Abstract
Galectin-1, an adhesion molecule, is expressed in macrophages and implicated in human immunodeficiency virus (HIV-1) viral adsorption. In this study, we investigated the effects of methamphetamine on galectin-1 production in human monocyte derived macrophages (MDM) and the role of galectin-1 in methamphetamine potentiation of HIV-1 infection. Herein we show that levels of galectin-1 gene and protein expression are significantly increased by meth-amphetamine. Furthermore, concomitant incubation of MDM with galectin-1 and methamphetamine facilitates HIV-1 infection compared to galectin-1 alone or methamphetamine alone. We utilized a nanotechnology approach that uses gold nanorod (GNR)-galectin-1 siRNA complexes (nanoplexes) to inhibit gene expression for galectin-1. Nanoplexes significantly silenced gene expression for galectin-1 and reversed the effects of methamphetamine on galectin-1 gene expression. Moreover, the effects of methamphetamine on HIV-1 infection were attenuated in the presence of the nanoplex in MDM.
Keywords: Macrophage, HIV-1, Galectin-1, Goldnanorod, siRNA
Introduction
Substance abuse is a global health concern. Methamphetamine is a widely abused addictive drug that suppresses both innate and adaptive immunity (Martinez et al. 2007; Tallóczy et al. 2008; Martinez et al. 2009). Methamphetamine is known to inhibit antigen processing and presentation in dendritic cells and macrophage (Tallóczy et al. 2008). Methamphetamine has also been shown to attenuate anti-ovalbumin antibody production in mice (Wey et al. 2008). Transmission of human immunodeficiency virus (HIV-1) infection is also a possible consequence of methamphetamine abuse. Approximately 33.3 million people worldwide are living with HIV-1/AIDS, and 2.5 million people were estimated to contract HIV-1 in 2009 (Hamamoto and Rhodus 2009). Addictive drugs potentiate HIV-1 replication in immunocompetent cells including macrophage, monocytes and peripheral blood mononuclear cells (PBMC) (Liang et al. 2008; Toussi et al. 2009, Roth et al. 2005). Methamphetamine treatment dose-dependently increased HIV-1 reverse transcriptase activity in human macrophages (Liang et al. 2008) and increased HIV-1 production by both HIV-1 infected monocytes and CD4 T lymphocytes in vitro (Toussi et al. 2009). Therefore, coincident use of addictive drugs by HIV-1 infected patients facilitates both initial infection and progression of disease.
During the budding process, HIV-1 incorporates host-derived molecules including ICAM-1, HLA-DR1, and MHC II (Gilbert et al. 2007; Fortin et al. 1998) and may include galectins. Galectins are a family of β-galactoside binding lectins that modulate cell to cell and cell to matrix interactions, cell adhesion and cell signaling by cross linking of cell surface glycol conjugates (Barondes et al. 1994; Chiariotti et al. 2004). Twelve different galectins exist (galectin 1–12). In particular, galectin-1 is a 14 kDa monomer that consists of 2 identical carbohydrate recognition domains (Chiariotti et al. 2004). In regards to HIV-1, galectin-1 acts as a soluble adhesion molecule that mediates direct cell-HIV-1 interactions (Ouellet et al. 2005; St. Pierre et al. 2010). Studies reveal that galectin-1 stabilizes virus-cell interactions and promotes virus replication in PBMC and CD4+ T cells (Ouellet et al. 2005). Mercier et al. (2008) shows that galectin-1 enhances HIV-1 infectivity and replication in macrophages by increasing adsorption of virus particles to the macrophages cell surface. Studies by our laboratory have shown that treatment of immature dendritic cells (IDC) with methamphetamine increases the expression of galectin-1 (Reynolds et al. 2007). Thus we propose that methamphetamine by regulating galectin-1 expression and release, facilitates HIV-1 attachment to human monocyte derived macrophages (MDM) thereby regulating HIV-infection.
HIV-1 infection is characterized by sustained activation of the immune system. Macrophages are a first line of defense against pathogens. Macrophages are permissive to HIV-1 infection and contribute to viral persistence (Le Douce et al. 2010; Herbein and Varin 2010; Bergamaschi and Pancino 2010). Consequently these cells can serve as vehicles for dissemination and reservoirs of HIV-1 infection (Gendelman et al. 1988; Gendelman et al. 1989; Crowe et al. 2003). Macrophages are a primary source of HIV-1 in the central nervous system (CNS) through transmigration across the blood brain barrier (Wu et al. 2000; Buckner et al. 2006; Eugenin et al. 2006). The virus invades the CNS within 1 to 2 weeks after initial viral infection (Davis et al. 1992; Williams and Hickey 2002). HIV-1 infected macrophages secrete toxic proteins that induce neuronal dysfunction (Kadiu et al. 2005; Persidsky and Gendelman 2003) and regulate the onset and progression of HIV-1 associated neuro-cognitive disorders (HAND) (Ciborowski and Gendelman 2006; Ricardo-Dukelow et al. 2007). Therefore macrophages impact the overall course of HIV-1 disease.
The application of nanotechnology to diagnose and treat various diseases has attracted considerable interest. Nanoparticles can be used as chromophores for molecular imaging as well as for delivering a wide range of payloads to targeted cells. A plethora of colloidal carriers, such as liposomes, nanocapsules and nanoparticles, have been developed to deliver small interfering (si)RNA resulting in silencing of specific genes (Mimi et al. 2011; Liu et al. 2011; Bonoiu et al. 2009; Mahajan et al. 2011, Reynolds et al. 2012). Recent studies have also used nanoparticles as delivery agents for siRNA in macrophage. Tumor necrosis factor α (TNFα) siRNA/polyethyleneimine loaded into polylactide nanoparticles covered with polyvinyl alcohol are efficiently taken up by macrophages and inhibit TNFα secretion (Laroui et al. 2011). Targeting TNFα in macrophages by intraperitoneal administration of chitosan/TNFα siRNA nanoparticles completely prevented radiation-induced fibrosis in CDF1 mice (Nawroth et al. 2010). Chitosan nanoparticles containing an unmodified anti-TNFα Dicer-substrate siRNA mediated TNFα knockdown in primary peritoneal macrophages (Howard et al. 2009). We have previously published an approach that combines the therapeutic potential of gene silencing technology with the imaging and site-specific delivery potential of nanotechnology using Gold nanorods (GNRs). We have shown that siRNA complexed to GNRs (nanoplexes) silenced target genes with efficiency surpassing that obtained using the commercial agent (Bonoiu et al. 2009). In this study we used GNRs as a platform for delivery of siRNA directed against galectin-1. We demonstrate that methamphetamine potentiates galectin-1 expression in human monocyte derived macrophages (MDM) which is attenuated by the galectin-1 siRNA/GNR nanoplex. Moreover, the nanoplexes reduced the effects of methamphetamine on HIV-1 infection in MDM. Further these studies reveal the potential part of galectin-1 in methamphetamine facilitation of HIV-1 infection in MDM.
Materials and methods
Human subjects
Blood donors were recruited at the University at Buffalo; consents were obtained consistent with the policies of University at Buffalo Health Sciences Institutional Review Board (HSIRB) and the National Institutes of Health. Peripheral blood samples from HIV-1 negative individuals were drawn into a syringe containing heparin (20 units/ml, Sigma-Aldrich, St. Louis, MO).
Isolation of human MDM
Due to the difficulties of assessing HIV-1 infection in tissue macrophages, we have used human MDM in this study. Human PBMC were separated by Ficoll-Paque (GE Health Care, Piscataway, NJ) gradient centrifugation. CD14+ cells were isolated from PBMC by direct positive isolation using Dynabeads CD14 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. CD14+ cells were cultured in complete medium [RPMI 1640, 10 % fetal calf serum, 5 % human AB serum, 10 mM/L HEPES, 1 % Penicillin-Streptomycin, 10 ng/ml macrophage colony-stimulating factor (Millipore, Billerica, MA)] for 7 days for differentiation into MDM.
Drug treatment
MDM were treated with methamphetamine hydrochloride (1–100 μM) (Sigma-Aldrich) for 2, 4, 6, 24 or 48 h. The concentrations of METH used were based on previous dose response studies that produced a maximum biological response without causing toxicity to the target cells and also were based on published in vitro studies (Reynolds et al. 2007; Tallóczy et al. 2008; Tipton et al. 2009). These concentrations are similar to levels found in blood, urine or tissue samples of METH users that range from ≤2 μM to 600 μM (Takayasu et al. 1995; Kalasinsky et al. 2001; Schepers et al. 2003; Klette et al. 2005; Morefield et al. 2011). For all experiments, cells treated with vehicle alone (media) were used as the untreated control.
