Abstract
Background
Activated protein C (aPC) mediates powerful cytoprotective effects through protease activated receptor (PAR)-1 that translate into reduced harm in mouse injury models. However, it remains elusive how aPC-activated PAR1 can mediate cytoprotective effects while thrombin activation does the opposite.
Objectives
We hypothesized that aPC and thrombin might induce distinct active conformations in PAR1 causing opposing effects.
Methods
We analyzed antibody binding to, and cleavage and signalling of PAR1 in either endogenously expressing endothelial or overexpressing 293T cells.
Results
In thrombin-cleaved PAR1 neither the tethered ligand nor the hirudin like domain were available for anti-PAR1 ATAP2 and WEDE15 binding unless the tethered ligand was quenched. In contrast, aPC irreversibly prevented ATAP2 binding while not affecting WEDE15 binding. Reporter constructs with selective glutamine substitutions confirmed R41 as the only thrombin cleavage site in PAR1, whereas aPC preferentially cleaved at R46. Similarly, we report distinct cleavage sites on PAR3, K38 for thrombin and R41 for aPC. A soluble peptide corresponding to R46-cleaved PAR1 enhanced the endothelial barrier function and reduced staurosporine toxicity in endothelial as well as in 293T cells if PAR1 was expressed. Overexpression of PAR1 variants demonstrated that cleavage at R46 but not R41 is required for cytoprotective aPC signaling.
Conclusions
We provide a novel concept on how aPC and thrombin mediate distinct effects. We propose that the enzyme specific cleavage sites induce specific conformations which mediate divergent downstream effects. This unexpected model of PAR1 signaling might lead to novel therapeutic options for treatment of inflammatory diseases.
Keywords: PAR1, thrombin, activated protein C
Introduction
Activated protein C (aPC) has powerful protective effects in systemic inflammation [1–6] and was approved to treat patients with severe sepsis [7]. However, efficiency is controversially discussed and the recently completed PROWESS-SHOCK trial failed to show a survival benefit [8], resulting in withdrawal of the drug from the market [9]. However, still no other specific treatment options for sepsis are currently available. Thus a more thorough understanding of how aPC mediates protective effects and what the pathways involved are could help to develop novel future therapeutic approaches.
Protease activated receptor-1 (PAR1) was shown to be efficiently cleaved and activated by aPC in an endothelial protein C receptor (EPCR) dependent way [10]: inducing protective gene expression [10, 11], enhancing the endothelial barrier function [12, 13], reducing cytokine secretion [14, 15] and protecting against apoptosis [11, 14, 16]. Similarly, in animal studies aPC-PAR1 was shown to have neuro- [1, 17–19] and nephro-protective effects [20], to enhance the vascular barrier function [3, 5], and to reduce cytokine secretion [4]. These effects translated into survival benefits [2–4, 6]. Thus, aPC-cleaved PAR1 has been shown to induce protective effects both in vitro and in vivo. In contrast, PAR1 activation by thrombin can result in proinflammatory effects such as disruption of endothelial barrier integrity [21]. PAR1 is therefore a Janus-faced receptor that mediates protective aPC signaling and pro-inflammatory thrombin signaling.
PAR1 is a 7-transmembrane domain receptor that couples to various G-proteins [13, 21–23], potentially explaining how a single receptor can mediate opposing effects. Since in related dopamine receptors ligand specific G-protein activation was shown to directly depend on ligand-specific conformations [24], we hypothesized that a similar model could apply to PAR1 as well. Current information indicates that thrombin and aPC cleave at arginine 41 uncovering the same tethered ligand. This cleavage site was found by screening and examining whether soluble peptides which are homologous to PAR1’s truncated N-terminus can induce calcium release in PAR1 overexpressing cells [25, 26]. Thus cleavage site(-s) promoting active conformations with favourable coupling towards non calcium inducing or calcium inhibiting pathways might have been missed.
Here we show that aPC-cleaved as compared to thrombin-cleaved PAR1 binds anti-PAR1 antibodies differently. Further, we discovered a novel aPC specific cleavage site at R46 in PAR1, indicating that alternatively (R46) cleaved PAR1 can mediate distinct biological effects.
Methods
Reagents
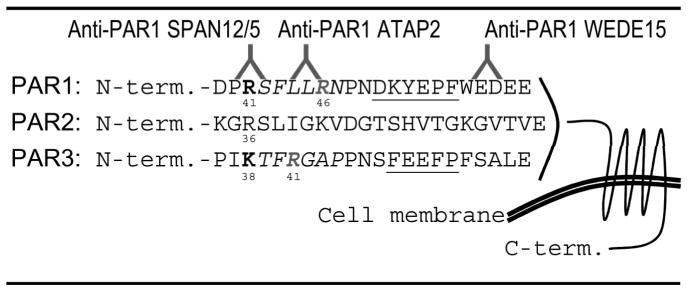
Clotting proteases were purchased from Haematologic Technologies (Essex Junction, VT, USA), Trypsin was from Gibco (Invitrogen). Peptides corresponding to the N-terminus of R41 cleaved PAR1 (R41PAR1pep, SFLLRNPN), R46 cleaved PAR1 (R46PAR1pep, NPNDKYEP) and a length matched mock peptide were custom made (Antagene; Sunnyvale, CA, USA). The small chemical PAR1 antagonist RWJ-58259 was a kind gift from Dr. Patricia Liaw (McMaster University, Hamilton, ON, Canada). All experiments involving agonist stimulation with clotting proteases other than thrombin included hirudin (Lepirudin, Schering, Berlin, Germany). Hirudin alone had no effect in any of our assays. Monoclonal anti-PAR1 SPAN12/5, ATAP2 and WEDE15 were used as described previously [27, 28].
