Abstract
Age-associated atrophy of the thymus with coincident reduction in thymopoeisis, decline in thymic output, and subsequent immune dysfunction has been reversed by the use of interleukin-7 (IL-7). In the earlier studies and in clinical trials, delivery of IL-7 has been by multiple injections over several days to maintain effective activity levels in the tissues. This is unlikely to meet with high compliance rates in future clinical use, and so we tested alternate routes of delivery using a technique involving tagging IL-7 with fluorescent dye that emits in the near-infrared region and whose fluorescence can be visualized within the tissues of live animals. We have shown that intratracheal instillation, enabling transfer through the lungs, provides an effective route for delivering IL-7 into the bloodstream and from there into the tissues in older animals. Delivery is rapid and widespread tissue distribution is seen. Comparison of administration either subcutaneously or by instillation reveals that IL-7 delivery by the pulmonary route provides significantly greater transmission to lymphoid tissues when compared with injection. In functional assessment studies, pulmonary administration led to significantly improved intrathymic T cell development in older animals when compared with IL-7 delivered by injection. Furthermore, in these older animals, delivery of IL-7 by intratracheal instillation was not accompanied by any apparent adverse events when compared with controls receiving saline vehicle by instillation or animals receiving IL-7 by subcutaneous injection.
Introduction
Interleukin-7 (IL-7) is a type 1 short-chain cytokine of the hematopoietin family and plays a central role in the development and maintenance of T cells. The human IL-7 gene consists of 6 exons on chromosome 8q12–13 that together code for a protein whose molecular weight is 25 kD after glycosylation.1 The protein can be detected in many stromal tissues, including epithelial cells in the thymus, bone marrow and gut, keratinocytes, liver, dendritic cells,1 and also astrocytes.2 The action of IL-7 is mediated through the binding of the protein to a cell-surface receptor composed of two chains, α (CD127) and γ (CD132). The first step in this process is the interaction of IL-7 with the IL-7 receptor α (IL-7Rα) chain, forming a stable 1:1 intermediate. This then interacts with the γ-chain, forming an active ternary complex, and the amalgamation of these two chains by IL-7 orients their cytoplasmic domains so that their respective kinases can phosphorylate sequence elements on the cytoplasmic domains. Transcriptional activators bind to the phosphorylated sequence elements of these chains and become phosphorylated, after which they dissociate from the chains, oligomerize, and move to the nucleus, where they initiate transcriptional activity.3
Decline in thymic activity with advancing age in animal models has been shown to be associated with a reduction in the level of expression in the thymus of IL-7.4,5 Some studies report that treatment of old animals with IL-7 reverses thymic atrophy, increases thymopoeisis, and improves thymic output, boosting immune function.6–9 However others report that IL-7 introduced into the thymus was unable to impact on thymic involution or increase T cell output, possibly because of reduced proliferation of the DN4 population of cells.10 These studies followed earlier animal studies, which provided promising results and suggested that IL- 7 could have significant potential in the clinic for assisting in the treatment of viral infections such as human immunodeficiency virus (HIV) and boosting immune recovery after bone marrow transplantation.
Clinical trials with IL-7 have been initiated and the treatment regimen is normally subcutaneous injection of IL-7 in a saline vehicle every 2 or 3 days over a 2- to 3-week period.11–17 Although this route of administration is efficient and necessary to achieve effective levels within the tissues, it has limitations associated with maintenance, distribution, and compliance.
If IL-7 is to be of therapeutic benefit to older individuals in reversing age-associated immune decline, its take up as a therapeutic agent will be hampered if it is to be injected repeatedly. This may be an acceptable form of delivery when patients are confined to hospital, but it would be unacceptable for any therapeutic agent designed for use in the community. Improved compliance would require finding alternatives to repeated parenteral delivery.
The aim of this study was to determine whether delivery of IL-7 by the pulmonary route would provide efficient systemic distribution as well as an effective means of targeting IL-7 to the thymus. We followed the trajectory of IL-7 after administration by conjugating IL-7 to a near-infrared dye that could be detected in deeper tissue sites after fluorescent activation and enabled real-time visualization of its movement, distribution, and kinetic turnover in vivo. Although the pulmonary route is often used for the delivery of proteins and peptides, there are safety concerns because of the possibility of immune-mediated reactions.18 Therefore, this study also aimed to note whether there were any adverse events associated with intratracheal delivery in this instance.
Materials and Method
Mice
Inbred C57BL/6 female mice were obtained from Charles Rivers and housed according to the local rules and regulations.
Conjugation of IL-7 to IRDye 800CW
Recombinant murine (rm) IL-7 (R&D Systems, Abingdon, UK) was conjugated to the infrared fluorescent dye IRDye 800CW (LI-COR Biosciences, Cambridge, UK) according to the manufacturer's instructions. Briefly, the lyophilized IL-7 was reconstituted with filter-sterilized phosphate-buffered saline (PBS) to a final concentration of 100 μg/mL, and 1 mg of rmIL-7 was mixed with 15.5 μL of IRDye 800CW and incubated in the dark at room temperature for 2 hr. Afte conjugation, residual unbound dye was removed using a Pierce Zeba™ Desalting Spin Column (Thermo Fisher Scientific, Northumberland, UK). Aliquots of the conjugated protein at concentration of 1 μg/25 μL were stored at −40°C until required.
As a control, we used a nonreactive carboxylated form of the IRDye 800CW (LI-COR Biosciences, Cambridge, UK), prepared by reconstitution of the dye with filter-sterilized PBS. The dye was subsequently stored at −40°C until required. The labeling ratio and the concentration of the conjugated protein (IL-7-800CW) were estimated by using the extinction coefficients of IL-7 (ε280nm=8,970 M−1cm−1) and CW800 (ε777nm=270,000 M−1cm−1) in PBS:methanol, with correction for the 3.0% of measured absorbance at 280 nm attributable to CW800: Labeling ratio=(Abs777nm/ε777nm)/[(Abs280nm − 0.03*Abs777nm)/ε280nm].
