Abstract
Recently we have shown that the human life span is influenced jointly by many common single-nucleotide polymorphisms (SNPs), each with a small individual effect. Here we investigate further the polygenic influence on life span and discuss its possible biological mechanisms. First we identified six sets of prolongevity SNP alleles in the Framingham Heart Study 550K SNPs data, using six different statistical procedures (normal linear, Cox, and logistic regressions; generalized estimation equation; mixed model; gene frequency method). We then estimated joint effects of these SNPs on human survival. We found that alleles in each set show significant additive influence on life span. Twenty-seven SNPs comprised the overlapping set of SNPs that influenced life span, regardless of the statistical procedure. The majority of these SNPs (74%) were within genes, compared to 40% of SNPs in the original 550K set. We then performed a review of current literature on functions of genes closest to these 27 SNPs. The review showed that the respective genes are largely involved in aging, cancer, and brain disorders. We concluded that polygenic effects can explain a substantial portion of genetic influence on life span. Composition of the set of prolongevity alleles depends on the statistical procedure used for the allele selection. At the same time, there is a core set of longevity alleles that are selected with all statistical procedures. Functional relevance of respective genes to aging and major diseases supports causal relationships between the identified SNPs and life span. The fact that genes found in our and other genetic association studies of aging/longevity have similar functions indicates high chances of true positive associations for corresponding genetic variants.
Introduction
Recent genome-wide association studies (GWAS) of life span and other complex traits1–3 have shown that such traits can be affected by the joint influence of large numbers of common single-nucleotide polymorphisms (SNPs), each having small individual effects and little predictive value. The results of these studies indicate an urgent need for advancing methods of evaluating the joint effects of SNP alleles on aging and longevity and investigating biological mechanisms of such joint influence.
Individual small-effect, low-significance SNP alleles have been observed in many GWAS of complex traits, including life span4–7; however, such variants were typically excluded from further analyses. This is because respective studies considered only individual (and not collective) SNP effects on the traits of interest, which required extremely low p value thresholds to reach a genome-wide significance level for the individual effects. The criteria for associating a single genetic variant with a trait in GWAS are based on comparison of p values resulting from testing the null hypotheses regarding the absence of the variant effect on life span, with a threshold that is supposed to take into account the need for multiple comparisons; this typically leads to p values below 10−7.
Addressing the joint influence of alleles, each with a small/low significant effect, could help both to significantly improve efficiency of the use of genome-wide SNP data and to explain a substantial part of so called “missing heritability” of complex traits,8 including the aging-related ones. Recent GWAS1–3,9,10 strongly support this idea demonstrating that life span and other complex traits, such as height and body mass index (BMI), are significantly influenced by large numbers of jointly acting, common SNPs, each with a small individual effect. However, the SNP alleles in these studies were typically selected using one or two statistical models, which might not be sufficient for meaningful interpretation of the study results, because different statistical models may potentially yield different numbers of SNPs influencing the trait of interest. This stresses the importance of advancing statistical approaches to analyses of joint genetic effects on aging and longevity. Indeed, the potential influence of a genetic variant on life span can be described by not just one but by many different statistical models. The use of different models might result in different estimates of the effect size and in different p values for the same SNP allele, so that the results of selection (i.e., composition of the set of longevity alleles), and hence conclusions about genetics of life span, may depend substantially on the statistical model used in the allele selection procedure. Controlling for different observed covariates may add variability to the results of analyses.
To address this problem and enhance the efficiency of studying joint genetic effects on aging/longevity traits in this paper, we first used six statistical models for allele selection procedures: (1) Normal linear (N), (2) Cox (C), and (3) logistic (L) regressions; (4) generalized estimation equation (GEE); (5) mixed model (MM); and (6) gene frequency (GF) method. Then we evaluated the joint influence of SNP alleles selected with each of the six methods on life span, as well as the effect on life span of the SNPs from the overlapping set of alleles selected with all of the six statistical procedures. In all regression methods, possible effects of observed covariates on the results of selection were taken into account. These include gender, birth cohort, and smoking status, taken from the original cohort data of the Framingham Heart Study (FHS). Then we used SNP alleles influencing life span in the FHS Original cohort to predict the relationship between the number of “longevity” alleles and life span in the FHS Offspring cohort.
Finally, we reviewed current literature and online information sources to explore functional properties of genes related to “longevity” SNPs in the overlapping set of SNPs selected in the six statistical procedures. The findings that corresponding genes have functional relevance to physiological aging and/or aging-associated health disorders would support a causal relationship between these SNPs and life span.
Data and Methods
Framingham Study Data
In this study, we used genetic and nongenetic data for the FHS Original and Offspring cohorts. The FHS Original cohort was launched at Exam 1 in 1948 (9/1948–4/1953) and has continued with biennial examinations to the present. The FHS Original cohort consists of 5,209 respondents (55% females) aged 28–62 years residing in Framingham, Massachusetts, between 1948 and 1951.11 Nearly all subjects were Caucasians. Examination included an interview, physical examination, and laboratory tests.
The Offspring cohort (FHSO) was launched at Exam 1 in 1971 (8/1971–9/1975) and has on average been examined every 3–4 years since enrollment. The FHSO dataset consists of a sample of 3,514 biological descendants of the Original cohort, 1,576 of their spouses, and 34 adopted Offspring for a total sample of 5,124 subjects (52% females).12,13 The FHSO subjects were enrolled in 1971–1975 using research protocols similar to those of the FHS, so that comparisons of the results from the FHSO and the FHS could be made.
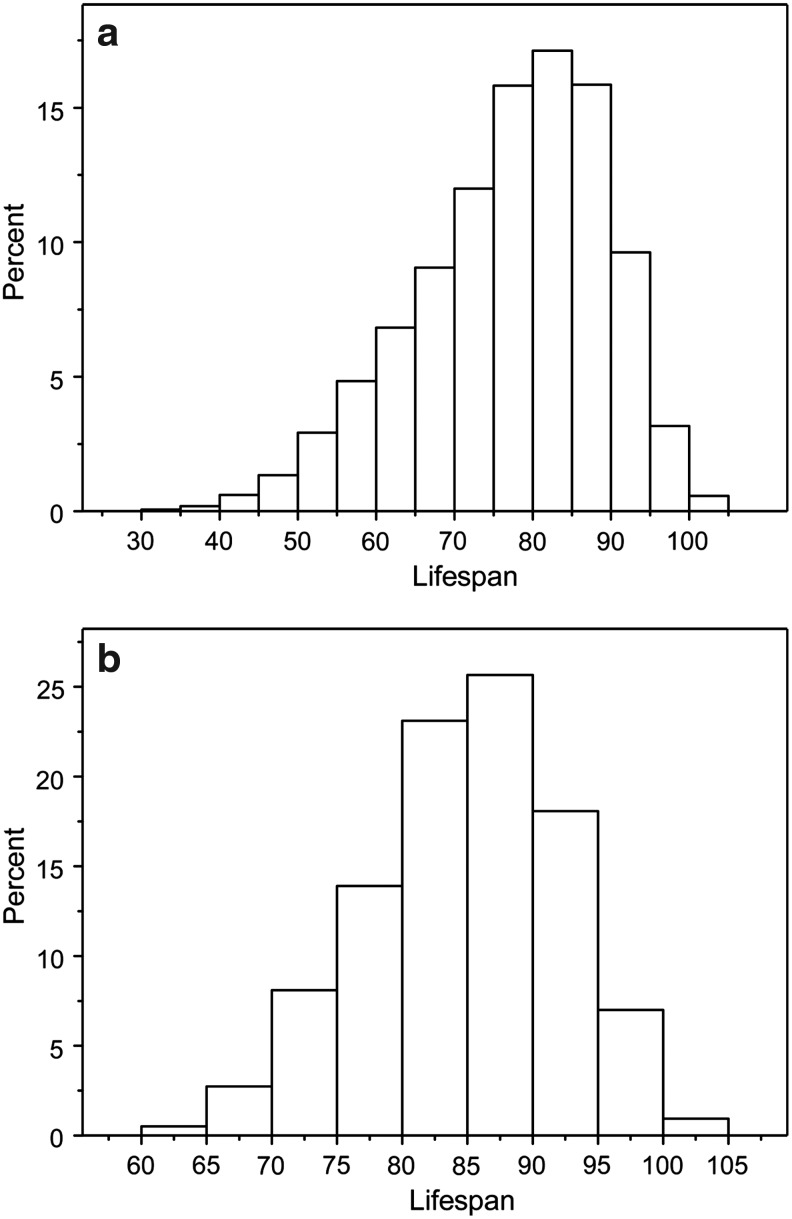
About 9,300 individuals from all generations of the FHS were genotyped for about 550,000 SNPs using Affymetrix 500K (genome-wide) and 50K (enriched with SNPs within genes) mapping arrays, overall representing a significant part of human genome variability. On average, 40% of SNPs were located within genes in the combined 550K set. In this study, we used information about 1,471 genotyped participants of the FHS Original cohort who passed quality control (QC), including 1,173 individuals for whom life spans were available. Information about 517 individuals (all deceased) from the FHSO cohort was used for validating the connection between life span and genetic variants in this cohort using “longevity” alleles obtained in the analyses on the original FHS cohort. Individual information on the SNP genotyping and phenotypic traits collected in the FHS was obtained through the dbGaP web site, upon request for controlled access for individual level data (www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000007.v3.p2/). The histograms of the life span distribution for two groups of deceased individuals (genotyped+nongenotyped and genotyped) from the original FHS cohort are shown in Fig. 1, a and b, respectively.
