Abstract
A modular approach to adipose tissue engineering was explored by embedding adipose-derived mesenchymal stromal cells (adMSC) in sub-mm-sized collagen rods or “modules” and coating with human microvascular endothelial cells (HMEC). After subcutaneous injection into a SCID/Bg mouse, HMEC on modules containing embedded adMSC appeared to detach from the modules to form vessels as early as day 3, as confirmed by the human EC-specific UEA-1 lectin stain, and these vessels persisted for up to 90 days. Vessel numbers decreased over 14 days, but vessel size increased suggesting a maturing of the vasculature. Vessel perfusion with the host was confirmed at 21 days by microCT. HMEC on modules without embedded adMSC remained attached to the module surface at day 3 and UEA-1 staining disappeared over 14 days suggesting cell death. It appeared that cotransplantation with adMSC had an anti-apoptotic and proangiogenic effect on HMEC. The early revascularization strategy may be successful in supporting adMSC viability and differentiation, as a preliminary study suggests progressive fat accumulation in the HMEC+adMSC implants: ∼60% of the implant area stained positive for Oil Red O by day 90. adMSC-embedded modules without HMEC surface coating did not show similar levels of Oil Red O staining. All implant volumes decreased over the time course of the experiment, yet HMEC+adMSC module implants were larger than adMSC-only implants at day 90. Collagen gel is mechanically weak and contracts in vivo making it unsuitable as a biomaterial for adipose tissue engineering where volume maintenance is critical. When combined with an appropriate biomaterial, the modular approach to adipose tissue engineering may represent a successful strategy to engineer soft tissue substitutes of clinical relevance.
Introduction
Vascularization of tissue constructs is a primary need for large implants where Fick's law is inadequate to supply nutrients and oxygen and remove metabolic wastes. Hence, our interests in using modular tissue engineering with endothelial cell-coated collagen gel modules to effect vascularized implant sites.1–5 Beyond nutrient supply, the endothelial cells also influence the fate of cotransplanted cells and the resulting tissue, here, for example, fat.
There is a critical need for soft tissue reconstruction for patients suffering from cancer, trauma, deep burns, and congenital defects. These patients suffer from contour defects due to loss of soft tissue, mainly comprised of subcutaneous adipose tissue. The American Society of Plastic Surgeons cites that over 5.2 million reconstructive procedures were performed in 2009. Yet, despite the increasing clinical demand in reconstructive, cosmetic, and correctional surgery, there remains no optimal strategy for the regeneration and replacement of adipose tissue. The use of autologous adipose tissues for soft tissue replacement is one clinical approach that has proven problematic due to absorption, subsequent volume loss, and fibrosis of the transplanted tissue.6,7
Adipose-derived mesenchymal stromal cells (adMSC) are found in the stromal vascular fraction (SVF) of adipose tissues. These adult stromal cells are similar in phenotype and multipotency to bone marrow-derived mesenchymal stromal cells (bmMSC) and have gained much interest in recent years because of the near limitless supply of adipose tissue and the relative ease with which it can be harvested via liposuction or abdominoplasty procedures. We use the term adMSC in a broad sense to encompass all adipose precursors found in the SVF that include cells such as preadipocytes and what others call adipose-derived stem cells. adMSC have been successfully cultured on a wide array of materials for adipose tissue regeneration including synthetic materials such as biodegradable poly(lactic-co-glycolic acid) (PLGA),8 protein-coated polytetrafluroethylene,9 and polyglycolic acid10 and natural scaffolds such as hyaluronan-based,11 collagen12 and acellular placenta.13 Previous attempts at adipose tissue reconstruction using either autologous tissue or tissue engineering strategies, however, have commonly failed as a result of implant resorption due to ischemic cell death. Native adipose tissue is highly vascular, with each mature fat cell in contact with at least one capillary.14 We presumed therefore that a successful adipose tissue engineering strategy will require revascularization of the transplanted adipose construct.
Many studies have shown that stromal cells from bone marrow,15 skeletal muscle,16 umbilical cord blood,17 and adipose tissue18 can be used to treat patients with ischemic conditions such as myocardial infarction, heart disease, and peripheral vascular disease. It is believed that these cells, likely the pericyte population within these tissues,19,20 promote revascularization through the release of both proangiogenic and anti-inflammatory bioactive molecules. We have used bmMSC to enhance the chimeric vasculature produced when rat aortic endothelial cells were transplanted in an allogenic rat model with immunosuppression.21 Without the bmMSC, the chimeric vasculature containing both host and donor cells was immature at 21 days and was well (although likely not completely) formed at 60 days; with bmMSC, a mature vasculature was produced by 21 days.21
We also transplanted Human Umbilical Vein Endothelial Cells (HUVEC) on modules after subcutaneous transplant into severely combined immunodeficient mice (SCID/bg). Like others,22,23 HUVEC rapidly underwent apoptosis and no HUVEC-derived vessels formed.3 Here, we have chosen to use adipose-derived MSC to support the transplanted cells and enable vascularization, as others have done before in similar but different circumstances.24–29 For example, adMSC were combined with endothelial progenitor cells (EPC) in collagen implants and subcutaneously implanted in SCID mice to form a functional vessel network.20
Here, adMSC (a heterogeneous mixture of adipose precursor cells harvested from the stromal vascular fraction of human adipose tissue) were embedded in small collagen hydrogel rods (0.5 mm diameter, 1 mm length: modules) coated with adipose-derived human microvascular endothelial cells (HMEC). Early time points were investigated (day 3, 7, 14, and 21) to study the fate of the endothelialized lining of the adMSC-embedded modules while later time points (day 30, 60, and 90) were focused on determining the characteristics of the tissue created in the presence of the HMEC.
Materials and Methods
Cells
Primary human adMSC and HMEC were harvested from sterile abdominal fat samples obtained from patients undergoing elective surgery, with consent as published30 with modifications to include magnetic bead separation for HMEC. Briefly, the tissue was minced and digested in a 2 mg/mL collagenase solution at 37°C, containing Kreb's Ringer bicarbonate buffer, supplemented with 3 mM glucose, 25 mM HEPES, and 20 mg/mL BSA (all Sigma). Upon digestion the sample was filtered to remove undigested tissue fragments and centrifuged to separate the desired stromal vascular cell population from the buoyant mature adipocytes. The cell suspension was gently agitated in erythrocyte lysing buffer and filtered again to remove red blood cell fragments. Several centrifugation steps with washes in complete medium finished the processing. Half of the resultant cell pellet was plated for adMSC culture in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F-12 nutrient mixture (DMEM:Ham's F-12; Sigma), supplemented with 10% fetal bovine serum (FBS; Sigma), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Gibco). A similar protocol resulted in cells that are CD29+, CD34+, CD44lo, CD45lo CD73+, CD90+, and CD105+ and capable of undergoing both adipogenesis and osteogenesis in vitro.31
The other half of the stromal vascular cell pellet was plated in MCDB-131 (VEC Technologies) for HMEC purification. HMEC were purified from contaminating adMSC by CD31 positive selection using MiniMACS™ magnetic bead separation columns and a CD31 MicroBead Kit (Miltenyi Biotech). Briefly, cells obtained from the harvest were preplated in T-150 flasks for 72 h to allow attachment and growth of HMEC before separation. Following manufacturer's instructions, cells were trypsin-released and centrifuged at 300 g for 3 min and resuspended to a maximum concentration of 107 cells/60 μL medium. Twenty microliter of FcR Blocking Reagent was then added and vortexed briefly before adding 20 μL of the CD31 magnetic beads. Following incubation at 4°C for 15 min the cells were washed in medium and resuspended in 1 mL medium and passed through 2 MS MACS® separation columns in series (to improve separation efficiency) in a MiniMACS™ separator. The purified cells were cultured in a T-25 flask and expanded until a confluent T-75 flask of cells was obtained. The magnetic bead separation procedure was conducted a second time to further purify the HMEC from any contaminating adMSC. Cytospin slides of the cells obtained from this procedure were immunostained for Von Willebrand factor and revealed a pure population of HMEC without adMSC contamination.
Primary HUVEC (Lonza), cultured in EGM-2 culture medium (Lonza), were also used in one set of implantations to compare different types of EC.
The growth medium on the cells was changed every 2–3 days. To passage the cells, cultures at 90% confluence were trypsin-released (0.25% trypsin/0.1% EDTA, Gibco), washed, counted, and replated in new flasks at 30,000 cells/cm2. adMSC at passage 2–3 and HMEC at passage 4–6 were used for in vivo implantations. HUVEC were used at passage 4.
