral fronto-parietal cortex
In contrast to the reward-related modulations observed in shift-related regions of dorsal fronto-parietal cortex, no modulations were found in shift-related regions of the ventral fronto-parietal cortex, including right temporo-parietal junction (TPJ), and bilateral dorso-lateral prefrontal cortex (DLPFC), which were similarly identified from the voxelwise map of cue type by time (Supplementary Fig. 2A). Although inspection of the time courses of activation in these regions (Supplementary Fig. 2B) suggested a trend in right TPJ and right DLPFC for greater activity for high reward transition cues than for the others conditions, none of the statistical analyses yielded significant effects of reward expectation. Specifically, results from regional ANOVAs indicated that none of these regions showed significant modulations due to changes in expected reward magnitude or modulations for signals for shifting attention between RSVP streams due to changes in expected reward magnitude [cue onset position (transition, stream) by expected reward magnitude (high, low) by time (10 time points), right TPJ: p=.3, right DLPFC: p=.9, left DLPFC: p=.8; cue onset position by expected reward magnitude by cue type by time, right TPJ: p=.7, right DLPFC: p=.5, left DLPFC: p=.3] [see Supplementary Fig. 2B and 2C for time courses of activity in these regions as a function of cue onset position, expected reward magnitude and cue type (same format of the time courses in Fig. 3B–C)].
The evidence that shift-related regions in dorsal and ventral fronto-parietal cortex showed distinct functional responses to manipulations of reward-related variables is consistent with the fact that they support partially segregated functions and do not form a functionally homogeneous network (Shulman et al., 2009).
3.2.5. No modulation by reward expectation in spatially-selective regions of dorsal fronto-parietal cortex
Along with the above analyses of reward-related signals in shift-related regions of dorsal and ventral fronto-parietal cortex, we examined the effects of reward-related signals in regions of the dorsal fronto-parietal cortex that showed sustained spatially selective signals and were likely involved in maintaining attention at a location (Shulman et al., 2009; Serences and Yantis, 2007; Silver and Kastner, 2009). As described in section 3.2.2. and shown in Supplementary Figure 1, spatially-selective regions of dorsal fronto-parietal cortex, which were identified from the voxelwise map of cue location by time, included bilateral regions of the IPS that were located lateral to the shift-related regions of the precuneus and small topographic regions in FEF. Using the same logic adopted for shift-related regions, we examined whether these regions were modulated by changes in expected reward magnitude in a particular direction and whether these modulations were associated with changes of spatial selectivity by testing the interaction between cue onset position (transition, stream) expected reward magnitude (high, low) and time (10 time points) and the interaction between cue onset position, expected reward magnitude, cue location (left, right) and time via regional ANOVAs (Supplementary Fig. 3, A-B-C). ANOVAs revealed that these regions were not significantly modulated either by a particular direction of change in expected reward magnitude or by the interaction of signals for directionally selective changes with spatially-selective signals (cue onset position by expected reward magnitude by time, right IPS: p=.9, left IPS: p=.7, left FEF: p=.1, right FEF: p=.2; cue onset position by expected reward magnitude by cue location by time, right IPS: p=.9, left IPS: p=.8, left FEF, right FEF: p=.6). For IPS regions, we additionally verified that the same null results were observed whether regions were selected from the voxelwise ANOVA comparing left and right cues (above results) or from the intersection of this voxelwise map with the co-registered average anatomical borders of human visual areas in the software suite Caret (Van Essen, 2004, 2005) (Supplementary Fig. 3D) (cue onset position by expected reward magnitude by time, right drawn IPS: p=.2, left drawn IPS: p=.5; cue onset position by expected reward magnitude by cue location by time, right drawn IPS: p=.7, left drawn IPS: p=.8).
3.2.6. Interaction between spatially selective signals and reward expectation in visual cortex
In contrast to the null effect of reward-related variables on the spatially selective signal in medial IPS and FEF, significant effects were observed in visual cortex. Regions of interest were selected in visual cortex based on a voxelwise ANOVA comparing left and right cues (averaging shift and stay cues) [see Fig. 4A for locations of selected regions of visual cortex in relation to the borders of human visual areas from the Caret software atlas (Van Essen, 2004, 2005)]. As no significant effects were found in these regions for the interaction of cue onset position, expected reward magnitude and time, regressors were collapsed across transition and stream cues and the time course of the BOLD response was analyzed in each ROI using ANOVAs with cue location (contralateral, ipsilateral), expected reward magnitude (high, low), cue type (shift, stay) and time as factors.
