Summary
Background
The development of inhibitory antibodies, referred to as inhibitors, against exogenous FVIII in a significant subset of patients with hemophilia A remains a persistent challenge to the efficacy of protein replacement therapy. Our previous studies using the transgenic approach provided proof-of-principle that platelet-specific expression could be successful for treating hemophilia A in the presence of inhibitory antibodies.
Objective
To investigate a clinically translatable approach for platelet gene therapy of hemophilia A with pre-existing inhibitors.
Methods
Platelet-FVIII expression in pre-immunized FVIIInull mice was introduced by transplantation of lentivirus-transduced bone marrow or enriched hematopoietic stem cells. FVIII expression was determined by a chromogenic assay. The transgene copy number per cell was quantitated by real time PCR. Inhibitor titer was measured by Bethesda assay. Phenotypic correction was assessed by the tail clipping assay and an electrolytic-induced venous injury model. Integration sites were analyzed by LAM-PCR.
Results
Therapeutic levels of platelet-FVIII expression were sustained long-term without evoking an anti-FVIII memory response in the transduced pre-immunized recipients. The tail clip survival test and the electrolytic injury model confirmed that hemostasis was improved in the treated animals. Sequential bone marrow transplants showed sustained platelet-FVIII expression resulting in phenotypic correction in pre-immunized secondary and tertiary recipients.
Conclusions
Lentivirus-mediated platelet-specific gene transfer improves hemostasis in hemophilic A mice with pre-existing inhibitors, indicating that this approach may be a promising strategy for gene therapy of hemophilia A even in the high-risk setting of pre-existing inhibitory antibodies.
Keywords: Hemophilia A, Inhibitor, FVIII, Gene therapy, Platelet
Introduction
Hemophilia A is an X-linked genetic bleeding disorder, resulting from a deficiency of factor VIII (FVIII). The current standard of care relies on protein replacement therapy by infusion of plasma-derived or recombinant FVIII. However, the development of inhibitory antibodies against exogenous FVIII in a significant subset of patients remains a persistent challenge to the efficacy of protein replacement therapy.[1]
Hemophilic disease is considered a prime candidate for gene therapy, as even low levels of the missing factor would have a significant beneficial therapeutic effect. Although the precise site(s) of FVIII biosynthesis are not fully characterized,[2,3] a wide spectrum of cell types and tissues can produce fully bioactive FVIII in vitro[4-6] and in vivo.[7-9] Genetic therapy of FVIII deficiency has been consistently successful in rodent and canine models of hemophilia A, resulting in long-term expression of therapeutic FVIII levels. However, these strategies have yet to be proven beneficial in clinical trials due to low expression levels or generation of immunity to the expressed protein or vectors.[10,11] The initial data from the first clinical trials of liver-specific codon-optimized FIX expression in hemophilia B gene therapy in humans are encouraging.[12] The same strategy has been applied to FVIII gene therapy in an experimental model, where impressive efficacy was observed after gene therapy of mice with hemophilia A.[9]
Restricted expression of recombinant B-domain-deleted FVIII in megakaryocytes and platelets, controlled by platelet-specific promoters [Glycoprotein (GP) Ib, GPIIb, or platelet factor 4 (PF4)], results in phenotypic correction of hemophilia using a transgenic approach.[13-15] Importantly, the storage and sequestration of FVIII in platelets appears not only sufficient to selectively deliver FVIII to sites of hemostatic activation, but it also protects FVIII from function-blocking inhibitory antibodies. As a result, platelet-targeted FVIII (2bF8) synthesis retains efficacy even in the presence of excessive inhibitor levels.[14] To apply this novel approach to realistic gene therapy model, we used an HIV-1-based lentiviral vector (LV) to deliver the 2bF8 expression cassette into hematopoietic stem cells (HSCs), resulting in expression of FVIII in platelets. We have demonstrated that 2bF8 lentiviral vectors can introduce therapeutic levels of platelet-FVIII expression in hemophilia A mice by performing bone marrow (BM) transduction and syngeneic transplantation.[16] This approach has not been studied in the clinical setting of pre-existing FVIII inhibitory antibodies to determine whether sufficiently high transduction efficiencies can practically be achieved to ensure transgene delivery to rare HSCs with long-term repopulation potential in immunologically primed individuals, and whether ectopic expression of FVIII would interfere with the ability of the transduced HSCs to competitively populate the patient’s hematopoietic system without the need for severe myelosuppression. Issues of safety that must be addressed include potential activation of immune responses by lentivirus-mediated platelet-expressed transgene products, and insertional mutagenesis.
Materials and methods
Mice
FVIIInull mice used in this study were in a 129/SV x C57BL/6 mixed genetic background, and were generated by targeted disruption of exon 17 of the FVIII gene.[17] Isoflurane or 2.5% avertin were used for anesthesia. All mice were kept in pathogen-free microisolator cages at the animal facilities operated by the Medical College of Wisconsin. Animal studies were performed according to a protocol approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. For the inhibitor model, six-to ten-week old FVIIInull mice were immunized by weekly injections of recombinant human B-domain deleted FVIII (rhFVIII, Refacto, Wyeth Pharmaceuticals, Collegeville, PA, USA) at 50 IU kg−1 via the retro-orbital venous plexus. One week after the fourth immunization, blood samples were collected for Bethesda assay to determine the titer of inhibitors.
Vector construction, production and purification
The 2bF8 lentiviral vector harboring human B-domain deleted FVIII under control of the platelet-specific integrin αIIb promoter was constructed as described previously.[16] Recombinant lentivirus production and purification were performed as described in our previous studies. [16]
Ex vivo transduction
BM cells were collected from femurs and tibia of pre-immunized FVIIInull (inhibitor model) or non-immunized (non-inhibitor model) donor mice. BM mononuclear cells (BMMCs) were isolated using Fico/Lite-LM (mouse) (Atlanta Biologicals, Lawrenceville, GA, USA) as previously described.[14] Sca-1+ HSCs were selected using Anti-Sca-1 MicroBead Kit (Miltenyi Biotec Inc., Auburn, CA, USA) following instructions provided by the manufacturer. Sca-1+ selected HSCs or unselected BMMCs were transduced with 2bF8 LV as described in our previous report.[16] Briefly, cells were pre-stimulated in complete X-VIVO 10 media with cytokine cocktail for 48 hours and then transduced with 2bF8 LV at MOI of approximately ten. After transduction, cells were harvested and resuspended in pre-stimulation media for transplantation.