FIG. 1.
The histograms of life span distribution for (a) 4,760 deceased (genotyped and nongenotyped) and (b) 1,173 deceased genotyped individuals from the original Framinghan Heart Study (FHS) cohort.
The use of the Shapiro–Wilk test indicates that the normal approximation of the life span distribution for genotyped individuals can be used (for more information about other descriptive statistics relevant to these analyses, see Supplemental Material (www.liebertpub.com/rej).
QC procedure
Following Lunetta et al.4 we applied a GWAS QC procedure (call rate≥80%; minor allele frequency [MAF]>1%; Hardy–Weinberg equilibrium [HWE]>10−7) to the data on genotyped (550K SNPs) individuals from the original cohort in the FHS. (See Table S1 and Fig. S3 in Supplemental Data for additional information concerning call rates and other information about selected SNPs; see also Discussion section).
Identifying “longevity” alleles
To identify “longevity” alleles, we applied six different statistical procedures to selecting individual SNP alleles for their positive effect on life span, using 550K SNP data and the FHS original cohort genotyped sample. These procedures use N regression, the GEE approach, MM, C regression, L regression, and GF method. Because the use of the different procedures is likely to result in different sets of “longevity” alleles, we will distinguish them using the notions of GEE-alleles, MM-alleles, N-alleles, C-alleles, L-alleles, and GF-alleles, meaning that the selection procedure used general estimation equation, mixed model, normal, Cox, logistic regressions, or the gene frequency method, respectively. In case of the N-, GEE, MM-, and C- models, life span was considered as a dependent variable. In the L-model, the indicator of the event that life span exceeded the age of 90 years, I(LS>90), was considered as a dependent variable. We use 90 years as the cutoff age for longevity to have enough individuals who survived the cutoff age. This also allowed us to compare the results of our analyses with those of other studies6 in which the same cutoff age has been used. In all five regression models, selection was performed controlling for gender, birth year, and smoking status (ever or never smoked).
Seven sets of “longevity” alleles
The six sets of “longevity” alleles identified using six different statistical procedures may consist of different numbers of such alleles. To be sure that our results were independent from the method of alleles selection, we also considered the set of SNP alleles at the intersection (I) of the six sets. Each allele in this set was thus selected using all of the six statistical procedures. We call such alleles “I-longevity alleles,” meaning “I” for “intersection.” Thus, altogether we obtained seven sets of longevity alleles.
Evaluating additive (polygenic) genetic effects on life span
For each genotyped individual in the original FHS cohort, and for each of the seven sets of longevity alleles, we calculated the value of polygenic score index—the number of genetic variants contained in a person's genome (genetic dose). Then we estimated parameters of seven linear regression models, considering life span as a dependent and the polygenic score as an independent variables.10
Validating polygenic influence on lifespan in the FHSO cohort
To be sure that “longevity” alleles from each of the six sets had a joint effect on life span, not only in population of the FHS original cohort but also in population of other individuals (whose genetic information was not used in the allele selection procedure), we calculated values of respective indices for each of the 517 individuals from the FHSO cohort. Then we estimated parameters of linear regressions considering life span as a function of respective indices.
Analyses and Results
Normal regression
The N regression procedure for selecting longevity alleles (with p value threshold ≤10−7) was applied to 1,173 members of the original FHS cohort who passed the QC procedure and for whom life span data were available. The implementation of this procedure resulted in 823 N-longevity alleles.
The GEE method
The GEE procedure for selecting longevity alleles (with p≤10−7) was applied to 1,173 members of the original FHS cohort, taking into account sib relationships among individuals. The implementation of this procedure resulted in 1217 GEE-longevity alleles.
Mixed model
The MM regression procedure for selecting longevity alleles (with p≤10−7) was applied to the data on 1,173 members of the original FHS cohort, taking into account sib relationships among individuals. In this model, the observed covariates, including SNP genotypes, were considered as fixed-effects components, and the random effect was modeled as a random variable having the multivariate normal distribution with zero mean and covariance matrix defined as Rσ, where R is the relationship matrix and σ is the additive genetic variance due to polygenic relationships among related individuals. The implementation of this procedure resulted in 1,202 MM-longevity alleles.
Cox regression
The C regression procedure for selecting longevity alleles was applied to 1,441 members of the original FHS cohort who passed the QC procedure. This number is larger than that used in normal regression because Cox regression allows for dealing with individuals which life spans are censored (e.g., some study participants are alive). The procedure selected SNP variants for which the estimate of the regression parameter was negative and significant with p≤10−7. This procedure resulted in selection of 3,683 C-longevity alleles.
Logistic regression
The L regression procedure for selecting longevity alleles considered survival to the age of 90 years as longevity phenotype. The procedure was applied to 1,331 individuals. This number differs from the two methods above because individuals censored before the age 90 were excluded from the analyses (e.g., alive individuals, whose age did not exceed 90 years). Similar to other regressions, this procedure also controlled for the same observed covariates. It is important to note that the use of the selection threshold p≤10−7 (as in four procedures above) did not produce any longevity alleles. An increase of this threshold up to p≤10−4 resulted in selection of 97 L-longevity alleles.
Gene frequency method
This method does not allow us to control for observed covariates. We compared gene frequencies in three age groups: 60–75, 75–85, and 85+. The SNP allele was assigned to be a longevity allele if its frequency increased at the interval of aging and if this increase was statistically significant. We used p≤10−4 as the level of significance for selecting longevity alleles. The use of smaller p values for selecting longevity alleles failed to select any genetic variant. As a result of this procedure, 52 GF-longevity alleles were selected.
I-longevity alleles
The intersection of the four sets of longevity alleles selected by each of four methods resulted in the new set of 27 I-longevity alleles. All alleles passed the test for the linkage disequilibrium. The list of respective SNPs and change in their MAFs over age is shown in Table 1. Note that altogether we obtained seven sets of longevity SNP alleles: Six model-specific sets and one intersection set.
Table 1.
Minor Allele Frequencies for 27 “Longevity” Single-Nucleotide Polymorphisms at Different Ages
| SNP | MAF (60–75) | MAF (75–85) | MAF (85–105) | P: 85–105 vs. 60–75 |
|---|---|---|---|---|
| rs3800358 | 25.90% | 28.10% | 31.60% | 5.41E-03 |
| rs1974676 | 35.40% | 38.80% | 41.60% | 4.96E-03 |
| rs1834497 | 44.70% | 47.20% | 50.40% | 9.79E-03 |
| rs2590504 | 37.30% | 38.90% | 43.20% | 8.54E-03 |
| rs2024714 | 26.80% | 30.00% | 35.30% | 7.43E-05 |
| rs41383 | 25.00% | 28.10% | 31.00% | 4.55E-03 |
| rs16975963 | 46.70% | 49.60% | 53.30% | 7.13E-03 |
| rs13008689 | 23.20% | 25.60% | 28.50% | 6.86E-03 |
| rs3120819 | 42.10% | 43.80% | 48.40% | 8.06E-03 |
| rs9876781 | 34.90% | 37.80% | 42.40% | 8.68E-04 |
| rs9517320 | 30.80% | 33.30% | 37.70% | 2.04E-03 |
| rs139170 | 34.70% | 37.70% | 41.40% | 3.32E-03 |
| rs4648884 | 27.70% | 30.50% | 33.00% | 9.92E-03 |
| rs739401 | 32.40% | 35.60% | 39.50% | 1.54E-03 |
| rs10256972 | 36.50% | 38.90% | 43.60% | 2.24E-03 |
| rs10937739 | 41.90% | 44.40% | 48.40% | 4.04E-03 |
| rs5771675 | 30.90% | 32.90% | 36.50% | 9.85E-03 |
| rs12623542 | 36.70% | 39.20% | 44.00% | 1.92E-03 |
| rs9616906 | 22.70% | 25.70% | 30.80% | 9.36E-05 |
| rs13053175 | 28.00% | 30.40% | 33.60% | 6.97E-03 |
| rs4148544 | 24.60% | 27.30% | 29.70% | 9.77E-03 |
| rs2370413 | 35.20% | 38.70% | 42.60% | 1.10E-03 |
| rs10819510 | 26.50% | 29.20% | 32.00% | 7.74E-03 |
| rs1205035 | 36.20% | 38.20% | 42.00% | 9.83E-03 |
| rs432203 | 38.00% | 40.90% | 44.30% | 7.79E-03 |
| rs1327533 | 25.30% | 28.90% | 33.70% | 3.56E-04 |
| rs2826891 | 36.50% | 40.00% | 43.10% | 4.02E-03 |
SNP, Single-nucleotide polymorphism; MAF, minor allele frequency.
The results of evaluating polygenic influence on life span obtained by applying linear regression without observed covariates to the life span data in the original FHS cohort are shown in Table 2 for each of seven sets of selected genetic variants. One can see that each relationship explains a substantial portion of the phenotypic variance. In all cases, the values of the slope (as well as the intercept) associated with polygenic effects were highly statistically significant (p≤10−15). We repeated these analyses controlling for observed covariates, including gender, birth cohort, and smoking status (ever, or never smoking). In all cases, the polygenic effects were highly statistically significant.