RNA extraction and real time quantitative PCR (Q-PCR)
Cytoplasmic RNA was isolated from MDM (1×105cells/ml) using TRIzol (Invitrogen) according to the manufacturer's specifications. The final RNA pellet was dried and resuspended in diethyl pyrocarbonate water and the concentration of RNA was determined using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington DE). Any DNA contamination in the RNA preparation was removed by treating the RNA with DNAse (1 IU/μg of RNA, Promega, Madison WI) for 2 h at 37 °C, followed by protein-ase K digestion at 37 °C for 15 min and subsequent extraction with phenol/chloroform and NH4OAc/ETOH precipitation. The isolated RNA was stored at –70 °C until used. Relative abundance of each mRNA species was assessed using the SYBR green master mix from Stratagene (La Jolla, CA) to perform Q-PCR. Differences in threshold cycle number were used to quantify the relative amount of PCR target contained within each tube. Relative mRNA species expression was quantitated and expressed as transcript accumulation index (TAI=2–(ΔΔCT)), calculated using the comparative CT method. All data were controlled for quantity of RNA input by performing measurements on an endogenous reference gene, β-actin (Bustin 2002; Radonić et al. 2004). All values were normalized to the constitutive expression of the housekeeping gene, β-actin (Mahajan et al. 2005).
Western blot
Briefly, 50 μg of protein was separated by electrophoresis using 4–20 % Tris–Hepes NuSep Longlife gels (Bioexpress, Kaysville, UT) and transferred to polyvinylidene fluoride (PVDF) membranes (Sigma-Aldrich). Membranes were blocked for 1 h with NAP-Blocker (G-Biosciences, Maryland Heights, MO) in Tris-buffered saline with Tween 20 [150 mM NaCl, 20 mM Tris, pH 7.5, 0.1 % Tween 20, TBST] and then incubated with primary antibodies overnight at 4 °C with gentle rocking. Primary antibodies used were anti-galectin-1 rabbit polyclonal antibody (cat# sc-28248, Santa Cruz Biotechnology, Santa Cruz, CA) and anti-β-actin rabbit polyclonal antibody (cat# sc-130656, Santa Cruz Biotechnology). After incubation with primary antibodies, membranes were washed and incubated with a biotin-conjugated secondary antibody (goat anti-rabbit IgG, cat# BAF017, R&D Systems, Minneapolis, MN). Membranes were washed 3 times, for 10 min each, in TBST and then incubated for 30 min with a streptavidin-alkaline phosphatase conjugate (Invitrogen) followed by colorimetric detection using NBT/BCIP reagent (Thermo Scientific, Rockford, IL). Densitometry analyses were done using Image-J (1.37c software version of Image J (NIH, Bethesda, MD). Data were normalized to protein expression levels of β-actin.
ELISA
Total galectin-1 concentrations were measured by ELISA (NovaTeinBio, Cambridge, MA). Absorbance values were read at 450 nm using a microtiter plate spectrophotometer, and the results are expressed relative units of protein release, whereas controls are set to a 0 value.
Immunofluorescence immunostaining
MDM (1×105cells/ml) were cultured on Lab-Tek chambered coverglasses (Nalgene Nunc International, Rochester, NY) were fixed and permeabilized in cold 70 % methanol for 30 min at 4 °C. Cells were washed in phosphate-buffered saline (PBS), incubated with Image-iT™ FX signal enhancer (Invitrogen) for 30 min at RT in a humid environment. Cells were washed in PBS then incubated with primary antibody (anti-galectin-1 goat polyclonal, 1:50, cat#: sc-19277, Santa Cruz Biotechnology) overnight at 4 °C. Cells were washed with PBS and incubated for 1 h at RT with a secondary antibody conjugated to ALEXA Flour 647 (Alexa Fluor® 647 Donkey Anti-Goat IgG, cat#: A21447, Invitrogen). Cells were then counterstained with 4′, 6-diamidino-2-phenylindole, dilactate (DAPI, Molecular Probes-Invitrogen). MDM were imaged by confocal microscopy using a Leica Confocal Laser Scanning Microscope (TCS SP2 AOBS, Leica Microsystems Heidelberg GmbH) with an Oil Immersion objective lens 63X. HeNe633 nm laser was applied to excite Alexa Fluor-647; argon ion laser was applied to excite DAPI. Quantification of immunostaining was performed by densitometry using Image-J (1.37c software version of Image J (NIH, Bethesda, MD). Expression of galectin-1 was quantified by determining the relative fluorescence units (RFU), by measuring the average value over the total image area per microscopy field (Trika and Jeremic 2011; Kumar et al. 1997).
Synthesis of GNRs
Gold seeds were synthesized by reducing gold salt in the presence of 99 %, hexadecyltrimethylammonium bromide (CTAB) as a capping agent (Nikoobakht et al. 2002; Prasad 2004). Briefly, 10 ml of 0.1 M CTAB solution was mixed with 200 μl of 25 mM HAuCl4. Then, 1 ml of ice-cold 0.01 M sodium borohydride (NaBH4) was quickly added, with vigorous stirring for 2 min. A light brown solution (seed solution) was formed, which was then kept in a water bath at 33 °C for further use. The average size of these Au seeds was 3–5 nm. Meanwhile, to synthesize GNRs, 10 ml of 0.1 M CTAB was mixed with 200 μl of 25 mM HAuCl4 in a separate vial, in the presence of a small amount of Ag+ ion (8×10−5 M). Then, the moderate reductant, 100 μl of 0.1 M ascorbic acid, was added at RT, resulting in the formation of colorless solution. This mixture is the so-called growth solution. Finally, the growth solution was heated to 33 °C in the water bath and 12 μl of seed solution was gently added to it. The rod formation was permitted undisturbed at 33 °C for at least 3 h. Then, to remove the excess CTAB from the prepared GNRs, the bilayer CTAB-coated GNRs were centrifuged at 9000 rpm for 15 min. GNRs were sequentially coated with two successive layers of polyelectrolytes, (a) the negatively charged poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDT/PSS), and (b) the positively charged poly(diallyldimethylammoniumchloride) (PDDAC).
Formation of GNR-siRNA nanoplexes and MDM transfection
Cationic GNRs were electrostatically attached to siRNA resulting in the formation of nanoplexes, and will be referred to as nanoplexes throughout the text (galectin-1 siRNA/GNR = nanoplexes). Briefly, galectin-1 siRNAFAM (FAM=6-carboxyfluorescein, fluorophore label, Invitrogen) or scrambled control (SCR) were reconstituted in DNase–RNase free water to a final concentration of 0.1 μM and mixed with GNR solution then incubated at RT for 15 min. We have shown that nanoplexes are stable for more than 1 month post-complexation (Bonoiu et al. 2009).
MDM transfection
1×105 cells/ml were seeded onto six-well plates in OPTI-MEM containing 4 % FBS with no antibiotics. Nanoplexes or SCR were used at a final concentration of 200 pmol of siRNA/per 30 μl. Transgene expression or cellular uptake was monitored at 4, 12, 24, 48, 72 or 168 h post transfection.
Characterization of GNR-siRNA nanoplexes
Transmission electron microscopy (TEM) images were obtained using JEOL model JEM-100CX microscope, operating with acceleration voltage 80 kV. The specimens were prepared by drop-coating the sample dispersion onto a holey carbon-coated 200 mesh copper grid, which was placed on filter paper to absorb excess solvent.
Dark-field microscopy imaging
The cellular uptake of nanoplexes was visualized using Dark-Field microscopy 4 h post-transfection. The light-scattering images were recorded using an upright Nikon Eclipse 800 microscope with a high numerical dark-field condenser (N.A. 1.20-1.43, oil immersion) and a 100/1.4 NA oil Iris objective (Cfi Plan Fluor). In the dark-field configuration, the condenser delivers a narrow beam of white light from a tungsten lamp and the high NA oil immersion objective collects only the scattered light from the samples. The dark-field imaging was captured using a QImaging Micropublisher 3.3 RTV color camera. The Qcapture software was used for image acquisition and has a feature for adjusting the white color balance for accurately capturing the color differences in samples.
Photoluminescence studies from cell lysates
MDM (1×105/ml) were incubated with GNR alone, 200 pmols of free siRNAFAM, and nanoplexes for 4 h, medium was removed and cells were lysed using Cell Extraction Buffer (Invitrogen). Lysate was analyzed for photoluminescence (PL) using FLx800 multi-detection fluorescence reader (Biotek, Winooski, VT) at emission wavelength of 520 nm. FAM has an emission maximum wavelength at 520 nm wavelength.
Phagocytosis assay
MDM (10,000 cells/well) were incubated with nanoplexes for 2, 24, 48 and 72 h; phagocytosis activity was subsequently measured. Phagocytosis: CytoSelect™ 96-Well Phagocytosis Assay, Zymosan Substrate assay was performed according to manufacturer's specifications (Cell Biolabs, San Diego, CA). Briefly, MDM were incubated with zymosan suspension for 2 h. Cells were fixed for 5 min, blocked, then permeabilized with 1X permeabilization solution. Detection reagent was added for 1 h followed by substrate incubation for 20 min. Stop Solution was added and the absorbance of each well was read at a wavelength of 405 nm. Cytochalsin D (0.2 mM): positive control.
