Abstract
Background
Characterization of long-term health trajectory in older individuals is important for proactive health management. However, the relative prognostic value of information contained in clinical profiles of nonfrail older adults is often unclear.
Methods
We screened 825 phenotypic and genetic measures evaluated during the Health, Aging, and Body Composition Study (Health ABC) baseline visit (3,067 men and women aged 70–79). Variables that best predicted mortality over 13 years of follow-up were identified using 10-fold cross-validation.
Results
Mortality was most strongly associated with low Digit Symbol Substitution Test (DSST) score (DSST<25; 21.9% of cohort; hazard ratio [HR]=1.87±0.06) and elevated serum cystatin C (≥1.30 mg/mL; 12.1% of cohort; HR=2.25±0.07). These variables predicted mortality better than 823 other measures, including baseline age and a 45-variable health deficit index. Given elevated cystatin C (≥1.30 mg/mL), mortality risk was further increased by high serum creatinine, high abdominal visceral fat density, and smoking history (2.52≤HR ≤3.73). Given a low DSST score (<25) combined with low-to-moderate cystatin C (<1.30 mg/mL), mortality risk was highest among those with elevated plasma resistin and smoking history (1.90≤HR≤2.02).
Conclusions
DSST score and serum cystatin C warrant priority consideration for the evaluation of mortality risk in older individuals. Both variables, taken individually, predict mortality better than chronological age or a health deficit index in well-functioning older adults (ages 70–79). DSST score and serum cystatin C can thus provide evidence-based tools for geriatric assessment.
Introduction
The identification of mortality risk factors is important for the care of older patients and for supporting informed decisions on treatment alternatives within clinical settings. Chronological age is one factor that correlates with mortality risk and functional limitation, but individuals of the same chronological age can exhibit heterogeneous aging trajectories.1 Indicators of “frailty” also predict health outcomes and mortality in older individuals (e.g., weight loss, low grip strength, lack of energy, slow walking speed, and low physical activity).2 However, among well-functioning older adults, trademark characteristics of frailty may occur at low frequency and thus provide little guidance for clinical decision-making.3 Furthermore, frailty indices have not accounted for or assigned special weight to predictors of long-term outcomes, such as smoking or diabetes status.3,4 In predominantly nonfrail populations, therefore, chronological age and frailty metrics may not accurately predict aging trajectory and long-term outcomes. At the same time, there is no consensus on which indicators are most sensitive to future health status in well-functioning adults, and few analyses have evaluated relative predictive validity of measures that might identify mortality risk in seemingly low-risk persons.3–5
The clinical profile of older adults can be extensive, and information with prognostic value can be drawn from several domains, including basic demographic data, family history, clinical or subclinical disease markers, measures of physical performance, and biochemical assay results. Genotype and genome sequencing data will further add to the size and complexity of geriatric profiles in coming years.6 A key challenge, in this context, is to identify parsimonious subsets of information that can predict mortality patterns as well as the rate of functional decline with increased age.3 Numerous variables have been associated with all-cause mortality in older adults.3,7–10 However, in relative terms, some variables may be more useful for prediction and warrant focused clinical attention.3 Moreover, relative value of predictors can vary among subgroups defined by shared risk factors. For example, serum vascular adhesion protein-1 (VAP-1) levels (elevated with chronic hyperglycemia) predict mortality among those with diabetes, but this association is not present among those without diabetes.11
We performed a comprehensive screen of 825 variables measured in year 1 of the Health, Aging, and Body Composition (Health ABC) Study (3,067 men and women aged 70–79 years). Health ABC participants were well-functioning, community-dwelling older adults without mobility-related disability at the time of enrollment. Therefore, we expected that measures most predictive of mortality in this cohort could provide metrics for geriatric assessment and indicators of aging trajectory. We highlight phenotypic measures that, relative to all other participant attributes, are most predictive of mortality in the Health ABC cohort. Additionally, we partition the Health ABC cohort into subgroups defined by shared risk factors and identify the strongest mortality predictors within each subgroup.
Methods
Study Design and Participants
Health ABC is a prospective cohort study designed to evaluate interrelationships between weight-related health conditions, body composition, and physical function in older adults. Participants included 3,075 initially well-functioning 70- to 79-year-old men and women (52% women and 42% black). These individuals were identified from a random sample of white Medicare beneficiaries and all age-eligible community-dwelling black residents in designated zip code areas surrounding Memphis, Tennessee, and Pittsburgh, Pennsylvania. Participants were eligible if they reported no difficulty in walking one quarter of a mile, going up 10 steps without resting, or performing basic activities of daily living. Participants were excluded if they reported a history of active treatment for cancer in the prior 3 years, planned to move out of the study area in the next 3 years, or were currently participating in a randomized trial of a lifestyle intervention. Baseline data, collected between April, 1997, and June, 1998, included an in-person interview and clinic-based examination, with evaluation of body composition, clinical and subclinical diseases, and physical functioning. Participant characteristics at the time of baseline examination are summarized in Supplemental Table 1 (supplemental data available at www.liebertpub.com/rej). Following enrollment, participants were contacted every 6 months by telephone or during scheduled clinic visits. Deaths were ascertained from family member proxies, local obituaries, or the Social Security death index. Immediate and underlying causes of death were assigned by an adjudication committee based upon review of medical records, death certificate, and decedent proxy interview. The most frequent (assigned) causes of death were cancer (28%), atherosclerotic cardiovascular disease (23%), dementia (10%), and cerebrovascular disease (10%). The current analysis is based upon all deaths that occurred through November, 2010 (1,430 deaths or 46.5% of the cohort).