Table 2.
Results of Analyses of Joint Effects of “Longevity” Alleles on Life Span
| GEE | Normal | Cox | Logistic | GF | MM | Intersection | |
|---|---|---|---|---|---|---|---|
| N ind | 1,173 | 1,173 | 1,471 | 1,331 | 1,471 | 1,173 | 1,471 |
| N SNPs | 1,217 | 823 | 3,683 | 97 | 52 | 1,202 | 27 |
| R2orig | 16.6% | 16.9% | 13% | 16% | 16.1% | 18.5% | 14.4% |
| R2off | 10.6% | 10.3% | 6% | 9% | 8.3% | 10.2% | 8.2% |
The first six columns show the effect of SNP alleles on life span for each of the six models used in the allele selection procedure. The last column shows this effect for the intersection set (of I-longevity alleles). The rows in the table denote: Nind, number of individuals from the FHS original cohort, which data were used in the alleles selection procedures; NSNPs, number of SNPs selected with respective model; R2orig, percent of life span variance in the FHS original cohort explained by respective model; R2off, percent of life span variance in the FHS offspring cohort explained by the respective model.
GEE, Generalized estimation equation; normal, normal linear regression; Cox, Cox regression; logistic, logistic regression; MM, mixed model; GF, gene-frequency method; SNP, single-nucleotide polymorphism.
To test whether longevity alleles obtained from the data on the FHS Original cohort jointly influence life span in the Offspring cohort, we calculated the values of the seven polygenic score indices for each of the 517 deceased individuals from the FHSO cohort. Then we estimated parameters of seven linear regressions considering life span as a function of each of the polygenic score indices. The percentages of life span variance in the FHSO cohort explained by each of seven sets of genetic variants selected from the data on the original cohort are shown in Table 2.
In each of seven cases and in both FHS cohorts, the estimates of the slope (as well as the intercept) parameter were positive and highly statistically significant (p≤10−9). We also observed that individuals in the FHSO cohort having larger numbers of longevity alleles taken from the I-set with 27 such alleles had better survival than those from the same cohort who have a smaller number of such alleles in their genome (see Fig. S1 in Supplemental Data). Note that the difference in survival functions for carriers of different numbers of longevity alleles does not prove that the 27 detected genetic variants are true positive longevity alleles. The fact that individuals from both FHS cohorts were genotyped on the same chip can lead to apparently robust false-positive findings.
Additional arguments confirming that detected genetic variants are linked to life span could come from three sources of information from: (1) The new GWAS performed with independent populations; (2) the results obtained in earlier GWAS on independent populations performed by other researchers; or (3) the fact that genes linked to detected variants belong to established metabolic pathways involved in the regulation of aging and life span. Such pathways have been identified in a number of earlier studies using independent populations. In this paper we investigated the third source of information mentioned above.
Genes closest to the 27 I-longevity SNPs: Their roles in aging and health
The fact that the 27 I-longevity alleles jointly significantly influenced life span, regardless of the statistical method used for their selection, indicates potential functional significance of respective genes in physiological aging and/or age-associated diseases, because both the aging and the diseases are major contributors to life span. To see if this is the case, we looked for where these 27 SNPs are located in the genome and conducted a review of current knowledge on biological functions (established or suggested) of genes closest to these SNPs. For this, we used peer-review publications available through PubMed, as well as online sources of genetic information, such as NCBI Entrez SNP, Entrez Gene, GeneCards, OMIM, KEGG, PANTHER, Your Favorite Gene (powered by Ingenuity), and some others. The results are summarized in Tables 3 and 4.
Table 3.
Summary Characteristics of the 27 I-Longevity Single-Nucleotide Polymorphisms and Functions (Known or Suggested) of Their Closest Genes
| SNP | Chr | Relation to gene | Distance to gene | Closest gene | Gene/protein description | Functions and phenotypic effects known or believed to be associated with this gene/protein | Major relevant biological process or health disorder |
|---|---|---|---|---|---|---|---|
| rs4648884 | 1 | Intronic | 0 | RUNX3: runt-related transcription factor 3 | Can either activate or suppress transcription; interacts with other transcription factors | Inhibits proliferation, induces apoptosis41; functions as a tumour suppressor; frequently deleted or silenced in cancer42; suppresses IL-4; role in allergy, asthma43–45 | Apoptosis; tumor suppression; immune response; asthma |
| rs3120819 | 1 | Intergenic | −84,906 | RP11-149P14.1 | Pseudogene | ||
| rs1974676 | 2 | Intronic | 0 | HPCAL1: hippocalcin-like 1 | Member of neuron-specific calcium-binding proteins family | May have relevance for neuronal signaling and be involved in calcium-dependent regulation of rhodopsin phosphorylation, hypertension and asthma46,47 | Brain information processing; cardiovascular disease, asthma (?) |
| rs432203 | 2 | Intronic | 0 | TGF-α | A mitogenic polypeptide able to bind to the EGF receptor and act synergistically with TGF-β to promote cell proliferation | Stimulates cell growth and proliferation, e.g., neural cell proliferation in the injured brain; may be protective in stroke48; inhibits apoptosis49; upregulated in some cancers | Cell proliferation; brain response to damage; cancer; stroke (?) |
| rs12623542 | 2 | Noncoding | 0 | AC131097.3 | |||
| rs13008689 | 2 | Intergenic | −153,466 | AC011747.3 | |||
| rs1834497 | 3 | Intronic | 0 | CLSTN2: calsyntenin 2 | Postsynaptic membrane proteins with highest levels in GABAergic neurons; expressed in the medial temporal lobe | Linked to synaptic plasticity; associated with episodic memory50; beneficial effect of the CLSTN2 C allele on memory and connectivity between brain regions51; associated with cognitive function in AD52 | Brain information processing; memory formation; AD |
| rs9876781 | 3 | Noncoding | 0 | RP11-24C3.2 | Closest gene: ATR interacting protein (ATRIP) | ATRIP phosphorylation regulates cell cycle arrest in response to DNA damage53; ATR may also trigger replicative senescence in absence of DNA damage.54 | Cell cycle arrest/ replicative senescence |
| rs10937739 | 4 | Intronic | 0 | PPP2R2C: protein phosphatase 2, regulatory subunit B, gamma | Serine/threonine phosphatase 2 is abundant in brain; can modify mitogen-activated protein kinase (MAPK activity | The negative control of cell growth and survival; induces apoptosis, neuronal differentiation; possibly involved in cancer55–57; role in synaptic plasticity, learning and memory; disrupted in intellectual disability and bipolar disorder58,59; myocardial calcium regulation; down-regulated in tachycardia60 | Apoptosis; memory; brain disorders; cancer, CVDs (?) |
| rs1205035 | 6 | Intergenic | −109,050 | RP1-223B1.1 | |||
| rs3800358 | 6 | 3 prime_UTR | 0 | BTBD9: BTB domain containing 9 | Involved in protein–protein interactions | Restless legs syndrome and possibly Tourette syndrome; iron regulation in the brain61 | Sleep disorders |
| rs10256972 | 7 | Intronic | 0 | C7orf50: chr7 open reading frame 50 | |||
| rs1327533 | 9 | Intronic | 0 | SVEP1: EGF and pentraxin domain containing 1 | A multidomain protein: Sushi, CCP, vWF-A, EGF/ EGF-like calcium binding, and pentraxin domains | Plays a role in cell adhesion and myogenesis; marker of activated satellite cells; regulation of muscle stem cell fate62 | Cell adhesion; muscle growth and regeneration |
| rs2590504 | 9 | Downstream | −6,153 | KIAA0649 | 1A6/DRIM ("Down-regulated in Metastasis") interacting protein with oncogenic characteristics | Enhances colony formation, allows anchorage-independent growth; may promote cell proliferation; has oncogenic potential63 | Cell proliferation; cancer |
| rs10819510 | 9 | Intergenic | −37,267 | RP11-65J3.3 | |||
| rs739401 | 11 | Intronic | 0 | CARS: cysteinyl-tRNA synthetase | Catalyzes tRNA aminoacylation; located near imprinted gene domain in a tumor-suppressor gene region | Alterations in this gene region have been associated with cancer and diabetic nephropathy64,65 | Cancer; diabetes |
| rs2370413 | 12 | Intronic | 0 | CACNA1C: alpha 1C subunit of L-type voltage-gated calcium channel | Mediates the entry of calcium ions into cells; involved in variety of calcium-dependent processes | Important role in smooth and cardiac muscle contraction, hormone or neurotransmitter release, gene expression; cell motility, division and death; polymorphisms are linked to bipolar disorder, schizophrenia, memory, grey matter volume, and response to hypertension treatment66–69 | Calcium-dependent processes; brain volume, memory; brain disorders; cardiovascular disease |
| rs9517320 | 13 | Intronic | 0 | STK24: serine/threonine kinase 24 | Participates in the MAPK cascade | Required for axon regeneration, synaptic plasticity70,71; possibly involved in cancer and PD71,72 | Brain regeneration; memory; possibly PD and cancer |
| rs4148544 | 13 | Intronic | 0 | ABCC4: adenosine triphosphate-binding cassette transporters family | Transport various molecules across extra and intracellular membranes | Involved in multidrug resistance; may play a role in cellular detoxification and survival from cancer73,74 | Transport of xenobiotics, detoxification; cancer |