Infection of MDM with HIV-1
In one series of experiments, MDM (1×105 cells/ml) were treated methamphetamine (10 μM) for 24 h and then recombinant galectin-1 (2 μM) for 30 min prior to infection with HIV-1. In another series of experiments, MDM (1×105 cells/ml) were treated with nanoplex for (24 h), then methamphetamine (10 μM) for 24 h prior to infection with HIV-1.
Cells were washed and subsequently infected with CCR5 (R5)-using virus HIV-1 BaL (Advanced Biotechnologies, Columbia, MD) at multiplicity of infection (MOI) of 0.05 for 2 h washed 3 times with Hank's balanced salt solution (Invitrogen) before being returned to culture for 5 and 10 days (Gaskill et al. 2009; Balkundi et al. 2010; Ciborowski et al. 2007; Kraft-Terry et al. 2011). The culture supernatants were assayed for p24 antigen using a p24 ELISA kit (ZeptoMetrix Corporation, Buffalo, NY) on day 5 and 10. Cells were harvest for RNA analyses at day 10 for quantification of HIV-LTR-R/U5 gene expression (Secchiero et al. 2000).
Statistics
Multiple comparisons were calculated using ANOVA followed by Bonferroni post-hoc test (SPSS). Data represent the mean±standard deviation.
Results
Galectin-1 expression is increased by methamphetamine
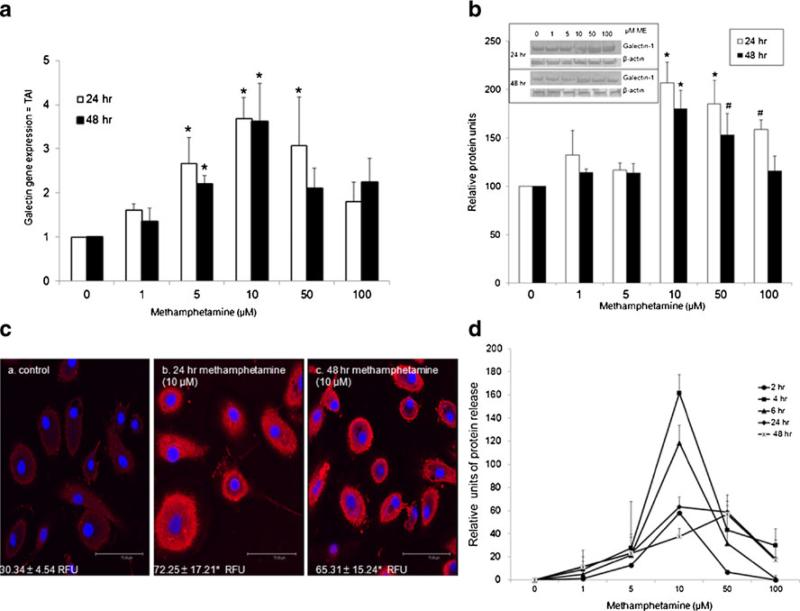
We sought to determine whether methamphetamine could modulate galectin-1 gene expression in human MDM. As shown in shown in Fig. 1a, methamphetamine significantly increased galectin-1 gene expression at 5, 10 and 50 μM at 24 h. Gene expression for galectin-1 was significantly increased at 5 and 10 μM at 48 h. These data demonstrate that methamphetamine modulates galectin-1 gene expression at various times and doses in MDM. We next performed western blotting to validate the changes in gene expression of galectin-1 in MDM. Shown in Fig. 1b, methamphetamine significantly increased galectin-1 protein expression at 10, 50, and 100 μM at 24 h. At 48 h, 10 and 50 μM methamphetamine significantly increased galectin-1 expression. The maximal effect of methamphetamine on galectin-1 protein expression occurred at 10 μM for both 24 and 48 h. A representative image of a western blot is shown in the inset, β-actin, as an internal control, showed an equivalent protein content among all samples. These data are consistent with our gene expression data. Since the maximal effects of methamphetamine on protein expression were observed at 10 μM, further validation in the changes in expression of galectin-1 were done using immunofluorescence immunostaining and subsequent confocal microscopy. Data demonstrate immunostaining for galectin-1 in MDM (red), with cell nuclei labeled blue (DAPI) (Fig. 1c, a–c). Relative fluorescent units (RFU) are shown in the corner of each panel. Data demonstrated that protein expression for galectin-1 was increased at 24 h (b) and 48 h (c) of methamphetamine incubation (10 μM) compared to control (a). These data confirm both Q-PCR and western blotting data. Since galectin-1 is secreted by macrophage (Rabinovich et al. 1999b) we next investigated the effects of methamphetamine on unbound galectin-1 in the supernatant. MDM were treated with methamphetamine (1–100 μM) for 2, 4, 6, 24 and 48 h then assessed for galectin-1 protein release in the supernatant by ELISA. As shown in Fig. 1d, meth-amphetamine significantly increased galectin-1 release at 10 μM at 2, 4, 6, 24 h and at 50 μM for 4 and 6 h compared to control. Data are normalized to the untreated control, being set at 0 values. Peak release of galectin-1 into the supernatant occurred at 4 h, with decreasing amounts being released thereafter. Collectively, Q-PCR, western blotting, immunostaining and ELISA demonstrate that methamphetamine enhances the expression and release of galectin-1 from human MDM.
Fig. 1.
Methamphetamine regulates galectin-1 gene and protein expression in MDM. MDM were incubated with methamphetamine (1, 5, 10, 50, 100 μM), for 2, 4, 6, 24 or 48 h. a Gene expression for galectin-1 was determined using Q-PCR, (n=4). Data are expressed as transcript accumulation index (2–(ΔΔCT)) or TAI. b) Galectin-1 protein expression was determined using western blotting (n=4). Immunore-active protein bands were semi-quantified by densitometric analysis. Data are expressed as relative protein levels. Representative western blot for both galectin-1 and β-actin are shown in inset. c) Representative confocal image of galectin-1 protein expression (red) following methamphetamine (10 μM) incubation (a) control (b) 24 methamphetamine (c) 48 h methamphetamine with DAPI nuclear staining (blue). Relative fluorescent units (RFU) are shown in each panel (n=4, scale bar=75.49 μM). d) Supernatants were assayed for galectin-1 protein using ELISA (n=3). Data are expressed as relative protein units, with control value set at 0. Control cells release 120±5.2 pg/ml. All statistical significance was calculated using ANOVA followed by Bonferroni post-hoc test, * p<0.001; # p<0.05. • 2 h; ■ 4 h; ▲ 6 h; ◇ 24 h; X 48 h
Uptake of nanoplexes in MDM
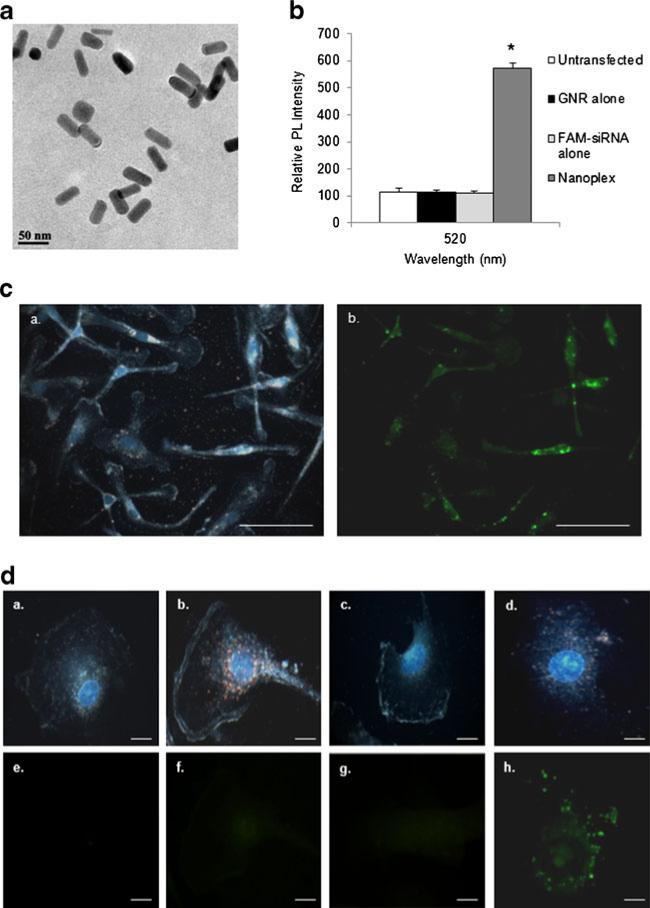
Cationic GNRs were electrostatically attached to anionic galectin-1 specific siRNA, resulting in the formation of nanoplexes. Analyses of the nanoplex using TEM (Fig. 2a) showed no aggregation of GNRs upon complexation with siRNA indicating the nanoplexes were still nanoscale in size. We next investigated intracellular uptake of the nanoplex using a spec-trofluorometer. Fluorescence intensity from MDM cellular lysates was measured 4 h post-transfection of siRNAFAM alone or of nanoplexes. Results (Fig. 2b) demonstrated that lysates from MDM incubated with GNR alone and free siRNAFAM show little or no fluorescence while intracellular fluorescence was significantly increased in MDM lysates 4 h post-transfection with the nanoplexes compared to control. These data indicate successful intracellular delivery of galectin siRNAFAM and that GNRs are a suitable carrier for siRNA. We further investigated the cellular delivery of the nanoplex using the strong orange-red plasmonic scattering associated with GNRs using dark-field microscopy (Ding et al. 2007; Sokolov et al. 2003) and corresponding fluorescent microscopy. Figure 2c shows a representative 40X dark field image of MDM 4 h post-transfection with nanoplexes and the corresponding fluorescent image. These images confirm the entry/cellular uptake of nanoplexes (a) and delivery of FAM labeled siRNA (b) in MDM. Figure 2d shows dark-field images (100X) and corresponding fluorescent images of (a) untransfected MDM, (b) GNRs alone, (c) free galectinsiRNAFAM and (d) nanoplexes 4 h post- transfection. Cellular uptake of GNR alone (b) and the nanoplexes (d) was observed as evidenced of the orange-red scattering in the image, as opposed to the untreated MDM (a). No orange-red scattering was seen in the (c) free galectin-siRNAFAM image, as there are no GNR present. Fluorescent microscopy was also used to confirm the cellular entry of the nanoplexes and FAM labeled siRNA. Data demonstrate uptake of nanoplex and delivery of siRNA as early as 4 h post-transfection (h) compared to untransfected control (e). No fluorescence was seen with GNR alone (f) demonstrating that GNR do not have intrinsic fluorescence. No fluorescence was seen with free galectinsiRNAFAM (g) indicating that free siRNA cannot penetrate the cell membrane without a carrier. Taken together, these data demonstrate that GNR serve as a competent platform for delivery of siRNA to MDM.