Health Deficit Index
A health deficit index was constructed from participant responses to 45 questions relating to diverse domains of individual health (Supplemental Table 2), where each question was scored as a deficit (value=1) or nondeficit (value=0). The final index comprises the total number of deficits among the 45 items normalized to range from 0 to 1.12 Applying guidelines from previous work,12 approximately 26% of participants were frail according to the 45-variable health deficit index (index >0.20) (Supplemental Fig. 1). In contrast, fewer than 1.5% of Health ABC participants were frail according to the five-component Fried frailty index developed within the Cardiovascular Health Study (CHS) or the three-component index developed within the Study of Osteoporotic Fractures (SOF) (data not shown).2,13
Data mining to identify mortality predictors: Preprocessing of Health ABC data
We applied a data mining procedure and screened 825 variables (498 categorical and 327 continuous) to determine which best predicted mortality in the Health ABC cohort (Supplemental Fig. 2). These 825 variables included: Information obtained from the baseline questionnaire (214 variables); participant responses and measurements obtained during a clinical visit (179 variables); medication inventory (52 variables), imaging data processed and interpreted by specialists (156 variables; e.g., soft tissue computed tomography, dual-energy X-ray absoptiometry [DXA], and electrocardiogram [ECG]); calculated variables derived from baseline measures (133 variables; e.g., body mass index [BMI]); biochemical assay results from blood samples (24 variables); and single-nucleotide polymorphism (SNP) genotypes (55 variables). Also included were age at baseline, study site, sex, race, the 45-variable deficit index described above, and the CHS and SOF frailty indices.2,13 The 825 variables represented a filtered subset derived from an initial set of 3,300 possible measures obtained from Health ABC participants at baseline (1,894 phenotypic variables and 1,406 genetic markers). We removed 2,427 variables from consideration because data were missing for more than 5% of men or 5% of women, and 54 measures were removed because they did not exhibit any interparticipant variability. Additionally, 8 participants were excluded because data were missing for more than 20% of the 825 variables satisfying inclusion criteria. These preprocessing steps yielded a data matrix consisting of 3,067 participants and 825 variables, with fewer than 1% missing values for each of the 825 variables (on average). This data matrix was used in cross-validation experiments (see below) and further preprocessed to impute missing entries and to generate a larger set of representative data vectors (i.e., indicators for each categorical variable and alternatively scaled versions of each continuous variable) (Supplemental Fig. 2). A partial listing of the 825 included variables is provided in Supplemental Table 3.
Missing values for phenotypic measures and genetic marker data were separately imputed using the nearest-neighbor approach, with imputed values calculated from the 20 participants most similar to any given participant with missing data.3 This imputation was only carried out as part of the data mining procedure and estimation of cross-validation area under the curve (AUC) values (Supplemental Fig. 2). For all other calculations, such as the estimation of hazard ratios, participants with missing data were simply excluded from analyses. Following imputation, data vectors representative of the 825 variables were generated. For each categorical variable with k levels, we generated k vectors where each vector was a 0-1 indicator. For example, with respect to sex, two vectors were generated—one with value of 1 if the participant was male (and 0 otherwise) and another with value 1 if the participant was female (and 0 otherwise). For continuous variables, we anticipated that prediction accuracy in simulation trials might be influenced by choice of scale. For each continuous variable, therefore, six data vectors were generated, including one vector identical to the original variable (same units and scale), along with five vectors generated by applying Box–Cox transformations to the original variable with power parameter λ equal to −2, −1, 0, 1 and 2, respectively. Following this preprocessing, we had generated 3,683 vectors (representative of 825 total variables), which were further screened in cross-validation analyses (see below and Supplemental Fig. 2).