| rs41383 | 16 | Intronic | 0 | NLRC5: NLR family, CARD domain containing 5 | IFN-γ-inducible nuclear transcriptional regulator of the NF-κB and type I interferon signalling | Transcriptional repression; key negative regulator of inflammatory pathways in innate immunity and antiviral response75,76 | Innate immunity; inflammation; viral infection |
| rs16975963 | 19 | Noncoding | 0 | AC016582.2 | |||
| rs2024714 | 20 | Intronic | 0 | CDH4: R-cadherin (retinal) | Participates in calcium-dependent cell–cell adhesion | Involved in axons outgrowth, kidney and muscle development, cell motility; may act as tumor suppressor; methylated in many cancers21,77; polymorphism associated with total cerebral brain volume17 | Cell adhesion; brain volume, brain aging; cancer |
| rs2826891 | 21 | Intronic | 0 | NCAM2: neural cell adhesion molecule 2 | Brain protein, superfamily of the immunoglobulin; one of plasma membrane-anchored proteins | Mediates cell adhesion; may play role in zone-to-zone projection of the primary olfactory axons, and in brain disorders78; mediates response to gene therapy in breast, prostate cancers79 | Neural cell adhesion; cancer, brain disorders (?) |
| rs139170 | 22 | Intronic | 0 | PARVG: parvin, gamma | Actin-binding proteins associated with focal adhesion | Involved in leukocyte-substrate interaction and leukocyte migration80; tumor suppressor feature81 | Cell adhesion; tumor suppression |
| rs5771675 | 22 | Intronic | 0 | FAM19A5: member of TAFA family | Related to MIP-1α, a member of the CC-chemokine family. | TAFA proteins are predominantly expressed in specific regions of the brain82; proposed to function as brain-specific chemokines or neurokines | Regulation of brain inflammation and neuronal survival (?) |
| rs9616906 | 22 | Upstream | −3,552 | AC000050.2 | |||
| rs13053175 | 22 | Upstream | −7,992 | RAC2: ras-related C3 botulinum toxin substrate 2 | GTPase of the RAS superfamily regulating cell growth, cytoskeleton, and protein kinases activation | T cell development83; B cell adhesion84; inflammation85; can modulate HSC adhesion, mobilization, and proliferation86 | Cell growth/ proliferation; cell adhesion; immunity, inflammation |
Sources of information: (1) Peer-reviewed research papers (references indicated); (2) if not indicated otherwise, online data bases and research tools for searching and systematizing genetic information, such as NCBI Entrez SNP, Entrez Gene, GeneCards, OMIM, KEGG, PANTHER, and Your Favorite Gene (powered by Ingenuity).
SNP, Single-nucleotide polymorphism; Chr, chromosome; IL-4, interleukin-4; TGF-α, transforming growth factor-α; EGF, epidermal growth factor; GABAergic, γ-aminobutyric acid–secreting; AD, Alzheimer disease; CVD, cardiovascular diseases; vWF, von Willebrand factor; PD, Parkinson disease; IFN, interferon; NF-κB, nuclear factor-κB; MIP-α, macrophage inflammatory protein 1 α; GTPase, guanosine triphosphatase; HSC, hematopoietic stem cells.
Table 4.
Examples of Similarity in Functions and Phenotypic Effects of “Longevity” or “Aging” Genes Found in Our and Other Studies
| Genes closest to I-longevity SNPs from our study | Biological processes/disorders associated with these genes | Examples of “longevity” or “aging” genes from other studies that have been linked to similar biological processes or health disorders as genes in our study |
|---|---|---|
| KIAA0649; STK24; PPP2R2C; TGFA; RAC2 | Cell growth; proliferation; cancer | IGF-187; MINPP16; LMO418 |
| RP11-24C3.2; PPP2R2C; RUNX3; CDH4; CARS; PARVG | Growth arrest; senescence; apoptosis; tumor suppression | p5325,88; p2189; FOXO3A90,91; p16Ink4a20,92 |
| CDH4; SVEP1; NCAM2; RAC2; PARVG; RUNX3 | Cell adhesion | p16Ink4a20; set of 478 aging- and 586 CR-related mouse genes19; CDH417; NCAM118 |
| CLSTN2; HPCAL1; CDH4; STK24; TGFA; FAM19A5; PPP2R2C; NCAM2, CACNA1C | Central nervous system information processing; brain aging; brain disorders; memory | p5393; Klotho94,95; CDH417; TOMM 40, APOE30; LMO4, GRIA1, NETO1, NCAM1, CACNA1C18 |
| NLRC5; FAM19A5; RAC2; RUNX3 | Immunity; inflammation | Set of 478 aging- and 586 CR-related mouse genes19; IL-1096 |
| ABCC4 | Detoxification; metabolism of xenobiotics | GSTT197,98 |
Some of the genes shown here are linked to multiple biological processes; therefore, the same gene name may appear in more than one row.
This review resulted in a number of important findings:
The majority (74%) of SNPs from the core set of 27 SNPs were located within genes, compared to only 40% of SNPs in the original 550K set. This supports potential functional relevance of these SNPs for achieving longevity.
A total of 80% (16 of 20) of those longevity SNPs that were located within genes are intronic. No longevity SNPs were found in exons. This finding is important because it suggests that the identified genetic effects on longevity might be linked to changes in protein concentrations rather than to the changes in protein structure. Indeed, introns may perform various jobs, such as alternative splicing or regulation of gene transcription levels with the help of an enhancer located in the intron. The latter is the reason why intronic polymorphisms may have functional effects, even when the introns are not translated. When activated by a transcription factor, the enhancer may increase the level of gene expression several-fold, which in turn will increase the yield of a respective protein. Therefore, polymorphisms in intronic enhancers may potentially significantly affect the balance of protein concentrations, without any changes in the protein structures, thus providing the possible mechanism of their influence on life span. To see if this is the case, it is necessary to connect the results of SNP genotyping with the results of the analyses of expression of respective genes and proteins in a further study. Currently, there is indirect evidence in support of such mechanism. One of the authors of this paper (Dr. Wu), in an earlier work on nematode worms,14 demonstrated a correlation between levels of gene–protein expression and survival, not related to the changes in gene–protein structure (the worms were genetically identical).
A review of functions and biological effects (known, as well as suggested) of genes closest to the 27 I-longevity SNPs revealed that biological processes most often associated with these genes are cell growth/proliferation, apoptosis, cell adhesion, and neural activities (such as information processing, response to damage, synaptic plasticity, and memory formation). Other (less common) processes were inflammation, detoxification, and replicative senescence. Health outcomes predominantly associated with these genes were cancer and brain disorders. Other (less common and mostly suggestive) associations were with CVDs and asthma.
Discussion
Potential mechanisms connecting genes and life span
A closer look on findings shown in Tables 3 and 4 allows for clarifying important features of mechanisms connecting genes and life span. The detected genes are typically multifunctional, with overlapping functions, so that each gene can contribute to several cell functions, and each function is supported by several genes. The latter provides support for the polygenic influence with potentially interchangeable and additive effects of the identified SNPs on life span.
A substantial part of the genes closest to the 27 longevity SNPs (26% of all loci, and 35% of the SNPs located within genes) are involved in regulating cell growth/proliferation and apoptosis (Tables 3 and 4). These cell responses and relevant pathways are thought to be the key players in physiological aging and linked to the decline in stress resistance. The pathways regulating growth/proliferation and apoptosis are known to be deeply interacting,15,16 so that the collective effects of genes involved in relevant pathways on life span could be expected.
Also a substantial number of the genes closest to I-longevity SNPs are involved in cell–cell and focal adhesion (CDH4, SVEP1, NCAM2, RAC2, PARVG, RUNX3). The portion of the SNPs related to the adhesion genes was surprisingly large (22% of all I-longevity SNPs, and 30% of the I-longevity SNPs located within genes). One of the genes (CDH4) was also identified in an earlier GWAS of brain aging involving Framingham participants.17 Another one (NCAM2) is a close relative of the NCAM1 neural adhesion gene featured in pathway analysis in the recent GWAS of life span by Walter et al.18
To understand mechanisms of the influence of cell adhesion genes on life span, it is important to stress that these genes may favor longevity through aging as well as cancer-related processes,19–21 and this influence may potentially involve significant tradeoffs; e.g., Hong et al.19 found that cell adhesion genes in mice were overactivated by the aging process, whereas calorie restriction (the only proved antiaging intervention so far) attenuated them. That is, the lower level of cell adhesion was associated with the slower physiological aging. In relation to interpretation of our study results, this would mean that in cases where the adhesion genes act together toward the lower level of the adhesion, this could favor longevity due to the slower aging. On the other hand, enhanced levels of cell adhesion were shown to be protective against cancer.20 In this case, longevity SNPs acting together toward the higher level of the cell adhesion could favor longevity due to a lower cancer risk. This means that to truly understand how the SNP alleles related to the adhesion genes favor survival, one needs at least to know if they down- or upregulate respective genes/proteins/pathways. It again stresses the importance of connecting the results of GWAS with the results of analyses of the genes/proteins expression.
