Abstract
Biodegradable nanofibers are important scaffolding materials for bone regeneration. In this paper, the basic concepts and recent advances of self-assembly, electrospinning and thermally induced phase separation techniques that are widely used to generate nanofibrous scaffolds are reviewed. In addition, surface functionalization and bioactive factor delivery within these nanofibrous scaffolds to enhance bone regeneration are also discussed. Moreover, recent progresses in applying these nanofiber-based scaffolds to deliver stem cells for bone regeneration are presented. Along with the significant advances, challenges and obstacles in the field as well as the future perspective are discussed.
Keywords: Bone tissue engineering, Nanofibrous scaffold, Osteogenic microenvironment, Bioactive agent delivery, Stem cell delivery
1. Introduction
Bone fracture healing engages both intramembranous and endochondral ossification processes, with the latter involving cartilaginous callus formation. The callus formation is dependent on the recruitment of progenitor cells from the surrounding tissues, and the formed callus undergoes vascularization, calcification and remodeling into normal bone restoring biomechanical properties [1]. The fracture healing process recapitulates many events of embryologic or postnatal bone development, which is biochemically and biophysically orchestrated by soluble factors, cell-cell and cell-matrix interactions [2]. However, fractures and defects greater than a critical size are unable to heal autogenously, where substitutionary materials have to be employed to fill the injured bone sites in the clinic. The current treatments using metallic stabilizers or permanent implants to serve as a passive physical support often have drawbacks such as stress shielding, infections and chronic pain, among other side effects [3]. Bone allografts from human donors provide an alternative solution, which can address some of the issues by taking advantage of the similarities in physical and biological properties between the host and donor tissues. However, there are risks of infection, immune rejection, and disease transmission associated with allografts [3, 4].
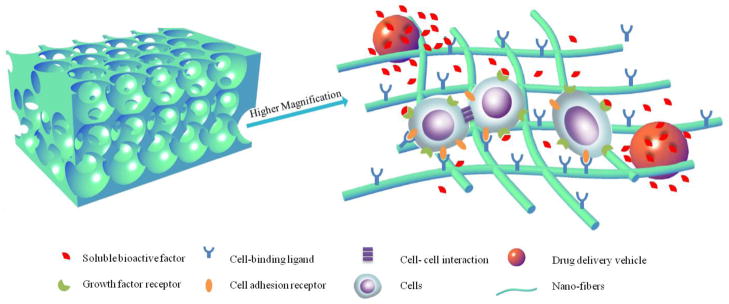
Bone tissue engineering, by using a biomimetic scaffold to construct a synthetic osteogenic microenvironment to facilitate the natural ossification processes, holds promise of developing an improved clinical therapy [5, 6]. An ideal biomimetic scaffold not only provides physical support, but preferably also delivers bioactive agents and/or stem cells, in a three-dimensionally (3D) controlled manner (Figure 1).
Figure 1.
Schematic illustration of a biomimetic nano-fibrous scaffold with integrated synthetic osteogenic microenvironment. The macro-porous scaffold can not only provide a physical structure that accommodates cells and tissue formation, but also serve as a ECM-mimicking matrix to enhance cell-scaffold interactions and delivery of bioactive agents and/or stem cells in a three-dimensionally controlled manner.
First, unlike a permanent implant, a scaffold provides temporary physical support before the neo-tissue takes over, which could be accomplished by using biodegradable materials [4, 7–9]. Some artificial features, such as a macro-porous structure with an interconnected pore network, are often incorporated into the scaffold to promote deep cell infiltration and accommodate neo-tissue formation [5, 10–13].
Second, the surface architecture and chemistry of the scaffold can be engineered to encourage its positive interactions with cells [14]. Collagen is the major organic component of the bone extracellular matrix (ECM), which is present in the form of a fibrous network with fiber diameters ranging from 50 to 500 nm [15]. Numerous studies showed that collagen fibers promoted osteogenesis [16, 17]. Therefore, scaffolds with nanofibrous (NF) architecture were developed to mimic the structural features and hopefully the pro-osteogenic properties of collagenous ECM of the bone [18–20]. These scaffolds were indeed found to enhance osteogenesis [6, 21–25]. In this review, fibers with diameters from a few nanometers to a few micrometers, intended for tissue engineering applications, are collectively called ‘nanofibers’. Three commonly used techniques to fabricate NF structure, namely, molecular self-assembly, electrospinning and thermally-induced phase separation will be discussed.
Third, the synthetic osteogenic microenvironment should contain bioactive agents and support osteogenic differentiation of stem cells. Many types of bioactive agents, including growth factors [26], nucleic acids [27] and integrin-binding ligands [28] have been incorporated into scaffolds. For example, growth factors are very important soluble signals in the osteogenic microenvironment, which are actively involved in tissue ingrowth, cell recruiting, proliferation, angiogenesis and osteogenesis.
In addition, the field of stem cell biology is rapidly evolving, with more and more available stem cell sources [29–37] to be used with scaffolds for bone regeneration [38, 39]. However, the delivery of bioactive agents and stem cells on nanofiber-based scaffolds is significantly affected by the specific type of nanofibrous scaffolds. There remain challenges in delivering them effectively for bone tissue engineering application. The delivery strategies for both bioactive agents and stem cells are a focus of this review article.
Collectively, this review is started with current techniques to fabricate NF scaffolds, followed by the delivery strategies for growth factors and other bioactive agents, and concluded with bone tissue engineering and translational studies that employ stem cells and the NF scaffolds.
2. Fabrication of NF Scaffolds
We will focus our discussion on three main techniques to fabricate nanofibers: molecular self-assembly, electrospinning and thermally induced phase separation.
2.1 Molecular self-assembly
Molecular self-assembly is the spontaneous organization of individual molecules into structurally-defined stable arrangements through preprogrammed noncovalent interactions, such as hydrogen bonds, van der Waals forces, hydrophobic interactions and electrostatic interactions [40–43]. Self-assembly is a bottom-up approach to create nanofibers from small building blocks, including small molecules, peptides and nucleic acids.
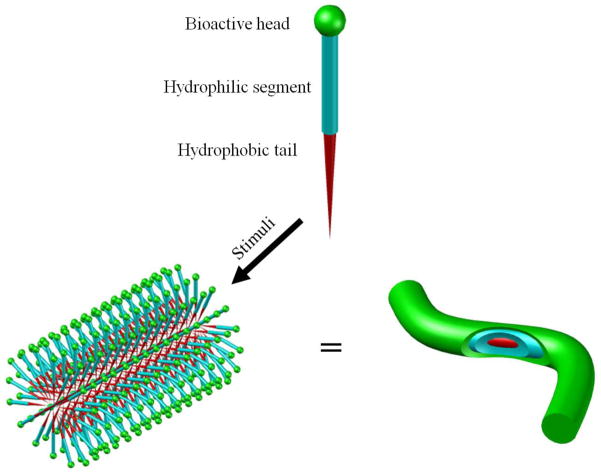
To mimic the triple helical structure of collagen, peptide-amphiphiles (PAs) were synthesized [44–46]. Such a PA consists of a collagen sequence Gly-Val-Lys-Gly-Asp-Lys-Gly-Asn-Pro-Gly-Trp-Pro-Gly-Ala-Pro connected to a long-chain mono- or di-alkyl ester lipid. The collagen sequence peptide head group forms the triple helical structure, while the lipophilic tails associate with one another via hydrophobic interactions to induce and/or stabilize the three-dimensional structure of the collagen sequence peptide head group [46]. The self-assembled triple helical structure of the PA promotes cell adhesion [47]. However, such lipids were not demonstrated to form supramolecular fibers [5].
Other types of peptide-amphiphiles (PAs) were later synthesized and employed to self-assemble into nanofibers. The self-assembly of such PAs into nanofibers can be driven either by pH change or the introduction of divalent ions (such as Ca2+), which neutralize the electrostatic repulsion between molecules to allow for clustering of the hydrophobic tails in the core and to expose the amino acids on the outer surface of the fibers (commonly a few nm in diameter) in an aqueous environment [48] (Figure 2). Under similar principles, synthetic diblock/triblock polypeptides and dendrimers can also self-assemble into nanofibrous structures [49–51].
Figure 2.
Schematic illustration showing the self-assembly approach to fabricate nanofibers with desired surface functionalities. Peptide amphiphiles are used as an example here.
Ionic self-complementary oligopeptides can also serve as building blocks to self-assemble into nanofibers [52–54]. The oligopeptides consist of repeating ionic hydrophilic and hydrophobic amino acids. In water, these oligopeptides form a beta-sheet with two surfaces: a polar surface of charged ionic chains and a non-polar surface of alanine. Upon exposure to monovalent alkaline cations or physiological conditions, the oligopeptides spontaneously assemble into hydrogels, composed of interwoven nanofibers with a diameter of 10–20 nm and pores of 50–200 nm [52–54].
The advantages of self-assembly for nanofiber fabrication include the simple fabrication process, easy cell encapsulation in a hydrogel, the injectable form for in situ scaffold formation. Although the mechanical properties of hydrogels self-assembled from PAs can be adjusted to a certain degree [55], they are often insufficient to provide a stable 3D geometry for bone tissue engineering. There are also other limitations such as the limited choice of self-assembly molecules, the often time-consuming and costly PA synthesis, the difficulty in controlling pore size and shape within the hydrogel scaffold, and the significantly smaller fiber diameter than that of collagen.
2.2 Electrospinning
2.2.1 Nanofiber fabrication
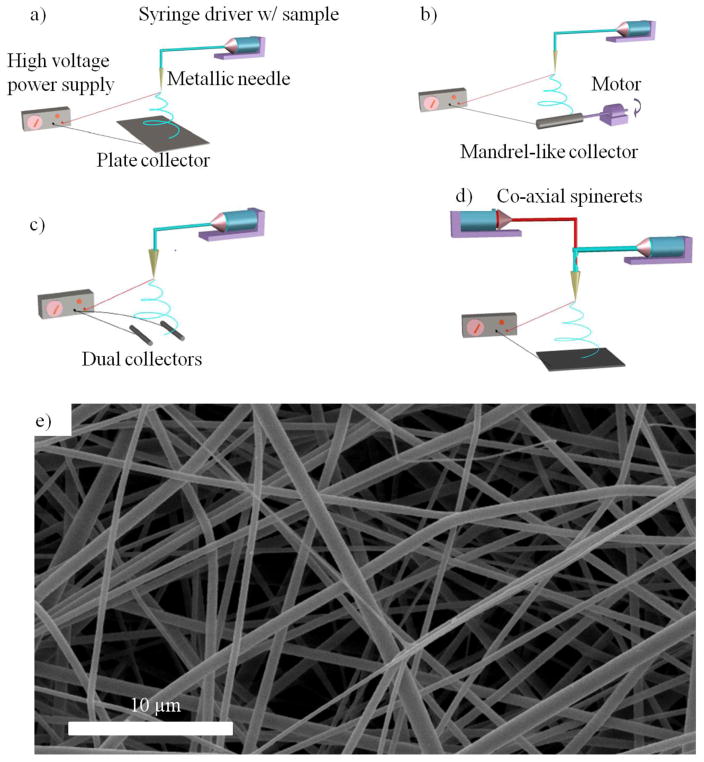
Electrospinning technique has been widely used for the fabrication of tissue engineering scaffolds [20, 56, 57]. The basic electrospinning setup includes a polymer solution or melt reservoir, grounded collector, and a high voltage electric field in between (Figure 3a) [58]. When the voltage is high enough to overcome the surface tension of the polymer solution/melt, a charged jet is generated towards the grounded collector, along which the solvent evaporates/melt solidifies to form solid-state thin fibers [59–61]. Electrospun NF scaffolds have been generated from a large variety of materials ranging from natural macromolecules such as collagen [56], chitosan [62] and silk fibroin [63], to synthetic biodegradable polymers such as poly(glycolic acid) (PGA) [64, 65], poly(lactic acid) (PLA) [65], and their copolymers (PLGA) [65]. Different polymers can also be mixed for electrospinning to generate blend nanofibers [64, 66, 67]. Recently, inorganic nanofibers were also created and employed in bone tissue engineering [68].