Data mining to identify mortality predictors: Cross-validation assessment of prognostic value
Ten-fold cross-validation was used to identify data vectors best able to predict mortality in the Cox regression model framework.3 In each simulation trial, 90% of participants were randomly assigned to a training set, with the remaining 10% of participants assigned to a testing set. A univariate Cox regression model was fit based upon vector values and the follow-up survival times of participants in the training set, including both censored and noncensored survival times. The fitted model was then applied to vector values of participants in the testing set to generate risk scores (linear predictors). Prediction accuracy was then estimated from correspondence between risk scores and the censored or noncensored survival times of participants in the testing set. This correspondence was quantified by an AUC-based concordance index representing a weighted average of time-specific AUC statistics.3,14 This composite AUC statistic was calculated in each of N cross-validation trials, and for each vector we assessed prognostic value based upon the average AUC statistic value across the N trials.3
A computationally efficient procedure was used to screen vectors based upon cross-validation performance (Supplemental Fig. 2). The approach first removes the weakest vectors from consideration using relatively few simulation trials per vector, and subsequently reevaluates top-ranking vectors using more simulations to obtain a more precise estimate of the average AUC.3 First, all 3,683 data vectors were evaluated based upon N=30 cross-validation trials each, and the 500 data vectors with highest average AUC were identified. These 500 vectors were further screened using N=300 cross-validation trials each, and the 50 vectors with highest average AUC were identified. The relative prognostic value of these 50 vectors was then established based upon N=30,000 cross-validation trials each. Given N=30,000 simulation trials, the standard error associated with the average AUC estimate was less than 0.001. In the final step, the 50 selected vectors were mapped back to their associated variables, yielding a ranked set of p variables (p≤50). If more than one vector mapped to the same variable, the largest mean AUC among such vectors was assigned to that variable. For instance, if one vector representing BMI on its original scale yielded an average AUC of 0.55, while a second vector representing BMI with Box–Cox transformation λ=2 yielded an average AUC of 0.56, then an AUC of 0.56 was assigned to the variable BMI. The complete procedure (Supplemental Fig. 2) was applied to the full Health ABC cohort (n=3,067) and subsequently to multiple subgroups, which were defined based upon those variables that best predicted mortality in the full Health ABC cohort.
Results
Digit Symbol Substitution Test score and serum cystatin C each predict mortality better than 823 other Health ABC baseline measures
We screened 825 variables evaluated during year 1 of the Health ABC study to determine which best predicted mortality over 13 years of follow-up (Supplemental Fig. 2; median follow-up=12.2 years; interquartile range [IQR]=5 years). This approach identified the Digit Symbol Substitution Test (DSST) score and serum cystatin C as the strongest overall predictors of mortality (AUC=0.599 and 0.587, respectively; N=30,000 cross-validation trials) (Table 1). Other top-ranked predictors included the Health ABC performance score (a composite metric based upon performance in chair stand, usual walk, narrow walk, and balance tasks; AUC=0.578),15 Modified Mini-Mental State (3MS) exam score (AUC=0.575), serum creatinine (AUC=0.573), and chronological age at baseline evaluation (AUC=0.566) (Table 1). DSST score and cystatin C remained the top two mortality predictors (AUC=0.599 and 0.581, respectively) when 179 deaths occurring during the first 3 years of follow-up were censored. Both variables were also among the top three predictors when added to models that already included both age and sex (DSST, AUC=0.619; cystatin C, AUC=0.613; Supplemental Table 4). Additionally, we screened all possible 4,950 bivariate models generated from the top 100 variables with highest AUC value, and identified the combination DSST score+cystatin C as the best-performing bivariate model (AUC=0.628).
Table 1.
Top-Ranking Variables That Best Predicted Mortality Over 13 Years in the Full Health ABC Cohort (n=3,067)
| Variable | Transformation | Mean AUC | SD (AUC) |
|---|---|---|---|
| DSST score (number of correct substitutions) a | λ=2 | 0.599 | 0.024 |
| Serum cystatin C (mg/L) b | λ=0 | 0.587 | 0.021 |
| Health ABC performance score (0– 4) c | λ=2 | 0.578 | 0.023 |
| 3MS score a | λ=2 | 0.575 | 0.020 |
| Serum creatinine (mg/dL) b | λ=0 | 0.573 | 0.021 |
| Chronological age at baseline exam | None | 0.566 | 0.023 |
| Time to walk 6 m (fastest of two trials) c | λ=–1 | 0.565 | 0.023 |
| Full standing balance test time 0–90 sec c | λ=2 | 0.565 | 0.023 |
| Pack-years exposure to cigarettes | λ=0 | 0.564 | 0.023 |
| EPESE performance battery score 0–12 c | λ=2 | 0.563 | 0.022 |
The table lists 10 variables that best predicted mortality as identified from the data mining and cross-validation procedure described in Supplemental Fig. 2. For each variable, the mean area under the curve (AUC) from the highest-ranking vector associated with that variable is listed. Data vectors for each variable were generated using Box–Cox transformations (see Methods). The “transformation” column indicates the Box–Cox parameter that yielded the highest-scoring mean AUC listed for each variable. If the highest-scoring vector for a given variable was an untransformed variable on its original scale, then “None” is listed in the transformation column. All mean AUC values were calculated using 30,000 cross-validation trials, with a standard error less than 0.001 in each case. The standard deviation (SD) among AUC statistics generated in the 30,000 simulation trials is listed for each variable in the final column.