Fig. 2.
Cellular uptake of nanoplexes. MDM were transfected with GNRs, free siRNA and nanoplexes for various time frames. a Representative Transmission electron microscopy (TEM) image of nanoplex. b The fluorescence intensity from cellular lysates was measured in untransfected, GNR alone, free siRNAFAM alone or nanoplex treated MDM 4 h post-transfection. PL = Photoluminescence. Statistical significance was calculated using ANOVA followed by Bonferroni post-hoc test, * compared to control (n=4). c Representative 40X dark field image of MDM 24 h post transfection with nanoplexes (gold) with DAPI nuclear staining (blue) (a) and the corresponding fluorescent image (b) (scale bar=100 μM). d Representative dark-field images (100X) (a-d) with DAPI nuclear staining (blue) and corresponding fluorescent images (e-h) of untransfected (a, e), GNRs alone (b, f), free galectin-siRNAFAM (c, g) and nanoplexes (d, h) 4 h-post transfection (scale bar=10 μM)
RNA interference
Since a primary function of macrophage is phagocytosis (Gordon and Mantovani 2011), we investigated if the nanoplex had any effect on the ability of macrophage to phagocytize zymosan particles. Our studies demonstrate that phagocytosis was inhibited by Cytochalasin D (positive control) while it was unaffected in the presence of the nanoplexes at 2, 24, 48, and 72 h post-transfection (Fig. 3a) in MDM. Studies have previously shown that GRN and nanoplexes have no effect on cell viability (Connor et al. 2005; Bonoiu et al. 2009). Since the nanoplex does not affect MDM function, we subsequently investigated RNA interference against galectin-1 at 12, 24, 48, 72 and 168 h post-transfection of nanoplexes in MDM. Gene expression for galectin-1 was determined using Q-PCR. As shown in Fig. 3b, the nanoplexes significantly decreased gene expression for galectin-1 at 24, 48 and 72 h post-transfection compared to control but not at 12 and 168 h post-transfection. Scrambled siRNA had no effect on galectin-1 gene expression. Since we have shown that methamphetamine potentiates galectin-1 gene expression (Fig. 1), we next investigated the effect of the nanoplexes on this potentiation of galectin-1 expression. MDM were transfected with nanoplexes, 24 h post-transfection MDM were treated with methamphetamine (10 μM) for 24 h. Gene expression for galectin-1 was then analyzed (48 h post-transfection, 24 h post methamphetamine administration). Data demonstrated that nanoplexes inhibited galectin-1 gene expression and methamphetamine potentiation of galectin-1 gene expression was reversed in the presence of nanoplex (Fig. 3c). These data were further validated using immunofluorescence immunostaining. Galectin-1 protein expression was analyzed (48 h post transfection, 24 h post methamphetamine administration (10 μM)). Shown in Fig. 3d are representative confocal fluorescent images: (a) scrambled control; (b) GNR; (c) control; (d) nanoplex; (e) methamphetamine (10 μM); (f) nanoplex plus methamphetamine. There was no significant change in protein expression in scrambled control (a), GNR alone (b) compared to control (c). The nanoplexes (d) attenuated galectin-1 protein expression at 48 h post-transfection compared to control (c). Methamphetamine (10 μM, 24 h) increased galectin-1 expression (e) compared to all controls (a–c). Further the increase in galectin-1 expression induced by methamphetamine (e) was reduced in the presence of the nanoplex (f) compared to methamphetamine alone. Our protein expression data confirm gene expression results.
Fig. 3.
Effect of nanoplexes on galectin-1 expression. MDM were transfected with nanoplexes for various time frames, phagocytosis activity, gene expression and protein expression were subsequently investigated. a Phagocytosis activity as determined by Zymosan Substrate CytoSelect™ 96-Well Phagocytosis Assay. Cyt D = Cytochalasin D (positive control, phagocytosis inhibitor). Data are presented as percent change in phagocytosis activity (n=4). b Gene expression for galectin-1 using Q-PCR (n=6). Data are expressed as transcript accumulation index (2–(ΔΔCT)) or TAI. c MDM were transfected with nanoplexes (24 h) then incubated in the presence of 10 μM methamphetamine (24 h). Gene expression for galectin-1 was examined using Q-PCR (n=4). Data are expressed as transcript accumulation index (2–(ΔΔCT)) or TAI. d Representative confocal image of galectin-1 protein expression (red) with DAPI nuclear staining (blue) following transfection of nanoplexes for 24 h then incubated in the presence of 10 μM methamphetamine for 24 h. a) scrambled control b) GNR c) control d) nanoplex e) methamphetamine f) nanoplex + methamphetamine. RFU are shown in each panel (n=4, scale bar=75.49 μM). All statistical significance was calculated using ANOVA followed by Bonferroni post-hoc test, * compared to control; † compared to methamphetamine alone. C = control; SCR = scramble; NP = nanoplex; ME = methamphetamine
HIV-1 analyses
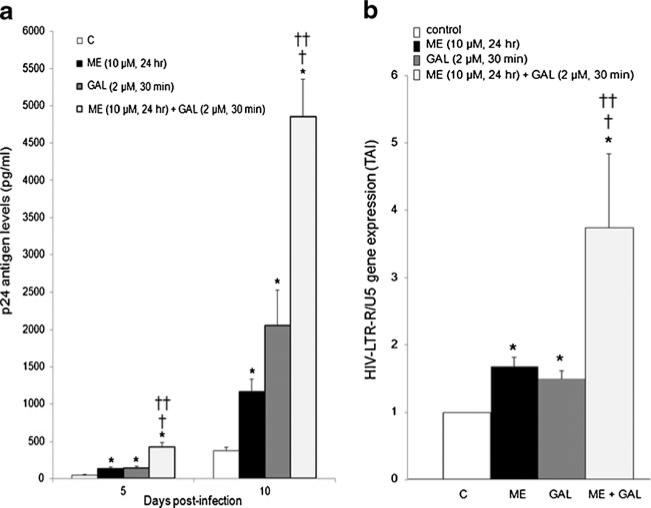
Previous reports demonstrate that individually both galectin-1 and methamphetamine enhance HIV-1 infection in MDM (St. Pierre et al. 2010; Mercier et al. 2008; Toussi et al. 2009; Liang et al. 2008). Therefore we investigated the effects of concomitant administration of exogenous galectin-1 and methamphetamine on HIV-1 p24 antigen production. As shown in Fig. 4a, 30 min incubation of MDM with exogenous recombinant galectin-1 (2 μM) prior to HIV-1 infection enhances p24 antigen production at both 5 and 10 days post infection. Methamphetamine (10 μM, 24 h) enhances HIV-1 p24 antigen production at both 5 and 10 days post-infection. Furthermore, concomitant incubation of methamphetamine (10 μM, 24 h) with recombinant galectin-1 (2 μM, 30 min) prior to infection had a synergistic effect on p24 antigen production by MDM at both 5 and 10 days post infection. Since the maximal effect of methamphetamine and galectin-1 on p24 antigen production occurred at day 10, we next investigated changes in amplification of the HIV-LTR-R/U5 region using Q-PCR on day 10 post-infection. This method is designed to detect early stages of reverse transcription of HIV-1 (Secchiero et al. 2000). Data demonstrate that meth-amphetamine alone and galectin-1 alone increased the expression of the HIV-LTR-R/U5 region at day 10 post-infection (Fig. 4b). Further, concomitant methamphetamine and galectin-1 enhanced gene expression for HIV-LTR-R/U5 region (Fig. 4b).