Cell culture and plasmid transfection and gene silencing
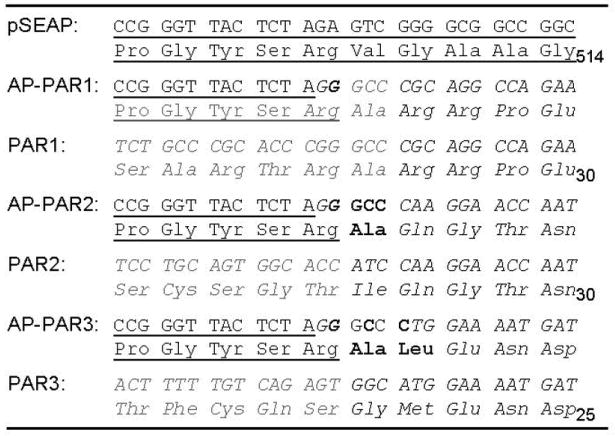
Endothelial EA.hy926 cells [29] and human embryonic kidney cell-derived 293T cells were cultivated and propagated as described previously [27, 28]. Gene silencing using Lipofectamin (Invitrogen) and siRNA was done as described previously [28]. Tagged and non-tagged PAR1 and EPCR were transiently overexpressed as described [28]. PAR2 and PAR3, originally obtained from Dr. Lawrence Brass (University of Pennsylvania, USA) were introduced into pcDNA3.1/Zeo+. For N-terminal alkaline phosphatase (AP) (Clontech, CA, USA) tags, the signal peptides were replaced by ApaI restriction sites allowing the exchange of mature PARs within the tagged PAR1 construct (Table 1). Mutations were obtained by site directed mutagenesis (Stratagene, CA and Phusion® Site-Directed Mutagenesis Kits, NEB, MA, USA). For fluorescent PAR1 the stop codon in our construct was replaced by enhanced green fluorescent protein (Clontech, CA, USA). All constructs were verified by sequencing. As mock construct an antibiotic was expressed in pcDNA3.1/Zeo+.
Table 1.
|
Cell surface immunoassays, SDS page and permeability assay
Cell surface PAR1 was quantified by cell surface enzyme-linked immunosorbent assay [27, 28] and analyzed by SDS page as described [27, 28]. In some experiments involving quenching of the tethered ligand with the PAR1 antagonist RWJ-58259 the cells were fixed for 40 instead of 30 minutes with PFA 2%. Macromolecular monolayer permeability was analyzed using a dual chamber system (#3402; Corning Inc., Corning, NY, USA) and Evans blue-labelled BSA [5, 30].
PAR1 cleavage reporter assay
293T cells transiently expressing AP-tagged PAR constructs (Table 1) were used [28]. Washed cells were agonist incubated (20 min), supernatants were removed, filtered (cellulose ester filter; pore size, 0.45 μm), and AP activity was quantified (1-Step PNPP; Thermo Scientific, Rockford, IL, USA). In all experiments, expression levels of AP-PAR were verified to be comparable among constructs by quantifying cell surface AP activity as well.
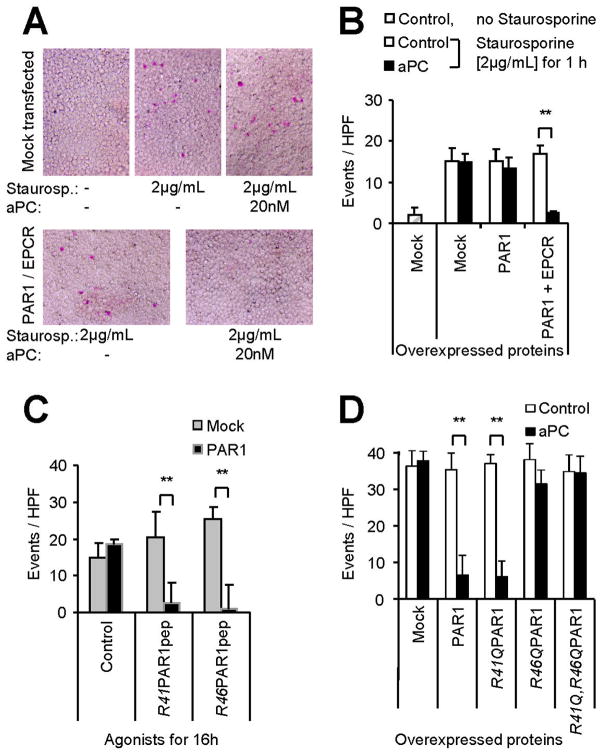
Apoptosis assay
Apoptosis was analyzed as described by Joyce et al. [11, 16]. Lower (1–8%) or higher (10%) amounts of fetal calf serum were used for experiments with EA.hy926 and transiently overexpressing 293T cells, respectively. This modification was required since lower serum levels resulted in detachment of 293T cells. In all experiments agonist incubation was 16 hours, staurosporine (2 μg/mL; Sigma, MO, USA) was added for the final 1 hour, and the colour dye from the APOPercentage™ kit (Biocolor, Carrickfergus, UK) was used at 5% for the final 30 minutes of incubation. Washing of the cells and quantitative analysis of stained cells by analytical digital photomicroscopy was performed according to the manufacturer’s instructions.
Statistics
Data analysis and presentation was performed using NCSS, SPSS software packages. A two-sample, two-tailed homoscedastic t-test was used to calculate the indicated P-values.
