Abstract
Adipose-derived stromal/stem cells (ASCs) are a promising cell source for vascular-based approaches to clinical therapeutics, as they have been shown to give rise to both endothelial and perivascular cells. While it is well known that ASCs can present a heterogeneous phenotypic profile, spontaneous interactions among these subpopulations that result in the formation of complex tissue structures have not been rigorously demonstrated. Our study reports the novel finding that ASCs grown in monolayers in the presence of angiogenic cues are capable of self-assembling into complex, three-dimensional vascular structures. This phenomenon is only apparent when the ASCs are seeded at a high density (20,000 cells/cm2) and occur through orchestrated interactions among three distinct subpopulations: CD31-positive cells (CD31+), α-smooth muscle actin-positive cells (αSMA+), and cells that are unstained for both these markers (CD31−/αSMA−). Investigations into the kinetics of the process revealed that endothelial vessel-like structures initially arose from individual CD31+ cells through proliferation and their interactions with CD31−/αSMA− cells. During this period, αSMA+ cells proliferated and appeared to migrate toward the vessel structures, eventually engaging in cell-cell contact with them after 1 week. By 2 weeks, the lumen-containing CD31+ vessels grew greater than a millimeter in length, were lined with vascular basement membrane proteins, and were encased within a dense, three-dimensional cluster of αSMA+ and CD31−/αSMA− cells. The recruitment of αSMA+ cells was largely due to platelet-derived growth factor (PDGF) signaling, as the inhibition of PDGF receptors substantially reduced αSMA+ cell growth and vessel coverage. Additionally, we found that while hypoxia increased endothelial gene expression and vessel width, it also inhibited the growth of the αSMA+ population. Together, these findings underscore the potential use of ASCs in forming mature vessels in vitro as well as the need for a further understanding of the heterotypic interactions among ASC subpopulations.
Introduction
Heterogeneity in adipose-derived stromal/stem cell (ASC) cultures can give rise to novel cellular interactions that may be useful for the regeneration of complex tissues which are comprised of multiple cellular phenotypes. However, the importance of cell population heterogeneity is an under-explored concept in tissue engineering. ASCs hold great promise as an autologous cell source for regenerative medicine due to their abundance, ease of harvest via liposuction, and multipotency.1 In addition to their well-studied potential toward the classic mesenchymal lineages (bone, cartilage, and fat),2 ASCs possess vascular properties and can give rise to both endothelial3–5 and perivascular cells6–10. Each of these populations is essential for the formation of stable vascular networks.11,12 Therefore, the potential to supply all tissue components from a single autologous cell source would serve as a powerful tool in engineering complex vascularized grafts.
Several studies have investigated the perivascular and endothelial potential of ASCs. Intriguingly, it was recently demonstrated that ASCs may be resident in perivascular sites in native adipose tissues.9,10 They also possess the ability to secrete pro-angiogenic factors and stabilize vascular networks.10,13–15 Additionally, a recent study utilized a two-dimensional co-culture model of vasculogenesis which demonstrated that ASCs can stimulate mature endothelial cells that assemble into vascular structures via direct contact and bi-directional paracrine signaling.16 However, the potential of ASCs alone to yield vascular networks is less clear. Approaches toward in vitro endothelial differentiation are typically growth-factor mediated,3,4,17 and involve Matrigel,3,18 hypoxia,19 shear stress,20,21 or spontaneous transformation.5,22 All these studies have successfully reported an increase in the overall endothelial phenotype in cultures, though the functional characteristics of these cells and the phenotypes of the nonendothelial cells remain unclear. There is also evidence that ASCs differentiate into endothelial cells in vivo and engraft into host vasculature.4,5 These studies have been counter-balanced by the assertion that the ASCs inherently have limited endothelial potential due to the epigenetic restrictions of endothelial gene promoters.23 Each of these studies raises important questions regarding the robustness of the endothelial potential of ASCs and whether it is possible to simultaneously obtain endothelial and perivascular subpopulations within the same culture.
These questions may be answered by studying their phenotypic profiles. ASCs are isolated from adipose tissue by a selection for the plastic-adherent population of the stromal vascular fraction.24,25 These cells have been extensively characterized by multiple laboratories that have reported on their phenotypes across multiple donors,4,21,26 passages,26,27 extraction methods,4,28 and clonal differentiation potentials.29 While they exhibit increasingly homogenous profiles with sustained in vitro cultivation, demonstrating greater than a 90% positive expression of mesenchymal surface markers CD13, CD29, CD73, and CD90 by passage 3,26,27 ASCs are still widely considered a heterogeneous population. There also often exists a minority subpopulation that expresses endothelial cell-associated markers, such as CD31. This CD31+ population can range between 1% and 25% in early-passage ASC cultures,4,5,23,26 but diminishes substantially with sustained in vitro cultivation. More interestingly, this inherent heterogeneity might even be intentionally utilized to induce the formation of complex tissue structures in engineered grafts.
The current study aims at investigating the ability of ASCs to form stable vascular structures in vitro in response to angiogenic growth factors and varying seeding densities. We use a combination of vascular endothelial growth factor (VEGF) and basic fibroblastic growth factor (bFGF), which have been shown to enhance the expression of endothelial genes.3,17,19 Given the appropriate conditions, we have found that multiple ASC subpopulations are able to closely interact and spontaneously self-assemble into three-dimensional vascular structures. By closely tracking these events, we have explored and defined some of the necessary cellular interactions that are required to generate vascular networks for use in tissue-engineered grafts and potentially harness the heterogeneity of ASCs.
Materials and Methods
ASC isolation and culture
ASCs were isolated at the Stem Cell Biology Laboratory, Pennington Biomedical Research Center, under an Institutional Review Board-approved protocol according to published methods.30 Briefly, fresh, human, subcutaneous, adipose lipoaspirates were obtained by informed consent from five healthy Caucasian female donors undergoing elective liposuction surgery with a mean age of 47.6±1.9 years and a mean body mass index of 27.1±0.6. The fat depots included (number of donors denoted) the following: thighs (3), abdomen (2), hips, and flanks (2). The lipoaspirate tissue was extensively washed with a warm phosphate buffer solution to remove erythrocytes and then digested in phosphate-buffered saline (PBS) supplemented with 0.1% Collagenase Type I (Worthington Biochemical Corp.), 1% bovine serum albumin, and 2 mM CaCl2 for 1 h at 37°C. After room temperature centrifugation at 300 g and resuspension in Stromal Medium (DMEM/Hams F-12 Medium supplemented with 10% fetal bovine serum [FBS; Hyclone] and 1% antibiotic/antimycotic), the stromal vascular pellet obtained from 35 mL of the lipoaspirate digest was plated in a T175 flask (0.2 mL/cm2). After 24 h of incubation at 37°C, 5% CO2, the adherent cells were washed with warm PBS and maintained in Stromal Medium until they were 80%–90% confluent. The adherent population (“passage 0”) was harvested by digestion with trypsin (0.05%)/EDTA (1 mM) at 37°C for 5 min, washed with Stromal Medium, and cryopreserved31 for shipment to Johns Hopkins University. Previous studies have demonstrated no deleterious effects on ASCs due to cryopreservation, such as loss of viability or multipotency.31–33 For expansion, the ASCs were thawed and cultured in expansion medium: high-glucose DMEM (GIBCO Invitrogen) with 10% FBS (Atlanta Biologicals), 1% penicillin/streptomycin (GIBCO Invitrogen), and 1 ng/mL bFGF (PeproTech). The cells at passage three were used for all the experiments. The ASCs from a single donor were used for the initial experiments, and the observations were subsequently confirmed with four additional donors.
