Abstract
The signal transduction mechanisms of pituitary adenylate cyclase activating polypeptide (PACAP) were investigated in lung cancer cells. Previously, PACAP-27 addition to NCI-H838 cells increased phosphatidylinositol turnover and intracellular cAMP leading to proliferation of lung cancer cells. Also, PACAP receptors (PAC1) regulated the tyrosine phosphorylation of ERK, focal adhesion kinase and paxillin. In this communication the effects of PACAP on cytosolic Ca2+ and PYK-2 tyrosine phosphorylation were investigated. PACAP-27 increased cytosolic Ca2+ within seconds after addition to FURA-2 AM loaded NCI-H838 cells. The increase in cytosolic Ca2+ caused by PACAP was inhibited by PACAP (6–38) (PAC1 antagonist), U73122 (phospholipase C inhibitor) or BAPTA (calcium chelator), but not H89 (PKA inhibitor). PACAP-38 but not vasoactive intestinal peptide (VIP), addition to NCI-H838 or H1299 cells significantly increased the tyrosine phosphorylation of PYK-2 after 2 min. The increase in PYK-2 tyrosine phosphorylation caused by PACAP was inhibited by PACAP(6–38), U73122 or BAPTA, but not H89. The results suggest that PAC1 regulates PYK-2 tyrosine phosphorylation in a calcium-dependent manner.
Introduction
Proline-rich tyrosine kinase (PYK-2), a member of the focal adhesion kinase (FAK) family, is a non-receptor tyrosine kinase which may play a role in cellular proliferation, differentiation and migration (Picasicia et al., 2002; Kuwabara et al., 2004; Lipinski et al., 2010). PYK-2 is activated by an increase in cytoplasmic Ca2+ which occurs after addition of vasopressin or platelet-derived growth factor (Lev et al., 1995). PYK-2 is a 116 KDa protein which is phosphorylated (Tyr402) after activation of the phospholipase C pathway (Zrihan-Licht et al., 2000). PYK-2 has a central catalytic domain flanked by an N-terminal which has SH2- and SH3-binding sites and a C-terminal, which contains two proline-rich domains (Hall et al., 2011). The C-terminal of PYK-2 interacts with paxillin, a scaffold protein, which coordinates Rho family GTPases regulating the actin skeleton (Bellis et al., 1997). Paxillin is phosphorylated by FAK or PYK-2 at Tyr118 and phosphorylated paxillin provides a docking site for recruitment of other proteins to focal adhesions (Schaller et al., 1992).
G-protein coupled receptors (GPCR) such as PAC1 regulate FAK and paxillin tyrosine phosphorylation (Moody et al., 2012). PAC1, which contains 467 amino acids, crosses the plasma membrane 7 times and has a 28 amino acid HOP1 splice variant (SV) and/or 28 amino acid HIP SV insert in the third cytosolic domain (Pisegna and Wank 1993, Spengler et al., 1993). All PAC1 SV interact with a stimulatory guanine nucleotide binding protein (Gs) causing elevated cAMP (Moody and Jensen, 2006). PAC1 HOP1 SV interacts strongly with Gq causing phosphatidylinositol (PI) turnover (Pisegna and Wank 1996). Because PACAP binds with high affinity to PAC1, PACAP addition to lung cancer cells increases cAMP and PI metabolites. The inositol-1,4,5-trisphosphate (IP3) released causes elevation of cytosolic Ca2+. In contrast VIP binds with low affinity to PAC1 but high affinity to VPAC1 and VPAC2 (Ishihara et al., 1992; Lutz et al., 1993). Addition of VIP to lung cancer cells increases cAMP but does not cause PI turnover (Lee et al., 1990).
Lung cancer is characterized by high densities of VPAC1 and PAC1 but not VPAC2 (Reubi et al 2000, Moody et al., 2003a). PACAP and VIP are autocrine growth factors for some lung cancer cells. The growth of NCI-H838 cells is stimulated by PACAP as well as VIP and inhibited by the receptor antagonists PACAP(6–38) as well as VIPhybrid (Moody et al., 2003b). VIP hybrid potentiates the cytotoxicity of chemotherapeutic drugs such as paclitaxel using lung cancer cells (Moody et al., 2001). Traditionally, lung cancer is treated with chemotherapeutic drugs but the 5 year patient survival rate is only 16% (Shedden et al., 2008). Lung cancer is comprised of the neuroendocrine small cell lung cancer (SCLC) and the epithelial non-SCLC (NSCLC). PYK-2 is expressed in high levels in 62% of the NSCLC tumors and higher expression of PYK-2 was present in lymph node metastases (Zhang et al., 2008). The results indicate that PYK-2 may be important in NSCLC.
