Abstract
Cilia are antenna-like organelles found on the surface of most cells. They transduce molecular signals and facilitate interactions between cells and their environment. Ciliary dysfunction has been shown to underlie a broad range of overlapping, clinically and genetically heterogeneous phenotypes, collectively termed ciliopathies. Literally, all organs can be affected. Frequent cilia-related manifestations are (poly)cystic kidney disease, retinal degeneration, situs inversus, cardiac defects, polydactyly, other skeletal abnormalities, and defects of the central and peripheral nervous system, occurring either isolated or as part of syndromes. Characterization of ciliopathies and the decisive role of primary cilia in signal transduction and cell division provides novel insights into tumorigenesis, mental retardation, and other common causes of morbidity and mortality, including diabetes mellitus and obesity. New technologies (“Next generation sequencing/NGS”) have considerably improved genetic research and diagnostics by allowing simultaneous investigation of all disease genes at reduced costs and lower turn-around times. This is undoubtedly a result of the dynamic development in the field of human genetics and deserves increased attention in genetic counselling and the management of affected families.
Keywords: Cilia/ciliopathies, Cystic kidneys, Polycystic kidney disease, ADPKD, ARPKD, Congenital hepatic fibrosis/ductal plate malformation, Nephronophthisis (NPHP), Ivemark syndrome, Meckel syndrome (MKS), Joubert syndrome (JBTS), Bardet–Biedl syndrome (BBS), Alstrom syndrome, Short-rib polydactyly syndromes, Jeune syndrome (ATD), Ellis-van Crefeld syndrome (EVC), Sensenbrenner syndrome, Primary ciliary dyskinesia (Kartagener syndrome), von Hippel-Lindau (VHL), Tuberous sclerosis (TSC), Oligogenic inheritance, Modifier, Mutational load
Introduction
Defective cellular organelles such as mitochondria, peroxisomes, and lysosomes are well-known causes of human disease. In line with a unifying disease concept, disorders often derive their name from the respective dysfunctional organelle, e.g., mitochondriopathies. The cilium, another cellular structure, currently arises much interest and an emerging number of diseases with a wide phenotypic spectrum have been shown to be related to ciliary dysfunction, now collectively termed “ciliopathies”.
In humans, four different types of cilia that differ in structure and motility are known (9+0 vs. 9+2 structure, immotile vs. motile; all combinations are known). All have in common an axoneme that is composed of nine microtubule doublets (some with an additional central pair = 9+2 structure and dyneins that power ciliary motility) derived from a modified centrosome, the basal body, in quiescent cells. The cilium–centrosome complex has been conserved throughout evolution and across organ systems [34]. Primary cilia are usually immotile with a 9+0 structure, as tiny hair-like projections cilia extend about 5–10 μm from the apical membrane of polarized cells into the extracellular environment [59] (Fig. 1). An elaborate, highly conserved process (intraflagellar transport/IFT) along the microtubule core organizes the transport of proteins into (anterograde) and out of (retrograde) the cilium, and this process is needed for formation, maintenance, and function of the cilium [53].
Fig. 1.
Schematic diagram of a primary cilium and associated processes. The inner ciliary structure is defined by the axoneme composed of nine microtubule doublets derived from the basal body and mother centriole of the centrosome (inset displays cross-section revealing 9+0 architecture). Along this microtubule core, the transport of proteins toward the tip of the cilium (anterograde, by kinesin-2 with its major component KIF3A) and in the retrograde direction towards the cell body (by dynein-2) is organized by an elaborate process called intraflagellar transport (IFT). Cilia are small antennae that detect a variety of different extracellular stimuli and orchestrate multiple signaling pathways with nuclear trafficking of some molecules
Over the past decade, it has become obvious that cilia are practically ubiquitously present in all organs which explain the broad range of phenotypes associated with defects in their function and/or structure [20]. They represent versatile tools for various cellular functions including proliferation, apoptosis, and planar cell polarity that have been shown to be crucial for proper epithelial function and normal diameters of tubular structures [25]. Overall, cilia can be best understood as environmental rheostats and cellular signaling centers that detect and orchestrate a bunch of different extracellular stimuli through specific ciliary receptors (e.g., fluid flow, light, smell, hormones, growth factors, and other chemokines). Cilia act as mechano-, chemo-, and osmosensors and mediate multiple pathways (Wnt, Hedgehog, Notch, JAK-STAT, etc.) essential for normal development, but, if disrupted, lead to early developmental defects and cancer. In line, cilia play a crucial role in cell cycle regulation responsible for the coordination of cancer-related signaling molecules with opposite effects on tumorigenesis, either repressing or stimulating depending on the context [29, 77].
Primary ciliary dyskinesia and Kartagener syndrome
Many people may know motile cilia from the respiratory tract and their function to generate flow-clearing mucus. In contrast to primary cilia with a typically 9+0 structure, these motile counterparts usually additionally contain a central microtubule pair (9+2 structure) that is thought to impart additional function. Motile cilia lining the upper and lower respiratory tract are defective in patients with primary ciliary dyskinesia (PCD), also known as immotile cilia syndrome. As a consequence of impaired mucociliary clearance, recurrent airway infections and lung damage such as bronchiectases occur [6]. Alterations in the left–right organization of the internal organ positioning such as situs inversus and situs ambiguous are observed in 50% of PCD patients (then called Kartagener's syndrome) and can be explained by dysfunctional nodal cilia during early embryogenesis. Less common are other heterotaxy features such as asplenia/polysplenia and congenital heart defects. Due to the fact that sperm tail axonemes (flagella) display a comparable ultrastructure as respiratory cilia, a considerable proportion of male PCD patients have reduced fertility. PCD is not only clinically, but also genetically heterogeneous (Table 1). In line with ultrastructural analyses that reveal defective outer dynein arms in most patients, autosomal recessive mutations have been described in genes encoding components of the outer dynein arms, radial spokes, and cytoplasmic pre-assembly factors of axonemal dyneins. Detailed characterization by electron microscopy, immunofluorescence, and high-speed videomicroscopy, usually only available in specialized centers, is most helpful in making a specific diagnosis.
Table 1.
Primary ciliary dyskinesia and Kartagener syndrome
| Disease | Main features | Gene (locus) | Corresponding protein |
|---|---|---|---|
| Primary ciliary dyskinesia (PCD)/Kartagener syndrome | Impaired mucociliary clearance = recurrent airway infections and lung damage (e.g., bronchiectases) | DNAI1/CILD1 (9p13.3) | DNAI1 |
| Reduced fertility in males | DNAH5/CILD3 (5p15.2) | DNAH5 | |
| Situs inversus (=Kartagener syndrome), heterotaxy features such as asplenia/polysplenia or congenital heart defects less common | TXNDC3/CILD6 (7p14.1) | TXNDC3 | |
| DNAH11/CILD7 (7p15.3) | DNAH11 | ||
| DNAI2/CILD9 (17q25.1) | DNAI2 | ||
| KTU (C14orf104)/CILD10 (14q21) | Kintoun | ||
| RSPH4A/CILD11 (6q22.1) | RSPH4A | ||
| RSPH9/CILD12 (6p21.1) | RSPH9 | ||
| LRRC50/CILD13 (16q24.1) | LRRC50 | ||
| CCDC39 (3q26.33) | CCDC39 | ||
| CCDC40 (17q25.3) | CCDC40 | ||
| DNAL1 (14q24.3) | DNAL1 |
A clear-cut distinction between motile and immotile cilia and their microtubule-based inner structure is not as easy as often thought, and there is increasing evidence for some overlap which invite additional scrutiny of motile cilia dysfunction in patients with primary cilia-related disorders and vice versa. For example, altered structure and function of motile cilia in airway epithelia have been demonstrated in patients with Bardet–Biedl syndrome [61]. Likewise, it has been shown that both motile and immotile cilia have sensory functions.
Polycystic kidney disease and other cystic kidney disorders
Polycystic kidneys initially paved the way for the meanwhile large group of cilia-related disorders, and it is becoming increasingly evident that most ciliopathies have a renal cystogenic component, making kidney cyst formation a hallmark feature of ciliopathies. The first link between cilia and cystic kidney disease was given by Pazour and colleagues in 2000 when they observed shortened cilia in the kidneys of orpk mice, a model for polycystic kidney disease bearing mutations in the gene encoding the intraflagellar transport protein IFT88 [50]. Polycystic kidney diseases (autosomal dominant polycystic kidney disease (ADPKD) and autosomal recessive polycystic kidney disease (ARPKD)) and other cystic kidney disorders including the nephronophthisis-medullary cystic kidney disease complex are clinically and genetically heterogeneous and may already manifest in utero. Progressive fibrocystic renal changes are often accompanied by hepatobiliary changes and sometimes other extrarenal abnormalities [19].