Flow cytometry
The phenotypic profile of ASCs for mesenchymal (CD73, CD105) and vascular markers (CD34, VEGFR2, CD31, and α-smooth muscle actin [αSMA]) was examined at passage 3. The detached cells were suspended in PBS containing 2% FBS and incubated with monoclonal antibodies conjugated to fluorescein isothiocyanate or phycoerythrin for 30 min at 4°C. The cells were analyzed with a flow cytometer (BD Accuri C6). All antibodies were purchased from BD Biosciences.
Cell seeding density
ASCs were seeded at 3,000, 10,000, or 20,000 cells/cm2 and cultured for 14 days in an endothelial induction medium (EIM). The composition of EIM was optimized in preliminary experiments (see Supplementary Figs. S1 and S2; Supplementary Data are available online at www.liebertonline.com/tea), consisting of Endothelial Basal Medium-2 (EBM2; Lonza) supplemented with 2% FBS, 1% penicillin/streptomycin, 2 ng/mL VEGF165 (PeproTech), 10 ng/mL bFGF, and 1 μg/mL L-ascorbic acid-2-phosphate (Sigma). Endothelial phenotypes were assessed via quantitative reverse transcription-polymerase chain reaction (RT-PCR) and immunocytochemistry. The DNA content was assessed at various time points to evaluate the growth patterns and final cell densities within the cultures.
Evaluation of vascular morphogenesis
ASCs were seeded at 20,000 cells/cm2 and cultured for approximately 14 days in EIM under either normoxia (20% O2) or hypoxia (2% O2). This nomenclature was adopted to maintain consistency with an abundance of published literature. Normoxic cultures were maintained in a 37°C incubator with 5% CO2, 95% ambient air. Hypoxic cultures were placed in a modular incubator chamber (Billups-Rothenberg) that was flushed with a hypoxic gas mixture (2% O2, 5% CO2, and 93% N2) and placed in a 37°C incubator. Quantitative RT-PCR and immunocytochemistry were used to evaluate endothelial and perivascular phenotypes and morphologies. Samples for immunostaining were fixed on days 0 (4 h after seeding), 3, 7, 10, and 14, while RNA was harvested on days 1, 7, and 14.
Bromodeoxyuridine incorporation
With the aim of identifying proliferating cells in the culture, 10 μM bromodeoxyuridine (BrdU; Sigma) was added to the culture medium for 24 h. A long incubation time was used because proliferation was low, particularly at later time points. This was followed by washing and fixation with 3.7% formaldehyde for 20 min. The samples were first immunostained for CD31 and αSMA in order to identify the subpopulations, followed by 10 min postfixation to preserve the signal. DNA was denatured by incubating cells with 4N HCl plus 0.5% triton X-100 in PBS for 15 min at room temperature. After extensive washing, the samples were incubated with mouse anti-BrdU conjugated to Alexa Fluor 647 (1:50; Invitrogen) overnight at 4°C. The nuclei were then counterstained with 4′,6-diamino-2-phenylindole (DAPI), and the samples were mounted for analysis.
Platelet-derived growth factor receptor inhibition
The effects of platelet-derived growth factor-B (PDGF-B) signaling on the vascular morphogenesis in our system were assessed by blocking its receptor. ASCs were seeded at 20,000 cells/cm2 and cultured for 14 days in EIM under either normoxia or hypoxia. For each oxygen tension group, the medium was supplemented with either vehicle only (dimethyl sulfoxide) or 50 μM tyrphostin AG1295 (Santa Cruz Biotechnology), a selective inhibitor of the PDGF receptor (PDGFR) tyrosine kinase activity. Morphological effects were evaluated via immunocytochemistry.
Quantitative RT-PCR
Cells were grown in 12-well plates (n=4 per group) for approximately 14 days. Total RNA was isolated using a TRIzol (Invitrogen) extraction method and quantified using a NanoDrop spectrophotometer (Thermo Scientific). Reverse transcription was performed with 1 μg of total RNA using the iScript cDNA Synthesis Kit (BioRAD). Complementary DNA was amplified using the SYBR Green PCR Master Mix (Applied Biosystems) and a StepOnePlus Real-Time PCR System (Applied Biosystems). The primer sequences used for PCR analysis are listed in Supplementary Table S1. Expression levels were calculated by the comparative CT method using either GAPDH (seeding density study) or β-actin (hypoxia studies) as endogenous reference genes.
DNA content
In order to evaluate proliferation, the cells were grown in 24-well plates for 0, 3, 7, 10, or 14 days and lysed with 250 μL of digestion buffer (10 mM Tris, 1 mM EDTA, 0.1% Triton X-100, and 0.1 mg/mL proteinase K). The samples were incubated overnight at 50°C, and DNA content was assessed using a PicoGreen dsDNA quantitation kit (Molecular Probes). Briefly, PicoGreen dye was added to the samples in duplicate in black, 96-well plates (50 μL sample, 50 μL 1× Tris-EDTA buffer, and 100 μL PicoGreen diluted 1:200) and read with a fluorescent plate reader (excitation 485 nm, emission 530 nm). DNA content was determined using a λDNA standard curve and compared against known cell numbers from day 0 samples to determine cell number.