As Table 3 shows, the health effects of the genes related to longevity SNPs focus mostly on cancer and brain disorders. Roughly, about half of all genes in the table are shown to be involved in cancer development, or at least in regulation of highly relevant cell responses (proliferation, senescence, apoptosis, and adhesion). Therefore, one reason why the joint contribution of respective genes is important for surviving old age could be that certain alleles of these genes, acting together, reduce cancer risk in individuals. Indeed, a majority of long-living people (at least nowadays) are those who have managed to avoid cancer until very old age; e.g., Newman et al.6 have been reported significantly less cancers among those who survived age 90 compared to those who did not.
Finally, about one-third of the genes closest to I-longevity SNPs are involved in brain activities that are highly relevant to brain aging (speed of information processing, brain response to damage, and memory formation), and also in brain disorders (Alzheimer disease [AD], Parkinson's disease [PD], bipolar disorder, and schizophrenia), most often through calcium signaling pathways. As Table 3 shows, the possible mechanisms of prolongevity effects of polymorphisms in these genes may involve: (1) Slower brain aging and decline in stress resistance (e.g., through delayed memory loss and better brain regenerative response to damage), and (2) lower susceptibility to brain disorders. It is not clear which mechanism is prevailing, or if there is a trade-off. Because brain aging starts early in life,22 typically long before the manifestation of aging in other organs and onsets of major diseases, including in the brain, the brain aging could be a causal factor contributing to brain disorders later in life.
Summarizing this part of the Discussion, the fact that many of the identified genes participate in regulating both aging and cancer, or both brain aging and brain disorders, raises a question about distinguishing between the aging and disease-related functional effects of SNP alleles that underline the increases in longevity. Do these alleles favor longevity mainly because they attenuate physiological aging changes in body (and respective decline in stress resistance), or mainly because they help to reduce risks and postpone onsets of common diseases with high fatality rates, such as cancer and AD? They may do both, but there may also be trade-offs. For example, it is well known that risks of many common diseases, including cancer and AD, typically decline at older ages, when the overall risk of death continues to increase.16,23 This indicates that there may be factors, including genetic ones, that affect the age-associated increase in vulnerability to death due to physiological aging (and related decline in stress resistance), and risks of major diseases, differently or even oppositely. For example, van Heemst et al.24 demonstrated that individuals with Pro/Pro genotype of p53 corresponding to a reduced apoptosis in cells had a significant increase in both overall survival (by 41%) and mortality from cancer. In another study, Ørsted et al.25 found that the overall 12-year survival was increased in p53 Pro/Pro versus Arg/Arg homozygotes despite potential cancer-favoring properties of the former genotype. Boquoi et al.26 have recently reported that expression of a key tumor suppressor protein p16 (loss of which is associated with many human cancers) induced premature aging in mice via blocking cell proliferation in normal tissues. This consideration emphasizes the importance of taking into account the possibility of trade-offs between genetic factors influencing aging and diseases (as well as between diseases), when trying to explain mechanisms of the observed genetic effects on life span.16
False positives and replication issues
The large numbers of SNP alleles selected using N-, GEE-, MM-, and C-methods, as well as substantial difference in these numbers, indicate high chances of having false-positive alleles in selected sets. The numbers of selected false-positive alleles depends on the QC procedure. In our study, we used the call rate≥80%, HWE>10−7, and MAF≥1%, which is likely to be not stringent enough. More stringent QC procedures (e.g., Fornage et al.27 and O'Seaghdha et al.28) may substantially reduce the number of false positives. Note that intersection of the six sets of selected SNP alleles may substantially reduce the chances of having false positives in the I-set and increase the chances of selecting true positive alleles. Table S1 in the Supplemental Data shows that 5 out of 27 SNPs (rs16975963, rs1327533, rs432203, rs41383, rs3120819) have a call rate between 80% and 90%, 2 out of remaining 22 SNPs (rs2370413, rs9616906) have a call rate between 90% and 95%, and 3 out of remaining 20 SNPs (rs12623542, rs13008689, rs1834497) have a call rate between 95% and 97%. The fact that 17 out of 27 selected SNPs passed through the most stringent QC with the call rate of 97% indicates high chances for them to be true positive alleles. It is important to note that the 27 SNPs selected in our paper passed through additional stringent QC procedure (which was not used in the studies discussed above). They have been selected in each of six different procedures based on different statistical models (see Fig. S6 and Fig. S7 in Supplemental Data concerning additional properties of selected 27 SNPs).
Note that replicating GWAS findings in a new study with an independent population might be a less reliable and cost-ineffective method of confirming true positive associations compared to testing the roles of the detected genes in known metabolic pathways. Indeed, with heritability in life span about 25%, the environmental factors contribute up to 75% of variability in this trait. Because environmental factors influence life span by activating appropriate genes from metabolic pathways involved in the regulation of aging, health, and longevity, distinct conditions might activate different genes from the same or different pathways. Therefore, it is likely that different sets of SNP alleles will be associated with life span in the two independent populations, reducing chances to replicate GWAS findings in independent populations. This conclusion is illustrated by the results of two recent GWAS produced by established research groups, where none of the previously identified longevity genes were found.29,30 Does it mean that earlier genetic studies of longevity produced false-positive results? Not necessarily. It is most likely that the idea of replication using independent population does not fully correspond to the multi-factorial nature of complex traits.
It is important to point out that the selected 27 longevity SNPs do not exhaust all SNP variants positively influencing life span in a given dataset. The complexity of life span as a phenotypic trait indicates that many other genes with small effects might be involved in the regulation of life span. Moreover, the same gene may play prolongevity or antilongevity roles dependent on the functioning of other genes, meaning that its effect may be opposite in the two individuals. They may be opposite in the same individual in different periods of his/her life and in different environments. Statistical models used in the allele selection procedures estimate average effect of genetic variants on life span. That is why the estimate of such an effect may be modified when the number of individuals in a sample changes (e.g., as a result of using different quality control procedure, or by adding a portion of newly genotyped individuals to the initial sample). Similarly, the use of different statistical models in the allele selection procedure provides researchers with different approximations of the real connection between genes and life span resulting in different subsets of selected genetic variants.
Lunetta et al.4 performed GWAS using genetic data on 100K SNPs (Affymetrix 100K GeneChip) collected in participants of the FHS Original and Offspring cohorts. The Cox proportional hazards model was used to perform the regression analysis of survival times from age at study entry to age at death. Models were adjusted for a number of observed covariates, including birth cohorts and behavioral and physiological characteristics. The authors concluded that longevity and aging traits are associated with a number of SNPs; however, none of the associations achieved genome-wide significance.
Newman et al.6 performed a meta-analysis of GWAS of longevity (defined as survival to age 90 years or older) in Caucasians from four prospective cohort studies. The authors found 273 SNPs associations with p<0.0001, but none reached the prespecified significance level of 5×10−8. The authors concluded that survival studies of larger size or more extreme or specific phenotypes may support or refine their findings.
Taking into account the difficulties in detecting individual longevity alleles with significant effects using traditional GWAS, as well as the results of earlier studies evaluating narrow-sense heritability of longevity between 15% and 30%,10,31,32 we recently tested the presence of the additive genetic influence on life span, using the FHS data.10 Such influence was demonstrated for other complex traits, such as height.3 To select respective alleles, Yashin et al.10 used a linear regression model without observed covariates and demonstrated that significant additive effects of common SNPs on life span do exist. The results of our analyses showed that detected sets of genetic variants depend on the statistical model used in allele selection procedure, but also have many genetic variants in common. These analyses also showed that SNP alleles from each of the six sets have substantial joint genetic influence on life span, which was also highly statistically significant. Thus, our study demonstrated that six different methods used in the allele selection procedure lead to the same conclusion: A substantial portion of variability in human life span is associated with the additive genetic component. This conclusion is in concert with the results of recent studies of genetic contribution to variability of complex traits.33
In the pregenomic era, the additive genetic contribution to complex traits was a major focus of genetic studies of such traits. Respective statistical models described phenotypic traits as a sum of independent genetic and environmental components without interaction. The genetic influence on a trait was characterized by the narrow-sense heritability, i.e., the proportion of phenotypic variance, explained by the additive genetic component. Although, using today's knowledge about phenotypes (e.g., life span) and factors affecting them, mathematical models used for heritability calculations look oversimplified, many conclusions derived from them have important practical meaning. For example, the estimates of heritability have been used to measure selection rate in breeding.34 The presence of additive genetic effects on phenotypic traits was also one of the key assumptions in evolutionary models of natural selection.35 However, despite recognition of the importance of additive genetic components in phenotypic variation of complex traits, and a number of publications33,36–38 clearly demonstrating the possibility of estimating this component from available genome-wide SNP data, as well as the benefits of using the estimate in analyses of genetic influence on complex traits (e.g., risks of diseases), the potential of this approach for studying life span, ages at disease onset, or other health-related durations remain largely underused.
The set of I-longevity alleles selected in these analyses differs from that selected in our earlier paper,10 although these sets have nine genetic variants in common. This is likely to be due to the differences in approaches to selecting genetic variants associated with life span.