Results
Antibody binding to cleaved PAR1 is agonist specific
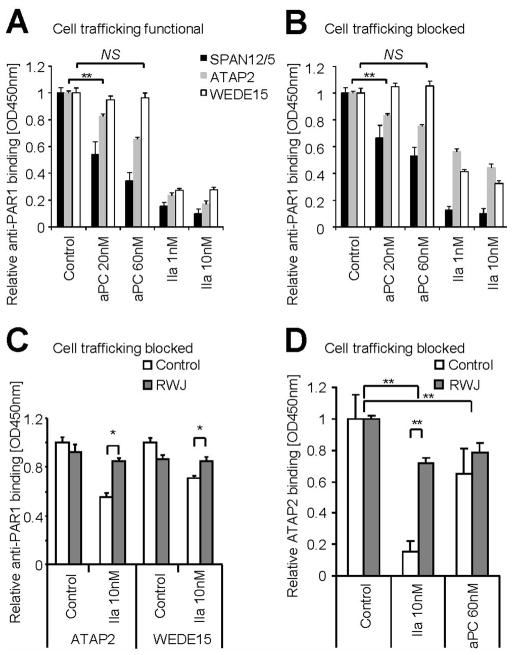
In order to test whether thrombin- and APC-cleaved PAR1 adopt different conformations, we studied the availability of anti-PAR1 epitopes on endogenous endothelial cell surfaces following agonist incubation. These antibodies have been extensively used and the epitopes have been mapped (Table 2). The SPAN12/5 epitope spans the arginine 41 (R41) cleavage site and is thus physically “cleavage sensitive” [5, 31]. SPAN12/5 binding is reduced upon agonist incubation with aPC and thrombin in both native cells (Figure 1A) and cells in which cell trafficking was blocked by fixation with paraformaldehyde (Figure 1B). This finding is consistent with efficient cleavage of PAR1 by APC as well as the fact that thrombin cleaves almost all apically expressed N-termini of PAR1. ATAP2 was raised against the thrombin receptor activator peptide and recognizes L44 to R46 within the tethered ligand. Given the L44-R46 epitope beeing C-terminal of the R41 cleavage site, one would not expect ATAP2 binding to be affected by proteolytic cleavage of PAR1. We however have previously shown that ATAP2 and PAR1’s ligand binding site compete for binding to the tethered ligand [27]. In accordance we found aPC and thrombin to significantly decrease ATAP2 binding in cells with functional and blocked receptor trafficking (Figure 1A–B). WEDE15 recognizes the hirudin-like domain (F55-E60) [32]. WEDE15 binding was not altered upon incubation with aPC, a finding that has been explained by delayed clearance of aPC cleaved PAR1 from the cell surface [23, 27]. In contrast, thrombin strongly reduced WEDE15 binding, consistent with rapid internalization of thrombin cleaved PAR1 [33]. However, internalization cannot explain why thrombin still decreases WEDE15 binding in fixed cells (Figure 1B). To further explore this unexpected finding, we used the antagonist RWJ-58259 which prevents the tethered ligand from interacting with PAR1’s ligand binding site [34]. If added after thrombin incubation, RWJ-58259 restored ATAP2 as well as WEDE15 binding (Figure 1C). Although efficient, recovery by RWJ-58259 was not complete. Possible explanations are only partial quenching of the tethered ligand, partial shedding of the N-terminus following quenching, or additional cleavage by thrombin C-terminal of the ATAP2 binding site. Successful quenching of PAR1 by RWJ-58259 restored anti-PAR1 antibody binding and supports a model of direct competition between ATAP2 and PAR1’s ligand binding site for tethered ligand binding. Further it suggests fundamental changes in PAR1’s conformation upon cleavage by thrombin that also render the hirudin like domain inaccessible to antibody binding. The reversibility of WEDE15 and ATAP2 binding following thrombin together with our previous finding that thrombin-cleaved PAR1 can be detected by Western blotting using WEDE15 [27] support conformational changes and not N-terminal shedding of PAR1 as the underlying mechanism. Interestingly, RWJ-58259 efficiently restored ATAP2 binding to thrombin- but not aPC-treated PAR1 (Figure 1D).
Table 2.
|
Figure 1.
Together these data indicate that anti-PAR1 antibody binding to cleaved PAR1 is agonist specific. The hirudin like domain becomes unavailable to WEDE15 binding following thrombin but can be uncovered upon quenching with RWJ-58259, consistent with conformational ‘hiding’ of the WEDE15 epitope following thrombin. In contrast WEDE 15 binds to aPC cleaved PAR1 suggesting a conformation different to the thrombin-cleaved receptor in which the hirudin like domain remains accessible. Further antibody binding to the tethered ligand (ATAP2) is also agonist specific, recoverable by RWJ-58259 following thrombin, but irreversibly lost after aPC.
