Abstract
In the experimental setting several investigators have recently reported exacerbations of the burden of axonal damage and other neuropathological changes following repetitive traumatic brain injuries (TBI) that were sustained at intervals from hours to days following the initial insult. These same studies also revealed that prolonging the interval between the first and second insult led to a reduction in the burden of neuropathological changes and/or their complete elimination. Although demonstrating the capability of repetitive TBI to evoke increased axonal and other neuropathological changes, these studies did not address the potential for concomitant microvascular dysfunction or damage, although vascular dysfunction has been implicated in the second-impact syndrome. In this study we revisit the issue of repetitive injury in a well-controlled animal model in which the TBI intensity was bracketed from subthreshold to threshold insults, while the duration of the intervals between the injuries varied. Employing cranial windows to assess vascular reactivity and post-mortem amyloid precursor protein (APP) analysis to determine the burden of axonal change, we recognized that subthreshold injuries, even when administered in repeated fashion over a short time frame, evoked neither axonal nor vascular change. However, with an elevation of insult intensity, repetitive injuries administered within 3-h time frames caused dramatic axonal damage and significant vascular dysfunction bordering on a complete loss of vasoreactivity. If, however, the interval between the repetitive injury was extended to 5 h, the burden of axonal change was reduced, as was the overall magnitude of the ensuing vascular dysfunction. With the extension of the interval between injuries to 10 h, neither axonal nor vascular changes were found. Collectively, these studies reaffirm the existence of significant axonal damage following repetitive TBI administered within a relatively short time frame. Additionally, they also demonstrate that these axonal changes parallel changes in the cerebral microcirculation, which also may have adverse consequences for the injured brain.
Key words: injury severity and timing, repetitive traumatic brain injury, rats, second-impact syndrome
Introduction
Over the last decade, our understanding of the pathobiology of repetitive brain injury has begun to come into focus, with compelling evidence from both the clinical and experimental settings, indicating that repeat injuries within specific time frames can cause exacerbated brain pathology and related behavioral morbidity. In the experimental setting, recent evidence suggests that singular mild injuries not associated with significant neuropathological change can be associated with significant pathological and/or behavioral abnormalities when the same injury is repeated one to several days after the initial insult (Friess et al., 2009; Huh et al., 2007; Longhi et al., 2005; Prins et al., 2010; Raghupathi et al., 2004; Shitaka et al., 2011). In the experimental setting, these abnormal pathological changes associated with repeat injury have been linked primarily to the increase in the ensuing axonal injury, its associated downstream wallerian degeneration, and/or other forms of reactive change reflected in microglial activation (Huh et al., 2007; Laurer et al., 2001; Prins et al., 2010; Shitaka et al., 2011). In the clinical setting, particularly that involving contact sports, evidence for neuropathological change is less complete (Biasca and Maxwell, 2007; Broglio et al., 2011; Eckner et al., 2011), although several studies have suggested that the exacerbation of axonal injury (Yuen et al., 2009), as well as neurodegenerative change possibly linked to axonal injury, is a key player in any ensuing morbidity (Gavett et al., 2011; McKee et al., 2009; Khurana and Kaye, 2012). Additionally, in the human setting, there is evidence that with repetitive brain injury vascular dysfunction may ensue and thereby contribute to further significant morbidity and even mortality (Cantu and Gean, 2010). To date, there has been no comprehensive study conducted in animals to determine if vascular changes occur with repetitive brain injuries, and what if any relation these vascular abnormalities have with other forms of concomitant neuropathological change associated primarily with axonal perturbation. Such studies would have merit, because they would improve our understanding of repetitive brain injury in animal models, and perhaps lead to more mechanistic studies exploring the potential interaction of these two events in the ensuing morbidity and mortality described in animals and humans. To this end, the current investigation utilized a well-established model of impact acceleration injury (IAI), with bracketing of the severity of injury below levels previously found to exert axonal and microvascular changes (Baranova et al., 2008; Marmarou et al., 1994). These studies incorporated various paradigms involving repetitive injury administered at various time points following the initial insult to determine the window of vulnerability to repetitive injury. As will be shown, these studies illustrate that relatively mild injuries, when given repetitively, can cause dramatic axonal and microvascular changes, which tend to rapidly resolve when the interval between the initial and second insult is prolonged.
Methods
Experimental design
All experimental procedures were performed with a protocol approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University. Thirty-five adult male Sprague-Dawley rats, weighing 350–500 g, were used in the current study. The animals were housed in individual cages on a 12-h light/dark cycle with free access to water and food.
In this study, both vascular function and the burden of axonal damage were assessed after repetitive mild IAI. To this end, the animals were arbitrarily divided into seven groups; each group contained five animals. There were no animals in which the vasoreactivity could not be assessed due to complications.
Group 1: 0.5-meter IAI administered twice
These animals were subjected to 0.5-meter (m) IAI twice at a 3-h interval. The vascular responses to the two concentrations of acetylcholine (ACh) were assessed at 3 and 4 h following the second impact injury (Fig. 1).
FIG. 1.
This chart shows the time course for each experimental group (IAI, impact acceleration injury).
Group 2: 0.5-meter IAI administered three times
These animals were subjected to 0.5-m IAI repeated three times at 1.5-h intervals. The vascular responses to ACh were assessed at 3 and 4 h following the third impact injury (Fig. 1).
Group 3: 0.75-m IAI administered twice
These animals were subjected to 0.75-m IAI repeated twice at a 3-h interval. The vascular responses to ACh were assessed at 3 and 4 h following the second impact injury.
Group 4: 1.0-m IAI administered once
These animals were subjected to only one 1.0-m IAI. Vascular reactivity to ACh was assessed hourly for 4 h post-injury.
Group 5: 1.0-m IAI administered twice at a 3-h interval
These animals were subjected to 1.0-m IAI repeated twice at a 3-h interval. The vascular responses to ACh were assessed at 3 and 4-hours following the second impact injury.
Group 6: 1.0-m IAI administered twice at a 5-h interval
These animals were subjected to 1.0-m IAI repeated twice at a 5-h interval. The vascular responses to ACh were assessed at 3 and 4 h following the second impact injury.