Figure 3.
Schematic illustration of the electrospinning setup with: a) a grounded plate collector; b) a grounded mandrel-like collector; c) parallel dual collectors; d) core-shell dual spinnerets; and e) an SEM image of electrospun nanofibers from a PLLA solution. e) is from He et al [203], Copyright © 2010 by John Wiley & Sons, reprinted with permission by John Wiley & Sons.
In addition to the flexibility of material choice, another advantage of electrospinning is the controllability over fiber morphology by adjusting the processing parameters, such as voltage, polymer solution concentration, distance between spinneret and collector, flow rate and spinneret diameter [59, 60, 69–71]. Fibers with diameters ranging from nanometer to micrometer scales (depending on the specific system) can be created. However, many electrospun “nanofibers” from biodegradable polymers often have diameters on the sub-micrometer to micrometer scales [20, 57]. By increasing polymer viscosity through increasing polymer molecular weight or solution concentration, the fiber diameter could be increased. Significant amounts of work have been carried out to study the relationships among fiber characteristics, processing and material parameters [72].
2.2.2 Nanofiber organization
In addition to the basic setup, the electrospinning device could have various configurations to advantageously organize fiber deposition (Figure 3). For example, a rotating collector (Figure 3b) could achieve an aligned electrospun fiber structure assisting cell and tissue orientation [73], which is beneficial for engineering anisotropic tissues [24]. The mandrel size can be tuned to control the diameter of the formed NF tubular scaffolds for vascular and nerve regeneration [74]. One issue associated with a rotating collector is that a very high rotating speed may break the fibers during deposition, presenting a challenge to obtaining highly aligned nanofibers. Dual collectors can also lead to the formation of aligned fibers [75]. The electrospun fibers tend to bridge over the two parallel collectors (Figure 3c), resulting a fiber length up to several centimeters [76]. Different from the rotating collector, the dual collectors generate separate and aligned fibers loosely suspended in the air, which allows for the characterization of the mechanical properties of and cell interactions with individual nanofibers. The employment of dual collectors to generate dense aligned nanofibers is challenging, since prolonged deposition time results in insulation of the collectors and the subsequent random fiber orientation.
The spinneret of the electrospinning device can also have different configurations. In one example known as co-axial electrospinning (Figure 3d), a dual syringe system is employed to generate nanofibers with a core-shell structure [77–81]. Although the fiber diameter is usually greater than 1 μm, the core-shell structure has the potential to deliver therapeutics, which will be discussed in the next section. Multiple separate spinnerets have also been employed to deposit different components simultaneously [82].
There are significant challenges in controlling the architecture of electrospun fibers, due to whipping instability of the jets [61]. One technique called ‘near-field electrospinning,’ which employed a short collection distance and a low voltage, was developed to achieve a straight liquid jet towards the substrate [83]. By employing a collector able to move in the x-y plane, researchers can draw a straight nanofiber towards the substrate to generate patterns [84].
2.2.3 Electrospun 3D scaffolds
There are challenges to fabricate 3D scaffolds using electrospinning techniques. The thickness of electrospun nanofibrous mat is normally on the order of 100 μm, because the electrospun fibers can insulate the collector when the thickness increases. In addition, the open-pore sizes could not be independently controlled, instead are associated with the fiber size. To generate fine nanofibers by electrospinning, the open-pore sizes are at best a few micrometers in size, while the minimum pore size for deep cell infiltration and vascularization is about 100 μm [85], thus accommodating limited tissue formation within the NF network. To address these issues, researchers employed sacrificial materials during the electrospinning process, which are removed afterwards to increase porosity. The sacrificial material can be co-electrospun [86] or introduced as particles (e.g., salt particles) [87] into the electrospun matrix, which can be leached out afterwards. Researchers have also electrospun fibers onto a hollow rotating drum filled with solid CO2, which could grow ice crystals off the collector during the fiber deposition. Water crystals were lyophilized afterwards to improve porosity [88]. However, the structural stability after the removal of the sacrificial materials needs to be improved, because distortion frequently occurs when the porosity is increased in such ways. Future goals associated with these strategies include better control over pore shape and interconnectivity.
Another strategy is to electrospin fibers onto a macro-porous structure. In one study, nanofibers were electrospun onto a microfiber mesh [89]. In this way, the microfiber architecture allows for cell penetration while the NF structure on the microfibers offers improved cell interaction. This technique, however, can only produce scaffolds with limited thickness. Alternatively, microfibers and nanofibers could be electrospun simultaneously to produce scaffolds with improved cell penetration [90]. In a recent report, an automated system was developed to produce ordered microstructure in a layer-by-layer fashion, allowing electrospun nanofibers to be deposited on each layer [91].
2.3 Thermally induced phase separation
2.3.1 Preparation of NF matrices
Phase separation is a thermodynamic process, in which a homogeneous multi-component system tends to separate into multiple phases to lower system free energy. For polymer solutions, phase separation can be initiated either thermally or by using a nonsolvent, forming polymer-rich and polymer-lean phases. Upon solvent removal, the polymer-rich phase solidifies to form the polymer skeleton while the polymer-lean phase becomes the void space [8]. Different processing parameters, such as polymer/solvent combination, polymer concentration and phase separation temperature, could be utilized to generate different pore morphologies for the aimed scaffolds. When phase separation is induced thermally, if the solvent crystallization temperature is higher than the phase separation temperature used, solid-liquid phase separation occurs, where the solvent crystallizes to form the pore shape after solvent sublimation. If the solvent crystallization temperature is lower than the phase separation temperature used, a liquid-liquid phase separation takes place as the temperature of the solution is decreased. Within certain polymer concentration range and temperature range, the liquid-liquid phase separation follows a spinodal decomposition pathway and forms a bicontinuous pattern where both polymer-rich and polymer-lean regions are interconnected [92]. A typical thermally induced phase separation (TIPS) process that generates NF structures involves five steps: polymer dissolution, liquid-liquid phase separation and gelation, solvent extraction, freezing, and freeze-drying [18, 93–95]. For example, poly(L-lactic acid) (PLLA) is dissolved in a selected solvent and thermally induced to phase separate and gel when the temperature is decreased. The solvent of choice should have a low crystallization temperature to allow liquid-liquid phase separation, e.g., tetrahydrofuran, tetrahydrofuran-methanol, dioxane-methanol, and dioxane-pyridine. Since PLLA is a semicrystalline polymer, the polymer can crystallize, which helps to stabilize the structure. After the removal of the solvent via extraction, sublimation or evaporation, a 3D continuous NF PLLA structure with a porosity as high as 98.5% can be generated [18]. The fiber diameter ranges from 50 to 500 nm, similar to the size of natural collagen fibers. Due to the extremely high surface area, the NF PLLA scaffold degrades significantly faster than the solid-walled PLLA scaffold [96], making the NF PLLA scaffold more suitable for tissue engineering applications.
In addition to PLLA, other synthetic and naturally-derived polymers have been fabricated into nanofibers by TIPS. For example, a series of biodegradable amphiphilic poly(hydroxyalkyl methacrylate)-graft-poly(L-lactic acid) copolymers were synthesized and fabricated into NF structures by TIPS [97]. These copolymers have functional groups allowing conjugation with bioactive moieties and degrade faster than the PLLA homopolymer. As another example, gelatin was fabricated into highly porous NF matrices by TIPS using either ethanol/water mixture or methanol/water mixture [94]. Gelatin is a mixture of peptide chains resulted from the irreversible hydrolysis of collagen, and thus has the chemical composition essentially the same as that of collagen. The denaturing hydrolysis process eliminates the possible resident pathogens. Thus through mimicking ECM collagen both in chemical composition and physical structure, the NF gelatin scaffold is more advantageous than the control gelatin scaffold without the NF feature [94].
2.3.2 NF 3D scaffolds with anatomical shapes and pre-designed macro-pores
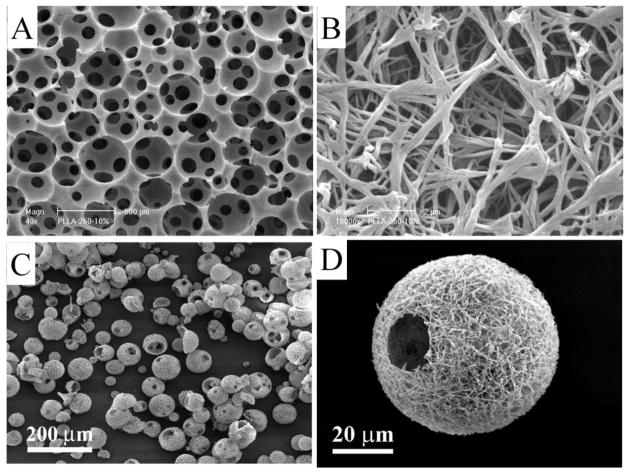
One of the distinct advantages of the TIPS technique is its capacity to integrate with other scaffold fabrication techniques to construct macro/micro pore/channel networks within the 3D NF scaffolds. In one example, sugar spheres (in the diameter range of 150–500 μm) were assembled into a mold (negative replica) to fabricate NF scaffolds with inter-connected spherical pores [98] (Figure 4a &b). In this process, water was used to extract the solvent from the system and to leach the sugar spheres to generate the predesigned spherical pore network. The pore size could be controlled by the sugar sphere size. Different degrees of inter-pore connectivity could be achieved by using different heat treatment temperatures and times. In another example, sugar fibers were assembled into a 3D template to build a tubular pore network [99]. Furthermore, solid free form (SFF) technique was employed to construct wax mold with computer-designed internal structural features, which was used as a negative replica to fabricate NF scaffolds with inter-connected micro-channels [93].
Figure 4.
SEM images of NF scaffolds generated by TIPS: Macro-porous scaffolds prepared from sugar sphere template leaching and TIPS techniques at a lower magnification (a) and at a higher magnification (b); NF hollow microspheres fabricated from a star-shaped PLLA, showing that almost every microsphere had one or more open hole(s) on the shell (c); and a representative NF hollow microsphere, showing the NF architecture and a hole of approximately 20 μm on the microsphere shell (d). Figures a) and b) are from Wei and Ma [98], Copyright © 2006 by John Wiley & Sons, reprinted with permission by John Wiley & Sons; Figures c) and d) are from Liu, Jin and Ma [108], Copyright © 2011 by Nature Publishing Group, reprinted with permission of Nature Publishing Group.
