Abstract
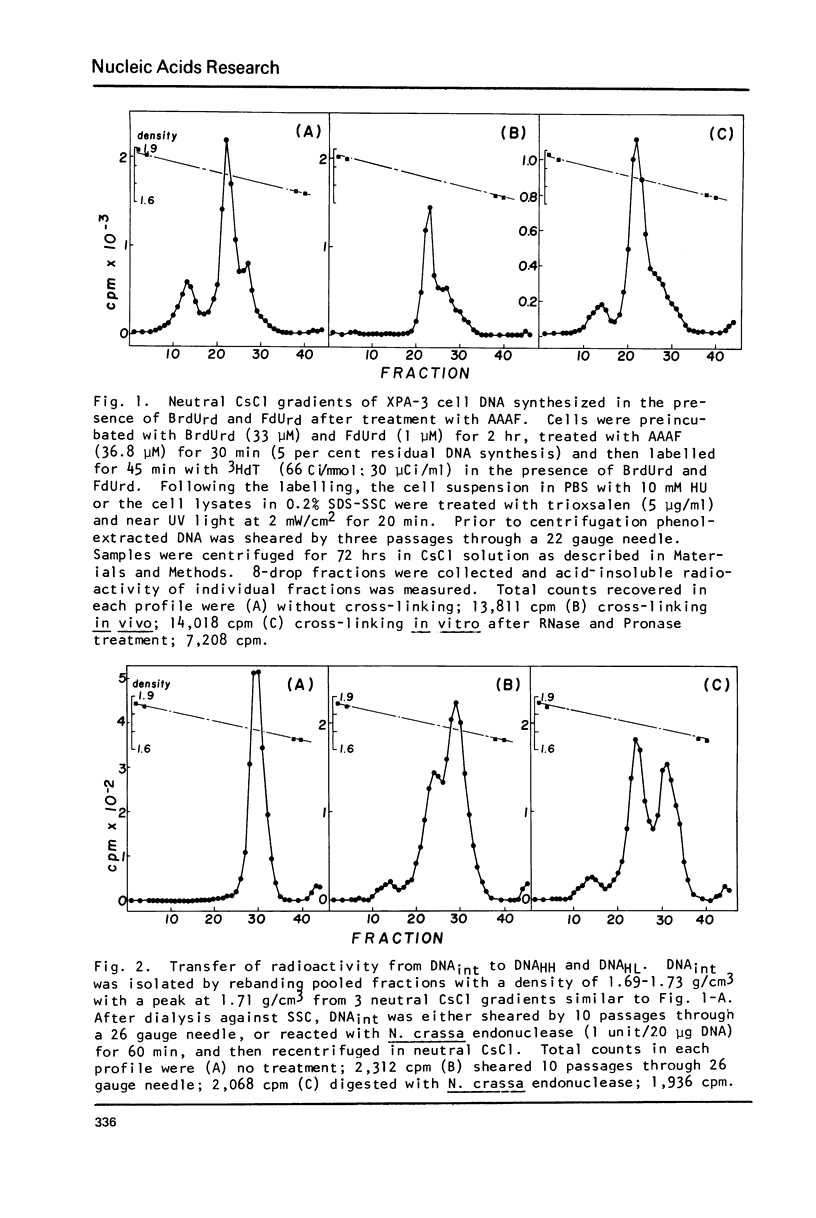
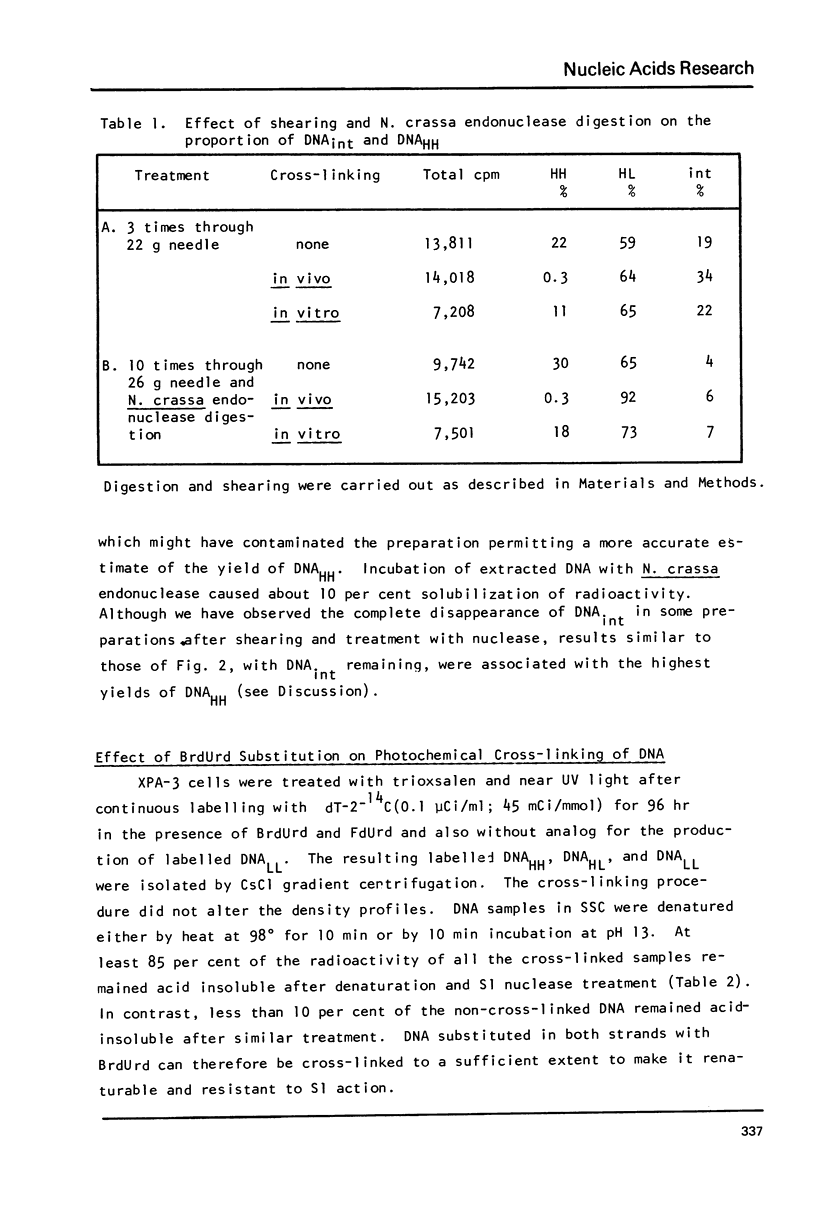
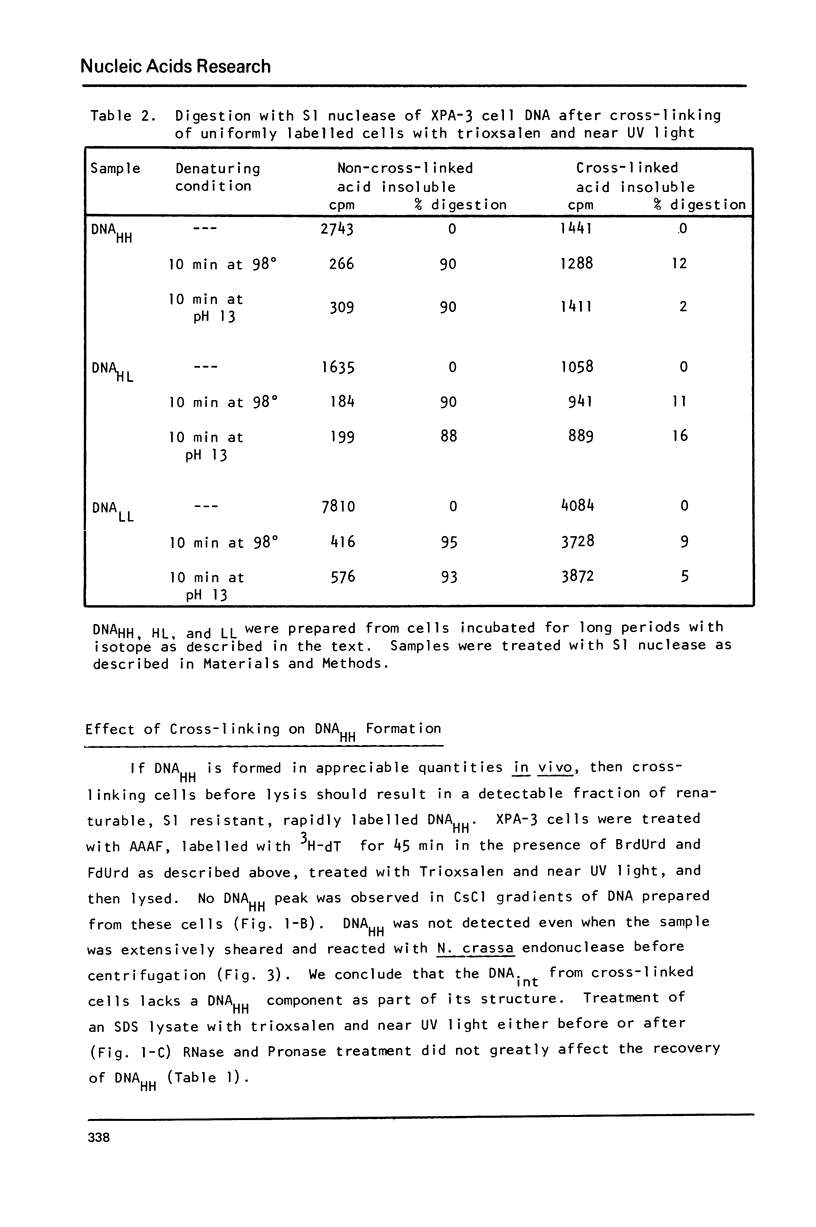
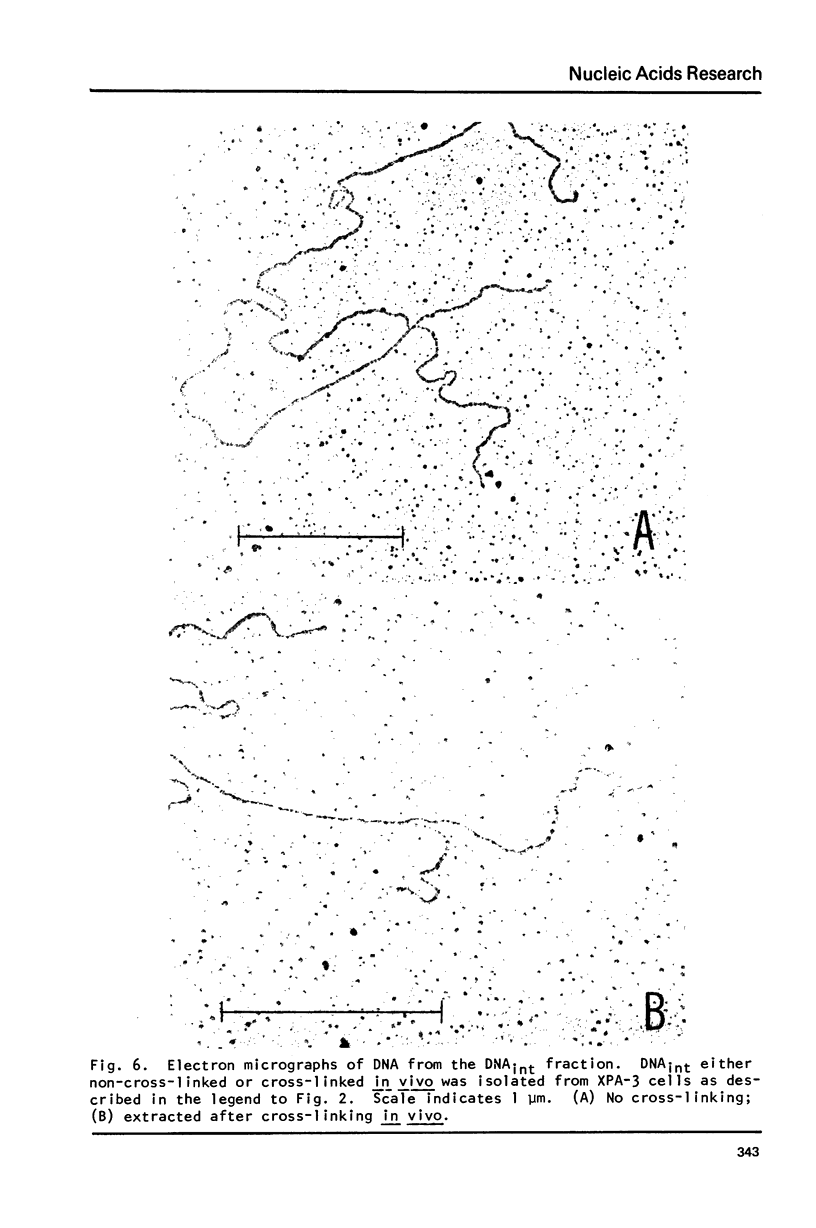
Incubation of human lymphoid cells with bromodeoxyuridine (BrdUrd) for short periods produces three classes of DNA containing analog: DNAHL (hybrid DNA, density approximately equal to 1.75 g/cm3), DNAint (intermediate density DNA, density approximately equal to 1.71 g/cm3), and DNAHH (DNA with both strands containing analog, density approximately equal to 1.80 g/cm3). Preparations of DNAint yield DNAHH after extensive