For the detection of even small cysts, ultrasound is usually the first step in the diagnostic process as it is cheap, readily available, noninvasive, and highly sensitive. In certain cases, computed tomography (CT) and magnetic resonance imaging (MRI) may be useful. Compared to ultrasound, CT and MRI are both known to allow for better and more accurate detection of smaller cysts, especially when an intravenous contrast agent is being used.
Autosomal dominant polycystic kidney disease
ADPKD is the most frequent life-threatening genetic disease with a prevalence of 1/400–1,000, affecting ∼12.5 million individuals worldwide. About 5–10% of adult patients who require renal replacement therapy are affected by ADPKD that is transmitted in an autosomal dominant, fully penetrant fashion. The majority (∼80–85%) carries a germline mutation in the PKD1 gene on chromosome 16p13, whereas about 15–20% harbor a mutation in the PKD2 gene on chromosome 4q21 [32]. The encoded proteins polycystin-1 and polycystin-2 are both glycosylated integral membrane proteins that interact via their C-terminal coiled-coil domains. Polycystin-2 is a member of the transient receptor potential protein superfamily and known to function as a divalent cation channel particularly involved in cellular Ca2+ signaling [69].
PKD1 sequencing is complicated by the presence of six pseudogenes in a duplicated region adjacent to the original PKD1 locus. It is still a matter of debate if these pseudogenes are merely “junk” or functional DNA. Several lines of evidence show that some pseudogenes are “alive” with functional roles in gene expression and regulation, e.g., by acting as microRNA decoys [51]. Mutation analysis in ADPKD has much improved during recent years, and it is now generally possible to detect the disease-causing mutation in most affected families. Sequencing of the large and structurally complex PKD1 gene is usually the first step; if negative, it is followed by PKD2 sequencing and finally MLPA (multiplex ligation-dependent probe amplification) analysis of both genes to detect larger deletions.
Diagnosis of ADPKD by ultrasound is established in at-risk individuals aged 15 to 39 years if three or more (unilateral or bilateral) renal cysts are detected. About 60% of children younger than 5 years of age and 75 to 80% of children aged 5–18 years with a known PKD1 mutation had renal cysts detectable by ultrasound. In general, the finding of even one renal cyst should alert a pediatrician to the possibility of ADPKD because simple cysts are otherwise extremely rare in childhood. In children with a 50% risk of ADPKD, the finding of one cyst can thus be considered diagnostic [52].
Clinical symptoms usually do not arise until adulthood; however, there is striking phenotypic variability even within the same family indicating that modifying genes, epigenetic mechanisms, and/or environmental factors considerably influence the clinical course in ADPKD. About 2% of ADPKD patients present with early clinical manifestations before age 15 years. Among these are cases with significant perinatal morbidity and mortality sometimes indistinguishable from severe autosomal recessive polycystic kidney disease (ARPKD). Notably, affected families with early-manifesting offspring have a high recurrence risk for the birth of a further child with similar clinical manifestation; an information that should be shared with afflicted families and that clearly hints at a common familial modifying background for early and severe disease expression [70].
Renal cysts can vary considerably in size and appearance arising from all segments of the nephron (Fig. 2). Usually, they show progressive enlargement and may become disconnected from the tubular space. The Consortium for Radiologic Imaging for the Study of Polycystic Kidney Disease recently showed that renal enlargement in ADPKD mimicked exponential growth. Over a 3-year period among more than 200 patients, the renal volume increased by a mean of 63.4 ml (5.3%) per year resulting from the expansion of cysts in a continuous process associated with the decline of renal function. While this leads to progressive destruction of the adjacent renal parenchyma and massive enlargement of the kidneys, renal function can long be preserved as functioning nephrons undergo compensatory hypertrophy [27]. Chronic renal failure presents in about 50% of patients by the age of 60 years [21]. PKD2 is usually significantly milder than PKD1 with a lower prevalence of arterial hypertension and urinary tract infections [39]. Patients with PKD1 develop end-stage renal disease (ESRD) approximately 20 years earlier than patients with PKD2, with a median age of onset of ESRD at 54.3 and 74.0 years, respectively [33]. The greater severity of PKD1 is due to the development of more cysts at an early age, not to faster cyst growth [30].
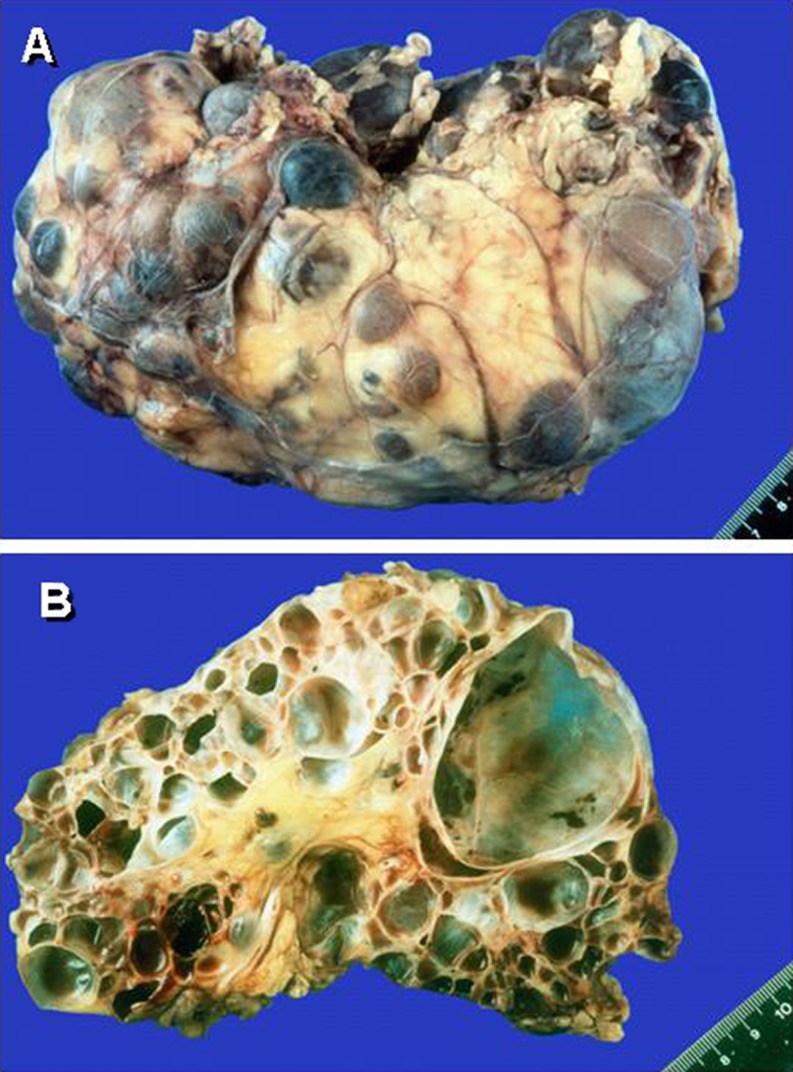
Fig. 2.
Enlarged kidney from a patient with autosomal dominant polycystic kidney disease (ADPKD). Multiple cysts, grossly varying in size, have massively destructed the renal parenchyma
While the kidneys are the main organ involved, ADPKD is clearly a systemic disorder with profound extrarenal disease burden. Polycystic liver disease is by far the most common extrarenal manifestation in ADPKD and usually a benign disease that may cause mechanical compression or irritation. Notably, polycystic liver disease can also occur isolated as an autosomal dominant trait with a mutation in either PRKCSH or SEC63. Liver cysts affect about 75% of ADPKD patients in their sixties [5] and usually gain in size and number as they do in the kidney. Women, especially those who have used hormones and/or have had multiple pregnancies, are more often and more severely affected suggesting that estrogen influences hepatic cyst growth [22, 63]. Estrogen is known to stimulate proliferation of cholangiocytes, and estrogen receptors are expressed in epithelial cells lining hepatic cysts [3]. Cysts in other epithelial organs (e.g., seminal vesicles, pancreas, arachnoid membrane) and diverticulosis are also common [43, 67]. Among cardiovascular co-morbidities, intracerebral aneurysms play a significant role and are present in about 8% of ADPKD patients. The prevalence is even higher in patients with a positive family history for intracerebral aneurysms and/or subarachnoid hemorrhage. Cardiac valve disease, particularly mitral valve prolapse, can be diagnosed in approximately 25% of patients with ADPKD [37].
