Abstract
Inducible nitric oxide synthase (iNOS) is a key mediator of inflammation and oxidative stress produced during pathological conditions, including neurodegenerative diseases and central nervous system (CNS) injury. iNOS is responsible for the formation of high levels of nitric oxide (NO). The production of highly reactive and cytotoxic NO species, such as peroxynitrite, plays an important role in secondary tissue damage. We have previously demonstrated that acute administration of iNOS antisense oligonucleotides (ASOs) 3 h after moderate contusive spinal cord injury (SCI) potently inhibits iNOS-mediated increases in NO levels, leading to reduced blood–spinal cord barrier permeability, decreased neutrophil accumulation, and less neuronal cell death. In the current study we investigated if iNOS ASOs could also provide long-term (10-week) histological and behavioral improvements after moderate thoracic T8 contusive SCI. Adult rats were randomly assigned to three groups (n=10/group): SCI alone, SCI and mixed base control oligonucleotides (MBOs), or SCI and iNOS ASOs (200 nM). Oligonucleotides were administered by spinal superfusion 3 h after injury. Behavioral analysis (Basso-Beattie-Bresnahan [BBB] score and subscore) was employed weekly for 10 weeks post-SCI. Although animals treated with iNOS ASOs demonstrated no significant differences in BBB scores compared to controls, subscore analysis revealed a significant improvement in foot positioning, trunk stability, and tail clearance. Histologically, while no gross improvement in preserved white and gray matter was observed, greater numbers of surviving neurons were present adjacent to the lesion site in iNOS ASO-treated animals than controls. These results support the effectiveness of targeting iNOS acutely as a therapeutic approach after SCI.
Key words: neuronal cell death, nitric oxide, nitric oxide synthase, oligonucleotides, spinal cord injury
Introduction
Spinal cord injury (SCI) results in the progressive death of neurons and glia for days to weeks after the initial mechanical trauma. The secondary phase of tissue damage occurs in response to a range of cytotoxic and degradative molecules, including proinflammatory cytokines, intracellular proteases, neurotransmitters, and free radicals, which leads to a further loss of tissue and function (Schwab and Bartholdi, 1996; Sipski and Pearse, 2006). Nitric oxide (NO) is a gaseous biomolecule that plays a role in neurotransmission, vasodilation, and modulation of inflammatory responses (Laroux et al., 2001; MacMicking et al., 1997; Taylor and Bishop, 1993); high and persistent NO generation, however, appears to be deleterious in central nervous system (CNS) injury and disease (Knowles and Moncada, 1994). The physiological and pathological actions of NO are often linked, but not limited to, the various isoforms of nitric oxide synthase (NOS), the enzyme responsible for the production of NO. There are three known isoforms of NOS: endothelial (eNOS), neuronal (nNOS), and inducible (iNOS) (Bredt and Snyder, 1994). Both eNOS and nNOS are constitutively expressed in the cells for which they are named, and are both calcium- and calmodulin-dependent (Garthwaite and Boulton, 1995). Unlike the constitutive forms of NOS, iNOS is not consistently expressed and is not calcium- or calmodulin-dependent. Instead, iNOS expression can be induced by lipopolysaccharide and other inflammatory cytokines, often leading to high and persistent increases in NO (Iadecola et al., 1997). Uncontrolled NO production can lead to tissue injury and damage to cellular constituents through the generation of reactive nitrogen species, such as peroxynitrite, which is involved in the nitration of proteins and lipids that prevents their normal functioning (Liu et al., 2001; Rubbo and Radi, 2008; Xiong et al., 2007). Peroxynitrite may also be a major trigger of lipid peroxidation (Radi et al., 1991); after CNS insults studies have shown a similar spatial and temporal localization of 3-nitrotyrosine (3-NT), a marker for protein nitration, and 4-hydroxynonenal (4-HNE), which is used to identify lipid peroxidation following both traumatic brain injury (Hall et al., 2004) and SCI (Carrico et al., 2009). Peroxynitrite has additionally been shown to inhibit mitochondrial respiration (Bolaños et al., 1995), as well as produce DNA strand breakage, which can trigger cell death through the activation of poly(ADP-ribose) polymerase (PARP; Szabó, 2003).