Group 7: 1.0-m IAI administered twice at a 10-h interval
These animals were subjected to 1.0-m IAI repeated twice at a 10-h interval. The vascular responses to ACh were assessed at 3 and 4 h following the second impact injury.
General preparation
The animals were anesthetized intraperitoneally with sodium pentobarbital at 60 mg/kg. The femoral artery was cannulated with a PE 50 catheter (Becton Dickinson, Sparks, MD) for continuous monitoring of arterial blood pressure (PowerLab; AD Instruments, Colorado Springs, CO), and periodic collection of blood samples for the determination of partial arterial oxygen tension (Pao2), partial arterial carbon dioxide pressure (Paco2), and pH values (Stat Profile® pHOx; Nova Biomedical, Waltham, MA). Arterial blood gas samples (100 μL) were taken 10 min prior to injury and every hour post-second injury. The femoral vein was cannulated with a PE 50 catheter for administration of medication. After completion of the tracheotomy, the animals were mechanically ventilated (Harvard Apparatus, Holliston, MA) on room air. Post-injury pancuronium bromide (3 mg/kg) was administrated intravenously to produce neuromuscular blockade. Sodium pentobarbital was administrated intravenously ad libitum to achieve the appropriate level of anesthesia. The resting Paco2 was maintained at a constant level, between 35 and 40 mm Hg, by adjusting the rate and/or volume of the respirator. Body temperature was maintained at 37°C with a heat lamp and/or a heating pad throughout the procedures.
For the animals in groups 1, 2, 3, 4, and 5, whose interval of repetitive TBI was less than 3 h, these general preparatory procedures were initiated at the beginning of the experiments. However, for those animals in groups 6 and 7, whose interval between injuries ranged from 5 to 10 h, all general preparative procedures other than the initial short-term anesthesia were initiated 1 h prior to the second injury.
Experimental traumatic brain injury
The rats were subjected to different intensities of mild IAI administered from one to three times. The mild IAI model was modified from a model described previously (Baranova et al., 2008; Fujita et al., 2011), wherein injuries involving a weight drop from the height of 2 m onto the intact skull evoked axonal injury within the brainstem, while also eliciting altered vascular responsiveness within the cerebral microcirculation. Typically these axonal and vascular abnormalities occurred without evidence of overt contusional change and/or intraparenchymal bleeding, suggesting the induction of a moderate injury. As will be explained below, this model was employed at significantly reduced levels of impact, 0.5–1 m, to reduce the burden of any ensuing morphophysiologic change, and to allow repetitive injury. In brief, the rats were placed in a stereotaxic frame under pentobarbital anesthesia; a mid-sagittal incision was performed to expose the skull. A 10-mm circular stainless steel helmet was installed over the sagittal suture between the bregma and the lambda and secured with dental acrylic. After the animal was placed prone on a foam pad, a 450-g weight was dropped from a height of either 0.5, 0.75, or 1.0 m through an acrylic glass tube on the metal helmet, which was centered under the lower end of the tube.
Visualization and assessment of the cerebral microcirculation
Visualization and assessment of the cerebral microcirculation was performed as described in our previous studies (Ellis et al., 1983; Fujita et al., 2011; Gao et al., 2010; Levasseur et al., 1975; Oda et al., 2011). Briefly, after removing the helmet for IAI, a 2×4-mm rectangular craniotomy was made in the skull over the left parietal cortex, and the underlying dura mater was incised and removed. Next, a cranial window was installed over the exposed brain surface, and fixed in place by bone wax and dental acrylic. The cranial window consisted of a stainless steel ring with three outlets and a circular glass plate inside a ring. Two of the outlets served as inflow and outflow paths for the perfusion and clearance of selected vasoactive agents, while the free end of the other outlet was set at a predetermined height to achieve an intracranial pressure (ICP) of 5 mm Hg. The space under the cranial window and the three outlets were then filled with artificial cerebrospinal fluid (CSF), and the pH was adjusted to 7.35 by equilibration with a gas mixture containing 6% oxygen and 6% carbon dioxide balanced with nitrogen. The underlying pial microcirculation was visualized with a microscope, and pial arteriolar diameters were measured with a Vickers image-splitting device (Vickers Instruments Inc., Maiden, MA). Typically, in each window preparation a minimum of four arteriolar segments were evaluated. Acetylcholine (ACh), which is known to elicit endothelial-dependent vasodilation, was used in two different concentrations to assess vascular dilation. ACh (Sigma-Aldrich, St. Louis, MO) was dissolved in artificial CSF to achieve the final concentrations of 10−7 M and 10−5 M, and was then applied via the cranial window to induce a vasodilatory response. After each application of ACh in the space under the window for 2–4 min, vessel diameter was measured. The vascular reactivity to ACh was expressed as the percent change from the baseline diameter at each measurement time point.
Tissue preparation
For evaluating the burden of axonal damage that followed the above-described repetitive TBI, we assessed axonal damage in brainstem projection axons using methods previously utilized in our laboratory (Fujita et al., 2011; Gao et al., 2010; Koizumi and Povlishock, 1998; Suehiro and Povlishock, 2001). In this study, we used the same animals evaluated in groups 1–7 for the conduct of detailed axonal analyses. At 4 h following the last injury, and after the measurement of vascular reactivity to ACh, the rats were sacrificed with an overdose of euthanasia solution under general anesthesia, and then transcardially perfused with 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M Millonig's phosphate buffer. After perfusion, the brains were removed, transferred to fixative, and stored overnight. They were placed in a sagittal brain-blocking device with 2 mm of the brain cut from the midsection, followed by further sagittal blocking to include the medulla, pons, and midbrain. This blocking strategy was based on the fact that in this model, numerous damaged axons can be found in the pyramids at the medullospinal junction in the descending corticospinal tracts (Povlishock et al., 1997). After harvesting the 2-mm-wide sagittal block, the tissue section was flat-mounted and serially sectioned on a vibratome at a thickness of 40 μm. Sagittal sections (n=60) were serially collected in alternating wells, with the wells containing adjacent sections. Systematic uniform sampling of sagittal sections was initiated from a random starting well, with every fourth section collected, for a total of 20 sections per animal.