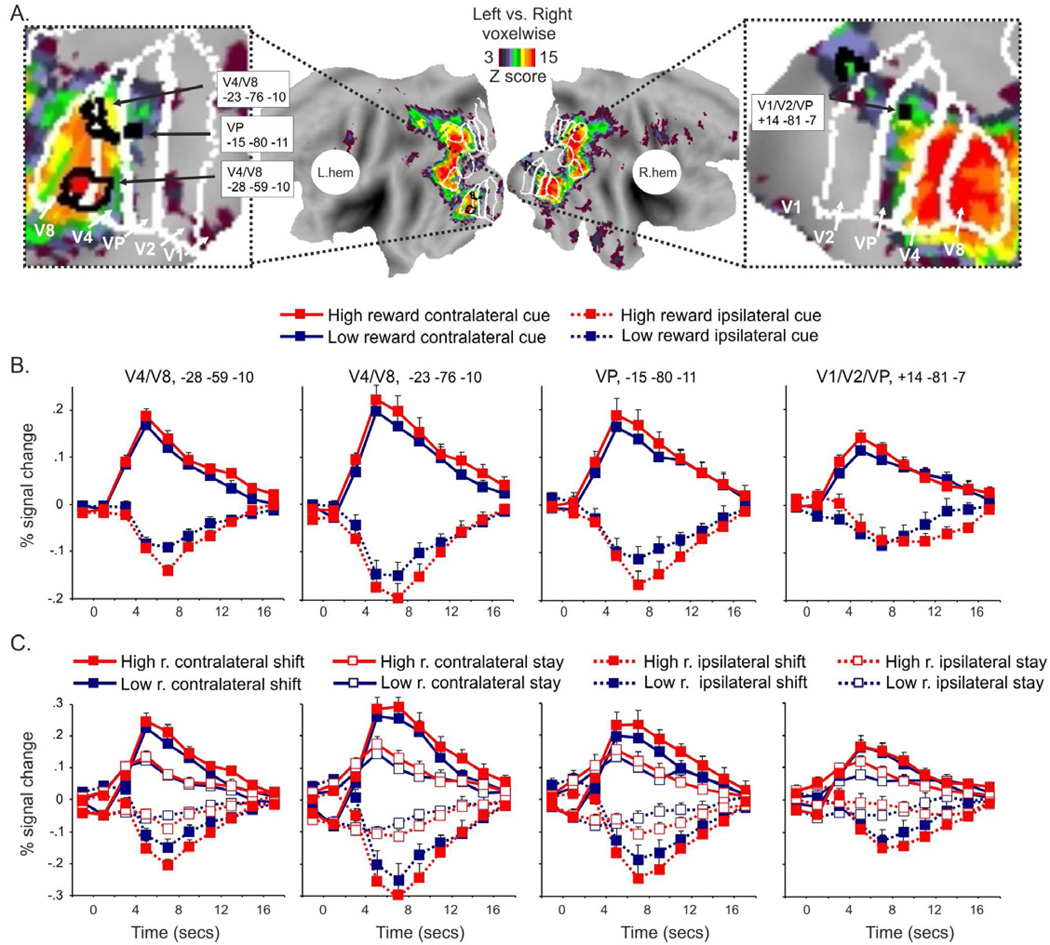
Figure 4. Effects of expected reward magnitude in visual cortex I.
A. Voxels that showed a significantly different activation following left and right cues (cue location by time ANOVA map, corrected for multiple comparisons) and four ROIs of visual cortex (black outlines) defined from the cue location by time map are superimposed over a flat representation of both hemispheres of the PALS atlas.
The graphs show the time course of the BOLD signal following high and low reward cues as a function of cue location (B) and cue location and cue type (C) in the four ROIs highlighted. Error bars represent within-subjects s.e.m calculated according to Cousineau, 2005.
As shown in Figure 4B, the four regions in ventral visual cortex that were identified from the voxelwise map of cue location by time showed an interaction between expected reward magnitude and the location of the attended stream. In particular, in clusters that approximately fell within the borders of areas VP/V4/V8 according to co-registered average anatomical borders of human visual areas in the software suite Caret (Van Essen, 2004, 2005), the difference between attended (contralateral) and unattended (ipsilateral) responses was significantly greater during periods of high than low reward expectation (interaction between expected reward magnitude, cue location and time, V4/V8, −28 −59 −10: F9,225=2.2 p=.02; V4/V8, −23 −76 −10: F9,225=2.1 p=.03; VP, −15 −80 −11: F9,225=1.8 p=.05; V1/V2/VP, +14 −81 −07: F9,225=1.7 p=.04) (Fig. 4B). Similar results were obtained when ROIs were drawn based on the intersection of the voxelwise activity map with the borders of human visual areas in the Caret software (Van Essen, 2004, 2005) (Supplementary Fig. 4A). This approach is not meant to substitute for individual retinotopic mapping of visual areas, but is a convenient way to identify large clusters of activity in visual cortex and it was used to confirm the effects of reward expectation on spatially-selective signals observed following regional ANOVAs on small regions in visual cortex selected on the voxelwise map of cue location by time. We drew five large regions in each hemisphere at the group level: V1/V2; VP/V4/V8; V3/V3A/V7; MT/LO.
Consistent with the results on regions of visual cortex identified from the cue location by time voxelwise map, a significant interaction between expected reward magnitude, cue location and time was observed in VP/V4/V8 (F9,225=2.3 p=.017) (Supplementary Fig. 4B). A significant interaction was also observed in right V1/V2 (F9,225=2.2 p=.02)(Supplementary Fig. 4C), while overall weaker effects were observed in MT/LO and V3/V3A/V7 (Supplementary Fig. 4D–E). The above findings indicate that high reward expectation modulated spatially-selective signals in visual cortex.