Digit Symbol Substitution Test (DSST) score (number of correct substitutions) was correlated with the Modified Mini-Mental State (3MS) score (rs=0.59; p<0.001).
Serum cystatin C was correlated with serum creatinine (rs=0.57; p<0.001).
Health, Aging, and Body Composition Study (Health ABC) performance score was correlated with full standing balance test time (rs=0.76), time taken to walk 6 m (rs=−0.70), and Established Populations for Epidemiological Study of the Elderly (EPESE) performance battery score (rs=0.69) (p<0.001 for each variable).
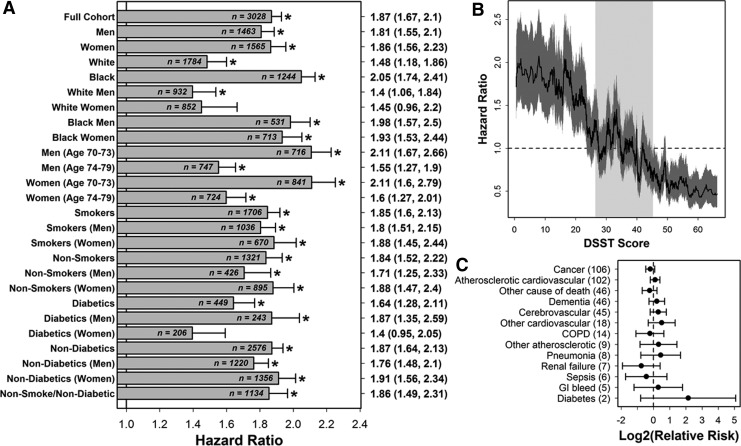
Low DSST score (<25) predicted mortality in the full Health ABC cohort and 23 of 25 subgroups (Fig. 1A; 1.40≤HR≤2.11; DSST≥25 in reference group). In the full Health ABC cohort, the association of low DSST score with mortality remained significant after adjustment for any other variable, including education, age, and 3MS exam score (i.e., bivariate Cox regression models). Mortality risk increased continuously with lower DSST scores, although risk was significantly heightened only among participants with a DSST score <25 (Fig. 1B; n=671 participants). Among participants with DSST<25 that died during follow-up, there was no significant increase in dementia as an assigned underlying cause of death (Fig. 1C).
FIG. 1.
Digit Symbol Substitution Test (DSST) score predicts mortality in the Health Aging, and Body Composition Study (Health ABC) cohort and multiple participant subgroups. (A) The hazard ratio (HR) associated with low DSST score (DSST <25) was estimated in the full Health ABC cohort and each of 25 subcohorts. Significant HRs are indicated by an asterisk symbol (*). Point estimates with 95% confidence intervals are listed in the right margin. Sample sizes used for each subgroup are listed at the end of each horizontal bar (participants with missing data were excluded from calculations). A 0-1 indicator was used as the independent variable in Cox regression models, where the value of the indicator was 1 for participants with low DSST (DSST <25) and 0 otherwise. HR estimates are adjusted for study site (Memphis or Pittsburgh). (B) The HR associated with low to high DSST score intervals (windows) was evaluated. Participants were sorted in ascending order according to DSST score (horizontal axis). A sliding window analysis was then performed in which the HR was estimated for a window of 100 participants relative to all other participants outside of the window. The solid black line represents the estimated HR for a given window of 100 participants, and the dark grey region outlines a 95% confidence interval (CI). The light grey vertical region in the background outlines the middle 50% of DSST scores among all participants (i.e., interquartile range). (C) The relative risk of (assigned) underlying causes of death was evaluated in participants with a DSST score <25 (n=414 deaths) and participants with a DSST score ≥25 (n=915 deaths). Assigned causes of death are sorted from most frequent to least frequent among those with a DSST score <25 (frequencies are given in parentheses).