Immunocytochemistry for axonal damage
These sections were processed for visualization of an antibody targeting amyloid precursor protein (APP), a marker of impaired axonal transport and axonal damage, using a protocol adapted in our laboratory (Stone et al., 2000). Briefly, the sections were reacted with 0.3% H2O2 in phosphate-buffered saline (PBS) for 30 min to block endogenous peroxidase activity, and microwaved twice in citric acid buffer while maintaining a 45°C maximum temperature for 5 min (Stone et al., 1999). After microwave processing, the sections were allowed to cool for 20 min each time. The sections were preincubated for 1 h in 10% normal goat serum (NGS) with 0.2% Triton X in PBS, and then incubated for 18 h with rabbit anti-β-APP NGS diluted 1:1500 in 1% NGS in PBS. On the following day, the sections were incubated for 1 h with biotinylated goat anti-rabbit immunoglobulin G (Vector Laboratories, Inc., Burlingame, CA) diluted 1:1200 in 1% NGS in PBS. Then the sections were visualized via incubation for 1 h in avidin biotinylated enzyme complex (Vectastain® ABC kit; Vector Laboratories), followed by 0.05% diaminobenzidine, 0.01% H2O2, and 0.3% imidazole in 0.1% mol/L sodium phosphate buffer for 15 min. The sections were mounted on 0.5% gelatin-coated glass slides, serially dehydrated, and cover-slipped.
Quantitative analysis of the axonal damage
After completion of the APP immunocytochemical procedures, the slides were transferred to an Eclipse 800 microscope (Nikon, Tokyo, Japan), interfaced with a computer-assisted imaging system (DP Controller, version 3.2; Olympus Corp., Tokyo, Japan). Consistent regions of the medulla at the medullospinal junction were enlarged to 10× magnification and saved as a TIFF files. Based on our previous experience (Gao et al., 2010), the images were viewed on a monitor using image analysis software (IPLab, version 3.7; BD Biosciences Bioimaging, Rockville, MD) and converted to grayscale. The APP-immunoreactive axonal profiles were outlined and overlaid in cyan, a strategy used to suppress background immunoreactivity. The sampling area in the medullospinal junction was delineated by a rectangle measuring 500×200 μm, which was superimposed over the specified region. Within this rectangle the number of damaged APP-immunoreactive axonal profiles that exceeded 0.968 μm2 in size were then counted. This number was expressed as the density of damaged axons per unit area. For the corticospinal tract, eight alternate serial sections from the same tissue block were analyzed in this fashion, together with the use of investigator blinding.
Statistical analysis
Statistical analysis was performed using the statistical software PASW Statistics 17.0 (SPSS Inc., Chicago, IL). All data were presented as mean±standard error of the mean (SEM). The physiological parameters and laboratory data, which were normally distributed, were analyzed by one-way analysis of variance (ANOVA). When a significant difference was found, multiple comparisons among time points in the same group and groups at each time point were performed using the Bonferroni correction. The vascular reactivity to ACh and the number of damaged axons, which were not normally distributed, were analyzed by the Kruskal-Wallis test, followed by multiple comparisons with the Bonferroni correction. Statistical significance was set at p<0.05.
Results
General physiological observations
There were no significant differences in baseline body weight and hematocrit among the groups. Table 1 shows the time course measurements of mean arterial blood pressure, rectal and temporal muscle temperature, and blood gas analyses, in groups 4, 5, 6, and 7. The rectal temperature was maintained at 37°C over the entire experimental period in all groups (Table 1). The temporalis muscle temperature was slightly lower than the rectal temperature. All physiological variables reported in Table 1 were within normal physiological limits. All physiological variables in groups 1, 2, and 3 were similar to those of the other groups (data not shown). There was no significant difference in any physiological variable among the groups.
Table 1.
Physiological parameters after the last injury
| |
|
Measurement period |
||||
|---|---|---|---|---|---|---|
| Variable | Group | 0 h | 1 h | 2 h | 3 h | 4 h |
| MAP (mm Hg) | 4 | 113±2 | 109±2 | 108±3 | 110±2 | 110±2 |
| 5 | 110±3 | 108±3 | 104±2 | 109±3 | 108±3 | |
| 6 | 107±3 | 106±2 | 103±5 | 105±3 | 106±3 | |
| 7 | 109±1 | 107±4 | 109±1 | 107±4 | 107±2 | |
| Rectal temperature (°C) | 4 | 36.7±0.1 | 36.9±0.1 | 36.9±0.1 | 36.8±0.1 | 36.9±0.1 |
| 5 | 36.8±0.1 | 36.8±0.1 | 36.7±0.1 | 37.0±0.1 | 36.8±0.1 | |
| 6 | 36.8±0.1 | 36.8±0.1 | 36.9±0.1 | 36.8±0.1 | 36.9±0.1 | |
| 7 | 36.7±0.1 | 36.8±0.1 | 36.8±0.1 | 36.8±0.1 | 36.8±0.1 | |
| Temporal temperature (°C) | 4 | 36.9±0.2 | 36.7±0.1 | 36.7±0.1 | 36.7±0.1 | 36.9±0.1 |
| 5 | 36.6±0.1 | 36.7±0.1 | 36.6±0.1 | 36.8±0.1 | 36.6±0.1 | |
| 6 | 36.8±0.1 | 36.7±0.1 | 36.7±0.1 | 36.7±0.1 | 36.7±0.1 | |
| 7 | 36.5±0.1 | 36.7±0.1 | 36.6±0.1 | 36.7±0.2 | 36.7±0.1 | |
| pH | 4 | 7.40±0.02 | 7.40±0.02 | 7.40±0.01 | 7.40±0.01 | 7.38±0.02 |
| 5 | 7.39±0.02 | 7.38±0.02 | 7.38±0.01 | 7.40±0.01 | 7.40±0.01 | |
| 6 | 7.38±0.01 | 7.37±0.01 | 7.37±0.01 | 7.38±0.01 | 7.38±0.01 | |
| 7 | 7.41±0.01 | 7.40±0.01 | 7.41±0.01 | 7.41±0.01 | 7.41±0.01 | |
| Pao2 (mm Hg) | 4 | 91±4 | 87±2 | 89±3 | 91±3 | 91±1 |
| 5 | 88±4 | 89±4 | 86±3 | 90±3 | 91±2 | |
| 6 | 85±2 | 82±4 | 81±3 | 86±3 | 86±3 | |
| 7 | 89±6 | 80±4 | 84±4 | 88±3 | 86±4 | |
| Paco2 (mm Hg) | 4 | 37±1 | 37±1 | 37±1 | 36±1 | 36±1 |
| 5 | 36±1 | 36±1 | 35±1 | 35±1 | 36±1 | |
| 6 | 36±1 | 36±1 | 37±1 | 37±1 | 36±1 | |
| 7 | 38±1 | 38±1 | 36±1 | 35±1 | 35±1 | |
MAP, mean arterial blood pressure; Pao2, partial arterial oxygen tension; Paco2, partial arterial carbon dioxide pressure.