Treatment regimens
The C57BL/6 female mice used in these experiments were separated according to age. Young mice (9–12 weeks of age) were used to compare delivery of IL-7 either by injection or instillation. Injections were carried out subcutaneously. Instillation into the lungs was performed using a microsprayer (Model FMJ-250, Penn-Century Inc., USA) inserted into the oropharyngeal tube in mice anesthetized with ketamine and xylazine where the trachea had been visualized with the mouse in a prone position as previously described.19 Animals were instilled with either the carboxylate dye or 1 μg of recombinant IL-7-800CW. All animals were visualized in real time for 48 hr using the LI-COR® Pearl imaging system before being culled and tissues collected for analysis.
Older animals (12 months of age) were used to compare the efficacy of the delivery. Animals were given three doses of either 1 μg of recombinant IL-7 or saline vehicle alone on alternate days by instillation to the lungs as described above or alternatively were given three doses of either 1 μg of recombinant IL-7 or saline vehicle alone by subcutaneous injection after receiving anesthesia similar to that given to animals receiving instillation. These groups of animals were culled 15 days after the initiation of treatment.
Tissue analysis
Tail blood was collected from animals at 1, 24, and 48 hr as described previously20 to determine the circulating concentration of IL-7-800CW. A total of 20 μL of blood was collected at each time point. The samples were frozen at −20°C until analyzed, at which point the blood samples were diluted at a ratio of 1:5 in PBS and 50 μL of the diluted samples were transferred to microtiter plates (Corning, UK) and imaged using the LI-COR Pearl imaging system to determine the fluorescent intensity reading.
Tissue distribution
The mice were killed by CO2 asphyxiation followed by cervical dislocation. To determine the tissue distribution of the IL-7-800CW, different organs were collected, including lungs, spleen, thymus, liver, kidney, and lymph nodes. The organs were weighed and then either homogenized immediately or for long-term storage placed in 2 mL of RNAlater (Ambion) solutions at 4°C prior to transfer to −80 °C according to the manufacturer's instructions.
The tissues were homogenized with the Precellys®24 homogenizer (Bertin-Cepheid, Derbyshire), briefly. The organ was placed into a 2-mL tube containing 1.5 mL of 10% Dulbecco modified Eagle medium (DMEM) in PBS and small ceramic beads (CK14) for the Precellys (Bertin-Cepheid, Derbyshire). The thymus was homogenized at 5,100 rpm for 5 sec and the spleen and lung at 5,000 rpm for 8 sec. The homogenized tissue was strained using a cell strainer to remove any cellular debris, and the concentration and cellular viability of the cellular suspension was determined using a Countess Automated Cell Counter (Invitrogen, UK). Aliquots of 1×107 cells were either viably stored in fetal calf serum (FCS) and 10% dimethylsulfoxide (DMSO) or the cells were stained with appropriate fluorescently labeled antibodies as described previously.7 Briefly, approximately 106 cells were incubated in a volume of 100 μL of PBS supplemented with FCS along with 1 μg of fluorescently tagged antibody or its appropriate isotype control for approximately 30 min at 4°C. The cells were then washed by centrifugation and resuspended in PBS and either analyzed immediately or fixed for analysis later. Analysis was carried out on an Accuri C6 flow cytometer.
Statistical analysis
The results of the descriptive tissue analysis are presented for numerical variables in the form of means±standard deviation, and percentage was calculated for categorical outcomes. Statistical tests used for the comparative analysis between treatment groups were chosen according to the type of variable, the sample size under consideration, and the number of groups compared. Thus, numerical outcomes were tested using the Mann–Whitney test; the Fisher exact test was chosen for categorical outcomes. Specifically, analysis of the relative fluorescent signal detected from different animal organs according to the delivery route (instilled vs. injected) and the treatment administrated (IL-7 vs. carboxylate) were computed with the procedure PROC MIXED for the generalized linear model using repeated data. Indeed, to reduce the variance of estimates of treatment effects, triplicate fluorescent signal detections analyses were performed for each organ and each subject irrespective of treatment or delivery route.
The level of significance was set at p=0.05. All analyses were performed using SAS software version 9.1 (SAS System, SAS Institute Inc., Cary, NC).
Results
Comparing delivery routes for IL-7
These experiments were carried out on young animals. Preliminary experiments were carried out to determine the distribution in the lungs of IL-7-800CW, 1 hr after instillation. Our results (Fig. 1) revealed that intratracheal instillation produces a clear and apparently even distribution of the labeled IL-7 throughout the lungs. Once there was evidence that instillation in the lungs could provide an effective means of delivery, comparative analysis of the routes of delivery was carried out. Animals were treated with a single dose of 1 μg of labeled IL-7 delivered either by intratracheal instillation or by subcutaneous injection. Comparisons of the scanned images from both methods of delivery are shown in Fig. 2. These suggest that IL-7 is disseminated to the tissues when delivered through the lungs and that much of the IL-7 remains at the site of delivery when injected subcutaneously.
FIG. 1.
