Abstract
Engineering functional cartilaginous grafts using stem cells isolated from osteoarthritic human tissue is of fundamental importance if autologous tissue engineering strategies are to be used in the treatment of diseased articular cartilage. It has previously been demonstrated that human infrapatellar fat pad (IFP)–derived stem cells undergo chondrogenesis in pellet culture; however, the ability of such cells to generate functional cartilaginous grafts has not been adequately addressed. The objective of this study was to explore how environmental conditions regulate the functional development of cartilaginous constructs engineered using diseased human IFP–derived stem cells (FPSCs). FPSCs were observed to display a diminished chondrogenic potential upon encapsulation in a three-dimensional hydrogel compared with pellet culture, synthesizing significantly lower levels of glycosaminoglycan and collagen on a per cell basis. To engineer more functional cartilaginous grafts, we next explored whether additional biochemical and biophysical stimulations would enhance chondrogenesis within the hydrogels. Serum stimulation was observed to partially recover the diminished chondrogenic potential within hydrogel culture. Over 42 days, stem cells that had first been expanded in a low-oxygen environment proliferated extensively on the outer surface of the hydrogel in response to serum stimulation, assembling a dense type II collagen–positive cartilaginous tissue resembling that formed in pellet culture. The application of hydrostatic pressure did not further enhance extracellular matrix synthesis within the hydrogels, but did appear to alter the spatial accumulation of extracellular matrix leading to the formation of a more compact tissue with superior mechanically functionality. Further work is required in order to recapitulate the environmental conditions present during pellet culture within scaffolds or hydrogels in order to engineer more functional cartilaginous grafts using human osteoarthritic FPSCs.
Introduction
Critical-sized defects to the articular surface of synovial joints generally do not heal and if left untreated lead to the development of osteoarthritis (OA).1 This has led to increased interest in cell-based therapies for articular cartilage regeneration. Autologous chondrocyte implantation (ACI) has been used with reasonable success to treat focal lesions in the knee joint2,3; however, it is still unclear whether ACI is superior to other treatment strategies.4–6 Further, the ACI technique suffers from a number of technical disadvantages, including the need to biopsy regions of the undamaged joint surface to isolate chondrocytes and the de-differentiation of these chondrocytes during monolayer expansion.7–9 This has led to increased interest in the use of mesenchymal stem cells (MSCs) for the treatment of damaged and diseased articular cartilage.10 MSCs have been isolated from multiple different tissues, including bone marrow,11–13 adipose tissue,14–17 synovium,18–21 and infrapatellar fat pad22–26 among others. These cells have been incorporated into different types of scaffolds27,28 and hydrogels29–38 in attempts to tissue engineer cartilaginous grafts, with different combinations of growth factors39,40 and biophysical cues, such as compression41 and hydrostatic pressure (HP),42 used to improve the functionality of the construct.
MSCs are beginning to be used clinically in the treatment of focal cartilage defects.43,44 While the use of MSCs has been shown to improve repair compared with untreated controls, critically normal hyaline cartilage does not regenerate, rather a hyaline-like or fibro-cartilagenous tissue forms.45–47 Chondrogenic predifferentiation of MSCs within collagen gels, leading to improved functionality of the engineered graft prior to implantation, has been shown to improve the outcome in long-term sheep studies,48 resulting in superior repair compared with undifferentiated MSCs or chondrocytes.49 While promising, the quality of repair observed in these animal model studies using predifferentiated MSCs is still variable,48,49 suggesting that engineering a more functional graft may be required prior to implantation. Further, it remains to be elucidated whether such predifferentiated cartilaginous grafts can be engineered using stem cells isolated from diseased human donors. If autologous MSCs are to be ultimately used for regeneration of damaged or diseased articular cartilage in osteoarthritic joints, then it will be necessary to first identify the optimal culture conditions that lead to the development of functional cartilaginous tissues using such cell sources.
Human infrapatellar fat pad (IFP) contains multipotent stem cells (FPSCs) that can be easily harvested arthroscopically,23 and that possess at least comparable chondrogenic capacity to chondrocytes isolated from adult articular cartilage.24 We have previously demonstrated that functional cartilage tissue can be engineered using immature porcine FPSCs32,33,35; however, it remains unclear whether such grafts can be engineered using stem cells isolated from human osteoarthritic IFP tissue. The IFP of patients with OA has been shown to have an inflammatory phenotype,50 which may impact the ability of stem cells isolated from this tissue to generate a functional cartilaginous tissue. The objective of this study was to first compare chondrogenesis of FPSCs in pellet culture to that in agarose hydrogels that are commonly used to engineer functional cartilaginous grafts. The influence of the oxygen tension during monolayer expansion of FPSCs on the subsequent properties of these cartilaginous grafts was assessed. We then explored how biochemical (serum) and biophysical (HP) cues would influence the functional development of cartilage tissues engineered using human FPSCs.
Materials and Methods
Cell isolation and expansion
Ethical approval for the study was obtained from the institutional review board of the Mater Misericordiae University Hospital with IFP tissue being obtained from five patients with knee OA at joint arthroplasty (1 male and 4 females, 50–79 years old). Fat pad was maintained in sterile phosphate-buffered saline (PBS) and transferred immediately to the Trinity Centre for Bioengineering for further processing. Fibrous tissue was carefully removed from the fat pad. Remaining tissue was weighed, washed thoroughly in PBS, and diced followed by incubation under constant rotation at 37°C with high-glucose Dulbecco's modified Eagle's medium (hgDMEM; GlutaMAX™) (GIBCO, Biosciences) containing 1% penicillin (100 U/mL)–streptomycin (100 μg/mL) and collagenase type II (4 mL solution/g tissue, 750 U/mL; Worthington Biochemical, LanganBach Services) for 4 h. Cells were filtered through serial cell sieves (Falcon, Sarstedt) with pore sizes from 100 μm, 70 μm, to 40 μm. The isolated FPSCs were seeded at a density of 5000 cells/cm2 and cultured in expansion medium [hgDMEM GlutaMax supplemented with 10% v/v fetal bovine serum (FBS) and penicillin (100 U/mL)–streptomycin (100 μg/mL) (all from Gibco, Biosciences)]. The expansion medium was also supplemented with 5 ng/mL fibroblast growth factor-2 (FGF-2) (Prospect-Tany TechnoGene Ltd.) that has previously been shown to enhance proliferation and chondrogenesis of porcine FPSCs.51 Cells were expanded to passage two (P2) and the oxygen level during cell expansion was maintained at either 20% or 5% O2. All cultures in this study were kept at 37°C. For the HP study (described below in 'HP Loading’ section), a superlot of FPSCs pooled from three different donors was used. For studies other than the HP Loading study, FPSCs isolated from individual donors were used, with replicate studies undertaken using FPSCs isolated from separate donors undertaken as described in the Results section.
Colony-forming unit–fibroblast assay
For the colony-forming unit–fibroblast (CFU-F) assay, freshly isolated cells were plated in 58 cm2 petri dishes at a density of 135 cells/cm2 and maintained with or without FGF-2 (5 ng/mL) at either 20% O2 or 5% O2. Triplicate dishes were plated for all conditions. After 12–14 days, cells were fixed with 2% paraformaldehyde (PFA), stained with 1% crystal violet (Sigma-Aldrich), and colony numbers (>50 cells) were counted. Total colony number and colony diameter were determined using ImageJ software (Rasband, W.S., Image J, U.S. National Institutes of Health, Bethesda, MD). For the purposes of comparison between groups, colony size was defined as the average diameter of the 10 largest colonies formed.
