Abstract
Organophosphates (OP) inhibit the enzyme cholinesterase and cause accumulation of acetylcholine, and are known to cause seizures and status epilepticus (SE) in humans. The animal models of SE caused by organophosphate analogs of insecticides are not well characterized. SE caused by OPs paraoxon and diisopropyl fluorophosphate (DFP) in rats was characterized by electroencephalogram (EEG), behavioral observations and response to treatment with the benzodiazepine diazepam administered at various stages of SE. A method for SE induction using intrahippocampal infusion of paraoxon was also tested. Infusion of 200 nmol paraoxon into the hippocampus caused electrographic seizures in 43/52 (82.7%) animals tested; and of these animals, 14/43 (30%) had self-sustaining seizures that lasted 4–18 hours after the end of paraoxon infusion. SE was also induced by peripheral subcutaneous injection of diisopropyl fluorophosphate (DFP, 1.25 mg/kg) or paraoxon (1.00 mg/kg) to rats pretreated with atropine (2 mg/kg) and 2-pralidoxime (2-PAM,50 mg/kg) 30 minutes prior to OP injection. SE occurred in 78% paraoxon–treated animals and in 79% of DFP-treated animals. Diazepam (10 mg/kg) was administered 10 min and 30 min after the onset of continuous EEG seizures induced by paraoxon and it terminated SE in a majority of animals at both time points. DFP-induced SE was terminated in 60% animals when diazepam was administered 10 minutes after the onset of continuous EEG seizure activity but diazepam did not terminate SE in any animal when it was administered 30 minutes after the onset of continuous seizures. These studies demonstrate that both paraoxon and DFP can induce SE in rats but refractoriness to diazepam is a feature of DFP induced SE.
Keywords: organophosphate, status epilepticus, EEG, seizures, benzodiazepine, diazepam
Introduction
Status epilepticus (SE) is a neurological emergency characterized by recurrent or continuous self- sustaining seizures. SE can contribute to morbidity, sustained neuronal injury and contribute to mortality(Fujikawa et al., 2000;Fujikawa, 2005). Understanding mechanisms of SE in humans is a particular challenge because patients have to be treated promptly and underlying neurological insult contributes to the pathology. Animal models have been used to understand the pathophysiology of SE and test novel therapies (Chen et al., 2007;Wasterlain et al., 1993). Current animals models of SE are based on chemical stimulation of cholinergic system by muscarinic agonists such as pilocarpine, agonists of glutamate receptors such as kainate and electrical stimulation of limbic structures (Turski et al., 1983)(Honchar et al., 1983;Lothman et al., 1981;Lothman et al., 1989;Mazarati et al., 1998b). However, there are few instances of these toxins causing human SE.
Organophosphates (OP) are potent inhibitors of the enzyme cholinesterase, and several these cause SE in humans and experimental animals. OPs such as parathion and malathion are used as insecticides and there are numerous reports of SE in humans induced by these insecticides (Garcia et al., 2003;Hoffmann and Papendorf, 2006). Extremely potent OPs, such as soman, sarin and VX are also used as nerve agents for chemical warfare and in civilian terrorist attacks, and they cause SE (McDonough, Jr. and Shih, 1997;Morita et al., 1995;Nozaki et al., 1995). However, OP nerve agents sarin, soman and VX etc are restricted use chemicals and SE induced by these agents has been studied in defense labs. Therefore, acceptable surrogate OP agents must be used for civilian research to understand the mechanisms, pathophysiology and treatment of OP induced SE.
Several organophophospates are available for civilian use including diisopropyl fluorophosphate (DFP), Paraoxon, chlorfenvinphos or dichlorvos. Chlorfenvinphos does not appear to cause seizures, and dichlorvos primarily causes fatalities by central respiratory depression (Bird et al., 2003;Gralewicz et al., 1989). On the other hand DFP and paraoxon models have been have been used to study neuropathology, drug response and calcium homeostasis neuropathology associated with OPs (Deshpande et al., 2010;Harrison et al., 2004;Kadriu et al., 2011;Li et al., 2011;Zaja-Milatovic et al., 2009;Zhu et al., 2010).. However, these studies did not characterize the evolution SE with EEG. Thus the time to initiation of seizures, duration of seizures and their EEG characteristics remain unknown. Furthermore, they did not test responsiveness to benzodiazepines, such as diazepam. Benzodiazepines are the mainstay of treatment of seizures and OP induced seizures (Alldredge et al., 2001;Treiman et al., 1998;Treiman, 2007). These drugs fail in 35–45% of cases and better treatments are needed.
We characterized SE induced by paraoxon and DFP by means of EEG and behavior. We also characterized the response to treatment with benzodiazepine diazepam at various stages of SE.
Materials and Methods
Surgery
All procedures on animals were performed according to a protocol approved by the institutional Animal Care and Use Committee. Adult male Sprague-Dawley rats (Taconic) weighing 175–300g were housed with food and water ad libitum. The animals were anesthetized with ketamine (50 mg/kg) and xylazine (10 mg/kg) for implantation. For animals undergoing intrahippocampal infusion, a bipolar electrode was implanted in the left ventral hippocampus (AP −5.3, ML −4.9, DV −5.0 to dura; incisor bar −3.3). A guide cannula (Plastics One, Roanoke, VA) was implanted into the right ventral hippocampus (AP −5.8, ML +4.6, DV −2.5 from dura; incisor bar −3.3), alongside a second bipolar electrode. A teflon coated 0.01 inch diameter stainless-steel wire positioned near the frontal sinus served as a ground electrode. For animals undergoing peripheral injection studies, three supra-dural cortical electrodes were placed over the cortex. In both procedures, the electrodes were inserted into a strip connector and then secured to the skull with dental acrylic as previously described (Lothman et al., 1988).