Values are expressed as the mean±standard error of the mean (SEM).
Brain arteriolar reactivity after repetitive impact acceleration injury
There was no significant impairment in the vascular reactivity to ACh in groups 1, 2, and 3, which were subjected to multiple IAI insults, all of which were below 1.0 m of impact severity (data not shown).
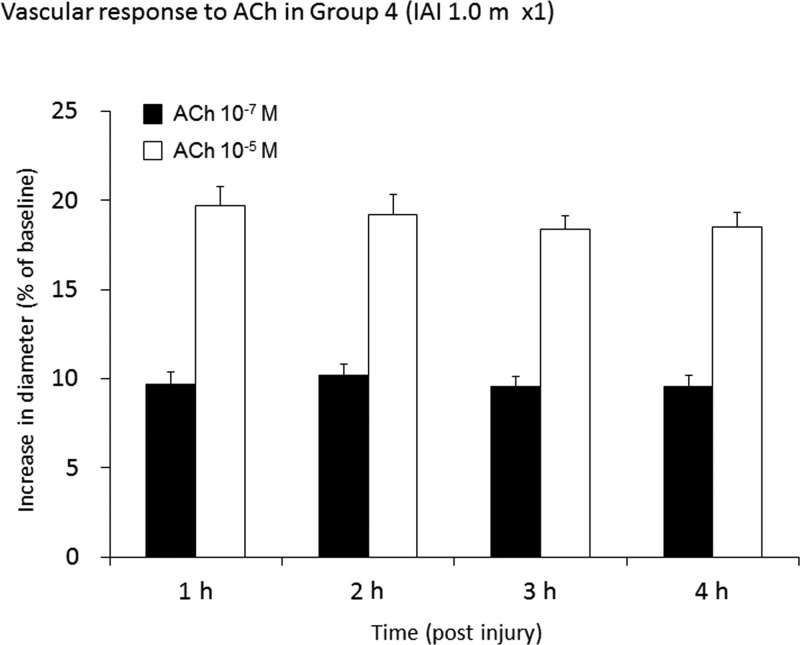
The vascular reactivity to ACh at 10−7 M and at 10−5 M in group 4, which was subjected to 1.0 m of IAI administered only once, is shown in Figure 2. Here the vascular dilation to ACh at 10−7 M and at 10−5 M post-IAI approximated 10% (9.7±0.7, 10.2±0.6, 9.5±0.5, and 9.5±0.6%, at 1, 2, 3, and 4 h post-injury, respectively), and 20% (19.7±1.1, 19.2±1.1, 18.4±0.8, and 18.5±0.8% at 1, 2, 3, and 4 h post-injury), respectively (Fig. 2). These values were essentially unchanged from those seen in groups 1, 2, and 3.
FIG. 2.
This bar graph shows the vascular reactivity to acetylcholine (ACh) at 10−7 M and at 10−5 M, in group 4 (a single 1.0-m impact acceleration injury [IAI]). Values are expressed as the mean±standard error of the mean.
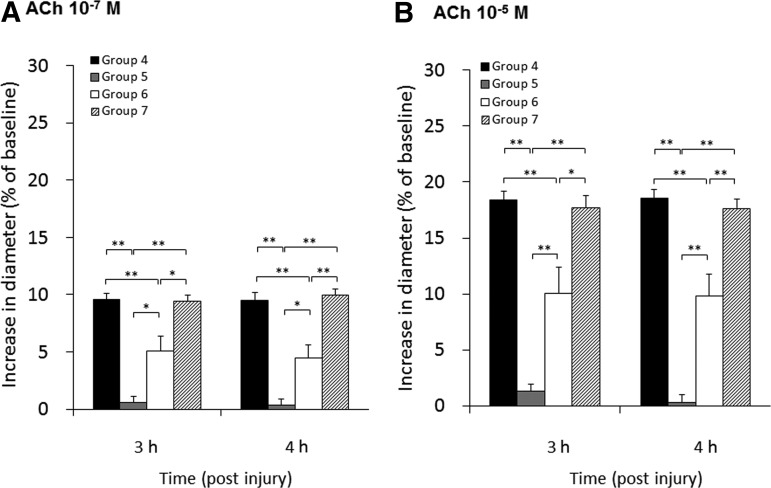
The vascular reactivity to ACh at 10−7 M and at 10−5 M in groups 4, 5, 6, and 7 at 3 and 4 h post-last injury is shown in Figure 3. There were significant differences in overall vascular reactivity for each ACh concentration at both 3 and 4 h post injury (p<0.01). In contrast in group 5, in which the animals were subjected to the same 1 m injury repeated 3 h following the initial insult, the vascular reactivity to both the 10−7 M and 10−5 M ACh doses was profoundly impaired (Fig. 3), and was significantly lower than that seen in group 4 at 3 and 4 h post-injury at 10−7 M and 10−5 M ACh (10−7 M ACh, 0.6±0.5 and 0.4±0.5% at 3 and 4 h post-injury, respectively; 10−5 M ACh, 1.3±0.6 and 0.3±0.7% at 3 and 4 h post-injury, respectively). In group 6, however, in which the interval between the repeated 1.0-m insults was 5 h, the vascular reactivity to ACh at 10−7 M and at 10−5 M was now only partially impaired, with values significantly lower than those of group 4 at 3 and 4 h post-injury at 10−7 M and 10−5 M ACh, yet was significantly higher than those of group 5 at 3 and 4 h post-injury at 10−7 M and 10−5 M ACh (10−7 M ACh, 5.1±1.3 and 4.5±1.2% at 3 and 4 h post-injury; 10−5 M ACh, 10.1±2.3 and 9.8±2.0% at 3 and 4 h post-injury; Fig. 3). In group 7, with the extension of the interval between the 1.0-m injuries to 10 h, the vascular reactivity to ACh at 10−7 M and 10−5 M was unimpaired, and was significantly higher than that seen in groups 5 and 6 at 3 and 4 h post-injury using 10−7 M and 10−5 M of ACh (10−7 M ACh, 9.4±0.6 and 10.0±0.6% at 3 and 4 h post-injury, respectively; 10−5 M ACh, 17.7±1.1 and 17.7±0.8% at 3 and 4 h post-injury, respectively; Fig. 3). No significant difference was found between the values reported in groups 4 and 7.