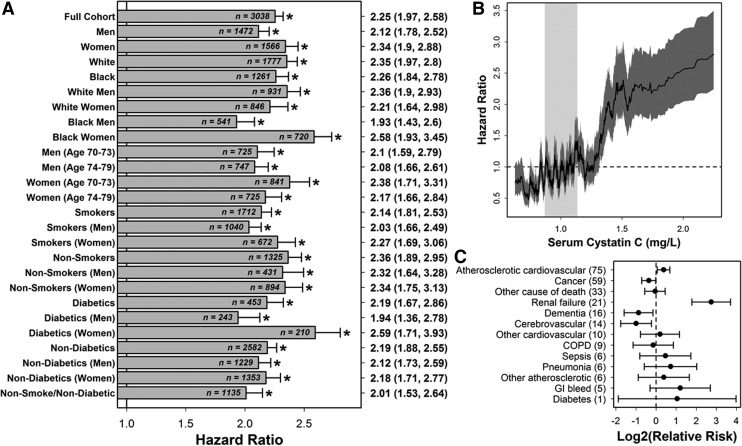
Elevated cystatin C (≥1.30 mg/L) was significantly associated with mortality in the full Health ABC cohort and each of 25 participant subgroups (Fig. 2A; 1.93≤HR≤2.59; cystatin C<1.30 in reference group). In the full cohort, this association remained significant in bivariate regression models that included any of the other 825 variables, such as creatinine, resistin, or C-reactive protein. Participants with low cystatin C (<1.30 mg/L; n=2,666) had slightly reduced mortality risk, and there was significantly increased mortality risk among those with elevated cystatin C (≥1.30 mg/L; n=372) (Fig. 2B). Among participants with elevated cystatin C (≥1.30 mg/L) who died, renal failure as an assigned underlying cause of death was over six-fold more likely (relative risk [RR]=6.70, 95% confidence interval [CI] 3.44, 13.03; n=261 deaths with an assigned cause; see Fig. 2C). Additionally, there was increased frequency of atherosclerotic cardiovascular disease and decreased frequency of dementia and cerebrovascular illness among these participants (Fig. 2C).
FIG. 2.
Serum cystatin C levels predict mortality in the Health, Aging, and Body Composition Study (Health ABC) cohort and are associated with renal failure and atherosclerotic cardiovascular disease. (A) The hazard ratio (HR) associated with high cystatin C (cystatin C≥1.30) was estimated in the full Health ABC cohort and each of 25 subcohorts. Significant HRs are indicated by an asterisk symbol (*). Point estimates with 95% confidence intervals are listed in the right margin. Sample sizes used for each subgroup are listed at the end of each horizontal bar (participants with missing data were excluded from calculations). A 0-1 indicator was used as the independent variable in Cox regression models, where the value of the indicator was 1 for participants with high cystatin C (cystatin C≥1.30) and 0 otherwise. HR estimates are adjusted for study site (Memphis or Pittsburgh). (B) The HR associated with low to high cystatin C intervals (windows) was evaluated. Participants were sorted in ascending order according to measured cystatin C (horizontal axis). A sliding window analysis was then performed in which the HR was estimated for a window of 100 participants relative to all other participants outside of the window. The solid black line represents the estimated HR for a given window of 100 participants, and the dark grey region outlines a 95% confidence interval. The light grey vertical region in the background outlines the middle 50% of cystatin C levels among all participants (i.e., interquartile range). (C) The relative risk of (assigned) underlying causes of death was evaluated in participants with cystatin C≥1.30 (n=261 deaths) and participants with cystatin C<1.30 (n=1,083 deaths). Assigned causes of death are sorted from most frequent to least frequent among those with cystatin C≥1.30 (frequencies are given in parentheses).
DSST score and serum cystatin C each predict mortality better than chronological age and an index of health deficits
Direct comparisons were performed to assess the relative predictive value of DSST score, serum cystatin C, chronological age, and an index of health deficits (Supplemental Fig. 3). Comparisons were carried out with respect to each of 25 subgroups of Health ABC participants (e.g., men, women, smokers, nonsmokers, etc.). In 16 of 25 subgroups, DSST score was the best predictor compared to cystatin C, age, and the deficit index. The relative advantage of DSST score was strongest among nondiabetics who did not smoke (Supplemental Fig. 3). In 9 of 25 subgroups, cystatin C was the best predictor, and the relative advantage of cystatin C was strongest among diabetic females (Supplemental Fig. 3). In all cases, both DSST score and cystatin C predicted mortality better than age and the health deficit index (Supplemental Fig. 3). DSST score and cystatin C also predicted mortality better than the deficit index in the context of three-variable models that included both age and sex (AUC≥0.613 versus AUC=0.588; see Supplemental Fig. 4).
Participants with elevated cystatin C: Serum creatinine, abdominal visceral fat density, and smoking history provide secondary indicators of mortality risk
Co-occurrence of elevated cystatin C with elevated creatinine was associated with significantly increased mortality risk (Fig. 3). The data mining procedure shown in Supplemental Fig. 2 was applied to 372 participants with elevated cystatin C (≥1.30 mg/L), and this identified serum creatinine as the strongest predictor of mortality within this subgroup (AUC=0.600; Fig. 3). Among the 372 participants, there were 43 with elevated cystatin C (≥1.30 mg/L) and elevated creatinine (≥2 mg/dL), and mortality risk within this group was higher than any other subgroup we evaluated (HR=3.73; 95% CI 2.69, 5.18; Fig. 3). In addition to elevated creatinine (≥2 mg/dL), we identified three other factors as most predictive of mortality among those with cystatin C≥1.30 mg/L, including high abdominal fat density (≥−81 HU), low DSST score (<25), and exposure to smoking (≥30 pack-years) (Fig. 3).
FIG. 3.