Intrahippocampal Infusion
Following a 1 week recovery, animals were connected via a cable to a data acquisition system (Stellate systems). Paraoxon was suspended in 4% hydroxypropyl-B-cyclodextrin (Sigma, St. Louis, MO). An injection needle connected to a 0.1 mL Hamilton syringe and driven by an infusion pump (KD scientific, Portland, OR) was back filled with paraoxon and inserted such that it extended 3 mm below the end of the cannula guide. Paraoxon was then infused at a rate of either 0.5 μL/min or 1 μl/min for a total volume of 20 μL. EEG and video monitoring began 10 minutes prior to paraoxon infusion and continued for 24 hours after infusion completion.
EEG recordings were subsequently reviewed for the presence of seizures as well as SE. Seizures were characterized by the appearance of high frequency (>2 Hz), rhythmic spike wave discharges with amplitudes at least three times that of the baseline EEG. Animals were considered to have SE if there was continuous epileptiform activity for 30 min, during which spike frequencies were more than 2 Hz, and spike amplitude was at least 3 times the background. Behavioral seizures were scored according to the Racine scale (Racine, 1972).
Peripheral injection
EEG recording was initiated prior to administration of 2 mg/kg atropine (Sigma, St. Louis, MO) and 50 mg/kg 2 pralidoxime (2-PAM) iodide (Sigma, St. Louis, MO) intraperitoneally. After 30 minutes, DFP (Sigma, St. Louis, MO) or paraoxon (Chem Service, West Chester, PA) was injected subcutaneously (SC). In some experiments animals were given 10 mg/kg diazepam 10 minutes or 30 minutes after onset of continuous SE activity. A non-diazepam injected group served as controls. 2-PAM and atropine were both dissolved in solution within one hour of injection. DFP and paraoxon were both mixed into cold saline immediately prior to injection. Animals were monitored via EEG and video for 24 hours following organophosphate injection.
Epileptiform activity was monitored and defined as follows. Seizures were defined as the appearance of high frequency (>2 Hz), rhythmic spike wave discharges with amplitudes at least 3 times that of the baseline EEG. The criterion for the onset of SE was the occurrence of continuous seizure activity for 10 minutes, during which spike frequencies do not drop below 2 Hz. The end of SE was characterized by non-uniform spike frequencies that remained lower than 1 Hz. EEG data was polled every 10 minutes after the onset of SE to determine SE termination during a 5 hour interval after SE began.
Immunohistochemistry for Fluorojade and NeuN
The procedures of tissue preparation were described in detail previously (Sun et al., 2004;Sun et al., 2007). Briefly, animals were anesthetized with an overdose of pentobarbitone sodium and perfused through the ascending aorta with 50–100 ml 0.9% NaCl followed by 350–450 ml 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). Brains were removed and post-fixed in the same fixative for 2 hours at 4°C. Brains were frozen by immersion in −70°C isopentane. Coronal sections from the anterior block were cut at 40 μm to collect dorsal sections.
In order to stain for NeuN and Fluoro-Jade B, immunohistochemical technique was modified as described previously (Jakab and Bowyer, 2002;Sun et al., 2007). Sections were incubated for 48 hours at 4°C in the anti-NeuN primary antibody mouse anti-NeuN, (diluted at 1:200, Millipore) followed by incubation with a secondary antibody conjugated to Alexa Fluor 594 (5μg/ml; molecular probes) for 60 minutes at room temperature. Sections were wet-mounted on glass slides, air-dried at 50°C for 15 minutes, and stained with Fluoro-Jade B. The slides were immersed in distilled water for 1 minute and oxidized in a 0.006% solution of KMnO4 for 5 minutes. After rinsing in distilled water twice for 30 seconds, sections were stained for 10 minutes in a 0.0003% solution of Fluoro-Jade B in 0.1% acetic acid. Finally, sections were rinsed in distilled water, air-dried, and cleared with xylene.
Results
Seizures and SE caused by paraoxon infusion
Infusion of 100 nmol paraoxon into the hippocampus caused electrographic seizures in 2/9 (22.2%) animals tested. None of the animals had seizures lasting beyond the end of infusion and 2 animals displayed intermittent seizures during paraoxon infusion which were not self-sustaining. No change in baseline EEG, and no behavioral seizures occurred in the remaining 7 animals (figure 1A).
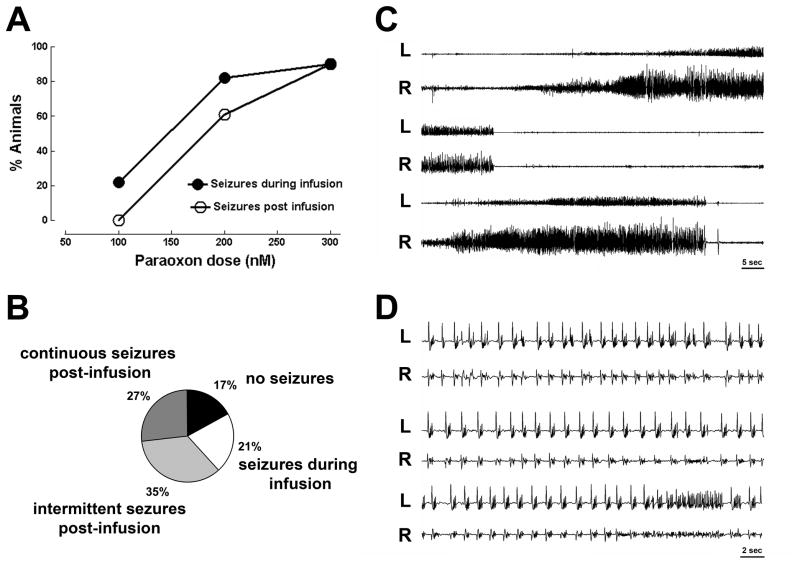
Figure 1.