The longevity alleles selected in this study differ from those selected in the studies of Lunetta et al.4 and Newman et al.6 discussed above. As results of our study show, one possible reason for this could be differences in the allele selection procedures. The control for observed covariates was also different. In contrast to Newman et al.,6 we did not control for observed covariates that could be considered as mediators of genetic influence on life span (e.g., physiological variables) in our study. Although controlling for such covariates may reduce effects of nongenetic factors affecting life span through these covariates, it may also reduce the number of selected genetic variants affecting this trait (see also Supplemental Data).
The SNPs identified in our study do not have exact overlaps with the SNPs found in other recent GWAS of longevity.5,7,18 While recognizing all the possible statistical reasons for such lack of replication (discussed above), it is important to stress that on the level of gene functions, pathways, cell responses, and health effects, in which the identified SNPs or related genes are involved, there are clear overlaps across respective studies, as well as with many other (not genome-wide) genetic association studies of aging and longevity (Table 4). For example, rs2024714 is one of the 27 I-longevity SNPs, which is located in the intronic region of CDH4, a cell adhesion gene involved in brain information processing and tumor suppression. In an earlier GWAS conducted by Framingham researchers using the Affymetrix 100K SNP array, a polymorphism (rs1970546) in the same CDH4 gene (and also located in the intronic region of this gene) was found to be significantly (p=3.7×10−8) associated with total cerebral brain volume used in that study as endophenotype of brain aging.17 Another example: Several of the 27 SNPs from our study are located within or close to genes involved in regulating cell proliferation (e.g., RAC2, TGF-α). Similarly, in Newman et al.,6 a gene (MINPP1) linked to one of the identified longevity SNPs with highest significance level (rs9664222, 6.77×10−7) is also involved in regulating cell proliferation (Table 4). In a more recent meta-analysis of nine GWA studies by Walter et al.,18 the authors identified 14 SNPs associated with time to death with a conventional level of significance (p<10−5), and also 862 SNPs with p<10−3, and evaluated biological processes associated with genes closest to these SNPs. Similar to our study, cell adhesion and neuronal activities (e.g., involving NCAM1 and CACNA1C genes) were overrepresented among the relevant processes (Table 4).
Taking into account that about 75% of phenotypic variation in life span could be due to nongenetic factors, and that these factors likely influence traits not directly but through genes involved in various pathways, one should not expect to exactly replicate the effect of a specific SNP allele in other populations. However, the fact that “longevity” or “aging” genes identified in different studies often represent the same molecular function, pathway, cell response, or biological process indicates that it may be reasonable to respectively address the issue of replication of gene-longevity associations,39 i.e., to do it on the level of gene functions, pathways, and their outcomes, rather than on the level of individual SNPs or genes.
Conclusions
The results of this study stress the importance of using several statistical approaches for identifying alleles in GWAS of complex traits, as well as the importance of using established metabolic pathways involved in regulation of these traits for confirming research findings. The latter creates an important link between population-based GWAS and the results of experimental studies focused on verifying molecular biological mechanisms, metabolic pathways, and protein interaction networks affecting phenotypic traits.
The fact that the majority of detected SNPs were located in genes and were intronic indicates a potentially pivotal role of changes in intronic enhancers and in gene/protein expression (not related to the changes in protein structure) in achieving longevity, as compared to the role of the changes in coding exons. This hypothesis needs further testing.
We also found that longevity genes from the intersecting set are not random, but are largely involved in regulating aging and cancer, as well as brain aging and brain disorders. Discovering the functional relevance of these genes to aging and major diseases supports a causal relationship underlying associations between the identified SNPs and life span, and reduces the chances for these alleles to be false positives.
Although the available information on functional effects of the detected longevity alleles is currently not comprehensive enough to draw conclusion about precise mechanisms of their influence on life span (e.g., it is in most cases unclear if the effects of these alleles result in up- or downregulating respective genes/proteins/pathways), discussing these mechanisms is necessary for formulating priority questions that need to be addressed in further studies, e.g., about relative contribution of genetically slower aging versus lower disease risks for achieving longevity, possibility of pleiotropic effects (e.g., trade-offs) of the same genetic factor on aging and diseases, as well as on different diseases.16 It is also crucial to connect the results of GWAS with the results of analyses of expression of respective genes and proteins.
Marked similarities in functions and phenotypic effects of many genes found in our and other genetic associations studies of aging/longevity indicate that replication of the results of genetic association studies can be achieved by using available information about the involvement of respective genes in certain pathways, cell responses, physiological processes, and health disorders.40
The next logical steps in studying the genetic influence on longevity could deal with analyses of genetic mechanisms underlying complex relationships among physiological aging changes, onsets of major diseases, and life span, taking into account the effects of both individual genes and polygenic influence (additive and nonadditive) on these traits, as well as the impact of nongenetic factors.
Supplementary Material
Acknowledgments
The FHS project is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University (N01 HC25195). The FHS data used for the analyses were obtained through dbGaP (phs000007.v3.p2). The authors acknowledge the investigators that contributed the phenotype and genotype data for this study. This manuscript was not prepared in collaboration with investigators of the FHS and does not necessarily reflect the opinions or views of the FHS, Boston University, or the NHLBI. This work was partly supported by NIH/NIA grant R01AG030612.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Yang J. Benyamin B. McEvoy BP, et al. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010;42:565–569. doi: 10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson RE. Maes HH. Holmans P, et al. Genetic risk sum score comprised of common polygenic variation is associated with body mass index. Hum Genet. 2011;129:221–230. doi: 10.1007/s00439-010-0917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen HL. Estrada K. Lettre G, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lunetta KL. D'Agostino RB., Sr. Karasik D, et al. Genetic correlates of longevity and selected age-related phenotypes: A genome-wide association study in the Framingham Study. BMC Med Genet. 2007;8(Suppl 1):S13. doi: 10.1186/1471-2350-8-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy J. Singleton A. Genomewide association studies and human disease. New Engl J Med. 2009;360:1759–1768. doi: 10.1056/NEJMra0808700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman AB. Walter S. Lunetta KL, et al. A meta-analysis of four genome-wide association studies of survival to age 90 years or older: The cohorts for heart and aging research in Genomic Epidemiology Consortium. J Gerontol A Biol Sci Med Sci. 2010;65:478–487. doi: 10.1093/gerona/glq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malovini A. Illario M. Iaccarino G, et al. Association study on long-living Individuals from Southern Italy identifies rs10491334 in the CAMKIV gene that regulates survival proteins. Rejuvenation Res. 2011;14:283–291. doi: 10.1089/rej.2010.1114. [DOI] [PubMed] [Google Scholar]