Bone marrow transplantation (BMT)
Eleven to twelve week old, pre-immunized FVIIInull recipient mice or non-immunized FVIIInull controls were conditioned for cellular transplantation with a single dose of 1100, 660, or 440 cGy total body irradiation using a cesium irradiator (Gammacell 40 Exactor, Best Theratronics Ltd, Ottawa, Canada). Busulfan (DSM Pharmacenticals, Inc. Greenville, NA, USA) was administrated intravenously either at 25 mg kg−1 or 12.5 mg kg−1 per day on days −2 and −1. Anti-(murine)-thymocyte globulin (ATG) (Fitzgerald, Acton, MA, USA) was administrated intravenously on day −2 at a dose of 10 mg kg−1. Twenty-four hours after irradiation, 10 × 106 transduced BMMCs (used only for lethally irradiated recipients) or 0.5 × 106 transduced Sca-1+ cells (transplanted into both lethal and sub-lethally irradiated, or busulfan conditioned recipients) were infused in a volume of 400 μL per mouse by retro-orbital vein injection. Untransduced cells were transplanted in controls. Mice were analyzed beginning 3 weeks after transplantation. At 6 months or later after transplantation, some animals were euthanized, BM cells were harvested, and sequential transplantation was carried out by transferring BMMCs into irradiated pre-immunized FVIIInull secondary, and in some cases from secondary into tertiary recipients. Blood samples were collected monthly by retro-orbital bleeds and plasma and platelets were isolated as previously described.[18]
PCR and qPCR analysis
Genomic DNA was purified from peripheral white blood cells (WBC) using QIAamp DNA Blood Mini Kit (QIAGEN Inc., Valencia, CA, USA) and amplified using PCR GoTaq Master Mix (Promega, Madison, WI, USA). A 650 bp fragment from the 2bF8 cassette was amplified as described in our previous report.[16] To confirm the FVIIInull background, primers amplifying a 150 bp fragment of the disrupted exon 17 of murine FVIII were used.[17] Another set of primer, 5′- GCA AGG GAA GTG ATA TCA CT-3′ and 5′- TCC TGT ACT GAC ACT TGT CTC-3′, were used to amplify a 233 bp fragment from the undisrupted wild type (WT) FVIII gene as a control to confirm the exon 17 disruption. DNA from a known 2bF8 transgenic mouse [14] served as a 2bF8 positive control and water was used as a negative control.
The average copy number of 2bF8 proviral DNA per cell in recipients was determined by quantitative real-time (qPCR) as described in our previous report.[18] Briefly, WBC-derived genomic DNA was analyzed for quantification of the 2bF8 expression cassette sequence, with normalization to the murine ApoB gene using iQ Supermix (BioRad, Hercules, CA, USA). DNA from 2bF8 transgenic, FVIIInull, and WT mice were used as controls.
FVIII assays
Functional FVIII activity (FVIII:C) assays of platelet lysates and plasma were performed to quantitate FVIII expression levels. Platelet pellets were lysed in 50 μL of assay buffer containing 0.5% CHAPS (the zwitterionic detergent, 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate) (MP Biomedicals, Solon, OH, USA) per 1 × 107 platelets. Dilutions of platelet lysates or plasma were assayed using a modified FVIII chromogenic assay as previously described.[14,18] Recombinant human B-domain deleted FVIII (rhFVIII, ReFacto) was used as the standard.
The titers of anti-FVIII inhibitory antibody in plasma at different time points were determined by a modified Bethesda assay as previously described.[14]
Assessment of phenotypic correction
To evaluate whether 2bF8 LV-mediated gene transfer can rescue hemostasis in hemophilia A mice with pre-existing anti-FVIII immunity, two in vivo assays were used; tail clip survival tests and an electrolytic venous injury model. For tail clip survival tests, a small wound was inflicted to the tail of anesthetized animals as previously described [14] and survival at 24 hours was used as an indicator of phenotypic correction. For the electrolytically induced venous injury model, femoral veins were exposed through an incision, and the procedures were performed as previously reported.[19] Briefly, 100 μL of 1 mM Rhodamine 6G (Sigma) was injected through the jugular vein to systemically label platelets. Fibrin was detected by infusion of an anti-fibrin monoclonal antibody (produced by our core laboratory) directly conjugated with Alexa 647. Thrombosis was induced in exposed femoral veins by placing a 70-μm blunt-end needle against the outer surface of the femoral vein for 30 seconds, with application of 1.5 volts of positive direct current. Fluorophore accumulation at the site of injury was recorded by time-lapse video for analysis.
Analysis of LV integration sites
2bF8 LV integration sites were analyzed by LAM-PCR to identify clones of 2bF8 lentiviral-mediated genetically modified HSCs in FVIIInull mice that received 2bF8 LV-transduced cells as reported.[20] Briefly, linear PCR was performed by repeated primer extension from a single biotinylated oligonucleotide positioned near the end of the 3′ LTR to allow extension into the flanking genomic DNA, followed by enrichment of the linear DNA products by capture with streptavidin-coated magnetic beads. Second-strand DNA was generated by random hexanucleotide priming using Klenow (exo-) (New England BioLabs), then digested with the restriction enzyme HpyCH4IV (New England BioLabs) to cleave the amplified gemomic DNA flanking LV insertion sites. A double stranded linker with compatible overhangs and containing PCR primer-binding sites was then ligated to the digested DNA. Finally, two rounds of nested exponential PCR amplifications were used to amplify the DNA that flanks the 5′ end of the lentiviral insertion site. The products were then cloned and sequenced to determine chromosomal vector integration sites.
Statistical analysis
The significance of differences between groups of mice was evaluated by 2-tailed Student t-test, except the data for survival rates between different groups of recipients, which was evaluated by Fisher’s Exact Test. A value of P < 0.05 was considered statistically significant.
Results
Introduction of therapeutic levels of platelet-FVIII expression in hemophilia A mice with inhibitors using lentiviral gene transfer
We engineered a lentiviral vector (2bF8 LV) that encodes human B-domain deleted FVIII under control of the platelet-specific αIIb promoter. Our previous studies demonstrated that 2bF8 LV can transduce HSCs.[16] In the present study, we wanted to explore whether pre-existing anti-FVIII immunity would alter efficacy of 2bF8 expression cassette transfer into hematopoietic cells by lentiviral-based transduction and whether engraftment of transduced cells following syngeneic transplantation is affected by immune status. As depicted in Fig. 1A, BMMCs were isolated from pre-immunized FVIIInull donors and transduced with 2bF8 LV, then transplanted into lethally irradiated (1100 cGy) pre-immunized littermates. Parallel experiments were performed using non-immunized donor and recipient mice. After at least three weeks of BM reconstitution, recipients were analyzed. Representative PCR analysis results are shown in Fig. 1B. The 2bF8 expression cassette was detected in all recipients [including primary (1°), secondary (2°), and tertiary (3°) recipients] that received 2bF8 LV-transduced BM cells, indicating viable long-term engraftment of pre-immunized BM cells genetically modified with the 2bF8 lentiviral transfer vector.