Seizures caused by intra-hippocampal infusion of the organophosphate paraoxon. A ) displays % fraction of animals having seizures in response to 100 nmols (n= 9), 200 nmols ( n= 52) and 300 nmols (n = 11)of intra-hippocampal paraoxon. B) A pie chart of four responses to 200 nmols paraoxon infused in the hippocampus: no seizures, seizures during infusion, intermittent post infusion seizures and continuous post infusion seizures. C) Displays EEG recordings from right (R) and left (L) hippocampus showing intermittent seizures following infusion of 200 nmol paraoxon solution into the right hippocampus. A seizure begins in the right hippocampus, spreads to the left hippocampus and ends several second later (middle two traces), and soon thereafter another seizure begins and spreads to the left hippocampus. D) Displays a continuous electrographic seizures occurring in right and left hippocampi, note faster time base. The seizure consists of continuous spike-wave discharges.
Infusion of 200 nmol paraoxon into the hippocampus caused electrographic seizures in 43/52 (82.7%) animals tested. In 32 animals seizures lasted beyond the end of infusion (61.5%) and 11 animals displayed intermittent seizures during paraoxon infusion which were not self-sustaining, and did not continue through the end of infusion (figure 1B). There was no change in baseline EEG in remaining 9 animals. These animals did not display behavioral characteristics indicative of seizure activity.
Among animals with seizures lasting beyond paraoxon infusion, two distinct types of electrographic seizure activity occurred: intermittent and continuous seizures. Intermittent seizures, which occurred in 18 animals, frequently appeared first in the paraoxon infusion site in the right hippocampus, and then spread to the contra-lateral (left) hippocampus(Figure 1C). Seizures consisted of rhythmic high frequency spike wave discharges that evolved in frequency and amplitude. There were brief periods of suppression of activity between seizures.
Continuous seizures occurred in 14 animals following paraoxon infusion, and these consisted of sustained bilateral discharges of repetitive spike patterns evolving over time (Fig. 1D). Continuous electrographic seizures started at approximately 15 minutes post infusion, and persisted for 4–18 hours. These prolonged self-sustaining seizures constituted SE. Paraoxon-induced SE was further confirmed upon observation of behavioral seizures. Animals experiencing SE exhibited freezing, staring, blinking, and hyper-exploratory movement, as well as wet-dog shaking movements for 4–18 hours.
Infusion of 300 nmol paraoxon into the hippocampus caused electrographic seizures in 10/11 (90.9%) of animals tested. The seizures in all of these animals continued beyond the end of infusion in form of SE with behavioral seizures ranging from 2–5 on the Racine scale. The majority of animals in SE (7) died of respiratory arrest, several of these exhibited symptoms of peripheral cholinergic stimulation, including muscle contractions and fasciculations. The remaining 3 animals survived, exhibiting seizures that lasted long beyond the end of infusion. Only 1 animal receiving paraoxon 300 nM infusion displayed neither behavioral characteristics indicative of seizure activity, nor EEG seizure activity (9.0%).
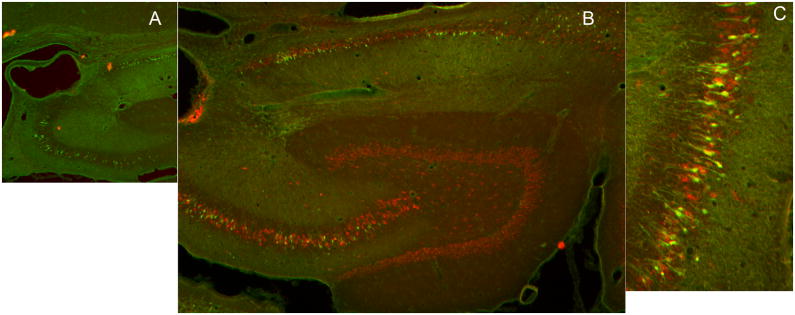
The location of the cannula was confirmed by sectioning the hippocampus. In many animals, the cannula and electrode tracts were localized by sectioning the brain in the plane of the electrode. In other animals, immunohistochemistry for neuronal stain Neun and staining for neuro-degeneration dye fluorojade J, was performed 3 days after SE caused by infusion of paraoxon. The site of infusion was at the ventricular border the CA3 layer of the hippocampus, (figure 2A). Fluorojade positive neurons were present in CA1and CA3 regions and of the hippocampus and the subiculum (figure 2B,C). Occasional Fluorojade positive cells were present in the hilus.
Figure 2.
Images of hippocampal sections from animals that had prolonged seizures following intra-hippocampal paraoxon administration, which were stained for neuronal injury stain FluoroJade B ( green) and neuronal marker NeuN (red). A) A low magnification image of the hippocampus showing drug infusion site close to the CA3 region of the hippocampus. B) A section of the hippocampus displaying green fluorojade positive CA3, CA1 pyramidal neurons and neurons in the subiculum. C) A higher magnification image of Fluorojade positive neurons in the CA3 region of the hippocampus.
Peripheral injection of Paraoxon
Preliminary experiments were performed to optimize the model. Paraoxon (0.35 mg/kg) administered by the intra-peritoneal route caused widespread muscle contractions, fasciculations, rare tonic convulsions, respiratory arrest and death in all four animals tested. Pretreatment of animals with scopolamine (4 mg/kg) protected against peripheral and systemic effects of paraoxon (n = 4 animals). Subsequently, based on the work of Deshpande et al. (Deshpande et al., 2010) oxime reactivator 2-PAM and muscarinic antagonist atropine was combined with peripheral paraoxon administration. In preliminary experiments we tested other doses of these agents and confirmed that most optimal doses were used.
