Abstract
Background
The intrarenal renin–angiotensin system contributes to hypertension by regulating sodium and water reabsorption throughout the nephron. Sex differences in the intrarenal components of the renin–angiotensin system have been involved in the greater incidence of high blood pressure and progression to kidney damage in males than females.
Objective
This study investigated whether there is a sex difference in the intrarenal gene expression and urinary excretion of angiotensinogen (AGT) during angiotensin II (Ang II)–dependent hypertension and high-salt (HS) diet.
Methods
Male and female Sprague-Dawley rats were divided into 5 groups for each sex: Normal-salt control, HS diet (8% NaCl), Ang II–infused (80 ng/min), Ang II–infused plus HS diet, and Ang II–infused plus HS diet and treatment with the Ang II receptor blocker, candesartan (25 mg/L in the drinking water). Rats were evaluated for systolic blood pressure (SBP), kidney AGT mRNA expression, urinary AGT excretion, and proteinuria at different time points during a 14-day protocol.
Results
Both male and female rats exhibited similar increases in urinary AGT, with increases in SBP during chronic Ang II infusion. HS diet greatly exacerbated the urinary AGT excretion in Ang II–infused rats; males had a 9-fold increase over Ang II alone and females had a 2.5-fold increase. Male rats displayed salt-sensitive SBP increases during Ang II infusion and HS diet, and female rats did not. In the kidney cortex, males displayed greater AGT gene expression than females during all treatments. During Ang II infusion, both sexes exhibited increases in AGT gene message compared with same-sex controls. In addition, HS diet combined with Ang II infusion exacerbated the proteinuria in both sexes. Concomitant Ang II receptor blocker treatment during Ang II infusion and HS diet decreased SBP and urinary AGT similarly in both sexes; however, the decrease in proteinuria was greater in the females.
Conclusion
During Ang II–dependent hypertension and HS diet, higher intrarenal renin-angiotensin system activation in males, as reflected by higher AGT gene expression and urinary excretion, indicates a mechanism for greater progression of high blood pressure and might explain the sex disparity in development of salt-sensitive hypertension.
Keywords: angiotensinogen, intrarenal RAS, mRNA, proteinuria, sex differences, Sprague-Dawley rats, systolic blood pressure
INTRODUCTION
Young female humans and animals are protected from the development of hypertension and the consequences of end-organ damage as compared with age-matched males.1,2 Because sex hormones have been found to influence blood pressure and the renin–angiotensin system (RAS), it has been suggested that differences in the intrarenal RAS of male and female rats contributes to the disparity in hypertension.3–5 In the intrarenal RAS, the principal source of angiotensinogen (AGT) is the proximal tubule cells.6 The size of the AGT protein (>50 kDa) minimizes filtration across the glomerular membrane unless damage has occurred.7 Increased AGT message and protein, along with AGT urinary excretion in male rats during angiotensin (Ang) II–dependent hypertension,8 suggest increased AGT availability for local generation of Ang I and conversion to Ang II.9–11 Increased intrarenal Ang II leads to additional sodium reabsorption because Ang II type 1 (AT1) receptors are present in the proximal and distal nephron.12 Urinary AGT measurements have been used in several models of hypertension and kidney disease (both rodents and humans) as an early biomarker of internal RAS activity.13 Urinary excretion of AGT provides an index of available AGT in the tubules.14 Sex differences in the intrarenal AGT expression have been reported during Ang II–dependent hypertension,15–17 but sex differences in the urinary excretion of AGT have not been investigated comprehensively.
A high-salt (HS) diet alone does not cause high blood pressure in healthy Sprague-Dawley rats; however, it can exacerbate existing hypertension.18–21 Sartori-Valinotti et al found in Sprague-Dawley rats that HS intake combined with chronic Ang II infusion, while blocking the endogenous RAS with angiotensin-converting enzyme (ACE) inhibition, increased mean arterial pressure in male but not female rats.15 In the same study, AGT protein expression increased in the kidney cortex of males but not females, suggesting that HS exacerbates the pathological responses of AGT to Ang II in males only.15 Lara et al21 reported that male Sprague-Dawley rats with chronic Ang II infusion that were subjected to HS diet exhibit exacerbation of blood pressure, urinary AGT excretion, and oxidative stress. Additional evidence suggesting that salt sensitivity is sex dependent comes from studies of sodium excretion reporting that female rats with chronic Ang II infusions excrete salt overload more efficiently.22,23 HS diet in the transgenic Cyp1a1Ren2 rat, a model of Ang II–dependent malignant hypertension, inappropriately increases plasma and intrarenal Ang II levels in male rats and exacerbates hypertension.24,25 In addition, mRen(2).Lewis rats, a congenic rat strain with Ang II–dependent hypertension and salt sensitivity has exhibited increases in urinary AGT. However, the effect of HS on urinary AGT in a salt-resistant rat model remains unresolved.26
Based on evidence from previous studies reporting that young females are protected from salt sensitivity,4,17 we hypothesized that male Sprague-Dawley rats have greater intrarenal RAS activation in response to salt during Ang II hypertension than female rats, which would exacerbate hypertension and kidney damage. The present study examined the effect of HS diet on blood pressure, intrarenal AGT expression, and urinary AGT excretion in male and female Sprague-Dawley rats during control normotensive conditions and chronic Ang II infusion. In addition, proteinuria was measured as a marker of renal damage to assess sex differences in the response of male and female rats to the Ang II–HS protocol. The angiotensin II receptor blocker (ARB), candesartan, was given to Ang II–salt male and female rats to elucidate the effects of AT1 receptor blockade on the individual parameters. Blood pressure measurements and 24-hour urine collections were taken multiple times during the 14-day study to examine the progression of hypertension, urinary excretion of AGT, and proteinuria. To assess the contribution of intrarenal AGT to the urinary AGT excretion, AGT mRNA levels were measured in the renal cortex.