shearing and/or treatment with single strand specific endonuclease. Cross-linking of pulse-labeled (BrdUrd + 3HdT) DNA in cells by treatment with trioxsalen and near UV light before lysis prevents the appearance of DNAHH.Cross-linking after lysis has little effect. A large fraction of DNAHH is obtained after incubation of cells with caffeine. Extraction of DNA at high salt concentration or cross-linking with trioxsalen and near UV light drastically reduced the amount of DNAHH obtained from caffeine-treated cells. We conclude that most DNAHH arises from in vitro branch migration in isolated DNA growing points.
Full text
PDF




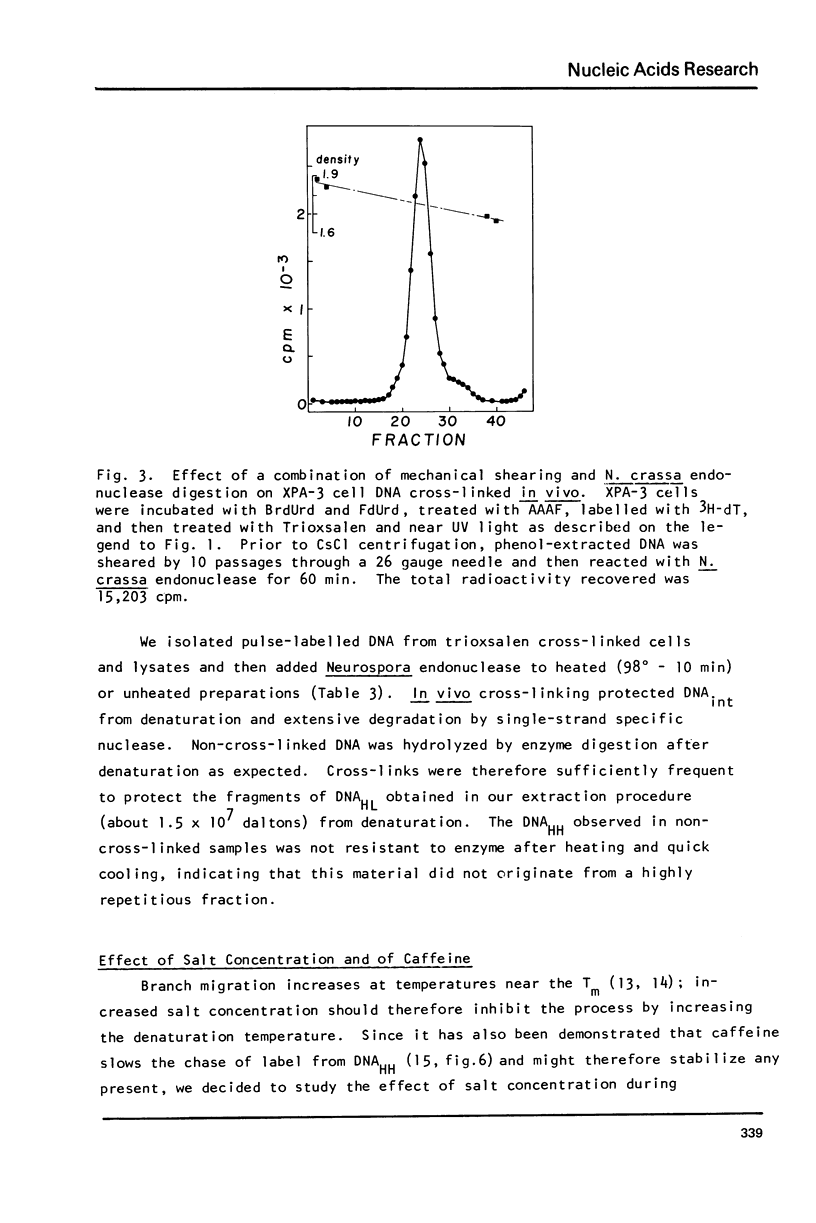
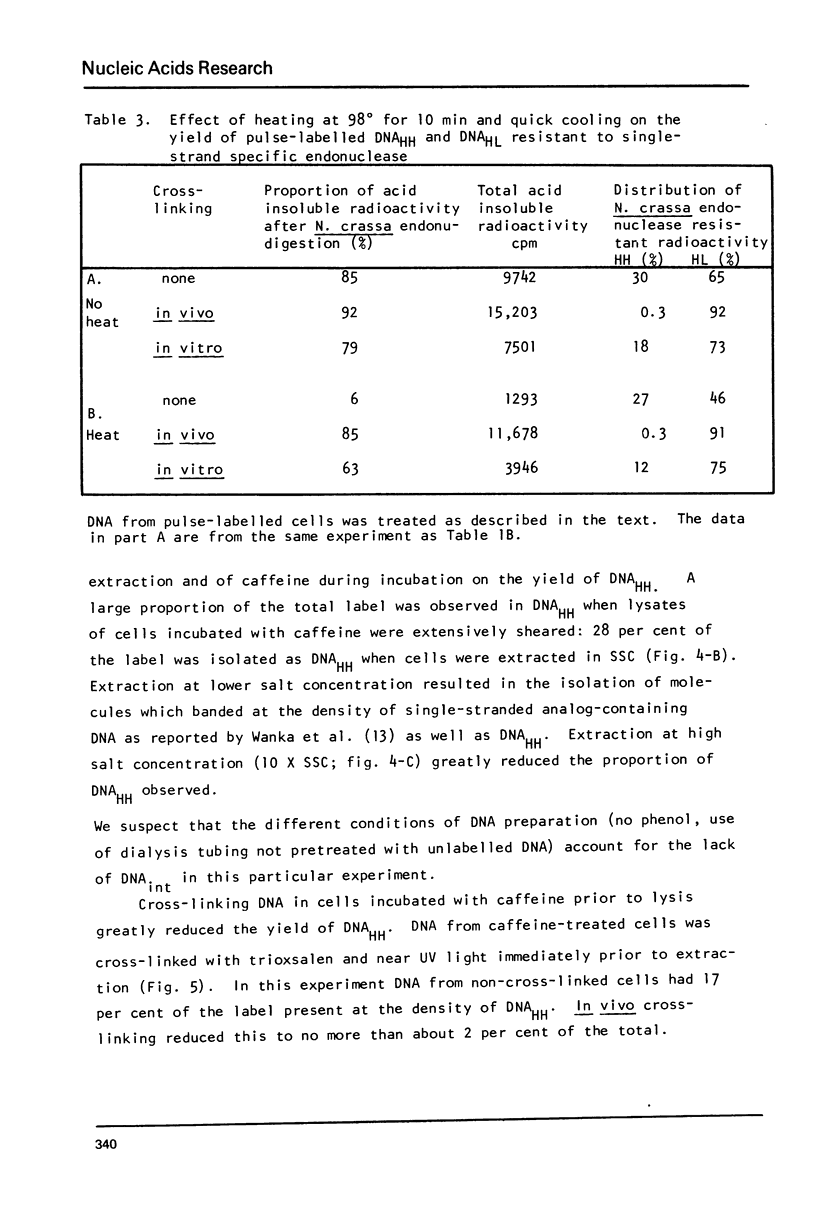
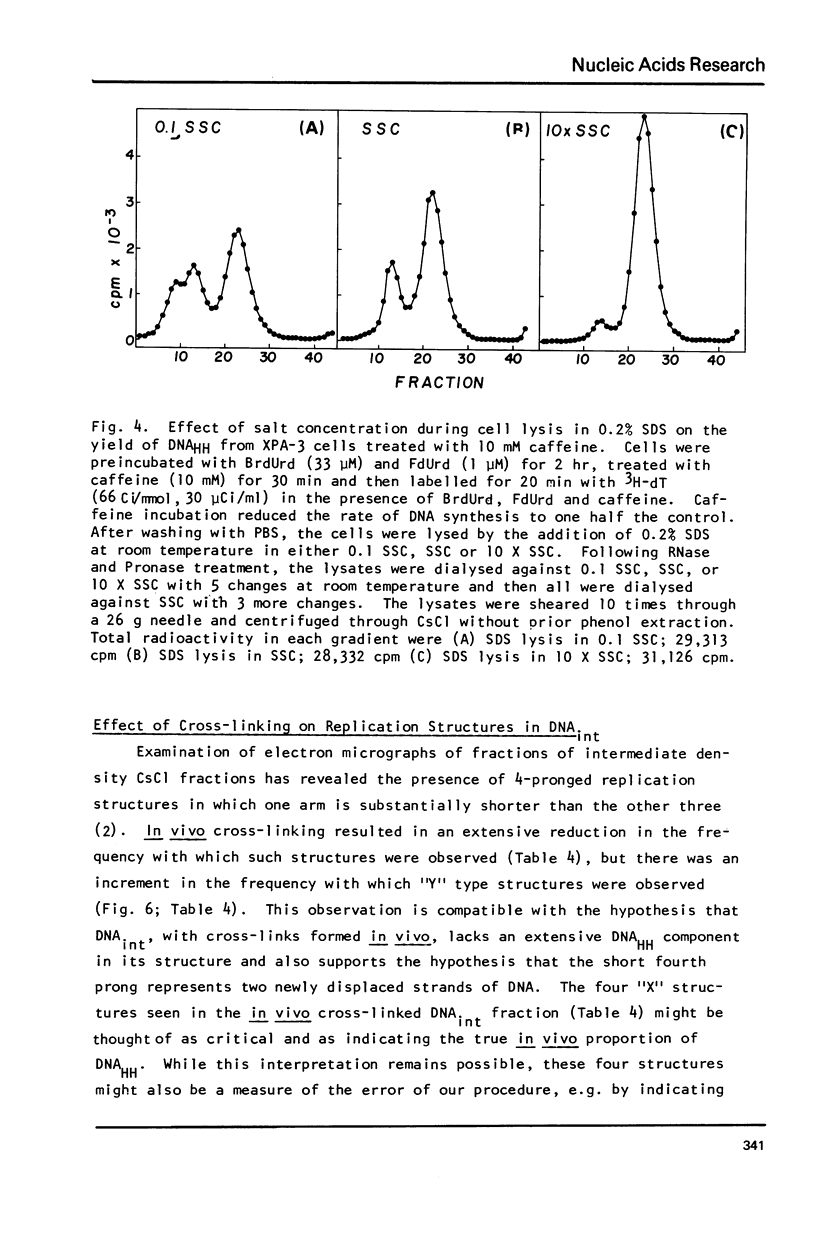
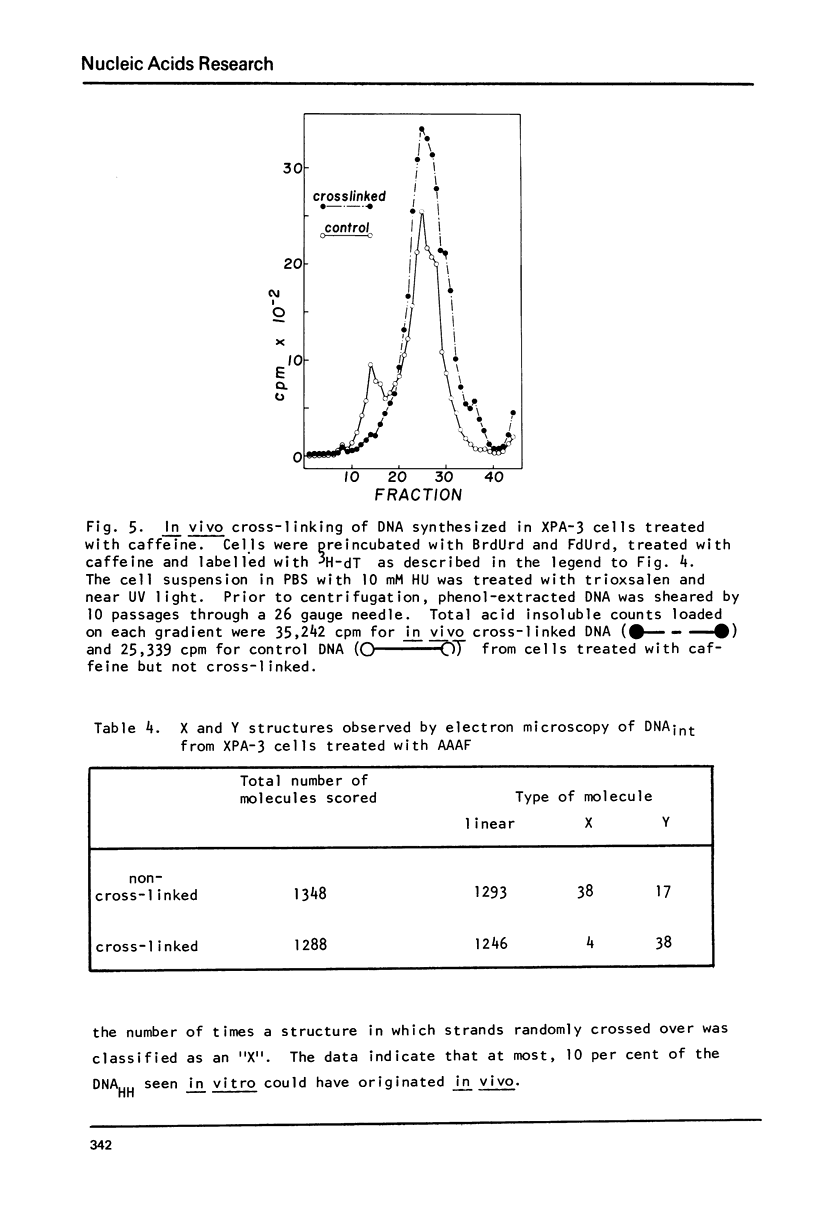




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews A. D., Robbins J. H., Kraemer K. H., Buell D. N. Xeroderma pigmentosum long-term lymphoid lines with increased ultraviolet sensitivity. J Natl Cancer Inst. 1974 Sep;53(3):691–693. doi: 10.1093/jnci/53.3.691. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Pardue M. L. Electron microscopy of DNA crosslinked with