FIG. 3.
This bar graph shows the vascular reactivity to 10−7 M (A) and 10−5 M (B) acetylcholine (ACh) at 3 and 4 h following the last injury. Values are expressed as the mean±standard error of the mean (*significant difference at p<0.05; **significant difference at p<0.01; group 4, 1.0 m IAI administered once; group 5, 1.0 m IAI administered twice at a 3-h interval; group 6, 1.0 m IAI administered twice at a 5-h interval; group 7, 1.0 m IAI administered twice at a 10-h interval; IAI, impact acceleration injury).
Evaluation of axonal injury after repetitive mild impact acceleration injury
Routine microscopic evaluation of the APP immunoreactivity in the medullospinal junction in groups 1, 2, and 3 revealed no evidence of axonal damage (data not shown), while comparable evaluations in groups 4, 5, 6, and 7 revealed a consistent pattern of axonal change (Fig. 4). No APP-immunoreactive axonal swellings could be identified in group 4, which was subjected to 1.0 m IAI administered once (Fig. 4A). In contrast, in group 5, subjected to two 1.0-m IAI insults at a 3-h interval, the pontomedullary junction revealed conspicuous axonal change, as reflected by the presence of numerous APP-immunoreactive profiles (Fig. 4B), which appeared as large axonal bulbs or swellings, comparable to those previously described in our laboratory (Povlishock and Christman, 1995; Povlishock and Pettus, 1996). Such APP-positive swellings were dramatically reduced in group 6, wherein the interval between injuries was 5 h (Fig. 4C), and they were virtually absent in group 7, wherein the interval between injuries was 10 h (Fig. 4D). In this model system axonal damage could also be identified in the hippocampus, neocortex, and thalamus (data not shown), but the distribution of this axonal damage was so sparse and variable as to preclude any meaningful qualitative or quantitative evaluation.
FIG. 4.
Shown are photomicrographs of sections of rat brains obtained 4 h after the last injury, showing the damaged/APP-immunoreactive axonal profiles within the corticospinal tract at the level of the pontomedullary junction. Panels A, B, C, and D show sections obtained from rats in groups 4, 5, 6, and 7, respectively (original magnification 100×; group 4, 1.0 m IAI administered once; group 5, 1.0 m IAI administered twice at a 3-h interval; group 6, 1.0 m IAI administered twice at a 5-h interval; group 7, 1.0 m IAI administered twice at a 10-h interval; IAI, impact acceleration injury; APP, amyloid precursor protein; IAI, impact acceleration injury).
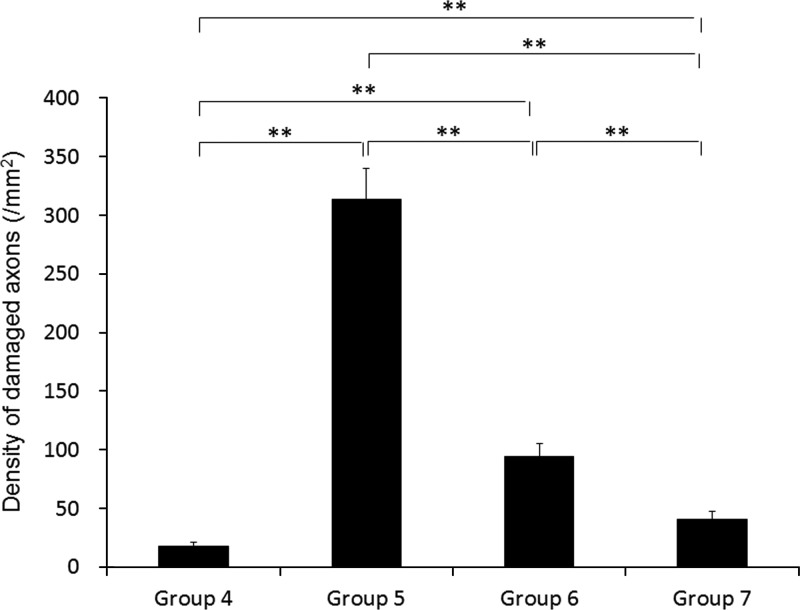
Quantitative analyses confirmed the previously-described qualitative observations. There was no evidence of an increase in the baseline axonal damage found in groups 1, 2, and 3, which were subjected to multiple IAIs below 1.0 m (data not shown). The number of damaged axons in group 4, which was subjected to 1.0 m of IAI administered once, was 18±4 per mm2 (Fig. 5), which was not significantly different than the values found in groups 1, 2, and 3. In group 5, which was subjected to two 1.0-m IAI insults at a 3-h interval, the number of damaged axons was striking, at 314±27 per mm2. These numbers were significantly higher than those found in group 4 (p<0.01; Fig. 5). In group 6, in which two 1.0-m IAI insults were administered over a 5-h interval, the number of damaged axons was significantly lower than that seen in group 5 (p<0.01), yet the number of damaged axons remained significantly higher than that found in group 4 (p<0.01; 94±11 per mm2; Fig. 5). In group 7, which was subjected to two 1.0-m IAI insults administered at a 10-h interval, the number of damaged axons was significantly lower than that reported in groups 5 and 6, while still remaining significantly higher than that reported in group 4 (41±6 per mm2; Fig. 5).
