Abstract
P-glycoprotein, encoded by the multidrug resistance gene MDR1, is an ATP-driven drug efflux pump which is highly expressed at the blood-brain barrier of vertebrates. Drug efflux of macrocyclic lactones by P-glycoprotein is highly relevant for the therapeutic safety of macrocyclic lactones, as thereby GABA-gated chloride channels, which are confined to the central nervous system in vertebrates, are protected from high drug concentrations that otherwise would induce neurological toxicity. A 4-bp deletion mutation exists in the MDR1 gene of many dog breeds such as the Collie and the Australian Shepherd, which results in the expression of a non-functional P-glycoprotein and is associated with multiple drug sensitivity. Accordingly, dogs with homozygous MDR1 mutation are in general prone to neurotoxicity by macrocyclic lactones due to their increased brain penetration. Nevertheless, treatment of these dogs with macrocyclic lactones does not inevitably result in neurological symptoms, since, the safety of treatment highly depends on the treatment indication, dosage, route of application, and the individual compound used as outlined in this review. Whereas all available macrocyclic lactones can safely be administered to MDR1 mutant dogs at doses usually used for heartworm prevention, these dogs will experience neurological toxicity following a high dose regimen which is common for mange treatment in dogs. Here, we review and discuss the neurotoxicological potential of different macrocyclic lactones as well as their treatment options in MDR1 mutant dogs.
Keywords: Dog, ivermectin, ivermectin-sensitive Collie, MDR1, milbemycin oxime, moxidectin, P-glycoprotein, pharmacogenetics.
P-GLYCOPROTEIN: A MULTIDRUG EFFLUX TRANSPORTER
The multidrug carrier P-glycoprotein (P-gp), encoded by the MDR1 (ABCB1) gene, belongs to the family of membrane bound ATP-binding cassette (ABC) transporters [1]. P-glycoprotein is an ATP-driven efflux pump that confers multidrug resistance (MDR) to cancer cells by actively extruding a wide range of structurally unrelated chemotherapeutic compounds from the cell. Juliano & Ling [2] first isolated P-gp as a membrane glycoprotein of approximately 170-kDa from chemotherapeutic drug-resistant Chinese hamster ovary cells that were selected for colchicine resistance and identified this protein as a major part of the functional multidrug resistance of these cells by limiting their permeability into the cell (P-gp, permeability glycoprotein). Many years later a cDNA was isolated from a multidrug-resistant carcinoma cell line, selected for its resistance to colchicine, vinblastine and doxorubicin, and was shown to encode P-gp [3,4]. Subsequently, the name MDR1 was established for the gene as well as for the encoded P-gp. Later on, by using bioinformatic approaches, the MDR1 gene was phylogenetically classified as member B1 of the ABC transporter superfamily [5]. The MDR1 (ABCB1) gene exist in all mammals analysed to date including the dog, with the peculiarity that this gene is duplicated in rodent genomes (referred to as mdr1a and mdr1b).
Many years of research on P-gp focused on the chemotherapeutic resistance of tumour cells and so the first P-gp substrates identified were cytostatic drugs [6,7]. Today, it is known that P-gp has a broader substrate specificity and transports a large number of structurally unrelated drugs and xenobiotics including anticancer drugs (e.g., vinca alkaloids, paclitaxel, doxorubicin), immunosuppressants (cyclosporine, tacrolimus), antiparasitic agents (ivermectin, moxidectin, selamectin, milbemycin oxime), antimicrobial agents (e.g., erythromycin, rifampicin, ketoconazole, levofloxacin), cardiac drugs (e.g., digoxin, verapamil, diltiazem, quinidine, talinolol, losartan), opioids (e.g., morphine, loperamide, butorphanol, fentanyl), steroid hormones (cortisol, dexamethasone, aldosterone) and many others (e.g., cimetidine, fexofenadine, acepromazine, domperidone, ondansetron) [8-11]. Most P-gp substrates are hydrophobic molecules and partition into the plasma membrane from where they are effluxed by P-gp. Accordingly, P-gp has been thought of as 'hydrophobic vacuum cleaner' for hydrophobic molecules which are embedded into the plasma membrane [12]. This type of substrate recognition makes P-gp a highly effective efflux pump, preventing the cellular entry of toxic compounds [13].