Chondrogenesis in pellet culture
Pellets were formed from culture-expanded cells by centrifuging 250,000 cells (P2) in 1.5 mL conical microtubes at 650 g for 5 min. The pellets were maintained in chondrogenic medium (CM): hgDMEM GlutaMax supplemented with penicillin (100 U/mL)–streptomycin (100 μg/mL) (Invitrogen), 100 μg/mL sodium pyruvate, 40 μg/mL L-proline, 50 μg/mL L-ascorbic acid-2-phosphate, 4.7 μg/mL linoleic acid, 1.5 mg/mL bovine serum albumin, 1× insulin-transferrin-selenium, and 100 nM dexamethasone (all from Sigma-Aldrich). The CM was additionally supplemented with 10 ng/mL recombinant human transforming growth factor-beta3 (TGF-β3; Prospect-Tany TechnoGene Ltd.) or 10 ng/mL TGF-β3+10% FBS (Lot no. 10270; Gibco). Each pellet was cultured in 1 mL of medium and the medium was changed twice a week. The oxygen level for pellet culture was maintained at either 20% O2 or 5% O2. All pellets were cultured for 3 weeks with pellets assessed at days 0 and 21.
Chondrogenesis in agarose hydrogels
FPSCs (P2) were suspended in 2% agarose hydrogels (type VII; Sigma-Aldrich). All hydrogels were seeded at a density of 10×106 cells/mL, except for the agarose gels subjected to HP (both free swelling [FS] controls and loaded constructs), which were seeded at a higher density of 20×106 cells/mL in an attempt to improve the overall functionality of the construct. The agarose cell suspension was cast in a stainless steel mould and cored using a biopsy punch to produce construct cylinders (Ø5mm×1.5 mm). Constructs were maintained in CM at 5% O2 for a period of 42 days with medium exchanges performed twice weekly. Gel samples were assessed at days 0, 21, and 42.
HP loading
Gel constructs were subjected to HP at a magnitude of 10 MPa and a frequency of 1 Hz for 2 h/day and 5 days/week for the final 4 weeks of the 6-week differentiation period in a custom HP bioreactor.42 Six cell-seeded gels were transferred to sterilized, heat-sealed bags filled with 15 mL of medium for the 2-h loading period. The bags for HP loading were placed into a water-filled pressure vessel while FS control groups (also contained in heat-sealed bags) were placed in an open water bath, both maintained at 37°C. After 2 h, all constructs were removed from heat-sealed bags and cultured in tissue culture polysterene plate at 37°C and 5% O2.
Mechanical analysis
Constructs were mechanically assessed using a protocol described previously.52 Briefly, constructs from each group were tested in unconfined compression between impermeable platens using standard Zwick testing machine with a 5 N load cell (Zwick Z005; Roell). A ramp and hold cycle with a ramp displacement of 1 μm/s until 10% strain was applied and maintained until equilibrium was reached (30 min). At this point, dynamic tests were performed with a cyclical strain amplitude of 1% at 1 Hz. Dynamic moduli were calculated as the ratio of the determined stress amplitude to the applied strain amplitude.
Biochemical analysis
Pellet and gel samples were digested in papain (125 μg/mL) in 0.1 M sodium acetate, 5 mM cysteine HCl, and 0.05 M EDTA (pH 6.0) (all from Sigma-Aldrich) at 60°C under constant rotation for 18 h. Total pellet DNA content was measured using a Quant-iT™ PicoGreen® dsDNA kit (Molecular Probes, Biosciences) with a lambda DNA standard and the DNA content of gels was quantified using the Hoechst Bisbenzimide 33258 dye assay (Sigma-Aldrich). Proteoglycan content was estimated by quantifying the amount of sulfated glycosaminoglycan (sGAG) in constructs using the dimethylmethylene blue dye-binding assay (Blyscan, Biocolor Ltd.), with a chondroitin sulfate standard. Total collagen content was determined by measuring the hydroxyproline content. Samples were hydrolyzed at 110°C for 18 h in concentrated HCL (38%) and assayed using a chloramine-T assay53 with a hydroxyproline-to-collagen ratio of 1:7.69.54
Histology and immunohistochemistry
Pellets and gels were fixed in 4% PFA, embedded in paraffin, and sectioned (5 μm). Sections were stained with 1% alcian blue 8GX (Sigma-Aldrich) in 0.1 M HCl for sGAG, and picrosirius red for collagen. The deposition of collagen types I and II was identified through immunohistochemistry.42,55 Briefly, sections were quenched of peroxidase activity for 20 min (PBS was used to rinse sections between steps) and treated with 0.25 U/mL chondroitinase ABC (Sigma) in a humidified environment at 37°C for 1 h to enhance permeability of the extracellular matrix by removal of chondroitin sulfate. After incubation with 10% goat serum to block nonspecific sites, the primary antibody of mouse monoclonal anti-collagen type I diluted 1:400 or mouse monoclonal anti-collagen type II diluted 1:100 (Abcam) was applied for 1 h at room temperature. Then, the secondary antibody (anti-mouse IgG biotin conjugate; Sigma-Aldrich) was added for another hour. Color was developed using the Vectastain ABC reagent (Vectastain ABC kit; Vector Laboratories) for 45 min and exposure to peroxidase DAB substrate kit (Vector laboratories) for 5 min. Slides were dehydrated through ethanol and xylene and mounted with Vectamount medium (Vector Laboratories). Human ligament and cartilage were included as controls for collagen type I and collagen type II, respectively.
Statistics
Numerical and graphical results are presented as mean±standard deviation (3–4 samples). Statistics were performed using R (The R Foundation for Statistical Computing, Vienna, Austria). Groups were analyzed for significant differences using a linear model for analysis of variance with multiple factors and interactions between these factors were also examined. Tukey's HSD test for multiple comparisons was used as post-tests. Significance was accepted at a level of p<0.05.