METHODS
Experimental Protocol
All protocols were evaluated and approved by the Tulane Institutional Animal Care and Use Committee and conformed to the guidelines of the National Institutes of Health on the care and use of laboratory animals. Male (n = 40) and female (n = 36) Sprague-Dawley rats, 7 (1) weeks of age (Charles River Laboratories, Wilmington, Massachusetts), were cage housed and maintained in a temperature-controlled room on a 12-hour light to dark cycle, with free access to tap water and rat chow during acclimation. Body weight (BW; in grams), food intake, water intake, and urine output were measured every 3 to 4 days (days −1, 3, 6, 9, and 13) after placing the rats in metabolic cages for 24 hours. After a 7-day training period, the systolic blood pressure (SBP) was monitored by tail-cuff plethysmography (Model 31; IITC Life-sciences, Woodland Hills, California). On day −1, rats of each sex were divided into 5 groups as follows: (1) males: normal salt (M normal salt; n = 9); (2) high salt (M HS; n = 9); (3) Ang II–infused (M Ang II; n = 8); (4) Ang II–infused plus HS diet (M Ang II + HS; n = 9); and (5) Ang II–infused plus HS and candesartan (M Ang II + HS + Cand; n = 5) and females: (1) normal salt (F normal salt; n = 8); (2) HS (F HS; n = 8); (3) Ang II–infused (F Ang II; n = 8); (4) Ang II–infused plus HS diet (F Ang II + HS; n = 7); and (5) Ang II-infused plus HS and candesartan (F Ang II + HS + Cand; n = 5). After baseline metabolic data were obtained on day −1, rats were anesthetized with Isoflurane and subcutaneous minipumps (Alzet 2002; Phoenix Pharmaceutical, Burlingame, California) were implanted to provide Ang II infusion at a rate of 80 ng/min for 14 days. The normal salt and HS rats received sham surgery. HS diet began on day 3 (8% NaCl; Dyets, Bethlehem, Pennsylvania). Rats not receiving the HS diet remained on normal-salt (0.7% NaCl) rat chow (Ralston Purina, St Louis, Missouri). Candesartan (AstraZeneca, Newark, Deleware) was administered in the drinking water at a concentration of 25 mg/L. At the end of the protocol, day 14, rats were euthanized by conscious decapitation. Trunk blood samples were collected and kidney tissue was preserved in RNAlater (Qiagen, Germantown, Maryland).
Plasma Renin Activity
For plasma rennin activity (PRA) determinations, blood samples were collected into a chilled tube containing 5 mM EDTA, centrifuged at 4000 rpm for 30 minutes at 4°C for plasma fractions separation. Measurements of the PRA were performed as indicated by the manufacturer (Diasorin, Stillwater, Minnesota) and expressed as ng/mL/h of generated Ang I as described previously.27
Plasma Sex Hormones
Testosterone levels were measured using the Correlate EIA kit (Assay Designs, Detroit, Michigan) in plasma samples preserved in 5 mM EDTA and diluted 15-fold with assay buffer, according to kit instructions. For 17β-estradiol levels, plasma samples preserved in similar conditions were assayed after liquid–liquid extraction using diethyl ether and rehydrated in 250 μL assay buffer. A 4-fold dilution was then prepared and measured according to instructions of the Serum/Plasma 17β-Estradiol EIA Kit (Assay Designs).
Urine Protein Excretion
Twenty-four–hour urine samples were collected from rats placed in metabolic cages on days −1, 3, 6, 9, and 13. Urine samples were centrifuged at 3000 rpm for 10 minutes to remove large particulates and assayed for protein content using the BioRad modified Bradford Assay (BioRad, Hercules, California). Urine samples were diluted 1:25 and proteinuria was measured by spectrophotometry and expressed as μg/d/BW(g).
Urine Angiotensinogen Excretion
Angiotensinogen excretion in urine samples was measured as described previously28 using the Rat Total Angiotensinogen Assay Kit (IBL Ltd, Fujioka, Japan) according to kit instructions and reported as ng/d/BW(g) excretion.
Renal ATG mRNA Expression
For AGT mRNA levels, 20 ng/well total RNA extracted from renal cortex samples was amplified using a Brilliant Mastermix II kit (Statagene, Santa Clara, California) and the Mx 3000p real time reverse transcription polymerase chain reaction machine (Statagene). The following primers: [Sense 5′-GAA GAT GAA CTT GCC ACTA GA-3′; Anti-sense 5′-AAG TGA ACG TAG GTG TTG AAA-3′] and the probe 5′-CAG CAC GGA CAG CAC CCT ATT-3′; in addition to coamplification of the glyceraldehyde 3-phosphate dehydrogenase gene labeled with 5′-HEX and 3′-black hole quencher-2. AGT mRNA levels were normalized based on glyceraldehyde 3-phosphate dehydrogenase mRNA levels.
Statistical Analysis
Results, expressed as mean (SEM), were analyzed with Prism GraphPad software (La Jolla, California). Data were evaluated both within sex and between sexes using one-way or two-way ANOVA, followed by Tukey or Bonferroni post test. The significance of differences among groups is defined at a value of P < 0.05.
RESULTS
Metabolic Variables and Sex Hormones in Male and Female Sprague-Dawley Rats
The BW, food intake/BW (mg/g), water intake/BW (mL/g), PRA, and hormonal levels achieved at the end of the study are summarized in the Table. Food consumption was similar when factored by BW, except in rats with chronic administration of Ang II (M Ang II: 0.07 [0.01] vs F Ang II: 0.10 [0.01] mg/g; P < 0.05). Food with HS did not affect food intake, as reported previously29,30; however, it did increase water consumption. Water intake when factored by BW was different in the control groups only (M normal salt 0.1 [0.01] vs F normal salt 0.6 [0.01] mL/g; P < 0.05). Male and female rats receiving candesartan in the drinking water also consumed comparable amounts of candesartan. Calculated candesartan ingestion per day was 5.7 (0.4) mg/kg BW for males and 6.2 (0.7) mg/kg BW for females (P = NS).