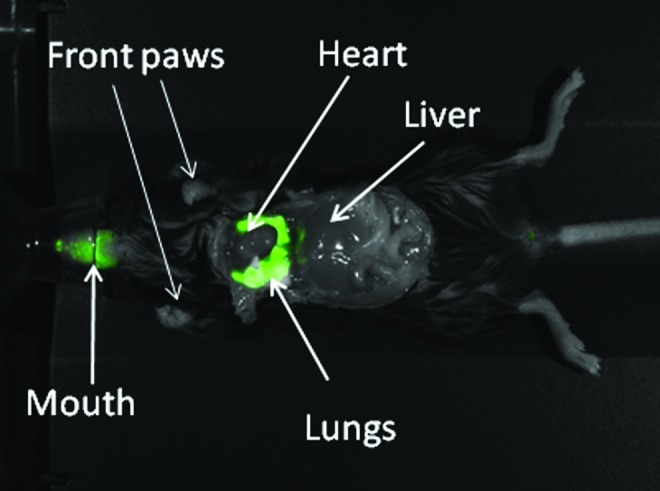
Fluorescent imaging of distribution of labeled interleukin-7 (IL-7) in a young animals within the lung approximately 60 min after intratracheal instillation of 500 ng of labeled IL-7.
FIG. 2.
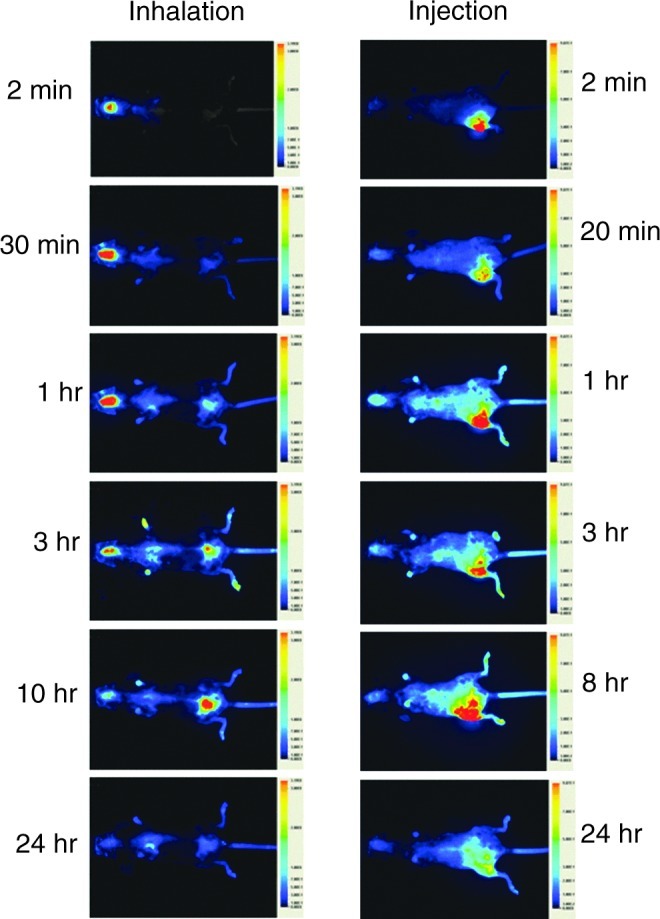
Detection of fluorescence in single young animal at timed intervals after the instillation or injection of labeled interleukin-7 (IL-7). Each image is a ventral view and the approximate time after administration is shown at the side of each image.
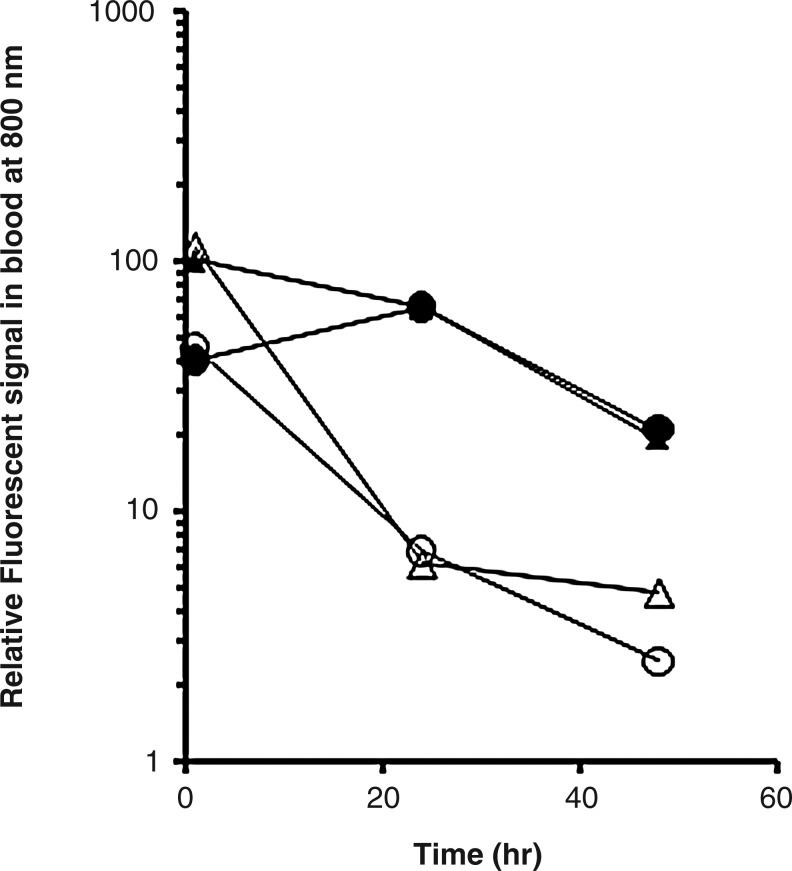
The administered IL-7 circulating in peripheral blood was determined by measuring the fluorescent signal obtained at 1, 24, and 48 hr postadministration. The results of fluorescent signal measurement in the blood (Fig. 3) show that at 1 hr and 48 hr after delivery there was no significant difference in the fluorescence in the blood between instillation versus injection delivery methods (p>0.05 at 1 hr and at 48 hr, where n=4 for inhalation and n=5 for injection at both time points). This would indicate that there was no significant decline in the amount of IL-7 in the blood over this time.