Here the ability of PAC1 to regulate PYK-2 tyrosine phosphorylation was investigated in NSCLC cells. PACAP-27, but not VIP, increased significantly PYK-2 tyrosine phosphorylation in a dose- and time-dependent manner. The increase in PYK-2 tyrosine phosphorylation was inhibited by PACAP(6–38) and U-73112 (phospholipase C inhibitor) but not H89 (protein kinase (PK) A inhibitor). Addition of PACAP to NCI-H838 cells increased cytosolic Ca2+ which was blocked by U-73112 but not H89. These results suggest that PAC1 regulates PI turnover and the resulting elevation in cytosolic Ca2+ is important for PYK-2 activation. Further BAPTA (calcium chelator) and PP2 (Src inhibitor) inhibited the ability of PACAP to cause PYK-2 phosphorylation. These results indicate that PYK-2 tyrosine phosphorylation in NSCLC cells is Ca2+-dependent.
Materials and Methods
Cell culture
NSCLC NCI-H157, A549, NCI-H727, NCI-H838 or NCI-H1299 cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium containing 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Grand Island, NY). Routinely, the cells were split weekly 1:20 with trypsin EDTA. The cells were mycoplasma free and were used when they were in exponential growth phase after incubation at 37°C in 5% CO2/95% air.
Western Blot
The ability of PACAP to stimulate tyrosine phosphorylation of PYK-2 was investigated by Western blot. NCI-H838 or H1299 cells were cultured in 10 cm dishes. When a monolayer of cells formed they were placed in RPMI-1640 containing 5 μg/ml insulin, 10 μg/ ml transferrin and 3 × 10−8M sodium selenite (SIT media) for 3 hr. NSCLC cells were treated with BAPTA, GF109203X, H89, PACAP(6–38), PP2, or U73122 (Sigma-Aldrich, St. Louis, MO) for 30 min. Then cells were treated with 100 nM PACAP-27 (Bachem, Torrence, CA) for 2 min, washed twice with PBS and lysed in buffer containing 50 mM Tris.HCl (pH 7.5), 150 mM sodium chloride, 1% Triton X-100, 1% deoxycholate, 1% sodium azide, 1 mM ethylene glycol tetraacetic acid (EGTA), 0.4 M EDTA, 1.5 μg/ml aprotinin, 1.5 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride and 0.2 mM sodium vanadate (Sigma-Aldrich, St. Louis, MO). The lysate was sonicated for 5 s at 4°C and centrifuged at 10000 x g for 15 min. Protein concentration was measured using the BCA reagent (Pierce Chemical Co., Rockford, IL), and 20 μg of protein was denatured in sample buffer. The samples were assayed by sodium dodecyl sulfate gel electrophoresis followed by transfer of the proteins from the gel to nitrocellulose. The nitrocellulose containing transferred proteins was washed with 12.5% non-fat milk containing 50 mM Tris/HCl (pH 8.0), 2 mM CaCl2, 80 mM sodium chloride, 0.05% Tween 20 and 0.02% sodium azide) and incubated for 16 h at 4°C with 1 μg/ml anti-PY402-PYK-2 antibody (Cell Signaling Technologies, Danvers, MA) followed by anti-rabbit immunoglobulin G-horseradish peroxidase conjugate (Upstate Biotechnologies, Lake Placid, NY). The membrane was washed for 10 min with blotto and twice for 10 min with washing solution (50 mM Tris/HCl (pH 8.0), 2 mM CaCl2, 80 mM sodium chloride, 0.05% Tween 20 and 0.02% sodium azide). The blot was incubated with enhanced chemiluminescence detection reagent (Pierce Chemical Co., Rockford, IL) for 5 min and exposed to Kodak XAR film. The intensity of the bands was determined using a densitometer. Alternatively blots were probed with anti-PYK2 or anti-tubulin antibody.
Cytosolic Ca2+
NCI-H838 cells were assayed for cytosolic Ca2+. NCI-H838 cells were rinsed in PBS and treated with trypsin. The cells were placed in an equal volume on RPMI-1640 containing 10% fetal bovine serum and counted using a hemocytometer. The cells centrifuged at 1000 x g for 3 min and the pellet resuspended in SIT media at a concentration of 2 × 106 cells/ml and incubated 1 μg/ml Fura-2 AM (Sigma Aldrich, St. Louis, MO) for 30 at 37°C for 30 min. The cells were centrifuged and resuspended in SIT media at a concentration of 2 × 106 cells/ml. The cells (2 ml) were put in a quartz cuvette and excited at 340 and 380 nm with an emission wavelength of 510 nm in a Photon Technology Corporation spectrophotometer. Drugs e.g. H89 were added at 37°C for 5 min, followed by the addition of PACAP-27 (100 nM). As a positive control, ionomycin (5 μg/ml) was added and the increase in cytosolic Ca2+ determined.
Results
The mechanism by which PACAP increases cytosolic Ca2+ in lung cancer cells was investigated. NCI-H838 cells were loaded with Fura-2 AM and 100 nM PACAP-27 added. Figure 1 (left) shows that relative fluorescence intensity rapidly increased within seconds after addition of PACAP to NCI-H838 cells. The fluorescence intensity peaked after about 15 sec and slowly declined, returning to basal levels after about 2 min. Using a second set of Fura-2 loaded cells, 50 μM H89 which inhibits protein kinase (PK) A, had no effect on the basal fluorescence intensity or that stimulated by PACAP-27 addition (Fig. 1, middle). Using a third set of NCI-H838 cells, 50 μM U73122 which inhibits phospholipase (PL) C, had little effect on basal fluorescence but abolished the ability of PACAP-27 to increase the fluorescence. In contrast, U73122 had little effect on the ability of 5 μM ionomycin (Ca2+ ionophore) to increase cytosolic Ca2+ (data not shown). Similar Ca2+ results were obtained using PACAP-38 but not VIP (data not shown). The results indicate that PLC but not PKA inhibitors impair the ability of PACAP to increase cytosolic Ca2+ in lung cancer cells.