Table.
Metabolic data and sex hormones.
| BW, g |
Food/BW, mg/g |
Water/BW, mL/g |
PRA, ng Ang I/mL/h |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Male | Female | Male | Female | Male | Female | Male | Female | Test, ng/mL Male | E2, pg/mL Female |
| NS | 330 (8) | 217 (12)* | 0.08 (0.01) | 0.09 (0.01) | 0.1 (0.01) | 0.6 (0.01)* | 5.5 (2) | 6.0 (0.4) | 9.7 (2) | 21.6 (21) |
| HS | 324 (7) | 225 (5)* | 0.06 (0.01) | 0.07 (0.01) | 0.2 (0.01) | 0.2 (0.01) | 0.14 (0.1)† | 2.2 (1)*,† | 10.1 (1) | 49.4 (5) |
| Ang II | 296 (7) | 209 (4)* | 0.07 (0.01) | 0.10 (0.01)* | 0.2 (0.01) | 0.3 (0.01) | 0.32 (0.2)† | 0.2 (0.1)† | 11.9 (5) | 55.3 (10) |
| Ang II + HS | 253 (9) | 196 (19)* | 0.06 (0.01) | 0.07 (0.02) | 0.4 (0.01) | 0.4 (0.01) | 0.22 (0.1)† | 1.9 (1)*,† | 4.2 (1)† | 36.5 (13) |
| Cand | 271 (11) | 224 (8)* | 0.06 (0.01) | 0.06 (0.01) | 0.3 (0.01) | 0.3 (0.01) | 41 (12)† | 0.3 (0.2)† | 7.3 (3) | 29.3 (7) |
Values are mean (SEM) for control (normal salt [NS]; n = 17), high salt (HS; n = 17), angiotensin II (Ang II; n = 16), Ang II + HS (n = 16), Ang II + HS + Candesartan (Cand; n = 10) rats of both sexes. Hypertension was induced with Ang II infusion via subcutaneous minipump at a rate of 80 ng/min for 14 days. Candesartan was administered in the drinking water (25 mg/L). High-salt diet was 8% NaCl in rat chow.
BW = body weight; Test = testosterone; E2 = 17β estradiol; PRA = plasma renin activity.
P < 0.05 compared with opposite sex, same treatment group.
P < 0.05 compared with same sex, normal salt control.
Male and female normal-salt rats exhibited similar levels of PRA. PRA was markedly suppressed by chronic Ang II infusion, HS, and the combination of Ang II and HS in the males, as it was in the Ang II–infused female rats (Table). However, in female rats with HS intake, both with and without Ang II infusion, PRA was only partially (65%) inhibited. PRA response to candesartan in Ang II + HS rats was markedly augmented in males; however, it was suppressed in female rats treated with candesartan.
Plasma testosterone in males and estradiol levels in females were measured from blood samples collected on day 14 to evaluate sexual maturity. As reported in the Table, testosterone levels were similar in all groups except the Ang II + HS rats, which had lower values, although still within the normal range (0.5 to 15 ng), as reported previously.31 Reduction of testosterone levels during hypertension has been reported in humans.32 Estradiol levels in all female rats indicated that these rats were sexually mature as described by others.33
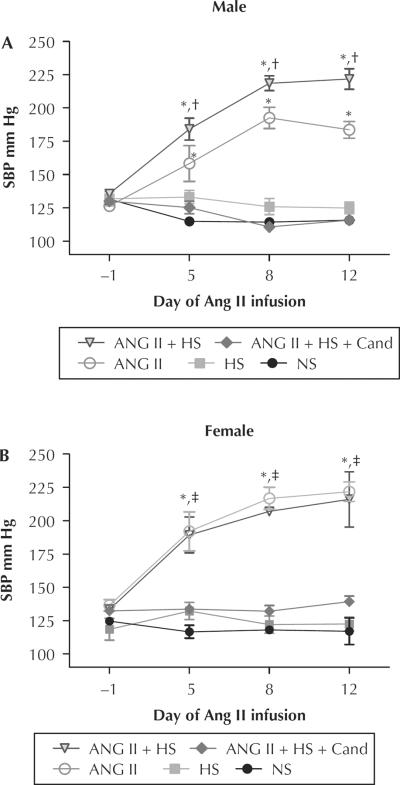
Baseline SBP values were similar between male and female rats (M: 131 [1] vs F: 129 [2] mm Hg) (Figure 1). After 14 days of chronic Ang II infusion, the SBP was increased in both sexes. Although HS diet alone did not change the SBP in either sex; in male rats, the coadministration of an HS diet with Ang II infusion augmented SBP values further from 184 [6] to 222 [8] mm Hg; P < 0.05), but did not augment the SBP further in female rats (222 [7] vs 216 [21] mm Hg; P = ns). Candesartan treatment prevented increases in SBP in rats of both sexes infused with Ang II and fed HS (Figure 1).
Figure 1.
Mean (SEM) systolic blood pressure (SBP) in mm Hg measured by tail-cuff method on day −1, day 5, day 8, and day 12 during 14-day study. (A) Male. (B) Female. Ang II = angiotensin II–infused (80 ng/min); Ang II + HS = angiotensin II infusion plus high-salt diet; Ang II + HS + Cand = angiotensin II infusion plus high-salt diet and candesartan (25 mg/L in the drinking water); HS = high salt (8% NaCl); NS = normal salt. *P < 0.05 compared with same-sex NS control. †P < 0.05 male Ang II compared with male Ang II + HS. ‡P < 0.05 compared with opposite-sex same treatment group.