The 3D anatomical shape of a scaffold can also be achieved by integrating TIPS with computer assisted design and computer assisted manufacture (CAD/CAM) techniques [93]. This approach enables the design and fabrication of patient-specific scaffolds. Computed-tomography scans or other medical images of human anatomical parts can be employed by CAD to design and construct the 3D shapes of wax molds, thereby controlling the anatomical shapes of the resulting scaffolds. By combining CAD, particulate leaching and TIPS, NF scaffolds with pre-designed pore structure as well as medical image-determined 3D anatomical shapes, such as a human mandible, ear [93] and hand digit bone [100], were successfully fabricated.
2.3.3 Preparation of injectable NF microspheres
For irregular shaped defect repair, injectable scaffolds can be advantageous in delivering bioactive agents and stem cells, allowing for minimally invasive procedures to minimize complications and pain. Microspheres are injectable and have been used as carriers to deliver cells for bone [101], cartilage [102], dermal [103], hepatic [104], and adipose [105] tissue regeneration. However, traditional microsphere fabrication techniques, such as emulsification [106] and emulsion polymerization [107], often lack control over the exterior and interior morphologies of the microspheres.
A novel technique has been recently developed to fabricate injectable NF hollow microspheres [108]. In this work, star-shaped PLLA (SS-PLLA) polymers were synthesized using poly(amidoamine) (PAMAM) dendrimers as initiators. The SS-PLLA polymers were dissolved in tetrahydrofuran, emulsified in glycerol under rigorous stirring, and quenched in liquid nitrogen to generate the NF hollow microspheres, where the NF structure formation was driven by TIPS. Upon solvent extraction and freeze-drying, highly porous hollow microspheres with open holes on the NF shells were obtained without using any template (Figure 4c &d). When a linear PLLA was processed with the same process, NF microspheres without a hollow core structure were generated. Thus, the star-shaped polymer architecture was a key feature to allow for the assembly of the NF hollow microspheres. The novel NF hollow microspheres were examined as an injectable scaffold for cartilage regeneration both in vitro and in vivo [108]. The hollow NF microspheres supported the regeneration of cartilage similar to native cartilage in structure, composition, and biomechanical properties. The engineered cartilage fully filled a critical-sized osteochondral defect in rabbits and smoothly integrated with the host cartilage.
3. Delivery of Bioactive Agents on NF Scaffolds
Bone regeneration is regulated by various bioactive agents such as growth factors [26], integrin-binding ligands [28] and nucleic acids [27]. Growth factors are important signals in the osteogenic microenvironment, whose concentration, temporal and spatial distributions play important roles in their osteogenic function. We will focus the discussion on growth factor delivery while the basic strategies involved can be applied to the delivery of other bioactive agents. While being potent, growth factors are expensive and have short half-lives in vivo. Therefore, scaffolds with controlled release capacity are desired to preserve their biological activity and prolong their function at the effective level over an extended time period [109].
Bioactive agent delivery strategies from nanofibers fabricated using electrospinning and TIPS techniques are discussed below and depicted in Figure 5. Self-assembled nanofibers are also used for growth factor delivery [110–115]. However, the bioactive agent delivery strategies and kinetics are very different [116, 117]. Detailed discussion is beyond the scope of this review. Interested readers are referred to more focused review papers on self-assembled nanofibers elsewhere [118, 119] or more general hydrogel-based therapeutic delivery systems [120].
Figure 5.
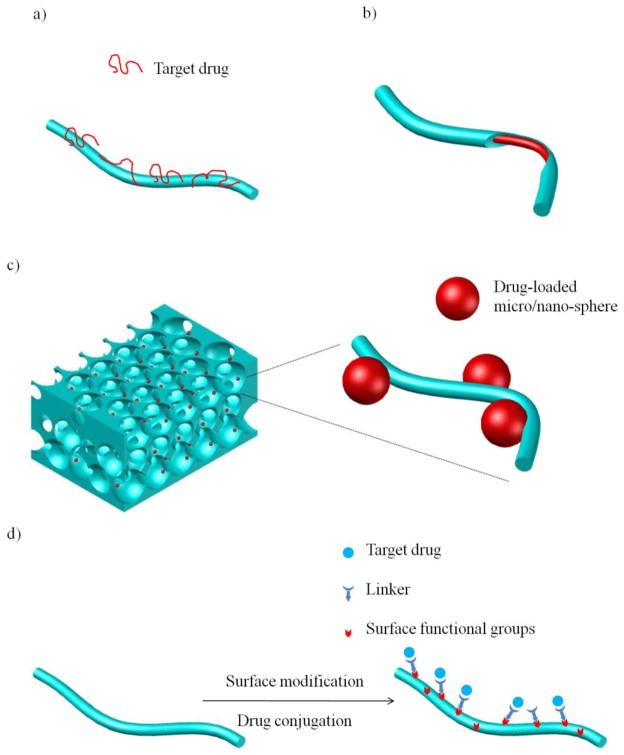
Schematic illustration showing the typical nanofiber-based drug (bioactive agent) delivery strategies: a) simple physical adsorption; b) coaxial electrospinning; c) pore surface immobilization of bioactive agent-loaded particles; and d) surface covalent immobilization of bioactive agents.
3.1 Physical immobilization and encapsulation
Physical absorption (Figure 5a) has been utilized to load drugs onto the surface of the nanofibers. NF scaffolds can adsorb significantly higher amounts of growth factors than control scaffolds due to the significantly larger surface areas of the NF materials. However, the passive adsorption method offers poor control over the factor retention, resulting in burst release and negligible biological function [121]. In addition, adsorption often involves electrostatic interactions, hydrogen bonding, and hydrophobic interactions between the proteins and scaffolds, leading to potential protein conformational change and loss of bioactivity. Growth factors could also be physically encapsulated inside the fibers for slow release. Blending a growth factor with the polymer solution for either electrospinning or TIPS is the simplest way to produce a factor-releasing NF scaffold [122, 123]. Limited growth factors can be well dispersed into a polymer solution, and their bioactivity may often be tempered even when they can. For example, drugs have been electrospun into nanofibers, but they may aggregate on the outer surface, causing undesired burst drug release. To avoid such undesired drug aggregation, additives were investigated to improve drug dispersion within the fibers in order to achieve more desirable release profile [124]. Certain naturally-derived macromolecules and their analogs have also been used to sustain growth factor release by taking advantage of affinity between the growth factors and the macromolecules [125, 126], which in principle could also be utilized for NF scaffolds to slowly release factors.
As discussed earlier, co-axial electrospinning has been developed, and this technique is used for growth factor or drug delivery. When a protein solution was incorporated as the core and the electrospinnable polymer as the shell, coaxial electrospinning process generated protein-encapsulated ultrafine fibers with reduced burst release and prolonged release duration [80] (Figure 5b). The release profile could be manipulated by adjusting the core/shell compositions and structures. In addition, the bioactivity of the protein in the core could be preserved because of its minimal contact with the organic solvent used in the shell. One concern might be the potential compromise of the fiber morphology and mechanical properties. Another is the possible factor leakage because it is often not easy to select the core/shell materials or to determine the processing parameters to obtain the perfect core-shell structure. Emulsion electrospinning were also used to generate core-shell nanofibers without using a complex spinneret system. When water-in-oil or oil-in-water emulsions were used for electrospinning, they were partially demulsified due to stretching and evaporation, ultimately leading to core-shell structure formation [127–129].
Microspheres and nanospheres have long been used for drug and protein deliveries, where various techniques have been developed to preserve the bioactivities and to control the release profile of the incorporated drugs and proteins [130]. One strategy is to integrate such microspheres (MS) or nanospheres (NS) into various NF scaffolds to achieve the desired controlled release of growth factors (Figure 5c), where an inter-connected macro pore network is advantageous [131]. Since NF scaffolds with such macro pores can be fabricated using the TIPS technique, the delivery of various bioactive agents have been achieved by immobilizing MS and NS onto such scaffolds [121, 132]. Drug-loaded biodegradable MS or NS were first fabricated from PLGA using a double emulsion technique [133]. Degradation pattern and therefore the release profile of the MS or NS were controlled through adjusting the lactic acid/glycolic acid ratio or the molecular weight of the PLGA. The bioactivity of the encapsulated proteins was maintained at a high level [121, 132]. The MS and NS were infiltrated into the scaffold evenly and immobilized onto the pore wall surface without compromising the scaffold pore network (Figure 6a, b &c). Sustained protein release over days to months was achieved. The MS/NS-immobilized scaffolds reduced the burst release and circumvented the uncontrollable spatial distribution due to coalescence and aggregation when MS/NS were used alone. In addition, multiple biological factors can be delivered in a spatially and temporally controlled fashion [134, 135], where the release kinetics of each factor can be individually controlled via a distinct MS/NS formulation.
Figure 6.
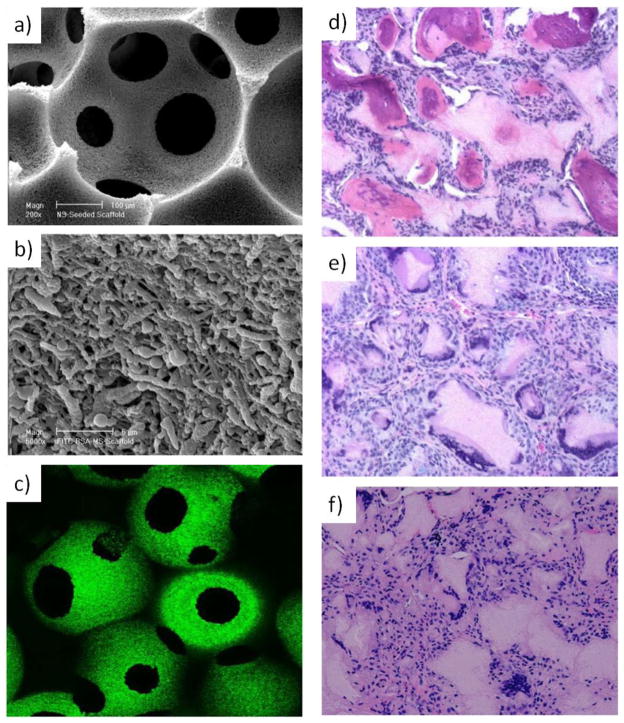
BMP-releasing macro-porous and NF PLLA scaffold for bone regeneration: a) SEM micrograph of PLGA nanosphere-immobilized on PLLA NF scaffolds at a lower magnification; b) at a higher magnification, showing the immobilized nanospheres on the surface of the internal pore walls; c) laser scanning confocal micrograph of PLGA nanosphere-immobilized PLLA NF scaffolds, where FITC-labeled bovine serum albumin was encapsulated into the PLGA nanospheres, showing green emission under confocal microscopy. New bone formation in rhBMP-7 incorporated PLLA NF scaffolds retrieved 6 weeks after subcutaneous implantation in rats: d) significant bone formation in the BMP7 nanosphere-containing scaffolds; e) fibrous tissue in the scaffolds without BMP7; f) fibrous tissue in scaffolds pre-soaked in a BMP7 solution (H&E staining with an original magnification of 100x). Sources: (a, b, c) from Wei et al [132]. Copyright © 2006 by Elsevier, reprinted with permission of Elsevier; (d, e, f) from Wei et al. [121] Copyright © 2007 by Elsevier, reprinted with permission of Elsevier.