Fig. 1.
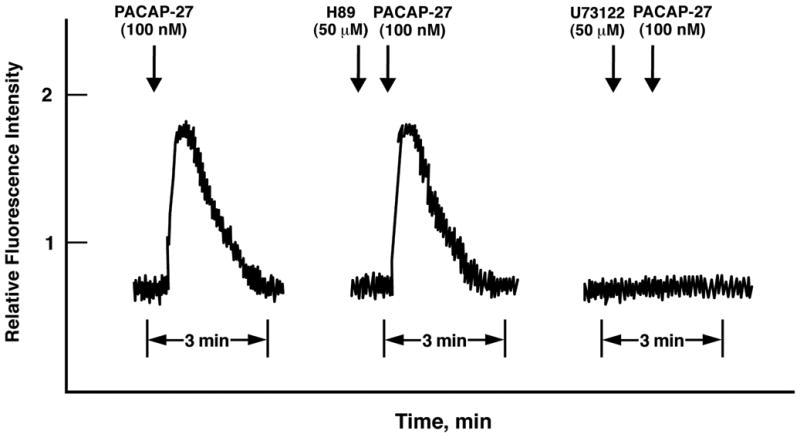
PACAP-27 causes increased cytosolic calcium. PACAP-27 (100 nM) addition to NCI-H838 cells loaded with Fura2-AM increased the cytosolic Ca2+ in the presence of no additions (left), 50 μM H89 (middle) or 50 μM U73122 (right). This experiment is representative of 2 others.
The ability of various inhibitors to impair the increase in cytosolic Ca2+ caused by PACAP was investigated. Table I shows that 1 μM BAPTA (Ca2+ chelator) or U73122 blocked the increase in the relative fluorescence caused by addition of 100 nM PACAP-27 to Fura-2 AM loaded NCI-H838 cells. In contrast, 5 μM GF109203X (PKC inhibitor), 1 μM PP2 (Src inhibitor) or H89 did not block the increase in cytosolic Ca2+ caused by the addition of PACAP-27 to NCI-H838 cells (Table I). Similar results were obtained using NCI-H1299 cells (data not shown). The results indicate that Ca2+ chelators and PLC inhibitors but not Src, PKC or PKA inhibitors impair the ability of PACAP to increase cytosolic Ca2+ in lung cancer cells.
Table I.
Ability to inhibit the PACAP cytosolic Ca2+ response and PYK-2 tyrosine phosphorylation.
| Addition | Ca2+ response | PYK-2 tyrosine phosphorylation |
|---|---|---|
| None | + | + |
| BAPTA, 1 μM | − | − |
| GF109203X, 5 μM | + | +/− |
| H89, 50 μM | + | + |
| PACAP(6–38), 10 μM | − | − |
| PP2, 1 μM | + | − |
| U73122, 50 μM | − | − |
PACAP-27 (100 nM) was added to Fura2-AM loaded NCI-H838 cells and the Ca2+ response determined after 15 sec. PACAP-27 (100 nM) was added to NCI-H838 cells and the PYK-2 tyrosine phosphorylation determined after 2 min; +, stimulation; +/−, partial inhibition; −, complete inhibition. These experiments were repeated 4 times.
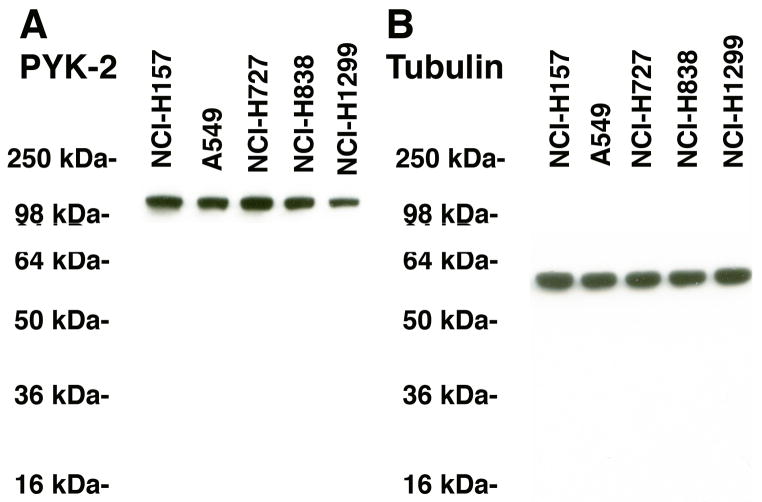
PYK-2 was investigated in NSCLC cells. Figure 2A shows that a major band of immunoreactive PYK-2 (116 kDa) was present in extracts derived from all cell lines tested (NCI-H157, A549, NCI-H727, NCI-H838 and NCI-H1299). Similarly, the housekeeping protein tubulin (55 kDa) was present in the 5 cell lines tested (Fig. 2B). Because high densities of PAC1 were present in NCI-H838 cells (Moody et al., 2003b), this line was used for further studies.