Fig. 4.
Concomitant galectin-1 and methamphetamine effects on HIV-1 infection. MDM were incubated with methamphetamine (10 μM, 24 h) alone or recombinant galectin-1 (2 μM, 30 min) alone or concomitantly prior to infection with HIV-1. a Supernatants were collected on day 5 and 10 post-infection as assayed for p24 antigen. Data are presented as p24 antigen levels (pg/ml) (n=6). b RNA was isolated on day 10 post-infection; HIV-LTR-R/U5 antigen gene expression was quantitated using Q-PCR (n=6). Data are expressed as transcript accumulation index (2–(ΔΔCT)) or TAI. All statistical significance was calculated using ANOVA followed by Bonferroni post-hoc test, * compared to control; † compared to methamphetamine alone; †† compared to recombinant galectin-1 alone
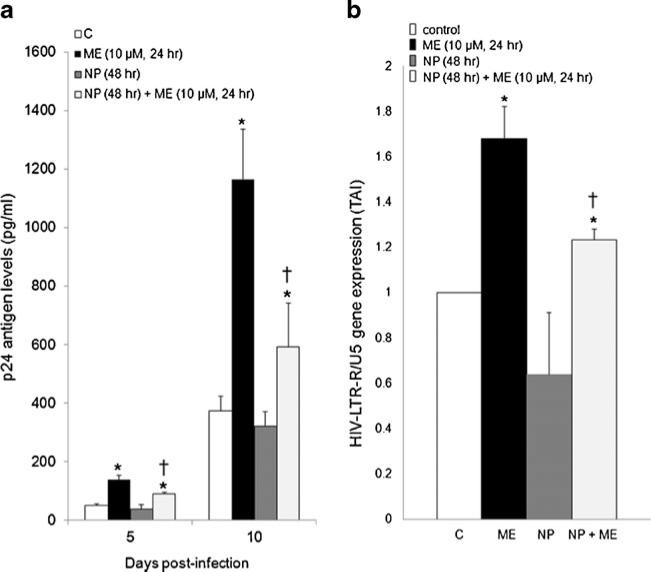
Since exogenous galectin-1 and methamphetamine have a synergistic effect on HIV-1 infection we next investigated whether a mechanism of enhanced HIV-1 infection by methamphetamine may be modulation of endogenous galectin-1. MDM were transfected with nanoplexes, then 24 h post-transfection were treated with methamphetamine (10 μM), for 24 h then were infected with HIV-1 for 2 h, washed and returned to culture for 5 and 10 days when levels of p24 antigen were measured. Shown in Fig. 5a, methamphetamine (10 μM) enhanced p24 antigen production compared to control MDM at day 5 and 10 post-infection. Incubation with the nanoplexes prior to methamphetamine treatment partially reversed the potentiation in p24 antigen production compared to methamphetamine alone at days 5 and 10 post infection. We next investigated changes in amplification of the HIV-LTR-R/U5 region using Q-PCR on day 10 post-infection. Data demonstrate that prior incubation with the nanoplexes before methamphetamine treatment (10 μM) partially reversed the increase in gene expression for the HIVLTR-R/U5 region compared to methamphetamine alone at day 10 post infection (Fig. 5b).
Fig. 5.
Effect of nanoplex on HIV-1 infection. MDM were transfected with nanoplexes, 24 h post-transfection MDM were treated with methamphetamine (10 μM, 24 h) then infected with HIV-1 for 2 h, washed and returned to culture. a p24 antigen levels were measured at day, 5 and 10 post-infection (n=6). Data are expressed as pg/ml. b RNA was isolated on day 10 post-infection; HIV-LTR-R/U5 antigen gene expression was quantitated using Q-PCR (n=6). Data are expressed as transcript accumulation index (2–(ΔΔCT)) or TAI. All statistical significance was calculated using ANOVA followed by Bonferroni post-hoc test, * compared to control; † compared to methamphetamine alone; †† compared to recombinant galectin-1 alone
Discussion
Since the drug abuse epidemic and HIV-1 epidemic are closely interrelated we proposed that increased expression of galectin-1 induced by methamphetamine may play a role in drug-induced facilitation of HIV-1 infection in MDM. Employing Q-PCR, western blotting, ELISA and immunofluorescence we characterized the effects of methamphetamine on galectin-1 expression in human MDM. We demonstrate that methamphetamine increased galectin-1 gene and protein expression and release in human MDM at various times and doses (Fig. 1). Together with previous studies (Liang et al. 2008; Toussi et al. 2009), we show that methamphetamine potentiates HIV-1 p24 antigen production and infection (HIV-LTR-RU gene expression) in MDM (Figs. 4 and 5). We further show that concomitant incubation of galectin-1 with methamphetamine has a synergistic effect on HIV-1 p24 antigen production in MDM and HIV-1 LTR/RU5 gene expression (Fig. 4). We further elucidated whether this modulation of galectin-1 is a mechanism by which methamphetamine facilities HIV-1 infection in MDM. We found that inhibition of galectin-1 expression, by using a nanotechnology approach utilizing GNRs complexed to galectin-1 siRNA (Figs. 2 and 3), partially prevented the effects of methamphetamine on p24 antigen production and gene expression for HIV-1 LTR/RU5 in MDM (Fig. 5). These observations, taken together provide new insights into methamphetamine modulation of HIV-1 infections.
Patients infected with HIV-1 can develop HAND, which consists of neurologic complications including motor and cognitive dysfunctions. Presumably infected peripheral immune cells migrating to the CNS are responsible for this development of HAND. After entering the CNS, infected monocytes, lymphocytes, and macrophages release various mediators including cytokines, reactive oxygen, and other molecules that have the potential to induce neurotoxicity (Ciborowski and Gendelman 2006; Ricardo-Dukelow et al. 2007). In addition to disrupting normal function of CNS cells these mediators also stimulate uninfected CNS monocytes, macrophages and lymphocytes to release potential neurotoxins (Kadiu et al. 2005; Persidsky and Gendelman 2003). Galectin-1 is an immunomodulater. Galectin-1 has been shown to increase production of monocyte chemoattractant protein-1 (MCP-1) (Masamune et al. 2006). MCP-1 is a potent chemoattractant and activator of monocytes/macrophages and has been suggested to play and important role in the pathogenesis of HAND (Gonzalez et al. 2002; Airoldi et al. 2012). Further, methamphetamine is known to activate macrophages (Tallóczy et al. 2008; Martinez et al. 2009) and activated macrophages secrete galectin-1 (Rabinovich et al. 1999a), these conditions are likely to exist in the drug using population blood milieu (i.e. methamphetamine + galectin-1) including the CNS. Increased release and production of galectin-1 by non-infected and infected macrophages may affect the course of HIV-1 disease and its progression of CNS disease. This study shows that methamphetamine increases the expression and release of galectin-1 from macrophages (Fig. 1). We also demonstrate that concomitant incubation of galectin-1 with methamphetamine has a synergistic effect on HIV-1 p24 antigen production in macrophages (Fig. 3). These studies suggest the potential effects that concomitant methamphetamine and galectin-1 have on HIV-1 infection in the drug using population and in the development of HAND in the drug using population. The current studies suggest that methamphetamine by modulating galectin-1 expression and release may contribute to the loss of control of the immune system over HIV-1 infection (Endharti et al. 2005; Rabinovich et al. 1999a; Romagnani 2000; Romagnani et al. 2000).