Inducible NOS has been implicated in potentiating the proinflammatory responses of immune cells, including neutrophils and monocytes, particularly after injury (Armstrong, 2001; Cifone et al., 1999; Satake et al., 2000). Studies have demonstrated that targeting iNOS following SCI using pharmacological, molecular, or transgenic knockout approaches is beneficial (Orihara et al., 2001; Paramentier-Batteur et al., 2001; Pearse et al., 2003; Isaksson et al., 2005). Previously we have shown that antisense oligonucleotides (ASOs), specific for iNOS mRNA, can reduce iNOS protein and activity, and inhibit neutrophil infiltration and myeloperoxidase (MPO) production, as well as reduce blood–spinal cord barrier (BSCB) permeability, astrogliosis, and neuron loss after SCI (Pearse et al., 2003). The iNOS ASOs were more effective than the pharmacological inhibitors 1400W or aminoguanidine in producing these beneficial effects. Therefore, as a continuation of our previous work, the present investigation sought to determine the long-term anatomical and functional benefit of acute iNOS ASO administration following contusive SCI.
Methods
Adult female Fischer rats (n=32; 180–200 g; Harlan Laboratories, Livermore, CA) were housed according to the National Institutes of Health (NIH) guide for the care and use of laboratory animals. The Institutional Animal Care and Use Committee of the University of Miami approved all animal procedures. Following anesthesia (45 mg/kg ketamine and 5 mg/kg xylazine), the animals received a moderate (12.5 mm) thoracic T8 contusion injury using the MASCIS weight drop device as described previously (Gruner, 1992). The contusion impact velocity and compression were monitored to guarantee consistency between animals. Animals (n=2) were excluded immediately when height or velocity errors exceeded 6%, or if the compression distance was not within the range of 1.35–1.75 mm. The iNOS ASOs used in this study were prepared as previously described (Parmentier-Batteur et al., 2001; Pearse et al., 2003). This ASO is a 21-mer oligodeoxyribonucleotide that consists of phosphothiolated (PT) bonds between the last three nucleotides on each end of the molecule: 5′-CTTCAGAGTCTGCCCATTGCT-3′. The random nonsense sequence of the oligodeoxynucleotide was used as a mixed base control oligonucleotide (MBO): 5′-TCTCAGTGAGCCCTCATTCTG-3′. At 3 h after SCI, the rats were anesthetized, the previous laminectomy site was re-exposed, and 200 nM iNOS ASOs or MBOs were delivered by spinal cord superfusion according to previously described methods (Pearse et al., 2003). After SCI and random allocation to treatment groups (n=10 SCI control; n=10 SCI+MBOs; n=10 SCI+iNOS ASOs), animals received weekly open-field locomotor testing for evaluation of hindlimb performance using the Basso-Beattie-Bresnahan (BBB) score developed by Basso and colleagues (1996). All behavioral scoring was carried out by two observers who were blinded to the experimental procedures performed on each animal. BBB subscore analysis was also employed to examine the recovery of hindpaw placement as described elsewhere (Pearse et al., 2007).
Rats were perfused at 10 weeks post-SCI, and the brain and spinal cord were removed for paraffin embedding and horizontal sectioning at 10 μm. Sections were collected in 10 series (each section was separated by a 100-μm space) onto Snowcoat X-tra slides (Surgipath, Winnipeg, Manitoba, Canada). One series of sections was stained with hematoxylin, eosin, and Luxol fast blue, while another was used for immunohistochemical staining for NeuN, a neuronal cell marker, as described previously (Pearse et al., 2004; 2005). All volume analysis and profile counts were conducted by individuals who were blinded to the experimental procedures performed on each animal. Hematoxylin, eosin, and Luxol fast blue-stained sections were used to quantify volumes of normal-appearing gray and white matter within the spinal cord section using computer-assisted microscopy and image analysis software (Neurolucida 5.04.3; MicroBrightField, Williston, VT) at a magnification of 400× as described previously (Pearse et al., 2005). Likewise neuronal counts at specific distances rostral and caudal to the injury were performed according to previously published methods (Pearse et al., 2003). Representative histological images from each group were taken with an Olympus BX51 microscope and processed with Meander scan software (MicroBrightField). The tonal range and sharpness (smart sharpen, 0.9 pixel) of the TIFF files were then normalized in range with Adobe Photoshop CS2 (Adobe Systems Inc., San Jose, CA). A one-way analysis of variance (ANOVA), and the Bonferroni post-hoc test was used for comparing tissue volumes and NeuN counts among the groups. For weekly analysis of BBB score and subscore data, a mixed factorial (repeated-measures) ANOVA followed by the Tukey-Kramer test was used (Scheff et al., 2002). Differences were accepted to be statistically significant at p<0.05* and p<0.01** compared to injury-only controls. All errors are reported as the standard error of the mean.