Factors that modify mortality risk given elevated serum cystatin C or low digit symbol substitution test (DSST) score. The algorithm shown in Supplemental Fig. 2 was applied to Health, Aging, and Body Composition Study (Health ABC) subgroups to identify dominant mortality predictors in each subgroup. For each subgroup, the variable associated with the highest area under the curve (AUC) value was identified, based upon cross-validation assessment and the mortality patterns among participants within the group. This top-ranking variable was then used to further divide the subgroup, as represented by the nested tree structure. If the top-ranking variable was continuous, an appropriate division point was chosen by inspecting sliding window plots of the hazard ratio (HR) versus variable values (e.g., see Figs. 1B and 2B). For each group at each level of the tree, the listed sample size (n) excludes any participants that could not be assigned to that group due to missing data. The HR value (±standard error) estimates the risk of mortality for individuals in that subgroup as compared to the complete Health ABC cohort. An asterisk symbol (*) is used to denote cases in which the HR is significantly different from 1 (p<0.05). The highlighted text indicates subgroups for which the estimated HR is significantly greater than 1 (p<0.05).
Participants with low DSST score and low-to-moderate cystatin C: Plasma resistin and smoking history provide secondary indicators of mortality risk
DSST score was identified as the strongest predictor of mortality among 2,666 participants with low-to-moderate cystatin C (<1.30 mg/L) (AUC=0.603; Fig. 3). DSST score appeared to predict mortality risk independently of cystatin C, because in bivariate Cox models both cystatin C and DSST score were significant predictors with nonsignificant interaction (n=2999; p=0.025 for cystatin C; p<0.001 for DSST score; p=0.40 for interaction). We further evaluated 571 participants with a DSST score less than 25, who were not at risk on the basis of cystatin C levels (cystatin C<1.30 mg/L) (Fig. 3). Within this subgroup, high plasma resistin (≥24 ng/mL) and smoking history (≥12 pack-years) were most predictive of increased mortality (Fig. 3).
Discussion
The phenotypic and genetic information available for predicting long-term outcomes in older individuals will continue to expand in coming decades. The relative prognostic value of such information, however, is not always clear. We conducted a large-scale evaluation of 825 measures taken at baseline in the Health ABC cohort, where each measure was obtainable from a single clinic visit (e.g., demographic data, physical and cognitive test performance, body composition measures, analysis of biospecimens, and genetic information). Our findings highlight DSST score and serum cystatin C as measures that warrant evaluation and priority consideration for health assessment of initially well-functioning older adults (aged 70–79). Each of these measures predicted mortality better than chronological age, an index of health deficits, and all other variables measured at baseline, including smoking history, diabetes status, and walking speed. These results point towards “leading indicators” that can be used to characterize aging trajectory or subclinical disease in older adults.
DSST score emerged from our analysis as the strongest overall predictor of all-cause mortality in the Health ABC cohort. This one variable was, in nearly all subgroups considered, able to predict mortality patterns better than baseline age or a health deficit index. This result is consistent with previous studies that have also reported an association between DSST score and long-term outcomes.16–19 DSST score provides an integrative measure of visuomotor coordination, processing speed, and short-term memory.20 It is possible that low DSST score denotes a deficit reflecting “slowness” of cognitive function, which might directly impact health to increase mortality risk (e.g., inability to follow medication schedules).16 Alternatively, low DSST score may signal the presence of other health problems possibly outside the central nervous system itself, such as vascular or metabolic disease.21 Among those with DSST scores less than 25 (also with cystatin C<1.30 mg/mL), high plasma resistin (≥24 ng/mL) was an indicator of increased mortality risk (Fig. 3). Because plasma resistin reflects the degree of systemic inflammation, elevated resistin may reflect underlying vascular or metabolic diseases that drive cognitive decline and development of other health problems.22
Cystatin C has been viewed as a marker of kidney function, atherosclerosis, and systemic inflammation.23–25 However, cystatin C is not routinely measured in geriatric health assessment. Previous studies of the Health ABC and CHS cohorts have identified cystatin C as a strong predictor of all-cause mortality and delineated high-risk groups as those with cystatin C above 1.19 mg/L (Health ABC) or above 1.28 mg/L (CHS).23,24 Our threshold value of 1.30 mg/L is in rough agreement with these prior estimates (see Fig. 2B). Our findings do not indicate that cystatin C is a specific predictor of renal failure, because atherosclerotic cardiovascular disease was the most frequent cause of death among those with cystatin C≥1.30 mg/L (Fig. 2C). However, relative to all other Health ABC participants, renal failure as an assigned underlying cause of death was 6.7 times more common in those with elevated cystatin C (≥1.30 mg/L) (8% versus 1.2%). For participants with both cystatin C≥1.3 mg/L and serum creatinine ≥2.0 mg/dL, renal failure as an assigned underlying cause of death was 16.8 times more common (29.7% versus 1.8%). Interestingly, for one-third of participants with elevated cystatin C (≥1.30 mg/L), mortality was not significantly increased (Fig. 3). Defining characteristics of this subgroup included low creatinine (<2.0 mg/dL), low abdominal visceral fat density (<− 81 HU), DSST score ≥25, and limited smoking history (<30 pack-years) (Fig. 3).