trimethylpsoralen: test of the secondary structure of eukaryotic inverted repeat sequences. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2644–2648. doi: 10.1073/pnas.73.8.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T., Pardue M. L. Cross-linking of DNA with trimethylpsoralen is a probe for chromatin structure. Cell. 1977 Jul;11(3):631–640. doi: 10.1016/0092-8674(77)90080-0. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Coyle M., McMahon M., Strauss B. Failure of alkylated HEp.2 cells to replicate newly synthesized DNA. Mutat Res. 1971 Aug;12(4):427–440. doi: 10.1016/0027-5107(71)90093-5. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Bick M. D. Thermal denaturation of DNA from bromodeoxyuridine substituted cells. Nucleic Acids Res. 1976 Jan;3(1):49–62. doi: 10.1093/nar/3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y., Tatsumi M. Replicative bypass repair of ultraviolet damage to DNA of mammalian cells: caffeine sensitive and caffeine resistant mechanisms. Mutat Res. 1976 Oct;37(1):91–110. doi: 10.1016/0027-5107(76)90058-0. [DOI] [PubMed] [Google Scholar]
- Hanson C. V., Shen C. K., Hearst J. E. Cross-linking of DNA in situ as a probe for chromatin structure. Science. 1976 Jul 2;193(4247):62–64. doi: 10.1126/science.935855. [DOI] [PubMed] [Google Scholar]
- Higgins N. P., Kato K., Strauss B. A model for replication repair in mammalian cells. J Mol Biol. 1976 Mar 5;101(3):417–425. doi: 10.1016/0022-2836(76)90156-x. [DOI] [PubMed] [Google Scholar]
- Kato K., Strauss B. Accumulation of an intermediate in DNA synthesis by HEp.2 cells treated with methyl methanesulfonate. Proc Natl Acad Sci U S A. 1974 May;71(5):1969–1973. doi: 10.1073/pnas.71.