In contrast to the interactive influence of reward expectation on spatially selective signals for maintaining attention and for sensory modulations, reward expectation had an additive effect on signals related to shifts of attention. Figure 4C shows that in spatially selective regions of visual cortex the greater activity for contralateral than ipsilateral cues was much stronger for shift than stay cues. This effect has been previously described (Shulman et al., 2009), and is consistent with an attenuation of the sensory modulation when subjects hold attention onto the same stream of sensory stimuli (stay cues), as compared to when they shift attention to a new stream (shift cues). The novel finding displayed in Figure 4C is that high reward expectation produced the same increase in contralateral versus ipsilateral activity following stay and shift cues. This result was quantitatively confirmed by the absence of an interaction between cue type, cue location, expected reward magnitude and time in the spatially-selective regions described above (V4/V8, −28 −59 −10: p=.4; V4/V8, −23 −76 −10: p=.9; VP, −15 −80 −11: p=.7; V1/V2/VP, +14 −81 −07: p=.2). Therefore, expected reward magnitude did not affect the spatially selective neural activity in visual cortex that was specifically associated with a shift of attention, similar to the result reported earlier in dorsal fronto-parietal regions.
The overall conclusion is that in visual cortex, a maintained state of high expected reward magnitude affected the steady-state spatially-selective signals associated with the maintenance of attention at a location rather than the dynamic signals associated with shifts of attention to a different location. This result in visual cortex contrasted with the absence of reward-related modulations in spatially selective regions of IPS. One possible explanation is that the reduced spatial selectivity of dorsal fronto-parietal regions compared to regions of visual cortex may have weakened our ability to detect modulations by reward expectation. Another possibility is that our paradigm did not involve the specific reward-related functions carried out in IPS (see discussion for more details).
3.2.7. Modulations by reward detection in the caudate
We observed reward-related signals in a structure that has been classically associated with reward acquisition or expectation, the caudate (Knutson et al., 2000, 2001a, 2001b, 2003, 2005; Watanabe et al., 2003), providing further evidence that our manipulation of reward magnitude was effective. Supplementary Figure 5A displays the caudate region that was localized in a voxelwise ANOVA that compared the time courses of BOLD activity following high and low reward targets. As expected, the time courses of BOLD activity indicated that activity was greater for detection of high relative to low reward targets (averaging reward valid and invalid conditions) (Supplementary Fig. 5B). We also examined whether the neural activity in this region was additionally modulated by changes in expected reward magnitude in a specific direction and by shifts of spatial attention by conducting regional ANOVAs with cue onset position, expected reward magnitude and cue type on the caudate ROI formed from the voxelwise comparison of reward target detection. The results showed that, unlike regions in the posterior cingulate and dorsal fronto-parietal cortex, the caudate was not significantly modulated by changes in expected reward magnitude (Supplementary Fig. 5C, cue onset position by expected reward magnitude by time, p=.3) or by shifts of attention (Fig. 5D, cue type by time, p=.9). Overall, the results indicate that reward-related modulations in the caudate were most strongly evident for reward detection.
3.3. Eye Movements
Two different measures were computed to determine whether subjects maintained accurate fixation. The first measure was the mean difference in eye position during periods of attending to the left versus right RSVP streams. In one subject, excluded from further analyses, the mean difference value was −1.5 degree, which indicated a bias to fixate closer to the attended than unattended streams (negative values refer to fixation to the left). The remaining subjects showed, on average, a −.15 degree value (standard deviation, .4 degree), which indicated that eye position during attention to the left and right streams was well aligned to the central fixation cross.
The second measure was based on the event-related time course of eye position time-locked to the presentation of shift and stay cues that appeared on the right and the left RSVP streams. The eye position in the 100 msec interval following each event onset was used as a baseline to determine the relative change in eye position during 7 time bins that spanned the 2 seconds following cue onset (100 msec to 325 msec, 325 to 550, 550 to 775, 775 to 1000, 1000 to 1250, 1500 to 1750, and 1750 to 2000). Averaged across intervals, the mean change of eye position was −.1 degree for cues to shift attention to the left stream and .03 degrees for shift cues to the right. These small values indicate that subjects accurately maintained fixation not only during periods of sustained attention to the left and the right stream, but also following the onset of a cue that shifted attention from one location to another.
4. Discussion
The results showed that signals due to shifts of spatial attention and increases in expected reward magnitude were largely separate. First, regions in the posterior cingulate, which previously have been linked with processing of reward, showed significant activations for changes in expected reward magnitude from low to high but were not modulated by shifts of spatial attention. Regions in the dorsal attention network such as precuneus and FEF, which were strongly modulated by shifts of attention, showed modest effects of increases in expected reward magnitude, but these effects were similar for cues that shifted and maintained attention. Therefore, in the dorsal attention network, signals for increases in expected reward magnitude did not affect the dynamic signals associated with shifts of attention.