Therapeutic approaches in ADPKD
The pathomechanism of ADPKD is closely related to defective intracellular calcium homeostasis and cAMP and mTOR signaling with increased rates of proliferation, apoptosis, and secretion along with remodeling of the extracellular matrix [9, 64]. Currently, several therapeutic clinical trials based on new pathophysiological insights are running. Hopes had been high that mTOR inhibitors would at least help delay progression of ADPKD; however, two separate clinical trials using everolimus and sirolimus recently failed to halt the decline in renal function [60, 74].
Increased expression of vasopressin V2 receptor (V2R), c-myc, and epidermal growth factor receptor (EGFR) in cystic kidneys has led to other therapeutic approaches [68]. Vasopressin is the major adenyl cyclase agonist in the principal cells of renal collecting ducts generating cAMP as a known promoter of renal cystic enlargement. Thus, V2R antagonists may have great potential for drug approval in cystic kidney diseases. Administration of the V2R antagonist OPC31260 in the pcy mouse even led to regression of already established renal cystic pathology with decreased proliferation, apoptosis, and interstitial fibrosis. Tolvaptan, another V2R antagonist, has proven its efficacy in different rodent models orthologous to human ADPKD, ARPKD, and nephronophthisis and is currently tested in advanced clinical trials in patients with ADPKD [68]. Besides the expected and well-tolerated mild to moderate thirst, no other significant adverse effect has been reported so far. Almost exclusive expression of the V2R protein in renal collecting ducts warrants specificity and safety of the drug but also implicates that extrarenal PKD manifestations will not be targeted.
Another promising approach might be intervention with the long-acting somatostatin-analog octreotide. Its therapeutic downstream effect is supposed to be based on inhibition of cAMP-generated chloride secretion by cyst-lining epithelia. Octreotide therapy was well tolerated in the majority of patients and found to inhibit renal and liver enlargement [14, 36, 54]. The drug might also be effective in other organs that show cystic alterations such as breast and ovaries [23]. Whether this therapy form proves beneficial in the long run has to be tested in further trials of longer duration with a greater number of patients.
“Neoplasia in disguise”
Jared J. Grantham was the first who called ADPKD “neoplasia in disguise” because the cystogenic process resembles profiles often observed in tumors [26, 31]. However, renal cell carcinoma or any other tumor only rarely develop in human PKD despite the frequency of hyperplastic polyps and microscopic adenomas in which aneuploid karyotypes with chromosomal extra-copies can often be determined. This phenomenon in cell growth and tumorigenesis has recently been explained by the so-called aneuploidy paradox which means that chromosome gains cause a proliferative disadvantage and not advantage as one may have assumed [76]. The integrity of cilia and the polycystin complex are required for the maintenance of mitotic spindle orientation and centrosome amplification [1, 7]. Interventions targeted at cell cycle regulation may thus be another smart drug approach as demonstrated by beneficial effects of the cyclin-dependent kinase (CDK) inhibitor roscovitine on cystic kidney disease in two PKD mouse models [13].
von Hippel–Lindau disease and tuberous sclerosis
The term “neoplasia in disguise” may also hint at some similarities between ADPKD and hereditary cancer syndromes such as von Hippel–Lindau (VHL) syndrome and tuberous sclerosis (TSC) (Table 2). VHL is an autosomal dominant condition caused by inactivating mutations of the VHL tumor suppressor gene and characterized by the development of hemangioblastomas in the brain, spinal cord, and retina often combined with renal clear cell carcinoma and pheochromocytoma [40]. Patients with VHL are also at increased risk for epididymal cystadenomas and cysts in the kidney and pancreas.
Table 2.
Polycystic kidney and liver disease, von Hippel–Lindau syndrome and tuberous sclerosis
| Disease | Main features | Gene (locus) | Corresponding protein |
|---|---|---|---|
| Autosomal recessive polycystic kidney disease (ARPKD) | In pre-/perinatal cases: Potter's sequence and respiratory distress, huge hyperechogenic kidneys with multiple tiny cysts throughout cortex and medulla, poor corticomedullary differentiation. Early arterial hypertension. Ductal plate malformation always present (leading to portal hypertension). Rarely, pancreatic cysts and/or fibrosis | PKHD1 (6p12.3-p12.2) | Polyductin/fibrocystin |
| Autosomal dominant polycystic kidney disease (ADPKD) | Typical disease onset in adulthood with arterial hypertension, proteinuria, hematuria, and/or renal insufficiency (rarely, early childhood manifestation). Generally enlarged kidneys with cysts of different size in all parts of the nephron. Liver cysts, rare in children. Intracranial aneurysms, familially clustered | PKD1 (16p13.3) | Polycystin-1 |
| PKD2 (4q22.1) | Polycystin-2 | ||
| Autosomal dominant polycystic liver disease (PCLD) | Numerous liver cysts (usually >20), only few kidney cysts. Symptoms arise from mass effect/hepatomegaly. Rarely, intracystic hemorrhage or rupture of cysts. More severe in females | PRKCSH (19p13.2) | Hepatocystin |
| SEC63 (6q21) | SEC63 | ||
| von Hippel–Lindau syndrome (VHL) (autosomal dominant) | Hemangioblastomas in brain/cerebellum, spinal cord, retina, often combined with renal clear cell carcinoma, pheochromocytoma, epididymal cystadenomas and cysts in kidneys and pancreas | VHL (3p25.3) | pVHL |
| Tuberous sclerosis (TSC) (autosomal dominant) | Epilepsy, cognitive impairment, angiomyolipomas, cystic kidneys, pulmonary lymphangioleiomyomatosis, skin manifestations (white spots, angiofibromas) | TSC1 (9q34.13) | Hamartin |
| TSC2 (16p13.3) | Tuberin |
Tuberous sclerosis (TSC) is caused by an autosomal dominant germline mutation in either TSC1 or TSC2 and can affect a broad range of organs with a birth incidence of approximately 1:6,000 [15]. About 90% of patients experience often intractable seizures and approximately every second patient shows cognitive impairment, autism, or other behavioral disorders. Renal manifestations are the leading cause of death in adult patients [62]; cystic kidney disease occurs in 50%, angiomyolipomas are diagnosed in even 80% of TSC patients. Many other parts of the body (e.g., heart and brain) can also be affected by primarily non-cancerous tumors. A third of all female patients display pulmonary involvement, specifically lymphangioleiomyomatosis. Frequent skin manifestations in TSC are white spots, facial angiofibromas, and peri-/subungual fibromas.
Both TSC and VHL intersect with the primary cilium and the mTOR signaling network known to be upregulated in PKD and several tumor types [38]. Activity of mTOR is down-regulated by polycystin-1 and the TSC1/TSC2 tumor suppressor complex, finally resulting in G1-cell cycle arrest and apoptosis. A synergistic role of polycystin-1 and the TSC2 gene product tuberin had already been suggested by the more severe and earlier-onset PKD phenotype in individuals harboring a deletion encompassing the adjacent TSC2 and PKD1 genes on chromosome 16p13 than in patients with a PKD1 mutation alone. A molecular explanation might be the fact that both proteins interact and tuberin traffics polycystin-1 to the plasma membrane [64].
Autosomal recessive polycystic kidney disease
ARPKD, the recessive counterpart of ADPKD, is caused by mutations in PKHD1, a large gene on chromosome 6p12 that extends over a genomic segment of almost 500 kb. The longest open reading frame (ORF) comprises 66 exons encoding polyductin/fibrocystin, a type I single-pass transmembrane protein of 4,074 amino acids [48, 75]. Due to allelic heterogeneity and a high level of missense mutations and private changes, mutation analysis for PKHD1 is laborious. Routine diagnostics has been largely alleviated by setting up an algorithm that allows the detection of 80% of PKHD1 mutations by screening only one third of the gene [11]. Patients with two truncating mutations generally display a severe phenotype with peri- or neonatal death, whereas patients surviving the neonatal period usually carry at least one hypomorphic missense mutation. PKHD1 is the main gene in ARPKD, but there is compelling evidence for locus heterogeneity why results that are merely based on linkage need to be handled with some care.
In contrast to ADPKD, ARPKD typically is a pediatric disease, although the clinical spectrum is much more variable than generally presumed and moderately affected adults have been described [2, 12]. Yet, the majority of cases are severely affected and identified late in pregnancy or at birth. Affected fetuses display a “Potter” oligohydramnios phenotype with massively enlarged kidneys, pulmonary hypoplasia, a characteristic facies, and contracted limbs with club feet. Approximately 30–50% of affected neonates die shortly after birth from respiratory insufficiency due to pulmonary hypoplasia and thoracic compression by the excessively enlarged kidneys (Fig. 3).
Fig. 3.