- 8.Manolio TA. Collins FS. Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purcell SM. Wray NR. Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yashin AI. Wu DQ. Arbeev KG. Ukraintseva SV. Joint influence of small-effect genetic variants on human longevity. Aging. 2010;2:612–620. doi: 10.18632/aging.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawber TR. Meadors GF. Moore FE. Epidemiological approaches to heart disease: The Framingham Study. Am J Public Health. 1951;41:279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kannel WB. Feinleib M. McNamara PM, et al. An investigation of coronary heart disease in families: The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 13.Splansky GL. Corey D. Yang Q, et al. The third generation cohort of the national heart, lung, and blood institute's Framingham Heart Study: Design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 14.Rea SL. Wu DQ. Cypser JR, et al. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet. 2005;37:894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine AJ. Feng ZH. Mak TW, et al. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 16.Ukraintseva SV. Arbeev KG. Akushevich I, et al. Trade-offs between cancer and other diseases: Do they exist and influence longevity? Rejuvenation Res. 2010;13:387–396. doi: 10.1089/rej.2009.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seshadri S. DeStefano AL. Au R, et al. Genetic correlates of brain aging on MRI and cognitive test measures: a genome-wide association and linkage analysis in the Framingham study. BMC Med. Genet 2007. 2007:8. doi: 10.1186/1471-2350-8-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walter S. Atzmon G. Demerath EW, et al. A genome-wide association study of aging. Neurobiol Aging. 2011;32:2109. doi: 10.1016/j.neurobiolaging.2011.05.026. .e2115–2109,e2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong S-E. Heo H-S. Kim DH, et al. Revealing system-level correlations between aging and calorie restriction using a mouse transcriptome. Age. 2010;32:15–30. doi: 10.1007/s11357-009-9106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seluanov A. Hine C. Azpurua J, et al. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proc Natl Acad Sci USA. 2009;106:19352–19357. doi: 10.1073/pnas.0905252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miotto E. Sabbioni S. Veronese A, et al. Frequent aberrant methylation of the CDH4 gene-promoter in human colorectal and gastric cancer. Cancer Res. 2004;64:8156–8159. doi: 10.1158/0008-5472.CAN-04-3000. [DOI] [PubMed] [Google Scholar]
- 22.Finch CE. The neurobiology of middle-age has arrived. Neurobiol Aging. 2009;30:515–520. doi: 10.1016/j.neurobiolaging.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Ukraintseva SV. Yashin AI. Individual aging and cancer risk: How are they related? Demographic Res. 2003;9:163–196. [Google Scholar]
- 24.van Heemst D. Mooijaart SP. Beekman M, et al. Variation in the human TP53 gene affects old age survival and cancer mortality. Exp Gerontol. 2005;40:11–15. doi: 10.1016/j.exger.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Orsted DD. Bojesen SE. Tybjaerg-Hansen A. Nordestgaard BG. Tumor suppressor p53 Arg72Pro polymorphism and longevity, cancer survival, and risk of cancer in the general population. J Exp Med. 2007;204:1295–1301. doi: 10.1084/jem.20062476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boquoi A. Chen T. Enders GH. Chemoprevention of mouse intestinal tumorigenesis by the cyclin-dependent kinase inhibitor SNS-032. Cancer Prev Res. 2009;2:800–806. doi: 10.1158/1940-6207.CAPR-09-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fornage M. Debette S. Bis JC, et al. Genome-wide association studies of cerebral white matter lesion burden: The CHARGE Consortium. Ann Neurol. 2011;69:928–939. doi: 10.1002/ana.22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Seaghdha CM. Yang Q. Glazer NL, et al. Common variants in the calcium-sensing receptor gene are associated with total serum calcium levels. Hum Mol Genet. 2010;19:4296–4303. doi: 10.1093/hmg/ddq342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nebel A. Kleindorp R. Caliebe A, et al. A genome-wide association study confirms APOE as the major gene influencing survival in long-lived individuals. Mech Ageing Dev. 2011;132:324–330. doi: 10.1016/j.mad.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Deelen J. Beekman M. Uh H-W, et al. Genome-wide association study identifies a single major locus contributing to survival into old age; the APOE locus revisited. Aging Cell. 2011;10:686–698. doi: 10.1111/j.1474-9726.2011.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGue M. Vaupel JW. Holm N. Harvald B. Longevity is moderately heritable in a sample of Danish twins born 1870–1880. J Gerontol. 1993;48:B237–B244. doi: 10.1093/geronj/48.6.b237. [DOI] [PubMed] [Google Scholar]
- 32.Herskind AM. McGue M. Holm NV, et al. The heritability of human longevity: A population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet. 1996;97:319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- 33.Hill WG. Goddard ME. Visscher PM. Data and theory point to mainly additive genetic variance for complex traits. PLoS Genet. 2008;4:e1000008. doi: 10.1371/journal.pgen.1000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falconer DS. Mackay TFC. Introduction to Quantitative Genetics. Addison Wesley Longman; Harlow, Essex, UK: 1996. [Google Scholar]
- 35.Fisher RA. The Genetical Theory of Natural Selection. Oxford: Clarendon Press; 1930. [Google Scholar]
- 36.Lee SH. Wray NR. Goddard ME. Visscher PM. Estimating missing heritability for disease from genome-wide association studies. Am J Hum Genet. 2011;88:294–305. doi: 10.1016/j.ajhg.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J. Lee SH. Goddard ME. Visscher PM. GCTA: A tool for genome-wide complex trait analysis. Am J Hum Genet 7. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visscher PM. Hill WG. Wray NR. Heritability in the genomics era—concepts and misconceptions. Nat Rev Genet. 2008;9:255–266. doi: 10.1038/nrg2322. [DOI] [PubMed] [Google Scholar]
- 39.Novelli V. Anselmi CV. Roncarati R, et al. Lack of replication of genetic associations with human longevity. Biogerontology. 2008;9:85–92. doi: 10.1007/s10522-007-9116-4. [DOI] [PubMed] [Google Scholar]
- 40.Torkamani A. Scott-Van Zeeland AA. Topol EJ. Schork NJ. Annotating individual human genomes. Genomics. 2011;98:233–241. doi: 10.1016/j.ygeno.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong D-d. Jiang Y. Li M, et al. RUNX3 inhibits cell proliferation and induces apoptosis by TGF-beta-dependent and -independent mechanisms in human colon carcinoma cells. Pathobiology. 2009;76:163–169. doi: 10.1159/000218332. [DOI] [PubMed] [Google Scholar]
- 42.Subramaniam MM. Chan JY. Yeoh KG, et al. Molecular pathology of RUNX3 in human carcinogenesis. Biochim Biophys Acta Rev Cancer. 2009;1796:315–331. doi: 10.1016/j.bbcan.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Fainaru O. Shseyov D. Hantisteanu S. Groner Y. Accelerated chemokine receptor 7-mediated dendritic cell migration in Runx3 knockout mice and the spontaneous development of asthma-like disease. Proc Natl Acad Sci USA. 2005;102:10598–10603. doi: 10.1073/pnas.0504787102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naoe Y. Setoguchi R. Akiyama K, et al. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. J Exp Med. 2007;204:1749–1755. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hollingsworth JW. Maruoka S. Boon K, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–3469. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Kamide K. Kokubo Y. Yang J, et al. Hypertension susceptibility genes on chromosome 2p24-p25 in a general Japanese population. J Hypertens. 2005;23:955–960. doi: 10.1097/01.hjh.0000166835.70935.3c. [DOI] [PubMed] [Google Scholar]
- 47.Imada Y. Fujimoto M. Hirata K, et al. Large scale genotyping study for asthma in the Japanese population. BMC Res Notes. 2009;2:54. doi: 10.1186/1756-0500-2-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guerra-Crespo M. Gleason D. Sistos A, et al. Transforming growth factor-alpha induces neurogenesis and behavioral improvement in a chronic stroke model. Neuroscience. 2009;160:470–483. doi: 10.1016/j.neuroscience.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 49.Cavin LG. Wang F. Factor VM, et al. Transforming growth factor-alpha inhibits the intrinsic pathway of c-myc-induced apoptosis through activation of nuclear factor-kappa B in murine hepatocellular carcinomas. Mol Cancer Res. 2005;3:403–412. doi: 10.1158/1541-7786.MCR-04-0186. [DOI] [PubMed] [Google Scholar]
- 50.Preuschhof C. Heekeren HR. Li S-C, et al. KIBRA and CLSTN2 polymorphisms exert interactive effects on human episodic memory. Neuropsychologia. 2010;48:402–408. doi: 10.1016/j.neuropsychologia.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 51.Jacobsen LK. Picciotto MR. Heath CJ, et al. Allelic variation of calsyntenin 2 (CLSTN2) modulates the impact of developmental tobacco smoke exposure on mnemonic processing in adolescents. Biol Psychiatry. 2009;65:671–679. doi: 10.1016/j.biopsych.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu F. Arias-Vasquez A. Sleegers K, et al. A genomewide screen for late-onset Alzheimer disease in a genetically isolated Dutch population. Am J Hum Genet. 2007;81:17–31. doi: 10.1086/518720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myers JS. Zhao R. Xu X, et al. Cyclin-dependent kinase 2-dependent phosphorylation of ATRIP regulates the G(2)-M checkpoint response to DNA damage. Cancer Res. 2007;67:6685–6690. doi: 10.1158/0008-5472.CAN-07-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toledo LI. Murga M. Gutierrez-Martinez P, et al. ATR signaling can drive cells into senescence in the absence of DNA breaks. Genes Dev. 2008;22:297–302. doi: 10.1101/gad.452308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eichhorn PJA. Creyghton MP. Wilhelmsen K, et al. A RNA interference screen identifies the protein phosphatase 2A subunit PR55 gamma as a stress-sensitive inhibitor of c-SRC. PLoS Genet. 2007;3:2381–2394. doi: 10.1371/journal.pgen.0030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banerjee AK. Read CA. Griffiths MH, et al. Clonal divergence in lung cancer development is associated with allelic loss on chromosome 4. Genes Chromosomes Cancer. 2007;46:852–860. doi: 10.1002/gcc.20472. [DOI] [PubMed] [Google Scholar]
- 57.Strack S. Overexpression of the protein phosphatase 2A regulatory subunit B gamma promotes neuronal differentiation by activating the MAP kinase (MAPK) cascade. J Biol Chem. 2002;277:41525–41532. doi: 10.1074/jbc.M203767200. [DOI] [PubMed] [Google Scholar]