Results
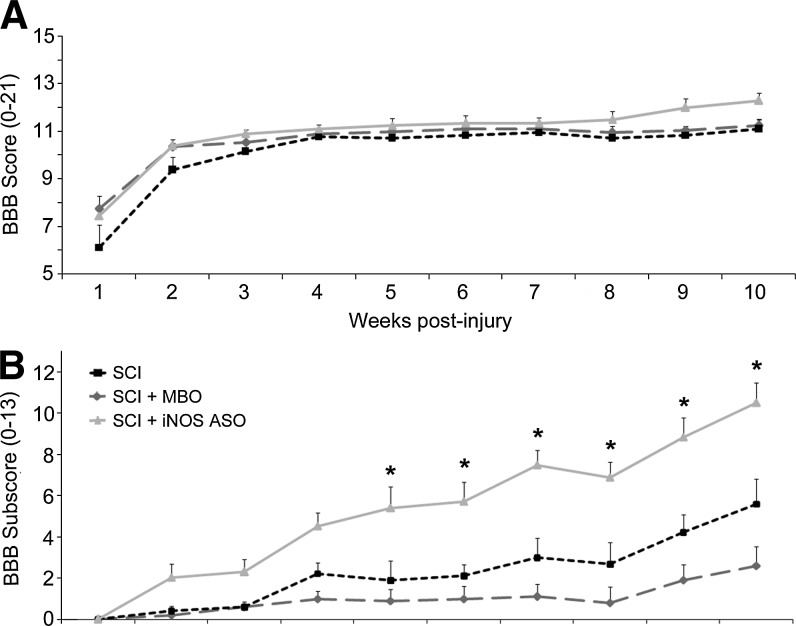
All animals exhibited a characteristic recovery in their locomotor behavior in the open-field over the course of the study as measured using the BBB score (Basso et al., 1996). The SCI control, MBO control, and iNOS ASO-treated animals showed joint movements of the hindlimbs with no weight support at 1 week post-injury (BBB scores: SCI, 6.1±0.9; MBO, 7.8±0.5 iNOS ASO, 7.5±0.3; p>0.05; Fig. 1A). By week 10, the SCI control and mixed base control animals had frequent to consistent weight-supported plantar steps with no forelimb-hindlimb (FL-HL) coordination (BBB scores: 11.1±0.4 and 11.3±0.3, respectively), while iNOS ASO-treated animals showed frequent to consistent weight-supported plantar steps with some FL-HL coordination (BBB score: 12.3±0.3; Fig. 1A). While no significant differences were found in end-point BBB scores between groups using a one-way ANOVA (p<0.05), the iNOS ASO group trended toward higher scores from weeks 8–10 post-SCI. BBB subscore analysis was employed to assess finer features of movement and hindpaw placement during open-field locomotion (Pearse et al., 2007). Although a temporal recovery of hindpaw placement was observed post-SCI, similarly to the BBB score, animals receiving iNOS ASOs demonstrated a significant improvement in BBB subscores over both control groups from 5–10 weeks post-injury (Fig. 1B). At week 10, animals receiving iNOS ASOs acutely exhibited a BBB subscore of 10.4±1.1, significantly higher than both the SCI only (5.6±0.9, p<0.05) and MBO controls (2.6±0.7, p<0.05).
FIG. 1.
The administration of iNOS ASOs improved locomotion in the open-field following SCI as measured by the BBB subscore. (A) Weekly assessment of open-field locomotion using the BBB score revealed no significant differences between SCI and MBO controls and iNOS ASO-treated animals from weeks 1–10 post-injury. (B) The use of BBB subscore analysis to examine hindpaw placement and positioning during open-field locomotion revealed a significant improvement in walking following iNOS ASO treatment versus controls from weeks 5 to 10 post-SCI. Statistical significance indicated at *p<0.05 (iNOS, inducible nitric oxide synthase; ASO, antisense oligonucleotide; SCI, spinal cord injury; BBB, Basso-Beattie-Bresnahan scale; MBO, mixed base control oligonucleotide).