Activated PC cleaves PAR1 at R46 and removes the canonical (R41) tethered ligand containing the ATAP2 epitope
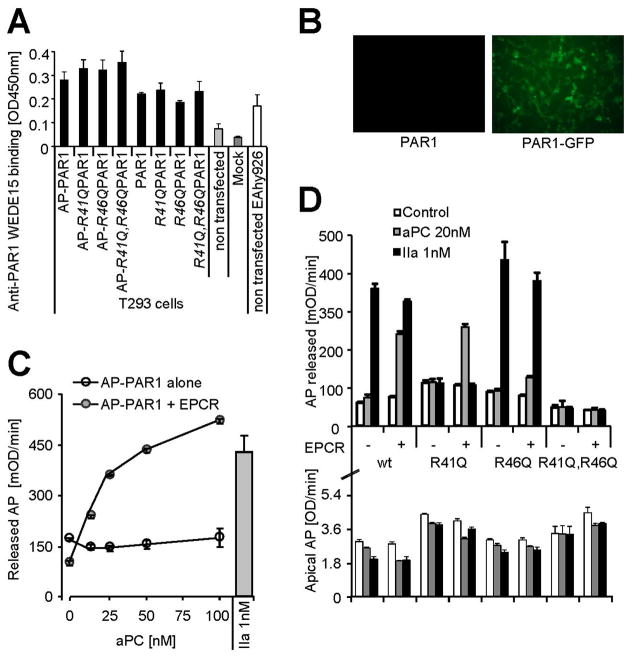
We next addressed why RWJ-58259 cannot uncover ATAP2 binding following aPC and tested if aPC sheds the tethered ligand potentially at R46 (Table 2). To test whether aPC cleaves at R46 we engineered PAR1 overexpression constructs [28]. We verified expression directly by cell surface ELISA (Figure 2A) and expression efficiency by expressing fluorescently tagged constructs (Figure 2B). Cells expressing PAR1 constructs with a N-terminal alkaline phosphatase (AP) tag acquired apical AP activity which was removed from the cell surface and released into the supernatant by aPC in a dose dependent manner if EPCR was co-expressed (Figure 2C). To map potential cleavage site(s) mutants with glutamine substitutes in place(s) 41 and/or 46 were engineered. Cell surface expression levels of these variants were comparable to the wildtype PAR1 (Figure 2D, lower panel). Thrombin only cleaved constructs with maintained arginine in position 41 (wild type PAR1 and the R46Q mutant), confirming R41 as the only thrombin cleavage site [26] (Figure 2D, upper panel). In contrast, if EPCR was co-expressed aPC efficiently cleaved wild type PAR1 and the R41Q variant, whereas R46Q PAR1 was less efficiently cleaved and R41Q; R46Q PAR1 resisted cleavage. Taken together these data implicate R46 as a novel and preferred cleavage site for aPC. Cleavage at this site sheds the tethered ligand together with the ATAP2 epitope (Table 2) and can explain why this epitope was irreversibly lost in endogenous endothelial PAR1 cleaved by aPC.
Figure 2.
Activated PC cleaves PAR3 at non canonical R41
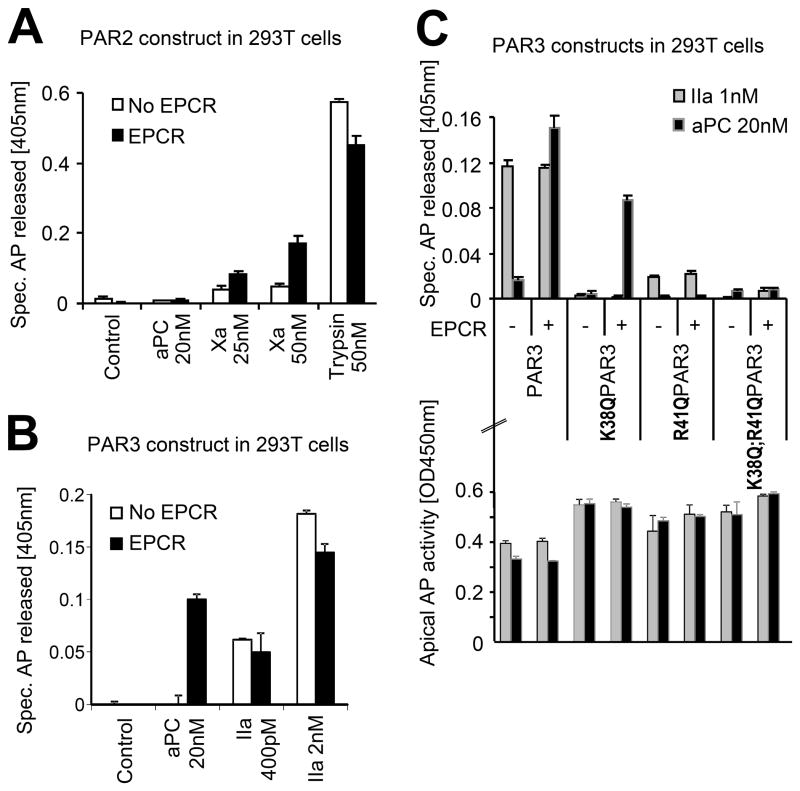
The protease activated receptors are highly homologous (Table 2) and some biological functions may be redundant [30]. PAR3 for example has also been implicated in protective effects of aPC [1, 35]. We thus hypothesized that aPC could cleave PARs other than PAR1 and that these receptors might harbour multiple cleavage sites as well. PAR2 and PAR3 constructs corresponding to AP-tagged PAR1 were engineered (Table 1) and expressed in 293T cells. Independent of the availability of EPCR, aPC failed to cleave the PAR2 reporter construct (Figure 3A). In contrast, trypsin as well as clotting factor Xa (Xa) cleaved PAR2. Of note, PAR2 cleavage by Xa was strongly enhanced in the presence of EPCR, similar to our previous results for PAR1 [28]. Tagged PAR3 was cleaved by thrombin and also very efficiently by aPC if EPCR was also available (Figure 3B). To identify the cleavage site(-s) in PAR3 we took the same approach as for PAR1. Analysis of K38Q, R41Q, and K38Q/R41Q PAR3 confirmed that K38 is the only thrombin cleavage site in PAR3 [36]. In contrast only constructs with arginine in position 41 were efficiently cleaved by aPC (Figure 3C). Taken together these data identify R41 as a novel and specific cleavage site in human PAR3 for aPC. These data also support the concept of clotting protease specific cleavage sites in PARs in general.