Fig. 1. PCR detection of 2bF8 expression cassette in the treated animals.
(A) The diagram depicts the overall experimental design. (B) PCR detection of 2bF8 transgene. DNA was purified from peripheral white blood cells after at least 3 weeks of bone marrow reconstitution. One set of primers was used to amplify a 650 bp fragment of 2bF8 transgene. Another set of primers was used to amplify a 233 bp fragment of the wild type mouse FVIII gene. A third set of primers was used to amplify a 150 bp fragment of exon 17-disrupted mouse FVIII gene (FVIIInull). Lane 1: DNA marker; Lane 2: FVIIInull; Lanes 3 & 4: 2bF8 LV-transduced pre-immunized primary recipients (1°); Lanes 5 & 6: 2bF8 LV-transduced pre-immunized secondary recipients (2°); Lanes 7 & 8: 2bF8 LV-transduced pre-immunized tertiary recipients (3°); Lane 9: dH2O; Lane 10: 2bF8 transgenic mouse; Lane 11: Wild type. Shown is one representative experiment that was performed three times.
The average copy number of 2bF8 expression cassette per cell in the treated animals was determined by qPCR analysis of DNA isolated from white blood cells. 2bF8 proviral DNA was measurable in all 2bF8 LV-transduced recipients. The average copy number of 2bF8 cassette per cell in the inhibitor model (0.20 ± 0.06, n = 10) was not significantly different from the non-inhibitor model (0.16 ± 0.05, n = 4) (P = 0.25) (Fig. 2A). To investigate whether hematopoietic cells that were genetically modified by 2bF8 LV would have an advantage or disadvantage in BM reconstitution or in self-renewal compared to untransduced cells, we monitored the copy number of 2bF8 proviral DNA in primary recipients up to 8 months after transplantation. There was no significant augmentation or reduction in the average copy number of 2bF8 proviral DNA per cell during this observation period (Fig. 2B), indicating that 2bF8 LV-mediated integration does not cause selective clonal expansion nor reduced clonal survival.
Fig. 2. Analysis of 2bF8 LV-transduced recipients.
FVIIInull mice with rhFVIII pre-immunization were conditioned with 1100 cGy TBI and received pre-immunized 2bF8 LV-transduced BMMCs (inhibitor model). Non-immunized FVIIInull mice were conditioned in parallel and received non-immunized 2bF8 LV-transduced BMMCs (non-inhibitor model). After at least 3 weeks of BM reconstitution, animals were analyzed. (A) Quantitative PCR determined the average copy number of 2bF8 proviral DNA per cell in 2bF8 LV-transduced recipients. DNA was purified from peripheral white blood cells (WBC) and analyzed for the 2bF8 transgene. Bars represent mean ± SD. For individual mice analyzed more than once over the study, the average copy number was calculated. (B) Average 2bF8 proviral DNA copy number per cell in WBC was measured at selected time points by real-time PCR. Bars represent mean ± SD. (C) Quantitative evaluation of FVIII:C levels in recipients’ platelets. FVIII:C was quantitated by chromogenic assay on platelet lysates. For individual mice analyzed more than once over the study, the average platelet FVIII:C was calculated. Bars represent mean ± SE. (D) Tail clip survival test assessing phenotypic correction of hemophilia A mice. The tail clip survival test was performed at least 3 months after BMT and the percentage of animals that survived beyond 24 hours was determined. Data shown are summarized from three BMT trials.
To measure FVIII expression in platelet lysates and plasma of 2bF8 LV-transduced recipients, we used a chromogenic FVIII:C assay. As shown in Fig. 2C, the average levels of platelet-FVIII:C in the transduced mice with pre-existing anti-FVIII inhibitory antibodies (inhibitor model) were 1.56 ± 0.56 mU per 108 platelets (ranging from 0.36 to 6.18 mU per 108 platelets over the study period, n = 10), which was not significantly different from a parallel non-inhibitor model (1.46 ± 0.43 per mU 108 platelets, ranging from 0.57 to 2.63 mU per 108 platelets, n = 4) (P = 0.92). As expected, FVIII:C was not detected in untransduced controls. FVIII:C could not be detected in the plasma of FVIIInull mice that received 2bF8 LV-transduced BM cells in the non-inhibitor model, which is consistent with our previous findings.[16] Since anti-FVIII antibodies inhibit the chromogenic assay, plasma FVIII:C could not be measured in the inhibitor model.
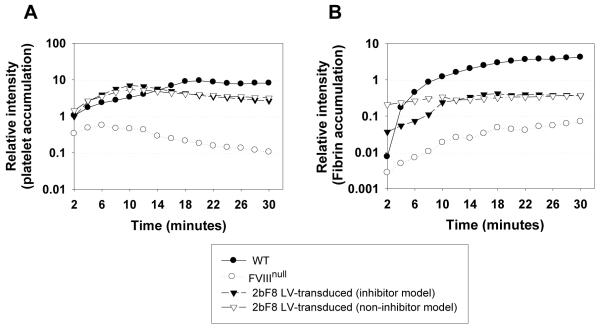
To assess whether lentivirus-mediated BM transduction and syngeneic transplantation could rescue the bleeding diathesis in hemophilia A mice with pre-existing anti-FVIII inhibitory antibodies, two in vivo injury models were used including the tail clip survival test and an electrolytic venous injury model. Nine of ten inhibitor model 2bF8 LV-transduced mice survived tail clipping with inhibitor titers of 40 – 600 BU mL−1 (Fig. 2D). The one animal that did not survive tail clipping had a relatively low level of platelet-FVIII (0.48 mU per 108 platelets) combined with the highest inhibitor titer (600 BU mL−1) in this group of mice. In contrast, all recipients survived tail clipping in the non-inhibitor model, while none survived in the untransduced control group. The electrolytic injury-mediated venous thrombosis model was used to further assess whether hemostasis is improved in 2bF8 LV-transduced mice, using the development of site-localized intravascular thrombosis as a measure of hemostasis.[19] Clot formation and growth was reflected by fluorophore accumulation representing both labeled platelets and fibrin localized to the site of injury. Platelet accumulation in 2bF8 LV-transduced animals, in both the inhibitor and non-inhibitor models, increased progressively with a peak at about 10 minutes after injury, earlier than in WT mice which peaked at about 20 minutes (Fig. 3A). Fibrin accumulation in the inhibitor model also reached its maximum at about 10 minutes (Fig. 3B). Interestingly, in both the inhibitor and non-inhibitor models significant (near peak) fibrin accumulation had already occurred 2 minutes after the injury, when the first images can be recorded. Notably, less fibrin had been deposited in WT mice at 2 minutes, implying that platelets containing FVIII may initiate hemostasis faster and more efficiently at sites of injury compared to plasma FVIII. Based on these results we conclude that hemostasis was improved in FVIIInull recipients that received 2bF8 LV-transduced BM cells, and importantly in recipients with pre-existing anti-FVIII immunity.