Baby with autosomal recessive polycystic kidney disease (ARPKD). a Distended abdomen due to voluminous kidneys that lead to respiratory problems. b Nephrectomized kidney of this girl. c–d Ultrasound showed symmetrically enlarged echogenic kidneys with fusiform dilations of collecting ducts and distal tubules arranged radially throughout the renal parenchyma from medulla to cortex
By ultrasound, children with ARPKD typically exhibit characteristic bilateral large echogenic kidneys with poor corticomedullary differentiation. Unlike ADPKD, the cysts are usually tiny, remain in contact with the urinary system, and are confined to the distal tubules and collecting ducts. With advancing clinical course and development of larger renal cysts accompanied by interstitial fibrosis, the kidney structure of ARPKD may increasingly resemble that of ADPKD. Arterial hypertension, often difficult to control despite multi-drug treatment, usually develops during the first months of life and affects up to 80% of children with ARPKD. Careful blood pressure monitoring is essential to prevent sequelae of hypertension (e.g., cardiac hypertrophy, congestive heart failure) and deterioration of the renal function [12].
While the early appearance of ARPKD is typically clearly dominated by renal manifestations and associated co-morbidities, histologic liver involvement is present in every ARPKD patient from early embryonic development on, reflected by the disease term “Polycystic Kidney and Hepatic Disease 1 (PKHD1)”. These obligatory liver changes are characterized by defective remodeling of the ductal plate with congenital hepatic fibrosis and hyperplastic biliary ducts [19, 71], defined as ductal plate malformation (DPM) which is also a frequent feature in other ciliopathies, e.g., Bardet–Biedl, Joubert, Meckel and Jeune syndrome.
Biliary anomalies may develop at any stage of the physiologic involution-remodeling process, and the timing or stage of development determines the resulting clinical and histological phenotype. Typically, cysts that arise from small interlobular bile ducts are detached from the biliary tree, while those that stem from malformation of medium- and large-sized bile ducts usually maintain connected. While there are overlaps between each subgroup, bile duct hamartomas, ARPKD, and other ciliopathies are mainly manifestations of DPM of the small interlobular bile ducts, whereas medium-sized intrahepatic ducts are generally afflicted in ADPKD and polycystic liver disease. Caroli's disease is usually the result of DPM of large intrahepatic bile ducts, while choledochal cysts are thought to represent large extrahepatic DPM [18].
Besides the portal tracts, the remaining liver parenchyma in ARPKD usually develops normally and liver enzymes, except for cholestasis parameters that might be elevated, are characteristically not increased. The hepatobiliary complications may dominate the clinical picture, particularly in older patients. Ductal plate malformation leads to progressive hepatic fibrosis and consecutive portal hypertension that causes hypersplenism with pancytopenia and oesophageal varices with upper gastrointestinal bleeding [12, 28]. Primary management of variceal bleeding includes endoscopic approaches, such as sclerotherapy or variceal banding. Occasionally, portosystemic shunting or liver–kidney transplantation (sequential or combined) has to be considered. ARPKD patients with liver cysts due to extensive dilatations of intra- and extrahepatic bile ducts (Caroli's disease and syndrome) are at increased risk of recurrent episodes of ascending bacterial cholangitis that may cause fulminant hepatic failure [41]. Occasionally, ARPKD patients present with an organ-specific phenotype, i. e., either an (almost) exclusive renal phenotype or a predominant or mere liver phenotype. In accordance, Pkhd1/PKHD1 mutations can cause isolated congenital hepatic fibrosis or Caroli's syndrome in mice and men [45].
Nephronophthisis and the medullary cystic kidney disease complex
Nephronophthisis (NPH) comprises a clinically and genetically heterogeneous group of autosomal recessive tubulo-interstitial cystic kidney disorders that represents the most frequent genetic cause of end-stage renal disease in children and young adults and typically initially presents with a urinary concentrating defect [35, 56]. The most common form, juvenile NPH, usually starts during the first decade with polyuria, polydipsia, and anemia. Patients usually do not show arterial hypertension before onset of renal failure. On ultrasound, NPH patients generally show normal or small-sized kidneys with increased echogenicity and also often loss of cortico-medullary differentiation. Cysts usually only occur secondarily after patients have progressed to end-stage renal failure and are typically located at the cortico-medullary junction. Histologically, tubular basement membrane thickening and disintegration, tubular atrophy, and disproportionate tubulo-interstitial fibrosis with minimal inflammation are observed [47].
Medullary cystic kidney disease (MCKD) is often regarded as the autosomal dominant counterpart of NPH (NPH–MCKD complex) with a usually later onset of renal failure than the recessive forms. The ciliary protein uromodulin (=Tamm–Horsfall glycoprotein), the most abundant protein in urine of healthy individuals, plays a major role and is encoded by UMOD on chromosome 16p12 [72]. Mutations in this gene can lead to different tubulo-interstitial nephropathies including MCKD2, glomerulocystic kidney disease, and familial juvenile hyperuricemic nephropathy, which may also be caused by mutations in TCF2 (HNF1ß) and REN (Renin).
To date, 12 genes (NPHP1-12) have been described for recessive NPH plus XPNPEP3 in which mutations cause an NPH-like phenotype (NPHP1L) (Table 3). Given that these genes only account for about the half of all NPH cases, further heterogeneity can be expected. NPHP1 on chromosome 2q13 is the most commonly mutated gene in NPH. In large series of patients, a homozygous deletion of NPHP1 was present in 20–40% of cases [35, 56]. Heterozygous deletions were found in another 6% of patients, harboring a concomitant point mutation on the other parental NPHP1 allele [56]. Other NPHP genes described so far may only contribute a minor part to the total mutational load of typical NPH. Mutations in NPHP2/INVS and NPHP3 lead to infantile and adolescent nephronophthisis, but often do not impress as typically NPH-like (e.g., cortically located microcysts) and may mimic polycystic kidney disease with enlarged kidneys and sometimes even prenatal manifestation of Potter's sequence.
Table 3.
Nephronophthisis and the medullary cystic kidney disease complex
| Disease | Main features | Gene (locus) | Corresponding protein |
|---|---|---|---|
| Nephronophthisis (NPH) and Senior-Loken syndrome (SLSN) | Autosomal recessive | NPHP1 (2q13) | Nephrocystin-1 |
| Hyperechogenic normal or small-sized kidneys with decreased corticomedullary differentiation | INVS/NPHP2 (9q31.1) | Inversin | |
| Tubulo-interstitial nephropathy | NPHP3 (3q22.1) | Nephrocystin-3 | |
| Polyuria, polydipsia, anemia | NPHP4 (1p36.31) | Nephrocystin-4 | |
| Senior-Loken syndrome: NPH + retinal dystrophy | IQCB1/NPHP5 (3q13.33) | IQCB1 | |
| CEP290/NPHP6 (12q21.32) | CEP290 | ||
| GLIS2/NPHP7 (16p13.3) | GLIS2 | ||
| RPGRIP1L/NPHP8 (16q12.2) | RPGRIP1L | ||
| NEK8/NPHP9 (17q11.2) | NEK8 | ||
| SDCCAG8/NPHP10 (1q43) | SDCCAG8 | ||
| TMEM67/NPHP11 (8q22.1) | Meckelin | ||
| TTC21B/NPHP12 (2q24.3) | IFT139 | ||
| XPNPEP3/NPHPL1 (22q13.2) | XPNPEP3 | ||
| Uromodulin-associated diseases: medullary cystic kidney disease (MCKD2), familial juvenile hyperuremic nephropathy (FJHN), glomerulocystic kidney disease (GCKD) | Autosomal dominant | UMOD (16p12.3) | Uromodulin/Tamm-Horsfall protein |
| Normal or reduced kidney volumen with usually small medullary or corticomedullary cysts | |||
| Tubulo-interstitial nephropathy | |||
| Hyperuricemia, gout |
The characterization of the different NPHP proteins (nephrocystins) have considerably supported the understanding of the cystogenesis process and of cilia-related disorders in general. As an example, inversin (NPHP2) has been demonstrated to be crucial in the regulation of Wnt signaling known to be involved in planar cell polarity (PCP). If inversin is defective, the canonical Wnt pathway will dominate over the non-canonical form with subsequent disruption of apical–basolateral polarity in renal epithelial cells. Likewise, a bunch of other signaling pathways as, e.g., hedgehog transduction have been shown to be altered in NPH and other ciliopathies [65].
As discussed, proper ciliary function at the embryonic node is responsible for the spatially controlled and coordinated process that leads to well-defined right- and left-sided positions of abdominal and thoracic structures within the body. Any organ arrangement other than situs solitus and situs inversus is referred to as situs ambiguous often associated with various other anomalies (heterotaxy). For example, patients with Ivemark syndrome and mutations in NPHP3 often show asplenia/polysplenia and cardiac defects in addition to renal-hepatic-pancreatic dysplasia [10].