Paraoxon (1 mg/kg) was administered SC to 23 animals 30 minutes after pre-treatment with 2 mg/kg atropine and 50 mg/kg 2-PAM and prolonged seizures were observed. SE occurred in 17 of the 23 animals (74%) with animals displaying a combination of chewing, head-bobbing, single and bilateral limb clonus and rearing, leading to constant full body tremors. In 3 animals there was no effect from the OP injection and 3 animals died from respiratory arrest without any seizures.
In these animals electrographic seizures either started as continuous long lasting seizures or evolved from discrete seizures to continuous seizures (Figure 3, panel control). The mean time for the onset of first electrographic seizure in animals treated with paraoxon was 6m 4s ± 42s. The first seizure was also the onset or continuous EEG activity in 29% (5/17) of the animals studied. In 7 of the 18 animals, a discrete electrographic seizure lasting 30–60 seconds was the first electrographic seizure, followed by a prolonged period of suppression. This was followed by the onset of continuous electrographic seizure activity. The remaining 35% (6/17) progressed from discrete seizure continuous EEG with a period of brief suppression (10–30 seconds) between 30–60 second seizure bursts. The mean time for onset of continuous electrographic seizure activity after paraoxon injection was 10m 3s ± 1m. At the onset of continuous EEG seizure activity, electrographic spiking occurred at a rate of 2–4 Hz, and frequency increased to 4–8 Hz within 10 minutes of onset.
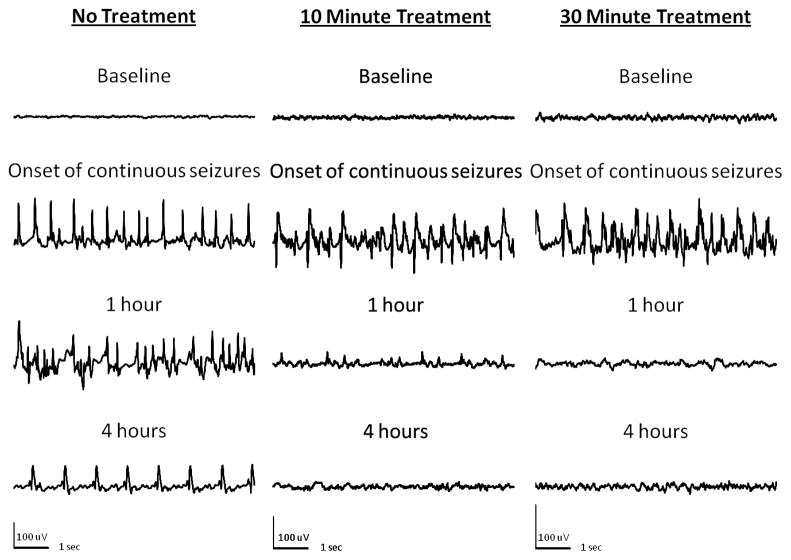
Figure 3.
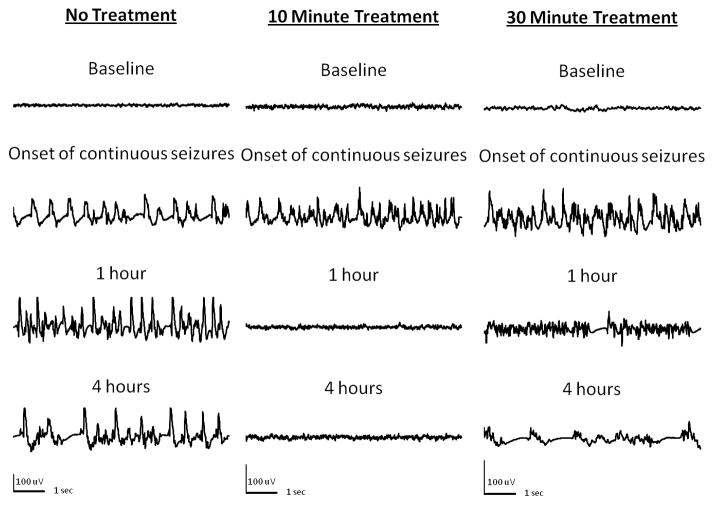
EEG recordings from hippocampi of animals given paraoxon subcutaneously after injection of atropine and 2-PAM. Left panel shows samples of recording from an animal in SE. Note continuous electrographic seizures for 4 hours with evolving morphology and frequency. Middle panel (10 minute treatment) shows EEG from an animal treated with diazepam 10 minutes after the onset of continuous electrographic seizures. Seizures were terminated promptly and animals stayed seizure free. Right panel (30 minute treatment) shows onset of continuous seizures and their termination when diazepam was administered 30 minutes after the onset of continuous electrographic seizures
One group of animals (n=6) was left untreated after the onset of continuous seizures. One animal died 2h 45m after the onset of continuous seizures. The mean SE duration was 10h 15m ± 1h 42m in the remaining five animals, ranging from 7–17 hours. The SE ended with the frequency of epileptiform activity falling below 1 Hz in all 5 of the animals within 18 hours of onset.
A group of animals (n =6) in paraoxon induced SE was treated with 10 mg/kg diazepam ten minutes after the onset of continuous seizure activity (Figure 3, middle panel). Diazepam terminated continuous seizures at this time point (Figure 4, middle panel). In two of these animals, SE was terminated within the first 10 minutes of treatment (figure 4). In these animals, spike frequency dropped from 4–6 Hz to 0 and baseline EEG was restored. Seizures did not recur. In two animals SE ended 90–150 minutes after onset (figure 4). The spike frequency gradually dropped from 4–6 Hz with poly-spikes to single spikes with a frequency of less than 1 Hz (figure 3). All animals were seizure free by seven hours.