Fig. 3. Electrolytically-induced femoral vein injury model.
The femoral vein was exposed and a vessel injury was induced by placing a blunt-end needle against the outer surface of the vein with application of positive direct current. Platelets were labeled in vivo using Rhodamine 6G and fibrin was labeled by anti-fibrin antibody conjugated with Alexa 647. (A) The relative fluorescence intensity from platelet accumulation in the thrombus formed at the site of injury. (B) The relative fluorescence intensity from fibrin accumulation in the thrombus formed at the site of injury. The data presented are the mean value of 4 - 6 mice in each group from three independent experiments.
To investigate the immune response in 2bF8 LV-mediated gene therapy of hemophilia A mice with pre-existing immunity, we monitored inhibitor titers in treated animals after BMT. We compared the levels of anti-FVIII inhibitory antibodies in pre-immunized FVIIInull mice that received either 2bF8 LV-transduced or untransduced FVIIInull BMMCs. The levels of anti-FVIII inhibitory antibodies in plasma decline with time in both 2bF8 LV-transduced and untransduced recipients (Fig. 4A). When reduction of the inhibitor titer over time was calculated as a half-life (t1/2), the t1/2 of inhibitory antibody titer in 2bF8 LV-transduced recipients was 33.65 ± 11.12 days, which was significantly shorter (P < 0.01) than the half-life in the untransduced group of recipients (t1/2 = 66.43 ± 22.24 days) (Fig. 4B). This calculation reflects both the continued level of antibody production as well as the rate of clearance.
Fig. 4. Immune response in 2bF8 LV-transduced FVIIInull mice with pre-existing inhibitors.
Pre-immunized FVIIInull recipients received whole body irradiation at 1100 cGy followed by BMT from 2bF8 LV-transduced or untransduced pre-immunized FVIIInull donors. Plasma was collected and inhibiter titer was determined by Bethesda assay at various time points. Bars represent mean ± SD. (A) Inhibitor titers at various time points. After one month of BM reconstitution, the inhibitor titers began to decline with time after BMT in both 2bF8 LV-transduced and untransduced pre-immunized recipients. (B) The half-life (t1/2) of inhibitor titers in BMT recipients. When the reduction of inhibitor titer was calculated as the half-life (t1/2) of inhibitor titers, the t1/2 from the 2bF8 LV-transduced group is significantly shorter than that obtained from the untransduced group. Data shown are summarized from four BMT trials.
Sustained platelet-FVIII expression is achieved in hemophilia A mice with inhibitors using 2bF8 LV-mediated gene transfer
We monitored the levels of platelet-FVIII:C in recipients after BMT for up to 6 months. Sustained platelet-FVIII expression was obtained in immunized recipients that received 2bF8 LV-transduced BM cells (Fig. 5A). To ascertain the sustained expression of platelet-FVIII, secondary and tertiary BM transplants into immunized FVIIInull recipients were carried out as depicted in Fig. 1A. The levels of platelet-FVIII:C in secondary and tertiary BM recipients were similar to those obtained from the primary recipients (Fig. 5B), indicating that long-term repopulating HSCs had been successfully modified by 2bF8 LV leading to sustained platelet-specific expression of FVIII in FVIIInull mice with pre-existing anti-FVIII antibodies. The average copy number of 2bF8 proviral DNA per cell in secondary and tertiary recipients were similar to copy numbers in primary recipient (Fig. 5C), indicating that there is no selective clonal expansion of 2bF8 LV-transduced HSCs, and conversely there was no indication of a selective disadvantage.
Fig. 5. Long-term engraftment was obtained in 2bF8 LV-transduced FVIIInull mice with pre-existing inhibitors.
(A) Platelet-FVIII expression in 2bF8 LV-transduced recipients. We monitored the level of platelet-FVIII activity in recipients after BMT. Platelets were collected from recipients at various time points and the platelet-FVIII:C was determined by chromogenic assay. Bars represent mean ± SE. Data shown are summarized from two BMT trials. (B) Platelet-FVIII expression in serial BMT recipients. Secondary and tertiary transplants into pre-immunized FVIIInull recipients were carried out using BMMCs from an inhibitor model primary recipient that had received 2bF8 LV-transduced pre-immunized BMMCs. Platelet-FVIII expression was determined by chromogenic assay. For individual mice analyzed more than once over the study, the average platelet FVIII:C was calculated. Bars represent mean ± SE. Shown is one representative serial BMT experiment that had been performed in three trials. (C) Copy number of 2bF8 proviral DNA in serial BMT recipients. DNA was purified from peripheral WBC and the average copy number of 2bF8 LV per cell was determined by quantitative real-time PCR. Bars represent mean ± SD. Shown is one representative serial BMT experiment that had been performed in three trials.
2bF8 gene therapy achieves therapeutic levels of platelet-FVIII in recipients given non-myeloablative conditioning
Another important question we addressed in this study is whether 2bFV LV gene therapy could still be successful when the intensity of pre-transplant conditioning was reduced to non-myeloablative levels. To this end, enriched HSCs (Sca 1+) were isolated from pre-immunized FVIIInull mice, transduced with 2bF8 LV, and then transplanted into sublethally irradiated (660 cGy) pre-immunized recipients. After transplantation the animals were analyzed for FVIII expression. The average levels of platelet-FVIII expression in the transduced mice conditioned with the 660 cGy non-myeloablative regimen ranged from 0.85 to 4.42 mU per 108 platelets over the study period (mean 2.01 ± 0.51 mU per 108 platelets, n = 6), which was not significantly different from the group conditioned with the 1100 cGy myeloablative regimen (2.02 ± 0.34 mU per 108 platelets, ranging from 1.16 to 3.07 mU per 108 platelets, n = 6) (P = 0.99) (Fig. 6A). Sustained platelet-FVIII expression was achieved in both groups of mice (Fig. 6B). The average copy number of 2bF8 proviral DNA per cell over the study period in the group conditioned with 660 cGy was 0.20 ± 0.10 (n = 6), which was not significantly different from the 1100 cGy myeloablative regimen group (0.21 ± 0.14 mU per 108 platelets, n = 6) (P = 0.98) (Fig. 6C). In the group conditioned with 660 cGy, 4 out of 5 mice with inhibitor titers between 36 to 1500 BU mL−1 survived the tail clip challenge. The one mouse that did not survive tail clipping had a modest level of platelet-FVIII (1.95 mU per 108 platelets), but this animal had the highest inhibitor titer (1500 BU mL−1) within that group. In the 1100 cGy irradiated group inhibitor titers ranged from 45 to 140 BU mL−1, and all of these mice survived tail clipping (Fig. 6D). To assess sustained platelet-FVIII expression derived from transduced HSC cell progeny, BMMCs harvested from 3 non-myeloablatively conditioned pre-immunized FVIIInull primary recipients that had received 2bF8 LV-transduced HSCs 11 months earlier were transplanted into secondary recipients. The levels of platelet-FVIII in secondary recipients were not significantly different from primary recipients (P = 0.49) (Fig. 6E).