Effects of Ang II Infusion and an HS Diet on Urinary AGT and Kidney Cortex AGT Gene Expression
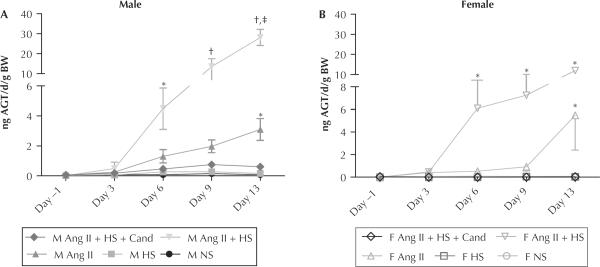
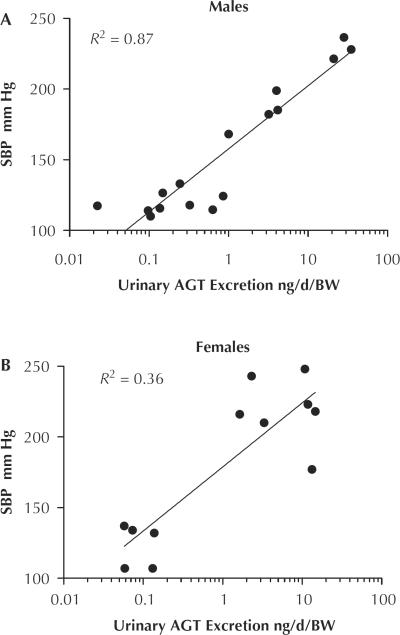
Urinary AGT excretion was measured in 24-hour urine samples collected on days −1, 3, 6, 9, and 13 of the study and normalized by BW measurements taken on the same day (fractional AGT excretion) (Figure 2). Fractional AGT excretion was similar between male and female normal-salt diet controls. HS diet alone did not increase the urinary AGT excretion in either sex. During Ang II infusion, both the male and female rats had significant increases in fractional urinary AGT excretion (male: normal salt 0.08 [0.03] vs Ang II 3.1 [0.7] ng/d/g BW; P < 0.01) (female: normal salt 0.08 [0.02] vs Ang II 4.7 [3.3] ng/d/g BW; P < 0.05). In addition, when HS was administered to Ang II–infused rats, there was an augmentation of urinary AGT excretion in both sexes; but this response was more pronounced in male rats: 28.1 [4] vs female: 12.0 [0.7] ng/d/g; P < 0.001). Candesartan treatment similarly ameliorated the increases in urinary AGT excretion in both sexes (Figure 2). Correlation analysis between urinary AGT excretion and SBP for each sex revealed a closer relationship in the male rats (R2 = 0.88; P < 0.0001) than the female rats (R2 = 0.33; P < 0.05) (Figure 3).
Figure 2.
Mean (SEM) angiotensinogen (AGT) in urine is expressed as ng/d/g body weight (BW) at baseline (day −1) and 4 times during the 14-day study (n = 3/per group/per day). (A) Male (M). (B) Female (F). Ang II = angiotensin II–infused (80 ng/min); Ang II + HS = angiotensin II infusion plus high-salt diet; Ang II + HS + Cand = angiotensin II infusion plus high-salt diet and candesartan (25 mg/L in the drinking water); HS = high salt (8% NaCl); NS = normal salt; HS = high salt diet (8% NaCl). *P < 0.05 and †P < 0.01 compared with same-sex normal salt control (NS). ‡P < 0.01 compared with opposite-sex same treatment group.
Figure 3.
Correlation of systolic blood pressure (SBP) and urinary angiotensinogen (AGT) excretion factored by body weight (BW) measured on day 13 of the study. (A) Males, n = 16. (B) females, n = 12.
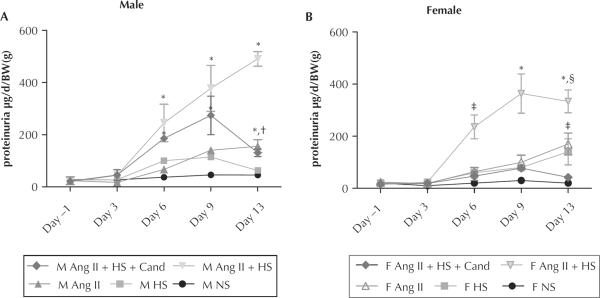
Figure 4 reports the 24-hour urinary protein excretion rates normalized by BW in male and female rats for days −1, 3, 6, 9, and 13. HS diet alone did not cause proteinuria in either male or female rats. By day 13, chronic Ang II infusion increased urinary protein excretion, regardless of sex (male: 156 [25]; female: 170 [42] μg/d/g; P < 0.05 compared with the same sex; normal salt). The combination of Ang II infusion and HS diet caused marked exacerbation of the proteinuria in both sexes, which was similar until day 13 (male: 491 [28] vs female: 334 [44] mg/d/g; P < 0.05). Also, the addition of HS to the Ang II infusions accelerated the onset of proteinuria, with both sexes reaching significance compared with their normal-salt controls by day 6. Candesartan treatment prevented proteinuria throughout the experimental protocol in females. In contrast, male rats subjected to chronic Ang II infusion plus HS diet and concomitant candesartan treatment exhibited similar levels of proteinuria to male rats under Ang II infusion and HS diet, except by day 13, when protein levels were significantly reduced (M Ang II + HS: 491 [28] vs M Cand: 130 [14] μg/d/BW(g); P < 0.01).
Figure 4.