The above conclusions were based on a task in which shifts of spatial attention and increases in expected reward magnitude occurred with different frequencies. While shifts of attention occurred on average every 6.18 seconds (as did ‘stay’ cues), transition cues indicating an increase in expected reward magnitude were more infrequent and occurred on average every 30 seconds (as did ‘high to low’ transition cues). It is unlikely, however, that the sensitivity of posterior cingulate to increases in expected reward magnitude but not to shifts of spatial attention was a consequence of their different frequencies. In a previous fMRI study that used the current RSVP paradigm but manipulated the frequency of shifts of attention (Shulman et al., 2009), the posterior cingulate did not manifest any modulation by shifts of attention irrespective of shift frequency. Our results in dorsal fronto-parietal cortex were also unlikely to be caused by differences in condition frequency. We observed reward-related effects in these regions that were smaller in magnitude than the effects related to shifts of attention, while to the extent that condition frequency affects the BOLD response, a rare condition might be expected to produced greater a response than a frequent condition. We cannot rule out the possibility, however, that an interaction of signals for shifting attention and increases in expected reward might have occurred in these regions if both were equally infrequent. Finally, in regions of ventral visual cortex high reward expectation produced an increase in spatially-selective signals related to the maintenance of attention and sensory-evoked modulations at a location. Because these regions are specialized for the perception and discrimination of visual features, these reward-related modulations may be useful for rapidly detecting target objects that confer high monetary reward.
4.1. Reward- and attention-related signals in the posterior cingulate
A major finding of our study is that neural activity in the posterior cingulate was particularly sensitive to changes in expected reward magnitude from low to high but not to shifts of spatial attention. These results clarify both the nature of reward-related representations in the posterior cingulate and its involvement in spatial attention, especially in relation to previous findings in humans and non-human primates.
Previous neurophysiological studies have suggested that the posterior cingulate is specifically implicated in monitoring reward outcomes that prompt changes of future behavior. In choice tasks in which a monkey’s behavior on a given trial was strongly influenced by the reward outcome experienced on previous trials, not only were neurons in the posterior cingulate sensitive to reward outcomes, but these outcome-contingent modulations persisted across subsequent trials and predicted a change of choice behavior (Hayden et al., 2008; Pearson, et al., 2009, 2011). Our results in humans are partly consistent with these findings, but also suggest a particular sensitivity to stimuli signaling an increase in expected reward. The posterior cingulate responded more strongly to high reward transition cues than to high reward stream cues, indicating that it was more associated with monitoring for events that signal increases in expected reward than a maintained state of high expected reward.
Our results also allow clear conclusions about the role of the posterior cingulate during shifts of spatial attention. The evidence that posterior cingulate activity is more strongly correlated with the behavioral benefit of anticipatory shifts of attention when a monetary incentive is expected and that it is anatomically located in a zone of transition between core limbic areas and fronto-parietal neocortex (Pandya et al., 1981; Baleydier and Mauguiere, 1980; Morecraft et al., 1993; Cavada and Goldman-Rakic, 1989; Vogt and Pandya, 1987), has led some authors to propose that it is a critical component of the attention network and mediates the motivational guidance of spatial attention (Mesulam, 1981; Small et al., 2003, 2005; Mohanty et al., 2008).
The view that the posterior cingulate encodes signals for shifts of spatial attention, however, is hard to reconcile both with the neurophysiological findings that posterior cingulate neurons are typically activated following saccades, which suggest that they monitor rather than control shifts of visual attention (Vogt et al., 1992), and with the evidence from human neuroimaging studies for activation of dorsal fronto-parietal cortex but not posterior cingulate during the cueing phase of spatial attention paradigms (Corbetta and Shulman, 2002; Corbetta et al., 2008).
In the original studies by Mesulam and colleagues, activation of the posterior cingulate was correlated with the speed of target detection during Posner cueing paradigms but was not significantly greater for shifts of attention than for baseline conditions in random-effect analyses (Mesulam et al., 2001). The correlation between detection speed and activation was enhanced by expectation of monetary incentives in more recent studies on the attention-motivation interaction (Small et al., 2005; Mohanty et al., 2008). However, these studies did not separate cue-related responses from evoked responses related to target detection or reward feedback, leaving unclear whether shifts of attention modulated the posterior cingulate. In particular, the observed correlations may have reflected non-specific effects of shorter RTs or processes active at target detection rather than during the preceding shift of spatial attention. In addition, because these studies always contrasted directional cues with neutral cues, the observed modulations of posterior cingulate may have reflected the differential motivational significance associated with the different sensory stimuli that cued a diffuse versus a focused distribution of attention. Consistent with this interpretation, a recent study that separated cue and target activity found weak or nearly absent responses of the posterior cingulate to cues that induced an anticipatory shift of attention relative to neutral cues (see Supplementary Figure in Mohanty et al., 2009). In our study, the temporal independence of cue and target stimuli and the use of identical shift and stay cues, both of which signaled a focused, peripheral distribution of attention, allowed a well-controlled measurement of any selective modulations specifically due to shifts of spatial attention. Moreover, our null effects of spatial attention were observed in posterior cingulate regions that showed significant reward-related modulations and had Talairach coordinates that corresponded well with those from previous studies (Small et al., 2005; Mohanty et al., 2008, 2009).
The predominant involvement of the posterior cingulate in reward-related variables rather than shifts of spatial attention is supported by the findings of a recent fMRI study demonstrating that activity in the posterior cingulate directly participates in the evaluation process of monetary rewards during inter-temporal choices (Kable and Glimcher, 2007). The observation that posterior cingulate encoded the subjective value of reward-related choices independently of the specific motor parameters used to communicate the choice have led to the proposal that it is part of a network of regions that evaluates choice options and then provide input to structures in the fronto-parietal cortex that convert the output of the evaluation process in action value i.e. the value of the particular actions used to communicate the choice.