FIG. 3.
Relative fluorescent signal detected from peripheral blood samples in young animals taken over a 48-hr time period using different routes of administration of labeled interleukin-7 (IL-7) or the free carboxylate dye control. Figures are the average values obtained from between two to five samples. Free carboxylate dye was instilled into the lungs (open triangles) or injected subcutaneously (open circles) and compared with tagged IL-7 instilled into the lungs (closed circles) or injected subcutaneously (closed triangles).
To eliminate the possibility that the detectable fluorescent signal within the circulation was due to unbound free dye, animals received a fluorescent nonreactive carboxylated free dye as a control. A relatively high level of signal was detected at 1 hr postadministration, and this diminished rapidly to only 15% of this value at 1 hr; by 48 hr it was just under 6% of the 1-hr value and considerably less than the labeled IL-7 value at 48 hr postadministration (Fig. 3).
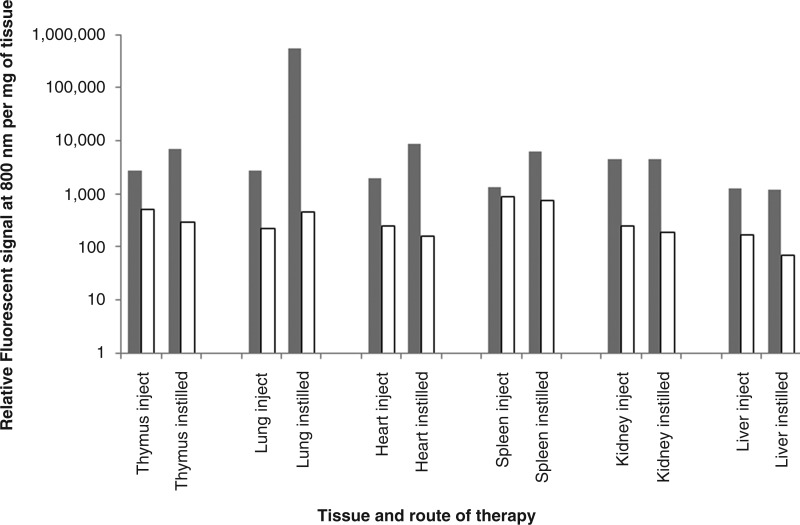
To compare different delivery routes on the accumulated tissue-specific concentrations of IL-7, organs, including thymus, lungs, heart, spleen, kidney, and liver, were tested at 48 hr after delivery. There was a significant difference in the relative fluorescence signal from the labeled IL-7 in the lungs (p=0.0005) when comparing instillation versus injection routes, as expected. However for other organs there was no significant difference (p>0.05) between instillation and injection in the nonlymphoid organs (heart, kidney, and liver), but significantly more signal in the thymus (p=0.02) and the spleen (p=0.007) following delivery by instillation when compared with delivery by injection (Fig. 4).
FIG. 4.
Relative fluorescent signal per milligram of tissue detected from different organs of young animals receiving labeled interleukin-7 (IL-7) (shaded columns) or carboxylate dye (open columns) by instillation or injection.
The carboxylate-treated control animals had lower detectable signals, indicating limited maintenance of the free dye and suggesting that the increased fluorescent signal in the IL-7–treated group was due to retention of IL-7. For example, the signal thymic tissue was significantly higher when labeled IL-7 was delivered either by injection (p=0.004) or instillation (p=0.004) when compared to delivery of carboxylate dye.
Comparison of functional effect of different delivery routes
Having established the feasibility of the instillation method for delivery of IL-7 and compared it with other delivery routes, the next stage was to establish the functional and physiological impact of the instilled intratracheal lung route compared to the injected route of delivery. These experiments were carried out on older animals.
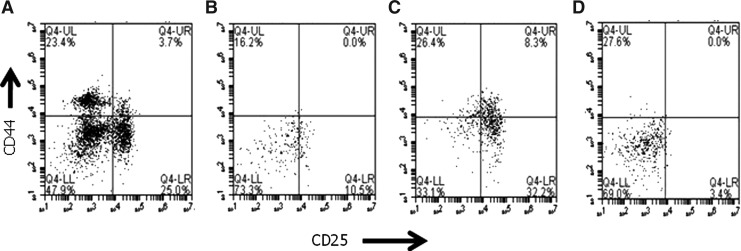
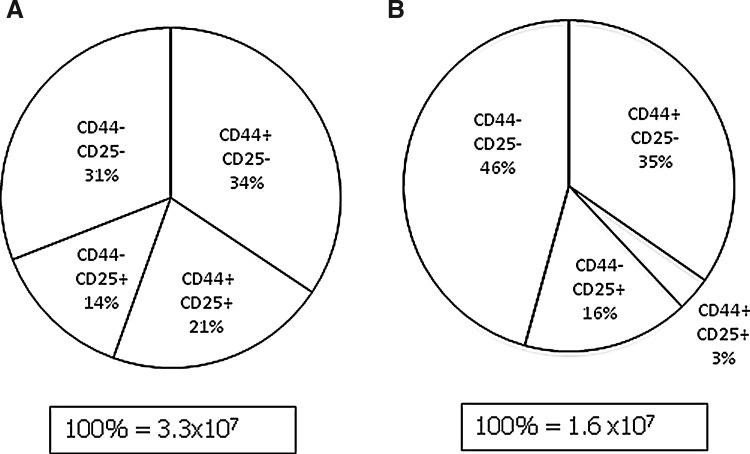
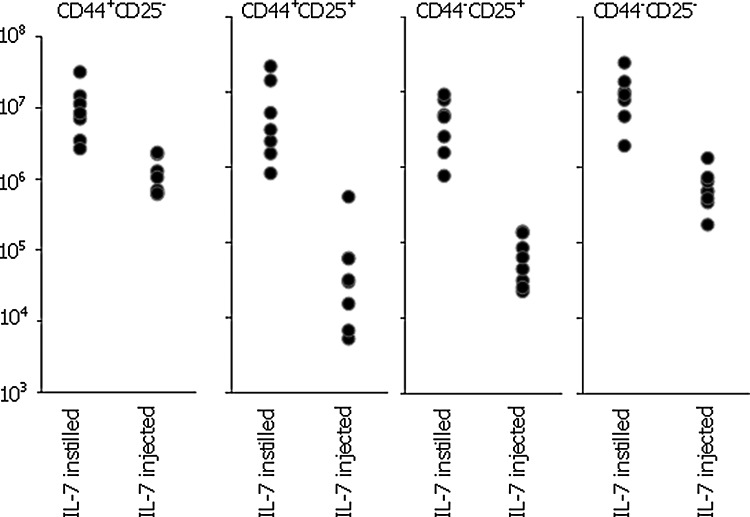
