Abstract
Autophagy is a housekeeping process which helps to maintain cellular energy homeostasis and remove damaged organelles. In the heart, autophagy is an adaptive process that is activated in response to stress including acute and chronic ischemia. Given the evidence that autophagy is suppressed in energy-rich conditions, the objective of this review is to examine autophagy and cardioprotection in the setting of the metabolic syndrome. Clinical approaches that involve the induction of cardiac autophagy pharmacologically to enhance the heart’s tolerance to ischemia are also discussed.
Introduction
Autophagy is a process whereby double-membrane vesicles (autophagosomes) form in the cytoplasm in response to cellular stress and ultimately sequester damaged cytoplasmic components including ubiquitinated protein aggregates, dysfunctional mitochondria, and endoplasmic reticulum. The machinery is activated by a number of stimuli including caloric restriction, oxidative stress, and brief episodes of ischemia and reperfusion.1 It begins with nucleation of autophagy proteins that give rise to a cup-shaped phagophore which surrounds the damaged cytoplasmic material and closes to form the autophagosome. This is regulated by the autophagy proteins Atg4, Atg7, LC3, and the complex of Atg12-Atg5-Atg16L. The outer membrane of the autophagosome fuses with a lysosome to form an autophagolysosome, where the cargo is degraded by lysosomal hydrolases yielding amino acids, simple sugars, and fatty acids which are exported back into the cytosol.
With respect to the heart, there is increasing evidence that autophagy is an adaptive response to ischemic stress. Studies in other tissues indicate that autophagy is deficient in the setting of nutritional excess. This could account for the heart’s increased susceptibility to ischemic injury in humans with metabolic syndrome (MetS). MetS is characterized by a constellation of factors which include obesity, insulin resistance, and dyslipidemia and is a known risk factor for cardiovascular disease, type 2 diabetes mellitus and all-cause mortality.2 MetS is a growing pandemic affecting 20% – 30% of the population in the United States.2, 3 The presence of MetS is associated with worse outcomes after myocardial infarction, more severe post-infarction remodeling, and more rapid progression to heart failure. Because MetS affects autophagy, which is an adaptive pathway in the heart, it is important to develop a better understanding of the relationship between cardiac autophagy, ischemia/reperfusion, myocardial protection and MetS.
Energy Status
The dynamic process of autophagy is tightly regulated by the availability of nutrients and the metabolic balance of the cell.1 A circadian rhythm of autophagy has been described in liver4, 5 and heart5 that show a peak in autophagic activity towards the end of the resting phase. Calorie intake quickly suppresses autophagy,6 whereas fasting dramatically enhances it.7–9 Short-term (4 weeks) and long-term (6 months) caloric restriction (CR) have been shown to improve the heart’s tolerance to ischemia.10–12 In contrast, autophagy is suppressed with obesity13 and cardioprotection is blunted.14 Thus, in conditions of sustained nutrient availability such as MetS, attenuation of autophagy may have profound consequences on the heart’s adaptability and cardioprotection.
Ischemic Stress
Myocardial infarction is one of the leading causes of death worldwide. It results in acute necrotic and apoptotic cell death and may lead to cardiac dysfunction and acute heart failure. Likewise, cardiac remodeling that occurs after an acute MI may also lead to heart failure and death. In an effort to increase the heart’s tolerance to ischemia a number of therapeutic strategies are under investigation. One approach that holds promise is the induction of cardiac autophagy. The rationale for this is based on the growing body of evidence that shows autophagy is an adaptive response to ischemia/reperfusion (I/R) injury.9, 15–27
While there is compelling evidence that autophagy is an adaptive process during ischemia and postinfarction remodeling there are reports that autophagy may be deleterious during reperfusion11, 12 and contributes to load induced heart failure 28. With respect to the former, Matsui et al. used Beclin1 (+/−) mice and found that they had decreased I/R injury.11 However, recent work from Diwan’s group showed that Beclin1 interferes with autophagy. Beclin1 (+/−) mice actually have higher flux (thus fewer autophagosomes at steady state), and the improved flux is responsible for their resistance to I/R injury. Zhai et al. showed that rapamycin increased I/R injury in mice overexpressing constitutively active GSK-3β.12 However, Khan et al. showed that rapamycin decreased infarct size in isolated perfused mouse hearts and cardiomyocytes29 and Das et al. showed a similar effect in db/db mice30 raising the possibility that Sadoshima’s genetically modified mice developed compensatory pathways that confounded interpretation of results. More recently, Sadoshima’s group observed that rapamycin enhanced autophagy abolished the effects of GSK-3β inhibition and protects against ischemia/reperfusion injury by modulating mTOR and autophagy.
With respect to pathological remodeling secondary to pressure overload, Zhu et al. reported that overexpression of Beclin 1 in mice subjected to aortic banding, accentuated pathological remodeling, whereas in Beclin 1+/− hearts, the pressure overload-induced decline in systolic function was attenuated.31 This may be explained by the dual role of Beclin 1 in the initiation of autophagy and interference with autophagosome-lysosome fusion.32 In other studies, cardiac deletion of mTOR resulted in a smaller increase in left ventricle (LV) wall thickness and a more dilated LV chamber 2 weeks after aortic constriction.21 Ablation of Raptor, a positive regulator of mTOR, impaired adaptive hypertrophy, altered metabolic gene expression, and caused heart failure in C57BL/6J mice. It also resulted in the activation of Capase 3, loss of mitochondria, and a robust increase in autophagy.23 These contradictory findings may be explained by the fact that the mTOR pathway affects not only autophagy, but several other mechanisms including protein synthesis, cell proliferation and metabolism.33 The complexity of interpreting studies of mTOR are partially addressed by Sciarretta’s work, in which mTOR inhibition with rapamycin fails to reverse deleterious effects of a high fat diet unless autophagy is restored by upregulation of Atg7. Overall, however, the findings to date suggest that optimal activation of autophagy results in effective cardioprotection and may lead to decreased long-term detrimental effects of post-infarction remodeling.