The MS/NS immobilized scaffolds have been evaluated for promoting osteogenesis and angiogenesis in animal models. In one study, bone morphogenetic protein-7 (BMP-7) encapsulated NS were immobilized onto NF scaffolds and then implanted in rats subcutaneously [121]. BMP-7 was released in a controlled fashion with high biological activity and induced ectopic bone formation (Figure 6d). In contrast, scaffolds without BMP-7 or with passively adsorbed BMP-7 were unable to induce osteogenesis (Figure 6e &f), probably due to the loss of bioactivity and insufficient release duration. In another study, platelet-derived growth factor (PDGF) was delivered by the NS-immobilized NF scaffolds implanted in rats [136]. The released PDGF not only induced tissue ingrowth, but also stimulated angiogenesis in a PDGF-dose-dependent manner [137].
3.2 Chemical immobilization
In addition to physical adsorption/encapsulation, bioactive agents can also be presented on the nanofibers through chemical immobilization, which are released by the cleavage of the linking bonds. As is exemplified in Figure 5d, the nanofiber surface should be activated first to present functional groups, which are subsequently employed to conjugate with bioactive agents through proper reactions.
3.2.1 Surface activation
In many cases, the nanofibers are fabricated from commercial polymers that lack functional groups to enable the conjugation reactions. There are various ways to activate the surface of nanofibers post fabrication. For example, plasma treatment and ultraviolet (UV) irradiation have been used to alter the polymer surface chemistry [138–143]. Plasma treatment, for instance, can create diverse functional groups including amine and carboxyl groups if the plasma source is properly configured [138–140]. These functional groups or free radicals can be utilized to initiate surface graft polymerization to introduce the desired chemistry [144–146]. It should be noted that these methods could only be applied to 2D films or very thin 3D constructs due to the limited plasma penetration depth. Chemical etching is another method for surface modification [147, 148]. For the widely used biodegradable polyesters, the exposed ester bonds in the polymer backbone partially degrade under acidic or basic conditions, resulting in carboxyl and hydroxyl groups on the surface. However, strong reaction conditions should be avoided to maintain the scaffold integrity.
3.2.2 Bioactive molecule immobilization
A variety of conjugation techniques have been developed [149]. The selection of a conjugation technique is dependent on the reactive groups on the bioactive agents. Primary amine, carboxyl, thiol, and hydroxyl groups can all be reactive sites for conjugation. While certain biomolecules can only be conjugated at specific site(s), most conjugation methods involve nonselective reactions between the functional groups of a protein and those on a biomaterial. There are more complicated techniques to achieve selective conjugation, which involve site-specific labeling of proteins and peptide tags [150].
Primary amine and carboxyl groups are the most frequently utilized reaction sites in biomolecules for conjugation. The reaction rate between amine and carboxyl groups could be accelerated by using the 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)/N-hydroxysuccimide (NHS) chemistry [139, 151–155]. Hydroxyl groups are also widely employed for the immobilization of biomolecules onto tissue engineering scaffolds. To improve the reactivity, hydroxyl groups can be activated, e.g., through oxidation by NaIO4, into aldehyde groups to react with amine groups [144]. The hydroxyl groups can also be treated by Ce(IV) to generate free radicals for graft copolymerization to introduce desired chemistry, which can be used in subsequent protein immobilization [156]. As compared to UV irradiation or plasma treatment, Ce(IV) treatment circumvents the use of strong reaction conditions. Recently, click chemistry is attracting much attention and shows great potential in protein immobilization onto nanofibers because the reactions are irreversible, quantitative and mild [157–160]. However, some widely used click reactions, such as the azide-alkyne Huisgen cycloaddition [157–160], employ metal catalysts with potential toxic effects. Catalyst-free click reactions [161] are more attractive for scaffold functionalization.
In summary, direct conjugation of bioactive agents onto the fiber surface can be achieved by amidation, esterification, or more contemporary techniques such as click reactions. Stimuli-responsive and cell-responsive release profile can be achieved when stimuli-responsive or cell-secreted enzyme-degradable linkers are employed [162, 163]. Again, a common concern is the inactivation of the immobilized biomolecules, especially when the active site of the biomolecule is involved in the conjugation reaction.
4. Delivery of Stem Cells on NF Scaffolds
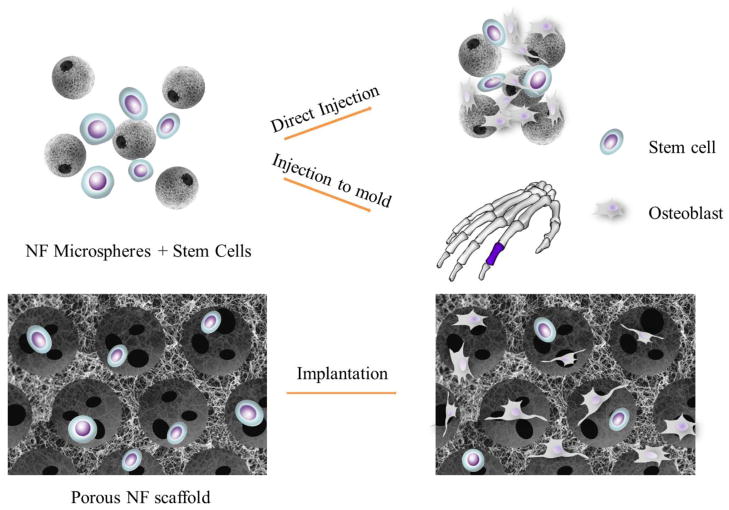
There are two strategies for nanofiber-based scaffolds to deliver stem cells to the site for bone regeneration, which are injection and implantation as illustrated in Figure 7. The injection strategy involves the use of injectable cell carriers with NF features, which are intended to facilitate cell attachment, differentiation and tissue regeneration in addition to their injectability to fill complex defect shapes. The cells and carriers can also be injected into a pre-designed mold to engineer tissue with a needed anatomical shape in vitro prior to implantation. With the general implantation strategy, cells are seeded onto a 3D NF scaffold and then either directly implanted or implanted after being cultured in vitro for a period of time. This approach is taken especially when a large bone defect needs to be regenerated. Self-assembled nanofibers are essentially hydrogels and can be used to encapsulate stem cells simply by mixing them into the solution before gelation occurs. Since there are few alternative ways and there are limited literature using these scaffolds to delivery stem cells for bone regeneration [164, 165], we will not separately discuss this category. As discussed above, electrospinning and TIPS techniques generate NF scaffolds with different pore structures, so the cell delivery strategies on NF scaffolds generated by electrospinning and macro-porous NF scaffolds generated by TIPS will be discussed separately.
Figure 7.
Schematic illustration of stem cell delivery strategies in bone tissue engineering. The injection strategy involves the use of injectable cell carriers, such as nano-fibrous microspheres to repair a defect with irregular shape. In addition, cell and carrier mixture can be injected into a pre-designed mold with a specific anatomical shape. The implantation strategy involves the use of a cell-seeded macro-porous block scaffold to repair a larger defect.
4.1 Electrospun NF scaffolds
The potential application of electrospun NF scaffolds in bone tissue engineering was initially explored with electrospun poly(ε-caprolactone) (PCL) fiber mesh to support osteogenic differentiation of rat bone marrow mesenchymal stem cells in vitro [57] and ectopic bone formation in vivo [166]. Electrospun scaffolds fabricated from a variety of biomaterials were then utilized to support osteogenic differentiation of various cell types in vitro, including osteoblasts and mesenchymal stem cells [23, 167–173]. The fiber diameter and orientation were also manipulated to enhance osteogenesis [174–176]. In one study, a layer-by-layer approach was proposed, where thin electrospun fiber meshes were alternately stacked with cells to fabricate a 3D cell-scaffold construct with 10 layers [177]. Another recent study employed a thin electrospun mesh to construct a stem cell-loaded layer mimicking periosteum (a thin layer of soft tissue that contains progenitor cells actively involved in bone formation [178]), which can then be used to support block bone formation via colonizing a cell-free macro-porous 3D scaffold [179]. In this study, human mesenchymal stem cells (MSCs) or amniotic fluid stem cells were seeded on electrospun NF meshes, which were found to be effective in colonizing a porous scaffold block in vitro [179]. However, few studies have used the electrospun scaffolds themselves to regenerate large bone defects in animal models except in thin calvarial defects [180, 181]. As discussed in Section 2.2.3, despite numerous efforts [182–185], it remains a major challenge to improve the porosity, pore size and shape control of electrospun fibrous scaffolds.
4.2 NF scaffolds by TIPS
As described earlier, TIPS technique offers more controllability over pore size and scaffold overall shape. Therefore, the geometry of NF scaffolds by TIPS can be easily designed [93, 95, 98] to repair a large defect with a specific shape. This was demonstrated by the construction of a patient-specific, anatomically shaped digit scaffold generated with a reverse solid free form fabrication technique based on reversed images of computed tomography scan of the digit [100]. Cell-NF scaffold interaction plays an important role in osteogenesis. The NF scaffolds greatly increased the total amount of serum proteins adsorbed on the NF scaffolds, especially the key ECM proteins involved in cell adhesion [186], and promoted the osteoblast attachment, growth, and differentiation [21, 186]. Because of the pluripotency and unlimited expansion capacity, embryonic stem (ES) cells [187] are becoming a desirable cell source for bone tissue regeneration [188, 189]. However, it is a great challenge to develop technologies to effectively induce the plastic cells into mature osteogenic phenotype prior to applying ES cells in bone therapy. It has been demonstrated that NF matrix can significantly facilitate the osteogenic differentiation of ES cells [190]. When ES cells were seeded on 3D NF scaffolds generated by TIPS, the nanofibers significantly increased the expression levels of osteogenic marker genes and calcium deposition, compared to solid-walled scaffolds without the NF features [191, 192]. Alternatively, MSC-like cells can be generated from human ES cells first via medium selection [193, 194] or cell sorting [195] and then the derived homogeneous cells can be used to regenerate bone tissues on 3D NF scaffolds [196]. The in vivo bone formation on NF scaffolds by TIPS has been further demonstrated by an implantation study. Multi-potent human amniotic fluid-derived stem cells were induced along osteogenic pathways in vitro. After subcutaneous implantation, the constructs with NF scaffolds showed significantly more robust bone formation than those with solid-walled scaffolds [197]. In a recent report, a porous poly(propylene carbonate) scaffold containing NF chitosan matrix inside its macro-pores was seeded with rabbit bone marrow MSCs and implanted into a rabbit femoral condyle defect [198]. After 16 weeks of implantation, the defect was reported to be fully repaired, showing the beneficial effect of NF matrix in supporting in situ osteogenesis.