Fig. 6. Platelet-FVIII expression in 2bF8 LV-transduced mice under a non-myeloablative conditioning regimen.
Immunized animals were conditioned with various conditioning regimens and received 2bF8 LV-transduced pre-immunized Sca-1+ cells. After at least 3 weeks of BM reconstitution, animals were analyzed. (A) Average platelet FVIII expression over the study period. Platelets were collected from recipients and the levels of platelet-FVIII:C were determined by chromogenic assay of platelet lysates. Bars represent mean ± SE. (B) Sustained platelet-FVIII expression in primary recipients under either a myeloablative 1100 cGy or non-myeloablative 660 cGy conditioning regimen. The data presented are the mean ± SE value of 5 - 6 mice in each group. (C) The average copy number of 2bF8 proviral DNA per cell over the study period. DNA was purified from peripheral WBC and the average copy number of 2bF8 LV was determined by quantitative real-time PCR. Bars represent mean ± SD. (D) The tail clip survival test assessing the FVIIInull coagulation defect phenotype. (E) Sustained platelet-FVIII expression in secondary recipients under the non-myeloablative (660 cGy TBI) conditioning regimen. Sequential BMT was carried out into 660 cGy TBI 2° recipients using total BMMCs from primary recipients that had been conditioned with 660 cGy TBI and transplanted with 2bF8 LV-transduced Sca-1+ cells. The levels of platelet-FVIII:C were determined by chromogenic assay of platelet lysates. Bars represent mean ± SE. (F) Average platelet FVIII expression over the study period for alternative non-myeloablative conditioning regimens. Bars represent mean ± SE. Data shown are summarized from five BMT trials.
We next tested other pre-transplant conditioning regimens, which included busulfan and/or relatively low total body irradiation (TBI), to further validate the clinical relevance of the 2bF8 LV-mediated gene therapy approach (Fig. 6F). In the inhibitor model, platelet-FVIII expression in the group conditioned with busulfan alone (50 mg kg−1) was almost undetectable (0.08 ± 0.07 mU per 108 platelets, n = 10). The average copy number of 2bF8 proviral DNA per cell was only barely detectable (0.05 ± 0.01 copy per cell). However, the FVIII expression levels were significantly increased to 1.08 ± 0.28 mU per 108 platelets (ranging from 0.34 to 2.63 mU per 108 platelets, n = 7) and the average copy number of 2bF8 expression cassette per cell was also significantly increased to 0.18 ± 0.04 when busulfan conditioning was supplemented with immunosuppressive anti-(murine)-thymocyte globulin (ATG, 10 mg kg−1). Platelet-FVIII levels of 1.52 ± 0.17 mU per 108 platelets (ranging from 1.10 to 1.94 mU per 108 platelets, n = 5) were achieved when a lower dose of busulfan (25 mg kg−1) was employed together with 440 cGy TBI. In contrast, in the non-inhibitor model, platelet-FVIII activity in the group conditioned with busulfan alone (50 mg kg−1) averaged 1.62 ± 0.36 mU per 108 platelets (ranging from 0.34 to 3.30 mU/ 108 platelets, n = 9). Functional platelet-FVIII was also detected in non-inhibitor recipients conditioned with 440 cGy TBI alone, ranging from 0.83 to 1.67 mU per 108 platelets (mean 1.16 ± 0.15 mU per 108 platelets, n = 5).
Insertion site analysis
Since insertional onco-mutagenesis is a major concern in integrating viral vector-mediated gene therapy, we used linear amplification-mediated PCR (LAM-PCR) to survey a selection of proviral DNA integration sites in 2bF8 LV-transduced recipients. In 6 primary recipients analyzed by LAM-PCR, 39 genomic insertion sites were identified. Among these 23 insertions were located in the introns of genes, 3 in exons, 9 in intergenic regions, and 4 matched satellite DNA sequences in mouse genome (Table 1). Integration of 2bF8 LV into the first intron of genes was seen at a higher frequency (26%) than other areas within genes. The frequency of sense versus anti-sense orientation of 2bF8 transgene within genes is 58% and 42%, respectively. We noted that one gene targeted by 2bF8 LV (Mds1) corresponds to a common integration site for γ-retroviral vectors[21] and another targeted gene, Arid1a, is a proto-oncogene presented in the Mouse Retrovirus-Tagged Cancer Gene Database (RTCGD) http://rtcgd.abcc.ncifcrf.gov).
Table 1. Linear amplification-mediated PCR (LAM-PCR) determined insertion sites.