Fig. 2.
PYK-2 and lung cancer. (A) PYK-2 was detected in NCI-H157, A549, NCI-H727, NCI-H838 and NCI-H1299 cellular extracts by Western blot. (B) Tubulin was present in all cell lines. This experiment is representative of 3 others.
The effects of PACAP-27 on PYK-2 tyrosine phosphorylation were investigated using NCI-H838 cells. Previous studies demonstrate that Y402-PYK-2 phosphorylation is essential for PYK-2 activation and recruitment of c-Src (Calaib et al, 1995). Figure 3A shows that addition of 100 nM PACAP-27 or PACAP-38, but not VIP or PACAP (6–38), to NCI-H838 cells increased Y402-PYK-2 phosphorylation. Total PYK-2 did not change with PACAP administration (Fig. 3A). Figure 3B shows that addition of PACAP-27 or PACAP-38 to NCI-H838 cells significantly increased phosphorylated PYK-2 by 106% and 75%, respectively. In contrast, PACAP(6–38) or VIP had little effect on phosphorylated PYK-2. Figure 3C shows that PACAP(6–38) antagonized the increase in PYK-2 tyrosine phosphorylation caused by PACAP-27 addition to NCI-H838 cells. PACAP(6–38) (0.01 μM) had little effect whereas 0.1 or 1 μM moderately and 10 μM PACAP(6–38) strongly decreased the ability of PACAP-27 to increase PYK-2 tyrosine phosphorylation. Fig. 3D shows that PACAP-27 addition to NCI-H838 cells increased significantly PYK-2 tyrosine phosphorylation by 78% and that PACAP(6–38) half-maximally inhibited the increase at a 0.2 μM concentration. The results suggest that PACAP increases PYK-2 tyrosine phosphorylation by interacting with PAC1 in NCI-H838 cells.
Fig. 3.
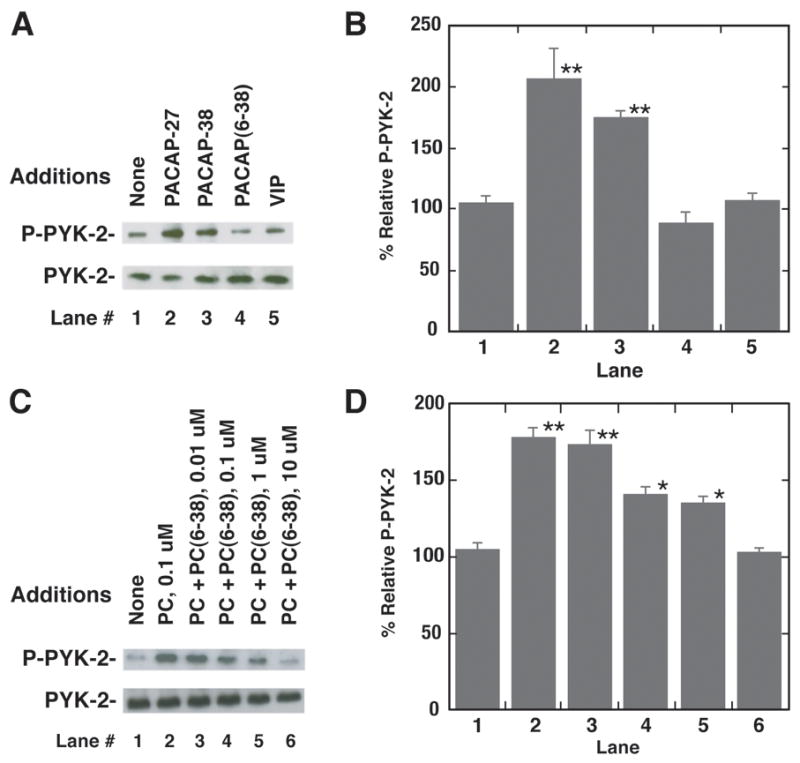
Phosphorylated PYK-2 and NCI-H838 cells. (A) PYK-2 tyrosine phosphorylation was determined 2 min after the addition of 100 nM PACAP-27, VIP, PACAP(6–38) or PACAP-38 (100 nM) to NCI-H838 cells (B) The mean value ± S.E. of 3 experiments is indicated; P < 0.01, ** using Student’s t-test. (C) The ability of varying doses of PACAP(6–38) to inhibit increases in PYK-2 tyrosine phosphorylation by PACAP-27 was investigated. (D) The mean value ± S.E. of 3 experiments is indicated; P < 0.05, *; P < 0.01, ** using Student’s t-test.