Results
The chondrogenic potential of FPSCs is diminished in hydrogel culture in comparison to pellet culture
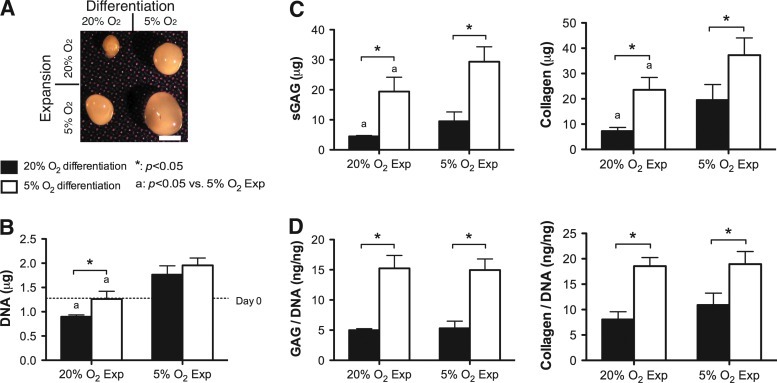
As we have reported previously,56 expansion of FPSCs in the presence of FGF-2 significantly reduced the CFU-F colony number and increased the colony diameter (Supplementary Fig. S1 A, B; Supplementary Data are available online at www.liebertonline.com/tea). Further, the colony diameter in FGF-2-expanded stem cells was significantly larger when maintained at 5% O2 compared with 20% O2. Following expansion to passage 2, stem cells expanded in the presence of FGF-2 were differentiated at either 5% O2 or 20% O2 using a pellet culture model. Stem cells expanded at 5% O2 formed larger pellets (Fig. 1A) and were more proliferative during chondrogenesis, as evident by a higher DNA content at day 21 (Fig. 1B), with total sGAG and collagen accumulation greatest for cells expanded and differentiated at 5% O2 (Fig. 1C). When normalized to DNA content the levels of sGAG and collagen within the pellets were comparable for stem cells expanded at either 5% O2 or 20% O2, but were always greatest for differentiation at 5% O2 (Fig. 1D). These general results were observed for stem cells isolated from other donors, with the only exception being that for some donors differentiation at 5% O2 was found to suppress total collagen production (see Supplementary Fig. S2).
FIG. 1.
The effect of oxygen tension during expansion and differentiation on chondrogenesis of infrapatellar fat pad–derived stem cells (FPSCs) in pellet culture for 21 days. Pellet morphology (A), DNA content (B), sulfated glycosaminoglycan (sGAG) and collagen content (C), and GAG/DNA and collagen/DNA (D). Scale bar, 1 mm. “*” indicates a significant difference between FPSCs undergoing chondrogenesis at either 5% or 20% O2, where all other culture conditions during differentiation were identical. “a” indicates a significant difference between FPSCs expanded at either 20% or 5% O2, where all other culture conditions during differentiation were identical. Color images available online at www.liebertonline.com/tea
In an attempt to engineer a cartilaginous graft, FPSCs were next seeded into agarose hydrogels and the levels of matrix accumulation (on a per cell basis) after 21 days in culture at 5% O2 were compared with that in pellets. Both sGAG and collagen syntheses were lower in hydrogel culture in comparison to pellet culture, with noticeably weak staining for both alcian blue and type II collagen, despite the attainment of a spherical cellular morphology within the hydrogel system (Fig. 2). This was observed for stem cells expanded at either 5% O2 or 20% O2. Further, the greater proliferative potential of stem cells expanded at 5% O2 that was observed in pellet culture was diminished in hydrogel culture.
FIG. 2.
A comparison of the chondrogenic potential of FPSCs maintained in pellets or in agarose hydrogels (10 million cells/mL) for 21 days. (A) DNA content, GAG/DNA, and collagen/DNA. (B) Alcian blue staining, picrosirius red staining, and collagen II immunostaining of the pellets and hydrogels expanded at 20% O2. Scale bar, 500 μm. “*” indicates a significant difference between FPSCs expanded at either 20% or 5% O2, where all other culture conditions during differentiation were identical. “b” indicates a significant difference between pellets and hydrogels maintained in otherwise identical environmental conditions. Color images available online at www.liebertonline.com/tea
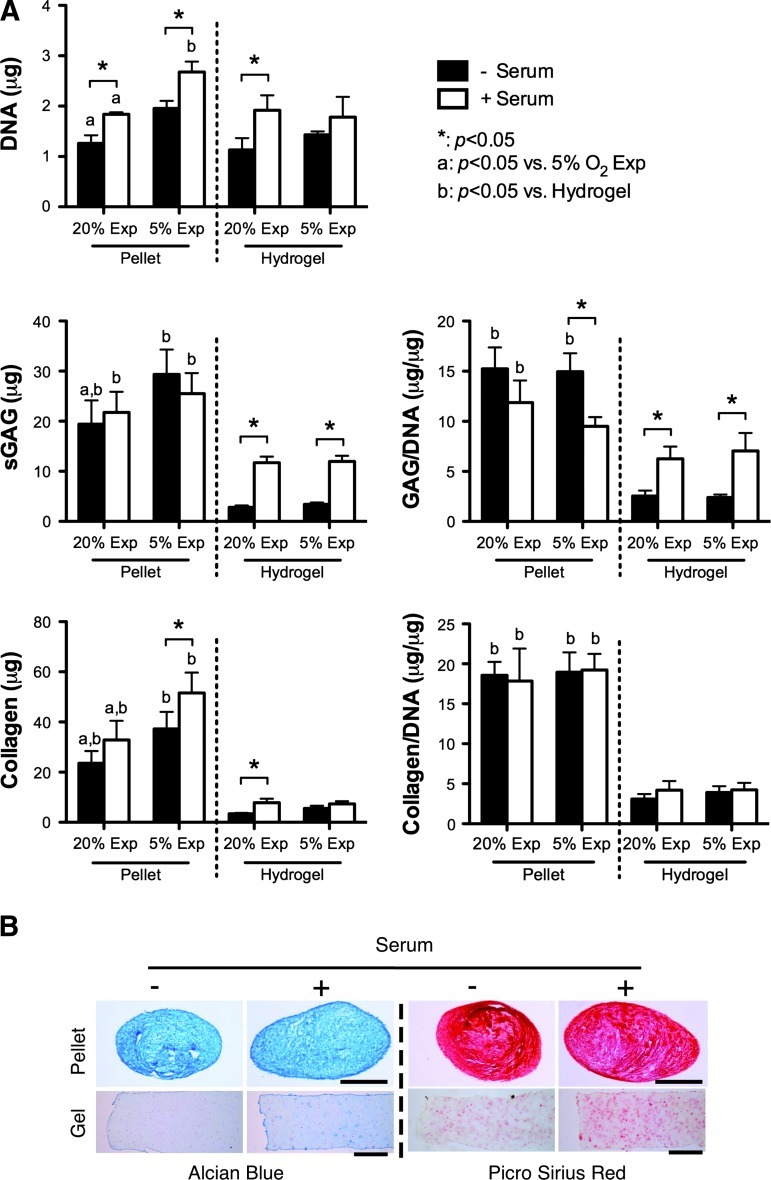
Serum stimulation promotes cell proliferation in both pellets and hydrogels and enhances matrix accumulation within hydrogels
In an attempt to provide a further stimulus to FPSCs embedded in agarose hydrogels to synthesize cartilage-specific extracellular matrix, constructs were additionally stimulated with 10% serum. In pellet culture this led to greater cell proliferation but no increase in matrix synthesis (Fig. 3A). In contrast, the presence of serum significantly enhanced sGAG synthesis within cell-seeded hydrogels, although synthesis levels were still lower than that observed in pellets. Collagen synthesis within hydrogels, when normalized to DNA content, was not affected by serum supplementation. Again, the oxygen levels during expansion did not appear to affect levels of matrix accumulation within cell-seeded hydrogels over 21 days of in vitro culture.
FIG. 3.