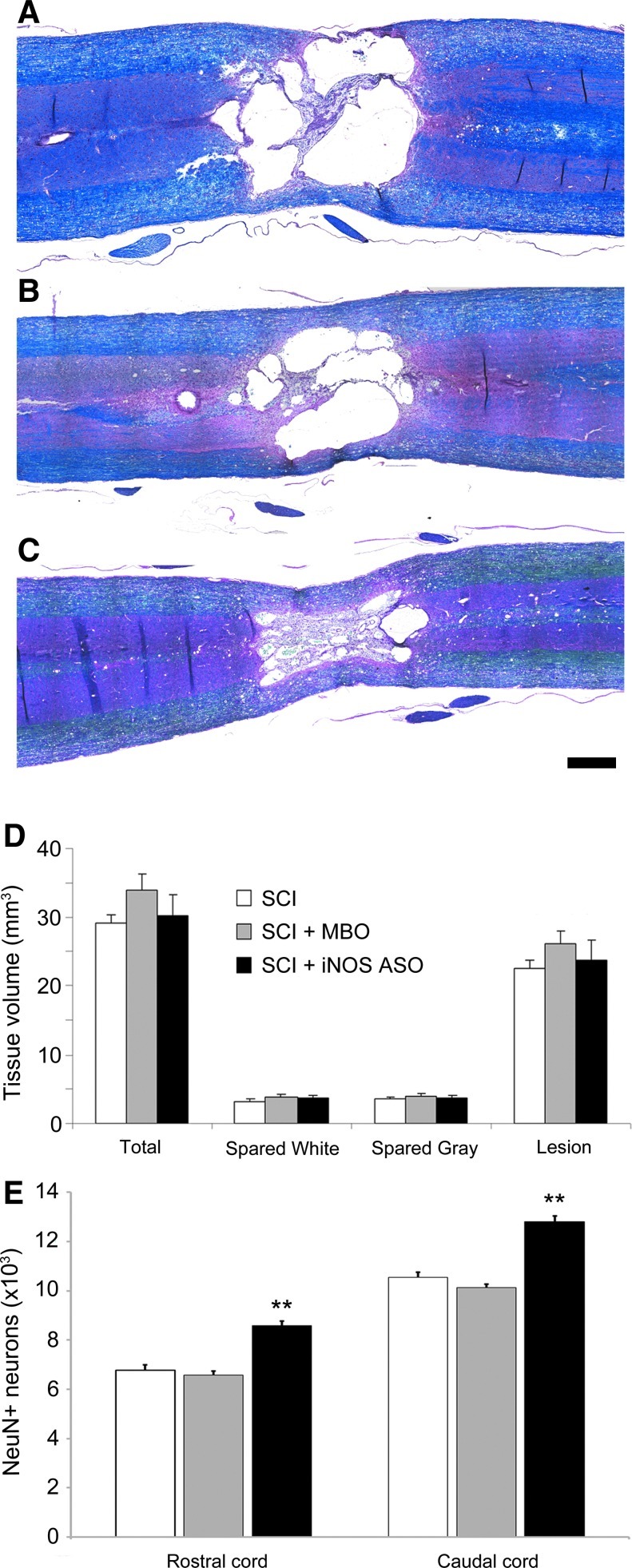
At 10 weeks post-SCI, the animals exhibited characteristic cyst/cavity formation with evidence of neuronal loss, immune cell infiltration, and demyelination, rostral and caudal to the injury epicenter, in hematoxylin-, eosin-, and Luxol fast blue-stained horizontal tissue sections (Fig. 2A–C). Comparison of tissue volumes (total, spared white and gray matter, and lesion) revealed no significant differences between the SCI control, MBO control, and iNOS ASO treatment groups (p>0.05; Fig. 2D). Specific examination of the numbers of NeuN-positive neurons at a distance of up to 3 mm rostral and caudal to the center of the injury cyst showed a significant increase (127% rostral and 121% caudal versus SCI only; Fig. 2E) in the number of preserved neurons in the iNOS ASO treatment group compared to the SCI and MBO controls (p<0.01 for both comparisons).