Joubert syndrome and related disorders
Additional clinical features are frequent in patients with NPH and lead to a variety of other ciliopathies including Senior–Loken (SLSN) and Joubert syndrome (JS/JBTS) and related disorders (JSRD) [35]. With the exception of rare X-linked recessive cases in JBTS10/OFD1, JSRDs follow autosomal recessive inheritance with 25% recurrence risk and constitute a heterogeneous group with currently 13 causative genes known (Table 4). Clinical hallmark is the so-called “molar tooth sign” (MTS), a complex mid-hindbrain malformation with cerebellar vermis hypoplasia visible on MRI (Fig. 4). Clinical correlates are developmental delay/mental retardation, neonatal irregular breathing, hypotonia, ataxia, and eye movement abnormalities [49]. Detection of the MTS should be followed by a multidisciplinary approach and diagnostic protocol to assess multiorgan involvement that often accompanies the neurological features and mainly includes polydactyly, retinal dystrophy, hepatic fibrosis, NPH, and other cystic kidney disease phenotypes. In neonates and young infants, particular attention should be paid to respiratory and feeding problems. Depending on the extent and severity of organ involvement, the prognosis varies considerably among patients with JSRD.
Table 4.
Other syndromic ciliopathies
| Disease | Main features | Gene (locus) | Corresponding protein |
|---|---|---|---|
| Bardet–Biedl syndrome (BBS) | Obesity, hypogonadism, retinal degeneration, polydactyly, cognitive impairment, cystic kidneys of different type, DPM, diabetes mellitus and other metabolic defects, less common other features (hearing loss, anosmia, etc.) | BBS1 (11q13.2) | BBS1 |
| BBS2 (16q12.2) | BBS2 | ||
| ARL6/BBS3 (3q11.2) | ARL6 | ||
| BBS4 (15q24.1) | BBS4 | ||
| BBS5 (2q31.1) | BBS5 | ||
| MKKS/BBS6 (20p12.2) | MKKS | ||
| BBS7 (4q27) | BBS7 | ||
| TTC8/BBS8 (14q31.3) | TTC8 | ||
| BBS9 (7p14.3) | PTHB1 | ||
| BBS10 (12q21.2) | BBS10 | ||
| TRIM32/BBS11 (9q33.1) | TRIM32 | ||
| BBS12 (4q27) | BBS12 | ||
| MKS1/BBS13 (17q22) | MKS1 | ||
| CEP290/BBS14 (12q21.32) | CEP290 | ||
| WDPCP/BBS15 (2p15) | FRITZ | ||
| SDCCAG8/BBS16 (1q43) | SDCCAG8 | ||
| Alstrom syndrome | Obesity, retinal dystrophy, sensorineural hearing loss, dilated cardiomyopathy, progressive pulmonary, hepatic and renal failure, endocrinological features (hypogonadism, diabetes mellitus, hypothyroidism, hyperlipidemia), usually normal intelligence | ALMS1(2p13.1) | ALMS1 |
| Ivemark syndrome | Renal-hepatic-pancreatic dysplasia with disturbed body symmetry (situs ambiguous/inversus) and heterotaxy features such as asplenia/polysplenia and congenital heart defects. | NPHP3 (3q22.1) | Nephrocystin-3 |
| Joubert syndrome (JBTS) and related disorders (JSRD) | Complex mid-hindbrain malformation with cerebellar vermis hypoplasia (Molar Tooth Sign/MTS). Clinical correlates are developmental delay/mental retardation, neonatal irregular breathing, hypotonia, ataxia, and eye movement abnormalities. Other frequent features are polydactyly, retinal dystrophy, DPM, nephronophthisis and other cystic kidney disease phenotypes | INPP5E/JBTS1 (9q34.3) | INPP5E |
| TMEM216/JBTS2 (11q12.2) | TMEM216 | ||
| AHI1/JBTS3 (6q23.3) | Jouberin | ||
| NPHP1/JBTS4 (2q13) | Nephrocystin-1 | ||
| CEP290/JBTS5 (12q21.32) | CEP290 | ||
| TMEM67/JBTS6 (8q22.1) | Meckelin | ||
| RPGRIP1L/JBTS7 (16q12.2) | RPGRIP1L | ||
| ARL13B/JBTS8 (3q11.1) | ARL13B | ||
| CC2D2A/JBTS9 (4p15.32) | CC2D2A | ||
| OFD1/JBTS10 (Xp22.2) | OFD1 | ||
| KIF7 (15q26.1) | KIF7 | ||
| TCTN2 (12q24.31) | Tectonic 2 | ||
| ATXN10 (22q13.31) | Ataxin 10 | ||
| Meckel syndrome (MKS) | Usually, lethal disorder with occipital meningoencephalocele, cystic kidneys, DPM, polydactyly, shortening and bowing of long tubular bones, congenital heart defects, microphthalmia, and cleft lip/palate | MKS1 (17q22) | MKS1 |
| TMEM216/MKS2 (11q13) | TMEM216 | ||
| TMEM67/MKS3 (8q22.1) | Meckelin | ||
| CEP290/MKS4 (12q21.32) | CEP290 | ||
| RPGRIP1L/MKS5 (16q12.2) | RPGRIP1L | ||
| CC2D2A/MKS6 (4p15.32) | CC2D2A | ||
| NPHP3 (3q22.1) | Nephrocystin-3 | ||
| TCTN2/MKS8 (12q24.31) | Tectonic 2 | ||
| B9D1 (17p11.2) | B9D1 | ||
| B9D2 (19q13.2) | B9D2 | ||
| Orofaciodigital syndrome 1 (OFD1) | X-linked dominant condition with embryonic male lethality. Malformations of oral cavity/teeth, face, and digits frequently associated with CNS abnormalities and cystic kidney disease (often PKD phenotype) | OFD1 (Xp22.2) | OFD1 |
| Short rib-polydactyly syndromes (incl. Jeune syndrome/asphyxiating thoracic dystrophy, ATD) | Severely constricted thoracic cage with short ribs often leading to respiratory insufficiency. Further skeletal findings include polydactyly, short long bones, trident acetabular roof. Diverse multisystem organ abnormalities (cystic kidneys, DPM, etc.) | IFT80 (3q25.33) | IFT80/WDR56 |
| DYNC2H1 (11q22.3) | DYNC2H1 | ||
| NEK1 (4q33) | NEK1 | ||
| TTC21B (2q24.3) | IFT139/THM1 | ||
| Ellis-van Creveld syndrome (EVC) | Chondroectodermal dysplasia with disproportionate short stature, acromesomelic shortening of limbs, short ribs, postaxial polydactyly, dysplastic nails and teeth with oral frenulae, congenital heart defects | EVC (4p16.2) | EVC |
| Milder allelic dominant disorder: Weyers acrodental dysostosis | EVC2 (4p16.2) | EVC2 | |
| Cranioectodermal dysplasia (CED/Sensenbrenner syndrome) | Rhizomelic dwarfism, dolichocephaly, ectodermal defects (sparse slowly growing hair, dental and nail hypo-/dysplasia), progressive tubulointerstitial nephropathy, DPM | IFT122 (3q21.3) | IFT122/WDR10 |
| WDR35 (2p24.1) | IFT121/WDR35 | ||
| C14orf179 (14q24.3) | IFT43 |
Most ciliopathies are primarily inherited in an autosomal recessive manner (or sometimes in an oligogenic way)
DPM ductal plate malformation, PKD polycystic kidney disease
Fig. 4.
Axial (a) and sagittal (b) MRI of a patient with Joubert syndrome demonstrating the complex mid-hindbrain malformation typical for JSRD with molar tooth sign (MTS) (a) and cerebellar vermis hypoplasia (b)
It is well known for ciliopathies that mutations in a specific gene do not always lead to a specific phenotype. Clinical severity might depend on the type of mutations present, such that severe loss-of-function mutations (e.g., truncating alleles) lead to severe disease, while more moderate mutations with residual protein function (e.g., hypomorphic missense changes) may give rise to milder phenotypes with later disease onset and possibly also slower progression. Overall, genetic, cellular, and biochemical data indicate that most ciliopathies rather represent a continuum in which the phenotypic outcome is determined by the nature of the mutation in conjunction with the genetic background of the respective patient and oligogenic inheritance patterns. While some genotype–phenotype correlations may allow for prioritization of genes, efficient testing has been largely alleviated by a new generation of sequencing technologies (Next-generation sequencing/NGS) and the possibility of simultaneous investigation of all genes to be discussed in a patient. This knowledge is particularly useful in sporadic, single cases from non-consanguineous marriages in which a direct approach is the only option.