Mean (SEM) proteinuria in (A) male (M) and (B) female (F) rats measured on days −1, 3, 6, 9, and 13 of the study and expressed as micrograms protein excreted per day per gram of body weight (BW). Ang II = angiotensin II infusion (80 ng/min for 14 d); Ang II + HS = angiotensin II infusion plus high-salt diet; Ang II + HS + Cand = angiotensin II infusion plus high-salt diet plus candesartan (25 mg/L in the drinking water); HS = high-salt diet (8% NaCl); NS = normal-salt diet. *P < 0.01, compared with same-sex normal-salt control (NS). †P < 0.05 M: Ang II + HS vs M Ang II + HS + Cand. ‡P < 0.05; §P < 0.05 compared with opposite sex same treatment group.
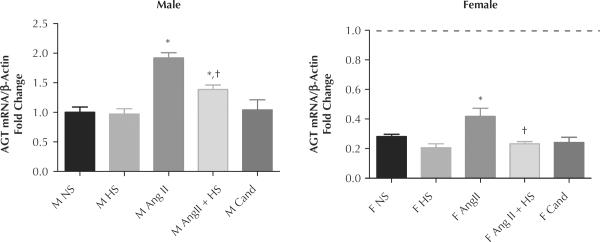
The AGT gene message was measured to determine intrarenal RAS activation. In all treatment groups the AGT mRNA expression levels were significantly higher in male than in female rats (Figure 5) (normal-salt M: 1.0 [0.09] vs normal-salt F: 0.28 [0.02]-fold change; P < 0.05). Both sexes had significant increases in AGT message during Ang II infusion; however, expression in the males was still greater (Ang II M: 0.95 [0.13] vs Ang II F: 0.25 [0.06] change; P < 0.05). The AGT expression in male rats with Ang II and HS diet remained significantly higher than normal-salt males, while the Ang II + HS female rats returned to baseline. Both sexes receiving candesartan exhibited AGT expression levels similar to controls.
Figure 5.
Mean (SEM) angiotensinogen (AGT) mRNA levels taken from cortex kidney samples. (A) Male, normalized to male control normal salt (NS) and presented as fold change. (B) Female, normalized to female NS. Dashed line represents male NS level. Ang II = angiotensin II infusion (80 ng/min for 14 d); Ang II + HS = angiotensin II infusion plus high-salt diet; Ang II + HS + Cand = angiotensin II infusion plus high-salt diet plus candesartan in the drinking water (25 mg/L); NS = normal-salt diet; HS = high-salt diet (8% NaCl). *P < 0.05 compared with same-sex NS. †P < 0.05 compared with same-sex Ang II.
DISCUSSION
The present study found that Ang II–salt hypertension displays sex differences because male Sprague-Dawley rats display a salt-sensitive augmentation in SBP during chronic Ang II infusion that is not apparent in female rats; and AGT urinary excretion increases to a greater extent in males than in females with Ang II and HS diet, even after factoring by BW. In addition, the present study reports that female rats have lower AGT gene expression in the kidney cortex than males, indicating that, in female rats, less activation of the intrarenal RAS occurs during normotensive and hypertensive states.
SBP was comparable in male and female sham rats in this study using the tail-cuff method of measurement, and others have found sex differences using 24-hour telemetry.15 Differences in sensitivity between these 2 methods might explain this discrepancy. In our study, females receiving Ang II exhibited higher SBP than males. This response could be explained by the higher dose of Ang II relative to BW in the females, perhaps leading to a maximum increase in blood pressure. The addition of an HS diet did not cause additional increases. However, similar findings have been reported in Sprague-Dawley rats chronically infused with Ang II subjected to endogenous RAS blockade with ACE inhibition.15 Regardless of the difference in the experimental protocol because we did not clamp the endogenous RAS, similar temporal patterns in blood pressure responses are noticed. Sartori-Valinotti et al15 infused Ang II at a dose of 150 ng/kg/min started 5 days after ACE inhibition. The female rats displayed higher mean arterial pressure than males during the following week. However, this sex difference was reversed after an HS diet was added to the protocol (day 16), and continued to increase in the males only until day 29 of the study. In the present study, we infused 80 ng/min for only 2 weeks, which increased SBP in the females to a greater extent than males, similar to Sartori-Valinotti et al's observation during the Ang II–administration phase in the presence of a normal-salt diet. In addition, as described by Sartori-Valinotti et al, after an HS diet (4% NaCl) was added, the male rats exhibited greater mean arte rial pressure and the females continued to exhibit values similar to the rats infused with Ang II and fed a normal-salt diet.15 In male mice during chronic Ang II infusion and ACE inhibition, reduced responses of intrarenal RAS have been reported to be associated with ameliorated augmentations in blood pressure and intrarenal Ang II content.34 However, this study was conducted during normal-salt diet conditions and no female subjects were assessed.