Cardioprotection
One of the most potent and well characterized cardioprotective mechanisms is the phenomenon of ischemic preconditioning (IPC). Recently, it has been shown that autophagy plays an important role in mediating the phenomenon in variety of animal models. Gurusamy et al. demonstrated upregulation of LC3 and Beclin 1 after IPC followed by 30 min global ischemia in isolated perfused rat hearts.34 In our laboratory, we have demonstrated induction of autophagy by IPC and that autophagy is required for the cardioprotective effects of IPC in a number of models.35 We have shown that Parkin, a protein necessary for selective removal of mitochondria by autophagy, translocates to mitochondria in response to IPC and that it is required for cardioprotection.36 Yan et al. showed that autophagy plays a role in the late phase of IPC in the hibernating myocardium, although they could not detect the induction of autophagy in the early phase of IPC.37 In this model of preconditioning pigs were subjected to IPC by 2×10 min coronary occlusion or 90 min 30% coronary stenosis every 12 hours for 3 days. An increase in autophagy was reflected by elevated expression of Beclin 1 and higher LC3-II/I ratio.37 Indirect evidence that autophagy plays an important role in IPC also comes from studies performed in senescent animals. It is generally accepted that cardioprotection by IPC is impaired in these animals.38, 39 However, after two weeks of CR, a strong inducer of autophagy was found to restore the cardioprotective effects of IPC in aged rats.12 CR also improved left ventricular function after acute MI in young and old animals. CR also increased phosphorylation of AMPK, which regulates autophagy as well as other potentially cardioprotective pathways.12
There are only limited data regarding the role of autophagy in preconditioning by pharmacological agents. We showed that autophagy is necessary for sulfaphenazole-induced cardioprotection.40 Rapamycin limited acute necrotic cell death and decreased infarct size in isolated mouse heart, although autophagy was not assessed.29 In swine, chloramphenicol administration before ischemia or at reperfusion afforded profound cardioprotection and was accompanied by elevated LC3 and Beclin 1 levels.41 Other agents such as biguanides induce cardioprotection and may involve induction of autophagy through activation of AMPK. The AMPK pathway can induce autophagy through direct phosphorylation of Ulk1.42 AMPK deficiency increased myocardial I/R injury as shown in mice with cardiomyocyte specific overexpression of a mutant alpha2 subunit of AMPK.43 Maintaining AMPK phosphorylation by the expression of constitutively active protein C also reduced infarct size.44 In C57BL/6J mice subjected to I/R, Matsui et al. found that AMPK was phosphorylated during ischemia and dephosphorylated at reperfusion in parallel with activation and suppression of autophagy. Autophagy during reperfusion, however, was accompanied by upregulation of Beclin 1. In mice expressing a dominant negative form of the AMPK protein they observed that ischemia-induced autophagy was attenuated.45 This suggests that autophagy may be mediated through AMPK-dependent mechanisms during ischemia, but through AMPK-independent mechanisms upon reperfusion. The importance of AMPK has been further elucidated in experiments where AMPK was modulated by metformin, AICAR and rosiglitazone. In Langendorff-perfused rat hearts metformin pretreatment reduced infarct size and improved heart function.46 In the same model metformin and AICAR given at reperfusion decreased infarct size.47 Mice receiving the AMPK-activator rosiglitazone at reperfusion also showed reduced infarct size.48 These findings suggest that activation of AMPK pathway before or after an acute ischemic insult plays a significant role in cardioprotection and is related to the induction of autophagy. It is important to note, however, that the AMPK system modulates many metabolic pathways49 which may also contribute to its cardioprotective effects. Other autophagy-related protective pathways include sequestration of damaged mitochondria via mitophagy, production of amino acids and other metabolites for energy production, and proton pumping into lysosomes to limit cytosolic acidification.
Remodeling
Cardiac autophagy may also play an important role in the setting of chronic ischemia and adverse remodeling. Metformin and AICAR have been reported to have a salutary effect in heart failure models as evidenced by an improvement in cardiac function and a reduction in pathological remodeling.50, 51 Rats treated with rapamycin for 4 weeks after MI exhibited less cardiac dysfunction, lower levels of atrial natriuretic factor, a marker of remodeling processes, and smaller infarct size. In the border zone of the infarction autophagy was induced and apoptosis was diminished.52 Interestingly, rapamycin treatment initiated 28 days after the induction of MI also attenuated pathologic remodeling and improved heart function.52 In mice, rapamycin significantly mitigated post-infarction left ventricular dilatation and both systolic and diastolic dysfunction. When bafilomycin A1 was used to arrest autophagy these beneficial effects were lost.53 It has also been reported that disruption of autophagy by cardiac specific deletion of atg5 leads to enhanced ER stress, contractile dysfunction, heart failure and cardiac hypertrophy.54
Metabolic Syndrome
There are substantial differences between genetic and diet-induced models of MetS.55, 56 This explains, in part, the seemingly contradictory findings that have been reported regarding the relationship between autophagy and myocardial protection in this setting. For example, ob/ob diabetic mice, which bear a mutation in their leptin gene, do not develop dyslipidemia, while db/db mice with a leptin receptor deficiency do. Neither strain develops hypertension. Similarly, since leptin supplementation has been shown to induce myocardial autophagy and confer protection against ischemic injury,57, 58 it is possible that the results obtained in models with a genetically disturbed leptin system (e.g. ob/ob, db/db mice, Zucker diabetic fatty rats) are at times at variance with the findings obtained using diet-induced obesity models. In regards to diet-induced models, a wide spectrum of "high-fat diets" are used in experimental studies and there is considerable variation in duration of the dietary intervention and carbohydrate and lipid content. Thus, it is important to establish whether a diet is isocaloric or hypercaloric and be aware that some, but not all high fat diets result in cardiac dysfunction.59, 60 In general, however, it now appears that rat and swine models based on a high carbohydrate, high fat diets are the preferable models for studying MetS since they develop many of the features of MetS that characterize the syndrome in humans.61 This is not always possible however given the complexity of MetS and the need to elucidate the effects of each of its components on autophagy.
For example, to assess the role of insulin, wild type C57BL/6 mice were placed on a high fat diet (HFD) for 8 weeks to develop obesity. This resulted in increased plasma insulin levels, and decreased insulin sensitivity without changes in fasting blood glucose levels. In these animals, hepatic autophagy was decreased as reflected by lower LC3-II/I ratios and increased Akt phosphorylation levels.13 When these animals were rendered hyperglycemic by streptozotocin, however, they exhibited increased hepatic autophagy manifested by an elevation in the LC3-II/I ratio and a decrease in p62 and polyubiquitinated protein levels.13 In contrast, 16 week old ob/ob mice and C57BL/6 mice placed on a HFD for 5 months had lower levels of hepatic LC3, Beclin 1, Atg5 and Atg7 proteins. These findings are consistent with the report that Atg7 protein expression is diminished in the liver of 15 week old db/db mice62 albeit autophagy protein expression levels in C57BL/6 mice rendered diabetic by a HFD were reportedly comparable to the levels measured in non-diabetic mice fed a normal diet.63 C57BL/6 mice on a HFD for 12 weeks that caused hepatic steatosis, however, was associated with increase in hepatic autophagy.64 In white adipose tissue of diabetic db/db mice, insulin stimulation resulted in a decrease in the phosphorylation of Akt compared to lean animals; although LC3 expression was unchanged.65 In omental adipose tissue obtained from obese patients, expression of LC3, p62 and Atg5 proteins was elevated. This increase in LC3 and Atg5 was even greater in patients with insulin resistance.66 C57BL/6 mice fed a HFD for 5 months exhibited a marked decrease in Atg5, Atg7 and LC3 protein in the hypothalamus.67 Indirect evidence of decreased autophagy has also observed in pancreatic, kidney, liver and hippocampal tissue samples obtained from 19 week-old Zucker diabetic fatty rats manifested by the accumulation of large polyubiquitinated protein aggregates, but not in muscle specimens.68 These results highlight the importance of glucose, insulin and lipid homeostasis in the regulation of autophagy in different tissues. Taken together, it would appear that insulin and the duration of hyperglycemia and obesity play an interactive role in modulating autophagy. The challenge is to dissect their various contributions in order to better understand the conditions in which autophagic activation can be optimized.