Figure 3.
R46-PAR1 N-terminal peptide and R46-cleaved PAR1 support cytoprotective signaling
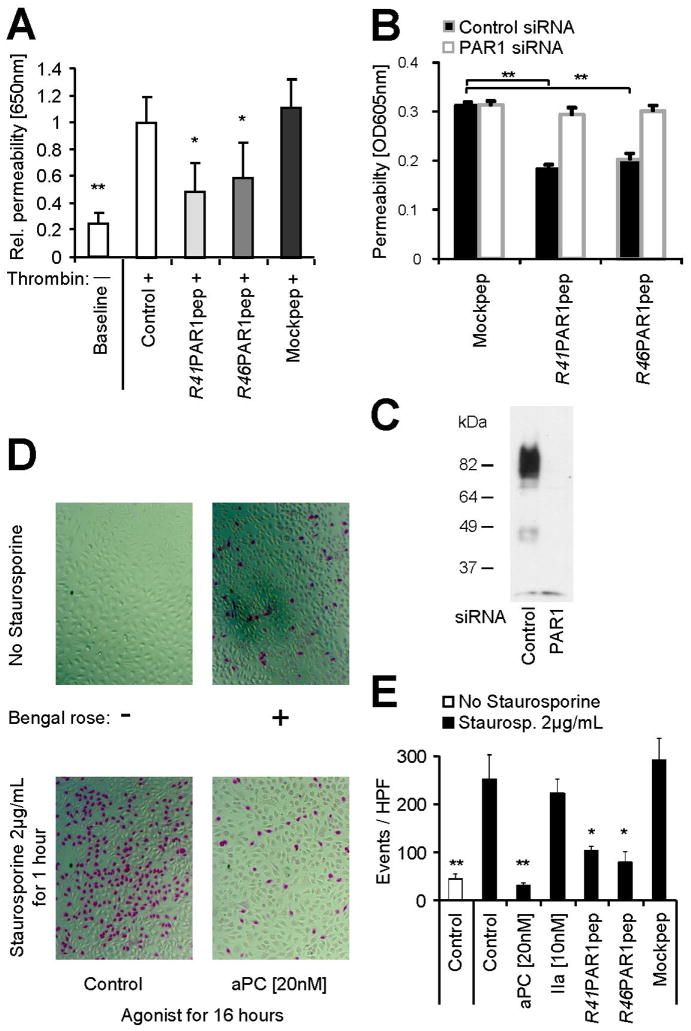
We next explored if the N-terminus of R46 cleaved PAR1 can mediate cytoprotective effects. Short peptides corresponding to the thrombin (R41) generated neo-N-terminus have been used to activate PAR1 albeit peptide and thrombin activated PAR1 have been shown to mediate distinct and different coupling to G-adaptor proteins [37]. Such differences might explain why the R41 peptide (SFLLRNPN) protects the endothelial cells from thrombin induced barrier disruption [30]. We confirmed barrier protective effects of the R41 peptide and found the R46 derived peptide (NPNDKYEP) to be similarly efficient whereas a length matched mock peptide did not have this effect (Figure 4A). Either of these peptides also directly enhanced the endothelial barrier function if endogenous PAR1 was available but not in PAR1 siRNA treated cells (Figure 4B). Control experiments showed that PAR1 was efficiently silenced in the experimental conditions used for the barrier assay (Figure 4C). Thus, PAR1 expression is required for the barrier protective effects of both peptides. We next analyzed protective effects of aPC and synthetic peptides on staurosporine toxicity. Both the R41 PAR1 as well as the R46 PAR1 peptide protected from toxicity, albeit either peptide was slightly less efficient than aPC (Figure 4D+E). Of note, cleavage at R41 by thrombin was not protective. These data demonstrate that a peptide corresponding to the new PAR1 N-terminus generated by R46 cleavage can mediate protective effects in endothelial cells. However they cannot answer the question whether the tethered ligand generated by aPC cleavage at R46 is cytoprotective.
Figure 4.
In order to directly test this question we developed an overexpression system. We adapted the staurosporine toxicity assay and used it in conjunction with the highly transfectable cell line 293T. In contrast to their maternal cell line HEK293 [11], we found the large T-antigen transformed cell line 293T to be unresponsive to aPC unless both EPCR and PAR1 were co-expressed (Figure 5A–B). Similar to endothelial cells, the R41- and R46-derived peptides reduced staurosporine toxicity if PAR1 was expressed (Figure 5C).
Figure 5.
Having established an overexpressing system we next addressed if R46 cleaved PAR1 directly mediates these protective effects. Variants of PAR1 with functional or substituted R41 and R46 cleavage sites were co-expressed with EPCR and tested for mediating protective effects of aPC. Only constructs with a maintained R46 cleavage site supported effects of aPC (Figure 5D). Absence of protective effects of R41Q PAR1 argues against indirect effects of a short SFLLR peptide released upon double cleavage. As in endothelial PAR1, thrombin could not reduce staurosporine toxicity in cells expressing any of the constructs (not shown). Taken together, these data demonstrate that the tethered R46 cleaved ligand of PAR1 directly mediates antitoxic effects in staurosporine treated cells. Since the corresponding soluble peptide can only mediate similar protective effects if PAR1 is available our data suggest that R46 cleaved PAR1 is a signalling competent receptor that directly mediates barrier protective and anti-apoptotic effects.