It has been suggested that young female rats are protected from salt-sensitive hypertension by estrogen,4,22 which might explain why males in this study displayed a salt-sensitive increase in SBP and the females did not. Harrison-Bernard et al4 reported that young ovariectomized rats had increased AT1 receptor protein levels in the kidney and suggested that salt sensitivity was via an Ang II mechanism.4 In the present study, female and male rats were young (10 weeks of age) when euthanized. Both male and female rats received Ang II infusion at a dose of 80 ng/min. Despite the smaller BW of the females and the possibility that they had greater systemic Ang II levels, they did not experience greater intrarenal RAS activation, as reflected by the diminished response of intrarenal AGT compared with males. This could be explained by the reduced AT1 receptors and AT1–Ang II binding as suggested by others.4,35,36
Urinary excretion of AGT in hypertensive male rats is currently of great interest in light of the significance that the presence of the intrarenal AGT can have for de novo intratubular Ang II formation, as well as for progression of hypertension and chronic renal diseases.28,37 Kobori et al13 proposed the use of urinary AGT excretion as a biological marker of elevated intrarenal Ang II levels in these conditions. The timeline in the present study provides evidence of the early response urinary AGT has to Ang II–salt augmentation. Urinary AGT excretion during Ang II–salt appeared by day 6 in both sexes. By day 14, the fractional urinary AGT excretion in Ang II–salt males increased 30-fold over controls, and the females increased about 10-fold. The greater exacerbation in males might be related to the influence of male hormones because testosterone is required for the full expression of the salt-sensitive hypertension phenotype in some rat models.38 Afsar39 recently evaluated hypertensive male patients and reported a negative correlation between testosterone levels and urinary sodium excretion,39 which could be a mechanism for increased hypertension. Yanes et al16 reported that castration in male rats reduced intrarenal expression of AGT, mean arterial blood pressure, and proteinuria during salt-sensitive hypertension in the Dahl SS model. The coexistence of proteinuria and augmented urinary AGT excretion observed in the present study and others17 suggests that the glomerular barrier might be disrupted and that some tissue injury occurred. Recent work by Lara et al21 reported that the combination of Ang II and HS diet in male rats exacerbated urinary excretion of AGT and renal injury compared with Ang II alone due to increased macrophage infiltration in the tubule-interstitial area, cell proliferation, and collagen deposition.21 An excess of AGT in the urine, from either source, systemic or intrarenal, indicates an augmented availability of the RAS substrate within the nephron lumen and greater intratubular Ang II de novo formation capability.10,40–42
This study found that female rats have lower levels of AGT gene expression than male rats under both control and Ang II–infused conditions. In addition, the data found that HS diet does not alter the AGT mRNA levels in the renal cortex or urinary AGT of male and female rats. Additionally, the increase in AGT gene expression seen in male rats during Ang II infusion is confirmed in females for the first time.8 Because urinary AGT excretion between male and females during chronic Ang II infusions is similar when normalized by BW, it might be that lower AGT expression levels in the female reflect their smaller size. Although the combination of Ang II infusion with HS diet did not increase AGT mRNA, it significantly exacerbated the urinary excretion of AGT, as described previously.8 These findings suggest that salt per se might not increase urinary AGT via intrarenal RAS activation; however, it might trigger other intracellular mechanisms, such as mitogen-activated protein kinase, transforming growth factor–β, and nitric oxide pathways,43–45 when it is combined with Ang II.21 Proteinuria levels in the Ang II–salt rats can also indicate that filtration of systemic AGT into the tubules could greatly contribute to the exacerbation of urinary AGT without changes in transcript levels; however, the origin of the AGT in the urine of the rats was not assessed in the present study.
Sex difference in the correlation of urinary AGT excretion with SBP correlation is consistent with the observation that the intrarenal RAS might be more directly involved in the control of SBP in male than in female rats. This seems likely considering that estrogens, by acting through receptors, reduce AT1 receptor density, primarily by inhibiting their translation at a ribosomal level35,36,46 and by decreasing AT1 receptor binding.35,36,46 The potential mechanism for the protective effects of estrogen has also been suggested by evidence found that after ovariectomy in salt-sensitive rats, there is a lower threshold for the hypertensinogenic effect of salt that is linked to an activation of Ang II.35,36,46
Candesartan, as a therapy for cardiovascular diseases, has been assessed frequently for its ability to lower SBP, improve cardiac remodeling, and protect renal function.47–49 Several studies have either found no sex differences in response to ARB therapy or suggested that ARB treatment might work better in women than in men.47,50 In the present study, we found similar blood pressure–lowering abilities for candesartan and urinary AGT excretion responses in male and female rats after factoring by BW (male: 98% vs female: 99% change). However, candesartan was better at preventing proteinuria in the female rats (male: 74 vs female: 85% change; P < 0.05). Male rats receiving candesartan had a rise in proteinuria that was only ameliorated at the end of the study. It has been previously reported that although candesartan prevents increases in reactive oxygen species in rats with chronic Ang II infusion and HS diet, it does not prevent collagen deposition or mesangial expansion, indicating that kidney damage was still present in the candesartan-treated males.21
We found suppression of PRA in the females receiving candesartan. This finding contrasts with previous studies reporting increased PRA during Ang II blockade by ACE inhibition or ARB therapy in male and female rats.5,51 In human volunteers of both sexes, Miller et al52 reported increased PRA levels during chronic Ang II infusion and irbesartan for 4 weeks. Accordingly, in the present study, male rats receiving coadministration of Ang II and HS diet and candesartan also displayed very high renin activity in the plasma. The difference in the present study from those previous reports is the coadministration of HS diet to the chronic Ang II infusions. Sartori-Valinotti et al15 reported significantly decreased PRA after ACE inhibition in both sexes of Sprague-Dawley rats chronically infused with Ang II and fed an HS diet. Susic et al53 also reported no rise in PRA of SHR rats fed HS and given losartan, while the normal-salt diet rats administered losartan did have increased PRA. Plasma AGT was not measured in the current study, but a reduction in the availability of the substrate, AGT, by chronic high Ang II and HS diet might have affected the female PRA levels. PRA is reported as nanograms of Ang I formed and the assay is performed without the addition of excess substrate, relying only on the naturally occurring renin and AGT in the plasma sample.27 Therefore, a lack of substrate would result in a reduced PRA response.
CONCLUSION
The present data indicate that males exhibit greater activation of the intrarenal RAS during chronic Ang II–salt hypertension. Female rats fed an HS diet exhibited no further increase in SBP and less augmentation of urinary AGT and proteinuria during Ang II–dependent hypertension. These data support the notion that the effects of HS consumption in the presence of hypertension exhibit sexual dimorphism in the kidneys of male and female Sprague-Dawley rats. Importantly, this study provides evidence that, in male rats, only short exposure to an HS diet is required to cause proteinuria or exacerbate urinary AGT excretion during Ang II–salt hypertension.