The mechanism by which PYK-2 is phosphorylated was investigated. Figure 4A shows that addition of BAPTA strongly and GF109203X moderately inhibited the increase in PYK-2 tyrosine phosphorylation caused by PACAP addition to NCI-H838 cells. Figure 4B shows that PACAP significantly increased PYK-2 tyrosine phosphorylation by 116%. BAPTA significantly decreased the ability of PACAP-27 to increase PYK-2 tyrosine phosphorylation. Figure 4C shows that addition of H89 to NCI-H838 cells had little effect on the ability of PACAP to cause PYK-2 tyrosine phosphorylation, whereas U73122 inhibited the increase in PYK-2 tyrosine phosphorylation caused by PACAP. Figure 4D shows that PACAP significantly increased PYK-2 tyrosine phosphorylation by 71%. U73122 but not H89, significantly inhibited the ability of PACAP to increase PYK-2 tyrosine phosphorylation. The results indicate that PLC activity and cytosolic Ca2+ are important in the ability of PAC1 to regulate PYK-2 tyrosine phosphorylation.
Fig. 4.
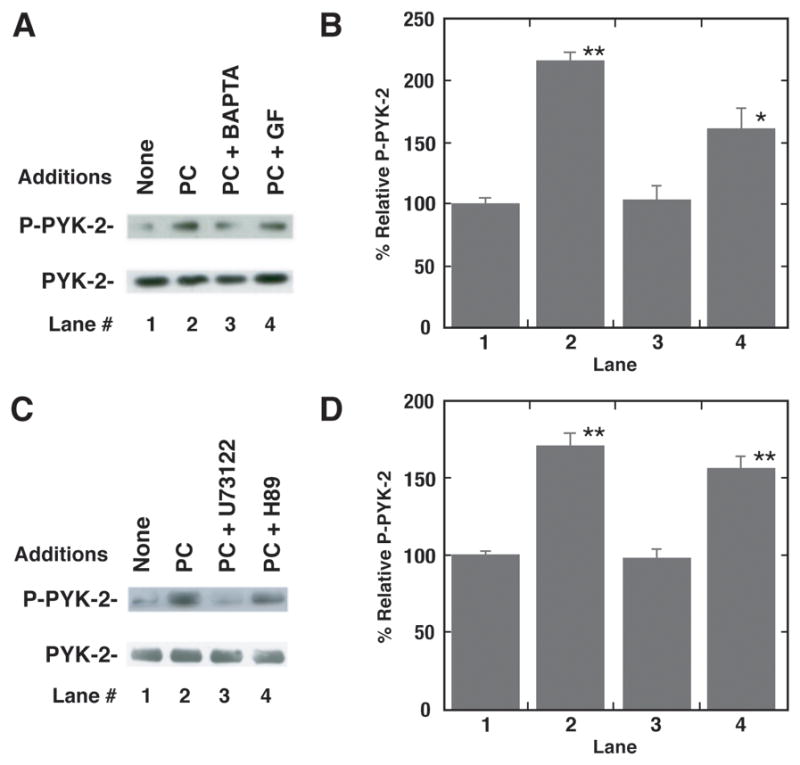
Inhibitors of PYK-2 tyrosine phosphorylation. (A) The ability of 1 μM BAPTA or 5 μM GF109203X to inhibit PYK-2 tyrosine phosphorylation caused by PACAP-27 was determined. (B) The mean value ± S.E. of 4 experiments is indicated; P < 0.01, ** using Student’s t-test. (C) The ability of H89 (50 μM) or U73122 (50 μM) to inhibit PYK-2 tyrosine phosphorylation induced by PACAP-27 was investigated. (D) The mean value ± S.E. of 3 experiments is indicated; P < 0.01, ** using Student’s t-test.
Discussion
GPCR are known to regulate PYK-2 tyrosine phosphorylation in SCLC cells (Roelle et al., 2008). Addition of 10 μM galanin (GAL) or bradykinin (BK) increased PYK-2 tyrosine phosphorylation and cytosolic Ca2+ in NCI-H69 and NCI-H510 SCLC cells. The increase in PYK-2 phosphorylation caused by GAL or BK addition to NCI-H69 cells was blocked by BAPTA. Similarly PP2 blocked the increase in PYK-2 and ERK tyrosine phosphorylation caused by addition of GAL or BK to NCI-H69 cells. These results suggest that GPCR regulate SCLC PYK-2 tyrosine phosphorylation in a Src- and Ca2+-dependent manner. Here the ability of GPCR to regulate PYK-2 tyrosine phosphorylation was investigated in NSCLC cells.
FAK, similar to PYK-2, phosphorylates downstream substrates and acts as a scaffolding protein in the assembly of signaling complexes (Hall et al., 2011). FAK is activated by growth factor receptors and integrin signaling, whereas PYK-2 is activated by growth factors and increases in cytosolic Ca2+ concentrations. PACAP caused PYK-2 tyrosine phosphorylation within 2 min after addition to NCI-H838 or NCI-H1299 NSCLC cells. The effects were Ca2+ dependent in that addition of PACAP to NCI-H838 cells treated with BAPTA had little effect on PYK-2 phosphorylation. PACAP-27 addition to NCI-H838 cells loaded with Fura2-AM strongly increased cytosolic Ca2+. Addition of BAPTA inhibited the increase in cytosolic Ca2+ caused by PACAP. The results suggest that PACAP increases PYK-2 tyrosine phosphorylation in a Ca2+-dependent manner.