Discussion
Although many receptors have been implicated in aPC mediated signaling [38–40], EPCR and PAR1 are believed to be pivotal for cytoprotective effects of aPC [10, 41, 42]. These have been shown to also translate into reduced harm in mouse injury models [1–5]. Currently EPCR is thought to recruit and to position aPC for efficient PAR1 cleavage and activation. This model however cannot explain why EPCR-aPC activated PAR1 is protective, whereas the same PAR1 mediates opposite effects when cleaved by thrombin [21].
Almost two decades ago, a first study provided convincing proof of concept for the existence of multiple agonist-specific states in G-protein coupled receptors [43]. PAR1 also exerts agonist specific G-protein coupling [33, 37, 44], and multiple agonist specific conformations thus exist as well. However, so far the conceptual difficulty in the case of PAR1 was that thrombin and APC were thought to cleave the same scissile bond in the receptor’s N-terminus, leading to the generation of the same tethered ligand.
For the first time our results directly support the conclusion that thrombin- and aPC-cleaved PAR1 are indeed in fundamentally different conformations. First, the WEDE15 binding epitope in PAR1’s hirudin like domain becomes inaccessible in thrombin-cleaved but not APC-cleaved PAR1. Second, APC can truncate PAR1 at R46, five residues downstream from the thrombin cleavage site at R41.
It remains unclear why the thrombin-induced active conformation of PAR1 binds WEDE15 only if PAR1 is denatured [27] or quenched with the antagonist RWJ-58259. One possibility is that thrombin remains docked to cleaved PAR1 through an interaction between the hirudin-like domain in PAR1 and the exosite I in thrombin [26], thus ‘hiding’ the WEDE15 epitope. The observation of a one-to-one stoichiometrical ratio of the thrombin-PAR1 interaction in a previous study [31] would support such stable binding of thrombin to cleaved PAR1. However, we could not obtain experimental evidence supporting this hypothesis (R. Schuepbach and M. Riewald; unpublished observations). Further research revealing either PAR1’s structure at high resolution or studies using fluorescence quencher techniques analyzing conformational changes upon PAR1 activation might in the future shed light on multiple agonist-specific states of PAR1.
Multiple lines of evidence support our conclusion that APC can generate a distinct PAR1 tethered ligand by cleaving at R46. In endothelial cells the ATAP2 binding site on native PAR1 can be recovered with RWJ-58259 from the thrombin- but not APC-cleaved receptors, consistent with the concept that this epitope is shed upon cleavage at R46. Quantification of cleavage efficiency of overexpressed PAR1 reporter constructs indicates that thrombin targets only R41 whereas EPCR-bound aPC either cleaves at R41 or R46, but favours the novel R46 site. Our data strongly support the conclusion that R46-cleaved PAR1 is signaling competent. A soluble peptide corresponding to the novel R-46 cleaved N-terminus was cytoprotective in the barrier function assay as well as in a staurosporine cytotoxicity assay if PAR1 was available. Consistent with earlier reports [12], the R41 PAR1 agonist peptide corresponding to the R41 cleavage site was also protective in both assays. Thus, the R41 peptide does not induce a thrombin-like active conformation of PAR1 which is in line with earlier observations that R41 derived peptides can exert distinct effects that differ from the ones induced by thrombin [37]. It is possible that the tethered ligand generated upon thrombin cleavage at R41 signals differently from the soluble peptide [33] as a result of its tethered nature. Another possibility is that the soluble R41 peptide is N-terminally degraded upon the prolonged incubation with the cells in the presence of fetal calf serum in the cytoprotection assays. Such degradation might also explain divergent reports on whether the PAR1 peptide is anti-apoptotic [16, 17]. To test if physiological, i.e. tethered, ligands of R41 and R46 cleaved PAR1 mediate distinct effects we directly tested cytoprotective signaling of aPC in 293T cells overexpressing PAR1 variants. Our results demonstrate that besides expression of EPCR aPC required a functional R46 cleavage site within over-expressed PAR1 in order to mediate protection from staurosporine induced toxicity. Cleavage at R41 could not prevent from staurosporine induced toxicity consistent with lack of antitoxic effects by thrombin. Thus, despite having lost the established R41 tethered ligand, R46 cleaved PAR1 remains signaling competent and mediates protective effects. Interestingly, a non-canonical cleavage site at D39 has been previously implicated in PAR1 signaling [45] and our results add to an unexpected complexity in PAR1 activation mechanisms.
Our data support that a soluble peptide corresponding to the R46 cleaved PAR1 mediates protective effects upon availability of PAR1 and that the corresponding tethered peptide from overexpressed PAR1 does the same. Although these data are suggestive and consistent with a simple model in which a tethered R46 ligand directly induces protective signaling through PAR1 we could not directly test this model with the tools available. The soluble and tethered R46 ligand might well have different proprieties and the novel tethered R46 ligand may activate receptors other than PAR1. Heterodimer formation with cross-activation has been described [46, 47] and activation in trans of a dimer partner is a possibility. The tethered R46 ligand might well activate PAR1, PAR2 or PAR3 dimer partners [32, 35] or even an adjacent yet to be identified non-PAR receptor. In view of the finding that aPC-cleaved PAR1 accumulates on the cell surface [23, 27] R46 ligands might thus accumulate as well and reach high local ligand density. Along these lines aPC could mediate protective effects in scaffolds in which R46-cleaved PAR1 is essential but in which the R46 cleaved N-terminus of PAR1 signals in trans through a yet to be identified receptor different form PAR1.
In addition to analyzing whether R46 cleaved PAR1 is signaling competent, we explored whether multiple cleavage sites are common among the PAR family. We found that PAR3 is preferably cleaved at R41 by aPC whereas PAR3 is only canonically (K38) cleaved by thrombin. Thus, PAR3 appears to be very similar to PAR1 in terms of its interaction with thrombin and aPC. This dichotomy carries the potential that PAR3 might also play a pivotal role in how aPC and thrombin counteract. This is further underlined by a recent study implicating aPC-PAR3 in mediating protective effects [35] in podocytes.