Module fabrication
To prepare modules,32 bovine Type 1 collagen (3 mg/mL, PureCol™, Inamed) was mixed with 10×MEM (10.1 g α-Minimum Essential Medium powder (Gibco, Invitrogen), and neutralized to pH ∼7.4 by adding the required volume of 0.8N NaHCO3. adMSC (106 cells/mL) were added to the collagen solution to prepare adMSC-embedded modules. This solution was cast into the inner diameter of sterile 0.71 mm ID polyethylene tubing (PE 60, Intramedic–BD Canada) and subsequently gelled for 60 min at 37°C. The tubing was cut into small pieces (∼2 mm long×0.6 mm diameter) using a custom automatic tube cutter and collected in DMEM:F12 Ham's media, where the modules were separated from the tubing by vortexing and cultured for 72 h at 37°C in a petri dish to allow contraction of the modules by adMSC. Settled modules (1 mL, produced using 3 m of tubing) with or without embedded adMSC, were dynamically seeded with HMEC or HUVEC (2×106) for 45 min on a low speed shaker and incubated overnight in a 50/50 mixture of DMEM:F12 Ham and MCDB-131 (HMEC) or EGM-2 (HUVEC). adMSC-only modules were prepared as above without endothelial cell seeding. HMEC-only modules were prepared as above with collagen solution without adMSC.
Module implants
Adult male (6 weeks of age) SCID/Bg mice (Charles River Laboratories) were individually housed in sterile cages and fed ad libitum under the approval by the University of Toronto animal care committee. Three different treatment groups were studied: modules coated with EC, without embedded adMSC; modules embedded with adMSC and coated with EC; modules with embedded adMSC, without EC. Approximately 250 modules (a packed volume of 40 mm3) suspended in PBS were subcutaneously injected (superficial to panniculus carnosus) using an 18G needle into each of two separate sites on the dorsum of mice. The mice were euthanized at various time points (3, 7, 14, 21, 30, 60, and 90 days) and the implants excised. The dimensions of each implant were carefully measured with calipers and the volume estimated using the volume of an ellipsoid. One implant was fixed in 4% neutral buffered formalin (Sigma Aldrich) for 48 h and one was placed in Tissue-Tek® O.C.T. compound and flash frozen in liquid nitrogen for frozen tissue processing (Oil Red O only). The number of animals tested at each time point varied depending on animal survival, particularly at later times. Images shown are representative of 12 implants or animals per treatment for times up to day 21; images are representative of 9 or 6 implants or animals for day 30 and day 60 or day 90 respectively. Sections were processed and stained for hematoxylin and eosin (H&E, Fisher), Masson Trichrome (Fisher), Oil Red O (Sigma), Ulex Europeus Agglutinin-1 (UEA-1, Vector, cat# B1201), and CD31 (Santa Cruz, cat# sc1506). Histology was performed by the Pathology Research Laboratory at Toronto General Hospital. All sections were viewed with a Zeiss Axiovert light microscope equipped with a CCD camera.
Histology quantification
UEA-1 stained positive vessels (donor cells) at day 3, 7, and 14 were counted using a Microvessel Density Count method as used before. At low power (2.5× Objective lens), the number and diameter of UEA-1 stained structures in the whole section (not hotspots) was counted using ImageJ (version 1.4.3). Three randomly selected sections were counted to give an average vessel count per implant and nine representative implants were counted for the experiment (n=9). The presence of a patent lumen or the presence of erythrocytes was not a prerequisite for the definition of a microvessel; yet, at this low magnification only larger vessel-like structures could be counted. Counts were expressed as the number of vessels per mm2 of implant.
The level of Oil Red O staining in implants at day 30, 60, and 90 was quantified using ImageJ. Low magnification images (2.5× objective) from three different animals (three sections per implant) at each time point for each treatment were taken. The sections were cut at regular intervals from a randomized start through the entire specimen to minimize selection bias. This preliminary experiment was conducted twice (n=6 animals for day 30 and 60, n=3 animals for day 90 due to animal death at this age). Again, at this low magnification the total area of the section containing the module implant was visualized. The area that was stained red by Oil Red O was calculated and was divided by the total area of the implant. The result was given as a% area of the implant stained positive for Oil Red O.
MicroCT study
The level of vascular perfusion in the implants was studied by microCT analysis at 21 days. Following a published protocol,33 mice were first heparinized (100 units, LEO Pharma, Inc.) by subcutaneous injection for 5 min before the procedure. Animals were then anesthetized with isofluorane and the descending aorta was cannulated and heparinized. PBS (5 U/mL) was perfused at a constant pressure of 100 mmHg to flush the blood from the vascular system. The mice were then perfused with 10 mL of Microfil® solution (MV-122; Flow-tech) and the solution was allowed to polymerize for 2 h. Implants were excised into 4% formalin (Sigma Aldrich), embedded into 1% agar, and visualized with a General Electric Medical Systems MS8 microCT.
Statistical analysis
All statistical analyses were performed using the software program STATISTICA Version 5.1 (Statsoft). Analysis of variance with Tukey's post-hoc analysis was used to compare the mean between groups analyzed and considered significant at p<0.05.
Results
Angiogenesis and remodeling
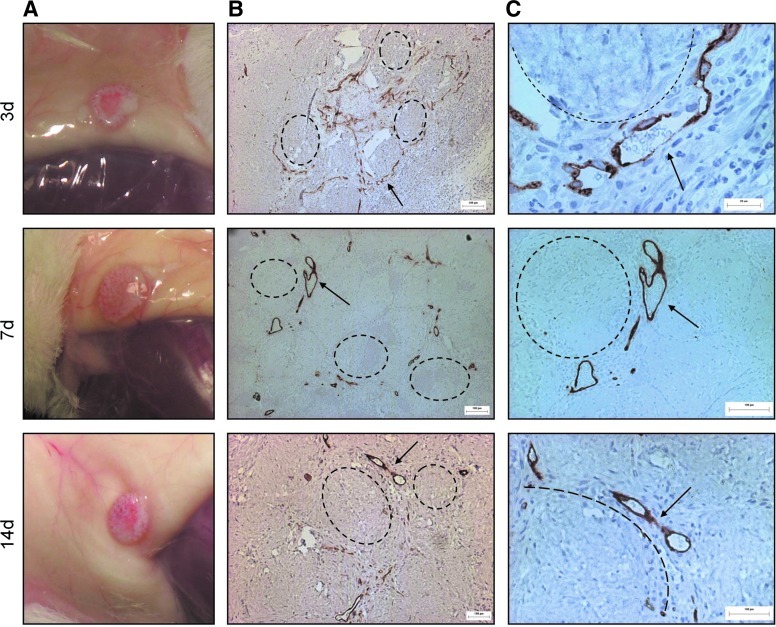
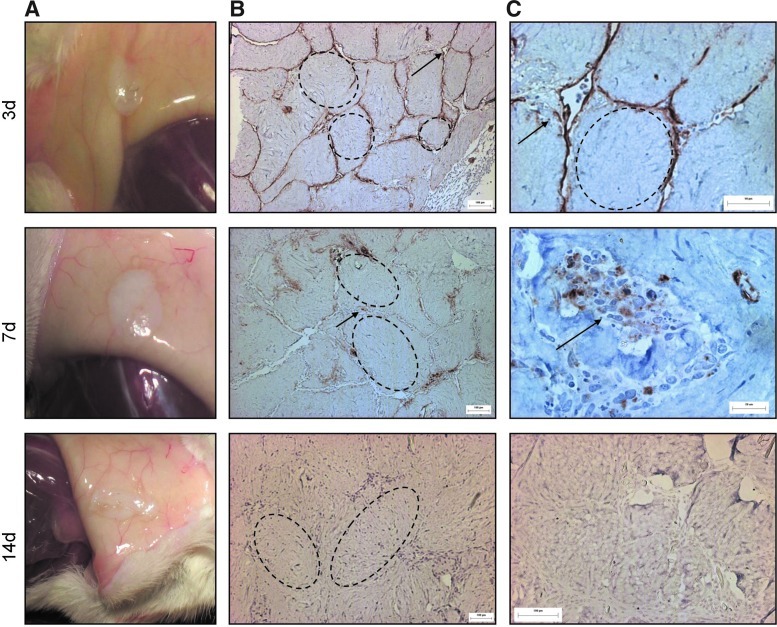
For implants taken out after 21 days, the focus was on the effect of embedded adMSC on the viability and angiogenic potential of the surface-coated HMEC. Upon visual inspection at explant, implants containing modules made with both surface-coated HMEC and embedded adMSC (HMEC+adMSC) formed a visible aggregate that was red and appeared vascularized as early as day 3 with what appeared to be blood vessels throughout the volume of the implant (Fig. 1A). By histological examination using the human-specific UEA-1 antibody, HMEC surrounding the modules containing adMSC had detached from the module surface and organized into vessels. At day 3, the vessels appeared to be disorganized and immature (Fig. 1B); yet, many contained erythrocytes (Fig. 1C) suggesting anastomosis with the host at this early time point. By day 7 and 14, the HMEC-derived vessels appeared organized, mature, and larger (Fig. 1B), again with vessels containing erythrocytes (Fig. 1C). With modules without embedded adMSC but with surface HMEC (HMEC-only), the module aggregate was opaque and avascular at all time points (Fig. 2A). UEA-1 lectin staining outlined individual modules at day 3, with staining diminished by day 7 and absent by day 14 indicating HMEC cell death over time (Fig. 2B, C). There were no HMEC-derived vessels found in the absence of adMSC cotransplantation. This is similar to what was seen with HUVEC in an earlier study where HUVEC (without the presence of a supporting cell) were found to undergo apoptosis.3
FIG. 1.