FIG. 2.
Spinal cord superfusion of iNOS ASOs increases numbers of preserved neurons after SCI. (A–C) Representative hematoxylin-, eosin-, and Luxol fast blue-stained horizontal sections from SCI only (A), SCI+MBO (B), and SCI+iNOS ASO (C) animals. (D) Tissue volume analysis showed no significant differences in total, spared white, spared gray, and lesion volumes among the three groups. (E) Quantification of NeuN-positive neurons within 3 mm of the center of the injury site rostral and caudal revealed greater neuron preservation with iNOS ASO treatment compared to SCI and MBO controls (scale bar=1 mm). Statistical significance indicated at **p<0.01 (iNOS, inducible nitric oxide synthase; ASO, antisense oligonucleotide; SCI, spinal cord injury; MBO, mixed base control oligonucleotide). Color image is available online at www.liebertonline.com/neu
Discussion
We have previously reported that molecular perturbation of iNOS with ASOs is an effective approach for antagonizing iNOS production and activity as well as decreasing BSCB permeability, neutrophil accumulation, astrogliosis, and neuron death after SCI (Pearse et al. 2003). The present study shows that the neuroprotection provided by acute iNOS ASO delivery is persistent, with increased neuron preservation through 10 weeks post-SCI, and leads to improved functional recovery as evidenced by an enhanced BBB subscore.
Acute spinal cord superfusion of iNOS PT ASOs, which exhibit high stability and long half-lives within the CNS compartment (Dash et al., 1987; Karras et al., 2007), ameliorates iNOS levels and activity after SCI (Pearse et al., 2003), during the peak of injury-induced iNOS production (Chatzipanteli et al., 2002). Accompanying this reduction is a significant enhancement of neuron preservation within the injury penumbra through perturbation of both necrotic and apoptotic cell death (Pearse et al., 2003), which we now demonstrate persists long-term after SCI. Inhibition of iNOS can produce neuroprotection by (1) dampening proinflammatory signaling in immune cells, and limiting subsequent activation of neuronal death receptors (Wen et al., 2006); (2) reducing hyperemia, microvascular injury, and BSCB permeability, and the ensuing leakage of toxic metabolites and degradative proteases from the blood, as well as the accumulation of circulating immune cells within the injured CNS (Belenky et al., 1993; Okamura et al., 2001); and (3) abating oxidative stress by preventing the formation of the toxic metabolite peroxynitrite (ONOO−), and its ensuing nitration and dysfunction of proteins and lipids (Cassina et al., 2002), as well as lipid peroxidation (Radi et al., 1991). In addition to exacerbating tissue damage and cell death, NO can also increase neurological dysfunction by inducing conduction block of demyelinated axons (Redford et al., 1997; Smith and Lassmann, 2002). The antagonism of NO generation by iNOS ASOs may also enhance function by improving axon conduction of these demyelinated axons.
Although modest, long-term improvements in neuronal preservation and behavioral outcome were observed following acute iNOS inhibition with ASOs, the effectiveness of the approach may have been limited by several factors. The length of iNOS inhibition was acute (<72 h), due to the use of a single administration paradigm and the limited stability of iNOS PT ASOs (previous work has shown that iNOS expression persists beyond 7 days post-SCI; Xu et al., 2001). Also, the restriction of iNOS antagonism to the spinal cord injury site may have had a limiting effect (greater efficacy might be possible with the use of systemic delivery to additionally target circulating immune cell populations before their accumulation at the site of SCI, as a major cellular source of iNOS in the acute injury phase is from invading polymorphonuclear leukocytes; Chatzipanteli et al., 2002). In addition, NO functions not only to generate peroxynitrite and oxidative stress, it can also act as a potent antioxidant, inhibiting lipid peroxidation by scavenging either lipid peroxyl (LOO*+*NO→LOONO), or lipid alkoxyl (LO*+*NO→LONO) radicals (O'Donnell et al., 1997; Rubbo et al., 1994). The use of approaches that specifically target peroxynitrite rather than NO, such as uric acid (Scott et al., 2005), or that enhance peroxynitrite decomposition (Genovese et al., 2009), could therefore prove to be more effective strategies. Indeed both of these approaches have been shown to be neuroprotective after SCI and significantly reduce functional deficits. Finally, if iNOS inhibition produces neuronal preservation but not white matter sparing after thoracic SCI, the modest improvements in BBB scores we observed might be limited due the lack of involvement of the thoracic motoneurons in direct control of the hindlimbs. These motoneurons innervate the chest and back muscles as well as abdominal organs. Examination of iNOS ASO animals after SCI of the cervical or lumbar cord may produce more pronounced behavioral improvements, as these neuronal pools are directly involved in limb function.