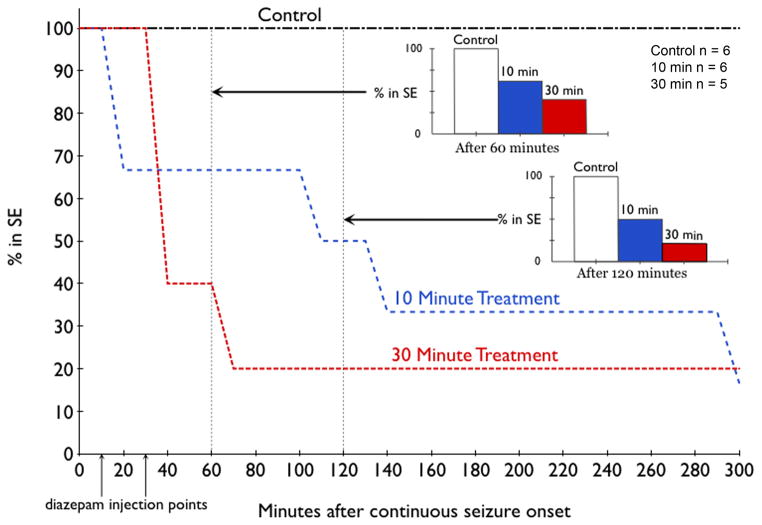
Figure 4.
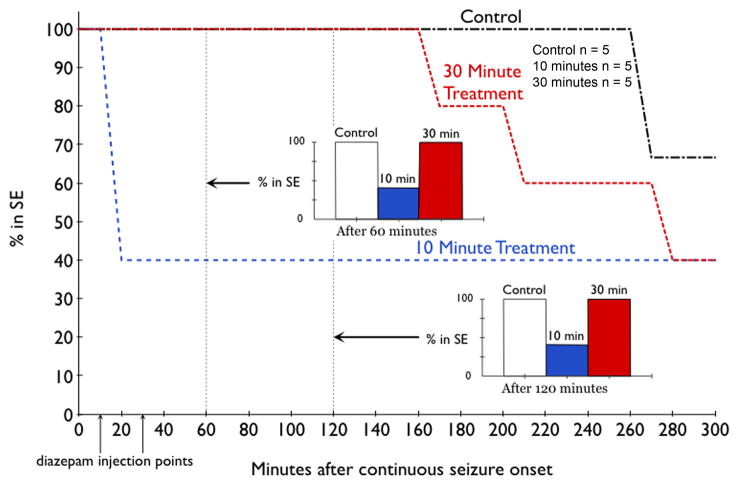
Time-course of SE induced by peripheral paraoxon injection in three groups: no further treatment, diazepam given 10 minutes after the onset of continuous seizures (Blue line) or 30 minutes after the onset of continuous seizures (Red line). In this study, 23 animals were studied, 17 developed continuous seizures, 6 were left untreated (control), 6 received diazepam 10 minutes after the onset of continuous seizures, and 5 received diazepam 30 minutes after the onset of continuous electrographic seizures. Diazepam effectively terminated SE in similar proportion of animals regardless of time of treatment (inset bar charts).
There was no mortality within 24 hours. Animals (n =5) treated with 10 mg/kg of diazepam 30 minutes after continuous EEG activity also responded to treatment, with (60%) becoming seizure free within 10 minutes of treatment (figure 3 right panel). Only one of the five animals was still having seizures 70 minutes after continuous seizures began, and continuous seizures lasted for 12 hours (figure 4). No animal in this group died during the 24 hour period.
DFP
Preliminary experiments were performed to optimize DFP model of SE. Rats that were pretreated with 1 mg/kg atropine 30 minutes and then given 1.25 mg/kg DFP SC (n=4) all died from respiratory arrest, whereas 50% of rats pretreated with 1 mg/kg atropine and 25 mg/kg 2-PAM (n=4) survived and exhibited prolonged seizure activity. Higher DFP dose of 1.5 mg/kg combined with a pretreatment of larger doses of atropine (2 mg/kg) and 2-PAM (50 mg/kg) caused death in 2 animals. These observations and previously published data suggested that 1.25 mg/kg DFP SC in cold saline with a pretreatment of 2 mg/kg atropine and 50 mg/kg 2-PAM were likely to produce SE in animals.
This combination was used to study DFP induced SE, and it caused continuous seizure activity and SE in 15 out of 19 (79%) of the animals studied, with the remainder dying of respiratory arrest. Animals in SE group exhibited a combination of chewing, head-bobbing, single and bi-limb clonus and rearing, leading to constant full body tremors and SE. The mean time to first EEG seizure in this group was 21m 43s ± 3m 25s. In 7 of 15 animals, the first electrographic seizure was also the onset of continuous seizure activity. In 7 other animals, seizure progression went from periods of discrete seizures, separated by normal EEG followed by seizures lasting 10 to 30 seconds with periods of suppression, after which seizures merged into continuous epileptiform activity. In one animal, a brief discrete seizure was followed by continuous seizure activity. Continuous epileptiform activity started at a frequency of 2 Hz and accelerated to 4–6 Hz within ten minutes. The latency to the development of continuous electrographic activity was 24m 41s ± 3m 48s after DFP injection.
In 5 animals left untreated after the DFP induced SE, 3 survived to 5 hours after continuous seizure onset and the remaining died. Seizures stopped in one animal 4.5 hours after the onset of continuous seizure activity, while the other two displayed electrographic seizures which lasted for more than 8 hours.