Adipose-derived mesenchymal stromal cells (adMSC) Cotransplantation drives HMEC vessel-like structure formation surrounding modules. Photographs of human microvascular endothelial cells (HMEC)+adMSC modular implants appear red, with blood vessels visible throughout the module clump at day 3, 7, and 14 (A). UEA-1 lectin staining (B) shows that the human-derived EC detach from the module surface to form vessel-like structures. These vessels appear to become larger and more organized at day 7 and 14. Higher magnification UEA-1 lectin images (C) show that these vessels contain erythrocytes suggesting anastomosis with the host. Arrows highlight some large UEA-1 stained vessels containing erythrocytes, with some modules highlighted by dashed lines. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
FIG. 2.
Without adMSC, HMEC do not organize into vessels and disappear over time. Photographs of HMEC-only explants appear opaque and avascular at day 3, 7, and 14 (A). UEA-1 lectin staining (B) shows that without adMSC, HMEC remain on the module surface (day 3) and the UEA-1 staining diminishes over time suggesting cell death (day 7). By day 14, no HMEC can be found. Higher magnification UEA-1 images (C) show HMEC disappearance over time. Arrows highlight some UEA-1 staining, with some modules highlighted by dashed lines. Color images available online at www.liebertpub.com/tea
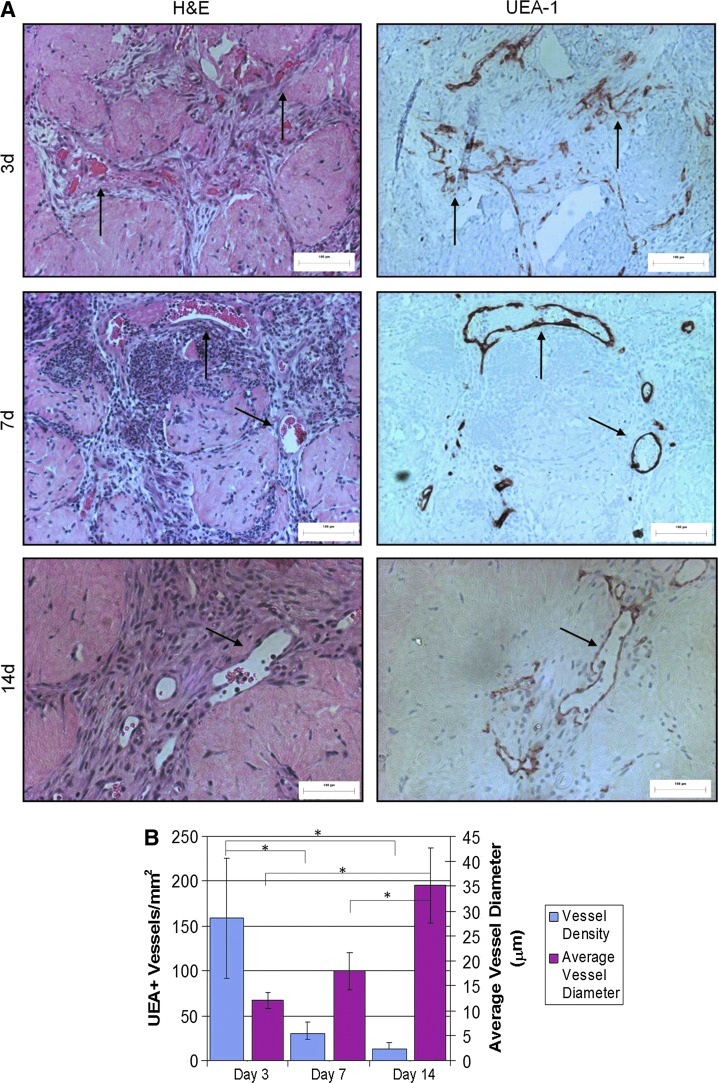
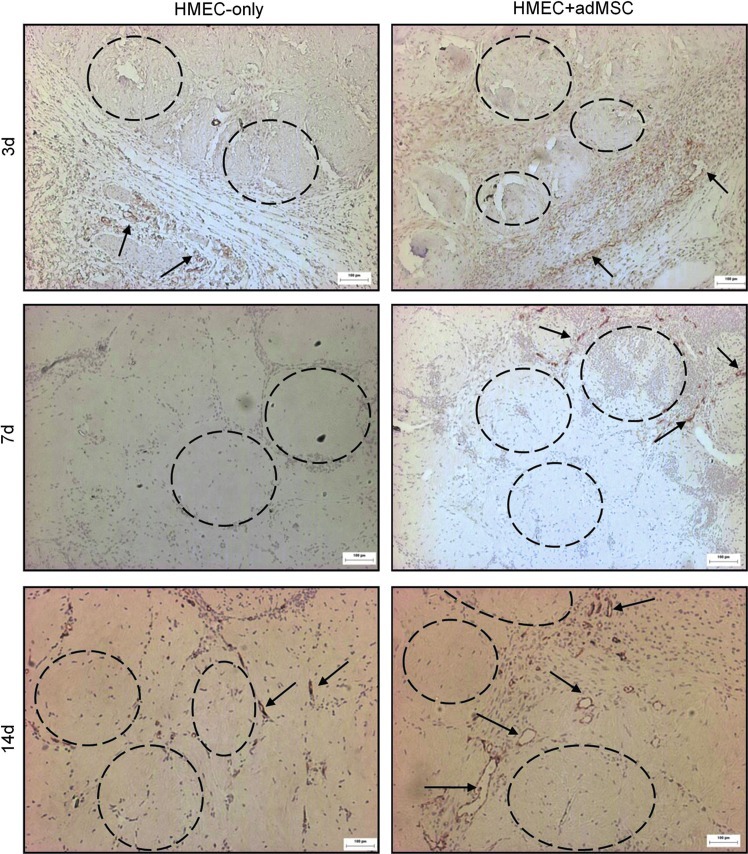
In the HMEC+adMSC implants there was initially a large number (>150 UEA-1+ structures present in the area of the implant (Fig. 3). Most of these structures were unorganized and small (<10 μm in diameter). By day 14, the total number of UEA-1+ structures had diminished considerably but those that remained were larger (>30 μm in diameter). These results suggest a typical angiogenic response that begins with a large number of small immature vessels that are then organized into larger functional vessels. As expected, it appeared that the adMSC also had an effect on the response of the host tissue to the implanted modules (Fig. 4). Murine blood vessels that stained positive for CD31 were seen surrounding both HMEC-only and HMEC+adMSC modular implants at day 3. By day 7, host blood vessels had infiltrated the outer edges of the HMEC+adMSC and by day 14, many CD31+ vessels were seen throughout the HMEC+adMSC implant. In contrast, HMEC-only implants showed no host blood vessel infiltration at day 7 with a few small blood vessels in the HMEC-only implants at day 14. Both HMEC (UEA-1+) and host-derived (CD31+) blood vessels were found surrounding the HMEC+adMSC modules by day 14 (Fig. 5).
FIG. 3.