Effect of serum on FPSCs maintained in pellets or in agarose hydrogels (10 million cells/mL) for 21 days. (A) DNA content, sGAG content, collagen content, GAG/DNA, and collagen/DNA. (B) Alcian blue staining and picrosirius red staining. Scale bar, 500 μm. “*” indicates a significant difference between cultures maintained in the presence or absence of serum, where all other culture conditions during differentiation were identical. “a” indicates a significant difference between FPSCs expanded at either 20% or 5% O2, where all other culture conditions during differentiation were identical. “b” indicates a significant difference between chondrogenesis in pellets and hydrogels for FPSCs maintained in otherwise identical environmental conditions. Color images available online at www.liebertonline.com/tea
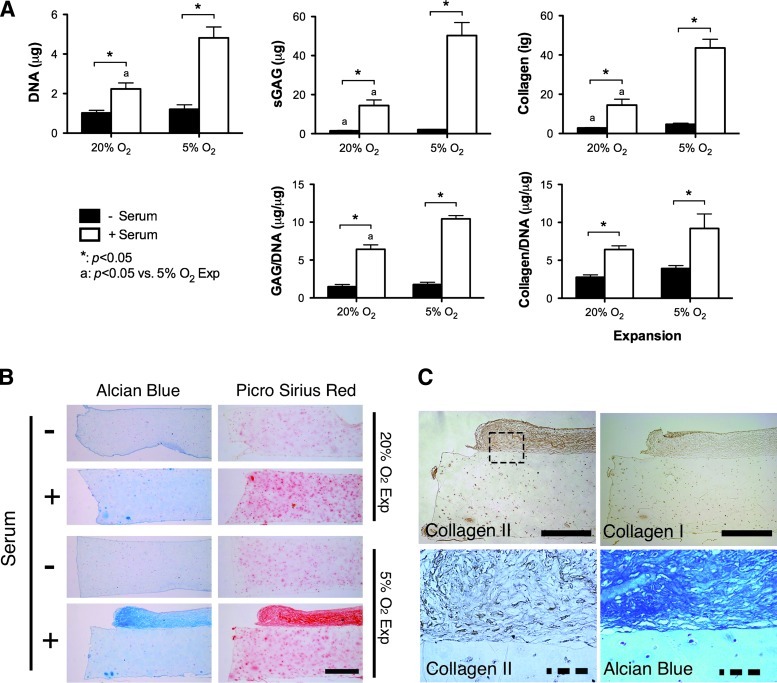
Oxygen conditions during the expansion of FPSCs influences the long-term development of cartilaginous grafts
We next explored the development of cartilaginous grafts engineered using FPSCs embedded in agarose hydrogels over a longer period of 42 days of in vitro culture. Unlike the findings for constructs cultured for 21 days, the DNA content within hydrogels dramatically increased for stem cells that were first expanded at 5% O2 and differentiated in the presence of serum (Fig. 4A), mirroring the findings first observed in pellet culture (Fig. 3). Expansion at 5% O2 also led to noticeably higher levels of total GAG and collagen accumulation within constructs supplemented with serum. Histological analysis of these constructs revealed that these higher levels of extracellular matrix accumulation were not due to dramatically greater synthesis within the hydrogels, but rather due to the formation of a dense layer of highly cellular cartilaginous tissue that formed on the surface of these constructs (Fig. 4B). This self-assembled tissue that formed on top of the hydrogels stained positively for type II collagen and weakly for type I collagen (Fig. 4C).
FIG. 4.
Effect of serum on the FPSCs maintained in agarose hydrogels (10 million cells/mL) for 42 days. (A) DNA, sGAG, collagen content, GAG/DNA, and collagen/DNA. (B) Alcian blue staining and picrosirius red staining. (C) A high-magnification view of a region (dashed-box) of a hydrogel seeded with FPSCs expanded at 5% O2 and differentiated in the presence of serum, demonstrating the formation of a dense layer of cartilaginous tissue on the surface of the hydrogel. Solid scale bar, 500 μm; dash scale bar, 100 μm. “*” indicates a significant difference between cultures maintained in the presence or absence of serum, where all other culture conditions during differentiation were identical. “a” indicates a significant difference between FPSCs expanded at either 20% or 5% O2, where all other culture conditions during differentiation were identical. Color images available online at www.liebertonline.com/tea
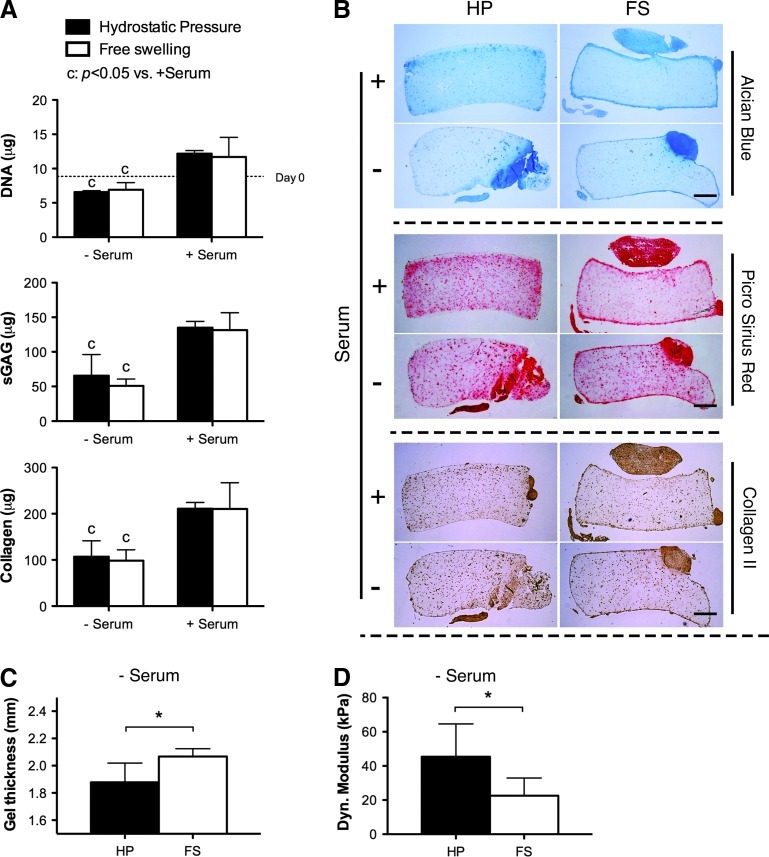
The application of HP enhances the mechanical functionality of cartilaginous grafts by generating a more compact tissue
In an attempt to further enhance chondrogenesis, cell-seeded hydrogels were subjected to 10 MPa of cyclic HP (1 Hz for 2 h/day, 5 days/week). HP did not influence total DNA, sGAG, or collagen content within the hydrogels, although it did appear to lead to a more homogeneous matrix distribution in gel constructs, with staining within the hydrogel appearing slightly more intense in constructs subjected to HP (Fig. 5B). Significant cartilaginous tissue outgrowth was observed on the surface of the hydrogels, particularly those additionally supplemented with serum. In the absence of serum, HP resulted in constructs of smaller height (Fig. 5C). When mechanically evaluated, these constructs were stiffer than controls maintained in FS conditions (Fig. 5D).
FIG. 5.