- 58.Backx L. Vermeesch J. Pijkels E, et al. PPP2R2C, a gene disrupted in autosomal dominant intellectual disability. Eur J Med Genet. 2010;53:239–243. doi: 10.1016/j.ejmg.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Borsotto M. Cavarec L. Bouillot M, et al. PP2A-B gamma subunit and KCNQ2 K+ channels in bipolar disorder. Pharmacogenom J. 2007;7:123–132. doi: 10.1038/sj.tpj.6500400. [DOI] [PubMed] [Google Scholar]
- 60.Hasdemir C. Aydin HH. Celik HA, et al. Transcriptional profiling of septal wall of the right ventricular outflow tract in patients with idiopathic ventricular arrhythmias. Pacing Clin Electrophysiol. 2010;33:159–167. doi: 10.1111/j.1540-8159.2009.02606.x. [DOI] [PubMed] [Google Scholar]
- 61.Jellen LC. Beard JL. Jones BC. Systems genetics analysis of iron regulation in the brain. Biochimie. 2009;91:1255–1259. doi: 10.1016/j.biochi.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shefer G. Benayahu D. SVEP1 is a novel marker of activated pre-determined skeletal muscle satellite cells. Stem Cell Rev Rep. 2010;6:42–49. doi: 10.1007/s12015-009-9106-9. [DOI] [PubMed] [Google Scholar]
- 63.Yang L. Zhao J. Lu WQ, et al. KIAA0649, a 1A6/DRIM-interacting protein with the oncogenic potential. Biochem Biophys Res Commun. 2005;334:884–890. doi: 10.1016/j.bbrc.2005.06.179. [DOI] [PubMed] [Google Scholar]
- 64.Debelenko LV. Arthur DC. Pack SD, et al. Identification of CARS-ALK fusion in primary and metastatic lesions of an inflammatory myofibroblastic tumor. Lab Invest. 2003;83:1255–1265. doi: 10.1097/01.lab.0000088856.49388.ea. [DOI] [PubMed] [Google Scholar]
- 65.Pezzolesi MG. Poznik GD. Mychaleckyj JC, et al. Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes. 2009;58:1403–1410. doi: 10.2337/db08-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moosmang S. Haider N. Klugbauer N, et al. Role of hippocampal Ca(v)1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Donovan MC. Craddock NJ. Owen MJ. Genetics of psychosis; insights from views across the genome. Hum Genet. 2009;126:3–12. doi: 10.1007/s00439-009-0703-0. [DOI] [PubMed] [Google Scholar]
- 68.Kempton MJ. Ruberto G. Vassos E, et al. Effects of the CACNA1C risk allele for bipolar disorder on cerebral gray matter volume in healthy undividuals. Am J Psychiatry. 2009;166:1413–1414. doi: 10.1176/appi.ajp.2009.09050680. [DOI] [PubMed] [Google Scholar]
- 69.Kamide K. Yang J. Matayoshi T, et al. Genetic polymorphisms of L-type calcium channel alpha 1C and alpha 1D subunit genes are associated with sensitivity to the antihypertensive effects of L-type dihydropyridine calcium-channel blockers. Circ J. 2009;73:732–740. doi: 10.1253/circj.cj-08-0761. [DOI] [PubMed] [Google Scholar]
- 70.Lorber B. Howe ML. Benowitz LI. Irwin N. Mst3b, an Ste20-like kinase, regulates axon regeneration in mature CNS and PNS pathways. Nat Neurosci. 2009;12:1407–1414. doi: 10.1038/nn.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zach S. Felk S. Gillardon F. Signal transduction protein array analysis links LRRK2 to Ste20 kinases and PKC zeta that modulate neuronal plasticity. PLoS One. 2010;5:e13191. doi: 10.1371/journal.pone.0013191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thalappilly S. Suliman M. Gayet O, et al. Identification of multi-SH3 domain-containing protein interactome in pancreatic cancer: A yeast two-hybrid approach. Proteomics. 2008;8:3071–3081. doi: 10.1002/pmic.200701157. [DOI] [PubMed] [Google Scholar]
- 73.Low S-K. Kiyotani K. Mushiroda T, et al. Association study of genetic polymorphism in ABCC4 with cyclophosphamide-induced adverse drug reactions in breast cancer patients. J Hum Genet. 2009;54:564–571. doi: 10.1038/jhg.2009.79. [DOI] [PubMed] [Google Scholar]
- 74.Moyer AM. Sun Z. Batzler AJ, et al. Glutathione pathway genetic polymorphisms and lung cancer survival after platinum-based chemotherapy. Cancer Epidemiol Biomarkers Prev. 2010;19:811–821. doi: 10.1158/1055-9965.EPI-09-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cui J. Zhu L. Xia X, et al. NLRC5 Negatively regulates the NF-kappa B and type I interferon signaling pathways. Cell. 2010;141:483–496. doi: 10.1016/j.cell.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benko S. Magalhaes JG. Philpott DJ. Girardin SE. NLRC5 Limits the activation of inflammatory pathways. J Immunol. 2010;185:1681–1691. doi: 10.4049/jimmunol.0903900. [DOI] [PubMed] [Google Scholar]
- 77.Andrews GL. Mastick GS. R-cadherin is a Pax6-regulated, growth-promoting cue for pioneer axons. J Neurosci. 2003;23:9873–9880. doi: 10.1523/JNEUROSCI.23-30-09873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kulahin N. Walmod PS. The neural cell adhesion molecule NCAM2/OCAM/RNCAM, a close relative to NCAM. Berezin V, editor. Structure and Function of the Neural Cell Adhesion Molecule Ncam. Adv Exp Med Biol. 2010;663(Part 8):403–420. doi: 10.1007/978-1-4419-1170-4_25. [DOI] [PubMed] [Google Scholar]
- 79.Takahashi S. Kato K. Nakamura K, et al. Neural cell adhesion molecule 2 as a target molecule for prostate and breast cancer gene therapy. Cancer Sci. 2011;102:808–814. doi: 10.1111/j.1349-7006.2011.01855.x. [DOI] [PubMed] [Google Scholar]
- 80.Yoshimi R. Yamaji S. Suzuki A, et al. The gamma-parvin-integrin-linked kinase complex is critically involved in leukocyte-substrate interaction. J Immunol. 2006;176:3611–3624. doi: 10.4049/jimmunol.176.6.3611. [DOI] [PubMed] [Google Scholar]
- 81.Lopez-Serra L. Ballestar E. Ropero S, et al. Unmasking of epigenetically silenced candidate tumor suppressor genes by removal of methyl-CpG-binding domain proteins. Oncogene. 2008;27:3556–3566. doi: 10.1038/sj.onc.1211022. [DOI] [PubMed] [Google Scholar]
- 82.Tom Tang Y. Emtage P. Funk WD, et al. TAFA: A novel secreted family with conserved cysteine residues and restricted expression in the brain. Genomics. 2004;83:727–734. doi: 10.1016/j.ygeno.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 83.Guo F. Cancelas JA. Hildeman D, et al. Rac GTPase isoforms Rac1 and Rac2 play a redundant and crucial role in T-cell development. Blood. 2008;112:1767–1775. doi: 10.1182/blood-2008-01-132068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arana E. Vehlow A. Harwood NE, et al. Activation of the small GTPase Rac2 via the B cell receptor regulates B cell adhesion and immunological-synapse formation. Immunity. 2008;28:88–99. doi: 10.1016/j.immuni.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 85.Dooley JL. Abdel-Latif D. St Laurent CD, et al. Regulation of inflammation by Rac2 in immune complex-mediated acute lung injury. Am J Physiol–Lung Cell Mol Physiol. 2009;297:L1091–L1102. doi: 10.1152/ajplung.90471.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williams DA. Zheng Y. Cancelas JA. Rho GTPases and regulation of hematopoietic stem cell localization. Methods Enzymol. 2008;439:365–393. doi: 10.1016/S0076-6879(07)00427-2. [DOI] [PubMed] [Google Scholar]
- 87.Albani D. Batelli S. Polito L, et al. A polymorphic variant of the insulin-like growth factor 1 (IGF-1) receptor correlates with male longevity in the Italian population: a genetic study and evaluation of circulating IGF-1 from the “Treviso Longeva (TRELONG)” study. BMC Geriatr. 2009;9:19. doi: 10.1186/1471-2318-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bojesen SE. Nordestgaard BG. The common germline Arg72Pro polymorphism of p53 and increased longevity in humans. Cell Cycle. 2008;7:158–163. doi: 10.4161/cc.7.2.5249. [DOI] [PubMed] [Google Scholar]
- 89.Gravina S. Lescai F. Hurteau G, et al. Identification of single nucleotide polymorphisms in the p21 (CDKN1A) gene and correlations with longevity in the Italian population. Aging-Us. 2009;1:470–480. doi: 10.18632/aging.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y. Wang W-J. Cao H, et al. Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum Mol Genet. 2009;18:4897–4904. doi: 10.1093/hmg/ddp459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Willcox BJ. Donlon TA. He Q, et al. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matheu A. Maraver A. Collado M, et al. Anti-aging activity of the Ink4/Arf locus. Aging Cell. 2009;8:152–161. doi: 10.1111/j.1474-9726.2009.00458.x. [DOI] [PubMed] [Google Scholar]
- 93.Di Giovanni S. Knights CD. Rao M, et al. The tumor suppressor protein p53 is required for neurite outgrowth and axon regeneration. EMBO J. 2006;25:4084–4096. doi: 10.1038/sj.emboj.7601292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15:437–441. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- 95.Imai M. Ishikawa K. Matsukawa N, et al. Klotho protein activates the PKC pathway in the kidney and testis and suppresses 25-hydroxyvitamin D-3 1 alpha-hydroxylase gene expression. Endocrine. 2004;25:229–234. doi: 10.1385/ENDO:25:3:229. [DOI] [PubMed] [Google Scholar]
- 96.Khabour OF. Barnawi JM. Association of longevity with IL-10-1082 G/A and TNF-alpha-308 G/A polymorphisms. Int J Immunogenet. 2010;37:293–298. doi: 10.1111/j.1744-313X.2010.00925.x. [DOI] [PubMed] [Google Scholar]
- 97.Taioli E. Mari D. Franceschi C, et al. Polymorphisms of drug-metabolizing enzymes in healthy nonagenarians and centenarians: Difference at GSTT1 locus. Biochem Biophys Res Commun. 2001;280:1389–1392. doi: 10.1006/bbrc.2001.4280. [DOI] [PubMed] [Google Scholar]
- 98.Onder G. Capoluongo E. Giovannini S, et al. Interaction between GSTM1 genotype and IL-6 on mortality in older adults: Results from the ilSIRENTE study. Cytokine. 2011;53:301–305. doi: 10.1016/j.cyto.2010.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.