Meckel syndrome
Meckel syndrome (MKS) is allelic to JSRD and related disorders and resides at the severe end of the broad phenotypic spectrum of ciliopathies [10]. MKS is an autosomal recessive, multisystemic disorder of early developmental rather than degenerative nature, and is considered to be the most frequent syndromic cause of neural tube defects. Classic disease manifestations comprise occipital meningoencephalocele, cystic kidney dysplasia, hepatobiliary ductal plate malformation, and postaxial polydactyly (Fig. 5). In addition, several other associated features such as shortening and bowing of long tubular bones, heart defects, microphthalmia, and cleft lip/palate have been reported (Table 4). Survival beyond birth or the neonatal period is unusual with the vast majority of cases dying in utero.
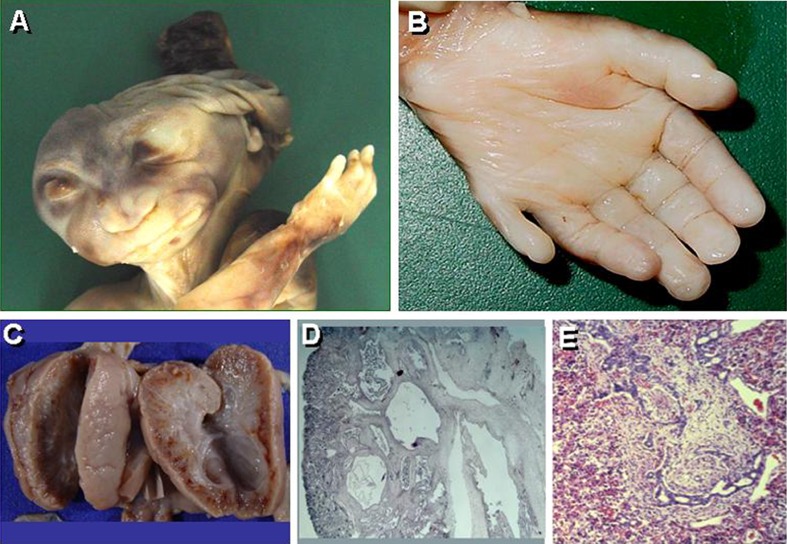
Fig. 5.
Fetus with Meckel syndrome. a Phenotype with occipital meningoencephalocele and a massively malformed brain resembling anencephaly. b Postaxial hexadactyly. c Bilateral considerably enlarged kidneys interspersed with small, pinhead-sized cysts. d Cystic kidney with considerable interstitial fibrosis. e Ductal plate malformation characterized by dysgenesis of the hepatic portal triad with hyperplastic biliary ducts and congenital hepatic fibrosis
To date, nine MKS genes have been identified (Table 4) plus NPHP3 in which mutations cause an MKS-like phenotype [57]. Currently, it is still hard to give exact figures on the contribution of each of these genes to the total mutational load in Meckel syndrome. It is becoming clear that there is even further genetic heterogeneity in MKS, while MKS1, MKS3/TMEM67, and MKS6/CC2D2A might be major MKS genes, followed by MKS4/CEP290. Mutations in MKS genes are mainly truncating, however, particularly in MKS3/TMEM67, missense mutations are also frequent.
Bardet–Biedl syndrome and Alström syndrome
Bardet–Biedl syndrome (BBS) is often called “model ciliopathy” due to its highly pleiotropic nature with multi-system involvement [78]. It is characterized by obesity, hypogonadism, retinal degeneration, polydactyly, mental retardation, and renal malformations (Fig. 6). Renal disease is a major cause of morbidity and mortality in BBS and can be very heterogeneous in phenotype. While some kidneys impress as NPH with tubulo-interstitial disease, others may exhibit findings resembling those usually seen in ARPKD and ADPKD with enlarged, hyperechogenic kidneys and loss of cortico-medullary differentiation, with or without macrocysts. The broad phenotypic spectrum also affects other organs, and a variety of additional features have been described in patients with BBS, such as hearing loss, anosmia, defects in thermosensory and nociceptive sensation, Hirschsprung disease, hepatic changes, metabolic defects, and diabetes mellitus.
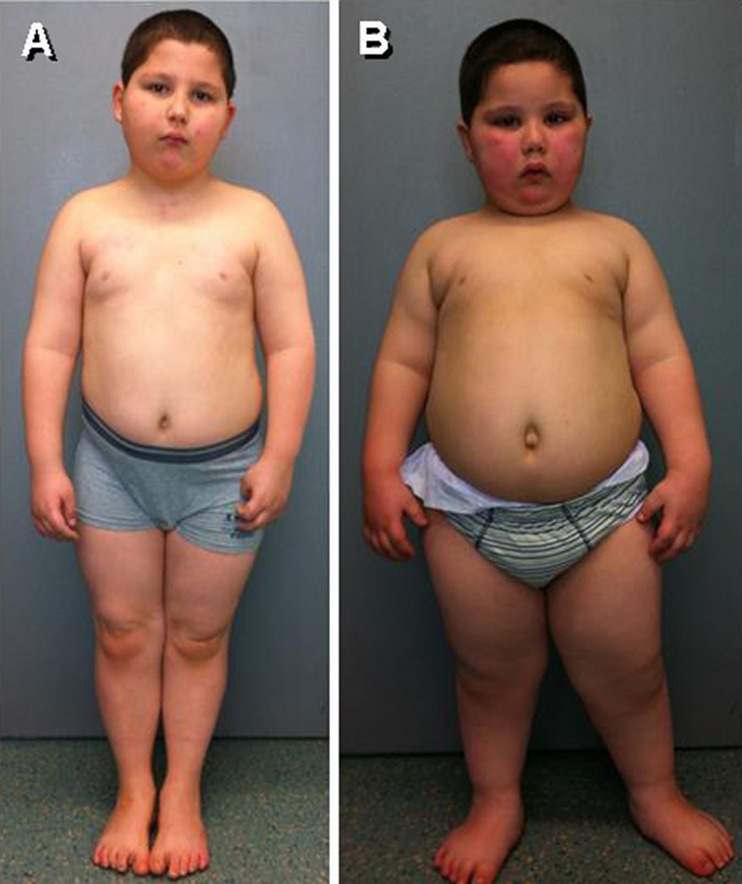
Fig. 6.
Two brothers affected with Bardet–Biedl syndrome
A total of 16 genes have been identified to date (Table 4). BBS1 and BBS10 are major genes in BBS and significantly contribute to the total mutational load. However, figures of about 20% for each of these genes often mentioned in this context might be too high. Two mutations, the M390R allele in BBS1 and the C91LfsX5 allele in BBS10, are especially frequent. While the former mutation is largely absent from non-European populations, the latter change can be found more or less worldwide. All other BBS loci are much rarer than BBS1 and BBS10 and make up only a minor part of the total mutational load [78].
Alström syndrome overlaps with BBS and typically displays obesity, retinal dystrophy, sensorineural hearing loss, and endocrinological features, such as, e.g., hypogonadism, diabetes mellitus, hypothyroidism, and hyperlipidemia. Other features that may point to the correct diagnosis are dilated cardiomyopathy and progressive pulmonary, hepatic, and renal failure. Importantly, most patients have normal intelligence in contrast to BBS patients who are often mentally retarded. The only gene known so far for Alström syndrome is ALMS1 on chromosome 2p13, and most individuals with a typical phenotype harbor two clearly pathogenic mutations in this recessive disease gene of which more than 80% are clustered in three (large) exons [44].
Short rib-polydactyly syndromes and other osteochondrodysplasias
In view of the various skeletal phenotypes in ciliopathies, it is not surprising that primary cilia play a crucial role in skeletal and chondral development. Clinical overlap between the different short rib-polydactyly syndromes and other osteochondrodysplasias clearly support allelism between some of these ciliopathies as part of a large disease spectrum.
Jeune syndrome, also known as asphyxiating thoracic dystrophy (ATD), belongs to the heterogeneous group of short rib-polydactyly syndromes frequently characterized by perinatal lethality due to short ribs with a narrow, bell-shaped chest which restricts the growth and expansion of the lungs and finally leads to respiratory insufficiency (Table 4). Further hallmarks are inconstant polydactyly, short long bones, a trident acetabular roof, and diverse multisystem organ abnormalities of the ciliopathy spectrum [17]. So far, autosomal recessive mutations have been identified in four genes; however, further genetic heterogeneity is certain given that many patients with a clear phenotype do not carry mutations in one of these genes. Three of the known genes encode proteins that are involved in intraflagellar transport: DYNC2H1 encodes the cytoplasmic dynein 2 heavy chain 1, IFT80 is part of the intraflagellar transport complex B and also known as WD repeat-containing protein 56 (WDR56), and TTC21B codes for the retrograde intraflagellar transport protein IFT139 with several tetratricopeptide (TPR) domains [16]. In addition, mutations have been described in the gene encoding the NIMA-related kinase NEK1 with a proposed function in cell-cycle regulation and ciliogenesis [66].