Previous studies have found that galectin-1 contributes to HIV-1 binding in T Cells and macrophage Ouellet et al. 2005; Mercier et al. 2008; St. Pierre et al. 2010). They report that galectin-1 facilitates HIV-1 infection by increasing the kinetics of HIV-1 binding to its target cell by facilitating attachment of HIV-1 to the cell surface (Mercier et al. 2008). They proposed that galectin-1 can cross-link HIV-1 and target cells to promote firmer adhesion of the virus to the cell surface, thereby augmenting the efficiency of the infection process (Ouellet et al. 2005; Mercier et al. 2008; St. Pierre et al. 2010). Their recent studies confirm that galectin-1 binds directly to HIV-1 through recognition of clusters of N-linked glycans on the viral envelope gp120. They suggest that HIV-1 exploits galectin-1 to enhance gp120-CD4 interactions, thereby promoting virus attachment and infection events (St. Pierre et al. 2011, 2012). Based on these findings we proposed that methamphetamine by regulating galectin-1 expression and release facilitates HIV-1 attachment to MDM. Since methamphetamine enhances gene expression for galectin-1 (Fig. 1) we used RNA interference to inhibit the expression of galectin-1 in MDM and investigated its effects on HIV-1 viral infection. We used GNRs as a delivery agent due to their biocompatibility and their ability to form a stable electrostatic complexation with siRNA, for the purpose of targeted gene delivery/silencing. By exploiting the phenomenon of SPR associated with GNRs, delivery and distribution of nanoplexes within MDM were monitored. We demonstrated uptake of the nanoplex (Fig. 2) and gene silencing of galectin-1 at 24, 48 and 72 h (Fig. 3). Moreover, we show that the potentiation of galectin-1 gene expression induced by methamphetamine was reversed in the presence of the nanoplex (Fig. 3). Since the nanoplex could attenuate the effects of methamphetamine of galectin-1 expression we next investigated the effects of the nanoplex on HIV-1 infection. In the presence of the nanoplex, the potentiation of HIV-1 infection induced by methamphetamine was reduced (Fig. 5). However, our data demonstrate that the effects of methamphetamine on HIV-1 infection were only partially reduced which suggests a redundancy in the regulation of HIV-1 infectivity, such that galectin-1 may act concomitantly with another protein or molecule to regulate HIV-1 infection.
There are several pharmaceutical challenges and limitations to effective siRNA delivery and activity in vivo, which must be overcome for achieving therapeutically relevant gene silencing. These include effective cell delivery, endosomal escape, off-target effects and competition with endogenous microRNAs for cellular miRNA-processing machinery (Tokatlian and Segura 2010; Aagaard and Rossi 2007). Nanotechnology offers an unprecedented opportunity to enhance the therapeutic applications of siRNA. The GNR used in this study have wide-ranging potential in medicine owing to their ease of bioconjugation, low toxicity, lack of immunogenicity, and excellent biocompatibility. Complexing siRNA with nanoparticles protects the siRNA from biological degradation and enhances it delivery across cell membranes (Bonoiu et al. 2009). Further the overall charge of the nanoplexes is sparsely negative, which is ideal for their avoidance of nonspecific interaction with physiological proteins and other biomolecules (Bonoiu et al. 2009; Reynolds et al. 2012). Although the study demonstrates the efficacy of this use of GNRs as an effective transport platform for siRNA, in vitro, their efficacy in vivo remains to be determined.
No single therapy is the “best therapy” for decreasing HIV-1 infectivity in drug abusing subjects. Several studies have shown that addictive drugs modulate the expression of HIV-1 co-receptors and enhanced expression of these coreceptors plays a role in increased HIV-1 infection in macrophage (Buch et al. 2011; Purohit et al. 2011; Mariani et al. 2011). We propose that concomitant silencing of galectin-1 and a co-receptor such as CCR5 or CXCR4 may be ideal candidates for therapeutic prevention of HIV-1 infection in addictive drug users. Further, when developing new therapeutic regimes to treat HIV-1 in drug abusers, simultaneous delivery of siRNA nanoparticles bound with sRNA and specific protein inhibitors may also be beneficial. St. Pierre et al. (2011) demonstrated that compounds that inhibit galectin-1 protein interactions reduced the galectin-1-mediated increase in HIV-1 attachment to target cells and also decreased the galectin-1-dependent enhancement of HIV-1 infection (St. Pierre et al. 2011). Moreover nanotherapy, combined with more traditional pharmacologic therapies such as highly active antiretroviral therapy (HAART) may also yield a more effective approach (Kadiu et al. 2011; Nowacek et al. 2011). HAART has been shown to be very beneficial in treating HIV-1 however there are several limitations such as development of drug resistance and long-term toxicity. Combine therapies may help to overcome these limitations. However, it is necessary for continual research on drug development to improve the overall health of HIV-1 in drug abusers.
These studies demonstrate that galectin-1 is an important mediator involved in immune-mediated responses observed in HIV-1 infected drug abusing subjects. We propose targeting galectin-1 will result in decreased cell talk and cell adhesion between viral components and host macrophages thereby decreasing HIV-1 infectivity of the macrophages in HIV-1 infected drug abusers.
Acknowledgements
This work was supported by the National Institutes of Health.
K01DA024577 (JR)
R01AI085569 (SAS)
R21DA030108 (SM)
Kaleida Health Foundation (SAS)
Footnotes
The authors declare that they have no conflict of interest.
Contributor Information
Jessica L. Reynolds, Department of Medicine, Division of Allergy, Immunology and Rheumatology, State University of New York at Buffalo, Innovation Center, 640 Ellicott Street, Buffalo, NY 14203, USA
Wing Cheung Law, Department of Chemistry, Institute for Lasers and Biophotonics, University of New York at Buffalo, Natural Science Complex, Amherst, NY 14203, USA.
Supriya D. Mahajan, Department of Medicine, Division of Allergy, Immunology and Rheumatology, State University of New York at Buffalo, Innovation Center, 640 Ellicott Street, Buffalo, NY 14203, USA
Ravikumar Aalinkeel, Department of Medicine, Division of Allergy, Immunology and Rheumatology, State University of New York at Buffalo, Innovation Center, 640 Ellicott Street, Buffalo, NY 14203, USA.
Bindukumar Nair, Department of Medicine, Division of Allergy, Immunology and Rheumatology, State University of New York at Buffalo, Innovation Center, 640 Ellicott Street, Buffalo, NY 14203, USA.
Donald E. Sykes, Department of Medicine, Division of Allergy, Immunology and Rheumatology, State University of New York at Buffalo, Innovation Center, 640 Ellicott Street, Buffalo, NY 14203, USA
Ken-Tye Yong, School of Electrical and Electronic Engineering, Nanyang Technological University, Nanyang Avenue, Singapore 639798, Singapore.
Rui Hui, Department of Chemistry, Institute for Lasers and Biophotonics, University of New York at Buffalo, Natural Science Complex, Amherst, NY 14203, USA.