In conclusion, based on our results, previous neurophysiological studies, and the above considerations of prior neuroimaging studies, we suggest that the posterior cingulate primarily encodes motivational variables rather than signals for shifts of spatial attention. Our results additionally suggested that the motivational signals encoded in the posterior cingulate are particularly related to environmental events that signal an increase in expected reward.
4.2. Reward and attention-related signals in shift-related regions of dorsal fronto-parietal cortex
We found that expectation of different levels of reward magnitude modulated the activity of regions of the dorsal attention network, consistent with other studies (Krawczyk et al., 2007; Leon and Shadlen, 1999; Hikosaka and Watanabe, 2000; Watanabe et al., 2002; Kobayashi et al., 2002; Mohanty et al., 2009; Engelmann et al., 2009).
The novelty of the current study is that the use of different types of cues allowed us to both determine the nature of the reward-related representations in these regions and to examine how signals for reward expectation affected the typical attention-related signals in these regions.
Regions in the FEF and precuneus that showed robust signals for shifting attention from one location to another were additionally modulated by reward-related variables such as stimuli that indicated a change of reward expectation from low to high.
These effects of expected reward magnitude, however, did not modulate the dynamic signals associated with shifts of attention. Instead, the BOLD signal was additively increased following cues to shift or maintain attention, in regions both ipsilateral and contralateral to the cued location. These results indicate that reward expectation and shifts of spatial attention were both encoded in these regions but independently from one another.
The independent modulations for shifts of attention and shifts of reward contingencies in the precuneus are reminiscent of the recent proposal that the precuneus is a source of domain-independent cognitive control. In particular, the evidence that precuneus is transiently activated by shifts of attention in multiple domains (shifts of spatial attention, shifts of feature attention, shifts between categorization rules and shifting attention in working memory) has suggested that it represents a common hub for the control of shift-related signals that reconfigure the brain for current task goals (Chiu and Yantis, 2009; Esterman et al., 2009; Greenberg et al., 2010). In our study, however, the activation peak in the precuneus was more anterior and dorsal than the common shift-related activations in these studies, and precuneus activations were observed for both shifts of spatial attention and shifts from low to high reward expectation, but not for shifts from high to low reward expectation. One possible explanation is that a pre-requisite of the transient response of the precuneus during across-domain shifts of attention is that the shifts are associated with a change of behavioral performance or attentional selection. Consistently, both the shift-related signals in the above mentioned studies and the shifts of spatial attention and reward contingencies from low to high reward expectation in our study involved a resetting of attentional selection or behavioral performance, which may not have occurred for shifts from high to low reward. Furthermore, the independence of signals for shifts of spatial attention and reward expectation observed in our study is consistent with the evidence that the across-domain region of activation in the precuneus contains multiple subpopulations of neurons, each specialized for shifting attention in a particular domain (Chiu and Yantis, 2009; Esterman et al., 2009; Greenberg et al., 2010). In summary, our findings distinguish the posterior cingulate, which specifically encodes signals for changes of reward contingencies in a direction that is relevant to behavior and is not modulated by shifts of spatial attention, from attention regions in dorsal fronto-parietal cortex, which encode independent signals for shifts of attention and expected reward magnitude. Based on the anatomical connectivity of posterior cingulate with both reward-related and oculomotor areas of the brain (Pandya et al., 1981; Baleydier and Mauguiere, 1980; Morecraft et al., 1993; Cavada and Goldman-Rakic, 1989; Vogt and Pandya, 1987), one possibility is that neurons in the posterior cingulate provide information about increases in reward expectation, irrespective of whether attention is concurrently shifted or maintained, to regions of dorsal fronto-parietal cortex that are involved in shifting attention.
4.3. Reward and attention-related signals in spatially-selective regions of the dorsal fronto-parietal cortex
In contrast to shift-related regions of dorsal fronto-parietal cortex, we found no evidence of reward modulations in regions of medial IPS that showed the most robust spatially-selective signals related to the maintenance of attention to the contralateral versus ipsilateral visual streams of objects. Therefore, expected reward magnitude did not affect the neural signals in dorsal fronto-parietal cortex that were specifically associated with either shifting attention or maintaining attention at a specific location.
Particularly for spatially-selective regions in the IPS, these results might appear to conflict with electrophysiological evidence of reward modulations in monkey area LIP (Platt and Glimcher, 1999; Sugrue et al., 2004). A recent study by Peck and colleagues (Peck et al., 2009), for example, examined neural responses in LIP to cues that predicted reward but not the location of targets at which a saccadic movement conferred the predicted reward. The authors showed that, although maladaptive to optimal performance, LIP activity indicated an attentional bias toward the location of cue stimuli that predicted the eventual receipt of reward, and concluded that LIP translated the results of reward evaluation into spatial behaviors. LIP neurons, moreover, have been implicated in the encoding the expected value of targets lying within their response field in other neurophysiological work that used either a cued or a decision-making saccade task (Platt and Glimcher, 1999; Sugrue et al., 2004).