| # | Chr. | Dir. of T.g. | Gene |
|---|---|---|---|
|
| |||
| 1 | 1 | ↓ | ↓ Ccdc93 (Coiled coil domain containing 93 iIsoform A) Intron 7 |
| 2 | 1 | ↓ | ↑ Cdc42bpa (CDC42 binding protein Kinase alpha) Intron 19 |
| 3 | 1 | ↓ | ↓ LOC677008 (Natural killer cell receptor 2B4 isoform 2) Intron 1 |
| 4 | 2 | ↓ | Intergenic |
| 5 | 2 | ↓ | ↑ Arhgap21 (Rho GTPase activator protein 21) Intron 17 |
| 6 | 2 | ↑ | ↑ Fbn1 (Fibrillin 1) Intron 17 (Just before Exon 18) |
| 7 | 2 | ↑ | ↑ Dpp4 (Dipeptidyl peptidase 4) Intron 14 |
| 8 | 3 | ↓ | ↓ 1700003P14 (α-1 type II collagen isoform 2 precursor) Intron 1 |
| 9 | 3 | ↑ | ↑ Hmm117608 (Calcium independent α-latroxin receptor homolog 2) Intron 1 |
| 10 | 3 | ↓ | ↑ Mds1 (Myelodysplasia sydrome 1 homolog) Intron 1 |
| 11 | 3 | ↑ | ↓ Tsc22d2 (TSC22 domain family 2) Intron 1 |
| 12 | 4 | ↑ | ↑ Arid1a (AT-rich interactive domain-containing protein 1A) Intron 4 |
| 13 | 5 | ↓ | Intergenic |
| 14 | 5 | ↑ | ↓ Tmprss11e (transmembrane protease serine 11E) Intron 3 |
| 15 | 6 | ↓ | ↑ Fam19a1 (TAFA1 protein precursor) Intron 2 |
| 16 | 8 | ↑ | ↑ Hook3 (Protein hook homolog 3) Intron 2 |
| 17 | 9 | ↓ | ↑ Prkcsh (Protein kinase C substrate 80K-H) Exon 8 |
| 18 | 9 | ↓ | ↑ Adam10 (Disintegrin and metalloproteinase domain-containing protein 10 precursor) Exon 5 |
| 19 | 9 | ↑ | Intergenic |
| 20 | 10 | ↓ | ↑ Lta4h (Leukotriene A-4 hydrolase) Intron 16 |
| 21 | 11 | ↑ | Intergenic |
| 22 | 12 | ↑ | Intergenic |
| 23 | 12 | ↑ | ↑ 1110034A24Rik (Dynein assembly factor 2 or axonemal) Intron 1 |
| 24 | 13 | ↓ | ↓ Elmo1 (Engulfment and cell motility isoform 1) Intron 1 |
| 25 | 13 | ↓ | ↑ Fcho2 (FCH domain only 3) Intron 8 |
| 26 | 13 | ↑ | Intergenic |
| 27 | 14 | ↑ | ↑ Dach1 (Dachshund homolog 1 isoform 2) Intron 6 |
| 28 | 15 | ↑ | ↑ Cpsf1 (cleavage and polyadenylation specificity factor subunit 1 isoform 1) Exon 3 |
| 29 | 16 | ↑ | ↑ Dzip3 (E3 ubiquitin protein ligase DAZ-interacting protein 3) Intron 1 |
| 30 | 16 | ↓ | Intergenic |
| 31 | 17 | ↑ | ↓ Ppard (Peroxisome proliferator receptor delta) Intron 1 |
| 32 | 19 | ↓ | ↓ Vti1a (Vessicle transport through Interaction with t-SNAREs homolog 1A) Intron 5 |
| 33 | X | ↓ | Intergenic |
| 34 | X | ↑ | Intergenic |
| 35 | X | ↑ | ↑ Cdkl5 (Cyclin-dependent kinase-like 5) Intron 1 |
| 36 | Satellite DNA | ||
| 37 | Satellite DNA | ||
| 38 | Satellite DNA | ||
| 39 | Satellite DNA | ||
“Chr.” means chromosome; “Dir. of T.g.” means the direction of transgene.
Discussion
The presence of anti-FVIII inhibitory antibodies could be a contraindication for a conventional gene therapy approach that relies on restoration of plasma FVIII because circulating inhibitory antibodies may inactivate functional FVIII if it is constitutively secreted into plasma. Pre-existing inhibitors might also complicate gene therapy based on a storage-with-release-on-demand model such as our platelet FVIII strategy. In the present study, we used a clinically translatable approach that includes lentivirus-mediated BM transduction and transplantation to assess the effects of pre-existing inhibitors on our platelet-specific FVIII expression strategy for genetic therapy of hemophilia A. Our studies demonstrate that HSCs from immunized FVIIInull mice can be transduced with 2bF8 LV and that transduced cells can efficiently engraft into FVIIInull mice even with pre-existing inhibitory antibodies. 2bF8 LV-mediated gene transfer efficiently introduced therapeutic levels of platelet-FVIII expression in hemophilia A mice with pre-existing anti-FVIII immunity under either a myeloablative or, more importantly, using a non-myeloablative conditioning regimen, resulting in sustained phenotypic correction.
HSCs, which represent a rare population in bone marrow with the unique capacity of both self-renewal and differentiation to all blood lineages including the platelet lineage,[22] should be a suitable target for gene transfer to induce long-term FVIII expression in platelets. Induction of 2bF8 expression in cells more differentiated than HSCs may provides short-term benefic, but does not result in long-term expression of FVIII because these cells are destined to further differentiate and ultimately die rather than self-renew. In the present study, we used total BMMCs, which contain 1 - 3% Sca-1+ HSCs, or a purified preparation of Sca-1+ selected BM HSCs for 2bF8 LV transduction and transplantation. Sustained therapeutic levels of platelet-FVIII expression in the recipients were achieved using either cell population, indicating that long-term repopulating HSCs were successfully modified genetically by 2bF8 LV in both cases. The average levels of platelet-FVIII expressed in the recipients that received 2bF8 LV-transduced unselected BMMCs over the study period were not significantly different from those obtained from recipients that received 2bF8 LV-transduced Sca-1+ cells.
Non-myeloablative conditioning regimens have more clinical relevance for gene therapy of hemophilia as compared to myeloablative conditioning. Our previous studies in a transgenic model showed that at two months post-transplantation, donor WBC reconstitution is about 94% in recipients conditioned with lethal irradiation (1100 cGy), 81% in recipients treated with 660 cGy, and only 27% in recipients conditioned with 440 cGy.[18] In the current study, we found that sublethal conditioning (660 cGy TBI) combined with transplantation of 2bF8 LV-transduced Sca-1+ selected HSCs for gene therapy of murine hemophilia A with inhibitors provides FVIII expression comparable to that achieved with lethal conditioning. The ability to reduce the level of conditioning significantly reduces radiation-associated toxicities. Since busulfan, an alkylating agent with potent effects on primitive hematopoietic cells, is an important component of many HSC transplantation preparative regimens in humans,[23] we evaluated the efficacy of busulfan conditioning regimens in 2bF8 LV gene therapy. We found that busulfan conditioning alone resulted in sustained therapeutic levels of platelet-FVIII expression in FVIIInull mice that received 2bF8 LV-transduced HSCs in the non-inhibitor model, but in animals with pre-existing immunity some form of additional immune suppression was required for efficacy (either ATG or low dose TBI in our model). Why pre-existing anti-FVIII immunity alters therapeutic engraftment in the system preconditioned with busulfan alone is still unknown and needs to be further investigated.
Immune responses to transgene or viral proteins are a concern in gene therapy.[24,25] Notably, no antibodies were detected in 2bF8 LV-treated non-immunized FVIIInull mice (non-inhibitor model). For the inhibitor model gene therapy study, we monitored the levels of platelet-FVIII expression and inhibitor titers in pre-immunized 2bF8 LV-transduced recipients for at least 6 months after BMT. After full BM reconstitution, which takes up to 12 weeks,[18,26] platelet-FVIII expression was sustained and inhibitor titers actually declined with time, indicating that platelet-derived FVIII does not provoke a memory response in FVIIInull mice that had previously mounted an immune response to rhFVIII. Interestingly, we found that the t1/2 of inhibitor disappearance in 2bF8 LV-transduced recipients was significantly shorter than in untransduced controls. Because the amount of circulating inhibitors at any given time is the result of equilibrium between continued antibody production and clearance, the shorter t1/2 in the 2bF8 LV-transduced group implies that there might be less antibody production in these mice since clearance in the two groups of mice should be similar. These data indicate that 2bF8 gene therapy may induce immune tolerance, even in hemophilia A with pre-existing immunity. The mechanisms responsible for the apparent loss in immunity to FVIII in our platelet-FVIII gene therapy needs to be further investigated.