Effect of hydrostatic pressure and serum on chondrogenesis of FPSCs maintained in agarose hydrogels (20 million cells/mL) for 6 weeks. Hydrostatic pressure loading was applied for the final 4 weeks of culture. (A) DNA, sGAG, and collagen content. (B) Alcian blue staining, picrosirius red staining, and collagen II immunostaining. Hydrogels subjected to hydrostatic pressure had a lower thickness (C) and higher dynamic modulus (D). Scale bar, 0.5 mm. The average dynamic modulus of acellular agarose hydrogels is 39 kPa. “c” indicates a significant difference between constructs maintained in the presence or absence of serum, where all other culture conditions during differentiation were identical. “*” indicates a significant difference between constructs subjected to hydrostatic pressure or maintained in free swelling conditions. Color images available online at www.liebertonline.com/tea
Discussion
Chondrogenic priming of MSCs to generate a more functional cartilage-like graft prior to implantation has been shown to improve the quality of repair in animal model studies of cartilage regeneration.48,49 Translating such therapies to treat damaged and diseased articular cartilage in man will most likely necessitate the development of tissue engineering strategies to generate functional cartilaginous grafts using autologous stem cells. The objective of this study was to explore how environmental factors during both the expansion and differentiation of diseased human FPSCs would influence their chondrogenic capacity following encapsulation into three-dimensional hydrogels. Despite previous findings that agarose hydrogels support robust chondrogenesis of stem cells isolated from skeletally immature animals,32,33,35 we found that the chondrogenesis of diseased human FPSCs was diminished in these hydrogels compared with pellet culture. Stimulation of the cell-seeded hydrogels with potent biochemical (serum) or biophysical (HP) stimuli did lead to improvements in cartilaginous matrix synthesis and construct functionality, respectively, although the inherent chondrogenic capacity observed in the pellet environment was never recapitulated.
When expanded in the presence of FGF-2, FPSCs maintained at 5% O2 formed larger colonies, suggesting a greater proliferative potential at this lower oxygen tension. Enhanced proliferation has previously been reported for MSCs maintained at a low oxygen tension.57–60 This proliferative potential during monolayer expansion was maintained during chondrogenic differentiation, both in pellet culture, but also in hydrogel culture once FPSCs were stimulated with serum. FPSCs did not appear to proliferate within the hydrogel, but rather on the construct surface. Cells cannot directly adhere to agarose, which coupled with the fact that the FPSCs are completely encapsulated within the hydrogel leads to limited proliferation. However, FPSCs can potentially migrate out onto the hydrogel surface, adhere to newly synthesized extracellular matrix, and then proliferate as they are no longer encapsulated in agarose, a process that appears to be accelerated in the presence of serum. Similar cellular outgrowth and capsule formation has been observed in chondrocyte-seeded hydrogels supplemented with serum.61,62 Interestingly, previous studies have reported that these layers are rich in type I collagen and therefore termed fibrous capsules, although the layers formed by the FPSCs stain positively for type II collagen, suggesting that specific experimental factors, such as the presence of TGF-β3 in the medium or maintenance at a low oxygen tension during differentiation, promoted a more cartilaginous phenotype.
Encapsulation of FPSCs in a three-dimensional hydrogel led to a diminished chondrogenic potential compared with pellet culture. It is well established that agarose can help support a chondrogenic phenotype for culture-expanded chondrocytes,7 and has been widely used as a hydrogel for cartilage tissue engineering using chondrocytes,63–65 bone-marrow-derived MSCs,66,67 adipose-derived stem cells,31 and FPSCs,33,35,55,68 although many of these studies used cells isolated from young animals. A key difference between the high-density pellet culture system11 and hydrogel encapsulation in agarose is the difference in cell–cell and cell–matrix interactions. Cellular condensation and expression of proteins associated with cell–cell interactions, such as N-cadherin, are known to be important at the onset of chondrogenesis.69,70 The pellet culture system also supports paracrine signaling important for chondrogenesis. The absence of ligands within the agarose hydrogel to facilitate integrin-mediated binding of FPSCs to their local substrate may also play a role in diminishing their chondrogenic potential. Alternative approaches to engineering functional cartilaginous grafts that recapitulate aspects of the pellet culture system, such as the self-assembly process,71 may provide promising approaches when using diseased human FPSCs.
Serum has previously been shown to influence the growth and development of engineered cartilaginous constructs.61,72–76 Supplementing the CM with serum was observed to enhance cartilaginous extracellular matrix accumulation in FPSC-seeded hydrogels, although synthesis levels on a per cell basis were still lower than that in pellet culture. The abundance of growth factors present in serum, such as TGF-β superfamily, insulin-like growth factor-1, and platelet-derived growth factor, may be playing a role in this enhanced biosynthetic activity. Serum also contains adhesive glycoproteins, such as fibronectin,77,78 which have numerous biological activities, including mediating cell–cell adhesion and enhancing cell–substrate anchoring and spreading.79 Further, by facilitating the proliferation of FPSCs on the surface of the hydrogels, serum may also be indirectly enhancing chondrogenesis by allowing greater cell–cell interactions in this region of the engineered tissue, thereby mimicking aspects of the pellet culture system known to support more robust chondrogenensis of diseased human FPSCs.
The application of HP did not enhance overall levels of matrix accumulation within FPSC-seeded constructs. This is in contrast to the results of previous studies demonstrating that cyclic HP can enhance chondrogenesis of bone marrow42,80–85 and adipose-derived86 stem cells. These discrepancies could potentially be explained by differences in phenotype between stem cells isolated from different tissues, or alternatively due to differences in cell–cell and cell–scaffold interactions between different studies that may play a central role in mechano-transduction of biophysical signals such as HP. The cell-seeding density can also impact the response to biophysical cues,87,88 and may potentially explain discrepancies in reported responses to HP. It should be noted, however, that while the application of HP did not increase overall levels of matrix accumulation, it did appear to influence the spatial distribution of matrix within the construct. HP has previously been shown to result in the formation of more compact pellets generated using bone-marrow-derived MSCs,84 and influence the spatial accumulation of matrix in MSC-seeded hydrogels.42 Histological analysis suggests that the application of HP preferentially supports the accumulation of matrix components within the hydrogel as opposed to on the construct surface. In hydrogels not additionally stimulated with serum, the application of HP was also observed to suppress outgrowth and/or swelling of the construct, as evident by a smaller construct thickness. This may explain why these constructs were stiffer than their FS counterparts, as compaction of an engineered cartilaginous tissue can lead to improvements in biomechanical functionality.89 Therefore the application of cues, such as HP, may play a key role in mechanically priming cartilaginous constructs engineered using FPSCs prior to implantation. Further optimization of these environmental conditions is required as the mechanical properties of these engineered grafts are still two orders of magnitude lower than normal articular cartilage.
In conclusion, we have demonstrated that encapsulation of diseased human FPSCs into agarose hydrogels in an attempt to engineer a functional cartilaginous graft can suppress their inherent chondrogenic potential. While this can be partially recovered through the application of biochemical or biophysical stimuli, their chondrogenic capacity is still diminished, and the resulting mechanical properties of the engineered grafts are still noticeably lower than native articular cartilage. Further work is required in order to recapitulate the environmental conditions present during pellet culture within hydrogels or scaffolds in order to engineer fully functional cartilaginous grafts using stem cells derived from human osteoarthritic IFP tissue.