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINN S., LEHMAN I. R. AN ENDONUCLEASE FROM NEUROSPORA CRASSA SPECIFIC FOR POLYNUCLEOTIDES LACKING AN ORDERED STRUCTURE. I. PURIFICATION AND PROPERTIES OF THE ENZYME. J Biol Chem. 1965 Mar;240:1287–1293. [PubMed] [Google Scholar]
- Moore P. D., Holliday R. Evidence for the formation of hybrid DNA during mitotic recombination in Chinese hamster cells. Cell. 1976 Aug;8(4):573–579. doi: 10.1016/0092-8674(76)90225-7. [DOI] [PubMed] [Google Scholar]
- Scudiero D., Henderson E., Norin A., Strauss B. The measurement of chemically-induced DNA repair synthesis in human cells by BND-cellulose chromatography. Mutat Res. 1975 Sep;29(3):473–488. doi: 10.1016/0027-5107(75)90066-4. [DOI] [PubMed] [Google Scholar]
- Thompson B. J., Camien M. N., Warner R. C. Kinetics of branch migration in double-stranded DNA. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2299–2303. doi: 10.1073/pnas.73.7.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wanka F., Brouns R. M., Aelen J. M., Eygensteyn A., Eygensteyn J. The origin of nascent single-stranded DNA extracted from mammalian cells. Nucleic Acids Res. 1977 Jun;4(6):2083–2097. doi: 10.1093/nar/4.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]