HMEC-derived vessels contain mouse erythrocytes. These vessels decrease in number but increase in size over 14 days with adMSC cotransplantation indicating vessel maturation. Hematoxylin and eosin (H&E) staining of micrographs of HMEC+adMSC modules shows vessels containing erythrocytes surrounding modules at day 3, 7, and 14. Corresponding serial sections stained with UEA-1 show that these vessels are of human origin (arrows highlight some vessels) (A). Microvessel density counts showed a large number of small vessels observed at early time points, while by day 14 the number of vessels has decreased and the average vessel diameter is larger (B). No HMEC vessels were counted for the HMEC-only case as this number was zero for all time points. Error bars represent±SD; n=9; * p<0.05. Color images available online at www.liebertpub.com/tea
FIG. 4.
adMSC cotransplantation enhances host tissue angiogenesis into the modular construct. Micrographs of HMEC-only and HMEC+adMSC modules stained for CD31(mouse) show the progressive infiltration of host-derived vessels into the modular construct. At day 3, CD31+ vessels (some highlighted with arrows) were seen at the edge of the modular construct in both the HMEC-only and HMEC+adMSC case (some modules highlighted by dashed circles). By day 7, CD31+ vessels were seen infiltrating into the HMEC+adMSC but not in the case without adMSC. By day 14, many larger CD31+ vessels have infiltrated into the HMEC+adMSC construct while the case without adMSC remains relatively avascular. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
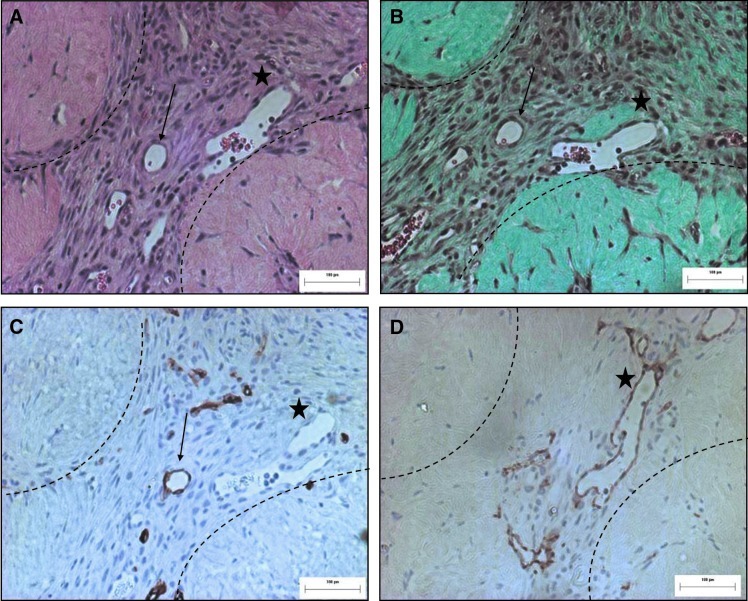
FIG. 5.
Higher magnification images show both human and mouse vessels surrounding HMEC+adMSC modules at day 14. Serial sections of HMEC-adMSC modular implants (A, H&E), (B, Trichrome) highlight two vessels (arrow, star) surrounding modules containing erythrocytes. Both UEA-1+ (C) (arrows, human) and CD31+ (D) (stars, mouse) blood vessels are observed surrounding the modules at day 14. Dashed line highlights module location. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
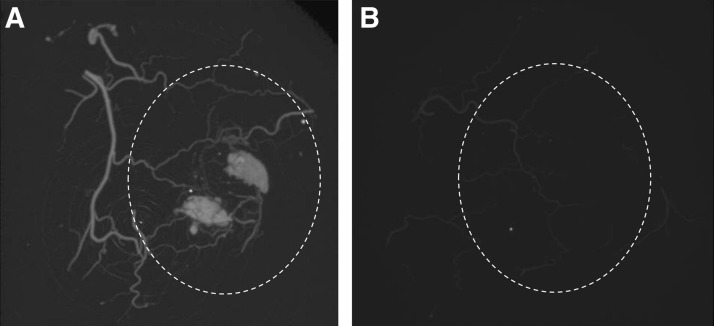
A microCT image of the implant (region marked by dashed line) under the mouse skin shows that the vessels were perfuseable in the HMEC+adMSC case at day 21 (Fig. 6A). In this sample, blood vessels are seen branching off from a large vessel in the skin to infiltrate the region of the implant. Two pools of microfil (the contrast agent) were seen within the implant and this may be the result of leaky vessels or vessel rupture caused due to high pressure during perfusion. Without adMSC cotransplantation, HMEC-only samples remained avascular with only the blood vessels in the skin visible (Fig. 6B). This is in accordance with the lack of vascularization seen in histology for the HMEC-only control case.
FIG. 6.
MicroCT demonstrates perfusion of the adMSC+HMEC implants by day 21. MicroCT images of HMEC+adMSC (A) and HMEC-only (B) module implants under mouse skin at day 21. Many blood vessels infiltrate the HMEC+adMSC implant (A), while the HMEC-only implant (B) remains avascular. Dashed lines show region of implant.
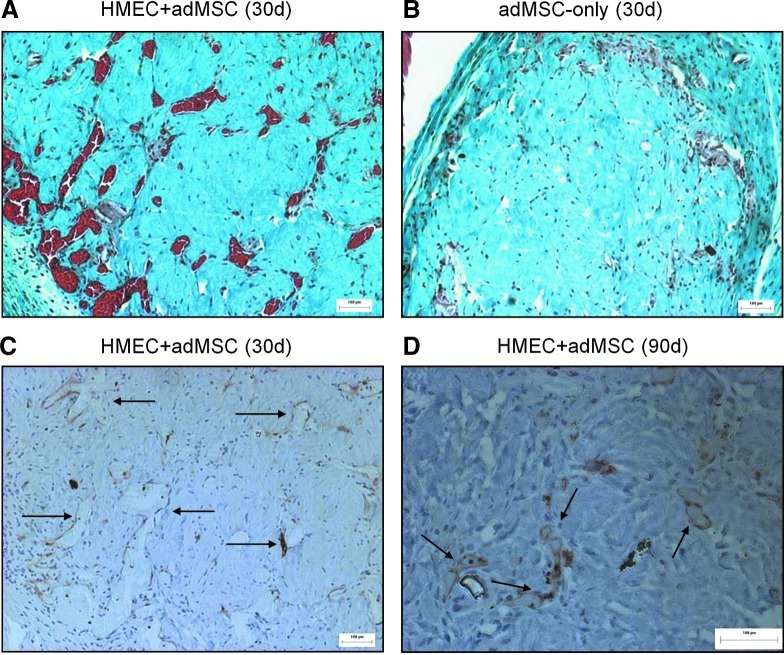
At longer time points, a clear difference in the level of vascularization between the HMEC+adMSC and adMSC-only modular implants is maintained. At day 30, HMEC+adMSC explants were rich in large red blood vessels filled with erythrocytes (Fig. 7A) while the adMSC-only explants were largely avascular (Fig. 7B). UEA-1 staining revealed that many of these large blood vessels were derived from the implanted HMEC (Fig. 7C) with UEA-1 lectin staining shown to persist in the HMEC+adMSC implants as long as 90 days post implantation (Fig. 7D).
FIG. 7.
HMEC+adMSC implants remain vascularized at day 30 with HMEC surviving out to 90 days. Trichrome micrographs of HMEC+adMSC (A) and adMSC-only (B) implants 30 days after implantation show that many erythrocyte-filled blood vessels are visible in the case with cotransplantation while the implant with adMSC-only modules appears avascular. UEA-1 micrographs of HMEC+adMSC modules show large HMEC-derived vessels (arrows) at day 30 (C). A higher magnification image at day 90 shows UEA-1+ staining demonstrating some HMEC survival up to 90 days in HMEC+adMSC module implants (D). Color images available online at www.liebertpub.com/tea
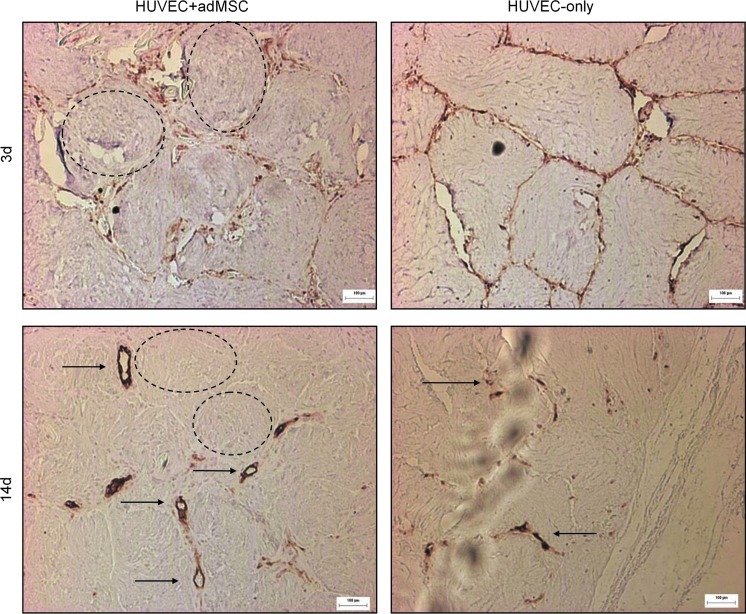
Similar results were also obtained with HUVEC. Similar to HMEC, HUVEC were seen to detach from the modules containing adMSC and form vessels, while HUVEC on modules without adMSC remained on the module surface with a decrease in UEA-1 staining over time suggesting cell death (Fig. 8). In contrast with the HMEC experiments, a few small HUVEC vessels were present at day 14 without adMSC, as was seen before.3
FIG. 8.