Supplementary Material
Acknowledgments
Funding for this study was provided by Irish Research Council for Science, Engineering & Technology under enterprise partner scheme with Sports Surgery Clinic Dublin (IRCSET-SSC-2010-01), a European Research Council Starter Grant (StemRepair–Project number: 258463), and a President of Ireland Young Researcher Award (08/Y15B1336). The authors would like to thank Andrew Steward for his technical support with HP loading.
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Schinhan M. Gruber M. Vavken P. Dorotka R. Samouh L. Chiari C. Gruebl-Barabas R. Nehrer S. Critical-size defect induces unicompartmental osteoarthritis in a stable ovine knee. J Orthop Res. 2012;30:214. doi: 10.1002/jor.21521. [DOI] [PubMed] [Google Scholar]
- 2.Brittberg M. Lindahl A. Nilsson A. Ohlsson C. Isaksson O. Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 3.Peterson L. Minas T. Brittberg M. Nilsson A. Sjögren-Jansson E. Lindahl A. Two-to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000:212. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Harris J.D. Siston R.A. Pan X. Flanigan D.C. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg. 2010;92:2220. doi: 10.2106/JBJS.J.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruano-Ravina A. Diííaz M.J. Autologous chondrocyte implantation: a systematic review. Osteoarthritis Cartilage. 2006;14:47. doi: 10.1016/j.joca.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Vasiliadis H.S. Wasiak J. Salanti G. Autologous chondrocyte implantation for the treatment of cartilage lesions of the knee: a systematic review of randomized studies. Knee Surg Sports Traumatol Arthrosc. 2010;18:1645. doi: 10.1007/s00167-010-1050-3. [DOI] [PubMed] [Google Scholar]
- 7.Benya P.D. Shaffer J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Romero J. Gaillard J.P. Grogan S.P. Nesic D. Trub T. Mainil-Varlet P. Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol. 2005;202:731. doi: 10.1002/jcp.20164. [DOI] [PubMed] [Google Scholar]
- 9.Barbero A. Grogan S. Schäfer D. Heberer M. Mainil-Varlet P. Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12:476. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Stoddart M. Grad S. Eglin D. Alini M. Cells and biomaterials in cartilage tissue engineering. Regen Med. 2009;4:81. doi: 10.2217/17460751.4.1.81. [DOI] [PubMed] [Google Scholar]
- 11.Johnstone B. Hering T.M. Caplan A.I. Goldberg V.M. Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 12.Yoo J.U. Barthel T.S. Nishimura K. Solchaga L. Caplan A.I. Goldberg V.M. Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg. 1998;80:1745. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 14.Pittenger M.F. Mosca J.D. McIntosh K.R. Human mesenchymal stem cells: progenitor cells for cartilage, bone, fat and stroma. Curr Top Microbiol Immunol. 2000;251:3. doi: 10.1007/978-3-642-57276-0_1. [DOI] [PubMed] [Google Scholar]
- 15.Zuk P.A. Zhu M. Ashjian P. De Ugarte D.A. Huang J.I. Mizuno H. Alfonso Z.C. Fraser J.K. Benhaim P. Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuk P.A. Zhu M. Mizuno H. Huang J. Futrell J.W. Katz A.J. Benhaim P. Lorenz H.P. Hedrick M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 17.Guilak F. Estes B.T. Diekman B.O. Moutos F.T. Gimble J.M. 2010 Nicolas Andry Award: Multipotent adult stem cells from adipose tissue for musculoskeletal tissue engineering. Clin Orthop. 2010;468:2530. doi: 10.1007/s11999-010-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Bari C. Dell'Accio F. Tylzanowski P. Luyten F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi Y. Sekiya I. Yagishita K. Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 20.Pei M. He F. Vunjak-Novakovic G. Synovium-derived stem cell-based chondrogenesis. Differentiation. 2008;76:1044. doi: 10.1111/j.1432-0436.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura K. Solchaga L.A. Caplan A.I. Yoo J.U. Goldberg V.M. Johnstone B. Chondroprogenitor cells of synovial tissue. Arthritis Rheum. 1999;42:2631. doi: 10.1002/1529-0131(199912)42:12<2631::AID-ANR18>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 22.Wickham M.Q. Erickson G.R. Gimble J.M. Vail T.P. Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003:196. doi: 10.1097/01.blo.0000072467.53786.ca. [DOI] [PubMed] [Google Scholar]
- 23.Dragoo J.L. Samimi B. Zhu M. Hame S.L. Thomas B.J. Lieberman J.R. Hedrick M.H. Benhaim P. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br. 2003;85:740. [PubMed] [Google Scholar]
- 24.English A. Jones E.A. Corscadden D. Henshaw K. Chapman T. Emery P. McGonagle D. A comparative assessment of cartilage and joint fat pad as a potential source of cells for autologous therapy development in knee osteoarthritis. Rheumatology. 2007;46:1676. doi: 10.1093/rheumatology/kem217. [DOI] [PubMed] [Google Scholar]
- 25.Khan W.S. Adesida A.B. Hardingham T.E. Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther. 2007;9:R55. doi: 10.1186/ar2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan W.S. Adesida A.B. Tew S.R. Andrew J.G. Hardingham T.E. The epitope characterisation and the osteogenic differentiation potential of human fat pad-derived stem cells is maintained with ageing in later life. Injury. 2009;40:150. doi: 10.1016/j.injury.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 27.Marsano A. Millward-Sadler S.J. Salter D.M. Adesida A. Hardingham T. Tognana E. Kon E. Chiari-Grisar C. Nehrer S. Jakob M. Martin I. Differential cartilaginous tissue formation by human synovial membrane, fat pad, meniscus cells and articular chondrocytes. Osteoarthritis Cartilage. 2007;15:48. doi: 10.1016/j.joca.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Cheng N.C. Estes B.T. Awad H.A. Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2009;15:231. doi: 10.1089/ten.tea.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mauck R.L. Yuan X. Tuan R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14:179. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama A. Sekiya I. Miyazaki K. Ichinose S. Hata Y. Muneta T. In vitro cartilage formation of composites of synovium-derived mesenchymal stem cells with collagen gel. Cell Tissue Res. 2005;322:289. doi: 10.1007/s00441-005-0010-6. [DOI] [PubMed] [Google Scholar]