ACKNOWLEDGMENTS
The authors express their thanks to the Tulane Hypertension and Renal Center of Excellence for facilitating the use of the Molecular and Analytical Cores. The data reported in this article are part of the research dissertation work of Vicky F. Rands, MS and were partially presented during the 2009 APS Conference on Sex and Gender in Cardiovascular-Renal Physiology and Pathophysiology and the 64th High Blood Pressure Research Conference in 2010. This work was partially supported by the National Institutes of Health (P20-RR-017659) from the Institutional Developmental Award (IdeA) program of NCRR. MCP is a scholar of the Tulane-BIRCWH Program (K12HD043451) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and a recipient of the American Heart Association Beginning Grant-In-Aid (09BGIA2280440). Dr. Rands and Mr. Seth were responsible for data collection and analyses. Drs. Rands and Prieto were responsible for data interpretation and preparation of the manuscript. Dr. Kobori provided ELISA Kits for determination of angiotensinogen in urine samples.
Footnotes
CONFLICTS OF INTEREST The authors have indicated that they have no conflicts of interest regarding the content of this article.
REFERENCES
- 1.Silbiger SR, Neugarten J. The impact of gender on the progression of chronic renal disease. Am J Kidney Dis. 1995;25:515–533. doi: 10.1016/0272-6386(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 2.Miller JA, Anacta LA, Cattran DC. Impact of gender on the renal response to angiotensin II. Kidney Int. 1999;55:278–285. doi: 10.1046/j.1523-1755.1999.00260.x. [DOI] [PubMed] [Google Scholar]
- 3.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 4.Harrison-Bernard LM, Schulman IH, Raij L. Postovariectomy hypertension is linked to increased renal AT1 receptor and salt sensitivity. Hypertension. 2003;42:1157–163. doi: 10.1161/01.HYP.0000102180.13341.50. [DOI] [PubMed] [Google Scholar]
- 5.Yanes LL, Romero DG, Iles JW, et al. Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R383–R390. doi: 10.1152/ajpregu.00510.2005. [DOI] [PubMed] [Google Scholar]
- 6.Ingelfinger JR, Zuo WM, Fon EA, et al. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haraldsson B, Nyström J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 8.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Von Thun AM, Vari RC, el-Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol Renal Physiol. 1994;266:F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 10.Casarini DE, Boim MA, Stella RC, et al. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol. 1997;272(3 Pt 2):F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 11.Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol. 1997;17:412–422. [PubMed] [Google Scholar]
- 12.Harrison-Bernard LM, Navar LG, Ho MM, et al. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol. 1997;273(1 Pt 2):F170–F177. doi: 10.1152/ajprenal.1997.273.1.F170. [DOI] [PubMed] [Google Scholar]
- 13.Kobori H, Alper AB, Shenava R, et al. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–350. doi: 10.1161/HYPERTENSIONAHA.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sartori-Valinotti JC, Iliescu R, Yanes LL, et al. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension. 2008;51:1170–1176. doi: 10.1161/HYPERTENSIONAHA.107.106922. [DOI] [PubMed] [Google Scholar]
- 16.Yanes LL, Sartori-Valinotti JC, Iliescu R, et al. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2009;296:F771–779. doi: 10.1152/ajprenal.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen JA, Lindsey SH, Pirro NT, et al. Influence of estrogen depletion and salt loading on renal angiotensinogen expression in the mRen(2).Lewis strain. AJP Renal. 2010;299:F35–F42. doi: 10.1152/ajprenal.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tobian L. Salt and hypertension. Lessons from animal models that relate to human hypertension. Hypertension. 1991;17(Suppl):I52–158. doi: 10.1161/01.hyp.17.1_suppl.i52. [DOI] [PubMed] [Google Scholar]
- 19.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346:913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 20.Meneton P, Jeunemaitre X, de Wardener HE, Macgregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 21.Lara LS, McCormack M, Semprun-Prieto L, et al. AT1 receptor mediated augmentation of angiotensinogen, oxidative stress and inflammation in Ang II-salt hypertension. Am J Physiol Renal Physiol. 2012;302:F85–F94. doi: 10.1152/ajprenal.00351.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chappell MC, Westwood BM, Yamaleyeva LM. Differential effects of sex steroids in young and aged female mren2.Lewis rats: a model of estrogen and salt-sensitive hypertension. Gend Med. 2008;5(Suppl A):S65–S75. doi: 10.1016/j.genm.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kittikulsuth W, Pollock JS, Pollock DM. Sex differences in renal medullary endothelin receptor function in angiotensin II hypertensive rats. Hypertension. 2011;58:212–218. doi: 10.1161/HYPERTENSIONAHA.111.172734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husková Z, Vanourková Z, Erbanová M, et al. Inappropriately high circulating and intrarenal angiotensin II levels during dietary salt loading exacerbate hypertension in Cyp1a1-Ren-2 transgenic rats. J Hypertens. 2010;28:495–509. doi: 10.1097/HJH.0b013e3283345d69. [DOI] [PubMed] [Google Scholar]
- 25.Williams DE, Prieto MC, Mullins JJ, et al. AT1 receptor blockade prevents the increase in blood pressure and the augmentation of intrarenal ANG II levels in hypertensive Cyp1a1-Ren2 transgenic rats fed with a high-salt diet. Am J Med Sci. 2010;339:356–361. doi: 10.1097/MAJ.0b013e3181d2b0a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chappell MC, Yamaleyeva LM, Westwood BM. Estrogen and salt sensitivity in the female mRen(2).Lewis rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1557–R1563. doi: 10.1152/ajpregu.00051.2006. [DOI] [PubMed] [Google Scholar]