We compared the effect of the different routes of treatment on the thymus and the results (Table 1) revealed that there was no significant difference between the total number of thymocytes in animals receiving treatment by instillation, nor between animals receiving treatment by injection, nor between those receiving IL-7 by inhalation and injection (p>0.05 in all cases). Furthermore, there was no significant difference between the number of double-positive cells within each of these categories (p>0.05 in all cases). However, the animals receiving IL-7 by inhalation showed more triple-negative cells than animals in the other groups. In view of this, we analyzed the early stages of T cell development in IL-7–instilled animals compared with PBS-instilled animals (Table 2). Representative samples of fluorescent profiles from each treatment (Fig. 5) suggest that instillation may provide the best route of delivery. These early stages are components of the triple-negative (CD3−CD4−CD8−) subset of the thymus whose numbers are increased in IL-7–instilled animals compared with the PBS controls (Fig. 6), where it is apparent that the contribution of the CD44+CD25+ subset toward the overall triple-negative population is increased after IL-7 instillation from 3% to 21% (p<0.0001). Comparison of the instilled versus injected route reveals significant improvements in all of the early subsets of the T cell development pathway in instilled animals compared with injected animals (Fig. 7).
Table 1.
Total Cell Number and Average Number of Cells in Each Population±1 Standard Deviation after Treatment of Older Animals
| Total cell number | Number of TNcells | Number of CD4+CD8+ cells | Number of CD4+CD8−cells | Number of CD4−CD8+ cells | |
|---|---|---|---|---|---|
| IL-7 instillation (n=7) | 2.76±1.82×108 | 3.31+2.42±107 | 1.56±1.01×108 | 7.63±5.16×107 | 1.68+1.21×107 |
| PBS instillation (n=8) | 3.36±1.19×108 | 1.6±0.99×107 | 2.49±1.12×108 | 5.6±0.8×107 | 1.29±0.43×107 |
| IL-7 injection (n=9) | 1.61±0.9×108 | 4.18±6.89×106 | 1.41±0.8×108 | 1.44±1.07×107 | 3.13±3.56×106 |
| PBS injection(n=9) | 3.33±2.29×108 | 8.97±6.12×106 | 2.59±1.9×108 | 4.84±3×107 | 1.29±0.76×107 |
TN, triple negative; IL-7, Interleukin-7; PBS, phosphate-buffered saline.
Table 2.
Average Number of Cells in Each Subpopulation of the TN Subset after Treatment of Older Animals
| |
Early stage T cell development subpopulations |
|||
|---|---|---|---|---|
| Treatment | CD44+CD25− | CD44+CD25+ | CD44−CD25+ | CD44−CD25− |
| IL-7 instilled (n=7) | 1.14×107 | 7.01×106 | 4.5×106 | 1.02×107 |
| PBS instilled (n=8) | 5.5×106 | 5.56×106 | 2.61×106 | 7.33×106 |
TN, triple negative; IL-7, interleukin-7; PBS, phosphate-buffered saline.
FIG. 5.
Representative examples of the fluorescent profiles obtained for the subsets defined by expression of CD44 and CD25 within the CD3−CD4−CD8− population from older animals who received interleukin-7 (IL-7) (A) or phosphate-buffered saline (PBS) (B) through the instillation route or IL-7 (C) or PBS (D) by injection.
FIG. 6.
Pie chart showing the distribution of the minor subsets within the CD3−CD4−CD8− population, showing the averages and the percentage contribution of n=7 (interleukin-7 [IL-7] instilled shown in A) and n=8 (phosphate-buffered saline [PBS] instilled shown in B) older animals. Total numbers of the CD3−CD4−CD8−population are shown below each pie chart.
FIG. 7.
Comparison between interleukin-7 (IL-7)–instilled and IL-7–injected older animals of the numbers of cells within each minor subset of the CD3−CD4−CD8− population.