The current study demonstrates that the acute inhibition of iNOS can lead to long-term anatomical and functional improvements after SCI. Future work to optimize iNOS ASO dosing through the examination of different ASO concentrations, the lengthening of the administration window, enhanced ASO stability, or the investigation of systemic administration to target circulating immune cell populations, may further enhance the effectiveness of this antisense therapeutic approach against iNOS after CNS injury. Determining the extent to which iNOS ASOs may abate protein nitration and lipid peroxidation, through the evaluation of 3-NT and 4-HNE levels during the acute injury phase, may improve our understanding of the mechanism by which iNOS ASOs are neuroprotective. Furthermore, comparative studies to investigate the neuroprotective and functional efficacy of targeting iNOS or peroxynitrite after SCI, or testing these agents in a cervical SCI contusion model (Pearse et al., 2005), could also aid in identifying the optimal therapeutic target for retarding oxidative damage and neurological dysfunction post-SCI.
Acknowledgments
We gratefully acknowledge the Miami Project Animal Core Facility for performing spinal cord contusion injuries and providing post-operative animal care and functional testing, in particular Alexander Marcillo, Paulo Diaz, Santiago Castro, Denise Koivisto, Kim Loor, and Rosa Abril. In addition, we thank Devin Bustin for help with histology, Raisa Puzis for immunohistochemistry, and Wai-Man Chan for proofreading. This work was supported by NIH National Institute of Neurological Disorders and Stroke (NINDS) grant POI NS38665, The Miami Project to Cure Paralysis, and The Buoniconti Fund.
Author Disclosure Statement
No competing financial interests exist.
References
- Armstrong R. The physiological role and pharmacological potential of nitric oxide in neutrophil activation. Int. Immunopharmacol. 2001;1:1501–1512. doi: 10.1016/s1567-5769(01)00094-7. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Belenky S.N. Robbins R.A. Rubinstein I. Nitric oxide synthase inhibitors attenuate human monocyte chemotaxis in vitro. J. Leukoc. Biol. 1993;53:498–503. doi: 10.1002/jlb.53.5.498. [DOI] [PubMed] [Google Scholar]
- Bolaños J.P. Heales S.J. Land J.M. Clark J.B. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J. Neurochem. 1995;64:1965–1972. doi: 10.1046/j.1471-4159.1995.64051965.x. [DOI] [PubMed] [Google Scholar]
- Bredt D.S. Snyder S.H. Nitric oxide: A physiologic messenger molecule. Annu. Rev. Biochemistry. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Carrico K.M. Vaishnav R. Hall E.D. Temporal and spatial dynamics of peroxynitrite-induced oxidative damage after spinal cord contusion injury. J. Neurotrauma. 2009;26:1369–1378. doi: 10.1089/neu.2008-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassina P. Peluffo H. Pehar M. Martinez-Palma L. Ressia A. Beckman J.S. Etévez A.G. Barbeito L. Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J. Neurosci. Res. 2002;67:21–29. doi: 10.1002/jnr.10107. [DOI] [PubMed] [Google Scholar]
- Chatzipanteli K. Garcia R. Marcillo A.E. Loor K.E. Kraydieh S. Dietrich W.D. Temporal and segmental distribution of constitutive and inducible nitric oxide synthases after traumatic spinal cord injury: Effect of aminoguanidine treatment. J. Neurotrauma. 2002;19:639–651. doi: 10.1089/089771502753754109. [DOI] [PubMed] [Google Scholar]
- Cifone M.G. D'Alo S. Parroni R. Millimaggi D. Biordi L. Martinotti S. Santoni A. Interleukin-2-activated rat natural killer cells express inducible nitric oxide synthase that contributes to cytotoxic function and interferon-gamma production. Blood. 1999;93:3876–3884. [PubMed] [Google Scholar]