Similar to the HMEC, adMSC causes HUVEC to detach from the module surface to form vessels. HUVEC on modules without adMSC remain on the module surface and the UEA-1 staining diminishes over time suggesting cell death. Unlike HMEC, some small HUVEC structures are present surrounding modules without adMSC at day 14. Some modules highlighted by dashed lines. Arrows highlight some large UEA-1 stained vessels containing erythrocytes. Scale bar 100 μm. n=4 implants. Color images available online at www.liebertpub.com/tea
Fat development
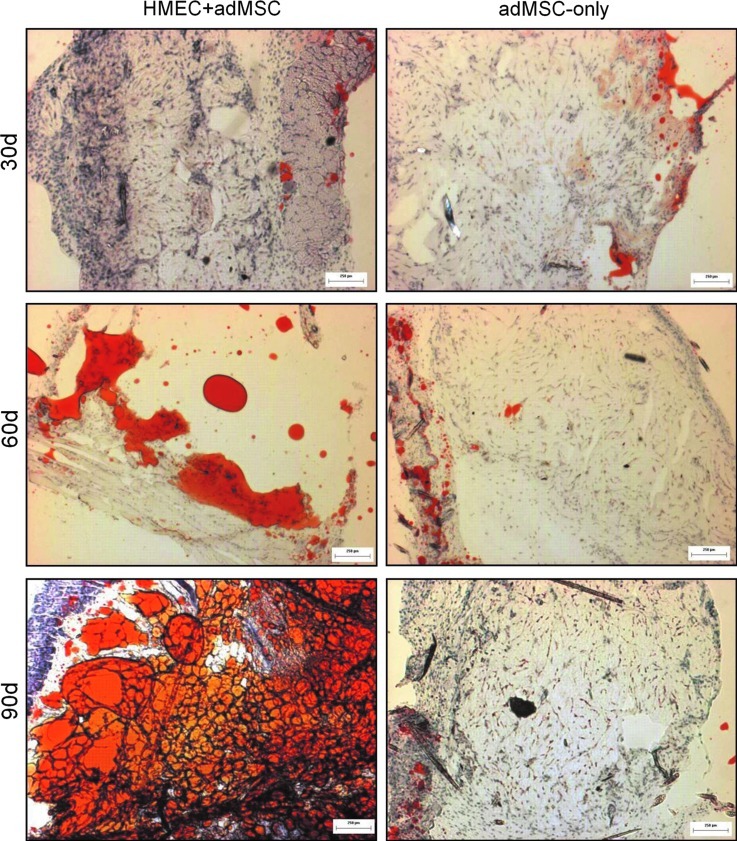
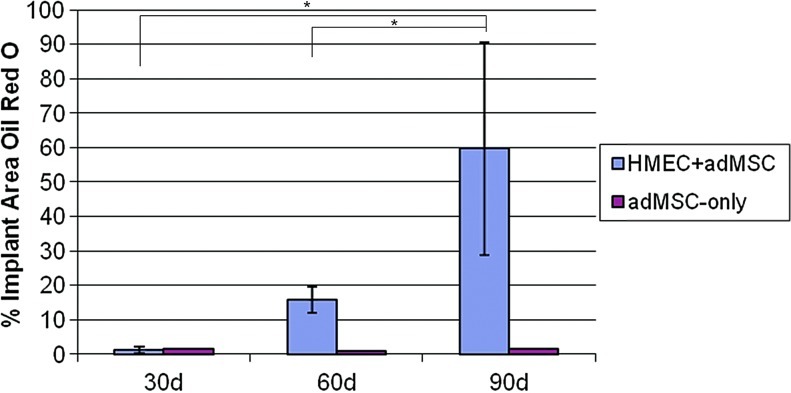
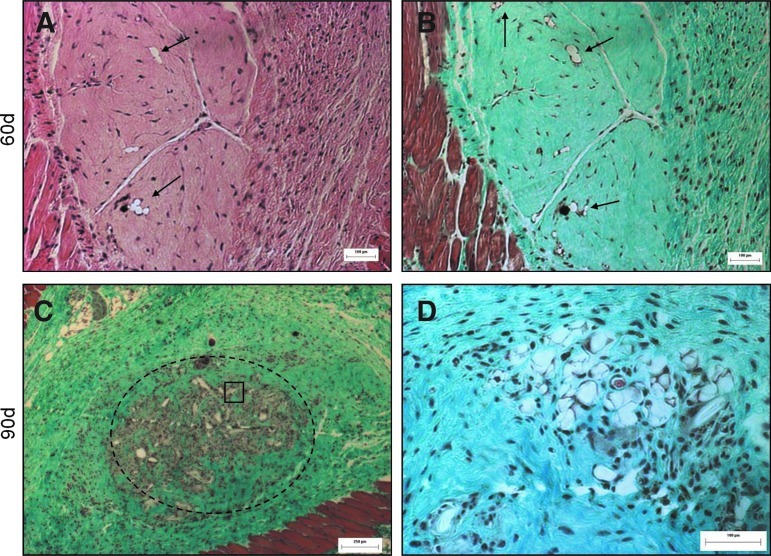
In a preliminary study (n=6, day 30 and 60; and n=3, day 90), Oil Red O staining on frozen sections of the modules at explant appeared to reveal progressive fat accumulation in the HMEC+adMSC modules over the 90-day implant period (Fig. 9), but not in the absence of the endothelial cells. At day 30 post implantation, no Oil Red O staining was evident in either the HMEC+adMSC or adMSC-only control case. By day 60, much red staining was seen in the region surrounding the HMEC+adMSC module implant with pockets of red staining within the module clump suggesting fat accumulation. By the 90 day time point, extensive Oil Red O staining was seen throughout the region of the HMEC+adMSC module clump suggesting a high level of fat differentiation and/or accumulation. In the adMSC-only case, a few small pockets of red staining could be observed by day 60 but much less than what was observed in the HMEC+adMSC case (Fig. 10). Image J analysis of the red staining (Fig. 10) showed that approximately 60% of the implant area stained positive for Oil Red O by day 90 in HMEC+adMSC implants. It appears that early vascularization, presumably driven by HMEC cotransplantation, may be beneficial for fat development at later time points. At least some of the Oil Red O staining in the HMEC+adMSC modular implants seen here was attributed to the transplanted adMSC differentiating into mature fat cells. This was confirmed with higher magnification images of H&E and Masson's Trichrome micrographs at day 60 that showed the typical unilocular appearance of fat cells within individual modules (Fig. 11A, B). At day 90, extensive clustering of adipose cells was seen within the implant region suggesting a maturation of adipose tissue (Fig. 11C, D).
FIG. 9.
HMEC and adMSC cotransplantation enhances Oil Red O staining in the modular implant. Oil Red O images of frozen sections from HMEC+adMSC and adMSC-only module implants at day 30, 60, and 90 post implantation. Little to no Oil Red O staining was seen in the adMSC-only case. Scale bar=250 μm. Color images available online at www.liebertpub.com/tea
FIG. 10.