In summary, we show specific differences between thrombin- and aPC-cleaved PAR1. Thrombin canonically cleaves PAR1 at R41 and induces an active conformation in which the hirudin like domain is not accessible. Activated PC in contrast cleaves PAR1 C-terminal from the canonical cleavage site at R46. This cleavage event also yields a signaling competent receptor which is characterized by a hirudin like domain accessible to antibody binding. These observations implicate that for PAR1, similarly to other G-coupled protein receptors, several agonist specific active conformations exist. This might explain agonist specific G-protein coupling and divergent signaling patterns. Further research will be required to directly link our novel observations to specific intracellular signaling pathways. Our finding carries a therapeutic potential for using specific PAR1 (ant-)agonists in the setting of inflammatory disorders in the future.
Acknowledgments
This work was made possible by the following Swiss grants to RAS: ‘Forschungskredit’ from the University of Zurich; ‘Hartmann Stiftung’, Zurich; the Swiss National Foundation (grant# PZ00P3_136639) and support from the Faculty of Medicine and the ‘Zentrum für Klinische Forschung’ of the University of Zurich. MR was supported by the National Institutes of Health (grant# HL 73318).
Footnotes
Disclosure of Conflict of Interest
The authors state that they have no conflict of interest.
References
- 1.Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, Griffin JH, Zlokovic BV. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–72. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 2.Kerschen E, Hernandez I, Zogg M, Jia S, Hessner MJ, Fernandez JA, Griffin JH, Huettner CS, Castellino FJ, Weiler H. Activated protein C targets CD8+ dendritic cells to reduce the mortality of endotoxemia in mice. J Clin Invest. 2010;120:3167–78. doi: 10.1172/JCI42629. 42629 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, Castellino FJ, Mackman N, Griffin JH, Weiler H. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204:2439–48. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, Derian CK, Andrade-Gordon P, Rosen H, Ruf W. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–8. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 5.Schuepbach RA, Feistritzer C, Fernandez JA, Griffin JH, Riewald M. Protection of vascular barrier integrity by activated protein C in murine models depends on protease-activated receptor-1. Thromb Haemost. 2009;101:724–33. doi: 10.1160/th08-10-0632. 09040724 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor FB, Chang A, Esmon CT, D’Angelo A, Vigano-D’Angelo S, Blick KE. Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. J Clin Invest. 1987;79:918–25. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 8.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gardlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration. 2012 www.fda.gov/Drugs/DrugSafety/DrugSafetyPodcasts/ucm277212.htm.
- 10.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–2. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 11.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276:11199–203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 12.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–84. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 13.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–93. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 14.Riewald M, Ruf W. Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J Biol Chem. 2005;280:19808–14. doi: 10.1074/jbc.M500747200. [DOI] [PubMed] [Google Scholar]
- 15.Joyce DE, Grinnell BW. Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit Care Med. 2002;30:S288–93. doi: 10.1097/00003246-200205001-00019. [DOI] [PubMed] [Google Scholar]
- 16.Mosnier LO, Griffin JH. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires protease-activated receptor-1 and endothelial cell protein C receptor. Biochem J. 2003;373:65–70. doi: 10.1042/BJ20030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9:338–42. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 18.Zhong Z, Ilieva H, Hallagan L, Bell R, Singh I, Paquette N, Thiyagarajan M, Deane R, Fernandez JA, Lane S, Zlokovic AB, Liu T, Griffin JH, Chow N, Castellino FJ, Stojanovic K, Cleveland DW, Zlokovic BV. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119:3437–49. doi: 10.1172/JCI3847638476. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo H, Singh I, Wang Y, Deane R, Barrett T, Fernandez JA, Chow N, Griffin JH, Zlokovic BV. Neuroprotective activities of activated protein C mutant with reduced anticoagulant activity. Eur J Neurosci. 2009;29:1119–30. doi: 10.1111/j.1460-9568.2009.06664.x. EJN6664 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13:1349–58. doi: 10.1038/nm1667. nm1667 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Sci STKE. 2007;2007:re8. doi: 10.1126/stke.4122007re8. stke.4122007re8 [pii] [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin JN, Mazzoni MR, Cleator JH, Earls L, Perdigoto AL, Brooks JD, Muldowney JA, 3rd, Vaughan DE, Hamm HE. Thrombin modulates the expression of a set of genes including thrombospondin-1 in human microvascular endothelial cells. J Biol Chem. 2005;280:22172–80. doi: 10.1074/jbc.M500721200. [DOI] [PubMed] [Google Scholar]