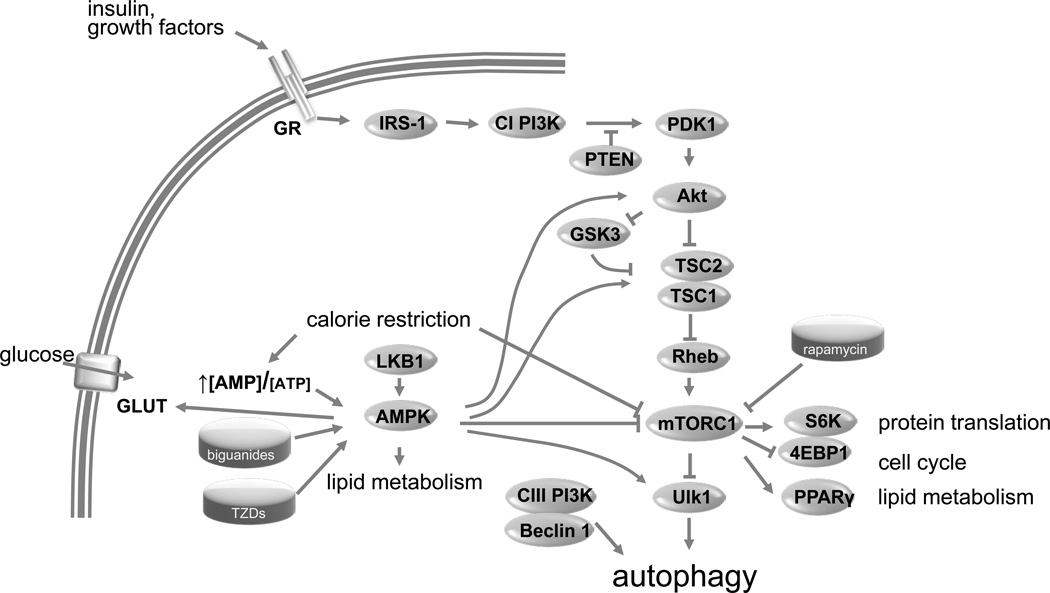
Much less is known about cardiac autophagy in the setting of MetS. Autophagy was reduced in the hearts of OVE26 diabetic mice as shown by a reduction in LC3-II. This reduction was abrogated by chronic treatment with the oral hypoglycemic agent metformin.17 In contrast, autophagy was upregulated in a C57BL/6J mouse model of pressure overload with transverse aortic constriction for 4 weeks. Whether this represents an adaptive or maladaptive process due to an accumulation of LC3-II as a result of impaired flux or an increase in autophagy per se is not known.69 It does appear however, that the mammalian target of rapamycin (mTOR)-pathway plays an important role in the regulation of autophagy in MetS (Figure 1). Insulin-induced phosphorylation of Akt, an upstream regulator of the mTOR pathway, is blunted in obese, hyperlipidemic and hypertensive rats fed a high cholesterol-high fructose diet for 15 weeks.70 Hyperactive cardiac mTOR signaling has been reported in Yucatan minipigs fed a hypercholesterolemic diet for 4 weeks that were not yet obese but had elevated total plasma cholesterol levels and LDL/HDL ratios. In these animals, mTOR signaling was assessed by measuring Akt and mTOR phosphorylation and upregulation of raptor.71 Similarly, Glazer et al. linked dyslipidemia to the dephosphorylation of AMP-activated protein kinase (AMPK), another known activator of autophagy,71 a finding consistent with a study reported in mice fed a HFD for 6 weeks.27 Although most of these upstream pathways show changes that suggest cardiac autophagy is suppressed in MetS and is related to mTOR hyperactivity, direct measurements of mTOR activation and the inducibility of autophagy and autophagic flux have yet to be performed. Nevertheless, just as MetS may suppress autophagy through direct signaling and downregulation of Atg7 through excessive mTOR signaling, advanced age is also associated with downregulation of rate-limiting autophagy proteins in aged animals and results in impaired autophagy/mitophagy. Thus, both aging and MetS may contribute to ischemia/reperfusion injury and post-infarction remodeling as a result of dysregulation of autophagy machinery.72
Figure 1. Autophagy and signaling pathways.
Insulin signaling activates the mTOR pathway via Akt and thus suppresses autophagy. The mTOR inhibitor rapamycin and its derivatives enhance autophagy. The AMPK pathway modulates the mTOR pathway via multiple mechanisms including interaction with Akt, TSC1, mTOR and Ulk1. Biguanides such as metformin and phenformin, and thiazolidinediones (TZDs), rosiglitazone and pioglitazone, enhance AMPK signaling.
Consequences of Impaired Autophagy
Insufficient autophagy as seen in many MetS-like conditions may also result in impaired quality control of organelles such as mitochondria and endoplasmic reticulum (ER) and exacerbate their dysfunction. Mitophagy, the selective removal of damaged or dysfunctional mitochondria, is required for normal metabolism and heart function.73, 74 Dysfunctional mitophagy may explain why mitochondrial dysfunction is associated with insulin resistance in various tissues including skeletal muscle, liver, fat, heart, and pancreas.75–81 Similarly markers of ER stress in the liver are increased in ob/ob mice.62 These observations are consistent with the notion that mitochondrial impairment leads to increased oxidative stress,82 higher levels of ROS,83 reduced antioxidant capacity84 and decreased fatty acid oxidation85 and that ER stress results in unfolded protein response86 and disturbed lipid metabolism.87 In summary, various conditions associated with MetS appear to interfere with autophagy and conversely its dysregulation may contribute to the adverse energy household that exists in MetS. The relative contributions of the individual components of the MetS and/or their interactions as it relates to the modulation of autophagy however remain to be determined.
Cardioprotection, Autophagy, and Metabolic Syndrome
The degree to which MetS or its components contribute to the injury after an acute and chronic ischemic insult is unknown. For the most part, however, the efficacy of cardioprotective interventions appears to be diminished in the presence of pathological conditions associated with MetS such as obesity, diabetes or dyslipidemia.14, 19, 25, 88 A majority of the studies indicate that the cardioprotection conferred by IPC is lost or diminished in diabetic dogs,22 sheep,89 rabbits,90 rats20, 91 and humans.92, 93 There is also evidence that IPC protection in diabetes can be restored by increasing the intensity of the IPC stimulus.94 Similarly, dyslipidemia has been reported to abrogate the cardioprotection afforded by IPC. In rats fed a HFD95 or high cholesterol diet,96 IPC failed to reduce infarct size. The effectiveness of late phase IPC also appears to be lost in cholesterol-fed rabbits.97 With respect to ischemic postconditioning (IPoC), the findings are less clear: in some cases dyslipidemia is associated with a blunting of IPoC,24 in others it has been reported to be effective.25 In ob/ob mice26 and in WOKW rats, cardioprotection by IPC is lost, possibly due to decreased phosphorylation of GSK3β98 or AMPK.26 In rats, cholesterol feeding interfered with cardioprotection afforded by pharmacologic conditioning by a cGMP analogue or by BNP.99 Taken together, these reports suggest a correlation between the loss of various cardioprotective interventions and the presence of various components of MetS. A cause and effect relationship however has yet to be demonstrated.