Percentage of total implant area on frozen sections stained red by Oil red O. Percentage of area stained red for Oil Red O on micrograph sections of HMEC+adMSC and adMSC-only module implants at day 30, 60, and 90. Increased Oil Red O staining is seen in the module implants containing both adMSC and HMEC, while little staining is observed in adMSC-embedded modules without HMEC. Error bars represent±SD. n=6 for day 30 and 60; n=3 for day 90; *p<0.05. Color images available online at www.liebertpub.com/tea
FIG. 11.
adMSC differentiate into fat cells within the HMEC+adMSC modules by day 60 with clusters of adipose cells visible by day 90. Serial sections of H&E (A) and Trichome (B) micrographs of HMEC+adMSC modules at day 60. Arrows highlight the unilocular appearance of adipose cells visible within the individual HMEC+adMSC modules. By day 90, trichrome (C) shows extensive fat accumulation within the implant region (dashed line). (D) Higher magnification image of the boxed region shows clustering of adipose cells. Color images available online at www.liebertpub.com/tea
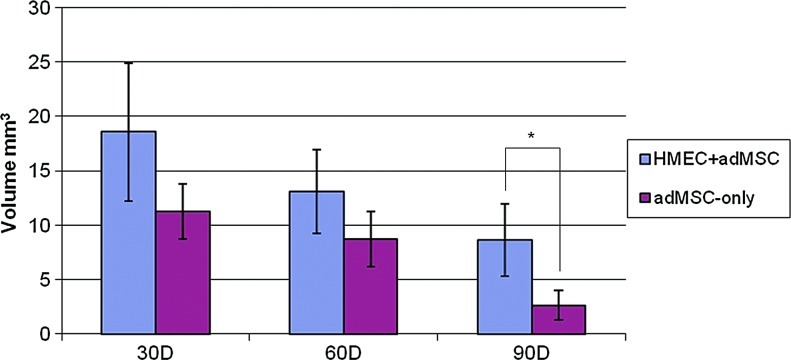
The explants shrank over time (Fig. 12, initial volume was 40 mm3) consistent with the degradation of the collagen gel. Most interestingly though, at day 90, HMEC+adMSC implants were larger than the adMSC-only implants (p<0.05). Presumably, the fat accumulation in HMEC+adMSC implants helped to reduce the loss of implant volume.
FIG. 12.
Implant Volume Measurements. At explant, both HMEC+adMSC and adMSC-only module implants show progressive shrinkage over 90 days. At day 90, HMEC+adMSC implants are statistically larger than adMSC-only implants, which was attributed to the observed Oil Red O staining in HMEC+adMSC implants. Initial volume at implantation=40 mm3. Error bars represent±SD. n=6 at day 30 and 60; n=3 at day 90; *p<0.05. Color images available online at www.liebertpub.com/tea
Discussion
adMSC support revascularization of modular constructs
HMEC transplantation as a revascularization strategy was successful in the implants with modules containing adMSC as a supporting cell. As early as day 3, HMEC were found to detach from the surface of the modules and organize into primitive vessels with lumens and many containing erythrocytes (Fig. 1B, C), suggesting rapid anastomosis with the host vasculature. By day 7 and 14, the number of vessels had reduced but the vessels that remained were large (∼200 μm diameter) suggesting that the initial leaky vessels had matured and organized during the angiogenic process (Fig. 2). Successful inosculation, at least by day 21, of the HMEC+adMSC implants with the host vasculature was confirmed by MicroCT (Fig. 6). Further, vessels derived from the transplanted HMEC (with embedded adMSC) were shown to persist in vivo for up to 90 days without drugs or the addition of anti-apoptotic genes (Fig. 7D). HMEC on modules without embedded adMSC, on the other hand, were found to remain on the surface of the modules at day 3 and UEA-1 lectin staining was shown to disappear over 14 days suggesting cell death without the presence of a supporting cell (Fig. 2).
adMSC are known to secrete many bioactive molecules, such as vascular endothelial growth factor (VEGF), hepatocyte growth factor, and granulocyte macrophage colony-stimulating factor that have been shown to promote EC survival and proliferation.34 adMSC may induce angiogenesis through paracrine support of EC35 and suppression of their apoptosis36,37 and may actually integrate into the host vasculature.37 Increasing evidence has shown that the adMSC in adipose tissue may reside in a perivascular location and that pericytes may actually be progenies of the adMSC.38,39 Further, adMSC may be able to aquire properties of pericytes both in vitro and in vivo following harvesting and expansion.19,20 Borges et al. and colleagues showed, using HDMEC spheroids and adMSC in a fibrin matrix in a chorioallantoic membrane model, a patent connection of tissue-engineered microvessels in adipose tissue to a host vessel system without applying exogenous angiogenic growth factors.40 More recently, adMSC and EPC were embedded in a collagen gel and subcutaneously implanted in SCID mice.20 Cotransplantation of both cells increased the density and complexity of the vascular networks many fold greater than in implants with each cell alone. Both direct contact and paracrine effects were thought responsible, with the former thought to be more important. Interestingly, the monocyte-derived endothelial progenitors were embedded with the adMSC in the literature case20 while we coat the collagen gel with mature EC in our case. It is not known whether these differences, particularly in terms of direct cell contact, are substantive. In related work with modules containing bone marrow-derived MSC in modules, there is considerable migration to a “pericyte-like” position and proliferation of the bmMSC in vitro41 and in vivo21 so that the direct cell contact emerges over time.
Alternative methods to achieve stability of donor EC-derived vessels include transfecting an anti-apoptotic gene into the EC or cotransplantation with supporting cells other than MSC. Bcl-2 is an anti-apoptotic gene that has been shown to extend EC survival in vitro42 and help establish stable HUVEC-lined microvessels in vivo.43,44 Cocultures of EC with pericytes (bovine retinal) in collagen gel45 or with fibroblasts (human dermal) in tissue-engineered skin46 and (human lung) in fibrin gel47 also stabilized tubular network formation. Perivascular precursors (10T1/2 cells) and embryonic fibrobasts have also been cocultured with HUVEC in collagen gel scaffolds48 and the resulting vascular network in SCID mice was shown to be functional and stable for 1 year in vivo. These precursor cells likely differentiated into smooth muscle cells that acted as a support for donor vessel maturation and stability.
We hypothesize that in addition to adMSC paracrine support of EC, adMSC “pericytes” help to stabilize vascular network formation via direct contact.19,20 While EC vessels may initially form, without this mural layer (of adMSC-derived pericytes or smooth muscle cells) these small vessels may degrade followed by EC death, which is what was observed in the case without adMSC cotransplantation. The next step is to study and track the fate of the adMSC in vivo to see whether adMSC/pericyte vessel stabilization is responsible for the formation (and maturation) of the vascular network.
The HMEC-driven vessels are expected to minimize ischemic conditions upon implantation and during adMSC differentiation and remodeling. There may yet be an advantage that both adMSC and HMEC are derived from the same patient, meaning that the adipose tissue-engineered construct can be an autologous transplant. This of course requires further evaluation especially in non-immune compromised animals. Nonetheless, it is worth noting that similar results were obtained with HUVEC.
Adipose tissue development
The formation of the vascular network appeared to support the transplanted cells. Only in the case of cotransplantation did we see significant amounts of Oil Red O staining inside the modules (Fig. 9) beginning at day 30 and progressing. until the end point of the study (90 days), where the average Oil Red O micrograph was stained ∼60% of total area for Oil Red O (Fig. 10). While we cannot be certain that all of this Oil Red O staining is derived from the transplanted adMSC, higher magnification images of H&E and Masson's Trichrome stained sections show the unilocular appearance of fat cells within individual modules (Fig. 11), suggesting that at least some of the transplanted adMSC had differentiated into mature adipocytes. These preliminary results are promising and further evaluation as to the ability of the HMEC+adMSC modules to support adMSC viability and differentiation in vivo is needed for definitive conclusions.