4.3 Advances in stem cells delivery on scaffolds
Stem cell biology and regenerative medicine are rapidly evolving. Although in most bone regeneration studies, the cell-scaffold constructs were induced along osteogenic route prior to implantation, recent studies showed ectopic bone formation can be achieved by an endochondral ossification process using the implanted cartilage constructs as a template [188, 199], leading to remodeled bone with good mechanical properties. As the bone is a highly vascularized tissue, incorporation of a vascular network perfusing the bone graft can potentially promote bone regeneration [200, 201]. In a recent report, MSCs and endothelial cells were co-delivered on porous scaffolds. The transplanted MSCs not only participated in bone mineralization, but also involved in the formation of a functional microvascular network [202]. The incorporation of the above-mentioned new concepts into studies of stem cell delivery on NF scaffolds, and specifically, the proper design of NF scaffolds to facilitate the process, holds great potential in the development of more efficient delivery strategies.
Conclusions and perspectives
The advances in stem cell biology and biomaterial science provide exciting opportunities for bone tissue engineering. Biomaterials can be more rationally designed to construct a synthetic osteogenic microenvironment to promote bone regeneration. Molecular self-assembly, electrospinning and TIPS techniques have produced ECM-mimicking nanofibers from a variety of synthetic and naturally-derived biodegradable materials. The nanofibrous features are being incorporated into 3D scaffolds by integrating and combining with other advanced micro/nano-technologies to generate biomimetic hierarchical structures. These novel NF scaffolds have also showed great promise in delivering bioactive agents and promoting stem cell differentiation.
Along with the significant progress in bone tissue engineering are also challenges. The parameters of nanofiber-based biomimetic scaffolds, including mechanical properties, bioactive agent release profile, and architectural and molecular features to accommodate or interact with cells, are all highly dependent on the fabrication technologies. While significant progresses have been made in nanofiber technologies and their application in bone regeneration, there are inadequate mechanistic understandings, limiting the pace of the advancement of the field. More systematic and quantitative studies are needed to establish the relationships between structural features, biologic delivery, stem cell function, and bone regeneration. The rapid advancing stem cell biology brings more cell types into the bone regeneration field and increases the complexity along with the new opportunities. Interdisciplinary and multidisciplinary approaches and collaborations are needed to more effectively tackle the challenges and advance the field.
Acknowledgments
The authors gratefully acknowledge the past and present grant support in these research areas from the Whitaker Foundation (RG-99-0137), NIH (DE014755, DE015384, GM075840, DE017689, DE022327) and DOD (W81XWH-12-2-0008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gerstenfeld LC, Alkhiary YM, Krall EA, Nicholls FH, Stapleton SN, Fitch JL, Bauer M, Kayal R, Graves DT, Jepsen KJ, Einhorn TA. Three-dimensional reconstruction of fracture callus morphogenesis. J Histochem Cytochem. 2006;54:1215–1228. doi: 10.1369/jhc.6A6959.2006. [DOI] [PubMed] [Google Scholar]
- 2.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 3.Mistry AS, Mikos AG. Tissue engineering strategies for bone regeneration. Advances in Biochemical Engineering/Biotechnology. 2005;94:1–22. doi: 10.1007/b99997. [DOI] [PubMed] [Google Scholar]
- 4.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 5.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials. 2011;32:9622–9. doi: 10.1016/j.biomaterials.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson RC, Yaszemski MJ, Powers JM, Mikos AG. Fabrication of biodegradable polymer scaffolds to engineer trabecular bone. J Biomater Sci, Polym, Ed. 1995;7:23–28. doi: 10.1163/156856295x00805. [DOI] [PubMed] [Google Scholar]
- 8.Ma PX. Scaffolds for tissue fabrication. Mater Today. 2004;7:30–40. [Google Scholar]
- 9.Ma PX. Tissue Engineering. In: Kroschwitz JI, editor. Encyclopedia of Polymer Science and Technology. 3. Vol. 12. John Wiley & Sons, Inc; Hoboken, NJ: 2005. pp. 261–291. [Google Scholar]
- 10.Mikos AG, Thorsen AJ, Czerwonka LA, Bao Y, Langer R, Winslow DN, Vacanti JP. Preparation and characterization of poly(l-lactic acid) foams. Polymer. 1994;35:1068–1077. [Google Scholar]
- 11.Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG. Evolution of bone transplantation: molecular, cellular and tissue strategies to engineer human bone. Biomaterials. 1996;17:175–185. doi: 10.1016/0142-9612(96)85762-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, Ma PX. Poly(alpha-hydroxyl acids)/hydroxyapatite porous composites for bone- tissue engineering, I. Preparation and morphology. Journal of Biomedical Materials Research. 1999;44:446–455. doi: 10.1002/(sici)1097-4636(19990315)44:4<446::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 13.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Holzwarth J, Ma PX. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromolecular Bioscience. 2012 doi: 10.1002/mabi.201100466. in press. [DOI] [PubMed] [Google Scholar]
- 15.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–37. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franceschi RT. The developmental control of osteoblast-specific gene expression: Role of specific transcription factors and the extracellular matrix environment. Crit Rev Oral Biol Med. 1999;10:40–57. doi: 10.1177/10454411990100010201. [DOI] [PubMed] [Google Scholar]
- 17.Xiao G, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res. 2002;17:101–110. doi: 10.1359/jbmr.2002.17.1.101. [DOI] [PubMed] [Google Scholar]
- 18.Ma PX, Zhang R. Synthetic nano-scale fibrous extracellular matrix. J Biomed Mater Res. 1999;46:60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Hartgerink JD, EBE, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 20.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 21.Woo KM, Jun JH, Chen VJ, Seo J, Baek JH, Ryoo HM, Kim GS, Somerman MJ, Ma PX. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28:335–343. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Hu J, Liu X, Ma PX. Induction of osteoblast differentiation phenotype on poly(l-lactic acid) nanofibrous matrix. Biomaterials. 2008;29:3815–3821. doi: 10.1016/j.biomaterials.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26:5158–5166. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Bashur CA, Shaffer RD, Dahlgren LA, Guelcher SA, Goldstein AS. Effect of fiber diameter and alignment of electrospun polyurethane meshes on mesenchymal progenitor cells. Tissue Eng Part A. 2009;15:2435–2445. doi: 10.1089/ten.tea.2008.0295. [DOI] [PubMed] [Google Scholar]
- 25.Jang JH, Castano O, Kim HW. Electrospun materials as potential platforms for bone tissue engineering. Adv Drug Deliv Rev. 2009;61:1065–1083. doi: 10.1016/j.addr.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 27.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 28.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 29.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 30.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 31.Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164–4173. [PubMed] [Google Scholar]
- 32.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 33.Gimble JM, Guilak F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 34.Thomson JA. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Carvajal-Vergara X, Sevilla A, Dsouza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, Ge Y, Cohen N, Edelmann LJ, Chang B, Waghray A, Su J, Pardo S, Lichtenbelt KD, Tartaglia M, Gelb BD, Lemischka IR. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465:808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–6. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 38.Petite H, Viateau V, Bensaid W, Meunier A, De Pollak C, Bourguignon M, Oudina K, Sedel L, Guillemin G. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959–963. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 39.Cowan C, SS, Alami O, Chou Y, TRMC, QN, WBM, LMT Adipose-derived Mesenchymal Stromal Cells Tissue Engineer Bone in Critical Sized Mouse Calvarial Defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 40.Whitesides GM, Mathias JP, Seto CT. Molecular self-assembly and nanochemistry: a chemical strategy for the synthesis of nanostructures. Science. 1991;254:1312–1319. doi: 10.1126/science.1962191. [DOI] [PubMed] [Google Scholar]
- 41.Lehn JM. Supramolecular chemistry. Science. 1993;260:1762–1763. doi: 10.1126/science.8511582. [DOI] [PubMed] [Google Scholar]
- 42.Ball P. Polymers made to measure. Nature. 1994;367:323–324. doi: 10.1038/367323a0. [DOI] [PubMed] [Google Scholar]
- 43.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 44.Yu YC, Tirrell M, Fields GB. Minimal lipidation stabilizes protein-like molecular architecture. Journal Of The American Chemical Society. 1998;120:9979–9987. [Google Scholar]
- 45.Berndt P, Fields GB, Tirrell M. Synthetic Lipidation Of Peptides And Amino-Acids - Monolayer Structure And Properties. Journal Of The American Chemical Society. 1995;117:9515–9522. [Google Scholar]
- 46.Yu YC, Roontga V, Daragan VA, Mayo KH, Tirrell M, Fields GB. Structure and dynamics of peptide-amphiphiles incorporating triple-helical proteinlike molecular architecture. Biochemistry. 1999;38:1659–68. doi: 10.1021/bi982315l. [DOI] [PubMed] [Google Scholar]
- 47.Fields GB, Lauer JL, Dori Y, Forns P, Yu YC, Tirrell M. Protein-like molecular architecture: biomaterial applications for inducing cellular receptor binding and signal transduction. Biopolymers. 1998;47:143–51. doi: 10.1002/(SICI)1097-0282(1998)47:2<143::AID-BIP3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 48.Beniash E, Hartgerink JD, Storrie H, Stendahl JC, Stupp SI. Self-assembling peptide amphiphile nanofiber matrices for cell entrapment. Acta Biomater. 2005;1:387–397. doi: 10.1016/j.actbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Yu M, Nowak AP, Deming TJ, Pochan DJ. Methylated mono- and diethyleneglycol functionalized polylysines: Nonionic, alpha-helical, water-soluble polypeptides. J Am Chem Soc. 1999;121:12210–12211. [Google Scholar]
- 50.Pochan DJ, Pakstis L, Ozbas B, NowaK AP, Deming TJ. SANS and cryo-TEM study of self-assembled diblock copolypeptide hydrogels with rich nano- through microscale morphology. Macromolecules. 2002;35:5358–5360. [Google Scholar]