DFP induced seizures were much more resistant to diazepam treatment when given at 30 minutes than when treated at 10 minutes (Figures 5,6). Three out of the five rats (60%) given diazepam after 10 minutes after continuous EEG activity demonstrated rapid drop in spike wave discharge frequency and amplitude with return of baseline EEG; the remaining two out of five animals (40%) continued to have seizures for more than 5 hours. No animal in this group died within the 24 hour after SE onset.
Figure 5.
SE induced by peripheral injection of DFP and response to diazepam treatment. Samples of recording from an animal in SE show continuous electrographic seizures for 4 hours. Recordings an animal treated with diazepam 10 minutes after the onset of continuous electrographic seizures (Middle panel, 10 minute treatment) demonstrate prompt termination of SE.. When diazepam was administered 30 minutes after the onset of continuous electrographic seizures (Right panel 30 minute treatment) SE continued with only a mild suppression of amplitude of spikes but continued high frequency spike-wave discharges (1 hour) and complex poly-spike-wave rhythmic discharges (4 hour).
Figure 6.
Time-course of SE induced by peripheral DFP injection in three groups: no further treatment, diazepam given 10 minutes after the onset of continuous seizures (Blue line) or 30 minutes after the onset of continuous seizures (Red line). Continuous seizures developed in 15 of 19 animals treated with DFP, and 5 were left untreated (control), 5 each were treated diazepam 10 or 30 minutes after the onset of continuous electrographic seizures. Diazepam effectively terminated SE in animals treated 10 minutes after the onset of continuous electrographic seizure but not in animals treated 30 minutes (inset bar charts).
Diazepam given 30 minutes after the start of continuous EEG activity did not stop SE within 60 and 120 minutes after onset of SE in any of the 5 animals tested (Figures 5, 6). First termination of SE in these animals first occurred 2.5 hours after treatment injection, with 60% (3/5) of rats coming out of SE within the first 5 hours of diazepam injection (Figure 6). In addition, while treatment at 30 minutes did eventually terminate SE, this termination was marked by appearance arrhythmic spikes and the baseline EEG was not restored in any animal.
Discussion
We have characterized SE induced by two different organophosphates, intrahippocampal infusion of paraoxon and peripheral injection of DFP or paraoxon following treatment of 2-PAM and atropine. Peripheral injection of OP agents has a higher incidence of producing self-sustaining seizures, while intrahippocampal infusion has the benefit of not requiring pretreatment with 2-PAM and atropine.
Direct injection of 200 nmol paraoxon into the hippocampus caused self-sustaining seizures without killing animals due to peripheral poisoning, but animals given 300 nmol paraoxon began to display peripheral OP poisoning effects and the majority of these animals did not survive. The mechanism of these peripheral effects is unknown, but they closely resembled those seen in preliminary trials when paraoxon was given IP without any pretreatment. Compared to peripheral dosing of paraoxon, intrahippocampal injections produced seizures less likely to become self-sustaining SE. Furthermore this model is cumbersome requiring cannula implantation and slow drug infusion. DFP was not tested using this method because of inconsistent development SE using intra-hippocampal infusion of paraoxon.
The volume of paraoxon infusion into the hippocampus was somewhat large ( 20 μL). However there are previous reports where 20 μL drug volume was infused into the hippocampus (Modol et al., 2011). Another study found that intracranial pressure was not increased by 10 μL infusion into the hippocampus (Drabek et al., 2011). Finally, in a previously published study we have infused up to 40 μL of saline into the hippocampus, and this did not cause seizures (Williamson et al., 2004).
In the case of both DFP and paraoxon given peripherally, both 2-PAM and atropine were required to prevent mortality due to respiratory arrest. A 30 minute pretreatment was decided on instead of treatment immediately after OP injection in order to minimize the effects on seizure activity of these agents. This allowed us to better understand the effects of diazepam given at various time points during SE. 2-PAM reactivates the enzyme cholinesterase, which does not penetrate the blood brain barrier. OPs inhibit the enzyme choline acetyl-transferase by acting as substrates for the enzyme and causing its phosphorylation rather than acetylation caused by natural substrate acetylcholine. The phosphorylated enzyme is stable and can no longer deacetylate acetylcholine. Oximes such as 2 PAM dephosphorylate the enzyme and reactivate it (Jokanovic and Stojiljkovic, 2006).
The methods for peripheral OP poisoning described above more accurately mimic those used for high dose pilocarpine experiments, allowing comparison with these models. The onset of continuous EEG seizure activity, which corresponds to EEG stage III as described by Treiman et al.(Treiman et al., 1990), was found to be a better marker of treatment refractoriness than behavioral seizures in the lithium-pilocarpine model of SE (Wang et al., 2009). The time of treatment was based on the onset of continuous seizure activity, because this most closely correlates with the time at which SE becomes refractory to diazepam.
Both DFP and paraoxon are OPs, which increase concentration of acetylcholine levels in the brain rapidly by inhibiting its breakdown by the enzyme cholinesterase (Shih and McDonough, Jr., 1997). This elevation in acetylcholine levels is likely to activate muscarinic and nicotinic receptors in the hippocampus (Harrison et al., 2004;Kozhemyakin et al., 2010). It was recently suggested that muscarinic receptor activation by paraoxon causes increased release of glutamate from presynaptic terminals (Kozhemyakin et al., 2010). In vitro models of recurrent bursting suggest that increased presynaptic release (frequency of EPSC) can contribute to the development of neuronal synchrony and seizures (Mangan and Kapur, 2004;Traub and Dingledine, 1990).