It important to note, however, that in all of these experiments the critical manipulation associated different reward magnitudes or probabilities with different locations in the visual field (whether inside or outside the receptive field of the neuron under study). This feature of the experimental design might foster the typical “salience” representation of the environment described in LIP neurons (Gottlieb, 2007; Bisley and Goldberg, 2010). In our experiment, however, although processes of selective spatial attention were robustly taxed by the presence of adjacent, irrelevant distracter RSVP streams (three on each side) and the brief presentation of the target stimuli, rewards only appeared at locations at which subjects were already attending, minimizing signals related to choice behavior. Therefore, although our paradigm activated visuo-spatial maps in parietal cortex, these maps may not have been needed specifically to encode reward–related variables for the purpose of guiding spatial attention. In other words, because rewards were not acquired through choice behavior but were conflated with the locus of attention, the use of parietal spatial maps to guide reward-related choice behavior may have been minimized.
4.4. Reward and attention-related signals in visual cortex
In visual cortex high expected reward magnitudes increased the sustained spatially-selective signals associated with the maintenance of spatial attention and sensory-evoked modulation, i.e. they increased the difference between attending to contralateral versus ipsilateral locations. The presence of reward modulation in areas such as VP/V4/V8, which are specialized for feature discrimination (Desimone and Schein, 1987; Reynolds and Chelazzi, 2004; Orban, 2008), is consistent with the heavy emphasis of our task on object recognition and is reminiscent of recent findings from electrophysiological and imaging studies showing that stimulus features that have been associated with higher reward magnitudes are more robustly represented in regions of the visual cortex that selectively encode those features (Franko et al., 2010; Hickey et al., 2010; Serences and Saproo, 2010).
What is less clear, however, is the mechanisms by which reward expectation affected the spatially-selective signals in these areas. The first and simplest explanation is that high reward expectation induced a stronger contralateral bias of attention that in turn produced a stronger spatially selective sensory evoked modulation in visual cortex. While we have no direct evidence against this view, it is puzzling that a similar modulation of spatially selective signals was not observed in the dorsal fronto-parietal regions that are often thought to control spatial attention.
An alternative explanation is that reward modulated a fronto-parietal signal for feature attention related to the representation of the target object, rather than spatial attention (Greenberg et al., 2010). This fronto-parietal signal, which might correspond to the reward-related activation we observed in FEF and precuneus that did not interact with spatial position, would be transmitted to visual cortex independently of whether attention was directed to the contralateral or ipsilateral hemifield (Serences and Boynton, 2007). Within visual cortex, the reward-enhanced signal for feature attention would be combined with the sustained, spatially-selective signals from attention-related regions of the IPS and FEF that are traditionally considered the source of modulatory signals for spatial attention in visual cortex (Blankenburg et al., 2010; Ruff et al., 2006, 2008; Ekstrom et al., 2008; Moore and Armstrong, 2003; Gregoriou et al., 2009; Bressler et al., 2008). In other words, another possible explanation for the observed reward-related enhancement of the spatially-selective signals in visual cortex was that a feature-attention signal related to the perceptual representation of the target object was increased under periods of high reward expectation, fed down to visual cortex, and interacted with spatial-attention signals that were also fed down.
We acknowledge that we cannot distinguish these two possibilities, nor do we have evidence that the reward-related signals observed in visual cortex involved modulations from dorsal fronto-parietal regions.
4.5. Conclusions
In this manuscript we found that signals for increases in reward expectation and shifts of spatial attention were largely independent. Regions of posterior cingulate that showed robust activations for increases in expected reward magnitude were not modulated by shifts of spatial attention. Therefore, posterior cingulate showed only reward-related signals. In contrast, regions in dorsal fronto-parietal cortex showed both reward-related and shift-related modulations, but the former were modest and smaller than the latter and the two sets of signals were largely independent. Increases in expected reward did not change the magnitude of the transient activations associated with shifting attention, relative to maintaining attention. High expected reward, relative to low expected reward, did increase the magnitude of the sustained activations in visual cortex associated with maintaining attention to the contralateral visual field, relative to the ipsilateral visual field. This effect was not observed, however, in spatially selective regions of dorsal fronto-parietal cortex, mirroring the null effect of increases in expected reward on shift-related signals. Therefore, while high reward expectation increased the modulatory effect of attention on spatially-selective signals in visual cortex, high reward expectation and spatial attention produced largely independent effects in higher level cortical regions.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arrington CM, Carr TH, Mayer AR, Rao SM. Neural mechanisms of visual attention: Object-based selection of a region in space. Journal of Cognitive Neuroscience. 2000;12(Suppl 2):106–117. doi: 10.1162/089892900563975. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Mauguiere F. The duality of the cingulate gyrus in monkey. Neuroanatomical study and functional hypothesis. Brain. 1980;103(3):525–554. doi: 10.1093/brain/103.3.525. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annual Review of Neuroscience. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Josephs O, Deichmann R, Driver J. Studying the role of human parietal cortex in visuospatial attention with concurrent tms-fmri. Cerebral Cortex. 