- 51.Nowak A, Breedveld V, Pakstis L, Ozbas B, Pine D, Pochan D, Deming TJ. Rapidly recovering hydrogel scaffolds from self-assembling diblock copolypeptide amphiphiles. Nature. 2002;417:424–428. doi: 10.1038/417424a. [DOI] [PubMed] [Google Scholar]
- 52.Zhang S, Holmes T, Lockshin C, Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc Natl Acad Sci USA. 1993;90:3334–3338. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang S, Holmes TC, DiPersio CM, Hynes RO, Su X, Rich A. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials. 1995;16:1385–1393. doi: 10.1016/0142-9612(95)96874-y. [DOI] [PubMed] [Google Scholar]
- 54.Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci U S A. 2000;97:6728–33. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dagdas YS, Tombuloglu A, Tekinay AB, Dana A, Guler MO. Interfiber interactions alter the stiffness of gels formed by supramolecular self-assembled nanofibers. Soft Matter. 2011;7:3524–3532. [Google Scholar]
- 56.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 58.Doshi J, Reneker DH. Electrospinning Process and Applications of Electrospun Fibers. J Electrostatics. 1995;35:151–160. [Google Scholar]
- 59.Huang Z-M, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 2003;63:2223–2253. [Google Scholar]
- 60.Teo WE, Ramakrishna S. A review on electrospinning design and nanofibre assemblies. Nanotechnology. 2006;17:R89–R106. doi: 10.1088/0957-4484/17/14/R01. [DOI] [PubMed] [Google Scholar]
- 61.Reneker DH, Yarin AL. Electrospinning jets and polymer nanofibers. Polymer. 2008;49:2387–2425. [Google Scholar]
- 62.Bhattarai N, Edmondson D, Veiseh O, Matsen FA, Zhang M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials. 2005;26:6176–6184. doi: 10.1016/j.biomaterials.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 63.Min BM, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 2004;25:1289–1297. doi: 10.1016/j.biomaterials.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 64.Park KE, Kang HK, Lee SJ, Min B-M, Park WH. Biomimetic Nanofibrous Scaffolds:Preparation and Characterization of PGA/Chitin Blend Nanofibers. Biomacromolecules. 2006;7:635–643. doi: 10.1021/bm0509265. [DOI] [PubMed] [Google Scholar]
- 65.Li WJ, Cooper JJA, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly([alpha]-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2:377–385. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 66.Jin HJ, Fridrikh SV, Rutledge GC, Kaplan DL. Electrospinning Bombyx mori Silk with Poly(ethylene oxide) Biomacromolecules. 2002;3:1233–1239. doi: 10.1021/bm025581u. [DOI] [PubMed] [Google Scholar]
- 67.Li M, Mondrinos MJ, Chen X, Gandhi MR, Ko FK, Lelkes PI. Co-electrospun poly(lactide-co-glycolide), gelatin, and elastin blends for tissue engineering scaffolds. J Biomed Mater Res, Part A. 2006;79A:963–973. doi: 10.1002/jbm.a.30833. [DOI] [PubMed] [Google Scholar]
- 68.Kim HW, Kim HE, Knowles JC. Production and potential of bioactive glass nanofibers as a next-generation biomaterial. Adv Funct Mater. 2006;16:1529–1535. [Google Scholar]
- 69.Liao S, Li B, Ma Z, Wei H, Chan C, Ramakrishna S. Biomimetic electrospun nanofibers for tissue regeneration. Biomed Mater. 2006;1:R45–R53. doi: 10.1088/1748-6041/1/3/R01. [DOI] [PubMed] [Google Scholar]
- 70.Schiffman JD, Schauer CL. A review: Electrospinning of biopolymer nanofibers and their applications. Polym Rev. 2008;48:317–352. [Google Scholar]
- 71.Sill TJ, von Recum HA. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 72.Cui W, Li X, Zhou S, Weng J. Investigation on process parameters of electrospinning system through orthogonal experimental design. J Appl Polym Sci. 2007;103:3105–3112. [Google Scholar]
- 73.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 74.Vaz CM, van Tuijl S, Bouten CVC, Baaijens FPT. Design of scaffolds for blood vessel tissue engineering using a multi-layering electrospinning technique. Acta Biomater. 2005;1:575–582. doi: 10.1016/j.actbio.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Dalton PD, Klee D, Moller M. Electrospinning with dual collection rings. Polymer. 2005;46:611–614. [Google Scholar]
- 76.Li D, Wang Y, Xia Y. Electrospinning of Polymeric and Ceramic Nanofibers as Uniaxially Aligned Arrays. Nano Lett. 2003;3:1167–1171. [Google Scholar]
- 77.Jiang H, Hu Y, Li Y, Zhao P, Zhu K, Chen W. A facile technique to prepare biodegradable coaxial electrospun nanofibers for controlled release of bioactive agents. J Controlled Release. 2005;108:237–243. doi: 10.1016/j.jconrel.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 78.Zhang YZ, Wang X, Feng Y, Li J, Lim CT, Ramakrishna S. Coaxial Electrospinning of (Fluorescein Isothiocyanate-Conjugated Bovine Serum Albumin)-Encapsulated Poly(epsilon-caprolactone) Nanofibers for Sustained Release. Biomacromolecules. 2006;7:1049–1057. doi: 10.1021/bm050743i. [DOI] [PubMed] [Google Scholar]
- 79.Liao IC, Chew SY, Leong KW. Aligned core-shell nanofibers delivering bioactive proteins. Nanomedicine. 2006;1:465–471. doi: 10.2217/17435889.1.4.465. [DOI] [PubMed] [Google Scholar]
- 80.Jiang H, Hu Y, Zhao P, Li Y, Zhu K. Modulation of protein release from biodegradable core–shell structured fibers prepared by coaxial electrospinning. J Biomed Mater Res, Part B. 2006;79B:50–57. doi: 10.1002/jbm.b.30510. [DOI] [PubMed] [Google Scholar]
- 81.Sun ZC, Zussman E, Yarin AL, Wendorff JH, Greiner A. Compound core-shell polymer nanofibers by co-electrospinning. Adv Mater. 2003;15:1929. [Google Scholar]
- 82.Stankus JJ, Guan J, Fujimoto K, Wagner WR. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27:735–744. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun D, Chang C, Li S, Lin L. Near-field electrospinning. Nano Lett. 2006;6:839–842. doi: 10.1021/nl0602701. [DOI] [PubMed] [Google Scholar]
- 84.Dalton PD, Joergensen NT, Groll J, Moeller M. Patterned melt electrospun substrates for tissue engineering. Biomed Mater. 2008;3 doi: 10.1088/1748-6041/3/3/034109. [DOI] [PubMed] [Google Scholar]
- 85.Nazarov R, Jin H-J, Kaplan DL. Porous 3-D Scaffolds from Regenerated Silk Fibroin. Biomacromolecules. 2004;5:718–726. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- 86.Baker BM, Gee AO, Metter RB, Nathan AS, Marklein RA, Burdick JA, Mauck RL. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008;29:2348–2358. doi: 10.1016/j.biomaterials.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nam J, Huang Y, Agarwal S, Lannutti J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng. 2007;13:2249–2257. doi: 10.1089/ten.2006.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simonet M, Schneider OD, Neuenschwander P, Stark WJ. Ultraporous 3D polymer meshes by low-temperature electrospinning: Use of ice crystals as a removable void template. Polym Eng Sci. 2007;47:2020–2026. [Google Scholar]
- 89.Tuzlakoglu K, Bolgen N, Salgado AJ, Gomes ME, Piskin E, Reis RL. Nano- and micro-fiber combined scaffolds: A new architecture for bone tissue engineering. J Mater Sci: Mater Med. 2005;16:1099–1104. doi: 10.1007/s10856-005-4713-8. [DOI] [PubMed] [Google Scholar]
- 90.Kim SJ, Jang DH, Park WH, Min BM. Fabrication and characterization of 3-dimensional PLGA nanofiber/microfiber composite scaffolds. Polymer. 2010;51:1320–1327. [Google Scholar]
- 91.Park SH, Kim TG, Kim HC, Yang DY, Park TG. Development of dual scale scaffolds via direct polymer melt deposition and electrospinning for applications in tissue regeneration. Acta Biomater. 2008;4:1198–1207. doi: 10.1016/j.actbio.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 92.Zhang R, Ma PX. Processing of polymer scaffolds: Phase separation. In: AtalaandLanza AR, editor. Methods of Tissue Engineering. Academic Press; San Diego, CA: 2001. pp. 715–724. [Google Scholar]
- 93.Chen VJ, Smith LA, Ma PX. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials. 2006;27:3973–3979. doi: 10.1016/j.biomaterials.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 94.Liu X, Ma PX. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials. 2009;30:4094–4103. doi: 10.1016/j.biomaterials.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen VJ, Ma PX. Nano-fibrous poly(L-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25:2065–2073. doi: 10.1016/j.biomaterials.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 96.Chen VJ, Ma PX. The effect of surface area on the degradation rate of nano-fibrous poly(l-lactic acid) foams. Biomaterials. 2006;27:3708–3715. doi: 10.1016/j.biomaterials.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 97.Liu X, Ma PX. The nanofibrous architecture of poly(l-lactic acid)-based functional copolymers. Biomaterials. 2010;31:259–269. doi: 10.1016/j.biomaterials.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wei G, Ma PX. Macroporous and nanofibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. J Biomed Mater Res, Part A. 2006;78:306–315. doi: 10.1002/jbm.a.30704. [DOI] [PubMed] [Google Scholar]
- 99.Zhang R, Ma PX. Synthetic nano-fibrillar extracellular matrices with predesigned macroporous architectures. J Biomed Mater Res, Part A. 2000;52:430–438. doi: 10.1002/1097-4636(200011)52:2<430::aid-jbm25>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 100.Wang P, Hu J, Ma PX. The engineering of patient-specific, anatomically shaped, digits. Biomaterials. 2009;30:2735–2740. doi: 10.1016/j.biomaterials.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang SW, Yang HS, Seo SW, Han DK, Kim BS. Apatite-coated poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for bone tissue engineering. J Biomed Mater Res, Part A. 2008;85:747–756. doi: 10.1002/jbm.a.31572. [DOI] [PubMed] [Google Scholar]
- 102.Mercier NR, Costantino HR, Tracy MA, Bonassar LJ. Poly(lactide-co-glycolide) microspheres as a moldable scaffold for cartilage tissue engineering. Biomaterials. 2005;26:1945–1952. doi: 10.1016/j.biomaterials.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 103.Huss FRM, Nyman E, Bolin JSC, Kratz G. Use of macroporous gelatine spheres as a biodegradable scaffold for guided tissue regeneration of healthy dermis in humans: An in vivo study. J Plast Reconstr Aes. 2010;63:848–857. doi: 10.1016/j.bjps.2009.01.068. [DOI] [PubMed] [Google Scholar]
- 104.Kawase M, Michibayashi N, Nakashima Y, Kurikawa N, Yagi K, Mizoguchi T. Application of glutaraldehyde-crosslinked chitosan as a scaffold for hepatocyte attachment. Biol Pharm Bull. 1997;20:708–710. doi: 10.1248/bpb.20.708. [DOI] [PubMed] [Google Scholar]
- 105.Chung HJ, Park TG. Injectable cellular aggregates prepared from biodegradable porous microspheres for adipose tissue engineering. Tissue Eng Part A. 2009;15:1391–1400. doi: 10.1089/ten.tea.2008.0344. [DOI] [PubMed] [Google Scholar]