Insertional mutagenesis is a concern in any integrating viral vector-mediated gene therapy. We identified 39 insertion sites in six 2bF8 LV-transduced recipients and found one insertion located within a common insertion site represented in the Retrovirus tagged Cancer Gene Database. This 2bF8 LV insertion was located in intron 4 with sense orientation relative to the Arid1a gene, a proto-oncogene that has been commonly found mutated in gastic cancers.[27] Another identified insertion sites was located in intron 1 with reverse orientation relative to the Mds1 gene, which has been reported to be a recurrent integration site for γ-retroviral vectors.[21] Mds 1 can be alternatively spliced, linking its coding sequence with that of Evi 1, giving rise to several alternative transcripts implicated in leukemogenesis.[28] Nevertheless, unlike Evi1 integrants,[29,30] no Mds1 integrants have been associated with development of leukemia or abnormalities in hematopoiesis in mice or humans receiving HSCs transduced with retrovirus vector,[21,31,32] Thus far, no overrepresented insertion sites have been reported in lentivirus transduced animals.[33,34] In the future, a higher throughput integration site analysis will be required to more thoroughly determine the clonal representation (repertoire) of our 2bF8 LV integrants.
In summary, our studies have demonstrated that induction of therapeutic levels of platelet-FVIII expression is successfully achieved using lentivirus-mediated transduction of HSCs and syngeneic transplantation even in the high-risk setting of pre-existing inhibitors. Thus, ex vivo transduction of patient-derived HSCs followed by autotransplantation appears to be a promising approach for gene therapy of hemophilia A patients with inhibitors as well as non-hemophilic patients with acquired inhibitory antibodies who can also have life-threatening clinical bleeding which is difficult to treat by conventional therapies.
Acknowledgments
We thank Haig H. Kazazian at the University of Pennsylvania School of Medicine for the FVIIInull mice. This work was supported by National Institutes of Health grants HL-102035 (QS), HL-44612 (RRM), and HL-68138 (DAW), American Heart Association National Center SDG 0730183N (QS), National Hemophilia Foundation CDA (QS), National Hemophilia Foundation JPG (YC), Hemophilia Association of New York grant (QS), Children’s Research Institute grant (QS), the Goerlich Foundation (RRM), and an American Heart Association Award from the Greater Midwest Affiliate 0755827Z (DAW).
Footnotes
The authors declared no conflict of interest.
Reference List
- 1.Hay CR, Brown S, Collins PW, Keeling DM, Liesner R. The diagnosis and management of factor VIII and IX inhibitors: a guideline from the United Kingdom Haemophilia Centre Doctors Organisation. Br J Haematol. 2006;133:591–605. doi: 10.1111/j.1365-2141.2006.06087.x. [DOI] [PubMed] [Google Scholar]
- 2.Kumaran V, Benten D, Follenzi A, Joseph B, Sarkar R, Gupta S. Transplantation of endothelial cells corrects the phenotype in hemophilia A mice. J Thromb Haemost. 2005;3:2022–2031. doi: 10.1111/j.1538-7836.2005.01508.x. [DOI] [PubMed] [Google Scholar]
- 3.Shahani T, Lavend’homme R, Luttun A, Saint-Remy JM, Peerlinck K, Jacquemin M. Activation of human endothelial cells from specific vascular beds induces the release of a FVIII storage pool. Blood. 2010;115:4902–4909. doi: 10.1182/blood-2009-07-232546. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman RJ, Wasley LC, Dorner AJ. Synthesis, processing, and secretion of recombinant human factor VIII expressed in mammalian cells. J Biol Chem. 1988;263:6352–6362. [PubMed] [Google Scholar]
- 5.Do H, Healey JF, Waller EK, Lollar P. Expression of factor VIII by murine liver sinusoidal endothelial cells. J Biol Chem. 1999;274:19587–19592. doi: 10.1074/jbc.274.28.19587. [DOI] [PubMed] [Google Scholar]
- 6.Shi Q, Wilcox DA, Fahs SA, Kroner PA, Montgomery RR. Expression of human factor VIII under control of the platelet-specific alphaIIb promoter in megakaryocytic cell line as well as storage together with VWF. Mol Genet Metab. 2003;79:25–33. doi: 10.1016/s1096-7192(03)00049-0. [DOI] [PubMed] [Google Scholar]
- 7.Fakharzadeh SS, Zhang Y, Sarkar R, Kazazian HH., Jr. Correction of the coagulation defect in hemophilia A mice through factor VIII expression in skin. Blood. 2000;95:2799–2805. [PubMed] [Google Scholar]
- 8.Xu L, Nichols TC, Sarkar R, McCorquodale S, Bellinger DA, Ponder KP. Absence of a desmopressin response after therapeutic expression of factor VIII in hemophilia A dogs with liver-directed neonatal gene therapy. Proc Natl Acad Sci U S A. 2005;102:6080–6085. doi: 10.1073/pnas.0409249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward NJ, Buckley SM, Waddington SN, Vandendriessche T, Chuah MK, Nathwani AC, McIntosh J, Tuddenham EG, Kinnon C, Thrasher AJ, McVey JH. Codon optimization of human factor VIII cDNAs leads to high-level expression. Blood. 2011;117:798–807. doi: 10.1182/blood-2010-05-282707. [DOI] [PubMed] [Google Scholar]
- 10.Roth DA, Tawa NE, Jr., O’Brien JM, Treco DA, Selden RF. Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N Engl J Med. 2001;344:1735–1742. doi: 10.1056/NEJM200106073442301. [DOI] [PubMed] [Google Scholar]
- 11.Powell JS, Ragni MV, White GC, Lusher JM, Hillman-Wiseman C, Moon TE, Cole V, Ramanathan-Girish S, Roehl H, Sajjadi N, Jolly DJ, Hurst D. Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion. Blood. 2003;102:2038–2045. doi: 10.1182/blood-2003-01-0167. [DOI] [PubMed] [Google Scholar]
- 12.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O’Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarovoi HV, Kufrin D, Eslin DE, Thornton MA, Haberichter SL, Shi Q, Zhu H, Camire R, Fakharzadeh SS, Kowalska MA, Wilcox DA, Sachais BS, Montgomery RR, Poncz M. Factor VIII ectopically expressed in platelets: efficacy in hemophilia A treatment. Blood. 2003;102:4006–4013. doi: 10.1182/blood-2003-05-1519. [DOI] [PubMed] [Google Scholar]