Immunocytochemistry
Cells were fixed with 3.7% formaldehyde for 20 min, permeabilized with 0.2% triton X-100 for 10 min, and then blocked with 10% normal donkey serum (Sigma) for 30 min. Briefly, the samples were then incubated overnight with antigen-specific primary antibodies at 4°C, followed by secondary antibodies for 1 h at room temperature. When using multiple primary antibodies from the same species (i.e., mouse), the antigens were stained sequentially with a slight modification of the first bound primary antibody: mouse IgG were masked with goat anti-mouse IgG monovalent Fab fragments (20 μg/mL) for 2 h at room temperature, and then detected with Cy3-conjugated donkey anti-goat IgG. Antibody complexes were lightly fixed with 1.85% formaldehyde for 10 min, and the cells were blocked with 10% normal goat serum (Sigma) for 30 min. The samples were then co-stained for the remaining antigens following standard protocol using species-specific secondary antibodies (DyLight 488- or 649-conjugated goat anti-mouse or anti-rabbit IgG, respectively). Coverslips were mounted in ProLong Gold Antifade Reagent (Invitrogen) containing DAPI, and imaged using a Zeiss Axio Observer inverted fluorescence microscope and a Zeiss LSM 510 confocal microscope with a 63x/1.4 oil objective. Primary antibodies were purchased from Santa Cruz Biotechnology [mouse anti-CD31 (1:50), mouse anti-vascular endothelial-cadherin (VE-Cad; 1:50), rabbit anti-desmin (1:50), and mouse anti-collagen IV (1:100)], Sigma [rabbit anti-von Willebrand factor (vWF; 1:200), rabbit anti-laminin (1:100)], and Invitrogen [mouse anti-αSMA (1:50)]. All secondary antibodies were purchased from Jackson Immunoresearch and used at a 1:200 dilution. Secondary-only controls are shown in Supplementary Figure S3.
Quantification of immunocytochemistry
Morphological measurements of immunostaining were performed using Image J software (NIH). At least eight vessels per group were used for the quantification of vessel length and width. Length was measured along the entire structure from tip to tip. The segment lengths were summed for branched vessels. The width of each vessel was averaged among discrete measurements (spaced ∼25 μm apart) along the entire length of the vessel. The area covered by the αSMA+ cells was averaged from five random view fields per sample, and across three samples per group. Images were thresholded to measure percent-positive areas. All groups were normalized to “day 0” values.
Statistical analysis
Quantitative data are expressed as mean±standard error. An unpaired t-test was used for two-group comparisons. Multi-group comparisons were determined by one-way analysis of variance with Tukey's test for post-hoc analysis. Significance levels were denoted as *p<0.05, **p<0.01, and ***p<0.001.
Results
Flow cytometry
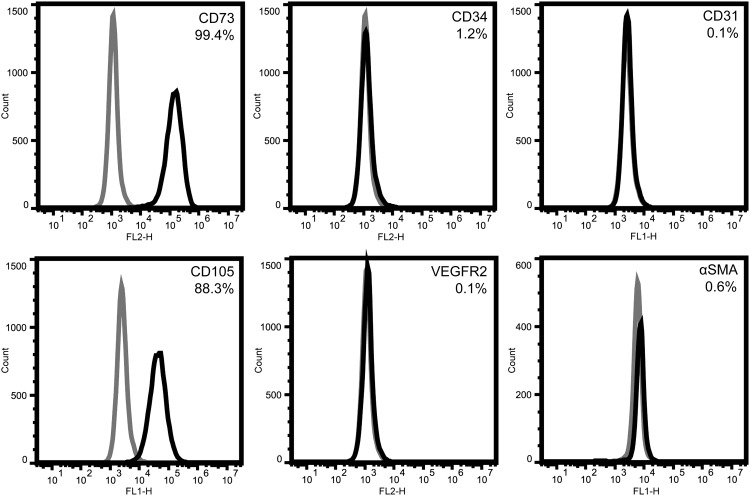
ASCs at passage 3 are primarily positive for mesenchymal markers CD73 (99.4%) and CD105 (88.3%) (Fig. 1). Very few cells express progenitor or mature markers for the endothelial lineage (1.2% CD34+; 0.1% VEGFR2+; 0.1% CD31+) or mature pericyte marker αSMA (0.6%).
FIG. 1.
Phenotypic profile of initial ASC population. Passage 3 ASCs are mostly positive for mesenchymal markers CD73 and CD105. A very small minority is positive for vascular markers, such as early and mature endothelial markers (CD34, VEGFR2, CD31) and the pericyte marker αSMA. ASCs, adipose-derived stromal/stem cells; αSMA, α-smooth muscle actin; VEGF, vascular endothelial growth factor.
Cell seeding density
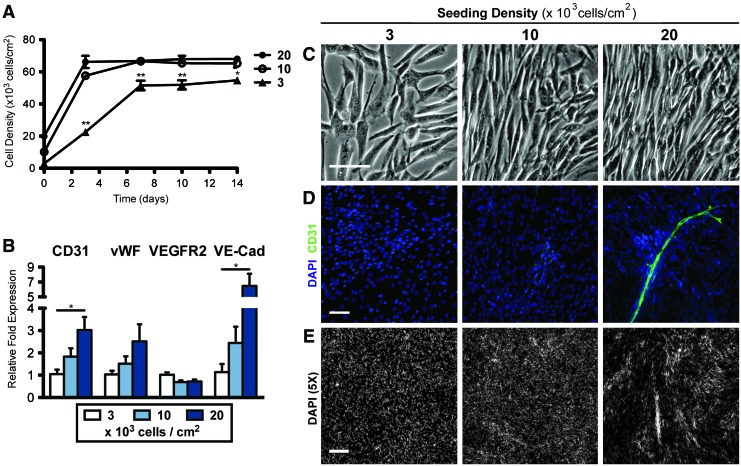
ASCs were cultured at 3000, 10,000, and 20,000 cells/cm2 for 14 days in EIM. The ASCs seeded at the two higher densities attained a final density of ∼65,000 cells/cm2, as determined by DNA content by day 3 (20,000 per cm2) or day 7 (10,000 per cm2), at which they stayed for the remainder of the differentiation period. Cells seeded at 3000 cells/cm2 grew to 50,000 cells/cm2 by day 7 and remained constant thereafter, never attaining the density of the other two groups (Fig. 2A). At higher seeding densities, there was an increased mRNA expression in CD31, vWF, and VE-cadherin by day 14. This may be due to the increased numbers of endothelial cells or cell maturation. VEGF receptor-2 (VEGFR-2) expression was not significantly affected by seeding density (Fig. 2B). After 14 days, the cells seeded at 10,000 to 20,000 cells/cm2 became densely packed with the cells aligning locally with neighboring cells, while those seeded at 3000 cells/cm2 remained sub-confluent with random cell orientation (Fig. 2C). Positive staining for CD31 was only observed when cells were seeded at 20,000 cells/cm2, with a specific staining of vessel-like structures enveloped within dense cell clusters (Fig. 2D). Low-magnification images of DAPI stains confirmed that the nonuniform aggregation occurred only in the 20,000 cells/cm2 group, while those seeded at 3000 or 10,000 cells/cm2 were uniformly distributed (Fig. 2E). In all subsequent experiments, cells were seeded at 20,000 cells/cm2.
FIG. 2.