DFP-induced SE became resistant to treatment with diazepam as SE progressed. This phenomenon has been described previously in SE induced by electrical stimulation, pilocarpine, lithium-pilocarpine and soman (Jones et al., 2002;Kapur and Macdonald, 1997;Mazarati et al., 1998a;Shih et al., 1999). However, the paraoxon model for SE does not exhibit the same phenomenon, making it less suitable as a surrogate for military OP poisoning studies. The current recommendation of 2-PAM, atropine and diazepam as a treatment for OP poisoning is incomplete because of the time dependent nature of its treatment. These studies demonstrate the importance of finding novel methods for dealing with and treating victims of OP poisoning.
Previous studies of seizures caused by organophosphate cholinesterase inhibitors have largely been carried out in defense labs using agents such as sarin VX and other nerve agents (McDonough, Jr. and Shih, 1997). However, organophosphate poisoning needs to be studied in civilian research laborites because organophosphate pesticide poisoning afflicts civilians and civilian populations can be targets of nerve agent attacks. A World Health Organization report suggested that there were more than 2 million cases of accidental or intentional organophosphate poisoning in the world each year (Jeyaratnam, 1990). Development of DFP and paraoxon models can lead to better treatments for OP poisoning.
Acknowledgments
The research is supported by the CounterACT Program, National Institutes Of Health Office of the Director, and the National Institute Neurological Disorders and Stroke, Grant Numbers NIH-NINDS UO1 NS58204 and RO1 NS040337 and also by the Department of Defense grant PR093963.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, Gottwald MD, O’Neil N, Neuhaus JM, Segal MR, Lowenstein DH. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345:631–637. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- Bird SB, Gaspari RJ, Dickson EW. Early death due to severe organophosphate poisoning is a centrally mediated process. Acad Emerg Med. 2003;10:295–298. doi: 10.1111/j.1553-2712.2003.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Chen JW, Naylor DE, Wasterlain CG. Advances in the pathophysiology of status epilepticus. Acta Neurol Scand Suppl. 2007;186:7–15. [PubMed] [Google Scholar]
- Deshpande LS, Carter DS, Blair RE, DeLorenzo RJ. Development of a Prolonged Calcium Plateau in Hippocampal Neurons in Rats Surviving Status Epilepticus Induced by the Organophosphate Diisopropylfluorophosphate. Toxicological Sciences. 2010;116:623–631. doi: 10.1093/toxsci/kfq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabek T, Janata A, Jackson EK, End B, Stezoski J, Vagni VA, Janesko-Feldman K, Wilson CD, van RN, Tisherman SA, Kochanek PM. Microglial depletion using intrahippocampal injection of liposome-encapsulated clodronate in prolonged hypothermic cardiac arrest in rats. Resuscitation. 2011 doi: 10.1016/j.resuscitation.2011.09.016. Epub ahead of print. http://dx.doi.org/10.1016/j.resuscitation.2011.09.016. [DOI] [PMC free article] [PubMed]
- Fujikawa DG. Prolonged seizures and cellular injury: understanding the connection. Epilepsy Behav. 2005;7(Suppl 3):S3–11. doi: 10.1016/j.yebeh.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Fujikawa DG, Itabashi HH, Wu A, Shinmei SS. Status epilepticus-induced neuronal loss in humans without systemic complications or epilepsy. Epilepsia. 2000;41:981–991. doi: 10.1111/j.1528-1157.2000.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Abu-Qare AW, Meeker-O’Connell WA, Borton AJ, Abou-Donia MB. Methyl parathion: a review of health effects. J Toxicol Environ Health B Crit Rev. 2003;6:185–210. doi: 10.1080/10937400306471. [DOI] [PubMed] [Google Scholar]
- Gralewicz S, Tomas T, Socko R. Effects of single exposure to chlorphenvinphos, an organophosphate insecticide, on electrical activity (EEG) of the rat brain. Pol J Occup Med. 1989;2:309–320. [PubMed] [Google Scholar]
- Harrison PK, Sheridan RD, Green AC, Scott IR, Tattersall JE. A guinea pig hippocampal slice model of organophosphate-induced seizure activity. J Pharmacol Exp Ther. 2004;310:678–686. doi: 10.1124/jpet.104.065433. [DOI] [PubMed] [Google Scholar]
- Hoffmann U, Papendorf T. Organophosphate poisonings with parathion and dimethoate. Intensive Care Medicine. 2006;32:464–468. doi: 10.1007/s00134-005-0051-z. [DOI] [PubMed] [Google Scholar]
- Honchar MP, Olney JW, Sherman WR. Systemic cholinergic agents induce seizures and brain damage in lithium-treated rats. Science. 1983;220:323–325. doi: 10.1126/science.6301005. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Bowyer JF. Parvalbumin neuron circuits and microglia in three dopamine-poor cortical regions remain sensitive to amphetamine exposure in the absence of hyperthermia, seizure and stroke. Brain Res. 2002;958:52–69. doi: 10.1016/s0006-8993(02)03439-x. [DOI] [PubMed] [Google Scholar]
- Jeyaratnam J. Acute pesticide poisoning: a major global health problem. World Health Stat Q. 1990;43:139–144. [PubMed] [Google Scholar]