FIG. 5.
This bar graph shows a comparison of the mean density of APP-immunoreactive damaged axons in the corticospinal tract at 4 h following the last injury. Values represent the means±standard error of the mean. Statistical differences were analyzed by the Kruskal-Wallis test, followed by the Bonferroni test for multiple comparisons (**significant difference at p<0.01; group 4, 1.0 m IAI administered once; group 5, 1.0 m IAI administered twice at a 3-h interval; group 6, 1.0 m IAI administered twice at a 5-h interval; group 7, 1.0 m IAI administered twice at a 10-h interval; APP, amyloid precursor protein; IAI, impact acceleration injury).
Discussion
In this article we have shown that relatively low levels of traumatic brain injury can generate significant axonal and vascular change when administered in a repetitive fashion over a specific temporal framework. Moreover, we also demonstrated that less intense injuries, when given in a repetitive fashion, cause neither axonal nor vascular changes. In aggregate, the reported findings both confirm and in some cases significantly extend findings existing in the current clinical and basic science literature. Specifically, we have demonstrated the damaging consequences of repetitive brain injury, confirming the widely-held belief that the exacerbation of axonal damage is a consistent feature of repetitive brain injury (Huh et al., 2007; Longhi et al., 2005; Prins et al., 2010; Shitaka et al., 2011). Importantly, in addition to confirming the potential exacerbation of the burden of axonal damage with repetitive mild injuries, the current study demonstrates for the first time that microvascular dysfunction parallels these axonal events, and as such may be a co-contributor to the ensuing morbidity that has been previously described in various animal models of repetitive injury (Longhi et al., 2005; Prins et al., 2010; Shitaka et al., 2011), as well as in humans, in whom repetitive injuries result in subsequent morbidity (Cantu and Gean, 2010).
As alluded to above, it is notable that the reported low levels of repetitive injuries, ranging from 0.5–0.75 m insult intensity, evoked neither axonal nor vascular change following the initial or subsequent repetitive insults. The choice of these lower levels of injury was based on our previous experience with this model system, wherein we appreciated that a 2.0-m injury was capable of generating consistent axonal and microvascular damage and dysfunction, without the common occurrence of contusional changes, mass lesion formation, or significant subarachnoid bleeding (Fujita et al., 2011). Thus it was reasonable and rational to assume that by lowering the intensity of the insult to below 2.0 m, we could reduce the burden of injury, and thereby allow for a meaningful assessment of a reduced injury intensity, and its overall implications for repetitive insults. In this vein, we were surprised that neither the 0.5-m nor the 0.75-m insult generated either vascular or axonal damage, even when the repetitive insults were administered relatively close together following the initial injury. Clearly, these studies indicate that there is a threshold for the generation of axonal and microvascular injury and its potential exacerbation by repeated traumatic insults. Although the current study demonstrates that relatively mild repetitive injuries can neither cause nor exacerbate the burden of axonal or microvascular dysfunction following TBI and/or repeated traumatic brain injuries, we are reluctant to categorize the threshold of injury evaluated as representing mild TBI. This is because although some have equated the onset of axonal change with the presence of mild concussive TBI (Longhi et al., 2005), others have recently reported that even in the absence of axonal change as assessed by the same immunocytochemical approach used in this study, that other forms of subtle neuropathological change can be seen via the use of silver salts to detect fine fiber and preterminal and terminal debris (Shitaka et al., 2011). Thus, there is now reasonable evidence that the finding of axonal swellings alone as detected by antibodies to APP may underestimate the potential for other ongoing structural and/or physiological changes in the same animal (Shitaka et al., 2011). Moreover, since we did not conduct concomitant behavioral studies, it is difficult to characterize these axonal injuries as the dominant factors involved in any subsequent behavioral deficits or enduring morbidity.
Despite the fact that our identification of axonal change may underestimate the overall burden of traumatically-induced perturbation in terms of other structural and physiological abnormalities, we believe our finding that these axonal changes were not manifest with repetitive subthreshold injuries is important, and speaks to the fact that under low-intensity loading, the brain can withstand repeated injuries spaced relatively closely together without any overt axonal or vascular dysfunction. Although in a sense this finding appears obvious, in our estimation it is a new contribution to the literature. In this regard, it is also of note that in the current study, injuries of 1.0 m impact intensity did not result in either axonal or microvascular change following the induction of just one traumatic injury. However, when this same injury was repeated over time, there was a dramatic demonstration of both axonal and microvascular changes. This observation is a departure from previous studies in that it illustrates that the brain can recover from one subthreshold injury, yet manifest full-blown pathological and physiological change in the presence of another injury administered soon after the first. Thus, while multiple investigators have shown the cumulative effect of repeated injuries, none have generated comparative data using an initial level of injury that did not generate pathological and physiological change following the initial traumatic insult. While the implications of these findings must be interpreted with caution, they do raise the possibility that even less severe injuries without overt anatomical and physiological changes place the brain at risk for repeated insults. Equally important in the current study is the observation that with the extension of the intervals between the initial TBI and the subsequent injury, a significant reduction, or in some cases full restoration/recovery of the observed axonal and microvascular changes, was seen. While similar findings have been reported by Longhi and colleagues (2005), using multiple end-points, the current studies extend these important observations, and now, for the first time, illustrate that the restoration/recovery associated with a delay before the second insult applies to changes both in the brain parenchyma, as well as its intrinsic circulation. The mechanisms responsible for the above-described axonal and microvascular changes associated with repetitive injuries sustained within a specific time frame are at the moment unknown. However, it is well appreciated that traumatic axonal injury involves multiple pathological processes, some of which evolve over time, and thereby may be capable of recovery (Yuen et al., 2009). Thus it is conceivable that the initial insult primes various intra-axonal cascades, which under normal circumstances would transition to recovery, but develop into pathology due to the secondary challenges associated with repeated insults. This issue will require detailed investigation, but in light of the current findings and the existing literature addressing this issue, these arguments appear to be reasonable and rational.