With respect to insulin resistance and diabetes, it was recently reported there was no change in basal cardiac autophagy 4 weeks after an MI induced by permanent coronary occlusion in diabetic mice.63 This suggests that modulation of autophagy might not be an effective method for salvaging diabetic myocardium. This is at variance however, with other reports that autophagic activation is beneficial. In OVE26 diabetic mice, which display reduced basal cardiac LC3-II expression, chronic metformin treatment attenuated AMPK dephosphorylation and alleviated cardiac dysfunction and severe cardiomyopathy. The beneficial effects paralleled the inhibition of mTOR signaling and suggest that activation of autophagy played an important role.17 In the same study, treatment with metformin improved cardiac function and reduced mortality in streptozotocin-induced diabetic mice. The salutary effects of metformin on cardiac function were abrogated in mice expressing dominant negative AMPKα2.17 Likewise, the administration of rosiglitazone, which augments Akt phosphorylation, reduced infarct size in Zucker diabetic fatty rats.100 Treatment with metformin, an AMPK activator, reduced infarct size in diabetic Goto-Kakizaki rats which were dependent upon Akt activity as well.101 Metformin as an acute pretreatment or given at reperfusion to diabetic db/db mice also has been shown to protect the myocardium against I/R injury.102 Other inducers of AMPK such as A-769662103 and the globular domain of adiponectin19 also proved to be cardioprotective. Resveratrol, a natural phenolic compound, has been reported to be cardioprotective in a number of animal models.104 Reportedly, it activates autophagy,105 via activation of AMPK.106 In Yorkshire miniswine on high cholesterol diet for 11 weeks, which induced glucose tolerance, dyslipidemia and hyperglycemia, resveratrol reversed the detrimental effects of 7 weeks ameroid constriction of the left circumflex coronary artery on myocardial function, while restoring glucose transporter-1 expression, decreasing PPAR gamma levels and increasing AMPK phosphorylation.107 These findings suggest that polyphenols may render the heart resistant against ischemic injuries in MetS disease-like models. Additional studies, however, are needed to confirm that resveratrol is cardioprotective and its effects are mediated by autophagy.
Therapy for MetS
Since MetS is a risk factor for heart disease and numerous studies show worse prognosis of and I/R injury in MetS-related conditions, a therapeutic goal could be to normalize preexisting metabolic abnormalities. Physical exercise and moderation of food intake, (calorie restriction or fasting) are potent inducers of autophagy in animal models.108 Should this be the case in humans, these lifestyle changes could help in moderating both the causes and consequences of MetS and render patients less susceptible to I/R injury.
Pharmacological approaches could include the administration of hypoglycemic agents, such as metformin, which have been shown to enhance to enhance autophagy. The majority of the existing data imply that activation of AMPK and its downstream targets by these biguanides renders the heart more resistant against I/R injury. Their cardioprotective effects may be related to the induction of autophagy.17, 46, 50, 101, 102 The thiazolidinedione rosiglitazone and pioglitazone which bind to PPAR gamma and improve insulin resistance have also been shown to activate autophagy, which may be mediated by the induction of the AMPK pathway. They have been shown to induce cardioprotection in non-diabetic and MetS animal models.48, 100
It is well known that the use of statins in diabetic patients with ischemic heart disease has been reported to decrease the risk of death.109 Interestingly, simvastatin was found to limit infarct size in diabetic-hypercholesterolemic rats subjected to I/R injury.110 A possible mechanism of action may be related to the activation of autophagy that has been observed in various cell types.111–113 However, evidence is lacking in intact animals or humans that cardioprotection conferred by statins is related to their effect on autophagy in the setting of MetS.
Another agent that has been shown to mimic both acute (early) and delayed (late phase) preconditioning is the nucleoside adenosine.114 It has been shown to have multiple beneficial effects during myocardial ischemia.115 We have shown that autophagy is required for the cardioprotection exhibited by a selective adenosine A1 receptor agonist,116 although its efficacy is yet to be tested in conditions mimicking MetS. Since adenosine receptor signaling mechanisms may interact with the AMPK pathway, there may be a role for the use of A1 agonists in patients with MetS, similar to that of the biguanides.
The mTOR pathway is a target for treatment of a variety of malignancies. Rapamycin analogues have been used with success in the prevention of in-stent coronary artery neointimal hyperplasia.117 However, its use in the setting of acute I/R injury has not been extensively studied, although there are a few reports that the inhibition of the mTOR pathway results in diminution of ventricular remodeling after I/R injury.21, 52, 53 Given the potential involvement of the mTOR pathway in the attenuation of cardioprotection in dyslipidemic animals,70, 71 the use of mTOR inhibitors may be useful for the treatment of adverse post-myocardial infarction remodeling in humans with MetS.
Conclusion
In summary there is persuasive evidence that some or all of the components of the metabolic syndrome adversely affect cardiac autophagy, its upstream signaling pathways, and the heart’s endogenous response to ischemia-reperfusion injury. While the mechanisms have yet to be identified, the elucidation of the role of autophagy in ischemia-reperfusion injury and MetS could lead to the development of more effective strategies to treat myocardial infarction, adverse post-infarction remodeling and heart failure in patients with dyslipidemia, insulin resistance, and obesity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dong Y, Undyala VV, Gottlieb RA, Mentzer RM, Jr, Przyklenk K. Autophagy: definition, molecular machinery, and potential role in myocardial ischemia-reperfusion injury. J Cardiovasc Pharmacol Ther. 2010;15(3):220–230. doi: 10.1177/1074248410370327. [DOI] [PubMed] [Google Scholar]
- 2.Prasad H, Ryan DA, Celzo MF, Stapleton D. Metabolic syndrome: definition and therapeutic implications. Postgrad Med. 2012;124(1):21–30. doi: 10.3810/pgm.2012.01.2514. [DOI] [PubMed] [Google Scholar]