Considerable overlap with ATD shows the chondroectodermal dysplasia Ellis-van Creveld (EVC) syndrome which is characterized by disproportionate short stature with acromesomelic shortening of limbs, short ribs, postaxial polydactyly, and dysplastic ectoderm-derived structures such as nails and teeth with oral frenulae [8]. About two thirds of all patients have a congenital heart defect, specifically abnormalities of atrial and atrioventricular septation. Autosomal recessive loss-of-function mutations in EVC and EVC2, located in a head-to-head configuration on chromosome 4p16, have been identified as causative in individuals affected with EVC. Both EVC genes have been shown to encode positive mediators of Hedgehog signal transduction and localize to the basal bodies of primary cilia. Allelic to EVC is the milder autosomal dominantly inherited phenotype Weyers acrodental dysostosis. Those patients usually carry a mutation in the last coding exon of EVC2 that lead to production of a truncated protein with potential gain-of-function characteristics [55].
The phenotype of individuals with ATD and EVC may overlap with that of patients with cranioectodermal dysplasia (CED, Sensenbrenner syndrome) characterized by dolichocephaly, rhizomelic dwarfism, ectodermal defects (sparse slowly growing hair, dental and nail hypo-/dysplasia), and tubulo-interstitial nephropathy which may lead to end-stage renal failure [42]. Mutations have been recently described for CED in three genes that all encode components of the intraflagellar transport (IFT43, IFT121, and IFT122) and are known to play a crucial role in the assembly and maintenance of primary cilia [24, 73] (Table 4).
Concluding remarks and rational genetic testing approaches
We have learned that there is huge clinical variability in cilia-related disorders even within the same family. How is it possible that one sibling is profoundly affected, whereas another one shows only mild clinical features? Classical Mendelian inheritance patterns with single-locus allelism are insufficient to explain phenomena as variable expressivity and incomplete penetrance. It is rather likely that stochastic, epigenetic, and environmental factors modify the phenotype. Furthermore, oligogenic inheritance with variations across multiple sites influences the clinical outcome and is consistent with a “mutational load theory” [4]. Such “additional” alleles, so-called second-site modifiers, may exert an aggravating effect in an epistatic way and contribute to early and severe disease expression.
Next-generation sequencing (NGS) provides rapidly growing insight in these challenging issues and has significantly improved genetic diagnostics. Conventional (Sanger) sequencing facilities are expensive with very limited capacities and thus only allow for a stepwise approach dependent on mutation frequencies and the ethnic origin and phenotype of the patient. In case of parental consanguinity and in multiplex pedigrees with more than one affected child, linkage analysis with subsequent gene sequencing in case of compatible haplotypes might be an option. However, in the end, all of these conventional approaches are usually more cost- and time-intensive than a primary NGS-based approach that allows for massively parallel sequencing of all disease genes which may have to be discussed in patients of the ciliopathy spectrum. Given overlapping disease phenotypes and extensive allelism, it is more the rule than the exception that multiple, often dozens of genes, have to be considered to be disease-relevant in one of these patients.
The elucidation of molecular mechanisms that explain some of the phenotypic variability has important implications for the understanding of cilia-related disorders. It can be hypothesized that the altered dosage of disease proteins severely disturbs converging pathways, network integrity, and finally cell homeostasis [46, 58]. It is to be expected that similar findings describe a general disease concept not confined to ciliopathies and also apply for unexplained variable disease expression in other conditions called monogenic so far. Those aspects deserve increased attention in genetic counselling and the management of affected families.
Acknowledgement
I would like to graciously acknowledge Anja and Rainer Büscher, Udo Vester, Metin Cetiner, Jens König, and Heymut Omran for their contribution of some of the clinical images. I received support from the Deutsche Forschungsgemeinschaft (DFG BE 3910/4-1, DFG ZE 205/14-1, and SFB/TRR57), Deutsche Nierenstiftung, and PKD Foundation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.AbouAlaiwi WA, Ratnam S, Booth RL, et al. Endothelial cells from humans and mice with polycystic kidney disease are characterized by polyploidy and chromosome segregation defects through survivin down-regulation. Hum Mol Genet. 2011;20:354–367. doi: 10.1093/hmg/ddq470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adeva M, El-Youssef M, Rossetti S (2006) Clinical and molecular characterization defines a broadened spectrum of autosomal recessive polycystic kidney disease (ARPKD). Medicine (Baltimore) 85(1):1–21 [DOI] [PubMed]
- 3.Alvaro D, Mancino MG, Onori P et al (2006) Estrogens and the pathophysiology of the biliary tree. World J Gastroenterol 12:3537–3545 [DOI] [PMC free article] [PubMed]
- 4.Badano JL, Mitsuma N, Beales PL, et al. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 5.Bae KT, Zhu F, Chapman AB, et al. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol. 2006;1:64–69. doi: 10.2215/CJN.00080605. [DOI] [PubMed] [Google Scholar]
- 6.Barbato A, Frischer T, Kuehni C et al (2009) Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J 34(6):1264–1276 [DOI] [PubMed]
- 7.Battini L, Macip S, Fedorova E, et al. Loss of polycystin-1 causes centrosome amplification and genomic instability. Hum Mol Genet. 2008;17:2819–2833. doi: 10.1093/hmg/ddn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baujat G, Le Merrer M. Ellis-van Creveld syndrome. Orphanet J Rare Dis. 2007;2:27. doi: 10.1186/1750-1172-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belibi FA, Reif G, Wallace DP et al (2004) Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int 66(3):964–973 [DOI] [PubMed]
- 10.Bergmann C, Fliegauf M, Bruchle NO, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel–Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008;82:959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergmann C, Küpper F, Dornia C (2005) Algorithm for efficient PKHD1 mutation screening in autosomal recessive polycystic kidney disease (ARPKD). Hum Mutat 25(3):225–231 [DOI] [PubMed]
- 12.Bergmann C, Senderek J, Windelen E (2005) Clinical consequences of PKHD1 mutations in 164 patients with autosomal recessive polycystic kidney disease (ARPKD). Kidney Int 67(3):829–848 [DOI] [PubMed]
- 13.Bukanov NO, Smith LA, Klinger KW, et al. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–952. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 14.Caroli A, Antiga L, Cafaro M et al (2010) Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide. Clin J Am Soc Nephrol 5(5):783–789 [DOI] [PMC free article] [PubMed]
- 15.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 16.Davis EE, Zhang Q, Liu Q et al (2011) TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat Genet 43:189–196 [DOI] [PMC free article] [PubMed]
- 17.de Vries J, Yntema JL, van Die CE, et al. Jeune syndrome: description of 13 cases and a proposal for follow-up protocol. Eur J Pediatr. 2010;169:77–88. doi: 10.1007/s00431-009-0991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desmet VJ (1998) Ludwig symposium on biliary disorders—part I. Pathogenesis of ductal plate abnormalities. Mayo Clin Proc 73(1):80–89 [DOI] [PubMed]
- 19.Drenth JP, Chrispijn M, Bergmann C. Congenital fibrocystic liver diseases. Best Pract Res Clin Gastroenterol. 2010;24:573–584. doi: 10.1016/j.bpg.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 21.Gabow PA, Johnson AM, Kaehny WD et al (1992) Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41(5):1311–1319 [DOI] [PubMed]
- 22.Gabow PA, Johnson AM, Kaehny WD et al (1990) Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology 11(6):1033–1037 [DOI] [PubMed]
- 23.Gambineri A, Patton L, De Iasio R, et al. Efficacy of octreotide-LAR in dieting women with abdominal obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:3854–3862. doi: 10.1210/jc.2004-2490. [DOI] [PubMed] [Google Scholar]