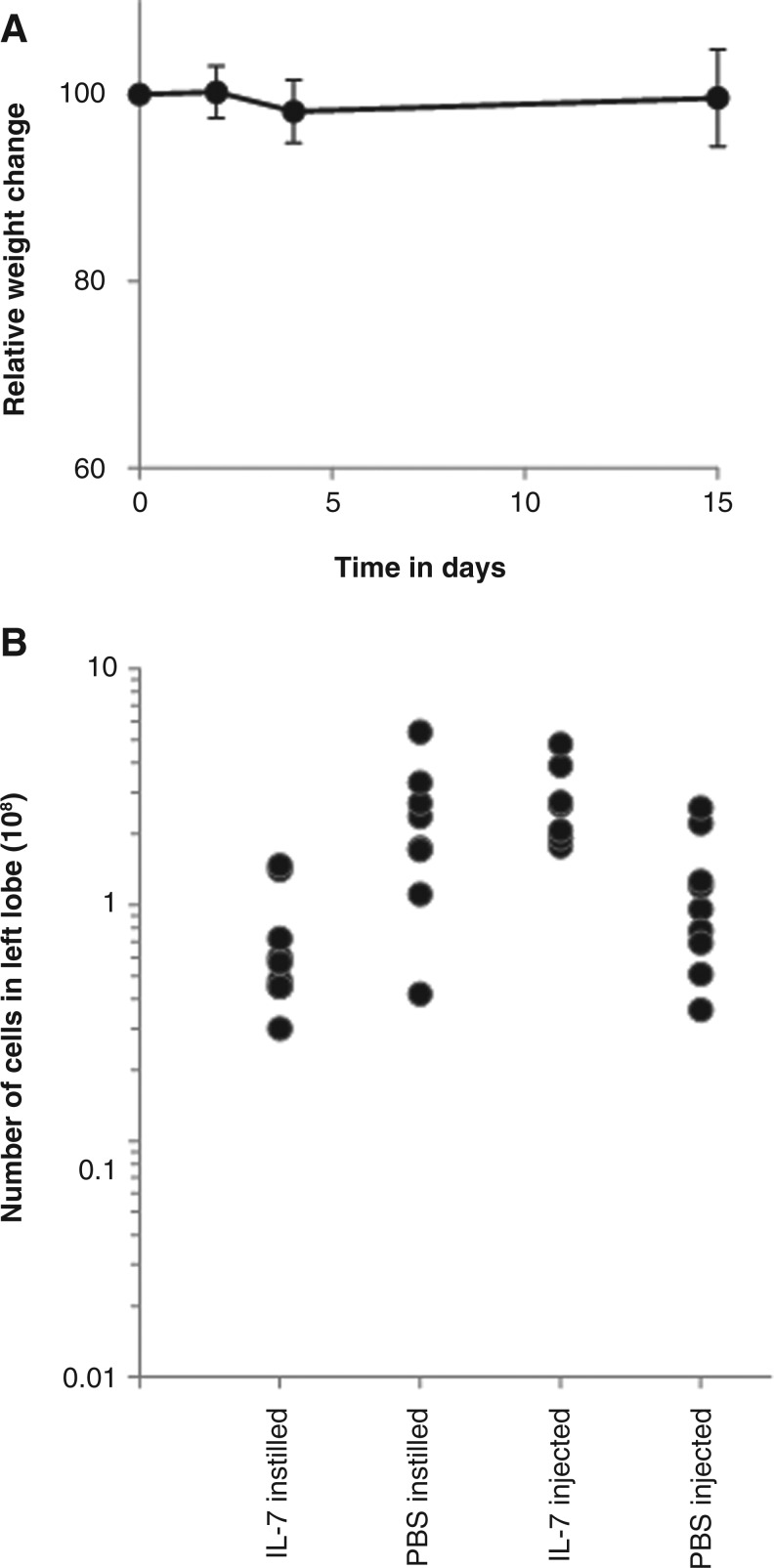
Finally, concern was raised over whether instillation of IL-7 in the lung would prove stressful for the animals and in addition could lead to attraction of inflammatory mononuclear cells to the lung or proliferation of resident mononuclear cells. Using weight change as a surrogate marker for stress, we saw no significant weight loss (p>0.05 at all time points when compared to the starting weight) in the animals throughout the treatment period (Fig. 8A). Analysis of the total number of cells in the larger left lobe of treated animals (Fig. 8B) revealed significantly fewer cells in the animals instilled with IL-7 compared with those receiving IL-7 injected subcutaneously (p=0.002).
FIG. 8.
Relative weight change in older animals receiving interleukin-7 (IL-7) by instillation (n=7) at times during the procedure (A) and comparison of the total number of mononuclear cells within the large left lung lobe of mice (B) treated by instillation or injection. PBS, Phosphate-buffered saline.
Discussion
Provision of proteins for therapeutic use by routes other than injection is limited to some extent by the efficiency with which the protein can cross the epithelial barrier. Here we have shown that IL-7, which is normally delivered in the clinic by repeated subcutaneous injection, can be effectively delivered as a therapeutic agent through the lungs. In terms of efficiency, our tagging experiments suggest that pulmonary delivery is at least as efficient as subcutaneous injection, whereas our functional experiments suggest that pulmonary delivery may be more efficient at producing an effect when compared with injection.
Previous studies to analyze the presence and distribution of IL-7 in the tissues after injection have distinguished between introduced and endogenously produced IL-7 on the basis of amount detected by enzyme-linked immunosorbent assay (ELISA)21 or have discriminated between these different sources of the IL-7 by radioactive labeling that which was injected.22 Tagging with dyes that fluoresce in the near-infrared region of the spectrum offers a safer alternative to studies involving radioactive labels and furthermore allowed us to follow the adsorption kinetics and distribution of the drug in real time. This provides considerable animal sparing when compared with other techniques.
A confounding factor in the delivery of IL-7 is its capacity to bind to heparin and heparin sulfate. Because heparin is produced by cells within connective tissue, it is found intracellularly in most tissues and also in serum; heparin sulfate is present in extracellular matrices, basement membranes, and cell surfaces.23 In addition, the amount of heparin varies with age,24 and the ability to bind IL-7 could alter the distribution profile of any IL-7 introduced into the body and its availability at the target organ. We noted prolonged maintenance of IL-7 at the site of subcutaneous injection, but such prolongation within the lungs was not as apparent.