- 23.Russo A, Soh UJ, Paing MM, Arora P, Trejo J. Caveolae are required for protease-selective signaling by protease-activated receptor-1. Proc Natl Acad Sci U S A. 2009;106:6393–7. doi: 10.1073/pnas.0810687106. 0810687106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gazi L, Nickolls SA, Strange PG. Functional coupling of the human dopamine D2 receptor with G alpha i1, G alpha i2, G alpha i3 and G alpha o G proteins: evidence for agonist regulation of G protein selectivity. Br J Pharmacol. 2003;138:775–86. doi: 10.1038/sj.bjp.0705116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–68. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 26.Vu TK, Wheaton VI, Hung DT, Charo I, Coughlin SR. Domains specifying thrombin-receptor interaction. Nature. 1991;353:674–7. doi: 10.1038/353674a0. [DOI] [PubMed] [Google Scholar]
- 27.Schuepbach RA, Feistritzer C, Brass LF, Riewald M. Activated protein C-cleaved protease activated receptor-1 is retained on the endothelial cell surface even in the presence of thrombin. Blood. 2008;111:2667–73. doi: 10.1182/blood-2007-09-113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuepbach RA, Riewald M. Coagulation factor Xa cleaves protease-activated receptor-1 and mediates signaling dependent on binding to the endothelial protein C receptor. J Thromb Haemost. 2010;8:379–88. doi: 10.1111/j.1538-7836.2009.03682.x. JTH3682 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A. 1983;80:3734–7. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feistritzer C, Lenta R, Riewald M. Protease-activated receptors-1 and -2 can mediate endothelial barrier protection: role in factor Xa signaling. J Thromb Haemost. 2005;3:2798–805. doi: 10.1111/j.1538-7836.2005.01610.x. [DOI] [PubMed] [Google Scholar]
- 31.Brass LF, Pizarro S, Ahuja M, Belmonte E, Blanchard N, Stadel JM, Hoxie JA. Changes in the structure and function of the human thrombin receptor during receptor activation, internalization, and recycling. J Biol Chem. 1994;269:2943–52. [PubMed] [Google Scholar]
- 32.Hoxie JA, Ahuja M, Belmonte E, Pizarro S, Parton R, Brass LF. Internalization and recycling of activated thrombin receptors. J Biol Chem. 1993;268:13756–63. [PubMed] [Google Scholar]
- 33.Shapiro MJ, Coughlin SR. Separate signals for agonist-independent and agonist-triggered trafficking of protease-activated receptor 1. J Biol Chem. 1998;273:29009–14. doi: 10.1074/jbc.273.44.29009. [DOI] [PubMed] [Google Scholar]
- 34.Zhang HC, Derian CK, Andrade-Gordon P, Hoekstra WJ, McComsey DF, White KB, Poulter BL, Addo MF, Cheung WM, Damiano BP, Oksenberg D, Reynolds EE, Pandey A, Scarborough RM, Maryanoff BE. Discovery and optimization of a novel series of thrombin receptor (par-1) antagonists: potent, selective peptide mimetics based on indole and indazole templates. J Med Chem. 2001;44:1021–4. doi: 10.1021/jm000506s. [DOI] [PubMed] [Google Scholar]
- 35.Madhusudhan T, Wang H, Straub BK, Grone E, Zhou Q, Shahzad K, Muller-Krebs S, Schwenger V, Gerlitz B, Grinnell BW, Griffin JH, Reiser J, Grone HJ, Esmon CT, Nawroth PP, Isermann B. Cytoprotective signaling by activated protein C requires protease-activated receptor-3 in podocytes. Blood. 2012;119:874–83. doi: 10.1182/blood-2011-07-365973. blood-2011-07-365973 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishihara H, Connolly AJ, Zeng D, Kahn ML, Zheng YW, Timmons C, Tram T, Coughlin SR. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–6. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- 37.McLaughlin JN, Shen L, Holinstat M, Brooks JD, Dibenedetto E, Hamm HE. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem. 2005;280:25048–59. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–21. doi: 10.1038/nm.2053. nm.2053 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang XV, Banerjee Y, Fernandez JA, Deguchi H, Xu X, Mosnier LO, Urbanus RT, de Groot PG, White-Adams TC, McCarty OJ, Griffin JH. Activated protein C ligation of ApoER2 (LRP8) causes Dab1-dependent signaling in U937 cells. Proc Natl Acad Sci U S A. 2009;106:274–9. doi: 10.1073/pnas.0807594106. 0807594106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Brien LA, Richardson MA, Mehrbod SF, Berg DT, Gerlitz B, Gupta A, Grinnell BW. Activated protein C decreases tumor necrosis factor related apoptosis-inducing ligand by an EPCR- independent mechanism involving Egr-1/Erk-1/2 activation. Arterioscler Thromb Vasc Biol. 2007;27:2634–41. doi: 10.1161/ATVBAHA.107.153734. ATVBAHA.107.153734 [pii] [DOI] [PubMed] [Google Scholar]
- 41.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–72. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 42.Weiler H, Ruf W. Activated protein C in sepsis: the promise of nonanticoagulant activated protein C. Curr Opin Hematol. 2008;15:487–93. doi: 10.1097/MOH.0b013e32830abdf400062752-200809000-00008. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–5. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 44.Soh UJ, Trejo J. Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through beta-arrestin and dishevelled-2 scaffolds. Proc Natl Acad Sci U S A. 2011;108:E1372–80. doi: 10.1073/pnas.1112482108. 1112482108 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trivedi V, Boire A, Tchernychev B, Kaneider NC, Leger AJ, O’Callaghan K, Covic L, Kuliopulos A. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137:332–43. doi: 10.1016/j.cell.2009.02.018. S0092-8674(09)00160-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Brien PJ, Prevost N, Molino M, Hollinger MK, Woolkalis MJ, Woulfe DS, Brass LF. Thrombin responses in human endothelial cells. Contributions from receptors other than PAR1 include the transactivation of PAR2 by thrombin-cleaved PAR1. J Biol Chem. 2000;275:13502–9. doi: 10.1074/jbc.275.18.13502. [DOI] [PubMed] [Google Scholar]
- 47.McLaughlin JN, Patterson MM, Malik AB. Protease-activated receptor-3 (PAR3) regulates PAR1 signaling by receptor dimerization. Proc Natl Acad Sci U S A. 2007;104:5662–7. doi: 10.1073/pnas.0700763104. [DOI] [PMC free article] [PubMed] [Google Scholar]