- 31.Awad H.A. Wickham M.Q. Leddy H.A. Gimble J.M. Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 32.Buckley C.T. Vinardell T. Thorpe S.D. Haugh M.G. Jones E. McGonagle D. Kelly D.J. Functional properties of cartilaginous tissues engineered from infrapatellar fat pad-derived mesenchymal stem cells. J Biomech. 2010;43:920. doi: 10.1016/j.jbiomech.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Buckley C.T. Vinardell T. Kelly D.J. Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived MSCs and articular chondrocytes. Osteoarthritis Cartilage. 2010;18:1345. doi: 10.1016/j.joca.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Erickson I.E. Huang A.H. Chung C. Li R.T. Burdick J.A. Mauck R.L. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15:1041. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinardell T. Buckley C.T. Thorpe S.D. Kelly D.J. Composition-function relations of cartilaginous tissues engineered from chondrocytes and mesenchymal stem cells isolated from bone marrow and infrapatellar fat pad. J Tissue Eng Regen Med. 2011;5:673. doi: 10.1002/term.357. [DOI] [PubMed] [Google Scholar]
- 36.Sheehy E.J. Buckley C.T. Kelly D.J. Chondrocytes and bone marrow-derived mesenchymal stem cells undergoing chondrogenesis in agarose hydrogels of solid and channelled architectures respond differentially to dynamic culture conditions. J Tissue Eng Regen Med. 2011;5:747. doi: 10.1002/term.385. [DOI] [PubMed] [Google Scholar]
- 37.Bahney C.S. Hsu C.W. Yoo J.U. West J.L. Johnstone B. A bioresponsive hydrogel tuned to chondrogenesis of human mesenchymal stem cells. FASEB J. 2011;25:1486. doi: 10.1096/fj.10-165514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buxton A.N. Zhu J. Marchant R. West J.L. Yoo J.U. Johnstone B. Design and characterization of poly(ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells. Tissue Eng. 2007;13:2549. doi: 10.1089/ten.2007.0075. [DOI] [PubMed] [Google Scholar]
- 39.Diekman B.O. Rowland C.R. Lennon D.P. Caplan A.I. Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: Induction by growth factors and cartilage-derived matrix. Tissue Eng Part A. 2010;16:523. doi: 10.1089/ten.tea.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang A.H. Stein A. Tuan R.S. Mauck R.L. Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A. 2009;15:3461. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang A.H. Farrell M.J. Kim M. Mauck R.L. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogels. Eur Cell Mater. 2010;19:72. doi: 10.22203/ecm.v019a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer E.G. Buckley C.T. Steward A.J. Kelly D.J. The effect of cyclic hydrostatic pressure on the functional development of cartilaginous tissues engineered using bone marrow derived mesenchymal stem cells. J Mech Behav Biomed Mater. 2011;4:1257. doi: 10.1016/j.jmbbm.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Wakitani S. Nawata M. Tensho K. Okabe T. Machida H. Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1:74. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]
- 44.Kuroda R. Ishida K. Matsumoto T. Akisue T. Fujioka H. Mizuno K. Ohgushi H. Wakitani S. Kurosaka M. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226. doi: 10.1016/j.joca.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Wakitani S. Goto T. Pineda S.J. Young R.G. Mansour J.M. Caplan A.I. Goldberg V.M. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Butnariu-Ephrat M. Robinson D. Mendes D.G. Halperin N. Nevo Z. Resurfacing of goat articular cartilage by chondrocytes derived from bone marrow. Clin Orthop. 1996:234. doi: 10.1097/00003086-199609000-00031. [DOI] [PubMed] [Google Scholar]
- 47.Dashtdar H. Rothan H.A. Tay T. Ahmad R.E. Ali R. Tay L.X. Chong P.P. Kamarul T. A preliminary study comparing the use of allogenic chondrogenic pre-differentiated and undifferentiated mesenchymal stem cells for the repair of full thickness articular cartilage defects in rabbits. J Orthop Res. 2011;29:1336. doi: 10.1002/jor.21413. [DOI] [PubMed] [Google Scholar]
- 48.Zscharnack M. Hepp P. Richter R. Aigner T. Schulz R. Somerson J. Josten C. Bader A. Marquass B. Repair of chronic osteochondral defects using predifferentiated mesenchymal stem cells in an ovine model. Am J Sports Med. 2010;38:1857. doi: 10.1177/0363546510365296. [DOI] [PubMed] [Google Scholar]
- 49.Marquass B. Schulz R. Hepp P. Zscharnack M. Aigner T. Schmidt S. Stein F. Richter R. Osterhoff G. Aust G. Josten C. Bader A. Matrix-associated implantation of predifferentiated mesenchymal stem cells versus articular chondrocytes: In vivo results of cartilage repair after 1 year. Am J Sports Med. 2011;39:1401. doi: 10.1177/0363546511398646. [DOI] [PubMed] [Google Scholar]
- 50.Klein-Wieringa I.R. Kloppenburg M. Bastiaansen-Jenniskens Y.M. Yusuf E. Kwekkeboom J.C. El-Bannoudi H. Nelissen R.G.H.H. Zuurmond A. Stojanovic-Susulic V. van Osch G.J.V.M. Toes R.E.M. Ioan-Facsinay A. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheum Dis. 2011;70:851. doi: 10.1136/ard.2010.140046. [DOI] [PubMed] [Google Scholar]
- 51.Buckley C.T. Kelly D.J. Expansion in the presence of FGF-2 enhances the functional development of cartilaginous tissues engineered using infrapatellar fat pad derived MSCs. J Mech Behav Biomed Mater. 2011 doi: 10.1016/j.jmbbm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Buckley C.T. Thorpe S.D. O'Brien F.J. Robinson A.J. Kelly D.J. The effect of concentration, thermal history and cell seeding density on the initial mechanical properties of agarose hydrogels. J Mech Behav Biomed Mater. 2009;2:512. doi: 10.1016/j.jmbbm.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Kafienah W. Sims T.J. Biochemical methods for the analysis of tissue-engineered cartilage. Methods Mol Biol. 2004;238:217. doi: 10.1385/1-59259-428-x:217. [DOI] [PubMed] [Google Scholar]
- 54.Ignat'eva N.Y. Danilov N.A. Averkiev S.V. Obrezkova M.V. Lunin V.V. Sobol E.N. Determination of hydroxyproline in tisses and evaluation of the collagen content of the tissues. J Anal Chem. 2006;62:51. [Google Scholar]
- 55.Thorpe S.D. Buckley C.T. Vinardell T. O'Brien F.J. Campbell V.A. Kelly D.J. The response of bone marrow-derived mesenchymal stem cells to dynamic compression following TGF-beta3 induced chondrogenic differentiation. Ann Biomed Eng. 2010;38:2896. doi: 10.1007/s10439-010-0059-6. [DOI] [PubMed] [Google Scholar]
- 56.O'hEireamhoin S. Buckley C.T. Schepens A. Jones E. McGonagle D. Mulhall K.J. Kelly D.J. The Effects of Growth Factor Supplementation and Oxygen Tension during Expansion on Subsequent Chondrogenesis of Human Infrapatellar Fat Pad Derived Mesenchymal Stem Cells. Presented at the Annual Meeting of the Orthopaedic Research Society; Long Beach, California, CA. 2011. [Google Scholar]
- 57.Xu Y. Malladi P. Chiou M. Bekerman E. Giaccia A.J. Longaker M.T. In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue Eng. 2007;13:2981. doi: 10.1089/ten.2007.0050. [DOI] [PubMed] [Google Scholar]
- 58.Das R. Jahr H. van Osch G.J.V.M. Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2010;16:159. doi: 10.1089/ten.TEB.2009.0296. [DOI] [PubMed] [Google Scholar]