Effects of cell seeding density on the endothelial phenotype of ASCs. (A) DNA content revealed that ASCs seeded at the two higher densities reached a final density of 65,000 cells/cm2 within three days, while those seeded at 3000 cells/cm2 took longer to reach a final density that was still significantly less. (B) mRNA expression of endothelial markers increased by increasing the seeding density by day 14. (C) Morphologically, the ASCs seeded at higher densities were more densely packed and aligned. (D) Only the ASCs seeded at 20,000 cells/cm2 gave rise to CD31+ vessels. (E) Cells remained uniformly distributed when seeded at 3000 and 10,000 cells/cm2, while those seeded at 20,000 cells/cm2 aggregated into dense clusters by day 14. Significance indicated as *p<0.05 or **p<0.01. Scale bars=100 μm (C, D) and 250 μm (E). DAPI, 4′,6-diamino-2-phenylindole. vWF, von Willebrand factor. Color images available online at www.liebertonline.com/tea
Vascular phenotypes and morphology
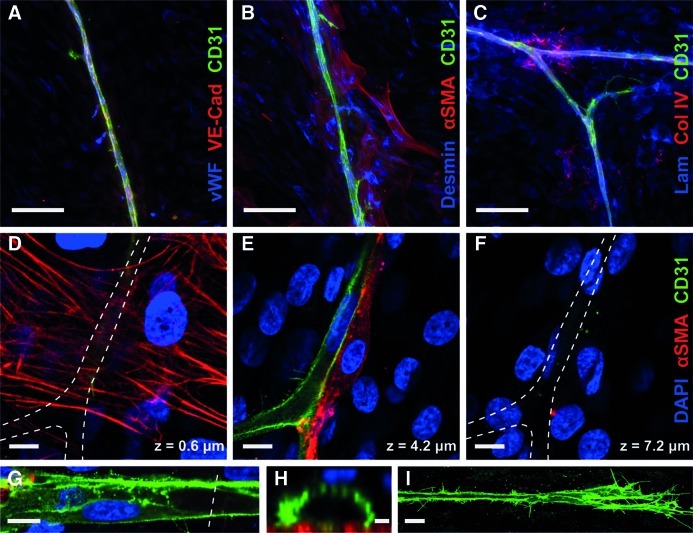
Vascular structures stained positively for three endothelial markers: CD31 located at cell junctions and filopodia, VE-cadherin located at cell junctions, and vWF with a granular pattern in the cytoplasm (Fig. 3A). Many of the cells surrounding the vessels were positive for pericyte markers (αSMA and desmin) (Fig. 3B). Vascular basement membrane proteins, collagen IV and laminin, were also present along vessel tracks (Fig. 3C). The pan-laminin antibody, which is reactive against all laminin isoforms, also faintly stained other cellular regions beyond the vessels.
FIG. 3.
Vascular phenotypes and morphology. (A) Vessels stained positively for CD31, VE-cadherin, and vWF (endothelial markers). (B) Cells clustered around the vessels were positive for αSMA and desmin (pericyte markers). (C) Vascular basement membrane proteins, collagen IV, and laminin were found along vessel tracks. (D–F) Optical slices from a confocal z-stack: (D) αSMA+ cells (red) stretched out against the glass beneath the vessels. (E) CD31+ vessels (green) propagated on top of this layer of cells, with some αSMA+ cells invested alongside. (F) Additional αSMA−/CD31− cells were located on top of the vessels (indicated by DAPI, blue), fully enveloping the vessel in a three-dimensional cellular environment. (G) Some vessels contained voids resembling patent lumens, demonstrated by the cross-section (dotted line) shown in (E). (F) The vessel tips were dense with filopodia. Scale bars=100 μm (A–C), 10 μm (D–G, I), and 2 μm (H). Color images available online at www.liebertonline.com/tea
Optical slices from 63× confocal z-stacks revealed the relative locations of the cells comprising the vascular structures (Fig. 3D–F). Many of the αSMA+ cells were stretched out against the glass (Fig. 3D), blanketing the area beneath the endothelial vessels. Some of the αSMA+ cells were also visible along the sides or on top of vessels, assuming a classic pericyte morphology (Fig. 3E). Additional CD31-/αSMA-cells were located on top of the vessels (Fig. 3F), indicating that the vessels were fully encased within a three-dimensional cellular microenvironment. Many vessels contained large voids that appeared to be patent lumens (Fig. 3G), as shown in a cross-sectional view of one representative vessel, which measured an internal width of 9.7 μm and a height of 5.4 μm, similar in size to a capillary (Fig. 3H). The vessel tips were dense with filopodia (Fig. 3I). The ASCs from four additional donors were cultured under the same conditions, and demonstrated similar vascular morphogenesis with the CD31+/vWF+ vessels surrounded by αSMA+ cells (Supplementary. Fig. S4).
Time course of morphogenesis
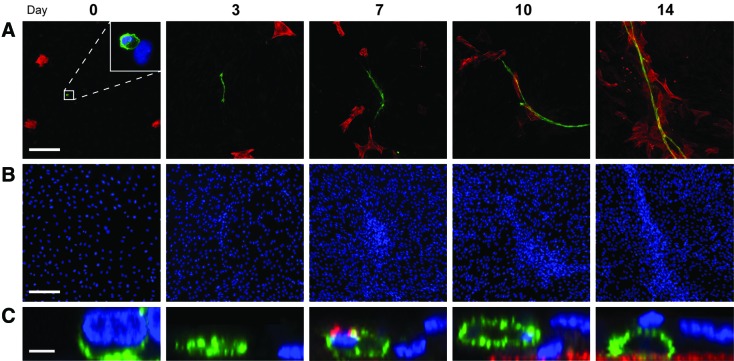
Four hours after seeding (“day 0”), the culture contained small numbers of αSMA+ cells (0.5%) and CD31+ cells (0.02%) (Fig. 4A), which similarly reflected the flow cytometry analysis. All CD31+ cells were in direct contact with other cells after attachment (Supplementary Fig. S5). During the first week, individual CD31+ cells began to elongate into multicellular vessels, while the spatially independent αSMA+ cells increased in number and appeared to migrate toward the vessels (Fig. 4A). In the second week, the αSMA+ cells co-localized with the vessels and continued to increase in number. DAPI stains revealed that the cells began forming dense clusters around the vessels very early on, even before contact with the αSMA+ cells (Fig. 4B). From an orthogonal view, the initially flat vessel (Day 3) increased in height over time, while additional cells in the surrounding monolayer migrated on top of one another to form a multilayered cluster of cells (Fig. 4C).
FIG. 4.
Time course of vascular morphogenesis. (A) Four hours postseeding (“day 0”), the culture contained small numbers of both αSMA+ cells (0.5%; red) and CD31+ cells (0.02%; green; higher magnification shown in inset). Over the course of several days, a multicellular CD31+ vessel emerged and elongated. αSMA+ cells increased in number over time, and began to cluster around the vessels in the second week. (B) DAPI counterstains (blue) of the same view fields illustrate the increasingly dense clustering of cells around the vessel over time. (C) Cross-sectional views of the vessels depict three-dimensional growth over time, accompanied by the migration of cells on top of the vessel. Scale bars=200 μm (A, B) and 5 μm (C). Color images available online at www.liebertonline.com/tea
BrdU incorporation studies revealed that the primary mode of vessel growth was proliferation (Fig. 5A), with most CD31+ cell nuclei staining positively for BrdU at days 3, 7, 10, and 14 (multiple time points not shown). The αSMA+ cells distant from the vessel were most often BrdU+, whereas those in direct contact were not (Fig. 5B).