Cholecystokinin (CCK) causes tyrosine phosphorylation of PYK-2 and FAK using pancreatic acini and elevates cytosolic Ca2+ (Pace et al., 2003). CCK increased PYK-2 phosphorylation at Y402, Y580 or Y881 within 2 min after addition to pancreatic acini. Addition of thapsigargin, which depletes the endoplasmic reticulum of Ca2+, inhibits the ability of CCK to increase cytosolic Ca2+ and increase Y402-PYK-2 phosphorylation. Thapsigargin had no effect, however, on the ability of CCK to increase phosphorylation of Y397-FAK (Pace et al., 2003). Also, PYK-2 was primarily localized to the membrane of pancreatic acinar cells, where FAK was in the cytosol (Tapia et al., 1999). Furthermore, the ability of CCK to increase phosphorylation of Y402-PYK-2 was partially inhibited by GF109203X addition to pancreatic acini, whereas FAK tyrosine phosphorylation was unaffected (Tapia et al., 1999). Our results indicate that the ability of PACAP to cause Y402-PYK-2 phosphorylation is totally dependent on Ca2+ and partially dependent on PKC activity.
The specificity of the PYK-2 tyrosine phosphorylation was investigated. PACAP-27 or PACAP-38, but not VIP, caused increased PYK-2 tyrosine phosphorylation 2 minutes after addition to NCI-H838 cells. Also, the increased in PYK-2 tyrosine phosphorylation caused by PACAP addition to NCI-H838 cells was antagonized by PACAP(6–38). Further PACAP-27 or PACAP-38, but not VIP, increased cytosolic Ca2+ within seconds after addition to NCI-H838 cells. The increase in cytosolic Ca2+ caused by PACAP was blocked PACAP(6–38). The results suggest that PAC1 regulates the increase in PYK-2 tyrosine phosphorylation and the increase in cytosolic Ca2+.
PYK-2 binds to SH2 and SH3 domains of Src kinases (Dikic et al., 1996). Tyr402 of PYK-2 is the major phosphorylation site of Src family SH2 binding sites. PP2 inhibited the increase in PYK-2 phosphorylation caused by PACAP addition to NCI-H838 cells (Table I). Previously Wittau et al., (2000) showed that Src co-immunoprecipitated with PYK-2 in SCLC cells. Src kinase activity is essential for GTP-loading of Ras and activation of extracellular signal-regulated kinases (ERK). Previously, we showed that PACAP caused ERK tyrosine phosphorylation in NCI-H838 cells which was inhibited by PD98059, a MEK inhibitor (Moody et al., 2002). In contrast, PD98059 had no effect on the ability of PACAP to cause PYK-2 tyrosine phosphorylation (data not shown). These results indicate that PACAP activation of PYK-2 is dependent upon Src; however, Src activity is not essential for elevation of cytosolic Ca2+. Furthermore, ERK activation is downstream from that of PYK-2 or Src.
Recently, we found that PACAP addition to NCI-H838 cells increased Y1068-epidermal growth factor receptor (EGFR) phosphorylation after 1 min (Moody et al., 2012). Because PP2 but not BAPTA inhibited the ability of PACAP to increase EGFR tyrosine phosphorylation, it is Src- but not Ca2+-dependent. Furthermore, the EGFR tyrosine kinase inhibitor gefitinib and PACAP(6–38) inhibited the ability of PACAP to increase EGFR tyrosine phosphorylation. In contrast, gefitinib had no effect on the ability of PACAP to increase PYK-2 tyrosine phosphorylation (data not shown). Both gefitinib and PACAP(6–38) inhibited the proliferation of NCI-H838 cells. PAC1 activates Akt leading to increase cellular survival (May et al., 2010).
Catalytic inhibitors for PYK-2 have been developed. TAE226 is a bis-anilino pyrimidine compound which inhibits the ATP binding site of PYK-2 and FAK with an IC50 of 5 nM (Liu et al., 2007). TAE226 impairs the PYK-2 and FAK reducing enzymatic activity. TAE226 inhibited ovarian and glioma tumor growth in animal models (Halder et al., 2007). PF-562,271 is a methane sulfonamide diaminopyrimidine which inhibits ATP binding to FAK and PYK-2 with IC50 values of 2 and 13 nM respectively (Roberts et al., 2008). PF-562,271 alters the protein conformation in FAK and PYK-2. PF-562,271 inhibited the growth of prostate, breast, pancreatic and colon tumors in nude mice. The results suggest that FAK and/or PYK-2 inhibitors reduce the proliferation and/or migration of cancer cells (Lipinski et al., 2005).