Some limitations of this study should be noted. First, we analyzed older individuals screened to exclude those with overt functional deficits. Our findings, therefore, may not apply to a more general population of older adults. Second, although our analysis included 825 variables, it is possible that other variables, either not measured in Health ABC or excluded due to missing data, may have provided better predictors of mortality than those we identified. Third, we have highlighted individual variables in our study that best predicted mortality, but it is possible that, in some cases, correlated measures could be substituted with only modest loss of prediction accuracy (e.g., 3MS score for DSST score; Table 1). In practical settings, the choice between alternative metrics would also depend upon the ease and cost of collecting information, which we have not factored into our analysis. Fourth, we have focused only on mortality outcomes in the Health ABC cohort. Variables that predict mortality may also be useful for predicting other long-term outcomes, such as accumulation of daily living impairments and the rate of physical strength decline with increased age.3 However, further work is required to determine whether mortality predictors we have identified are also informative with regard to health span and maintenance of physical function. Finally, the AUC statistics generated from univariate models were modest in magnitude in the context of 10-fold cross validation (e.g., AUC values <0.600; see Table 1). More accurate tools for prediction of mortality patterns can be generated by development of multivariate risk score indices, although cross-validation prediction accuracy can also be improved by the analysis of larger prospective cohort datasets.3
Attention to geriatric conditions in the clinical setting is often insufficient, and older individuals are, as a group, at risk for not receiving care that could otherwise prolong life or improve its quality.26,27 Comprehensive geriatric assessment has been viewed as one avenue leading toward better recognition of disease states and improved care.28 Comprehensive geriatric assessment, however, carries substantial time and resource demands.28 In this context, there is a need to: (1) Deemphasize elements of a geriatric profile only weakly associated with long-term outcomes and (2) better emphasize assessments that provide a stronger basis for long-term decision making. This study has identified measures most informative for predicting 13-year mortality in older adults. The priority consideration of these measures in clinical settings may facilitate geriatric assessment that is both efficient and evidence-based.
Supplementary Material
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institute of Aging, National Institutes of Health, Bethesda, MD (contracts N01-AG-6-2101, N01-AG-6- 2103, and N01-AG-6-2106). We thank two anonymous reviewers for providing constructive comments and suggestions on this manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Loewy EH. Age discrimination at its best: Should chronological age be a prime factor in medical decision making? Health Care Anal. 2005;13:101–117. doi: 10.1007/s10728-005-4474-z. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP. Tangen CM. Walston J. Newman AB. Hirsch C. Gottdiener J. Seeman T. Tracy R. Kop WJ. Burke G. McBurnie MA Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Swindell WR. Ensrud KE. Cawthon PM. Cauley JA. Cummings SR. Miller RA Study Of Osteoporotic Fractures Research Group. Indicators of “healthy aging” in older women (65–69 years of age). A data-mining approach based on prediction of long-term survival. BMC Geriatr. 2010;10:55. doi: 10.1186/1471-2318-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke GL. Arnold AM. Bild DE. Cushman M. Fried LP. Newman A. Nunn C. Robbins J Group, Cardiovascular Health Study Collaborative Research Group. Factors associated with healthy aging: The cardiovascular health study. J Am Geriatr Soc. 2001;49:254–262. doi: 10.1046/j.1532-5415.2001.4930254.x. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB. Boudreau RM. Naydeck BL. Fried LF. Harris TB. A physiologic index of comorbidity: Relationship to mortality and disability. J Gerontol A Biol Sci Med Sci. 2008;63:603–609. doi: 10.1093/gerona/63.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn SD. On the future of genomic data. Science. 2011;331:728–729. doi: 10.1126/science.1197891. [DOI] [PubMed] [Google Scholar]