FIG. 5.
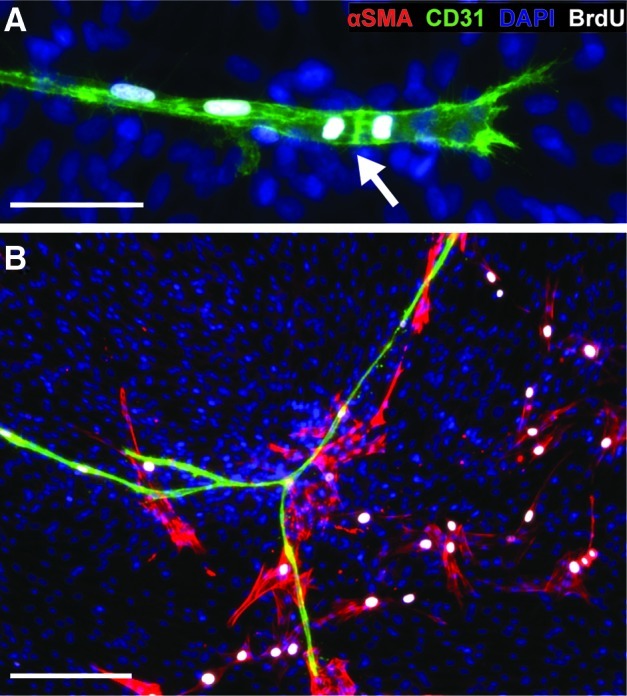
Proliferation of ASC subpopulations. ASCs were incubated with BrdU at multiple stages of vascular formation. (A) CD31+ vessels were BrdU+ at all stages of growth, depicted here at day 7. The arrow highlights a tip cell during division. (B) αSMA+ cells distant from the vessel were often BrdU+, while those directly contacting the vessel were BrdU-, depicted here at day 14. Scale bars=50 μm (A) and 200 μm (B). BrdU, bromodeoxyuridine. Color images available online at www.liebertonline.com/tea
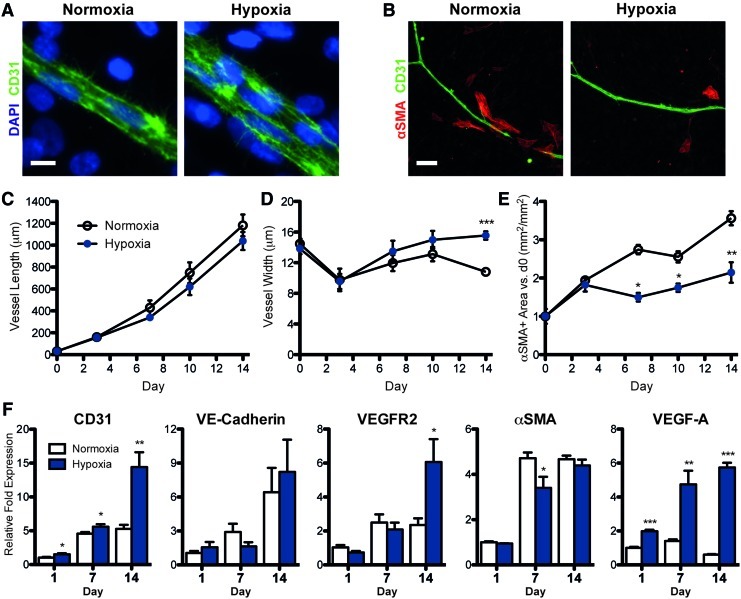
Hypoxia
Cultures in hypoxia yielded wider vessels with more CD31+ cells across the diameter of the vessel, as compared with those in normoxia (Fig. 6A). The quantification of vessel morphology indicated that vessel length was not affected by oxygen tension (Fig. 6C), while vessel width was significantly wider in hypoxic samples after 14 days in culture (Fig. 6D). There were substantially fewer αSMA+ cells surrounding the vessels in hypoxia (Fig. 6B). The quantification of αSMA+ area revealed that the αSMA+ population steadily increased over time in normoxia, whereas in hypoxia, there was significantly less growth of this population past day 3 (Fig. 6E). After 14 days of prolonged hypoxia, endothelial gene expression (CD31, VEGFR-2) increased significantly more than in normoxia (Fig. 6F). The expression of VEGF-A also increased over time under prolonged hypoxia, with a nearly 10-fold higher expression than in normoxia. The αSMA expression was significantly less in hypoxia than in normoxia at day 7, but increased to similar levels by day 14.
FIG. 6.
Effects of hypoxia on vessel morphology and pericyte coverage. The vessels in hypoxia were significantly wider than in normoxia (A, D), while lengths were similar (C). In addition, in hypoxia, there were significantly less αSMA+ cells surrounding vessels (B) and throughout the culture (E). (F) mRNA expression of endothelial markers was higher in hypoxia than in normoxia by day 14, while expression of αSMA was less in hypoxia at day 7. Significance indicated as *p<0.05, **p<0.01, or ***p<0.001 versus normoxia. Scale bars=10 μm (A) and 100 μm (B). Color images available online at www.liebertonline.com/tea
PDGFR inhibition
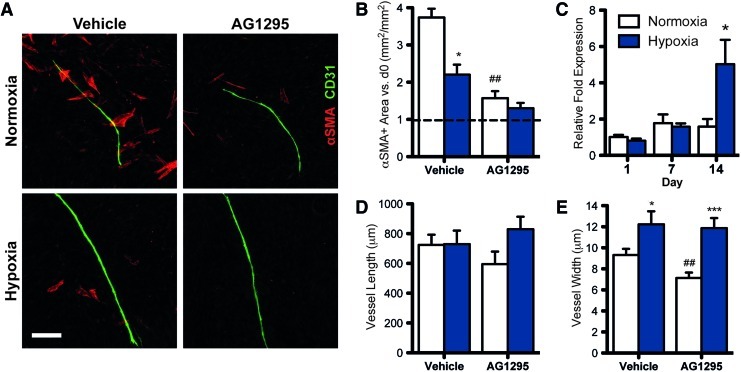
In both normoxia and hypoxia, the addition of a PDGFR inhibitor (AG1295) to the culture medium significantly reduced the αSMA+ cell vessel coverage (Fig. 7A) and their overall presence in the culture (Fig. 7B). Both the vehicle control and AG1295 groups in hypoxia had less αSMA+ area than their normoxic counterparts. However, mRNA expression of PDGF-B is significantly higher in hypoxia than in normoxia after 14 days (Fig. 7C). The vessel length was not significantly affected by AG1295 (Fig. 7D). In normoxia, the vessels were significantly thinner due to AG1295, while in hypoxia, the width was not affected by the inhibitor (Fig. 7E).