- Dash P. Lotan I. Knapp M. Kandel E.R. Goelet P. Selective elimination of mRNAs in vivo: Complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proc. Natl. Acad. Sci. USA. 1987;84:7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. Boulton C.L. Nitric oxide signaling in the central nervous system. Annu. Rev. Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Genovese T. Mazzon E. Esposito E. Di Paola R. Murthy K. Neville L. Bramanti P. Cuzzocrea S. Effects of a metalloporphyrinic peroxynitrite decomposition catalyst, ww-85, in a mouse model of spinal cord injury. Free Radic. Res. 2009;43:631–645. doi: 10.1080/10715760902954126. [DOI] [PubMed] [Google Scholar]
- Gruner J.A. A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. discussion 126–128. [DOI] [PubMed] [Google Scholar]
- Hall E.D. Detloff M.R. Johnson K. Kupina N.C. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J. Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Zhang F. Casey R. Nagayama M. Ross M.E. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J. Neurosci. 1997;17:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson J. Farooque M. Olsson Y. Improved functional outcome after spinal cord injury in iNOS-deficient mice. Spinal Cord. 2005;43:167–170. doi: 10.1038/sj.sc.3101672. [DOI] [PubMed] [Google Scholar]
- Karras J.G. Crosby J.R. Guha M. Tung D. Miller D.A. Gaarde W.A. Geary R.S. Monia B.P. Gregory S.A. Anti-inflammatory activity of inhaled IL-4 receptor-alpha antisense oligonucleotide in mice. Am. J. Respir. Cell Molec. Biol. 2007;36:276–285. doi: 10.1165/rcmb.2005-0456OC. [DOI] [PubMed] [Google Scholar]
- Knowles R.G. Moncada S. Nitric oxide synthases in mammals. Biochemical J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroux F.S. Pavlick K.P. Hines I.N. Kawachi S. Harada H. Bharwani S. Hoffman J.M. Grisham M.B. Role of nitric oxide in inflammation. Acta Physiologica Scandinavica. 2001;173:113–118. doi: 10.1046/j.1365-201X.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- Liu C. Jin A. Zhou C. Chen B. Gene expression of inducible nitric oxide synthase in injured spinal cord tissue. Chin. J. Traumatol. 2001;4:231–233. [PubMed] [Google Scholar]
- MacMicking J. Xie Q.W. Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- O'Donnell V.B. Chumley P.H. Hogg N. Bloodsworth A. Darley-Usmar V.M. Freeman B.A. Nitric oxide inhibition of lipid peroxidation: kinetics of reaction with lipid peroxyl radicals and comparison with alpha-tocopherol. Biochemistry. 1997;36:15216–15223. doi: 10.1021/bi971891z. [DOI] [PubMed] [Google Scholar]
- Okamura T. Ayajiki K. Toda N. Hypothermia on NO-mediated neurogenic relaxation and on hypoxic inhibition in the response of canine cerebral arteries. Hypertens. Res. 2001;24:47–53. doi: 10.1291/hypres.24.47. [DOI] [PubMed] [Google Scholar]
- Orihara Y. Ikematsu K. Tsuda R. Nakasono I. Induction of nitric oxide synthase by traumatic brain injury. Forensic Sci. Int. 2001;123:142–149. doi: 10.1016/s0379-0738(01)00537-0. [DOI] [PubMed] [Google Scholar]
- Parmentier-Batteur S. Bohme G.A. Lerouet D. Zhou-Ding L. Beray V. Margaill I. Plotkine M. Antisense oligodeoxynucleotide to inducible nitric oxide synthase protects against transient focal cerebral ischemia-induced brain injury. J. Cereb. Blood Flow Metab. 2001;21:15–21. doi: 10.1097/00004647-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Pearse D.D. Chatzipanteli K. Marcillo A.E. Bunge M.B. Dietrich W.D. Comparison of iNOS inhibition by antisense and pharmacological inhibitors after spinal cord injury. J. Neuropathol. Exp. Neurol. 2003;62:1096–1107. doi: 10.1093/jnen/62.11.1096. [DOI] [PubMed] [Google Scholar]
- Pearse D.D. Lo T.P., Jr. Cho K.S. Lynch M.P. Garg M.S. Marcillo A.E. Sanchez A.R. Cruz Y. Dietrich W.D. Histopathological and behavioral characterization of a novel cervical spinal cord displacement contusion injury in the rat. J. Neurotrauma. 2005;22:680–702. doi: 10.1089/neu.2005.22.680. [DOI] [PubMed] [Google Scholar]