- 24.Gilissen C, Arts HH, Hoischen A, et al. Exome sequencing identifies WDR35 variants involved in Sensenbrenner syndrome. Am J Hum Genet. 2010;87:418–423. doi: 10.1016/j.ajhg.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grantham JJ. Polycystic kidney disease: neoplasia in disguise. Am J Kidney Dis. 1990;15:110–116. doi: 10.1016/s0272-6386(12)80507-5. [DOI] [PubMed] [Google Scholar]
- 27.Grantham JJ, Torres VE, Chapman AB et al (2006) Volume progression in polycystic kidney disease. N Engl J Med: 354(20):2122–2130 [DOI] [PubMed]
- 28.Guay-Woodford LM, Desmond RA (2003) Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics 11(5 Pt 1):1072–1080 [DOI] [PubMed]
- 29.Han YG, Kim HJ, Dlugosz AA et al (2009) Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med 15(9):1062–1065 [DOI] [PMC free article] [PubMed]
- 30.Harris PC, Bae KT, Rossetti S, et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2006;17:3013–3019. doi: 10.1681/ASN.2006080835. [DOI] [PubMed] [Google Scholar]
- 31.Harris PC, Torres VE (2006) Understanding pathogenic mechanisms in polycystic kidney disease provides clues for therapy. Curr Opin Nephrol Hypertens 15(4):456–463 [DOI] [PubMed]
- 32.Harris PC, Torres VE (2009) Polycystic kidney disease. Annu Rev Med 60:321–337 [DOI] [PMC free article] [PubMed]
- 33.Hateboer N, van Dijk MA, Bogdanova N et al (1999) Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 353(9147):103–107 [DOI] [PubMed]
- 34.Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol. 2007;18:1855–1871. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- 36.Hogan M, Masyuk T, Page L et al (2010) Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol 21(6):1052–1061 [DOI] [PMC free article] [PubMed]
- 37.Hossack KF, Leddy CL, Johnson AM, et al. (1988) Echocardiographic findings in autosomal dominant polycystic kidney disease. N Engl J Med 319:907–912 [DOI] [PubMed]
- 38.Huber TB, Walz G, Kuehn EW (2010) mTOR and rapamycin in the kidney: signaling and therapeutic implications beyond immunosuppression. Kidney Int 79(5):502-11 [DOI] [PubMed]
- 39.Johnson AM, Gabow PA (1997) Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J Am Soc Nephrol 8:1560–1567 [DOI] [PubMed]
- 40.Kaelin WG., Jr The von Hippel–Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 41.Kashtan CE, Primack WA, Kainer G (1999) Recurrent bacteremia with enteric pathogens in recessive polycystic kidney disease. Pediatr Nephrol 13(8):678–682 [DOI] [PubMed]
- 42.Konstantinidou AE, Fryssira H, Sifakis S, et al. Cranioectodermal dysplasia: a probable ciliopathy. Am J Med Genet A. 2009;149A:2206–2211. doi: 10.1002/ajmg.a.33013. [DOI] [PubMed] [Google Scholar]
- 43.Kumar S, Adeva M, King BF et al (2006) Duodenal diverticulosis in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 21(12):3576–3578 [DOI] [PubMed]
- 44.Marshall JD, Beck S, Maffei P, et al. Alstrom syndrome. Eur J Hum Genet. 2007;15:1193–1202. doi: 10.1038/sj.ejhg.5201933. [DOI] [PubMed] [Google Scholar]
- 45.Moser M, Matthiesen S, Kirfel J (2005) A mouse model for cystic biliary dysgenesis in autosomal recessive polycystic kidney disease (ARPKD). Hepatology 41(5):1113–1121 [DOI] [PubMed]
- 46.Ocbina PJ, Eggenschwiler JT, Moskowitz I, et al. Complex interactions between genes controlling trafficking in primary cilia. Nat Genet. 2011;43:547–553. doi: 10.1038/ng.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Omran H (2008) Nephronophthis and medullary cystic kidney disease. In: Geary DF, Schaefer F (eds) Comprehensive pediatric nephrology. Elsevier, Amsterdam, pp 143–154
- 48.Onuchic LF, Furu L, Nagasawa Y (2002) PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple Immunoglobulin-Like Plexin-Transcription-Factor Domains and Parallel Beta-Helix 1 Repeats. Am J Hum Genet 70(5):1305–1317 [DOI] [PMC free article] [PubMed]
- 49.Parisi MA. Clinical and molecular features of Joubert syndrome and related disorders. Am J Med Genet C Semin Med Genet. 2009;151C:326–340. doi: 10.1002/ajmg.c.30229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pazour GJ, Dickert BL, Vucica Y, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poliseno L, Salmena L, Zhang J et al (2010) A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465(7301):1033–1038 [DOI] [PMC free article] [PubMed]
- 52.Ravine D, Gibson RN, Walker RG et al (1994) Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343(8901):824–827 [DOI] [PubMed]
- 53.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 54.Ruggenenti P, Remuzzi A, Ondei P, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68:206–216. doi: 10.1111/j.1523-1755.2005.00395.x. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz-Perez VL, Goodship JA. Ellis-van Creveld syndrome and Weyers acrodental dysostosis are caused by cilia-mediated diminished response to hedgehog ligands. Am J Med Genet C Semin Med Genet. 2009;151C:341–351. doi: 10.1002/ajmg.c.30226. [DOI] [PubMed] [Google Scholar]
- 56.Salomon R, Saunier S, Niaudet P. Nephronophthisis. Pediatr Nephrol. 2009;24:2333–2344. doi: 10.1007/s00467-008-0840-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salonen R, Kestilä M, Bergmann C (2011) Clinical utility gene card for Meckel syndrome. Eur J Hum Genet (in press) [DOI] [PMC free article] [PubMed]
- 58.Sang L, Miller JJ, Corbit KC, et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–528. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123:499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 61.Shah AS, Farmen SL, Moninger TO et al (2008) Loss of Bardet–Biedl syndrome proteins alters the morphology and function of motile cilia in airway epithelia. Proc Natl Acad Sci U S A 105(9):3380–3385 [DOI] [PMC free article] [PubMed]
- 62.Shepherd CW, Gomez MR, Lie JT, et al. Causes of death in patients with tuberous sclerosis. Mayo Clin Proc. 1991;66:792–796. doi: 10.1016/s0025-6196(12)61196-3. [DOI] [PubMed] [Google Scholar]
- 63.Sherstha R, McKinley C, Russ P et al (1997) Postmenopausal estrogen therapy selectively stimulates hepatic enlargement in women with autosomal dominant polycystic kidney disease. Hepatology 26(5):1282–1286 [DOI] [PubMed]
- 64.Shillingford J, Murcia N, Larson C et al (2006) The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 103(14):5466–5471 [DOI] [PMC free article] [PubMed]
- 65.Simons M, Walz G. Polycystic kidney disease: cell division without a c(l)ue? Kidney Int. 2006;70:854–864. doi: 10.1038/sj.ki.5001534. [DOI] [PubMed] [Google Scholar]
- 66.Thiel C, Kessler K, Giessl A, et al. NEK1 mutations cause short-rib polydactyly syndrome type majewski. Am J Hum Genet. 2011;88:106–114. doi: 10.1016/j.ajhg.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torra R, Sarquella J, Calabia J et al (2008) Prevalence of cysts in seminal tract and abnormal semen parameters in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 3(3):790–793 [DOI] [PMC free article] [PubMed]
- 68.Torres VE. Treatment strategies and clinical trial design in ADPKD. Adv Chronic Kidney Dis. 2010;17:190–204. doi: 10.1053/j.ackd.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Torres VE, Harris PC (2009) Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int 76(2):149–168 [DOI] [PMC free article] [PubMed]
- 70.Torres VE, Harris PC, Pirson Y (2007) Autosomal dominant polycystic kidney disease. Lancet 369:1287–1301 [DOI] [PubMed]
- 71.Turkbey B, Ocak I, Daryanani K (2009) Autosomal recessive polycystic kidney disease and congenital hepatic fibrosis (ARPKD/CHF). Pediatr Radiol 39(2):100–111 [DOI] [PMC free article] [PubMed]
- 72.Vylet’al P, Kublova M, Kalbacova M, et al. Alterations of uromodulin biology: a common denominator of the genetically heterogeneous FJHN/MCKD syndrome. Kidney Int. 2006;70:1155–1169. doi: 10.1038/sj.ki.5001728. [DOI] [PubMed] [Google Scholar]
- 73.Walczak-Sztulpa J, Eggenschwiler J, Osborn D, et al. Cranioectodermal Dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am J Hum Genet. 2010;86:949–956. doi: 10.1016/j.ajhg.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walz G, Budde K, Mannaa M et al (2010) Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363(9):830–840 [DOI] [PubMed]
- 75.Ward CJ, Hogan MC, Rossetti S et al (2002) The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30(3):259–269 [DOI] [PubMed]
- 76.Weaver BA, Cleveland DW. The aneuploidy paradox in cell growth and tumorigenesis. Cancer Cell. 2008;14:431–433. doi: 10.1016/j.ccr.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong SY, Seol AD, So PL et al (2009) Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med 15(9):1055–1061 [DOI] [PMC free article] [PubMed]
- 78.Zaghloul NA, Katsanis N (2009) Mechanistic insights into Bardet–Biedl syndrome, a model ciliopathy. J Clin Invest 119(3):428–437 [DOI] [PMC free article] [PubMed]