FIG. 7.
Inhibition of PDGFR signaling. The addition of tyrphostin AG1295 (a selective inhibitor of PDGFR) to the medium significantly reduced the amount of αSMA+ cells near the vessels (A) and throughout the culture (B). (C) PDGF-B expression is significantly higher in hypoxia at day 14. The inhibition has insignificant effects on vessel length (D), and reduces vessel width in normoxia, but not hypoxia (E). Significance indicated vs. normoxia (*p<0.05, ***p<0.001) or vehicle (#p<0.05, ##p<0.01). Scale bar=200 μm. PDGF-B, platelet-derived growth factor-B; PDGFR, PDGF receptor. Color images available online at www.liebertonline.com/tea
Discussion
The vascular potential of ASCs makes them a viable and unique cell source for clinical therapeutics. This study demonstrates that the characterized ASC populations26,34 are fully capable of spontaneously self-assembling to form long, three-dimensional endothelial tubes with lumens and invested by perivascular cells. The heterogeneity of ASCs plays an essential role, as multiple subpopulations seem to coordinate these events. This vascular self-assembly only occurs when ASCs are seeded at a high density (20,000 cells/cm2), with most CD31+ cells positioned in direct contact with other cells for hours after seeding. These findings indicate that paracrine factors and direct physical interactions among subpopulations may be critical to induce morphogenesis. It also seems that signaling and direct cellular interactions at the earliest stages of culture may be crucial; while cells seeded at 10,000 cells/cm2 eventually attained the same final cell density as those at 20,000 cells/cm2 and presumably contained half the number of CD31+ cells at the start of the culture period, they did not exhibit CD31+ staining or vessel formation after 14 days of culture.
Intriguingly, the various subpopulations of ASCs appeared to recapitulate some aspects of classic vasculogenesis. During development, mesodermal progenitors are induced toward the endothelial lineage in response to bFGF and VEGF.35 These endothelial cells then migrate, proliferate, form nascent vessels, and recruit pericyte progenitors via a PDGF-B-mediated mechanism.35–37 In our system, heterogeneous ASCs were bathed in a medium containing bFGF and VEGF. In the first few days after seeding, CD31+ cells elongated and proliferated to form multicellular vessel-like structures, while the proliferating αSMA+ cells were initially spatially independent. As the vascular structures elongated, both αSMA+ and CD31−/αSMA− cells appeared to migrate toward the vessel, forming dense clusters of cells around the vessel stalks. These cells fully enveloped the vessel, such that the endothelial cells within experienced a three-dimensional microenvironment. It was not evident from these findings whether CD31−/SMA− cells could directly differentiate into CD31+ cells and integrate into the vessel structure. The vessel tracks were lined with vascular basement membrane proteins, collagen IV, and laminin, and the vessels themselves exhibited a classic morphology, with a patent lumen, a thin, multicellular endothelial wall, and cells at sprouting tips containing numerous filopodia.
Our studies using a PDGFR inhibitor demonstrate that PDGF-B signaling plays a crucial role in the proliferation and migration of the αSMA+ subpopulation toward the growing endothelial vessels. PDGF-B is primarily secreted by proliferating endothelial cells, while its receptor, PDGFR-β, is expressed by pericytes.35–37 With the addition of a selective inhibitor of PDGFR to the culture medium, this specific mode of communication between the CD31+ and αSMA+ cells in the culture was effectively blocked and ultimately resulted in thinner vessels and a substantial reduction of αSMA+ cell vessel coverage.
This spontaneous organization of endothelial and pericyte-like populations from ASCs has significant implications for their therapeutic applications. Multiple tissue-engineering therapies have focused on generating capillary networks to vascularize grafts. However, the key to facilitating long-term, stable vascular regeneration may lie in the ability to recruit perivascular cells to endothelial networks that provide support and vasoresponsive properties.38 In skeletal muscle vascular remodeling, vessel expansion beyond capillary diameter is accompanied by investment by αSMA+ cells.39 The interaction between endothelial cells and pericytes elicits the deposition of a supportive vascular basement membrane,40,41 and, ultimately, leads to stabilization, junctional integrity, and maturation.36,41,42 Pericytes can also promote survival and resistance to vascular regression.43–45 ASCs, it seems, may be able to perform both roles concurrently through the spontaneous assembly of endothelial and pericyte subpopulations; however, seeding cell density appears to be a critical variable influencing this outcome. The investment of vessels by perivascular cells in tissues is a multicomponent process. For example, in rats, mesentery VEGF can induce endothelial vessel structures, but not αSMA+ cell recruitment; however, the triple delivery of VEGF, angiopoietin-1, and endothelial nitric oxide synthase induces perivascular cell-invested vessels.46 The formation of vascular structures here suggests that the heterogeneous ASC culture supplies these or other similar factors that produce the coordinated multicellular morphogenesis.
We also investigated the effects of prolonged hypoxia on cellular responses. Hypoxia has been previously shown to have a positive effect on the pro-angiogenic phenotype of several types of adult stem cells.47–50 Specifically, ASCs have been shown to upregulate the production of VEGF under hypoxia,6,13,19,51 as well as to increase the number of cells expressing VEGFR-2.19 Our current study confirmed these findings under prolonged hypoxia, and additionally demonstrated a thickening of vascular structures, likely due to a VEGF-induced increase in endothelial cell proliferation.52 Similarly, under chronic hypoxia in vivo, capillaries in all regions of the brain markedly increase growth and diameter in order to reduce the oxygen deficit in the tissue.53 Another novel finding in this study was that while prolonged hypoxia appeared to enhance the endothelial subpopulation, it had a negative effect on pericyte growth and vessel coverage. Within the first week of hypoxic exposure, the overall growth of αSMA+ cells in the culture halted, resulting in significantly less co-localization of αSMA+ cells with endothelial vessels compared with normoxic samples by day 14. While the expression of PDGF-B increases in hypoxia, it is somewhat delayed, and may not be sufficient to overcome the seemingly inhibitory effects of hypoxia in the studied time frame. The inhibition of PDGFR activity under hypoxia further reduces αSMA+ cell growth to levels similar to those at day 0. These observations are supported by a recently published study demonstrating that preconditioning ASCs in hypoxia delays the expression of αSMA in vitro and reduces recruitment in vivo.54 Taken together, the results of the current study indicate that the effects of hypoxia on the vascular properties of ASCs may be more complex than our previous understanding.