We were aware that some of the IL-7 could be maintained on the air side of the epithelium either through binding to intracellular components in the epithelium or by virtue of its molecular size, which may inhibit its rapid transepithelial transit.25 Prolonged maintenance within the lung could lead to enzymic degradation of the tagged IL-7 through the action of proteases or peptidases.25 However, the observation that 48 hr after instillation the amounts of fluorescence in the bloodstream were similar to that seen via the injection route, which was considerably more than that seen with the free dye, and the reports showing that more than 95% of proteins such as albumin instilled into the airways may reach the circulation (reviewed in ref. 25) would suggest that the IL-7 from pulmonary delivery found in the bloodstream was as intact as that delivered by injection.
Whether IL-7 delivered through the lungs was functional was tested in older animals. Previous work has shown that IL-7 production in the epithelial component of the thymus declines with age4 and that introduction of IL-7 can lead to a reversal of thymic atrophy with increases in the minor and major subsets of the thymus.6,7,26 IL-7 when instilled into the lungs appeared to be able to rapidly transit into the bloodstream and remain at levels commensurate with subcutaneous injection. Organ distribution measured by fluorescent signal appeared to significantly favor instillation over injection when these two routes were compared. However, use of fluorescence alone may hide the possibility of degeneration of the attached IL-7 to a nonfunctional state. Experiments in older animals to assess functional capability again revealed that instillation proved to be significantly better than injection. This was shown by the effect on the early stages of T cell development within the CD3−CD4−CD8− population. These populations, defined by their expression of CD44 and CD25, express the IL-7 receptor, and this would account for the increase in the animals instilled with IL-7 compared with the PBS controls. The differences between the values obtained from the instilled versus injected IL-7 would suggest that instillation is more effective than injection at delivering functional amounts of IL-7 to the thymus.
Because IL-7 is a growth and survival factor for lymphocytes, which in their turn may produce factors engendering inflammation, it was possible that instillation could induce expansion or recruitment of mononuclear cells associated with inflammation in the lung. However, our analysis revealed that following a single instillation there was no major differences between mice receiving the IL-7 by instillation and the control groups, suggesting that rapid transit of IL-7 through the epithelium may have prevented any build-up within the lungs.
Use of the pulmonary system as a delivery route for mammalian proteins provides opportunities for rapid dissemination into the bloodstream, provided the molecule can cross the epithelial barriers into the lungs and then enter the organs and tissues from the bloodstream. Although in general molecules above 6 kD should be able to transit the lung epithelium,27 there is some variability based on susceptibility to peptidase and molecular size. We have shown here that IL-7 can transit the lungs, enter the bloodstream, and have effector function in the relevant organs. This has implications on the future therapeutic uses of IL-7 because inhalation may provide a means of extending the place of drug delivery from the clinic to the community. IL-7 has been shown to broaden responses to vaccination, assisting in the generation and maintenance of effector cells,28 and so may be of benefit during influenza infection. However, the amount of fluid in the lung during influenza and in other lung infections may inhibit its passage into the body.
Acknowledgments
This work was supported by a grant from Research into Ageing (Grant Number 324), We would like to thank Licor Biosciences for their assistance with the imaging.
Author Disclosure statement
The authors have no conflict of interest.
References
- 1.Fry TJ. Mackall CL. Interleukin-7: From bench to clinic Blood. 2002;99:3892–3904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 2.Michaelson MD. Mehler MF. Xu H. Gross RE. Kessler JA. Interleukin-7 is trophic for embryonic neurons and is expressed in developing brain. Dev Biol. 1996;179:251–263. doi: 10.1006/dbio.1996.0255. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Q. Li WQ. Aiello FB. Mazzucchelli R. Asefa B. Khaled AR. Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Andrew D. Aspinall R. Age-associated thymic atrophy is linked to a decline in IL-7 production. Exp Gerontol. 2002;37:455–463. doi: 10.1016/s0531-5565(01)00213-3. [DOI] [PubMed] [Google Scholar]
- 5.Ortman CL. Dittmar KA. Witte PL. Le PT. Molecular characterization of the mouse involuted thymus: Aberrations in expression of transcription regulators in thymocyte and epithelial compartments. Int Immunol. 2002;14:813–822. doi: 10.1093/intimm/dxf042. [DOI] [PubMed] [Google Scholar]
- 6.Aspinall R. Pido-Lopez J. Imami N. Henson SM. Ngom PT. Morre M. Niphuis H. Remarque E. Rosenwirth B. Heeney JL. Old rhesus macaques treated with interleukin-7 show increased TREC levels and respond well to influenza vaccination. Rejuvenation Res. 2007;10:5–18. doi: 10.1089/rej.2006.9098. [DOI] [PubMed] [Google Scholar]