- 14.Shi Q, Wilcox DA, Fahs SA, Weiler H, Wells CW, Cooley BC, Desai D, Morateck PA, Gorski J, Montgomery RR. Factor VIII ectopically targeted to platelets is therapeutic in hemophilia A with high-titer inhibitory antibodies. J Clin Invest. 2006;116:1974–1982. doi: 10.1172/JCI28416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damon AL, Scudder LE, Gnatenko DV, Sitaraman V, Hearing P, Jesty J, Bahou WF. Altered bioavailability of platelet-derived factor VIII during thrombocytosis reverses phenotypic efficacy in haemophilic mice. Thromb Haemost. 2008;100:1111–1122. doi: 10.1160/th08-04-0242. [DOI] [PubMed] [Google Scholar]
- 16.Shi Q, Wilcox DA, Fahs SA, Fang J, Johnson BD, DU LM, Desai D, Montgomery RR. Lentivirus-mediated platelet-derived factor VIII gene therapy in murine haemophilia A. J Thromb Haemost. 2007;5:352–361. doi: 10.1111/j.1538-7836.2007.02346.x. [DOI] [PubMed] [Google Scholar]
- 17.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH., Jr. Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10:119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 18.Shi Q, Fahs SA, Wilcox DA, Kuether EL, Morateck PA, Mareno N, Weiler H, Montgomery RR. Syngeneic transplantation of hematopoietic stem cells that are genetically modified to express factor VIII in platelets restores hemostasis to hemophilia A mice with preexisting FVIII immunity. Blood. 2008;112:2713–2721. doi: 10.1182/blood-2008-02-138214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooley BC. In vivo fluorescence imaging of large-vessel thrombosis in mice. Arterioscler Thromb Vasc Biol. 2011;31:1351–1356. doi: 10.1161/ATVBAHA.111.225334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt M, Glimm H, Wissler M, Hoffmann G, Olsson K, Sellers S, Carbonaro D, Tisdale JF, Leurs C, Hanenberg H, Dunbar CE, Kiem HP, Karlsson S, Kohn DB, Williams D, von KC. Efficient characterization of retro-, lenti-, and foamyvector-transduced cell populations by high-accuracy insertion site sequencing. Ann N Y Acad Sci. 2003;996:112–121. doi: 10.1111/j.1749-6632.2003.tb03239.x. [DOI] [PubMed] [Google Scholar]
- 21.Calmels B, Ferguson C, Laukkanen MO, Adler R, Faulhaber M, Kim HJ, Sellers S, Hematti P, Schmidt M, von KC, Akagi K, Donahue RE, Dunbar CE. Recurrent retroviral vector integration at the Mds1/Evi1 locus in nonhuman primate hematopoietic cells. Blood. 2005;106:2530–2533. doi: 10.1182/blood-2005-03-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Italiano JE, Jr., Shivdasani RA. Megakaryocytes and beyond: the birth of platelets. J Thromb Haemost. 2003;1:1174–1182. doi: 10.1046/j.1538-7836.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- 23.McPherson ME, Hutcherson D, Olson E, Haight AE, Horan J, Chiang KY. Safety and efficacy of targeted busulfan therapy in children undergoing myeloablative matched sibling donor BMT for sickle cell disease. Bone Marrow Transplant. 2011;46:27–33. doi: 10.1038/bmt.2010.60. [DOI] [PubMed] [Google Scholar]
- 24.Ponder KP. Gene therapy for hemophilia. Curr Opin Hematol. 2006;13:301–307. doi: 10.1097/01.moh.0000239700.94555.b1. [DOI] [PubMed] [Google Scholar]
- 25.Margaritis P, High KA. Gene therapy in haemophilia--going for cure? Haemophilia. 2010;16(Suppl 3):24–28. doi: 10.1111/j.1365-2516.2010.02256.x. [DOI] [PubMed] [Google Scholar]
- 26.Tomita Y, Sachs DH, Sykes M. Myelosuppressive conditioning is required to achieve engraftment of pluripotent stem cells contained in moderate doses of syngeneic bone marrow. Blood. 1994;83:939–948. [PubMed] [Google Scholar]
- 27.Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, Chan TL, Kan Z, Chan AS, Tsui WY, Lee SP, Ho SL, Chan AK, Cheng GH, Roberts PC, Rejto PA, Gibson NW, Pocalyko DJ, Mao M, Xu J, Leung SY. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43:1219–1223. doi: 10.1038/ng.982. [DOI] [PubMed] [Google Scholar]
- 28.Soderholm J, Kobayashi H, Mathieu C, Rowley JD, Nucifora G. The leukemia-associated gene MDS1/EVI1 is a new type of GATA-binding transactivator. Leukemia. 1997;11:352–358. doi: 10.1038/sj.leu.2400584. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Dullmann J, Schiedlmeier B, Schmidt M, von KC, Meyer J, Forster M, Stocking C, Wahlers A, Frank O, Ostertag W, Kuhlcke K, Eckert HG, Fehse B, Baum C. Murine leukemia induced by retroviral gene marking. Science. 2002;296:497. doi: 10.1126/science.1068893. [DOI] [PubMed] [Google Scholar]
- 30.Kustikova O, Fehse B, Modlich U, Yang M, Dullmann J, Kamino K, von NN, Schlegelberger B, Li Z, Baum C. Clonal dominance of hematopoietic stem cells triggered by retroviral gene marking. Science. 2005;308:1171–1174. doi: 10.1126/science.1105063. [DOI] [PubMed] [Google Scholar]
- 31.Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, Glimm H, Kuhlcke K, Schilz A, Kunkel H, Naundorf S, Brinkmann A, Deichmann A, Fischer M, Ball C, Pilz I, Dunbar C, Du Y, Jenkins NA, Copeland NG, Luthi U, Hassan M, Thrasher AJ, Hoelzer D, von KC, Seger R, Grez M. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 32.Metais JY, Dunbar CE. The MDS1-EVI1 gene complex as a retrovirus integration site: impact on behavior of hematopoietic cells and implications for gene therapy. Mol Ther. 2008;16:439–449. doi: 10.1038/sj.mt.6300372. [DOI] [PubMed] [Google Scholar]
- 33.Beard BC, Dickerson D, Beebe K, Gooch C, Fletcher J, Okbinoglu T, Miller DG, Jacobs MA, Kaul R, Kiem HP, Trobridge GD. Comparison of HIV-derived lentiviral and MLV-based gammaretroviral vector integration sites in primate repopulating cells. Mol Ther. 2007;15:1356–1365. doi: 10.1038/sj.mt.6300159. [DOI] [PubMed] [Google Scholar]
- 34.Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, Sergi SL, Benedicenti F, Ambrosi A, Di SC, Doglioni C, von KC, Naldini L. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]