- 3.Shamseddeen H, Getty JZ, Hamdallah IN, Ali MR. Epidemiology and economic impact of obesity and type 2 diabetes. Surg Clin North Am. 2011;91(6):1163–1172. doi: 10.1016/j.suc.2011.08.001. vii. [DOI] [PubMed] [Google Scholar]
- 4.Ma D, Panda S, Lin JD. Temporal orchestration of circadian autophagy rhythm by C/EBPbeta. EMBO J. 2011 doi: 10.1038/emboj.2011.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeifer U, Strauss P. Autophagic vacuoles in heart muscle and liver. A comparative morphometric study including circadian variations in meal-fed rats. J Mol Cell Cardiol. 1981;13(1):37–49. doi: 10.1016/0022-2828(81)90227-3. [DOI] [PubMed] [Google Scholar]
- 6.Pfeifer U. Inverted diurnal rhythm of cellular autophagy in liver cells of rats fed a single daily meal. Virchows Arch B Cell Pathol. 1972;10(1):1–3. doi: 10.1007/BF02899710. [DOI] [PubMed] [Google Scholar]
- 7.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15(3):1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, Ishida H, Fukuda K. Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol. 2011;50(1):117–127. doi: 10.1016/j.yjmcc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Wohlgemuth SE, Julian D, Akin DE, Fried J, Toscano K, Leeuwenburgh C, Dunn WA., Jr Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10(3):281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- 10.Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116(24):2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 11.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295(6):H2348–H2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinmura K, Tamaki K, Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol Cell Cardiol. 2005;39(2):285–296. doi: 10.1016/j.yjmcc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Liu HY, Han J, Cao SY, Hong T, Zhuo D, Shi J, Liu Z, Cao W. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem. 2009;284(45):31484–31492. doi: 10.1074/jbc.M109.033936. Epub 32009 Sep 31416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59(4):418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 15.Varga O, Harangi M, Olsson IA, Hansen AK. Contribution of animal models to the understanding of the metabolic syndrome: a systematic overview. Obes Rev. 2009 doi: 10.1111/j.1467-789X.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- 16.French CJ, Tarikuz Zaman A, McElroy-Yaggy KL, Neimane DK, Sobel BE. Absence of altered autophagy after myocardial ischemia in diabetic compared with nondiabetic mice. Coron Artery Dis. 2011 doi: 10.1097/MCA.0b013e32834a3a71. [DOI] [PubMed] [Google Scholar]
- 17.Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60(6):1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malik SA, Marino G, BenYounes A, Shen S, Harper F, Maiuri MC, Kroemer G. Neuroendocrine regulation of autophagy by leptin. Cell Cycle. 2011;10(17):2917–2923. doi: 10.4161/cc.10.17.17067. [DOI] [PubMed] [Google Scholar]
- 19.Yi W, Sun Y, Gao E, Wei X, Lau WB, Zheng Q, Wang Y, Yuan Y, Wang X, Tao L, Li R, Koch W, Ma XL. Reduced cardioprotective action of adiponectin in high-fat diet-induced type II diabetic mice and its underlying mechanisms. Antioxid Redox Signal. 2011;15(7):1779–1788. doi: 10.1089/ars.2010.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravingerova T, Stetka R, Volkovova K, Pancza D, Dzurba A, Ziegelhoffer A, Styk J. Acute diabetes modulates response to ischemia in isolated rat heart. Mol Cell Biochem. 2000;210(1–2):143–151. doi: 10.1023/a:1007129708262. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, Dalton ND, Peterson KL, Guan KL, Brown JH, Chen J, Sonenberg N, Condorelli G. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120(8):2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kersten JR, Toller WG, Gross ER, Pagel PS, Warltier DC. Diabetes abolishes ischemic preconditioning: role of glucose, insulin, and osmolality. Am J Physiol Heart Circ Physiol. 2000;278(4):H1218–H1224. doi: 10.1152/ajpheart.2000.278.4.H1218. [DOI] [PubMed] [Google Scholar]
- 23.Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, Zorzato F, Krishnan J, Lerch R, Hall MN, Ruegg MA, Pedrazzini T, Brink M. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123(10):1073–1082. doi: 10.1161/CIRCULATIONAHA.110.977066. [DOI] [PubMed] [Google Scholar]
- 24.Kupai K, Csonka C, Fekete V, Odendaal L, van Rooyen J, Marais de W, Csont T, Ferdinandy P. Cholesterol diet-induced hyperlipidemia impairs the cardioprotective effect of postconditioning: role of peroxynitrite. Am J Physiol Heart Circ Physiol. 2009;297(5):H1729–H1735. doi: 10.1152/ajpheart.00484.2009. [DOI] [PubMed] [Google Scholar]
- 25.Zhao H, Wang Y, Wu Y, Li X, Yang G, Ma X, Zhao R, Liu H. Hyperlipidemia does not prevent the cardioprotection by postconditioning against myocardial ischemia/reperfusion injury and the involvement of hypoxia inducible factor-1alpha upregulation. Acta Biochim Biophys Sin (Shanghai) 2009;41(9):745–753. doi: 10.1093/abbs/gmp063. [DOI] [PubMed] [Google Scholar]
- 26.Bouhidel O, Pons S, Souktani R, Zini R, Berdeaux A, Ghaleh B. Myocardial ischemic postconditioning against ischemia-reperfusion is impaired in ob/ob mice. Am J Physiol Heart Circ Physiol. 2008;295(4):H1580–H1586. doi: 10.1152/ajpheart.00379.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko HJ, Zhang Z, Jung DY, Jun JY, Ma Z, Jones KE, Chan SY, Kim JK. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes. 2009;58(11):2536–2546. doi: 10.2337/db08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Circ Res. 2008;103(12):1363–1369. doi: 10.1161/CIRCRESAHA.108.186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan S, Salloum F, Das A, Xi L, Vetrovec GW, Kukreja RC. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol. 2006;41(2):256–264. doi: 10.1016/j.yjmcc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Das A, Durrant D, Salloum FN, Koka S, Kukreja RC. Abstract 14374: Chronic Treatment with Rapamycin Attenuates Diabetes-Associated Adverse Effects and Protects Against Myocardial Ischemia/Reperfusion Injury in Type II Diabetic Mice. Circulation. 2011;124:A14374. [Google Scholar]
- 31.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117(7):1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foyil SA, Ma X, Hill JA, Dorn GW. BNIP3 Induced Autophagy Contributes to Adverse Ventricular Remodeling. J Card Fail. 2010;16(8):S35–S35. [Google Scholar]