- 59.Grayson W.L. Zhao F. Bunnell B. Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 60.Krinner A. Zscharnack M. Bader A. Drasdo D. Galle J. Impact of oxygen environment on mesenchymal stem cell expansion and chondrogenic differentiation. Cell Prolif. 2009;42:471. doi: 10.1111/j.1365-2184.2009.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mauck R.L. Wang C.C.-B. Oswald E.S. Ateshian G.A. Hung C.T. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11:879. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Kisiday J.D. Kurz B. DiMicco M.A. Grodzinsky A.J. Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng. 2005;11:141. doi: 10.1089/ten.2005.11.141. [DOI] [PubMed] [Google Scholar]
- 63.Buschmann M.D. Gluzband Y.A. Grodzinsky A.J. Kimura J.H. Hunziker E.B. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 64.Lee D.A. Bader D.L. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997;15:181. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]
- 65.Mauck R.L. Soltz M.A. Wang C.C. Wong D.D. Chao P.H. Valhmu W.B. Hung C.T. Ateshian G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 66.Huang C.Y. Hagar K.L. Frost L.E. Sun Y. Cheung H.S. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells. 2004;22:313. doi: 10.1634/stemcells.22-3-313. [DOI] [PubMed] [Google Scholar]
- 67.Mauck R.L. Byers B.A. Yuan X. Tuan R.S. Regulation of Cartilaginous ECM Gene Transcription by Chondrocytes and MSCs in 3D Culture in Response to Dynamic Loading. Biomech Model Mechanobiol. 2006;6:113. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 68.Meyer E.G. Buckley C.T. Thorpe S.D. Kelly D.J. Low oxygen tension is a more potent promoter of chondrogenic differentiation than dynamic compression. J Biomech. 2010;43:2516. doi: 10.1016/j.jbiomech.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 69.Oberlender S.A. Tuan R.S. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development. 1994;120:177. doi: 10.1242/dev.120.1.177. [DOI] [PubMed] [Google Scholar]
- 70.Tavella S. Raffo P. Tacchetti C. Cancedda R. Castagnola P. N-CAM and N-cadherin expression during in vitro chondrogenesis. Exp Cell Res. 1994;215:354. doi: 10.1006/excr.1994.1352. [DOI] [PubMed] [Google Scholar]
- 71.Hu J.C. Athanasiou K.A. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 72.Masuda K. Sah R.L. Hejna M.J. Thonar E.J.-M.A. A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: the alginate-recovered-chondrocyte (ARC) method. J Orthop Res. 2003;21:139. doi: 10.1016/S0736-0266(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 73.Glowacki J. Yates K.E. Maclean R. Mizuno S. In vitro engineering of cartilage: effects of serum substitutes, TGF-beta, and IL-1alpha. Orthod Craniofac Res. 2005;8:200. doi: 10.1111/j.1601-6343.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 74.Kelly T.-A.N. Fisher M.B. Oswald E.S. Tai T. Mauck R.L. Ateshian G.A. Hung C.T. Low-serum media and dynamic deformational loading in tissue engineering of articular cartilage. Ann Biomed Eng. 2008;36:769. doi: 10.1007/s10439-008-9476-1. [DOI] [PubMed] [Google Scholar]
- 75.Wang C. Chen L. Kuo P. Chang J. Wang Y. Hung S. Apoptosis in chondrogenesis of human mesenchymal stem cells: effect of serum and medium supplements. Apoptosis. 2010;15:439. doi: 10.1007/s10495-009-0431-x. [DOI] [PubMed] [Google Scholar]
- 76.Bobacz K. Gruber R. Soleiman A. Erlacher L. Smolen J.S. Graninger W.B. Expression of bone morphogenetic protein 6 in healthy and osteoarthritic human articular chondrocytes and stimulation of matrix synthesis in vitro. Arthritis Rheum. 2003;48:2501. doi: 10.1002/art.11248. [DOI] [PubMed] [Google Scholar]
- 77.Yamada K.M. Olden K. Fibronectins—adhesive glycoproteins of cell surface and blood. Nature. 1978;275:179. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]
- 78.Pena S.D. Hughes R.C. Fibronectin-plasma membrane interactions in the adhesion and spreading of hamster fibroblasts. Nature. 1978;276:80. doi: 10.1038/276080a0. [DOI] [PubMed] [Google Scholar]
- 79.Mosesson M.W. Amrani D.L. The structure and biologic activities of plasma fibronectin. Blood. 1980;56:145. [PubMed] [Google Scholar]
- 80.Elder B.D. Athanasiou K.A. Hydrostatic pressure in articular cartilage tissue engineering: From chondrocytes to tissue regeneration. Tissue Eng Part B Rev. 2009;15:43. doi: 10.1089/ten.teb.2008.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo Z.J. Seedhom B.B. Light and low-frequency pulsatile hydrostatic pressure enhances extracellular matrix formation by bone marrow mesenchymal cells: An in-vitro study with special reference to cartilage repair. Proc Inst Mech Eng Part H J Eng Med. 2007;221:499. doi: 10.1243/09544119JEIM199. [DOI] [PubMed] [Google Scholar]
- 82.Wagner D.R. Lindsey D.P. Li K.W. Tummala P. Chandran S.E. Smith R.L. Longaker M.T. Carter D.R. Beaupre G.S. Hydrostatic pressure enhances chondrogenic differentiation of human bone marrow stromal cells in osteochondrogenic medium. Ann Biomed Eng. 2008;36:813. doi: 10.1007/s10439-008-9448-5. [DOI] [PubMed] [Google Scholar]
- 83.Miyanishi K. Trindade M.C. Lindsey D.P. Beaupre G.S. Carter D.R. Goodman S.B. Schurman D.J. Smith R.L. Dose- and time-dependent effects of cyclic hydrostatic pressure on transforming growth factor-beta3-induced chondrogenesis by adult human mesenchymal stem cells in vitro. Tissue Eng. 2006;12:2253. doi: 10.1089/ten.2006.12.2253. [DOI] [PubMed] [Google Scholar]
- 84.Miyanishi K. Trindade M.C.D. Lindsey D.P. Beaupre G.S. Carter D.R. Goodman S.B. Schurman D.J. Smith R.L. Effects of hydrostatic pressure and transforming growth factor-beta 3 on adult human mesenchymal stem cell chondrogenesis in vitro. Tissue Eng. 2006;12:1419. doi: 10.1089/ten.2006.12.1419. [DOI] [PubMed] [Google Scholar]
- 85.Angele P. Yoo J.U. Smith C. Mansour J. Jepsen K.J. Nerlich M. Johnstone B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res. 2003;21:451. doi: 10.1016/S0736-0266(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 86.Ogawa R. Mizuno S. Murphy G.F. Orgill D.P. The effect of hydrostatic pressure on three-dimensional chondroinduction of human adipose-derived stem cells. Tissue Eng Part A. 2009;15:2937. doi: 10.1089/ten.tea.2008.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu J.C. Athanasiou K.A. Low-density cultures of bovine chondrocytes: effects of scaffold material and culture system. Biomaterials. 2005;26:2001. doi: 10.1016/j.biomaterials.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 88.Bian L. Zhai D.Y. Zhang E.C. Mauck R.L. Burdick J.A. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng Part A. 2012;18:715. doi: 10.1089/ten.tea.2011.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nagel T. Kelly D.J. Mechanically induced structural changes during dynamic compression of engineered cartilaginous constructs can potentially explain increases in bulk mechanical properties. J R Soc Interface. 2012;9:777. doi: 10.1098/rsif.2011.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.