- 7.Henson SM. Snelgrove R. Hussell T. Wells DJ. Aspinall R. An IL-7 fusion protein that shows increased thymopoietic ability. J Immunol. 2005;175:4112–4118. doi: 10.4049/jimmunol.175.6.4112. [DOI] [PubMed] [Google Scholar]
- 8.Aspinall R. Andrew D. Age-associated thymic atrophy is not associated with a deficiency in the CD44(+)CD25(−)CD3(−)CD4(−)CD8(−) thymocyte population. Cell Immunol. 2001;212:150–157. doi: 10.1006/cimm.2001.1848. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrini M. Calzascia T. Toe JG. Preston SP. Lin AE. Elford AR. Shahinian A. Lang PA. Lang KS. Morre M. Assouline B. Lahl K. Sparwasser T. Tedder TF. Paik JH. DePinho RA. Basta S. Ohashi PS. Mak TW. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Phillips JA. Brondstetter TI. English CA. Lee HE. Virts EL. Thoman ML. IL-7 gene therapy in aging restores early thymopoiesis without reversing involution. J Immunol. 2004;173:4867–4874. doi: 10.4049/jimmunol.173.8.4867. [DOI] [PubMed] [Google Scholar]
- 11.Levy Y. Lacabaratz C. Weiss L. Viard JP. Goujard C. Lelievre JD. Boue F. Molina JM. Rouzioux C. Vettand-Fenoel V. Croughs T. Beq S. Thiebaut R. Chene G. Morre M. Delfraissy JF. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moniuszko M. Fry T. Tsai WP. Morre M. Assouline B. Cortez P. Lewis MG. Cairns S. Mackall C. Franchini G. Recombinant interleukin-7 induces proliferation of naive macaque CD4+ and CD8+ T cells in vivo. J Virol. 2004;78:9740–9749. doi: 10.1128/JVI.78.18.9740-9749.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nugeyre MT. Monceaux V. Beq S. Cumont MC. Ho TFR. Chene L. Morre M. Barre-Sinoussi F. Hurtrel B. Israel N. IL-7 stimulates T cell renewal without increasing viral replication in simian immunodeficiency virus-infected macaques. J Immunol. 2003;171:4447–4453. doi: 10.4049/jimmunol.171.8.4447. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg SA. Sportes C. Ahmadzadeh M. Fry TJ. Ngo LT. Schwarz SL. Stetler-Stevenson M. Morton KE. Mavroukakis SA. Morre M. Buffet R. Mackall CL. Gress RE. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sereti I. Dunham RM. Spritzler J. Aga E. Proschan MA. Medvik K. Battaglia CA. Landay AL. Pahwa S. Fischl MA. Asmuth DM. Tenorio AR. Altman JD. Fox L. Moir S. Malaspina A. Morre M. Buffet R. Silvestri G. Lederman MM. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113:6304–6314. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sportes C. Babb RR. Krumlauf MC. Hakim FT. Steinberg SM. Chow CK. Brown MR. Fleisher TA. Noel P. Maric I. Stetler-Stevenson M. Engel J. Buffet R. Morre M. Amato RJ. Pecora A. Mackall CL. Gress RE. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin Cancer Res. 2010;16:727–735. doi: 10.1158/1078-0432.CCR-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sportes C. Hakim FT. Memon SA. Zhang H. Chua KS. Brown MR. Fleisher TA. Krumlauf MC. Babb RR. Chow CK. Fry TJ. Engels J. Buffet R. Morre M. Amato RJ. Venzon DJ. Korngold R. Pecora A. Gress RE. Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agu RU. Ugwoke MI. Armand M. Kinget R. Verbeke N. The lung as a route for systemic delivery of therapeutic proteins and peptides. Respir Res. 2001;2:198–209. doi: 10.1186/rr58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bivas-Benita M. Zwier R. Junginger HE. Borchard G. Non-invasive pulmonary aerosol delivery in mice by the endotracheal route. Eur J Pharm Biopharm. 2005;61:214–218. doi: 10.1016/j.ejpb.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Hoff J. Methods of blood collection in the mouse. Lab Animal. 2000;29:47–53. [Google Scholar]
- 21.Dereuddre-Bosquet N. Vaslin B. Delache B. Brochard P. Clayette P. Aubenque C. Morre M. Assouline B. Le GR. Rapid modifications of peripheral T-cell subsets that express CD127 in macaques treated with recombinant IL-7. J Med Primatol. 2007;36:228–237. doi: 10.1111/j.1600-0684.2007.00240.x. [DOI] [PubMed] [Google Scholar]
- 22.Bui T. Faltynek C. Ho RJ. Differential disposition of soluble and liposome-formulated human recombinant interleukin-7: Effects on blood lymphocyte population in guinea pigs. Pharm Res. 1994;11:633–641. doi: 10.1023/a:1018955708443. [DOI] [PubMed] [Google Scholar]
- 23.Clarke D. Katoh O. Gibbs RV. Griffiths SD. Gordon MY. Interaction of interleukin 7 (IL-7) with glycosaminoglycans and its biological relevance. Cytokine. 1995;7:325–330. doi: 10.1006/cyto.1995.0041. [DOI] [PubMed] [Google Scholar]
- 24.Komosinska-Vassev KB. Winsz-Szczotka K. Kuznik-Trocha K. Olczyk P. Olczyk K. Age-related changes of plasma glycosaminoglycans. Clin Chem Lab Med. 2008;46:219–224. doi: 10.1515/CCLM.2008.048. [DOI] [PubMed] [Google Scholar]
- 25.Kim KJ. Malik AB. Protein transport across the lung epithelial barrier. Am J Physiol Lung Cell Mol Physiol. 2003;284:L247–L259. doi: 10.1152/ajplung.00235.2002. [DOI] [PubMed] [Google Scholar]
- 26.Andrew D. Aspinall R. Il-7 and not stem cell factor reverses both the increase in apoptosis and the decline in thymopoiesis seen in aged mice. J Immunol. 2001;166:1524–1530. doi: 10.4049/jimmunol.166.3.1524. [DOI] [PubMed] [Google Scholar]
- 27.Patton JS. Fishburn CS. Weers JG. The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc. 2004;1:338–344. doi: 10.1513/pats.200409-049TA. [DOI] [PubMed] [Google Scholar]
- 28.Melchionda F. Fry TJ. Milliron MJ. McKirdy MA. Tagaya Y. Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8(+) memory cell pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]