- 33.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurusamy N, Lekli I, Gorbunov NV, Gherghiceanu M, Popescu LM, Das DK. Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein. J Cell Mol Med. 2009;13(2):373–387. doi: 10.1111/j.1582-4934.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C, Yitzhaki S, Perry CN, Liu W, Giricz Z, Mentzer RM, Jr, Gottlieb RA. Autophagy induced by ischemic preconditioning is essential for cardioprotection. J Cardiovasc Transl Res. 2010;3(4):365–373. doi: 10.1007/s12265-010-9189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning Involves Selective Mitophagy Mediated by Parkin and p62/SQSTM1. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0020975. e20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan L, Sadoshima J, Vatner DE, Vatner SF. Autophagy in ischemic preconditioning and hibernating myocardium. Autophagy. 2009;5(5):709–712. doi: 10.4161/auto.5.5.8510. [DOI] [PubMed] [Google Scholar]
- 38.Schulman D, Latchman DS, Yellon DM. Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;281(4):H1630–H1636. doi: 10.1152/ajpheart.2001.281.4.H1630. [DOI] [PubMed] [Google Scholar]
- 39.Lee TM, Su SF, Chou TF, Lee YT, Tsai CH. Loss of preconditioning by attenuated activation of myocardial ATP-sensitive potassium channels in elderly patients undergoing coronary angioplasty. Circulation. 2002;105(3):334–340. doi: 10.1161/hc0302.102572. [DOI] [PubMed] [Google Scholar]
- 40.Huang C, Liu W, Perry CN, Yitzhaki S, Lee Y, Yuan H, Tsukada YT, Hamacher-Brady A, Mentzer RM, Jr, Gottlieb RA. Autophagy and Protein Kinase C Are Required for Cardioprotection by Sulfaphenazole. Am J Physiol Heart Circ Physiol. 2010;298(2):H570–H579. doi: 10.1152/ajpheart.00716.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sala-Mercado JA, Wider J, Undyala VV, Jahania S, Yoo W, Mentzer RM, Jr, Gottlieb RA, Przyklenk K. Profound cardioprotection with chloramphenicol succinate in the swine model of myocardial ischemia-reperfusion injury. Circulation. 2010;122(11 Suppl):S179–S184. doi: 10.1161/CIRCULATIONAHA.109.928242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bach M, Larance M, James DE, Ramm G. The Serine/Threonine Kinase ULK1 is a target of multiple phosphorylation events. Biochem J. 2011 doi: 10.1042/BJ20101894. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Gao E, Tao L, Lau WB, Yuan Y, Goldstein BJ, Lopez BL, Christopher TA, Tian R, Koch W, Ma XL. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation. 2009;119(6):835–844. doi: 10.1161/CIRCULATIONAHA.108.815043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Yang L, Rezaie AR, Li J. Activated protein C protects against myocardial ischemic/reperfusion injury through AMP-activated protein kinase signaling. J Thromb Haemost. 2011;9(7):1308–1317. doi: 10.1111/j.1538-7836.2011.04331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100(6):914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 46.Solskov L, Lofgren B, Kristiansen SB, Jessen N, Pold R, Nielsen TT, Botker HE, Schmitz O, Lund S. Metformin induces cardioprotection against ischaemia/reperfusion injury in the rat heart 24 hours after administration. Basic Clin Pharmacol Toxicol. 2008;103(1):82–87. doi: 10.1111/j.1742-7843.2008.00234.x. [DOI] [PubMed] [Google Scholar]
- 47.Paiva MA, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, Mocanu MM. Transitory activation of AMPK at reperfusion protects the ischaemic-reperfused rat myocardium against infarction. Cardiovasc Drugs Ther. 2010;24(1):25–32. doi: 10.1007/s10557-010-6222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrison A, Yan X, Tong C, Li J. Acute rosiglitazone treatment is cardioprotective against ischemia-reperfusion injury by modulating AMPK, Akt, JNK signaling in nondiabetic mice. Am J Physiol Heart Circ Physiol. 2011;301(3):H895–H902. doi: 10.1152/ajpheart.00137.2011. [DOI] [PubMed] [Google Scholar]
- 49.Hardie DG. Energy sensing by the AMP-activated protein kinase and its effects on muscle metabolism. Proc Nutr Soc. 2011;70(1):92–99. doi: 10.1017/S0029665110003915. [DOI] [PubMed] [Google Scholar]
- 50.Wang XF, Zhang JY, Li L, Zhao XY, Tao HL, Zhang L. Metformin improves cardiac function in rats via activation of AMP-activated protein kinase. Clin Exp Pharmacol Physiol. 2011;38(2):94–101. doi: 10.1111/j.1440-1681.2010.05470.x. [DOI] [PubMed] [Google Scholar]
- 51.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104(3):403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, Bea F, Bekeredjian R, Schinke-Braun M, Izumo S, Katus HA, Hardt SE. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2009;54(25):2435–2446. doi: 10.1016/j.jacc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 53.Kanamori H, Takemura G, Goto K, Maruyama R, Tsujimoto A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T, Fujiwara T, Fujiwara H, Seishima M, Minatoguchi S. The role of autophagy emerging in postinfarction cardiac remodelling. Cardiovasc Res. 2011;91(2):330–339. doi: 10.1093/cvr/cvr073. [DOI] [PubMed] [Google Scholar]
- 54.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13(5):619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 55.Panchal SK, Brown L. Rodent models for metabolic syndrome research. J Biomed Biotechnol. 2011;2011 doi: 10.1155/2011/351982. 351982. Epub 352010 Dec 351930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varga O, Harangi M, Olsson IA, Hansen AK. Contribution of animal models to the understanding of the metabolic syndrome: a systematic overview. Obes Rev. 2010;11(11):792–807. doi: 10.1111/j.1467-789X.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- 57.Malik SA, Marino G, Benyounes A, Shen S, Harper F, Maiuri MC, Kroemer G. Neuroendocrine regulation of autophagy by leptin. Cell Cycle. 2011;10(17):2917–2923. doi: 10.4161/cc.10.17.17067. Epub 2011 Sep 2911. [DOI] [PubMed] [Google Scholar]
- 58.Smith CC, Dixon RA, Wynne AM, Theodorou L, Ong SG, Subrayan S, Davidson SM, Hausenloy DJ, Yellon DM. Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol. 2010;299(4):H1265–H1270. doi: 10.1152/ajpheart.00092.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galvao TF, Brown BH, Hecker PA, O'Connell KA, O'Shea KM, Sabbah HN, Rastogi S, Daneault C, Des Rosiers C, Stanley WC. High intake of saturated fat, but not polyunsaturated fat, improves survival in heart failure despite persistent mitochondrial defects. Cardiovasc Res. 2012;93(1):24–32. doi: 10.1093/cvr/cvr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okere IC, Chess DJ, McElfresh TA, Johnson J, Rennison J, Ernsberger P, Hoit BD, Chandler MP, Stanley WC. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive dahl salt-sensitive rat. Clin Exp Pharmacol Physiol. 2005;32(10):825–831. doi: 10.1111/j.1440-1681.2005.04272.x. [DOI] [PubMed] [Google Scholar]
- 61.Panchal SK, Poudyal H, Iyer A, Nazer R, Alam MA, Diwan V, Kauter K, Sernia C, Campbell F, Ward L, Gobe G, Fenning A, Brown L. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J Cardiovasc Pharmacol. 2011;57(5):611–624. doi: 10.1097/FJC.0b013e31821b1379. [DOI] [PubMed] [Google Scholar]