- 27.Prieto-Carrasquero MC, Botros FT, Pagan J, et al. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip Goldblatt hypertensive rats. Hypertension. 2008;51:1590–1596. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobori H, Ohashi N, Katsurada A, et al. Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens. 2008;2:349–354. doi: 10.1016/j.jash.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stricker EM, Hoffmann ML, Riccardi CJ, Smith JC. Increased water intake by rats maintained on high NaCl diet: analysis of ingestive behavior. Physiol Behav. 2003;79:621–631. doi: 10.1016/s0031-9384(03)00172-0. [DOI] [PubMed] [Google Scholar]
- 30.Coelho MS, Passadore MD, Gasparetti AL, et al. High-or low-salt diet from weaning to adulthood: effect on body weight, food intake and energy balance in rats. Nutr Metab Cardiovasc Dis. 2006;16:148–155. doi: 10.1016/j.numecd.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Williams TM, Cattley RC, Borghoff SJ. Alterations in endocrine responses in male Sprague-Dawley rats following oral administration of methyl tert-butyl ether. Toxicol Sci. 2000;54:168–176. doi: 10.1093/toxsci/54.1.168. [DOI] [PubMed] [Google Scholar]
- 32.Fogari R, Preti P, Zoppi A, et al. Serum testosterone levels and arterial blood pressure in the elderly. Hypertens Res. 2005;28:625–630. doi: 10.1291/hypres.28.625. [DOI] [PubMed] [Google Scholar]
- 33.Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20:631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Villalobos RA, Satou R, Ohashi N, et al. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol. 2010;298:F150–F157. doi: 10.1152/ajprenal.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Z, Maric C, Roesch DM, et al. Estrogen regulates adrenal angiotensin AT1 receptors by modulating AT1 receptor translation. Endocrinology. 2003;144:3251–261. doi: 10.1210/en.2003-0015. [DOI] [PubMed] [Google Scholar]
- 36.Rogers JL, Mitchell AR, Maric C, et al. Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R794–R799. doi: 10.1152/ajpregu.00424.2006. [DOI] [PubMed] [Google Scholar]
- 37.Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting duct renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens. 2009;3:96–104. doi: 10.1016/j.jash.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalid M. Testosterone dependence of salt-induced hypertension in Sabra rats and role of renal alpha 2-adrenoceptor subtypes. J Pharmacol Exp Ther. 2002;300:43–49. doi: 10.1124/jpet.300.1.43. [DOI] [PubMed] [Google Scholar]
- 39.Afsar B. Testosterone and blood pressure: is the decreased sodium excretion the missing link? Hypertension. 2012;59:e41. doi: 10.1161/HYPERTENSIONAHA.112.192351. [DOI] [PubMed] [Google Scholar]
- 40.Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol. 2009;296:F1067–F1071. doi: 10.1152/ajprenal.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komlosi P, Fuson AL, Fintha A, et al. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 42.Liu L, Gonzalez AA, McCormack M, et al. Increased renin excretion is associated with augmented urinary angiotensin II levels in chronic angiotensin II-infused hypertensive rats. Am J Physiol Renal Physiol. 2011;301:F1195–F1201. doi: 10.1152/ajprenal.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Böger RH, Bode-Böger SM, Szuba A, et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842–1847. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 44.Ying W-Z, Sanders PW. Dietary salt intake activates MAP kinases in the rat kidney. FASEB J. 2002;16:1683–1684. doi: 10.1096/fj.02-0982fje. [DOI] [PubMed] [Google Scholar]
- 45.Ying WZ, Sanders PW. Dietary salt enhances glomerular endothelial nitric oxide synthase through TGF-beta1. Am J Physiol. 1998;275(1 Pt 2):F18–F24. doi: 10.1152/ajprenal.1998.275.1.F18. [DOI] [PubMed] [Google Scholar]
- 46.Harrison-Bernard LM, Schulman IH, Raij L. Postovariectomy hypertension is linked to increased renal AT1 receptor and salt sensitivity. Hypertension. 2003;42:1157–1163. doi: 10.1161/01.HYP.0000102180.13341.50. [DOI] [PubMed] [Google Scholar]
- 47.Hudson M, Rahme E, Behlouli H, et al. Sex differences in the effectiveness of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in patients with congestive heart failure—a population study. Eur J Heart Fail. 2007;9:602–609. doi: 10.1016/j.ejheart.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Burgess E, Muirhead N, de Cotret PR, et al. Supra-maximal dose of candesartan in proteinuric renal disease. J Am Soc Nephrol. 2009;20:893–900. doi: 10.1681/ASN.2008040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nixon RM, Müller E, Lowy A, Falvey H. Valsartan vs. other angiotensin II receptor blockers in the treatment of hypertension: a meta-analytical approach. Int J Clin Pract. 2009;63:766–775. doi: 10.1111/j.1742-1241.2009.02028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabi DM, Khan N, Vallee M, et al. Reporting on sex-based analysis in clinical trials of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker efficacy. Can J Cardiol. 2008;24:491–496. doi: 10.1016/s0828-282x(08)70624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grima M, Ingert C, Michel B, et al. Renal tissue angiotensins during converting enzyme inhibition in the spontaneously hypertensive rat. Clin Exp Hypertens. 1997;19:671–685. doi: 10.3109/10641969709083178. [DOI] [PubMed] [Google Scholar]
- 52.Miller JA, Cherney DZ, Duncan JA, et al. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol. 2006;17:2554–2560. doi: 10.1681/ASN.2005101095. [DOI] [PubMed] [Google Scholar]
- 53.Susic D, Frohlich ED, Kobori H, et al. Salt-induced renal injury in SHRs is mediated by AT1 receptor activation. J Hypertens. 2011;29:716–723. doi: 10.1097/HJH.0b013e3283440683. [DOI] [PMC free article] [PubMed] [Google Scholar]