- 106.O’Donnell PB, McGinity JW. Preparation of microspheres by the solvent evaporation technique. Adv Drug Delivery Rev. 1997;28:25–42. doi: 10.1016/s0169-409x(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 107.Piirma I. Colloids. In: Mark HF, Bikales NM, Overberger CG, Menges G, Kroschwitz JI, editors. Encyclopedia of Polymer Science and Engineering. 2. New York: John Wiley and Sons; 1985. [Google Scholar]
- 108.Liu X, Jin X, Ma PX. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat Mater. 2011;10:398–406. doi: 10.1038/nmat2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2000;97:11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schneider A, Garlick JA, Egles C. Self-assembling peptide nanofiber scaffolds accelerate wound healing. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hsieh PCH, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Segers VFM, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 113.Davis ME, Hsieh PCH, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci USA. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hosseinkhani H, Hosseinkhani M, Khademhosseini A, Kobayashi H. Bone regeneration through controlled release of bone morphogenetic protein-2 from 3-D tissue engineered nano-scaffold. J Controlled Release. 2007;117:380–386. doi: 10.1016/j.jconrel.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 115.Hosseinkhani H, Hosseinkhani M, Khademhosseini A, Kobayashi H, Tabata Y. Enhanced angiogenesis through controlled release of basic fibroblast growth factor from peptide amphiphile for tissue regeneration. Biomaterials. 2006;27:5836–5844. doi: 10.1016/j.biomaterials.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 116.Zhao Y, Tanaka M, Kinoshita T, Higuchi M, Tan T. Self-assembling peptide nanofiber scaffolds for controlled release governed by gelator design and guest size. J Controlled Release. 2010;147:392–399. doi: 10.1016/j.jconrel.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 117.Nagai Y, Unsworth LD, Koutsopoulos S, Zhang S. Slow release of molecules in self-assembling peptide nanofiber scaffold. J Controlled Release. 2006;115:18–25. doi: 10.1016/j.jconrel.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 118.Stupp SI. Self-Assembly and Biomaterials. Nano Lett. 2010;10:4783–4786. doi: 10.1021/nl103567y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Branco MC, Schneider JP. Self-assembling materials for therapeutic delivery. Acta Biomater. 2009;5:817–831. doi: 10.1016/j.actbio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 121.Wei G, Jin Q, Giannobile WV, Ma PX. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28:2087–2096. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 123.Nie H, Wang CH. Fabrication and characterization of PLGA/HAp composite scaffolds for delivery of BMP-2 plasmid DNA. J Controlled Release. 2007;120:111–121. doi: 10.1016/j.jconrel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 124.Wang H, Song HR, Chen XS, Deng YJ. Release of ibuprofen from PEG-PLLA electrospun fibers containing poly(ethylene glycol)-b-poly(α-hydroxy octanoic acid) as an additive. J Polym Sci. 2010;28:417–425. [Google Scholar]
- 125.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000;69:149–58. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 126.Manning CN, Kim HM, Sakiyama-Elbert S, Galatz LM, Havlioglu N, Thomopoulos S. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J Orthop Res. 2011;29:1099–105. doi: 10.1002/jor.21301. [DOI] [PubMed] [Google Scholar]
- 127.Xu X, Zhuang X, Chen X, Wang X, Yang L, Jing X. Preparation of core-sheath composite nanofibers by emulsion electrospinning. Macromol Rapid Commun. 2006;27:1637–1642. [Google Scholar]
- 128.Yang Y, Li X, Cui W, Zhou S, Tan R, Wang C. Structural stability and release profiles of proteins from core-shell poly (DL-lactide) ultrafine fibers prepared by emulsion electrospinning. J Biomed Mater Res, Part A. 2008;86:374–385. doi: 10.1002/jbm.a.31595. [DOI] [PubMed] [Google Scholar]
- 129.Xu X, Yang L, Xu X, Wang X, Chen X, Liang Q, Zeng J, Jing X. Ultrafine medicated fibers electrospun from W/O emulsions. J Controlled Release. 2005;108:33–42. doi: 10.1016/j.jconrel.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 130.Langer RS. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 131.Hu J, Ma PX. Nano-fibrous tissue engineering scaffolds capable of growth factor delivery. Pharm Res. 28:1273–81. doi: 10.1007/s11095-011-0367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wei G, Jin Q, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J Controlled Release. 2006;112:103–110. doi: 10.1016/j.jconrel.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wei G, Pettway GJ, McCauley LK, Ma PX. The release profiles and bioactivity of parathyroid hormone from poly(lactic-co-glycolic acid) microspheres. Biomaterials. 2004;25:345–352. doi: 10.1016/s0142-9612(03)00528-3. [DOI] [PubMed] [Google Scholar]
- 134.Wei G. Growth factor- delivering Nano-fibrous scaffolds for bone tissue regeneration. University of Michigan; Ann Arbor: 2006. [Google Scholar]
- 135.Wei G, Ma PX. Biomimetic nano-fibrous scaffold for multiple factor delivery. Trasactions of the 31st Annual Meeting of the Society For Biomaterials; 2006; 2006. p. 1. [Google Scholar]
- 136.Prabhakaran MP, Venugopal J, Chan CK, Ramakrishna S. Surface modified electrospun nanofibrous scaffolds for nerve tissue engineering. Nanotechnology. 2008;19 doi: 10.1088/0957-4484/19/45/455102. [DOI] [PubMed] [Google Scholar]
- 137.Jin Q, Wei G, Lin Z, Sugai JV, Lynch SE, Ma PX, Giannobile WV. Nanofibrous scaffolds incorporating PDGF-BB microspheres induce chemokine expression and tissue neogenesis in vivo. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Baker SC, Atkin N, Gunning PA, Granville N, Wilson K, Wilson D, Southgate J. Characterisation of electrospun polystyrene scaffolds for three-dimensional in vitro biological studies. Biomaterials. 2006;27:3136–3146. doi: 10.1016/j.biomaterials.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 139.Ma Z, He W, Yong T, Ramakrishna S. Grafting of gelatin on electrospun poly(caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell orientation. Tissue Eng. 2005;11:1149–1158. doi: 10.1089/ten.2005.11.1149. [DOI] [PubMed] [Google Scholar]
- 140.Nitschke M, Schmack G, Janke A, Simon F, Pleul D, Werner C. Low pressure plasma treatment of poly(3-hydroxybutyrate): Toward tailored polymer surfaces for tissue engineering scaffolds. J Biomed Mater Res. 2002;59:632–638. doi: 10.1002/jbm.1274. [DOI] [PubMed] [Google Scholar]
- 141.Kavc T, Kern W, Ebel MF, Svagera R, Polt P. Surface Modification of Polyethylene by Photochemical Introduction of Sulfonic Acid Groups. Chem Mater. 2000;12:1053–1059. [Google Scholar]
- 142.Chanunpanich N, Ulman A, Strzhemechny YM, Schwarz SA, Janke A, Braun HG, Kraztmuller T. Surface Modification of Polyethylene through Bromination. Langmuir. 1999;15:2089–2094. [Google Scholar]
- 143.Mathieson I, Bradley RH. Improved adhesion to polymers by UV/ozone surface oxidation. Int J Adhes Adhes. 1996;16:29–31. [Google Scholar]
- 144.Ma Z, Kotaki M, Yong T, He W, Ramakrishna S. Surface engineering of electrospun polyethylene terephthalate (PET) nanofibers towards development of a new material for blood vessel engineering. Biomaterials. 2005;26:2527–2536. doi: 10.1016/j.biomaterials.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 145.Mori M, Uyama Y, Ikada Y. Surface modification of polyethylene fiber by graft polymerization. J Polym Sci, Part A: Polym, Chem. 1994;32:1683–1690. [Google Scholar]
- 146.Kou RQ, Xu ZK, Deng HT, Liu ZM, Seta P, Xu Y. Surface modification of microporous polypropylene membranes by plasma-induced graft polymerization of [alpha]-allyl glucoside. Langmuir. 2003;19:6869–6875. [Google Scholar]
- 147.Gao J, Niklason L, Langer R. Surface hydrolysis of poly(glycolic acid) meshes increases the seeding density of vascular smooth muscle cells. J Biomed Mater Res. 1998;42:417–24. doi: 10.1002/(sici)1097-4636(19981205)42:3<417::aid-jbm11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 148.Park GE, Pattison MA, Park K, Webster TJ. Accelerated chondrocyte functions on NaOH-treated PLGA scaffolds. Biomaterials. 2005;26:3075–3082. doi: 10.1016/j.biomaterials.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 149.Hermanson GT. Bioconjugate Techniques. San Diego: Academic Press, Inc; 1996. [Google Scholar]
- 150.Duckworth BP, Xu J, Taton TA, Guo A, Distefano MD. Site-Specific, Covalent Attachment of Proteins to a Solid Surface. Bioconjugate Chem. 2006;17:967–974. doi: 10.1021/bc060125e. [DOI] [PubMed] [Google Scholar]
- 151.Li W, Guo Y, Wang H, Shi D, Liang C, Ye Z, Qing F, Gong J. Electrospun nanofibers immobilized with collagen for neural stem cells culture. J Mater Sci: Mater Med. 2008;19:847–854. doi: 10.1007/s10856-007-3087-5. [DOI] [PubMed] [Google Scholar]
- 152.Kim HS, Yoo HS. MMPs-responsive release of DNA from electrospun nanofibrous matrix for local gene therapy: In vitro and in vivo evaluation. J Controlled Release. 2010;145:264–271. doi: 10.1016/j.jconrel.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 153.Ji Suk C, Hyuk Sang Y. Electrospun nanofibers surface-modified with fluorescent proteins. J Bioact Ccompat Pol. 2007;22:508–524. [Google Scholar]
- 154.Kim TG, Park TG. Surface functionalized electrospun biodegradable nanofibers for immobilization of bioactive molecules. Biotechnol Prog. 2006;22:1108–13. doi: 10.1021/bp060039t. [DOI] [PubMed] [Google Scholar]
- 155.Yoo HS, Kim TG, Park TG. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv Drug Deliv Rev. 2009;61:1033–42. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 156.Yao C, Li X, Neoh KG, Shi Z, Kang ET. Surface modification and antibacterial activity of electrospun polyurethane fibrous membranes with quaternary ammonium moieties. J Membr Sci. 2008;320:259–267. [Google Scholar]
- 157.Bock VD, Hiemstra H, van Maarseveen JH. CuI-Catalyzed Alkyne-Azide “Click” Cycloadditions from a Mechanistic and Synthetic Perspective. Eur J Org Chem. 2006;2006:51–68. [Google Scholar]
- 158.Shi Q, Chen X, Lu T, Jing X. The immobilization of proteins on biodegradable polymer fibers via click chemistry. Biomaterials. 2008;29:1118–1126. doi: 10.1016/j.biomaterials.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 159.Chang Z, Fang Y, Zhang Q, Chen D. “Click” Chemistry for Facile Immobilization of Iron Phthalocyanines onto Electrospun Nanofiber Surface. Chem Lett. 2009;38:1144–1146. [Google Scholar]
- 160.Fu GD, Xu LQ, Yao F, Li GL, Kang ET. Smart nanofibers with a photoresponsive surface for controlled release. ACS applied materials & interfaces. 2009;1:2424–2427. doi: 10.1021/am900526u. [DOI] [PubMed] [Google Scholar]
- 161.Killops KL, Campos LM, Hawker CJ. Robust, efficient, and orthogonal synthesis of dendrimers via thiol-ene “click” chemistry. J Am Chem Soc. 2008;130:5062–5064. doi: 10.1021/ja8006325. [DOI] [PubMed] [Google Scholar]