The major barriers that are involved in cultivating complex tissues in vitro are the incomplete understanding of the kinetics of cellular differentiation which result in stable, terminally differentiated phenotypes and of the subsequent cross-talk and synergy that arises between these newly differentiated cells and neighboring stem/progenitor cells. This study demonstrates the remarkable ability of a single population of ASCs to spontaneously generate multicellular vascular structures through the coordination of its subpopulations. While donor-to-donor variability is a valid concern with ASCs, we have also demonstrated the ability of four additional donors to give rise to similar vascular structures. Given that cellular heterogeneity was a key component of these findings, it will be important to understand how these findings differ depending on a wider variety of donor demographics and harvest techniques. There may also be implications for methods used to culture and expand ASCs: Is the trend toward homogeneity with continued passaging beneficial? Ultimately, it may affect clinical application, as the ability to harvest all the necessary cells from a single procedure could potentially reduce cost and recovery time for the patient. Nevertheless, additional studies are required to address these considerations.
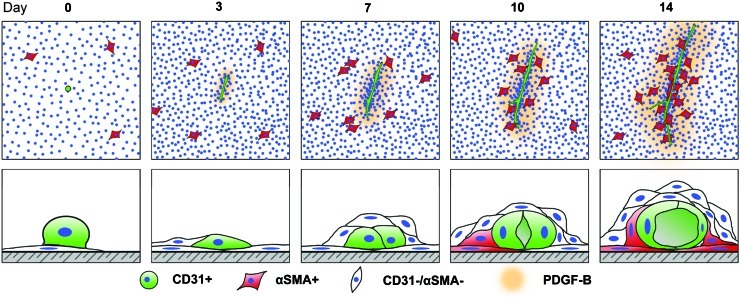
In summary, we have demonstrated that high seeding density is crucial for encouraging the heterotypic interaction and self-assembly of ASCs into vascular structures. While the cells were initially seeded onto two-dimensional substrates in the absence of exogenous extracellular matrix proteins, the cells were able to migrate, organize, and form three-dimensional vessels (Fig. 8). This coordination seems innately programmed, and may be harnessed for vascularizing three-dimensional tissue grafts, possibly explaining recent findings.22 Understanding the driving forces behind these events may ultimately guide our approaches toward efficiently recapitulating these events in three dimensions.
FIG. 8.
Schematic of the heterotypic interactions that mediate ASC vascular assembly. The top row depicts the growth and assembly of the vascular structures over time, with αSMA+ cells being recruited by PDGF-B secreted by the growing vessels. The bottom row is an interpretation of the evolution of these structures from a two-dimensional monolayer into a complex, three-dimensional structure. The recruitment of the supportive cells drives migration and multilayered, cell-on-cell clustering around the vessel. Color images available online at www.liebertonline.com/tea
Supplementary Material
Acknowledgments
This work was supported by a TEDCO grant from the Maryland Stem Cell Research Fund (2010-MSCRFE-0150-00). Use of the Zeiss LSM 510 confocal microscope was made possible thanks to the Microscopy and Imaging Core Module of the Wilmer Core Grant, EY001765.
Disclosure Statement
No competing financial interests exist.
References
- 1.Gimble J.M. Katz A.J. Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuk P.A. Zhu M. Mizuno H. Huang J. Futrell J.W. Katz A.J., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y. Sun Z. Liao L. Meng Y. Han Q. Zhao R.C. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 4.Miranville A. Heeschen C. Sengenes C. Curat C.A. Busse R. Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 5.Planat-Benard V. Silvestre J.S. Cousin B. Andre M. Nibbelink M. Tamarat R., et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 6.Rehman J. Traktuev D. Li J. Merfeld-Clauss S. Temm-Grove C.J. Bovenkerk J.E., et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 7.Zannettino A.C. Paton S. Arthur A. Khor F. Itescu S. Gimble J.M., et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 8.Amos P.J. Shang H. Bailey A.M. Taylor A. Katz A.J. Peirce S.M. IFATS collection: the role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of a perivascular phenotype. Stem Cells. 2008;26:2682. doi: 10.1634/stemcells.2008-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crisan M. Yap S. Casteilla L. Chen C.W. Corselli M. Park T.S., et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Traktuev D.O. Merfeld-Clauss S. Li J. Kolonin M. Arap W. Pasqualini R., et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 11.Chen R.R. Silva E.A. Yuen W.W. Mooney D.J. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24:258. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 12.Cao R. Brakenhielm E. Pawliuk R. Wariaro D. Post M.J. Wahlberg E., et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen J.G. Frobert O. Pilgaard L. Kastrup J. Simonsen U. Zachar V., et al. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy. 2011;13:318. doi: 10.3109/14653249.2010.506505. [DOI] [PubMed] [Google Scholar]
- 14.Verseijden F. Posthumus-van Sluijs S.J. Pavljasevic P. Hofer S.O. van Osch G.J. Farrell E. Adult human bone marrow- and adipose tissue-derived stromal cells support the formation of prevascular-like structures from endothelial cells in vitro. Tissue Eng Part A. 2010;16:101. doi: 10.1089/ten.TEA.2009.0106. [DOI] [PubMed] [Google Scholar]
- 15.Basu J. Genheimer C.W. Guthrie K.I. Sangha N. Quinlan S.F. Bruce A.T., et al. Expansion of the human adipose-derived stromal vascular cell fraction yields a population of smooth muscle-like cells with markedly distinct phenotypic and functional properties relative to mesenchymal stem cells. Tissue Eng Part C Methods. 2011;17:843. doi: 10.1089/ten.tec.2010.0697. [DOI] [PubMed] [Google Scholar]
- 16.Merfeld-Clauss S. Gollahalli N. March K.L. Traktuev D.O. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng Part A. 2010;16:2953. doi: 10.1089/ten.tea.2009.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ning H. Liu G. Lin G. Yang R. Lue T.F. Lin C.S. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tissue-derived stem cells. J Sex Med. 2009;6:967. doi: 10.1111/j.1743-6109.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suga H. Matsumoto D. Eto H. Inoue K. Aoi N. Kato H., et al. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18:1201. doi: 10.1089/scd.2009.0003. [DOI] [PubMed] [Google Scholar]
- 19.Thangarajah H. Vial I.N. Chang E. El-Ftesi S. Januszyk M. Chang E.I., et al. IFATS collection: adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells. 2009;27:266. doi: 10.1634/stemcells.2008-0276. [DOI] [PubMed] [Google Scholar]