In summary, PAC1 regulates the tyrosine phosphorylation of PYK-2 using NCI-H838 NSCLC cells. Because BAPTA strongly inhibited PYK-2 tyrosine phosphorylation induced by PACAP, PYK-2 phosphorylation is dependent upon Ca2+. Also, the ability of PAC1 to regulate PYK-2 is dependent on PLC, Src and partially dependent on PKC. It remains to be determined if selective PYK-2 inhibitors will inhibit the growth and/or metastasis of NSCLC cells.
Acknowledgments
This research is supported in part by intramural funds of the NCI and NIDDK of NIH.
Abbreviations used
- PACAP
pituitary adenylate cyclase activating polypeptide
- PYK-2
proline-rich tyrosine kinase 2
- FAK
focal adhesion kinase
- R
receptor
- SCLC
small cell lung cancer
- NSCLC
Non-SCLC
- VIP
vasoactive intestinal peptide
- Ca2+
calcium
- GPCR
G-protein coupled receptor
- pY
phosphotyrosine
References
- Bellis SL, Perotta JA, Curtis MS, Turner CE. Adhesion of fibroblasts to fibronectin stimulates both serine and tyrosine phosphorylation of paxillin. Biochem J. 1997;325:375–381. doi: 10.1042/bj3250375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaib MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- Halder J, Lin YG, Merritt WM, Spannuth WA, Nick AM, Honda T, Kamat AA, Han LY, Kim TJ, Lu C, Tari AM, Bornmann W, Fernandez A, Lopez-Berestein G, Sood AK. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. 2007;67:10976–10983. doi: 10.1158/0008-5472.CAN-07-2667. [DOI] [PubMed] [Google Scholar]
- Hall JE, Fu W, Schaller MD. Focal adhesion kinase: Exploring FAK structure to gain insight into function. Int Rev Cell Mol Biol. 2011;388:185–225. doi: 10.1016/B978-0-12-386041-5.00005-4. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Functional expression and tissue distribuiton of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Kuwabara K, Nakaoka T, Sato K, Nishishita T, Sasaki T, Yamashita N. Differential regulation of cell migration and proliferation through proline-rich tyrosine kinase 2 in endothelial cells. Endocrinology. 2004;145:3324–3330. doi: 10.1210/en.2003-1433. [DOI] [PubMed] [Google Scholar]
- Lee M, Jensen RT, Bepler G, Korman LY, Moody TW. Vasoactive intestinal polypeptide binds with high affinity to non-small cell lung cancer cells and elevates cAMP” levels. Peptides. 1990;11:1205–1210. doi: 10.1016/0196-9781(90)90153-v. [DOI] [PubMed] [Google Scholar]
- Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger JA. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Tran NL, Menashi E, Rohl C, Kloss J, Bay RC, Berens ME, Loftus JC. The tyrosine kinase PYK2 promotes migration and invasion of glioma cells. Neoplasia. 2005;7:435–445. doi: 10.1593/neo.04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski CA, Loftus JC. Targeting Pyk2 for theraepeutic intervention. Expert Opin Ther Targets. 2010;14:95–108. doi: 10.1517/14728220903473194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TJ, LaFortune T, Honda T, Ohmori O, Hatakeyama S, Meyer T, Jackson D, deGroot J, Yung WK. Inhibition of both focal adhesion kinase and insulin-like growth factor-I receptor kinase suppresses glioma proliferation in vitro and in vivo. Mol Cancer Ther. 2007;6:1357–1367. doi: 10.1158/1535-7163.MCT-06-0476. [DOI] [PubMed] [Google Scholar]
- Lutz EM, Sheward WJ, West KM, Morrow JA, Harmar AJ. The VIP2 receptor molecular characterization of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- May V, Lutz E, MacKenzie C, Schutz K, Dozark K, Braas K. Pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates multiple neurotophic signaling pathways. J Biol Chem. 2010;285:9749–9761. doi: 10.1074/jbc.M109.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody TW, Chan D, Fahrenkrug J, Jensen RT. Neuropeptides as autocrine growth factors in cancer cells. Current Pharmaceutical Design. 2003a;9:495–509. doi: 10.2174/1381612033391621. [DOI] [PubMed] [Google Scholar]
- Moody TW, Hill JM, Jensen RT. VIP as a trophic factor in the CNS and cancer cells. Peptides. 2003b;24:163–177. doi: 10.1016/s0196-9781(02)00290-5. [DOI] [PubMed] [Google Scholar]
- Moody TW, Leyton J, Casibang M, Pisegna J, Jensen RT. PACAP-27 tyrosine phosphorylates mitogen activated protein kinase and increases VEGF mRNAs in human lung cancer cells. Regul Pept. 2002;109:135–140. doi: 10.1016/s0167-0115(02)00196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody TW, Leyton J, Chan D, Brenneman DC, Fridkin M, Gelber E, Levy A, Gozes I. VIP receptor antagonists potentiate the action of chemotherapeutic drugs on breast cancer cells. Breast Cancer Res and Treatment. 2001;68:55–64. doi: 10.1023/a:1017994722130. [DOI] [PubMed] [Google Scholar]