Perhaps the most interesting finding in the current study focuses on the microvascular changes seen with repeated injury and their relationship to the ensuing axonal pathology. To our knowledge, this is the first description in an animal model of any form of microvascular change following repeat injury, and as such suggests that repetitive injuries can evoke significant vascular abnormalities. Similarly to the axonal injury, it is of note that these microvascular changes were only seen with repeated injuries given within a short time frame, with a reduction in microvascular dysfunction seen when the interval between the injuries was increased. In this sense, the pattern of microvascular dysfunction tended to parallel that of the axonal injury, with the important caveat that the magnitude of the observed microvascular changes far exceeded our expectations. Specifically, the vessels assessed following repetitive injury at a 3-h interval with a 1.0-m insult revealed virtually complete vascular failure of the normal vasodilatory effect of ACh. This observation was the most significant example of microvascular dysfunction that we have ever reported in multiple studies focusing on traumatic injury wherein vascular dysfunction but not complete paralysis was observed (Baranova et al., 2008). From a mechanistic perspective it is unclear what factors were at work behind this profound vascular dysfunction; however, once again, it is most likely that the vascular events launched by the initial traumatic brain insult that normally would revert and lead to normality are exacerbated, leading to the overt dysfunction described here. In this context, it is quite possible that the repeated injuries result in the recruitment of significant radical-mediated damage capable of affecting both endothelial and smooth muscle elements, thereby contributing to the profound vascular dysfunction we found (Fujita et al., 2011). However, as with the axonal injury mechanisms, this issue remains to be fully evaluated.
Another issue relevant to the vascular abnormalities seen with repetitive brain injuries are their functional consequences for the animal. As noted above, the observation of dramatic vasoparalysis, at least in terms of acetylcholine-mediated dilation, is an unprecedented finding, and theoretically would place the brain at considerable risk should a secondary insult involving either hypotension, hypertension, or hypoxia occur; this issue requires further investigation in this model system. This observation of profound vascular abnormality and dysfunction, however, is also consistent with some of the features of the second-impact syndrome described in humans (Saunders and Harbaugh, 1984), which indicates that secondary insults are associated with vasoparalysis, vascular engorgement leading to ICP elevations, and significant morbidity and mortality (Cantu and Gean, 2010). Although it is uncertain if the same mechanisms are at work in animals and humans, the current observation of microvascular dysfunction merits continued investigation to better appreciate the overall biological mechanisms at work in this devastating condition.
One of the major limitations of the current investigation was the fact that these intriguing findings were not explored with behavioral correlates. While we appreciate this shortcoming, we were constrained in our ability to conduct the necessary behavioral experiments. First and foremost, for the conduct of the vascular analyses, we avoided inhalation anesthetics because of their vasodilatory affects and instead used barbiturates, which made the assessment of behavioral responses virtually impossible. Moreover, given the limitations imposed by our animal care and use committee, we were not authorized to move in this direction. Most likely in the near future, we will conduct in a parallel population of injured animals, an evaluation of the behavioral consequences in the context of the injury intensities used in this report.
In sum, we believe that this work represents a significant contribution to the literature. As noted, it extends our previous work, but more importantly, it provides significant new insight into our understanding of repetitive brain injury, particularly via our demonstration of the fact that repetitive injuries are intimately linked to increased microvascular dysfunction. Equally important is the parallel finding that low-intensity injuries, when given in a repetitive fashion, do not evoke overt axonal damage and/or physiological vascular dysfunction in the cerebral microcirculation, thus suggesting that all ranges of impact may not necessarily be damaging, even when given repetitively.
Author Disclosure Statement
No competing financial interests exist.
Acknowledgments
We thank Susan Walker and Lynn Davis for their excellent technical assistance. This study was supported by National Institutes of Health grants HD055813 and NS047463.
References
- Baranova A.I. Wei E.P. Ueda Y. Sholley M.M. Kontos H.A. Povlishock J.T. Cerebral vascular responsiveness after experimental traumatic brain injury: the beneficial effects of delayed hypothermia combined with superoxide dismutase administration. J. Neurosurg. 2008;109:502–509. doi: 10.3171/JNS/2008/109/9/0502. [DOI] [PubMed] [Google Scholar]
- Biasca N. Maxwell W.L. Minor traumatic brain injury in sports: a review in order to prevent neurological sequelae. Prog. Brain Res. 2007;161:263–291. doi: 10.1016/S0079-6123(06)61019-4. [DOI] [PubMed] [Google Scholar]
- Broglio S.P. Echner J.T. Martini D. Sosnoff J.J. Kuktcher J.S. Randolph C. Cumulative head impact burden in high school football. J. Neurotrauma. 2011;28:2069–2078. doi: 10.1089/neu.2011.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu R.C. Gean A.D. Second-impact syndrome and a small subdural hematoma: An uncommon catastrophic result of repetitive head injury with a characteristic imaging appearance. J. Neurotrauma. 2010;27:1557–1564. doi: 10.1089/neu.2010.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner J.T. Sabin M. Kutcher J.S. Broglio S.P. No evidence for a cumulative impact effect on concussion injury threshold. J. Neurotrauma. 2011;28:2079–2090. doi: 10.1089/neu.2011.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis E.F. Wei E.P. Cockrell C.S. Choi S. Kontos H.A. The effect of PGF2 alpha on in vivo cerebral arteriolar diameter in cats and rats. Prostaglandins. 1983;26:917–923. doi: 10.1016/0090-6980(83)90154-5. [DOI] [PubMed] [Google Scholar]