Interestingly, adMSC-embedded modules without the surface coating of HMEC were not shown to stain positive by Oil Red O (Fig. 9). This is in contrast with several studies that have shown that adMSC can differentiate into adipocytes even when implanted alone,8,49 although the extent of this conversion is not large. While others have focused on predifferentation of adMSC in vitro and growth factor delivery (i.e., basic fibroblast growth factor) to enhance adipose tissue formation,49 we focused on a revascularization strategy. The lack of adMSC differentiation when implanted alone without HMEC may be simply the result of cell death due to lack of revascularization seen without HMEC cotransplantation. The density of adMSC in the cell-contracted collagen modules may have been too high in our case for the cells to survive by simple diffusion of oxygen and nutrients alone. Paracrine signaling between the HMEC and adMSC likely encouraged the development of Oil Red O positive staining in the cotransplantation case. In vitro work has shown that extracellular matrix factors secreted by microvascular endothelial cells, derived from adipose tissue, stimulate both adMSC proliferation50 and differentiation51 into mature fat cells in primary culture. In an in vivo study, inhibition of angiogenesis by a VEGFR2 blocking antibody not only reduced angiogenesis and tissue growth, but also inhibited 3T3-F442A (a preadipocyte cell line) differentiation into fat as well.52
Simple delivery of adMSC without attention to the mechanical properties of the implant and a suitable revascularization strategy is unlikely to produce a sizeable volume of adipose tissue. adMSC in fibrin gel subcutaneously implanted in athymic mice, for example, did not show extensive adipose tissue formation without the use of a support structure at 6 weeks.53 The successful correction of soft tissue defects represents a challenge in plastic surgery. While many current tissue engineering strategies are successful in initially generating an accumulation of adipose tissue in vivo, they fail to engineer an adipose tissue graft that will remain viable and retain its volume over the long term (>3 months). In one of the few published long-term studies, the volume of adipose tissues engineered with adMSC seeded onto a PLGA scaffold and subcutaneously implanted into a rat was found to be maximal at 2 months, decreased by 3 months, and completely resorbed by months 5–12.54 This reduction in implant volume was similar to what was observed here. However, the amount of adipose tissue development in the more slowly degrading PLGA scaffolds was only ∼4% by 3 months, which was only a small (albeit significant) improvement compared with the ∼2% in the acellular controls54 and much less than the amount (60%) of Oil Red O staining that we saw at this time point. In another study, adMSC seeded onto collagen scaffolds subcutaneously implanted into mice decreased in mass by approximately 50% after 3 weeks implantation and then remained stable for up to 2 months, yet with no statistically significant difference for implant weight or thickness between control and adMSC groups at the longer time point.12 The volume loss of adipose tissue may be the result of inadequate mechanical/degradation properties of the biomaterial and also the lack of sufficient neovascularization upon implantation. Necrosis due to insufficient blood supply after cell or tissue transplantation results in considerable volume loss. This is likely also the reason why autologous fat transfers have limited clinical outcomes.6
Here, volume measurements of the explants showed less shrinkage with cotransplantation of HMEC and adMSC than with adMSC alone, although both lost considerable volume over time, presumably in part due to collagen degradation. While the lower volume loss is promising, the observed reduction in size of an HMEC containing tissue construct is not clinically acceptable. It would be interesting to alter the degradation rate of the collagen gel say by cross-linking the gels with transglutaminase.55 The modified collagen modules would likely degrade more slowly with cross-linking so that maturation of the fat happens before the support structure has degraded so as to promote a long-term volume stable mass of adipose tissue.
Conclusions
Cotransplantation of adMSC and HMEC in collagen gel modules subcutaneously implanted into SCID/Bg mice rapidly created a vascular network. With the presence of adMSC, HMEC detached from the surface of the modules to form vessels that contained erythrocytes as early as day 3. HMEC-derived vessels were shown to decrease in number but increase in size over the first 14 days suggesting vessel maturation and persisted for up to 90 days in vivo. With successful early vascularization, HMEC+adMSC modules may have supported adMSC viability and perhaps even differentiation to create a construct that maintained more of its volume over 90 days than did adMSC module implants without HMEC.
Acknowledgments
The authors acknowledge the financial support of the U.S. National Institutes of Health (EB006903) and the Canadian Institutes of Health Research (MOP-89864). MJB thanks the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Advanced Regenerative Tissue Engineering Centre (ARTEC) for fellowship support. We are grateful to Dr. John Semple and Women's College Hospital for the donations of adipose tissue, to Chuen Lo for his technical expertise in animal surgeries, and to Kim Woodhouse for her guidance and support. Also, we thank Lisa Yu (Dr. R. M. Henkelman) for microCT imaging and Toronto General Hospital's Pathology research group for all histology and immunostaining services.
Disclosure Statement
No competing financial interests exist.
References
- 1.Chamberlain M.D. Gupta R. Sefton M.V. Chimeric vessel tissue engineering driven by endothelialized modules in immunosuppressed Sprague-Dawley rats. Tissue Eng Part A. 2011;17:151. doi: 10.1089/ten.tea.2010.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chamberlain M.D. Butler M.J. Ciucurel E.C. Fitzpatrick L.E. Khan O.F. Leung B.M., et al. Fabrication of micro-tissues using modules of collagen gel containing cells. J Vis Exp. 2010;46:2177. doi: 10.3791/2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper T.P. Sefton M,V. Fibronectin coating of collagen modules increases in vivo HUVEC survival and vessel formation in SCID mice. Acta Biomater. 2011;7:1072. doi: 10.1016/j.actbio.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta R. Van Rooijen N. Sefton M.V. Fate of endothelialized modular constructs implanted in an omental pouch in nude rats. Tissue Eng Part A. 2009;15:2875. doi: 10.1089/ten.tea.2008.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta R. Sefton M.V. Application of an endothelialized modular construct for islet transplantation in syngeneic and allogeneic immunosuppressed rat models. Tissue Eng Part A. 2011;17:2005. doi: 10.1089/ten.tea.2010.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ersek R.A. Transplantation of purified autologous fat: a 3-year follow-up is disappointing. Plast Reconstr Surg. 1991;87:219. [PubMed] [Google Scholar]
- 7.Niechajev I. Sevcuk O. Long-term results of fat transplantation: clinical and histologic studies. Plast Reconstr Surg. 1994;94:496. doi: 10.1097/00006534-199409000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Patrick C.W., Jr. Chauvin P.B. Hobley J. Reece G.P. Preadipocyte seeded PLGA scaffolds for adipose tissue engineering. Tissue Eng. 1999;5:139. doi: 10.1089/ten.1999.5.139. [DOI] [PubMed] [Google Scholar]
- 9.Kral J.G. Crandall D.L. Development of a human adipocyte synthetic polymer scaffold. Plast Reconstr Surg. 1999;104:1732. doi: 10.1097/00006534-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Fischbach C. Spruss T. Weiser B. Neubauer M. Becker C. Hacker M., et al. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp Cell Res. 2004;300:54. doi: 10.1016/j.yexcr.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 11.Halbleib M. Skurk T. de Luca C. von Heimburg D. Hauner H. Tissue engineering of white adipose tissue using hyaluronic acid-based scaffolds. I: in vitro differentiation of human adipocyte precursor cells on scaffolds. Biomaterials. 2003;24:3125. doi: 10.1016/s0142-9612(03)00156-x. [DOI] [PubMed] [Google Scholar]
- 12.von Heimburg D. Zachariah S. Heschel I. Kuhling H. Schoof H. Hafemann B., et al. Human preadipocytes seeded on freeze-dried collagen scaffolds investigated in vitro and in vivo. Biomaterials. 2001;22:429. doi: 10.1016/s0142-9612(00)00186-1. [DOI] [PubMed] [Google Scholar]
- 13.Flynn L. Prestwich G.D. Semple J.L. Woodhouse K.A. Adipose tissue engineering in vivo with adipose-derived stem cells on naturally derived scaffolds. J Biomed Mater Res A. 2009;89:929. doi: 10.1002/jbm.a.32044. [DOI] [PubMed] [Google Scholar]