- Moody TW, Jensen RT. VIP and PACAP as autocrine growth factors in breast and lung cancer. In: Kastin A, editor. Handbook of Biologically active peptides. Elsevier Press; 2006. pp. 493–498. [Google Scholar]
- Moody TW, Leyton J, Jensen RT. Pituitary adenylate cyclase-activating polypeptide causes increased tyrosine phosphorylation of focal adhesion kinase and paxillin. J Mol Neurosci. 2012;46:68–74. doi: 10.1007/s12031-011-9639-7. [DOI] [PubMed] [Google Scholar]
- Moody TW, Osefo N, Nuche-Berenguer B, Ridnour L, Wink D, Jensen RT. Pituitary adenylate cyclase activating polypeptide causes tyrosine phosphorylation of the EGF receptor in lung cancer cells. J Pharm Exp Ther. 2012 doi: 10.1124/jpet.111.190033. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace A, Garcia-Marin LJ, Tapia JA, Bragado MJ, Jensen RT. Phosphospecific site tyrosine phosphorylation of p125FAK and proline-rich kinase 2 is differentially regulated by cholecystokinin receptor type A activation in pancreatic acini. J Biol Chem. 2003;278:19008–19016. doi: 10.1074/jbc.M300832200. [DOI] [PubMed] [Google Scholar]
- Picascia A, Stanzione R, Chieffi P, Kisslinger A, Dikic I, Tramontano D. Prolie-rich tyrosine kinase 2 regulates proliferation and differentiation of prostate cells. Mol Cell Endocrinol. 2002;186:81–87. doi: 10.1016/s0303-7207(01)00667-0. [DOI] [PubMed] [Google Scholar]
- Pisegna J, Wank SA. Molecular cloning and functional expression of the pituitary adenylate cyclase activating polypeptide type I receptor. Proc Natl Acad Sci USA. 1993;90:6345–6349. doi: 10.1073/pnas.90.13.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisegna JR, Wank SA. Cloning and characterization of the signal transduction of four splice variants of the human pituitary adenylate cyclase activating polypeptide receptor. Evidence for dual coupling to adenylate cyclase and phospholipase C. J Biol Chem. 1996;271:17267–17274. doi: 10.1074/jbc.271.29.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi JC, Laderach U, Waser B, Gebbers JD, Robberecht P, Laissue JA. Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide receptor subtypes in human tumors and their tisssues of origin. Cancer Res. 2000;60:3105–3112. [PubMed] [Google Scholar]
- Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, Richter D, Emerson E, Lin J, Kath J, Coleman K, Yao L, Martinez-Alsina L, Lorenzen M, Berliner M, Luzzio M, Patel N, Schmitt E, LaGreca S, Jani J, Wessel M, Marr E, Griffor M, Vasdos F. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68:1935–1944. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- Rolle S, Grosse R, Buech T, Chubanov V, Gudermann T. Essential role of Pyk2 and Src kinase activation in neuropeptide-induced proliferation of small cell lung cancer cells. Oncogene. 2008;27:1737–1748. doi: 10.1038/sj.onc.1210819. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Cobb BS, Vaines RR, Reynolds AB, Parsons JT. P125FAK, a structurally distinctive protein tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89:192–196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheddon K, Taylor JM, Endemann SA. Gene expression-based survival prediction in lung adenocarcinoma: A multi-site blinded validation study. Nature Med. 2008;14:822–827. doi: 10.1038/nm.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Tapia JA, Ferris HA, Jensen RT, Garcia LJ. Cholecystokinin activates PYK2/CAKβ by a phospholipase C-dependent mechanism and its association with the mitogen-activated protein kinase signaling pathway in pancreatic acinar cells. J Biol Chem. 1999;274:31261–31271. doi: 10.1074/jbc.274.44.31261. [DOI] [PubMed] [Google Scholar]
- Wittau N, Grosse R, Kalkbrenner F, Gohla A, Schultz G, Gudermann T. The galanin receptor type 2 initiates multiple signaling pathways in small cell lung cancer cells by coupling to Gq, Gi and G12 proteins. Oncogene. 2000;19:1318–1328. doi: 10.1038/sj.onc.1203777. [DOI] [PubMed] [Google Scholar]
- Zhang S, Qiu X, Gu Y, Wang E. Up-regulation of proline-rich tyrosine kinase 2 in non-small cell lung cancer. Lung Cancer. 2008;62:295–301. doi: 10.1016/j.lungcan.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Zia F, Fagarasan M, Bitar K, Coy DH, Pisegna J, Wank S, Moody TW. PACAP receptors regulate the growth of non-small cell lung cancer cells. Cancer Res. 1995;55:4886–4891. [PMC free article] [PubMed] [Google Scholar]
- Zrihan-Licht S, Fu Y, Settleman J, Schinkmann K, Shaw L, Keydar I, Avraham S, Avraham H. RAFTK/PYK2 tyrosine kinase mediates the association of P190 RhoGAP with RasGAP and is involved in breast cancer cell invasion. Oncogene. 2000;19:1318–28. doi: 10.1038/sj.onc.1203422. [DOI] [PubMed] [Google Scholar]