- Jones DM, Esmaeil N, Maren S, Macdonald RL. Characterization of pharmacoresistance to benzodiazepines in the rat Li-Pilocarpine model of status epilepticus. Epilepsy Res. 2002;50:301–312. doi: 10.1016/s0920-1211(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Kadriu B, Guidotti A, Costa E, Davis JM, Auta J. Acute imidazenil treatment after the onset of DFP-induced seizure is more effective and longer lasting than midazolam at preventing seizure activity and brain neuropathology. Toxicol Sci. 2011;120:136–145. doi: 10.1093/toxsci/kfq356. [DOI] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL. Rapid Seizure-Induced Reduction of Benzodiazepine and Zn2+ Sensitivity of Hippocampal Dentate Granule Cell GABAA Receptors. J Neurosci. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhemyakin M, Rajasekaran K, Kapur J. Central Cholinesterase Inhibition Enhances Glutamatergic Synaptic Transmission. J Neurophysiol. 2010;103:1748–1757. doi: 10.1152/jn.00949.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lein PJ, Liu C, Bruun DA, Tewolde T, Ford G, Ford BD. Spatiotemporal pattern of neuronal injury induced by DFP in rats: a model for delayed neuronal cell death following acute OP intoxication. Toxicol Appl Pharmacol. 2011;253:261–269. doi: 10.1016/j.taap.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, Bekenstein JW, Perlin JB. Self-sustaining limbic status epilepticus induced by ‘continuous’ hippocampal stimulation: electrographic and behavioral characteristics. Epilepsy Res. 1989;3:107–119. doi: 10.1016/0920-1211(89)90038-7. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Collins RC, Ferrendelli JA. Kainic acid-induced limbic seizures: electrophysiologic studies. Neurology. 1981;31:806–812. doi: 10.1212/wnl.31.7.806. [DOI] [PubMed] [Google Scholar]
- Mangan PS, Kapur J. Factors underlying bursting behavior in a network of cultured hippocampal neurons exposed to zero magnesium. J Neurophysiol. 2004;91:946–957. doi: 10.1152/jn.00547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Baldwin RA, Sankar R, Wasterlain CG. Time-dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res. 1998a;814:179–185. doi: 10.1016/s0006-8993(98)01080-4. [DOI] [PubMed] [Google Scholar]
- Mazarati AM, Wasterlain CG, Sankar R, Shin D. Self sustaining status epilepticus after brief electrical stimulation of the perforant path. Brain Res. 1998b;801:251–253. doi: 10.1016/s0006-8993(98)00606-4. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–579. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- Modol L, Darbra S, Pallares M. Neurosteroids infusion into the CA1 hippocampal region on exploration, anxiety-like behaviour and aversive learning. Behav Brain Res. 2011;222:223–229. doi: 10.1016/j.bbr.2011.03.058. [DOI] [PubMed] [Google Scholar]
- Morita H, Yanagisawa N, Nakajima T, Shimizu M, Hirabayashi H, Okudera H, Nohara M, Midorikawa Y, Mimura S. Sarin poisoning in Matsumoto, Japan. Lancet. 1995;346:290–293. doi: 10.1016/s0140-6736(95)92170-2. [DOI] [PubMed] [Google Scholar]
- Nozaki H, Aikawa N, Shinozawa Y, Hori S, Fujishima S, Takuma K, Sagoh M. Sarin poisoning in Tokyo subway. Lancet. 1995;345:980–981. [PubMed] [Google Scholar]
- Shih TM, McDonough JH., Jr Neurochemical mechanisms in soman-induced seizures. J Appl Toxicol. 1997;17:255–264. doi: 10.1002/(sici)1099-1263(199707)17:4<255::aid-jat441>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Shih T, McDonough JH, Jr, Koplovitz I. Anticonvulsants for soman-induced seizure activity. J Biomed Sci. 1999;6:86–96. doi: 10.1007/BF02256439. [DOI] [PubMed] [Google Scholar]
- Sun C, Sieghart W, Kapur J. Distribution of alpha1, alpha4, gamma2, and delta subunits of GABAA receptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Mtchedlishvili Z, Bertram EH, Erisir A, Kapur J. Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulation-based animal model of temporal lobe epilepsy. J Comp Neurol. 2007;500:876–893. doi: 10.1002/cne.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Dingledine R. Model of synchronized epileptiform bursts induced by high potassium in CA3 region of rat hippocampal slice. Role of spontaneous EPSPs in initiation. J Neurophysiol. 1990;64:1009–1018. doi: 10.1152/jn.1990.64.3.1009. [DOI] [PubMed] [Google Scholar]
- Treiman DM. Treatment of convulsive status epilepticus. Int Rev Neurobiol. 2007;81:273–285. doi: 10.1016/S0074-7742(06)81018-4. [DOI] [PubMed] [Google Scholar]
- Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, Handforth A, Faught E, Calabrese VP, Uthman BM, Ramsay RE, Mamdani MB. A Comparison of four treatments for generalized convulsive status epilepticus. New England Journal of Medicine. 1998;339:792–798. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- Treiman DM, Walton NY, Kendrick C. A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Res. 1990;5:49–60. doi: 10.1016/0920-1211(90)90065-4. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Wang NC, Good LB, Marsh ST, Treiman DM. EEG stages predict treatment response in experimental status epilepticus. Epilepsia. 2009;50:949–952. doi: 10.1111/j.1528-1167.2008.01911.x. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG, Fujikawa DG, Penix L, Sankar R. Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia. 1993;34(Suppl 1):S37–S53. doi: 10.1111/j.1528-1157.1993.tb05905.x. [DOI] [PubMed] [Google Scholar]
- Williamson J, Mtchedlishvili Z, Kapur J. Characterization of the convulsant action of pregnenolone sulfate. Neuropharmacology. 2004;46:856–864. doi: 10.1016/j.neuropharm.2003.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Gupta RC, Aschner M, Milatovic D. Protection of DFP-induced oxidative damage and neurodegeneration by antioxidants and NMDA receptor antagonist. Toxicol Appl Pharmacol. 2009;240:124–131. doi: 10.1016/j.taap.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, O’Brien JJ, O’Callaghan JP, Miller DB, Zhang Q, Rana M, Tsui T, Peng Y, Tomesch J, Hendrick JP, Wennogle LP, Snyder GL. Nerve agent exposure elicits site-specific changes in protein phosphorylation in mouse brain. Brain Res. 2010;1342:11–23. doi: 10.1016/j.brainres.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]