Paras N. Prasad, Department of Chemistry, Institute for Lasers and Biophotonics, University of New York at Buffalo, Natural Science Complex, Amherst, NY 14203, USA
Stanley A. Schwartz, Department of Medicine, Division of Allergy, Immunology and Rheumatology, State University of New York at Buffalo, Innovation Center, 640 Ellicott Street, Buffalo, NY 14203, USA
References
- Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59(2–3):75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airoldi M, Bandera A, Trabattoni D, Tagliabue B, Arosio B, Soria A, Rainone V, Lapadula G, Annoni G, Clerici M, Gori A. Neurocognitive impairment in HIV-infected naïve patients with advanced disease: the role of virus and intrathecal immune activation. Clin Dev Immunol. 2012:467154. doi: 10.1155/2012/467154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkundi S, Nowacek AS, Roy U, Martinez-Skinner A, McMillan J, Gendelman HE. Methods development for blood borne macrophage carriage of nanoformulated antiretroviral drugs. J Vis Exp. 2010 doi: 10.3791/2460. doi:10.3791/2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269(33):20807–20810. [PubMed] [Google Scholar]
- Bergamaschi A, Pancino G. Host hindrance to HIV-1 replication in monocytes and macrophages. Retrovirology. 2010 doi: 10.1186/1742-4690-7-31. doi:10.1186/1742-4690-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonoiu AC, Mahajan SD, Ding H, Roy I, Yong KT, Kumar R, Hu R, Bergey EJ, Schwartz SA, Prasad PN. Nanotechnology approach for drug addiction therapy: gene silencing using delivery of gold nanorod-siRNA nanoplex in dopaminergic neurons. P Natl Acad Sci USA. 2009;106(14):5546–5550. doi: 10.1073/pnas.0901715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S, Yao H, Guo M, Mori T, Su TP, Wang J. Cocaine and HIV-1 interplay: molecular mechanisms of action and addiction. J Neuroimmune Pharmacol. 2011;6(4):503–515. doi: 10.1007/s11481-011-9297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner CM, Luers AJ, Calderon TM, Eugenin EA, Berman JW. Neuroimmunity and the blood-brain barrier: molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on neuroAIDS. J Neuroimmune Pharm. 2006;1(2):160–181. doi: 10.1007/s11481-006-9017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29(1):23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- Chiariotti L, Salvatore P, Frunzio R, Bruni CB. Galectin genes: regulation of expression. Glycoconjugate J. 2004;19(7–9):441–449. doi: 10.1023/B:GLYC.0000014073.23096.3a. [DOI] [PubMed] [Google Scholar]
- Ciborowski P, Gendelman HE. Human immunodeficiency virus-mononuclear phagocyte interactions: emerging avenues of biomarker discovery, modes of viral persistence and disease pathogenesis. Curr HIV Res. 2006;4(3):279–291. doi: 10.2174/157016206777709474. [DOI] [PubMed] [Google Scholar]
- Ciborowski P, Kadiu I, Rozek W, Smith L, Bernhardt K, Fladseth M, Ricardo-Dukelow M, Gendelman HE. Investigating the human immunodeficiency virus type 1-infected monocyte-derived macrophage secretome. Virology. 2007;363(1):198–209. doi: 10.1016/j.virol.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1(3):325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- Crowe S, Zhu T, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukocyte Biol. 2003;74(5):635–641. doi: 10.1189/jlb.0503204. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42(9):1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Ding H, Yong KT, Roy I, Pudavar HE, Law WC, Bergey EJ, Prasad PN. Gold nanorords coated with multilayer polyelectrolyte as contrast agent for multimodal imaging. J Phys Chem C. 2007;34(111):12552–12557. [Google Scholar]
- Endharti AT, Zhou YW, Nakashima I, Suzuki H. Galectin-1 supports survival of naive T cells without promoting cell proliferation. Eur J Immunol. 2005;35(1):86–97. doi: 10.1002/eji.200425340. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26(4):1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin JF, Cantin R, Tremblay MJ. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J Virol. 1998;72(3):2105–2112. doi: 10.1128/jvi.72.3.2105-2112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol. 2009;175(3):1148–1159. doi: 10.2353/ajpath.2009.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE, Leonard JM, Dutko F, Koenig S, Khillan J, Meltzer MS. Immunopathogenesis of human immunodeficiency virus infection in the central nervous system. Ann Neurol. 1988;23S:S78–81. doi: 10.1002/ana.410230721. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Orenstein JM, Baca LM, Weiser B, Burger H, Kalter DC, Meltzer MS. The macrophage in the persistence and pathogenesis of HIV infection. AIDS. 1989;3(8):475–495. doi: 10.1097/00002030-198908000-00001. [DOI] [PubMed] [Google Scholar]
- Gilbert C, Cantin R, Barat C, Tremblay MJ. Human immunodeficiency virus type 1 replication in dendritic cell-T-cell cocultures is increased upon incorporation of host LFA-1 due to higher levels of virus production in immature dendritic cells. J Virol. 2007;81(14):7672–7682. doi: 10.1128/JVI.02810-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, Mummidi S, Kulkarni H, Bamshad MJ, Telles V, Anderson SA, Walter EA, Stephan KT, Deucher M, Mangano A, Bologna R, Ahuja SS, Dolan MJ, Ahuja SK. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci U S A. 2002;99(21):13795–800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Mantovani A. Diversity and plasticity of mononuclear phagocytes. Eur J Immunol. 2011;41(9):2470–2472. doi: 10.1002/eji.201141988. [DOI] [PubMed] [Google Scholar]
- Hamamoto DT, Rhodus NL. Methamphetamine abuse and dentistry. Oral Dis. 2009;15(1):27–37. doi: 10.1111/j.1601-0825.2008.01459.x. [DOI] [PubMed] [Google Scholar]
- Herbein G, Varin A. The macrophage in HIV-1 infection: from activation to deactivation? Retrovirology. 2010 doi: 10.1186/1742-4690-7-33. doi:10.1186/1742-4690-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard KA, Paludan SR, Behlke MA, Besenbacher F, Deleuran B, Kjems J. Chitosan/siRNA nanoparticle-mediated TNF-alpha knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model. Mol Ther. 2009;17(1):162–168. doi: 10.1038/mt.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiu I, Glanzer JG, Kipnis J, Gendelman HE, Thomas MP. Mononuclear phagocytes in the pathogenesis of neurodegenerative diseases. Neurotox Res. 2005;8(1–2):25–50. doi: 10.1007/BF03033818. [DOI] [PubMed] [Google Scholar]
- Kadiu I, Nowacek A, McMillan J, Gendelman HE. Macrophage endocytic trafficking of antiretroviral nanoparticles. Nanomedicine (Lond) 2011;6(6):975–994. doi: 10.2217/nnm.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalasinsky KS, Bosy TZ, Schmunk GA, Reiber G, Anthony RM, Furukawa Y, Guttman M, Kish SJ. Regional distribution of methamphetamine in autopsied brain of chronic human meth-amphetamine users. Forensic Sci Int. 2001;116(2–3):163–169. doi: 10.1016/s0379-0738(00)00368-6. [DOI] [PubMed] [Google Scholar]
- Klette KL, Jamerson MH, Morris-Kukoski CL, Kettle AR, Snyder JJ. Rapid simultaneous determination of amphetamine, methamphetamine, 3,4-methylenedioxyamphetamine, 3,4-methylenedioxymethamphetamine, and 3,4-methylenedioxyethylamphetamine in urine by fast gas chromatography-mass spectrometry. J Anal Toxicol. 2005;29(7):669–74. doi: 10.1093/jat/29.7.669. [DOI] [PubMed] [Google Scholar]
- Kraft-Terry SD, Engebretsen IL, Bastola DK, Fox HS, Ciborowski P, Gendelman HE. Pulsed stable isotope labeling of amino acids in cell culture uncovers the dynamic interactions between HIV-1 and the monocyte-derived macrophage. J Proteome Res. 2011;10(6):2852–2862. doi: 10.1021/pr200124j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar U, Laird D, Srikant CB, Escher E, Patel YC. Expression of the five somatostatin receptor (SSTR1-5) subtypes in rat pituitary somatotrophes: quantitative analysis by double-label immunofluorescence confocal microscopy. Endocrinology. 1997;138(10):4473–4476. doi: 10.1210/endo.138.10.5566. [DOI] [PubMed] [Google Scholar]
- Laroui H, Theiss AL, Yan Y, Dalmasso G, Nguyen HT, Sitaraman SV, Merlin D. Functional TNFα gene silencing mediated by polyethyleneimine/TNFα siRNA nanocomplexes in inflamed colon. Biomaterials. 2011;32(4):1218–1228. doi: 10.1016/j.biomaterials.2010.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douce V, Herbein G, Rohr O, Schwartz C. Molecular mechanisms of HIV-1 persistence in the monocyte-macrophage lineage. Retrovirology. 2010 doi: 10.1186/1742-4690-7-32. doi:10.1186/1742-4690-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Wang X, Chen H, Song L, Ye L, Wang SH, Wang YJ, Zhou L, Ho WZ. Methamphetamine enhances HIV infection of macrophages. Am J Pathol. 2008;172(6):1617–1624. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HA, Liu YL, Ma ZZ, Wang JC, Zhang Q. A lipid nanoparticle system improves siRNA efficacy in RPE cells and a laser-induced murine CNV model. Invest Ophthalmol Vis Sci. 2011;52(7):4789–4794. doi: 10.1167/iovs.10-5891. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Aalinkeel R, Chawda RP, Sykes DE, Nair MP. Morphine modulates chemokine gene regulation in normal human astrocytes. Clin Immunol. 2005;115(3):323–332. doi: 10.1016/j.clim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, Reynolds JL, Nair B, Sykes DE, Bonoiu A, Roy I, Yong KT, Law WC, Bergey EJ, Prasad PN, Schwartz SA. Suppression of MMP-9 expression in brain microvascular endothelial cells (BMVEC) using a gold nanorod (GNR)-siRNA nanoplex. Immunol Invest. 2011 doi: 10.3109/08820139.2011.604863. doi:10.3109/08820139.2011.604863. [DOI] [PubMed] [Google Scholar]