- 62.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11(6):467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.French CJ, Tarikuz Zaman A, McElroy-Yaggy KL, Neimane DK, Sobel BE. Absence of altered autophagy after myocardial ischemia in diabetic compared with nondiabetic mice. Coron Artery Dis. 2011;22(7):479–483. doi: 10.1097/MCA.0b013e32834a3a71. [DOI] [PubMed] [Google Scholar]
- 64.Mei S, Ni HM, Manley S, Bockus A, Kassel KM, Luyendyk JP, Copple BL, Ding WX. Differential Roles of Unsaturated and Saturated Fatty Acids on Autophagy and Apoptosis in Hepatocytes. J Pharmacol Exp Ther. 2011 doi: 10.1124/jpet.111.184341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou L, Zhang J, Fang Q, Liu M, Liu X, Jia W, Dong LQ, Liu F. Autophagy-mediated insulin receptor down-regulation contributes to endoplasmic reticulum stress-induced insulin resistance. Mol Pharmacol. 2009;76(3):596–603. doi: 10.1124/mol.109.057067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kovsan J, Bluher M, Tarnovscki T, Kloting N, Kirshtein B, Madar L, Shai I, Golan R, Harman-Boehm I, Schon MR, Greenberg AS, Elazar Z, Bashan N, Rudich A. Altered autophagy in human adipose tissues in obesity. J Clin Endocrinol Metab. 2011;96(2):E268–E277. doi: 10.1210/jc.2010-1681. [DOI] [PubMed] [Google Scholar]
- 67.Meng Q, Cai D. Defective Hypothalamic Autophagy Directs the Central Pathogenesis of Obesity via the I{kappa}B Kinase {beta} (IKK{beta})/NF-{kappa}B Pathway. J Biol Chem. 2011;286(37):32324–32332. doi: 10.1074/jbc.M111.254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaniuk NA, Kiraly M, Bates H, Vranic M, Volchuk A, Brumell JH. Ubiquitinated-protein aggregates form in pancreatic beta-cells during diabetes-induced oxidative stress and are regulated by autophagy. Diabetes. 2007;56(4):930–939. doi: 10.2337/db06-1160. [DOI] [PubMed] [Google Scholar]
- 69.Georgescu SP, Aronovitz MJ, Iovanna JL, Patten RD, Kyriakis JM, Goruppi S. Decreased Metalloprotease 9 Induction, Cardiac Fibrosis and Higher Autophagy after Pressure Overload in Mice Lacking the Transcriptional Regulator p8. Am J Physiol Cell Physiol. 2011 doi: 10.1152/ajpcell.00211.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng JY, Huang JP, Lu LS, Hung LM. Impairment of cardiac insulin signaling and myocardial contractile performance in high-cholesterol/fructose-fed rats. Am J Physiol Heart Circ Physiol. 2007;293(2):H978–H987. doi: 10.1152/ajpheart.01002.2006. [DOI] [PubMed] [Google Scholar]
- 71.Glazer HP, Osipov RM, Clements RT, Sellke FW, Bianchi C. Hypercholesterolemia is associated with hyperactive cardiac mTORC1 and mTORC2 signaling. Cell Cycle. 2009;8(11):1738–1746. doi: 10.4161/cc.8.11.8619. Epub 2009 Jun 1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gottlieb RA, Mentzer RM, Jr, Linton PJ. Impaired mitophagy at the heart of injury. Autophagy. 2011;7(12) doi: 10.4161/auto.7.12.18175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gottlieb RA, Gustafsson AB. Mitochondrial turnover in the heart. Biochim Biophys Acta. 2011;1813(7):1295–1301. doi: 10.1016/j.bbamcr.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gottlieb RA, Finley KD, Mentzer RM., Jr Cardioprotection requires taking out the trash. Basic Res Cardiol. 2009;104(2):169–180. doi: 10.1007/s00395-009-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 76.Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology. 2006;147(6):2643–2649. doi: 10.1210/en.2006-0057. [DOI] [PubMed] [Google Scholar]
- 77.Hojlund K, Mogensen M, Sahlin K, Beck-Nielsen H. Mitochondrial dysfunction in type 2 diabetes and obesity. Endocrinol Metab Clin North Am. 2008;37(3):713–731. doi: 10.1016/j.ecl.2008.06.006. x. [DOI] [PubMed] [Google Scholar]
- 78.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116(4):434–448. doi: 10.1161/CIRCULATIONAHA.107.702795. [DOI] [PubMed] [Google Scholar]
- 79.Abdul-Ghani MA, DeFronzo RA. Mitochondrial dysfunction, insulin resistance, and type 2 diabetes mellitus. Curr Diab Rep. 2008;8(3):173–178. doi: 10.1007/s11892-008-0030-1. [DOI] [PubMed] [Google Scholar]
- 80.De Pauw A, Tejerina S, Raes M, Keijer J, Arnould T. Mitochondrial (dys)function in adipocyte (de)differentiation and systemic metabolic alterations. Am J Pathol. 2009;175(3):927–939. doi: 10.2353/ajpath.2009.081155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Finocchietto PV, Holod S, Barreyro F, Peralta JG, Alippe Y, Giovambattista A, Carreras MC, Poderoso JJ. Defective Leptin-AMP-Dependent Kinase Pathway Induces Nitric Oxide Release and Contributes to Mitochondrial Dysfunction and Obesity in ob/ob Mice. Antioxid Redox Signal. 2011;15(9):2395–2406. doi: 10.1089/ars.2010.3857. [DOI] [PubMed] [Google Scholar]
- 82.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hansel B, Giral P, Nobecourt E, Chantepie S, Bruckert E, Chapman MJ, Kontush A. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. 2004;89(10):4963–4971. doi: 10.1210/jc.2004-0305. [DOI] [PubMed] [Google Scholar]
- 84.Karamouzis I, Pervanidou P, Berardelli R, Iliadis S, Papassotiriou I, Karamouzis M, Chrousos GP, Kanaka-Gantenbein C. Enhanced oxidative stress and platelet activation combined with reduced antioxidant capacity in obese prepubertal and adolescent girls with full or partial metabolic syndrome. Horm Metab Res. 2011;43(9):607–613. doi: 10.1055/s-0031-1284355. [DOI] [PubMed] [Google Scholar]
- 85.Flachs P, Ruhl R, Hensler M, Janovska P, Zouhar P, Kus V, Macek Jilkova Z, Papp E, Kuda O, Svobodova M, Rossmeisl M, Tsenov G, Mohamed-Ali V, Kopecky J. Synergistic induction of lipid catabolism and anti-inflammatory lipids in white fat of dietary obese mice in response to calorie restriction and n-3 fatty acids. Diabetologia. 2011;54(10):2626–2638. doi: 10.1007/s00125-011-2233-2. [DOI] [PubMed] [Google Scholar]
- 86.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 87.Iqbal J, Dai K, Seimon T, Jungreis R, Oyadomari M, Kuriakose G, Ron D, Tabas I, Hussain MM. IRE1beta inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 2008;7(5):445–455. doi: 10.1016/j.cmet.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paulson DJ. The diabetic heart is more sensitive to ischemic injury. Cardiovasc Res. 1997;34(1):104–112. doi: 10.1016/s0008-6363(97)00018-7. [DOI] [PubMed] [Google Scholar]
- 89.del Valle HF, Lascano EC, Negroni JA, Crottogini AJ. Absence of ischemic preconditioning protection in diabetic sheep hearts: role of sarcolemmal KATP channel dysfunction. Mol Cell Biochem. 2003;249(1–2):21–30. [PubMed] [Google Scholar]
- 90.Nieszner E, Posa I, Kocsis E, Pogatsa G, Preda I, Koltai MZ. Influence of diabetic state and that of different sulfonylureas on the size of myocardial infarction with and without ischemic preconditioning in rabbits. Exp Clin Endocrinol Diabetes. 2002;110(5):212–218. doi: 10.1055/s-2002-33069. [DOI] [PubMed] [Google Scholar]