- Pearse D.D. Marcillo A.E. Oudega M. Lynch M.P. Wood P.M. Bunge M.B. Transplantation of Schwann cells and olfactory ensheathing glia after spinal cord injury: Does pretreatment with methylprednisolone and interleukin-10 enhance recovery? J. Neurotrauma. 2004;21:1223–1239. doi: 10.1089/neu.2004.21.1223. [DOI] [PubMed] [Google Scholar]
- Pearse D.D. Sanchez A.R. Pereira F.C. Andrade C.M. Puzis R. Pressman Y. Golden K. Kitay B.M. Blits B. Wood P.M. Bunge M.B. Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: Survival, migration, axon association, and functional recovery. Glia. 2007;55:976–1000. doi: 10.1002/glia.20490. [DOI] [PubMed] [Google Scholar]
- Radi R. Beckman J.S. Bush K.M. Freeman B.A. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophysics. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- Redford E.J. Kapoor R. Smith K.J. Nitric oxide donors reversibly block axonal conduction: Demyelinated axons are especially susceptible. Brain. 1997;120:2149–2157. doi: 10.1093/brain/120.12.2149. [DOI] [PubMed] [Google Scholar]
- Rubbo H. Radi R. Protein and lipid nitration: role in redox signaling and injury. Biochim. Biophys. Acta. 2008;1780:1318–1324. doi: 10.1016/j.bbagen.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Rubbo H. Radi R. Trujillo M. Telleri R. Kalyanaraman B. Barnes S. Kirk M. Freeman B.A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- Satake K. Matsuyama Y. Kamiya M. Kawakami H. Iwata H. Adachi K. Kiuchi K. Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Brain Res. Molec. Brain Res. 2000;85:114–122. doi: 10.1016/s0169-328x(00)00253-9. [DOI] [PubMed] [Google Scholar]
- Scheff S.W. Saucier D.A. Cain M.E. A statistical method for analyzing rating scale data: The BBB locomotor score. J Neurotrauma. 2002;19:1251–1260. doi: 10.1089/08977150260338038. [DOI] [PubMed] [Google Scholar]
- Schwab M.E. Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiological Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- Scott G.S. Cuzzocrea S. Genovese T. Koprowski H. Hooper D.C. Uric acid protects against secondary damage after spinal cord injury. Proc. Natl. Acad. Sci. USA. 2005;102:3483–3488. doi: 10.1073/pnas.0500307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipski M.L. Pearse D.D. Methylprednisolone and other confounders to spinal cord injury clinical trials. Nat. Clin. Pract. Neurol. 2006;2:402–403. doi: 10.1038/ncpneuro0221. [DOI] [PubMed] [Google Scholar]
- Smith K.J. Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002;1:232–241. doi: 10.1016/s1474-4422(02)00102-3. [DOI] [PubMed] [Google Scholar]
- Szabó C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol. Lett. 2003;140–141:105–112. doi: 10.1016/s0378-4274(02)00507-6. [DOI] [PubMed] [Google Scholar]
- Taylor W.F. Bishop V.S. A role for nitric oxide in active thermoregulatory vasodilation. Am. J. Physiol. 1993;264:H1355–H1359. doi: 10.1152/ajpheart.1993.264.5.H1355. [DOI] [PubMed] [Google Scholar]
- Wen W. Sanelli T. Ge W. Strong W. Strong M.J. Activated microglial supernatant induced motor neuron cytotoxicity is associated with upregulation of the TNFR1 receptor. Neurosci. Res. 2006;55:87–95. doi: 10.1016/j.neures.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Xiong Y. Rabchevsky A.G. Hall E.D. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J. Neurochem. 2007;100:639–649. doi: 10.1111/j.1471-4159.2006.04312.x. [DOI] [PubMed] [Google Scholar]
- Xu J. Kim G.M. Chen S. Yan P. Ahmed S.H. Ku G. Beckman J.S. Xu X.M. Hsu C.Y. iNOS and nitrotyrosine expression after spinal cord injury. J. Neurotrauma. 2001;18:523–532. doi: 10.1089/089771501300227323. [DOI] [PubMed] [Google Scholar]