- 162.Huang C, Soenen SJ, Rejman J, Lucas B, Braeckmans K, Demeester J, De Smedt SC. Stimuli-responsive electrospun fibers and their applications. Chem Soc Rev. 2011;40:2417–2434. doi: 10.1039/c0cs00181c. [DOI] [PubMed] [Google Scholar]
- 163.Ehrbar M, Rizzi SC, Hlushchuk R, Djonov V, Zisch AH, Hubbell JA, Weber FE, Lutolf MP. Enzymatic formation of modular cell-instructive fibrin analogs for tissue engineering. Biomaterials. 2007;28:3856–66. doi: 10.1016/j.biomaterials.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 164.Yoshimi R, Yamada Y, Ito K, Nakamura S, Abe A, Nagasaka T, Okabe K, Kohgo T, Baba S, Ueda M. Self-assembling peptide nanofiber scaffolds, platelet-rich plasma, and mesenchymal stem cells for injectable bone regeneration with tissue engineering. J Craniofac Surg. 2009;20:1523–30. doi: 10.1097/SCS.0b013e3181b09b7e. [DOI] [PubMed] [Google Scholar]
- 165.Kohgo T, Yamada Y, Ito K, Yajima A, Yoshimi R, Okabe K, Baba S, Ueda M. Bone regeneration with self-assembling peptide nanofiber scaffolds in tissue engineering for osseointegration of dental implants. Int J Periodontics Restorative Dent. 2011;31:e9–16. [PubMed] [Google Scholar]
- 166.Shin M, Yoshimoto H, Vacanti JP. In Vivo Bone Tissue Engineering Using Mesenchymal Stem Cells on a Novel Electrospun Nanofibrous Scaffold. Tissue Eng. 2004;10:33–41. doi: 10.1089/107632704322791673. [DOI] [PubMed] [Google Scholar]
- 167.Jin HJ, Chen J, Karageorgiou V, Altman GH, Kaplan DL. Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials. 2004;25:1039–1047. doi: 10.1016/s0142-9612(03)00609-4. [DOI] [PubMed] [Google Scholar]
- 168.Phipps MC, Clem WC, Catledge SA, Xu Y, Hennessy KM, Thomas V, Jablonsky MJ, Chowdhury S, Stanishevsky AV, Vohra YK, Bellis SL. Mesenchymal stem cell responses to bone-mimetic electrospun matrices composed of polycaprolactone, collagen I and nanoparticulate hydroxyapatite. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0016813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang Y, Venugopal JR, El-Turki A, Ramakrishna S, Su B, Lim CT. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials. 2008;29:4314–4322. doi: 10.1016/j.biomaterials.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 170.Kim HW, Yu HS, Lee HH. Nanofibrous matrices of poly(lactic acid) and gelatin polymeric blends for the improvement of cellular responses. J Biomed Mater Res, Part A. 2008;87:25–32. doi: 10.1002/jbm.a.31677. [DOI] [PubMed] [Google Scholar]
- 171.Venugopal J, Low S, Choon AT, Sampath Kumar TS, Ramakrishna S. Mineralization of osteoblasts with electrospun collagen/hydroxyapatite nanofibers. J Mater Sci: Mater Med. 2008;19:2039–2046. doi: 10.1007/s10856-007-3289-x. [DOI] [PubMed] [Google Scholar]
- 172.Ngiam M, Liao S, Patil AJ, Cheng Z, Chan CK, Ramakrishna S. The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering. Bone. 2009;45:4–16. doi: 10.1016/j.bone.2009.03.674. [DOI] [PubMed] [Google Scholar]
- 173.Zhang Y, Reddy VJ, Wong SY, Li X, Su B, Ramakrishna S, Lim CT. Enhanced biomineralization in osteoblasts on a novel electrospun biocomposite nanofibrous substrate of hydroxyapatite/collagen/chitosan. Tissue Eng Part A. 2010;16:1949–1960. doi: 10.1089/ten.TEA.2009.0221. [DOI] [PubMed] [Google Scholar]
- 174.Badami AS, Kreke MR, Thompson MS, Riffle JS, Goldstein AS. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27:596–606. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 175.Ma J, He X, Jabbari E. Osteogenic Differentiation of Marrow Stromal Cells on Random and Aligned Electrospun Poly(l-lactide) Nanofibers. Ann Biomed Eng. 2011;39:14–25. doi: 10.1007/s10439-010-0106-3. [DOI] [PubMed] [Google Scholar]
- 176.Wang Y, Gao R, Wang PP, Jian J, Jiang XL, Yan C, Lin X, Wu L, Chen GQ, Wu Q. The differential effects of aligned electrospun PHBHHx fibers on adipogenic and osteogenic potential of MSCs through the regulation of PPARy signaling. Biomaterials. 2012;33:485–493. doi: 10.1016/j.biomaterials.2011.09.089. [DOI] [PubMed] [Google Scholar]
- 177.Yang X, Shah J, Wang H. Nanofiber enabled layer-by-layer approach toward three-dimensional tissue formation. Tissue Eng Part A. 2009;15:945–956. doi: 10.1089/ten.tea.2007.0280. [DOI] [PubMed] [Google Scholar]
- 178.Matsushima S, Isogai N, Jacquet R, Lowder E, Tokui T, Landis WJ. The Nature and Role of Periosteum in Bone and Cartilage Regeneration. Cells Tissues Organs. 2011;194:320–325. doi: 10.1159/000324642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kolambkar YM, Peister A, Ekaputra AK, Hutmacher DW, Guldberg RE. Colonization and osteogenic differentiation of different stem cell sources on electrospun nanofiber meshes. Tissue Eng Part A. 2010;16:3219–3230. doi: 10.1089/ten.tea.2010.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim KH, Jeong L, Park HN, Shin SY, Park WH, Lee SC, Kim TI, Park YJ, Seol YJ, Lee YM, Ku Y, Rhyu IC, Han SB, Chung CP. Biological efficacy of silk fibroin nanofiber membranes for guided bone regeneration. J Biotechnol. 2005;120:327–339. doi: 10.1016/j.jbiotec.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 181.Seol YJ, Kim KH, Young MK, In AK, Rhee SH. Bioactivity, pre-osteoblastic cell responses, and osteoconductivity evaluations of the electrospun non-woven SiO2-CaO gel fabrics. J Biomed Mater Res, Part B. 2009;90:679–687. doi: 10.1002/jbm.b.31334. [DOI] [PubMed] [Google Scholar]
- 182.Telemeco TA, Ayres C, Bowlin GL, Wnek GE, Boland ED, Cohen N, Baumgarten CM, Mathews J, Simpson DG. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibrils produced by electrospinning. Acta Biomater. 2005;1:377–385. doi: 10.1016/j.actbio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 183.Balguid A, Mol A, Van Marion MH, Bank RA, Bouten CVC, Baaijens FPT. Tailoring fiber diameter in electrospun poly(epsilon-Caprolactone) scaffolds for optimal cellular infiltration in cardiovascular tissue engineering. Tissue Eng Part A. 2009;15:437–444. doi: 10.1089/ten.tea.2007.0294. [DOI] [PubMed] [Google Scholar]
- 184.Guimaires A, Martins A, Pinho ED, Faria S, Reis RL, Neves NM. Solving cell infiltration limitations of electrospun nanofiber meshes for tissue engineering applications. Nanomedicine. 2010;5:539–554. doi: 10.2217/nnm.10.31. [DOI] [PubMed] [Google Scholar]
- 185.Whited BM, Whitney JR, Hofmann MC, Xu Y, Rylander MN. Pre-osteoblast infiltration and differentiation in highly porous apatite-coated PLLA electrospun scaffolds. Biomaterials. 2010;32:2294–2304. doi: 10.1016/j.biomaterials.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 186.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res, Part A. 2003;67A:531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 187.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 188.Jukes JM, Both SK, Leusink A, Sterk LM, Blitterswijk CAv, Boer Jd. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci USA. 2007;105:6840–6845. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Handschel J, Naujoks C, Langenbach F, Berr K, Depprich RA, Ommerborn MA, Kübler NR, Brinkmann M, Kögler G, Meyer U. Comparison of ectopic bone formation of embryonic stem cells and cord blood stem cells in vivo. Tissue Eng Part A. 2010;16:2475–2483. doi: 10.1089/ten.TEA.2009.0546. [DOI] [PubMed] [Google Scholar]
- 190.Smith LA, Liu X, Hu J, Wang P, Ma PX. Enhancing osteogenic differentiation of mouse embryonic stem cells by nanofibers. Tissue Eng Part A. 2009;15:1855–1864. doi: 10.1089/ten.tea.2008.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Smith LA, Liu X, Hu J, Ma PX. The influence of three-dimensional nanofibrous scaffolds on the osteogenic differentiation of embryonic stem cells. Biomaterials. 2009;30:2516–2522. doi: 10.1016/j.biomaterials.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Smith LA, Liu X, Hu J, Ma PX. The Enhancement of human embryonic stem cell osteogenic differentiation with nano-fibrous scaffolding. Biomaterials. 2010;31:5526–5535. doi: 10.1016/j.biomaterials.2010.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brown SE, Tong W, Krebsbach PH. The derivation of mesenchymal stem cells from human embryonic stem cells. Cells Tissues Organs. 2008;189:256–260. doi: 10.1159/000151746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hwang N, Varghese S, Zhang Z, Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate modified hydrogels. Tissue Eng. 2006;12:2695–2706. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 195.Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:554–560. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hu J, Smith LA, Feng K, Liu X, Sun H, Ma PX. Response of human embryonic stem cell-derived mesenchymal stem cells to osteogenic factors and architectures of materials during in vitro osteogenesis. Tissue Eng Part A. 2010;16:3507–3514. doi: 10.1089/ten.tea.2010.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sun H, Feng K, Hu J, Soker S, Atala A, Ma PX. Osteogenic differentiation of human amniotic fluid-derived stem cells induced by bone morphogenetic protein-7 and enhanced by nanofibrous scaffolds. Biomaterials. 2010;31:1133–1139. doi: 10.1016/j.biomaterials.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhao J, Han W, Chen H, Tu M, Huan S, Miao G, Zeng R, Wu H, Cha Z, Zhou C. Fabrication and in vivo osteogenesis of biomimetic poly(propylene carbonate) scaffold with nanofibrous chitosan network in macropores for bone tissue engineering. J Mater Sci: Mater Med. 2012;23:517–525. doi: 10.1007/s10856-011-4468-3. [DOI] [PubMed] [Google Scholar]
- 199.Scottia C, Tonnarellia B, Papadimitropoulosa A, Scherbericha A, Schaerena S, Schauertec A, Lopez-Riosc J, Zellerc R, Barberoa A, Martina I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci USA. 2010;107:7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rouwkema J, Boer Jd, Blitterswijk CV. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006;12:2685–2693. doi: 10.1089/ten.2006.12.2685. [DOI] [PubMed] [Google Scholar]
- 201.Rouwkema J, Westerweel P, Boer Jd, Verhaar M, Blitterswijk Cv. The use of endothelial progenitor cells for prevascularized bone tissue engineering. Tissue Eng Part A. 2009;15:2015–2027. doi: 10.1089/ten.tea.2008.0318. [DOI] [PubMed] [Google Scholar]
- 202.Tsigkou O, Pomerantseva I, Spencer J, Redondo P, Hart A, O’Doherty E, Lin Y, Friedrich C, Daheron L, Lin C, Sundback C, Vacanti J, Neville C. Engineered vascularized bone grafts. Proc Natl Acad Sci USA. 2010;107:3311–3316. doi: 10.1073/pnas.0905445107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.He C, Xiao G, Jin X, Sun C, Ma PX. Electrodeposition on nanofibrous polymer scaffolds: Rapid mineralization, tunable calcium phosphate composition and topography. Advanced Functional Materials. 2010;20:3568–3576. doi: 10.1002/adfm.201000993. [DOI] [PMC free article] [PubMed] [Google Scholar]