- Mariani SA, Vicenzi E, Poli G. Asymmetric HIV-1 co-receptor use and replication in CD4(+) T lymphocytes. J Transl Med. 2011;9(Suppl 1):S8. doi: 10.1186/1479-5876-9-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Kim JH, Krystal J, Abi-Dargham A. Imaging the neurochemistry of alcohol and substance abuse. Neuroimaging Clin N Am. 2007;17(4):539–555. doi: 10.1016/j.nic.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Martinez LR, Mihu MR, Gácser A, Santambrogio L, Nosanchuk JD. Methamphetamine enhances histoplasmosis by immunosuppression of the host. J Infect Dis. 2009;200(1):131–41. doi: 10.1086/599328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamune A, Satoh M, Hirabayashi J, Kasai K, Satoh K, Shimosegawa T. Galectin-1 induces chemokine production and proliferation in pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2006;290(4):G729–736. doi: 10.1152/ajpgi.00511.2005. [DOI] [PubMed] [Google Scholar]
- Mercier S, St. Pierre C, Pelletier I, Ouellet M, Tremblay MJ, Sato S. Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology. 2008;371(1):121–129. doi: 10.1016/j.virol.2007.09.034. [DOI] [PubMed] [Google Scholar]
- Mimi H, Ho KM, Siu YS, Wu A, Li P. Polyethyleneimine-based core-shell nanogels: a promising siRNA carrier for argininosuccinate synthetase mRNA knockdown in HeLa cells. J Control Release. 2011 doi: 10.1016/j.jconrel.2011.10.035. doi:10.1016/j.jconrel.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Morefield KM, Keane M, Felgate P, White JM, Irvine RJ. Pill content, dose and resulting plasma concentrations of 3,4-methylendioxymethamphetamine (MDMA) in recreational ‘ecstasy’ users. Addiction. 2011;106(7):1293–300. doi: 10.1111/j.1360-0443.2011.03399.x. [DOI] [PubMed] [Google Scholar]
- Nawroth I, Alsner J, Behlke MA, Besenbacher F, Overgaard J, Howard K, Kjems J. Intraperitoneal administration of chitosan/DsiRNA nanoparticles targeting TNFα prevents radiation-induced fibrosis. Radiother Oncol. 2010;97(1):143–148. doi: 10.1016/j.radonc.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Nikoobakht B, Burda C, Braun M, Hun M, El-Sayed MA. The quenching of CdSe quantum dots photoluminescence by gold nanoparticles in solution. Photochem Photobiol. 2002;75(6):591–597. doi: 10.1562/0031-8655(2002)075<0591:tqocqd>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Nowacek AS, Balkundi S, McMillan J, Roy U, Martinez-Skinner A, Mosley RL, Kanmogne G, Kabanov AV, Bronich T, Gendelman H. Analyses of nanoformulated antiretroviral drug charge, size, shape and content for uptake, drug release and antiviral activities in human monocyte-derived macrophages. J Control Release. 2011;150(2):204–211. doi: 10.1016/j.jconrel.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet M, Mercier S, Pelletier I, Bounou S, Roy J, Hirabayashi J, Sato S, Tremblay MJ. Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J Immunol. 2005;174(7):4120–4126. doi: 10.4049/jimmunol.174.7.4120. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukoc Biol. 2003;5:691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- Prasad PN. Introduction to biophotonics. Wiley; New York: 2004. [Google Scholar]
- Purohit V, Rapaka R, Shurtleff D. Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Mol Neurobiol. 2011;44(1):102–110. doi: 10.1007/s12035-011-8195-z. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Ariel A, Hershkoviz R, Hirabayashi J, Kasai KI, Lider O. Specific inhibition of T-cell adhesion to extracellular matrix and proinflammatory cytokine secretion by human recombinant galectin-1. Immunology. 1999a;97(1):100–106. doi: 10.1046/j.1365-2567.1999.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich GA, Riera CM, Sotomayor CE. Galectin-1, an alternative signal for T cell death, is increased in activated macrophages. Braz J Med Biol Res. 1999b;32(5):557–567. doi: 10.1590/s0100-879x1999000500009. [DOI] [PubMed] [Google Scholar]
- Radonić A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Co. 2004;313(4):856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- Reynolds JL, Mahajan SD, Sykes DE, Schwartz SA, Nair MP. Proteomic analyses of methamphetamine (METH)-induced differential protein expression by immature dendritic cells (IDC). Biochim Biophys Acta. 2007;1774(4):433–442. doi: 10.1016/j.bbapap.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JL, Law WC, Mahajan SD, Aalinkeel R, Nair B, Sykes DE, Mammen MJ, Yong KT, Hui R, Prasad PN, Schwartz SA. Morphine and galectin-1 modulate HIV-1 infection of human monocyte-derived macrophages. J Immunol. 2012;8:3757–3765. doi: 10.4049/jimmunol.1102276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo-Dukelow M, Kadiu I, Rozek W, Schlautman J, Persidsky Y, Ciborowski P, Kanmogne GD, Gendelman HE. HIV-1 infected monocyte-derived macrophages affect the human brain microvascular endothelial cell proteome: new insights into blood-brain barrier dysfunction for HIV-1-associated dementia. J Neuroimmunol. 2007;185(1–2):37–46. doi: 10.1016/j.jneuroim.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. T-cell subsets (Th1 versus Th2). Ann Allergy Asthma Im. 2000;85:9–21. doi: 10.1016/S1081-1206(10)62426-X. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Annunziato F, Piccinni MP, Maggi E, Romagnani S. Th1/Th2 cells, their associated molecules and role in pathophysiology. Eur Cytokine Netw. 2000;11(3):510–511. [PubMed] [Google Scholar]
- Roth MD, Whittaker KM, Choi R, Tashkin DP, Baldwin GC. Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J Leukoc Biol. 2005;78(6):1198–1203. doi: 10.1189/jlb.0405219. [DOI] [PubMed] [Google Scholar]
- Schepers RJ, Oyler JM, Joseph RE, Jr, Cone EJ, Moolchan ET, Huestis MA. Methamphetamine and amphetamine pharmacokinetics in oral fluid and plasma after controlled oral methamphetamine administration to human volunteers. Clin Chem. 2003;49(1):121–32. doi: 10.1373/49.1.121. [DOI] [PubMed] [Google Scholar]
- Secchiero P, Zella D, Curreli S, Mirandola P, Capitani S, Gallo RC, Zauli G. Engagement of CD28 modulates CXC chemokine receptor 4 surface expression in both resting and CD3-stimulated CD4+ T cells. J Immunol. 2000;164(8):4018–24. doi: 10.4049/jimmunol.164.8.4018. [DOI] [PubMed] [Google Scholar]
- Sokolov K, Follen M, Aaron J, Pavlova I, Malpica A, Lotan R, Richards-Kortum R. Real-time vital optical imaging of precancer using antiepidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63(9):1999–2004. [PubMed] [Google Scholar]
- St. Pierre C, Ouellet M, Tremblay MJ, Sato S. Galectin-1 and HIV-1 infection. Method Enzymol. 2010;480:267–294. doi: 10.1016/S0076-6879(10)80013-8. [DOI] [PubMed] [Google Scholar]
- St. Pierre C, Manya H, Ouellet M, Clark GF, Endo T, Tremblay MJ, Sato S. Host-soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J Virol. 2011;85(22):11742–11751. doi: 10.1128/JVI.05351-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Pierre C, Ouellet M, Giguère D, Ohtake R, Roy R, Sato S, Tremblay MJ. Galectin-1-specific inhibitors as a new class of compounds to treat HIV-1 infection. Antimicrob Agents Chemother. 2012;56(1):154–162. doi: 10.1128/AAC.05595-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayasu T, Ohshima T, Nishigami J, Kondo T, Nagano T. Screening and determination of methamphetamine and amphetamine in the blood, urine and stomach contents in emergency medical care and autopsy cases. J Clin Forensic Med. 1995;2(1):25–33. doi: 10.1016/1353-1131(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Tallóczy Z, Martinez J, Joset D, Ray Y, Gácser A, Toussi S, Mizushima N, Nosanchuk JD, Goldstein H, Loike J, Sulzer D, Santambrogio L. Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog. 2008 doi: 10.1371/journal.ppat.0040028. doi:10.1371/annotation/bd02ad26-a081-4c61-88c2-ebda285b8bca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton DA, Legan ZT, Dabbous MK. Methamphetamine cytotoxicity and effect on LPS-stimulated IL-1beta production by human monocytes. Toxicol In Vitro. 2009;24(3):921–927. doi: 10.1016/j.tiv.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Tokatlian T, Segura T. siRNA applications in nanomedicine. Nanomed Nanobiotechnol. 2010;22(3):305–15. doi: 10.1002/wnan.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussi SS, Joseph A, Zheng JH, Dutta M, Santambrogio L, Goldstein H. Short communication: methamphetamine treatment increases in vitro and in vivo HIV replication. AIDS Res Hum Retroviruses. 2009;25(11):1117–1121. doi: 10.1089/aid.2008.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trika S, Jeremic AM. Clustering and internalization of toxic amylin oligomers in pancreatic cells requires plasma membrane cholesterol. J Biol Chem. 2011;286(41):36086–36097. doi: 10.1074/jbc.M111.240762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wey SP, Wu HY, Chang FC, Jan TR. Methamphetamine and diazepam suppress antigen-specific cytokine expression and antibody production in ovalbumin-sensitized BALB/c mice. Toxicol Lett. 2008;181(3):157–162. doi: 10.1016/j.toxlet.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- Wu DT, Woodman SE, Weiss JM, McManus CM, D'Aversa TG, Hesselgesser J, Major EO, Nath A, Berman JW. Mechanisms of leukocyte trafficking into the CNS. J Neurovirol. 2000;6(S1):S82–85. [PubMed] [Google Scholar]