- 7.Hu D. Pawlikowska L. Kanaya A. Hsueh WC. Colbert L. Newman AB. Satterfield S. Rosen C. Cummings SR. Harris TB. Ziv E Health, Aging, Body Composition Study. Serum insulin-like growth factor-1 binding proteins 1 and 2 and mortality in older adults: The Health, Aging, and Body Composition Study. J Am Geriatr Soc. 2009;57:1213–1218. doi: 10.1111/j.1532-5415.2009.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poehls J. Wassel CL. Harris TB. Havel PJ. Swarbrick MM. Cummings SR. Newman AB. Satterfield S. Kanaya AM Health, Aging, Body Composition Study. Association of adiponectin with mortality in older adults: The Health, Aging, and Body Composition Study. Diabetologia. 2009;52:591–595. doi: 10.1007/s00125-009-1261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman AB. Simonsick EM. Naydeck BL. Boudreau RM. Kritchevsky SB. Nevitt MC. Pahor M. Satterfield S. Brach JS. Studenski SA. Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 10.Shahar DR. Yu B. Houston DK. Kritchevsky SB. Lee JS. Rubin SM. Sellmeyer DE. Tylavsky FA. Harris TB Health, Aging, Body Composition Study. Dietary factors in relation to daily activity energy expenditure and mortality among older adults. J Nutr Health Aging. 2009;13:414–420. doi: 10.1007/s12603-009-0077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li HY. Jiang YD. Chang TJ. Wei JN. Lin MS. Lin CH. Chiang FT. Shih SR. Hung CS. Hua CH. Smith DJ. Vanio J. Chuang LM. Serum vascular adhesion protein-1 predicts 10-year cardiovascular and cancer mortality in individuals with type 2 diabetes. Diabetes. 2011;60:993–999. doi: 10.2337/db10-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Searle SD. Mitnitski A. Gahbauer EA. Gill TM. Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ensrud KE. Ewing SK. Cawthon PM. Fink HA. Taylor BC. Cauley JA. Dam TT. Marshall LM. Orwoll ES. Cummings SR Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–498. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heagerty PJ. Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61:92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- 15.Simonsick EM. Newman AB. Nevitt MC. Kritchevsky SB. Ferrucci L. Guralnik JM. Harris T Health, Aging, Body Composition Study. Measuring higher level physical function in well-functioning older adults: Expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 16.Swan GE. Carmelli D. LaRue A. Performance on the digit symbol substitution test and 5-year mortality in the Western Collaborative Group Study. Am J Epidemiol. 1995;141:32–40. doi: 10.1093/oxfordjournals.aje.a117342. [DOI] [PubMed] [Google Scholar]
- 17.Rosano C. Newman AB. Katz R. Hirsch CH. Kuller LH. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc. 2008;56:1618–1625. doi: 10.1111/j.1532-5415.2008.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavlik VN. de Moraes SA. Szklo M. Knopman DS. Mosley TH., Jr Hyman DJ. Relation between cognitive function and mortality in middle-aged adults: the atherosclerosis risk in communities study. Am J Epidemiol. 2003;157:327–334. doi: 10.1093/aje/kwf209. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP. Kronmal RA. Newman AB. Bild DE. Mittelmark MB. Polak JF. Robbins JA. Gardin JM. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 20.Matarazzo JD. Herman DO. Base rate data for the WAIS-R: Test-retest stability and VIQ-PIQ differences. J Clin Neuropsychol. 1984;6:351–366. doi: 10.1080/01688638408401227. [DOI] [PubMed] [Google Scholar]
- 21.Knopman DS. Mosley TH. Catellier DJ. Coker LH Atherosclerosis Risk in Communities Study Brain MRI Study. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: The ARIC MRI Study. Alzheimers Dement. 2009;5:207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Filková M. Haluzík M. Gay S. Senolt L. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin Immunol. 2009;133:157–170. doi: 10.1016/j.clim.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Shlipak MG. Wassel Fyr CL. Chertow GM. Harris TB. Kritchevsky SB. Tylavsky FA. Satterfield S. Cummings SR. Newman AB. Fried LF. Cystatin C and mortality risk in the elderly: The health, aging, and body composition study. J Am Soc Nephrol. 2006;17:254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 24.Shlipak MG. Sarnak MJ. Katz R. Fried LF. Seliger SL. Newman AB. Siscovick DS. Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 25.Keller CR. Odden MC. Fried LF. Newman AB. Angleman S. Green CA. Cummings SR. Harris TB. Shlipak MG. Kidney function and markers of inflammation in elderly persons without chronic kidney disease: the health, aging, and body composition study. Kidney Int. 2007;71:239–244. doi: 10.1038/sj.ki.5002042. [DOI] [PubMed] [Google Scholar]
- 26.Wenger NS. Solomon DH. Roth CP. MacLean CH. Saliba D. Kamberg CJ. Rubenstein LZ. Young RT. Sloss EM. Louie R. Adams J. Chang JT. Venus PJ. Schnelle JF. Shekelle PG. The quality of medical care provided to vulnerable community-dwelling older patients. Ann Intern Med. 2003;139:740–747. doi: 10.7326/0003-4819-139-9-200311040-00008. [DOI] [PubMed] [Google Scholar]
- 27.Higashi T. Shekelle PG. Adams JL. Kamberg CJ. Roth CP. Solomon DH. Reuben DB. Chiang L. MacLean CH. Chang JT. Young RT. Saliba DM. Wenger NS. Quality of care is associated with survival in vulnerable older patients. Ann Intern Med. 2005;143:274–281. doi: 10.7326/0003-4819-143-4-200508160-00008. [DOI] [PubMed] [Google Scholar]
- 28.Solomon DH. Geriatric assessment: Methods for clinical decision making. JAMA. 1988;259:2450–2452. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.