- Friess S.H. Ichord R.N. Ralston J. Ryall K. Helfaer A. Smith C. Marguiles S.S. Repeated traumatic brain injury affects composite cognitive function in piglets. J. Neurotrauma. 2009;26:1111–1121. doi: 10.1089/neu.2008.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M. Oda Y. Wei E.P. Povlishock J.T. The combination of either tempol or FK506 with delayed hypothermia: Implications for traumatically induced microvascular and axonal protection. J. Neurotrauma. 2011;28:1209–1218. doi: 10.1089/neu.2011.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Oda Y. Wei E.P. Povlishock J.T. The adverse pial arteriolar and axonal consequences of traumatic brain injury complicated by hypoxia and their therapeutic modulation with hypothermia in rat. J. Cereb. Blood Flow Metab. 2010;30:628–637. doi: 10.1038/jcbfm.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavett B.E. Stern R.A. McKee A.C. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin. Sports Med. 2011;30:179–188. doi: 10.1016/j.csm.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh J.W. Widing A.G. Raghupathi R. Repetitive mild non-contusive brain trauma in immature rats exacerbates traumatic axonal injury and axonal calpain activation: A preliminary report. J. Neurotrauma. 2007;24:15–27. doi: 10.1089/neu.2006.0072. [DOI] [PubMed] [Google Scholar]
- Koizumi H. Povlishock J.T. Posttraumatic hypothermia in the treatment of axonal damage in an animal model of traumatic axonal injury. J. Neurosurg. 1998;89:303–309. doi: 10.3171/jns.1998.89.2.0303. [DOI] [PubMed] [Google Scholar]
- Khurana V.G. Kaye A.H. An overview of concussion in sport. J. Clin. Neurosci. 2012;19:1–11. doi: 10.1016/j.jocn.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Laurer H.L. Bareyre F.M. Lee V.M. Trojanowski J.Q. Longhi L. Hoover R. Saatman K.E. Raghupathi R. Hoshino S. Grady M.S. McIntosh T.K. Mild head injury increases the brain's vulnerability to a second concussive impact. J. Neurosurg. 2001;95:859–870. doi: 10.3171/jns.2001.95.5.0859. [DOI] [PubMed] [Google Scholar]
- Levasseur J.E. Wei E.P. Raper A.J. Kontos A.A. Patterson J.L. Detailed description of a cranial window technique for acute and chronic experiments. Stroke. 1975;6:308–317. doi: 10.1161/01.str.6.3.308. [DOI] [PubMed] [Google Scholar]
- Longhi L. Saatman K.E. Fujimoto S. Raghupathi R. Meaney D.F. Davis J. McMillan B.S.A. Conte V. Laurer H.L. Stein S. Stocchetti N. McIntosh T.K. Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery. 2005;56:364–374. doi: 10.1227/01.neu.0000149008.73513.44. [DOI] [PubMed] [Google Scholar]
- McKee A.C. Cantu R.C. Mowinski C.J. Hedley-Whyte E.T. Gavett B.E. Budson A.E. Santini V.E. Lee H.S. Kubilus C.A. Stern R.A. Chronic traumatic encephalopathy in athletes: Progressive tauopathy following repetitive head injury. J. Neuropathol. Exp. Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmarou A. Foda M.A. van den Brink W. Campbell J. Kita H. Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J. Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- Oda Y. Gao G. Wei E.P. Povlishock J.T. Combinational therapy using hypothermia and the immunophilin ligand FK506 to target altered pial arteriolar reactivity, axonal damage, and blood-brain barrier dysfunction after traumatic brain injury in rat. J. Cereb. Blood Flow Metab. 2011;31:1143–1154. doi: 10.1038/jcbfm.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock J.T. Christman C.W. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J. Neurotrauma. 1995;12:555–564. doi: 10.1089/neu.1995.12.555. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Marmarou A. McIntosh T. Trojanowski J.Q. Moroi J. Impact acceleration injury in the rat: evidence for focal axolemmal change and related neurofilament sidearm alteration. J. Neuropathol. Exp. Neurol. 1997;56:347–359. [PubMed] [Google Scholar]
- Povlishock J.T. Pettus E.H. Traumatically induced axonal damage: evidence for enduring changes in axolemmal permeability with associated cytoskeletal change. Acta Neurochir. Suppl. 1996;66:81–86. doi: 10.1007/978-3-7091-9465-2_15. [DOI] [PubMed] [Google Scholar]
- Prins M.L. Hales A. Reger M. Giza C.C. Hovda D.A. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev. Neurosci. 2010;32:510–518. doi: 10.1159/000316800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathi R. Mehr M.F. Helfaer M.A. Margulies S.S. Traumatic axonal injury is exacerbated following repetitive closed head injury in the neonatal pig. J. Neurotrauma. 2004;21:307–316. doi: 10.1089/089771504322972095. [DOI] [PubMed] [Google Scholar]
- Saunders R.L. Harbaugh R.E. Second impact in catastrophic contact-sports head trauma. JAMA. 1984;252:538–539. [PubMed] [Google Scholar]
- Shitaka Y. Tran H.T. Bennett R.E. Sanchez L. Levy M.A. Dikranian K. Brody D.L. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J. Neuropathol. Exp. Neurol. 2011;70:551–567. doi: 10.1097/NEN.0b013e31821f891f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J.R. Singleton R.H. Povlishock J.T. Antibodies to the C-terminus of the beta-amyloid precursor protein (APP): a site specific marker for the detection of traumatic axonal injury. Brain Res. 2000;871:288–302. doi: 10.1016/s0006-8993(00)02485-9. [DOI] [PubMed] [Google Scholar]
- Stone J.R. Walker S.A. Povlishock J.T. The visualization of a new class of traumatically injured axons through the use of a modified method of microwave antigen retrieval. Acta Neuropathol. 1999;97:335–345. doi: 10.1007/s004010050996. [DOI] [PubMed] [Google Scholar]
- Suehiro E. Povlishock J.T. Exacerbation of traumatically induced axonal injury by rapid post-hypothermic rewarming and attenuation of axonal change by cyclosporin A. J. Neurosurg. 2001;94:493–498. doi: 10.3171/jns.2001.94.3.0493. [DOI] [PubMed] [Google Scholar]
- Yuen T.J. Browne K.D. Iwata A. Smith D.H. Sodium channelopathy induced by mild axonal trauma worsens outcome after repeat injury. J. Neurosci. Res. 2009;87:3620–3625. doi: 10.1002/jnr.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]