- 14.Katz A.J. Llull R. Hedrick M.H. Futrell J.W. Emerging approaches to the tissue engineering of fat. Clin Plast Surg. 1999;26:587. [PubMed] [Google Scholar]
- 15.Assmus B. Schachinger V. Teupe C. Britten M. Lehmann R. Dobert N., et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 16.Menasche P. Hagege A.A. Scorsin M. Pouzet B. Desnos M. Duboc D., et al. Myoblast transplantation for heart failure. Lancet. 2001;357:279. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 17.Phillips M.I. Tang Y.L. Pinkernell K. Stem cell therapy for heart failure: the science and current progress. Future Cardiol. 2008;4:285. doi: 10.2217/14796678.4.3.285. [DOI] [PubMed] [Google Scholar]
- 18.Madonna R. De Caterina R. Adipose tissue: a new source for cardiovascular repair. J Cardiovasc Med (Hagerstown), 2010;11:71. doi: 10.2459/JCM.0b013e328330e9be. [DOI] [PubMed] [Google Scholar]
- 19.Traktuev D.O. Merfeld-Clauss S. Li J. Kolonin M. Arap W. Pasqualini R., et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 20.Traktuev D.O. Prater D.N. Merfeld-Clauss S. Sanjeevaiah A.R. Saadatzadeh M.R. Murphy M., et al. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res. 2009;104:1410. doi: 10.1161/CIRCRESAHA.108.190926. [DOI] [PubMed] [Google Scholar]
- 21.Chamberlain M.D. Gupta R. Sefton M.V. Bone marrow-derived mesenchymal stromal cells enhance chimeric vessel development driven by endothelial cell coated microtissues. Tissue Eng Part A. 2012;18:285. doi: 10.1089/ten.tea.2011.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nor J.E. Peters M.C. Christensen J.B. Sutorik M.M. Linn S. Khan M.K., et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81:453. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 23.Skovseth D.K. Yamanaka T. Brandtzaeg P. Butcher E.C. Haraldsen G. Vascular morphogenesis and differentiation after adoptive transfer of human endothelial cells to immunodeficient mice. Am J Pathol. 2002;160:1629. doi: 10.1016/S0002-9440(10)61110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhang S.H. Cho S.W. La W.G. Lee T.J. Yang H.S. Sun A.Y., et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 25.Hong S.J. Traktuev D.O. March K.L. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Curr Opin Organ Transplant. 2010;15:86. doi: 10.1097/MOT.0b013e328334f074. [DOI] [PubMed] [Google Scholar]
- 26.Kloeters O. Berger I. Ryssel H. Megerle K. Leimer U. Germann G. Revitalization of cortical bone allograft by application of vascularized scaffolds seeded with osteogenic induced adipose tissue derived stem cells in a rabbit model. Arch Orthop Trauma Surg. 2011;131:1459. doi: 10.1007/s00402-011-1306-5. [DOI] [PubMed] [Google Scholar]
- 27.Kondo K. Shintani S. Shibata R. Murakami H. Murakami R. Imaizumi M., et al. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:61. doi: 10.1161/ATVBAHA.108.166496. [DOI] [PubMed] [Google Scholar]
- 28.Ohmura Y. Tanemura M. Kawaguchi N. Machida T. Tanida T. Deguchi T., et al. Combined transplantation of pancreatic islets and adipose tissue-derived stem cells enhances the survival and insulin function of islet grafts in diabetic mice. Transplantation. 2010;90:1366. doi: 10.1097/TP.0b013e3181ffba31. [DOI] [PubMed] [Google Scholar]
- 29.Zhu M. Zhou Z. Chen Y. Schreiber R. Ransom J.T. Fraser J.K., et al. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010;64:222. doi: 10.1097/SAP.0b013e31819ae05c. [DOI] [PubMed] [Google Scholar]
- 30.Flynn L. Semple J.L. Woodhouse K.A. Decellularized placental matrices for adipose tissue engineering. J Biomed Mater Res A. 2006;79:359. doi: 10.1002/jbm.a.30762. [DOI] [PubMed] [Google Scholar]
- 31.Yu G. Wu X. Dietrich M.A. Polk P. Scott L.K. Ptitsyn A.A., et al. Yield and characterization of subcutaneous human adipose-derived stem cells by flow cytometric and adipogenic mRNA analyzes. Cytotherapy. 2010;12:538. doi: 10.3109/14653241003649528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGuigan A.P. Sefton M.V. Design and fabrication of sub-mm-sized modules containing encapsulated cells for modular tissue engineering. Tissue Eng. 2007;13:1069. doi: 10.1089/ten.2006.0253. [DOI] [PubMed] [Google Scholar]
- 33.Marxen M. Thornton M.M. Chiarot C.B. Klement G. Koprivnikar J. Sled J.G., et al. MicroCT scanner performance and considerations for vascular specimen imaging. Med Phys. 2004;31:305. doi: 10.1118/1.1637971. [DOI] [PubMed] [Google Scholar]
- 34.Merfeld-Clauss S. Gollahalli N. March K.L. Traktuev D.O. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng Part A. 2010;16:2953. doi: 10.1089/ten.tea.2009.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rehman J. Traktuev D. Li J. Merfeld-Clauss S. Temm-Grove C.J. Bovenkerk J.E., et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 36.Miranville A. Heeschen C. Sengenes C. Curat C.A. Busse R. Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 37.Planat-Benard V. Silvestre J.S. Cousin B. Andre M. Nibbelink M. Tamarat R., et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 38.Lin C.S. Xin Z.C. Deng C.H. Ning H. Lin G. Lue T.F. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- 39.Cai X. Lin Y. Hauschka P. Grottkau B.E. Adipose stem cells originate from perivascular cells. Biol Cell. 2011;103:435. doi: 10.1042/BC20110033. [DOI] [PubMed] [Google Scholar]
- 40.Borges J. Mueller M.C. Padron N.T. Tegtmeier F. Lang E.M. Stark G.B. Engineered adipose tissue supplied by functional microvessels. Tissue Eng. 2003;9:1263. doi: 10.1089/10763270360728170. [DOI] [PubMed] [Google Scholar]
- 41.Khan O.F. Chamberlain M.D. Sefton M.V. Towards an in vitro vasculature: differentiation of mesenchymal stromal cells within an endothelial cell-seeded modular construct in a microfluidic flow chamber. Tissue Eng Part A. 2012;18:744. doi: 10.1089/ten.tea.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nor J.E. Christensen J. Mooney D.J. Polverini P.J. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enis D.R. Shepherd B.R. Wang Y. Qasim A. Shanahan C.M. Weissberg P.L., et al. Induction, differentiation, and remodeling of blood vessels after transplantation of Bcl-2-transduced endothelial cells. Proc Natl Acad Sci U S A. 2005;102:425. doi: 10.1073/pnas.0408357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schechner J.S. Nath A.K. Zheng L. Kluger M.S. Hughes C.C. Sierra-Honigmann M.R., et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci U S A. 2000;97:9191. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saunders W.B. Bohnsack B.L. Faske J.B. Anthis N.J. Bayless K.J. Hirschi K.K., et al. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175:179. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tremblay P.L. Hudon V. Berthod F. Germain L. Auger F.A. Inosculation of tissue-engineered capillaries with the host's vasculature in a reconstructed skin transplanted on mice. Am J Transplant. 2005;5:1002. doi: 10.1111/j.1600-6143.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 47.Chen X. Aledia A.S. Ghajar C.M. Griffith C.K. Putnam A.J. Hughes C.C., et al. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A. 2009;15:1363. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koike N. Fukumura D. Gralla O. Au P. Schechner J.S. Jain R.K. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 49.Cho S.W. Kim I. Kim S.H. Rhie J.W. Choi C.Y. Kim B.S. Enhancement of adipose tissue formation by implantation of adipogenic-differentiated preadipocytes. Biochem Biophys Res Commun. 2006;2:588. doi: 10.1016/j.bbrc.2006.04.089. [DOI] [PubMed] [Google Scholar]
- 50.Hutley L.J. Herington A.C. Shurety W. Cheung C. Vesey D.A. Cameron D.P., et al. Human adipose tissue endothelial cells promote preadipocyte proliferation. Am J Physiol Endocrinol Metab. 2001;281:E1037. doi: 10.1152/ajpendo.2001.281.5.E1037. [DOI] [PubMed] [Google Scholar]
- 51.Varzaneh F.E. Shillabeer G. Wong K.L. Lau D.C. Extracellular matrix components secreted by microvascular endothelial cells stimulate preadipocyte differentiation in vitro. Metabolism. 1994;43:906. doi: 10.1016/0026-0495(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 52.Fukumura D. Ushiyama A. Duda D.G. Xu L. Tam J. Krishna V., et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho S.W. Kim S.S. Rhie J.W. Cho H.M. Choi C.Y. Kim B.S. Engineering of volume-stable adipose tissues. Biomaterials. 2005;26:3577. doi: 10.1016/j.biomaterials.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 54.Patrick C.W., Jr. Zheng B. Johnston C. Reece G.P. Long-term implantation of preadipocyte-seeded PLGA scaffolds. Tissue Eng. 2002;8:283. doi: 10.1089/107632702753725049. [DOI] [PubMed] [Google Scholar]
- 55.Orban J.M. Wilson L.B. Kofroth J.A. El Kurdi M.S. Maul T.M. Vorp D.A. Crosslinking of collagen gels by transglutaminase. J Biomed Mater Res A. 2004;68:756. doi: 10.1002/jbm.a.20110. [DOI] [PubMed] [Google Scholar]