- 20.Fischer L.J. McIlhenny S. Tulenko T. Golesorkhi N. Zhang P. Larson R., et al. Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force. J Surg Res. 2009;152:157. doi: 10.1016/j.jss.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang P. Moudgill N. Hager E. Tarola N. Dimatteo C. McIlhenny S., et al. Endothelial differentiation of adipose-derived stem cells from elderly patients with cardiovascular disease. Stem Cells Dev. 2011;20:977. doi: 10.1089/scd.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natesan S. Zhang G. Baer D.G. Walters T.J. Christy R.J. Suggs L.J. A bilayer construct controls adipose-derived stem cell differentiation into endothelial cells and pericytes without growth factor stimulation. Tissue Eng Part A. 2011;17:941. doi: 10.1089/ten.tea.2010.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boquest A.C. Noer A. Sorensen A.L. Vekterud K. Collas P. CpG methylation profiles of endothelial cell-specific gene promoter regions in adipose tissue stem cells suggest limited differentiation potential toward the endothelial cell lineage. Stem Cells. 2007;25:852. doi: 10.1634/stemcells.2006-0428. [DOI] [PubMed] [Google Scholar]
- 24.Bunnell B.A. Flaat M. Gagliardi C. Patel B. Ripoll C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods. 2008;45:115. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gimble J.M. Bunnell B.A. Guilak F. Human adipose-derived cells: an update on the transition to clinical translation. Regen Med. 2012;7:225. doi: 10.2217/rme.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell J.B. McIntosh K. Zvonic S. Garrett S. Floyd Z.E. Kloster A., et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 27.Suga H. Shigeura T. Matsumoto D. Inoue K. Kato H. Aoi N., et al. Rapid expansion of human adipose-derived stromal cells preserving multipotency. Cytotherapy. 2007;9:738. doi: 10.1080/14653240701679873. [DOI] [PubMed] [Google Scholar]
- 28.Wosnitza M. Hemmrich K. Groger A. Graber S. Pallua N. Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation. 2007;75:12. doi: 10.1111/j.1432-0436.2006.00110.x. [DOI] [PubMed] [Google Scholar]
- 29.Guilak F. Lott K.E. Awad H.A. Cao Q. Hicok K.C. Fermor B., et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 30.Dubois S.G. Floyd E.Z. Zvonic S. Kilroy G. Wu X. Carling S., et al. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. Methods Mol Biol. 2008;449:69. doi: 10.1007/978-1-60327-169-1_5. [DOI] [PubMed] [Google Scholar]
- 31.Goh B.C. Thirumala S. Kilroy G. Devireddy R.V. Gimble J.M. Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen Med. 2007;1:322. doi: 10.1002/term.35. [DOI] [PubMed] [Google Scholar]
- 32.Gonda K. Shigeura T. Sato T. Matsumoto D. Suga H. Inoue K., et al. Preserved proliferative capacity and multipotency of human adipose-derived stem cells after long-term cryopreservation. Plast Reconstr Surg. 2008;121:401. doi: 10.1097/01.prs.0000298322.70032.bc. [DOI] [PubMed] [Google Scholar]
- 33.Liu G. Zhou H. Li Y. Li G. Cui L. Liu W., et al. Evaluation of the viability and osteogenic differentiation of cryopreserved human adipose-derived stem cells. Cryobiology. 2008;57:18. doi: 10.1016/j.cryobiol.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Yu G. Wu X. Dietrich M.A. Polk P. Scott L.K. Ptitsyn A.A., et al. Yield and characterization of subcutaneous human adipose-derived stem cells by flow cytometric and adipogenic mRNA analyzes. Cytotherapy. 2010;12:538. doi: 10.3109/14653241003649528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirschi K.K. Skalak T.C. Peirce S.M. Little C.D. Vascular assembly in natural and engineered tissues. Ann N Y Acad Sci. 2002;961:223. doi: 10.1111/j.1749-6632.2002.tb03090.x. [DOI] [PubMed] [Google Scholar]
- 36.Armulik A. Abramsson A. Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 37.Hellstrom M. Kalen M. Lindahl P. Abramsson A. Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 38.Frontini M.J. Nong Z. Gros R. Drangova M. O'Neil C. Rahman M.N., et al. Fibroblast growth factor 9 delivery during angiogenesis produces durable, vasoresponsive microvessels wrapped by smooth muscle cells. Nat Biotechnol. 2011;29:421. doi: 10.1038/nbt.1845. [DOI] [PubMed] [Google Scholar]
- 39.Mac Gabhann F. Peirce S.M. Collateral capillary arterialization following arteriolar ligation in murine skeletal muscle. Microcirculation. 2010;17:333. doi: 10.1111/j.1549-8719.2010.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz-Flores L. Gutierrez R. Madrid J.F. Varela H. Valladares F. Acosta E., et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 41.Stratman A.N. Malotte K.M. Mahan R.D. Davis M.J. Davis G.E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergers G. Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benjamin L.E. Golijanin D. Itin A. Pode D. Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benjamin L.E. Hemo I. Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 45.Enge M. Bjarnegard M. Gerhardt H. Gustafsson E. Kalen M. Asker N., et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benest A.V. Stone O.A. Miller W.H. Glover C.P. Uney J.B. Baker A.H., et al. Arteriolar genesis and angiogenesis induced by endothelial nitric oxide synthase overexpression results in a mature vasculature. Arterioscler Thromb Vasc Biol. 2008;28:1462. doi: 10.1161/ATVBAHA.108.169375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chacko S.M. Ahmed S. Selvendiran K. Kuppusamy M.L. Khan M. Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010;299:C1562. doi: 10.1152/ajpcell.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ong L.L. Li W. Oldigs J.K. Kaminski A. Gerstmayer B. Piechaczek C., et al. Hypoxic/normoxic preconditioning increases endothelial differentiation potential of human bone marrow CD133+ cells. Tissue Eng Part C Methods. 2010;16:1069. doi: 10.1089/ten.TEC.2009.0641. [DOI] [PubMed] [Google Scholar]
- 49.Reyes M. Dudek A. Jahagirdar B. Koodie L. Marker P.H. Verfaillie C.M. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang P. Baxter J. Vinod K. Tulenko T.N. Di Muzio P.J. Endothelial differentiation of amniotic fluid-derived stem cells: synergism of biochemical and shear force stimuli. Stem Cells Dev. 2009;18:1299. doi: 10.1089/scd.2008.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Efimenko A. Starostina E. Kalinina N. Stolzing A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med. 2011;9:10. doi: 10.1186/1479-5876-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pak O. Aldashev A. Welsh D. Peacock A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J. 2007;30:364. doi: 10.1183/09031936.00128706. [DOI] [PubMed] [Google Scholar]
- 53.Boero J.A. Ascher J. Arregui A. Rovainen C. Woolsey T.A. Increased brain capillaries in chronic hypoxia. J Appl Physiol. 1999;86:1211. doi: 10.1152/jappl.1999.86.4.1211. [DOI] [PubMed] [Google Scholar]
- 54.Amos P.J. Mulvey C.L. Seaman S.A. Walpole J. Degen K.E. Shang H., et al. Hypoxic culture and in vivo inflammatory environments affect the assumption of pericyte characteristics by human adipose and bone marrow progenitor cells. Am J Physiol Cell Physiol. 2011;301:C1378. doi: 10.1152/ajpcell.00460.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.