2010;20(11):2702–2711. doi: 10.1093/cercor/bhq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. The Journal of Neuroscience. 2008;28(40):10056–10061. doi: 10.1523/JNEUROSCI.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. The Journal of Comparative Neurology. 1989;287(4):393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Chiu YC, Yantis S. A domain-independent source of cognitive control for task sets: Shifting spatial attention and switching categorization rules. The Journal of Neuroscience. 2009;29(12):3930–3938. doi: 10.1523/JNEUROSCI.5737-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, Mcavoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3(3):292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Buchel C, Nobre AC. Orienting attention in time: Behavioural and neuroanatomical distinction between exogenous and endogenous shifts. Neuropsychologia. 2000;38(6):808–819. doi: 10.1016/s0028-3932(99)00132-3. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC. Where and when to pay attention: The neural systems for directing attention to spatial locations and to time intervals as revealed by both pet and fmri. Journal of Neuroscience. 1998;18(18):7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within subject designs: A simpler solution to Loftus and Masson's method. Tutorial in Quantitative Methods for Psychology. 2005;1(1):42–45. [Google Scholar]
- Desimone R, Schein SJ. Visual properties of neurons in area v4 of the macaque: Sensitivity to stimulus form. Journal of Neurophysiology. 1987;57(3):835–868. doi: 10.1152/jn.1987.57.3.835. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nature Neuroscience. 2000;3(3):277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Ekstrom LB, Roelfsema PR, Arsenault JT, Bonmassar G, Vanduffel W. Bottom-up dependent gating of frontal signals in early visual cortex. Science. 2008;321(5887):414–417. doi: 10.1126/science.1153276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Damaraju E, Padmala S, Pessoa L. Combined effects of attention and motivation on visual task performance: Transient and sustained motivational effects. Frontiers in Human Neuroscience. 2009;3:4. doi: 10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Chiu YC, Tamber-Rosenau BJ, Yantis S. Decoding cognitive control in human parietal cortex. Proceeding of the National Academy of Sciences of the United States of America. 2009;106(42):17974–17979. doi: 10.1073/pnas.0903593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fmri): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Franko E, Seitz AR, Vogels R. Dissociable neural effects of long-term stimulus-reward pairing in macaque visual cortex. Journal of Cognitive Neuroscience. 2010;22(7):1425–1439. doi: 10.1162/jocn.2009.21288. [DOI] [PubMed] [Google Scholar]
- Gottlieb J. From thought to action: The parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53(1):9–16. doi: 10.1016/j.neuron.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Esterman M, Wilson D, Serences JT, Yantis S. Control of spatial and feature-based attention in frontoparietal cortex. The Journal of Neuroscience. 2010;30(43):14330–14339. doi: 10.1523/JNEUROSCI.4248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324(5931):1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, JR, Sereno MI. Spatial maps in frontal and prefrontal cortex. Neuroimage. 2006;29(2):567–577. doi: 10.1016/j.neuroimage.2005.08.058. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Nair AC, Mccoy AN, Platt ML. Posterior cingulate cortex mediates outcome-contingent allocation of behavior. Neuron. 2008;60(1):19–25. doi: 10.1016/j.neuron.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward changes salience in human vision via the anterior cingulate. The Journal of Neuroscience. 2010;30(33):11096–11103. doi: 10.1523/JNEUROSCI.1026-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cerebral Cortex. 2000;10(3):263–271. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- Jack AI, Patel GH, Astafiev SV, Snyder AZ, Akbudak E, Shulman GL, Corbetta M. Changing human visual field organization from early visual to extra-occipital cortex. PLoS One. 2007;2(5):e452. doi: 10.1371/journal.pone.0000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley TA, Serences JT, Giesbrecht B, Yantis S. Cortical mechanisms for shifting and holding visuospatial attention. Cerebral Cortex. 2008;18(1):114–125. doi: 10.1093/cercor/bhm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DL, Gusnard DA, Snyder AZ, Raichle ME. Effect of practice on reading performance and brain function. Neuroreport. 2004;15(4):607–610. doi: 10.1097/00001756-200403220-00007. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience. 2001a;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fmri. Neuroreport. 2001b;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event-related fmri. Neuroimage. 2003;18(2):263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. The Journal of Neuroscience. 2005;25(19):4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Lauwereyns J, Koizumi M, Sakagami M, Hikosaka O. Influence of reward expectation on visuospatial processing in macaque lateral prefrontal cortex. Journal of Neurophysiology. 2002;87(3):1488–1498. doi: 10.1152/jn.00472.2001. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Gazzaley A, D'esposito M. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Research. 2007;1141:168–177. doi: 10.1016/j.brainres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN. Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron. 1999;24(2):415–425. doi: 10.1016/s0896-6273(00)80854-5. [DOI] [PubMed] [Google Scholar]