- 91.Tosaki A, Pali T, Droy-Lefaix MT. Effects of Ginkgo biloba extract and preconditioning on the diabetic rat myocardium. Diabetologia. 1996;39(11):1255–1262. doi: 10.1007/s001250050567. [DOI] [PubMed] [Google Scholar]
- 92.Sivaraman V, Hausenloy DJ, Wynne AM, Yellon DM. Preconditioning the diabetic human myocardium. J Cell Mol Med. 2010;14(6B):1740–1746. doi: 10.1111/j.1582-4934.2009.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ravingerova T, Stetka R, Pancza D, Ulicna O, Ziegelhoffer A, Styk J. Susceptibility to ischemia-induced arrhythmias and the effect of preconditioning in the diabetic rat heart. Physiol Res. 2000;49(5):607–616. [PubMed] [Google Scholar]
- 94.Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM. Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes. 2005;54(8):2360–2364. doi: 10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- 95.Yadav HN, Singh M, Sharma PL. Modulation of the cardioprotective effect of ischemic preconditioning in hyperlipidaemic rat heart. Eur J Pharmacol. 2010;643(1):78–83. doi: 10.1016/j.ejphar.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 96.Giricz Z, Lalu MM, Csonka C, Bencsik P, Schulz R, Ferdinandy P. Hyperlipidemia attenuates the infarct size-limiting effect of ischemic preconditioning: role of matrix metalloproteinase-2 inhibition. J Pharmacol Exp Ther. 2006;316(1):154–161. doi: 10.1124/jpet.105.091140. [DOI] [PubMed] [Google Scholar]
- 97.Tang XL, Takano H, Xuan YT, Sato H, Kodani E, Dawn B, Zhu Y, Shirk G, Wu WJ, Bolli R. Hypercholesterolemia abrogates late preconditioning via a tetrahydrobiopterin-dependent mechanism in conscious rabbits. Circulation. 2005;112(14):2149–2156. doi: 10.1161/CIRCULATIONAHA.105.566190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wagner C, Kloeting I, Strasser RH, Weinbrenner C. Cardioprotection by postconditioning is lost in WOKW rats with metabolic syndrome: role of glycogen synthase kinase 3beta. J Cardiovasc Pharmacol. 2008;52(5):430–437. doi: 10.1097/FJC.0b013e31818c12a7. [DOI] [PubMed] [Google Scholar]
- 99.Giricz Z, Gorbe A, Pipis J, Burley DS, Ferdinandy P, Baxter GF. Hyperlipidaemia induced by a high-cholesterol diet leads to the deterioration of guanosine-3',5'-cyclic monophosphate/protein kinase G-dependent cardioprotection in rats. Br J Pharmacol. 2009;158(6):1495–1502. doi: 10.1111/j.1476-5381.2009.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yue TL, Bao W, Gu JL, Cui J, Tao L, Ma XL, Ohlstein EH, Jucker BM. Rosiglitazone treatment in Zucker diabetic Fatty rats is associated with ameliorated cardiac insulin resistance and protection from ischemia/reperfusion-induced myocardial injury. Diabetes. 2005;54(2):554–562. doi: 10.2337/diabetes.54.2.554. [DOI] [PubMed] [Google Scholar]
- 101.Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103(3):274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- 102.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57(3):696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 103.Paiva MA, Rutter-Locher Z, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, Mocanu MM. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;300(6):H2123–H2134. doi: 10.1152/ajpheart.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu JM, Hsieh TC. Resveratrol: a cardioprotective substance. Ann N Y Acad Sci. 2011;1215:16–21. doi: 10.1111/j.1749-6632.2010.05854.x. [DOI] [PubMed] [Google Scholar]
- 105.Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease. Neurosignals. 2011;19(3):163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gurusamy N, Lekli I, Mukherjee S, Ray D, Ahsan MK, Gherghiceanu M, Popescu LM, Das DK. Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc Res. 2010;86(1):103–112. doi: 10.1093/cvr/cvp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Robich MP, Osipov RM, Chu LM, Han Y, Feng J, Nezafat R, Clements RT, Manning WJ, Sellke FW. Resveratrol modifies risk factors for coronary artery disease in swine with metabolic syndrome and myocardial ischemia. Eur J Pharmacol. 2011;664(1–3):45–53. doi: 10.1016/j.ejphar.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ogura Y, Iemitsu M, Naito H, Kakigi R, Kakehashi C, Maeda S, Akema T. Single bout of running exercise changes LC3-II expression in rat cardiac muscle. Biochem Biophys Res Commun. 2011 doi: 10.1016/j.bbrc.2011.09.152. [DOI] [PubMed] [Google Scholar]
- 109.Hippisley-Cox J, Coupland C. Effect of statins on the mortality of patients with ischaemic heart disease: population based cohort study with nested case-control analysis. Heart. 2006;92(6):752–758. doi: 10.1136/hrt.2005.061523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Adameova A, Harcarova A, Matejikova J, Pancza D, Kuzelova M, Carnicka S, Svec P, Bartekova M, Styk J, Ravingerova T. Simvastatin alleviates myocardial contractile dysfunction and lethal ischemic injury in rat heart independent of cholesterol-lowering effects. Physiol Res. 2009;58(3):449–454. doi: 10.33549/physiolres.931751. [DOI] [PubMed] [Google Scholar]
- 111.Araki M, Motojima K. Hydrophobic statins induce autophagy in cultured human rhabdomyosarcoma cells. Biochem Biophys Res Commun. 2008;367(2):462–467. doi: 10.1016/j.bbrc.2007.12.166. [DOI] [PubMed] [Google Scholar]
- 112.Cheng J, Ohsaki Y, Tauchi-Sato K, Fujita A, Fujimoto T. Cholesterol depletion induces autophagy. Biochem Biophys Res Commun. 2006;351(1):246–252. doi: 10.1016/j.bbrc.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 113.Ghavami S, Mutawe MM, Sharma P, Yeganeh B, McNeill KD, Klonisch T, Unruh H, Kashani HH, Schaafsma D, Los M, Halayko AJ. Mevalonate cascade regulation of airway mesenchymal cell autophagy and apoptosis: a dual role for p53. PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0016523. e16523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol. 2008;103(3):203–215. doi: 10.1007/s00395-007-0687-7. [DOI] [PubMed] [Google Scholar]
- 115.McIntosh VJ, Lasley RD. Adenosine Receptor-Mediated Cardioprotection: Are All 4 Subtypes Required or Redundant? J Cardiovasc Pharmacol Ther. 2011 doi: 10.1177/1074248410396877. [DOI] [PubMed] [Google Scholar]
- 116.Yitzhaki S, Huang C, Liu W, Lee Y, Gustafsson AB, Mentzer RM, Jr, Gottlieb RA. Autophagy is required for preconditioning by the adenosine A1 receptor-selective agonist CCPA. Basic Res Cardiol. 2009;104(2):157–167. doi: 10.1007/s00395-009-0006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gabardi S, Baroletti SA. Everolimus: a proliferation signal inhibitor with clinical applications in organ transplantation, oncology, and cardiology. Pharmacotherapy. 2010;30(10):1044–1056. doi: 10.1592/phco.30.10.1044. [DOI] [PubMed] [Google Scholar]