- Mccoy AN, Platt ML. Expectations and outcomes: Decision-making in the primate brain. Journal of Comparative Physiology A Neuroethology, Sensory, Neural, and Behavioral Physiology. 2005;191(3):201–211. doi: 10.1007/s00359-004-0565-9. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10(4):309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Nobre AC, Kim YH, Parrish TB, Gitelman DR. Heterogeneity of cingulate contributions to spatial attention. Neuroimage. 2001;13(6 Pt 1):1065–1072. doi: 10.1006/nimg.2001.0768. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Egner T, Monti JM, Mesulam MM. Search for a threatening target triggers limbic guidance of spatial attention. The Journal of Neuroscience. 2009;29(34):10563–10572. doi: 10.1523/JNEUROSCI.1170-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A, Gitelman DR, Small DM, Mesulam MM. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cerebral Cortex. 2008;18(11):2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421(6921):370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Architecture of connectivity within a cingulo-fronto-parietal neurocognitive network for directed attention. Archives of Neurology. 1993;50(3):279–284. doi: 10.1001/archneur.1993.00540030045013. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001a;13(1):218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001b;13(1):210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Orban GA. Higher order visual processing in macaque extrastriate cortex. Physiological Review. 2008;88(1):59–89. doi: 10.1152/physrev.00008.2007. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Van Hoesen GW, Mesulam MM. Efferent connections of the cingulate gyrus in the rhesus monkey. Experimental Brain Research. 1981;42(3–4):319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Hayden BY, Raghavachari S, Platt ML. Neurons in posterior cingulate cortex signal exploratory decisions in a dynamic multioption choice task. Current Biology. 2009;19(18):1532–1537. doi: 10.1016/j.cub.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML. Posterior cingulate cortex: Adapting behavior to a changing world. Trends in Cognitive Science. 2011;15(4):143–151. doi: 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck CJ, Jangraw DC, Suzuki M, Efem R, Gottlieb J. Reward modulates attention independently of action value in posterior parietal cortex. The Journal of Neuroscience. 2009;29(36):11182–11191. doi: 10.1523/JNEUROSCI.1929-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The videotoolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Platt Ml, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400(6741):233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annual Review of Neuroscience. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Bestmann S, Blankenburg F, Bjoertomt O, Josephs O, Weiskopf N, Deichmann R, Driver J. Distinct causal influences of parietal versus frontal areas on human visual cortex: Evidence from concurrent tms-fmri. Cerebral Cortex. 2008;18(4):817–827. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent tms-fmri and psychophysics reveal frontal influences on human retinotopic visual cortex. Current Biology. 2006;16(15):1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades. The Journal of Neuroscience. 2006;26(19):5098–5108. doi: 10.1523/JNEUROSCI.5330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Glimcher P, Heeger DJ. Topographic organization for delayed saccades in human posterior parietal cortex. Journal of Neurophysiology. 2005;94(2):1372–1384. doi: 10.1152/jn.01290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80(1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Current Opinion in Neurobiology. 2004;14(2):139–147. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Serences JT, Boynton GM. Feature-based attentional modulations in the absence of direct visual stimulation. Neuron. 2007;55(2):301–312. doi: 10.1016/j.neuron.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Serences JT, Saproo S. Population response profiles in early visual cortex are biased in favor of more valuable stimuli. Journal of Neurophysiology. 2010;104(1):76–87. doi: 10.1152/jn.01090.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Spatially selective representations of voluntary and stimulus-driven attentional priority in human occipital, parietal, and frontal cortex. Cerebral Cortex. 2007;17(2):284–293. doi: 10.1093/cercor/bhj146. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294(5545):1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Franke D, Pope DL, Snyder AZ, Mcavoy MP, Corbetta M. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. The Journal of Neuroscience. 2009;29(14):4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends in Cognitive Science. 2009;13(11):488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. Journal of Neurophysiology. 2005;94(2):1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage. 2003;18(3):633–641. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman D, Simmons K, Bloise SM, Parrish T, Mesulam MM. Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cerebral Cortex. 2005;15(12):1855–1865. doi: 10.1093/cercor/bhi063. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. A componential analysis of the erp elicited by novel events using a dense electrode array. Psychophysiology. 1999;36(3):409–414. doi: 10.1017/s0048577299981180. [DOI] [PubMed] [Google Scholar]
- Stevens AA, Skudlarski P, Gatenby JC, Gore JC. Event-related fmri of auditory and visual oddball tasks. Magnetic Resonance Imaging. 2000;18(5):495–502. doi: 10.1016/s0730-725x(00)00128-4. [DOI] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science. 2004;304(5678):1782–1787. doi: 10.1126/science.1094765. [DOI] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Toward a modern theory of adaptive networks: Expectation and prediction. Psychological Review. 1981;88(2):135–170. [PubMed] [Google Scholar]
- Swisher JD, Halko MA, Merabet LB, Mcmains SA, Somers DC. Visual topography of human intraparietal sulcus. The Journal of Neuroscience. 2007;27(20):5326–5337. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, editors. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publisher, Inc; 1988. [Google Scholar]
- Van Essen DC. Towards a quantitative, probabilistic neuroanatomy of cerebral cortex. Cortex. 2004;40(1):211–212. doi: 10.1016/s0010-9452(08)70954-7. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (pals) atlas of human cerebral cortex. Neuroimage. 2005;28(3):635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: The anterior executive and posterior evaluative regions. Cerebral Cortex. 1992;2(6):435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Vogt BA, PANDYA DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. The Journal of Comparative Neurology. 1987;262(2):271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Lauwereyns J, Hikosaka O. Neural correlates of rewarded and unrewarded eye movements in the primate caudate nucleus. The Journal of Neuroscience. 2003;23(31):10052–10057. doi: 10.1523/JNEUROSCI.23-31-10052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Hikosaka K, Sakagami M, Shirakawa S. Coding and monitoring of motivational context in the primate prefrontal cortex. The Journal of Neuroscience. 2002;22(6):2391–2400. doi: 10.1523/JNEUROSCI.22-06-02391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nature Neuroscience. 2002;5(10):995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.