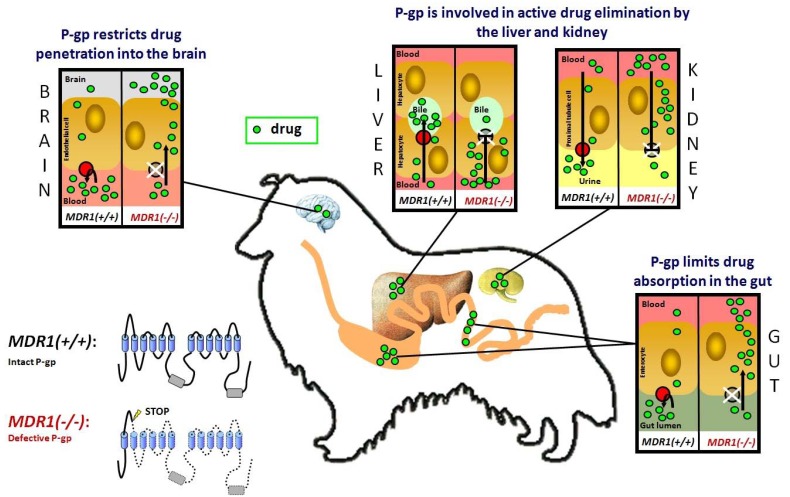
Apart from neoplastic tissues, P-gp shows high expression in the apical (luminal) membranes of epithelial cells lining the lower gastrointestinal tract, in the brush border of renal proximal tubules, in the canalicular membrane of hepatocytes and in capillary endothelial cells in the brain and testes. Furthermore, P-gp expression was found in the placenta, the adrenal cortex and CD34+ hematopoietic stem cells [14-20]. According to this expression pattern, it has been shown that P-gp limits drug absorption in the gastrointestinal tract and promotes drug elimination in the liver, kidney and intestine. Furthermore, P-gp restricts drug uptake into cells and tissues, in particular their permeation across the blood-brain barrier Fig. (1). Taken altogether, P-gp has an important protective function for the organism by eliminating potentially toxic compounds from the body and preventing their entry into the brain and organs of reproduction [21-23].
Fig. (1).
The role of P-gp in drug disposition. P-glycoprotein (shown in red) is an ATP-driven efflux transporter which pumps its substrates out of the cell. The intact P-gp limits drug entry into the organism after oral administration, promotes drug elimination into bile and urine, and restricts drug penetration across the blood-brain barrier. In MDR1(-/-) dogs which do not express a functional P-gp, enteral drug absorption is enhanced, biliary and urinary drug elimination is reduced, and the permeation of blood-tissue barriers is increased at the blood-brain barrier, blood-testis barrier and blood-placenta barrier. As a consequence, P-gp transported drugs can cause an increase in adverse effects in these dogs. This particularly applies to macrocyclic lactones, which would normally be efficiently transported by P-gp.
The important role of P-gp in protecting the brain from the penetration of drugs across the blood-brain barrier is highly relevant for the treatment of mammals with macrocyclic lactones. In parasitic lower organisms, macrocyclic lactones bind with high affinity to glutamate-gated and GABA-gated chloride ion channels which are widespread in the nervous system of arthropods and nematodes, resulting in an inhibition of nerve activity, flaccid paralysis and death [24,25]. However, the situation is completely different in mammals where neuronal glutamate-gated chloride channels are absent and GABA-gated chloride channels are confined to the central nervous system [26-28]. Here, these channels are protected from the binding of macrocyclic lactones by the highly effective P-gp mediated drug efflux at the blood-brain barrier which restricts drug penetration into the brain [21]. Therefore, and given the expression of a functionally active P-gp at the blood-brain barrier, macrocyclic lactones generally have a wide margin of safety in mammals at therapeutic doses [29].
BRAIN PENETRATION OF MACROCYCLIC LACTONES IN P-GP DEFICIENT MICE
Several experimental models have been developed to analyse drug interactions with P-gp. In vitro models include the Caco-2 cell line, which shows, among numerous other carriers, a high expression of P-gp, and cell lines stably transfected with P-gp such as Madin-Darby canine kidney cells [7]. In these cellular systems interactions with P-gp have been demonstrated for a large number of drugs including ivermectin, selamectin, moxidectin, eprinomectin, abamectin and doramectin [30,31]. Furthermore, in 1994 a genetically engineered knockout mouse was established in which first only the mdr1a gene and later on both murine mdr1 genes (mdr1a and mdr1b) were disrupted by insertional mutagenesis [32,33]. Despite the broad tissue expression of P-gp, loss of either or both genes did not result in an obvious phenotype or any physiological abnormality. The knockout mice were viable and fertile and almost indistinguishable from their wild-type littermates in a range of histological, hematological, serum-chemical and immunological parameters, but spontaneously develop colitis with age [33,34]. However, it has to be emphasised that laboratory mice grow up in a well-controlled and generally toxic-free environment where the importance of the protective function of P-gp may be less relevant. In these mice, the highly important role of P-gp for the safety of treatment with macrocyclic lactones was identified by serendipity.
Following a mite infection of the generated mdr1a knockout mice, the mice were sprayed with a dilute solution of ivermectin which is routine in mite infections in an animal facility and is normally well tolerated by the mice even though they ingest part of the drug due to grooming activities. Following the ivermectin application, however, a number of mdr1a(-/-) knockout mice, but not the mdr1a(+/+) wild-type mice, died with paralytic symptoms including immobilization, inability to right themselves, recumbency, decreased breathing frequency, and finally, onset of a comatose state. After a more detailed toxicity analysis the researchers demonstrated that mdr1a(-/-) mice were 50- to 100-fold more sensitive to orally administered ivermectin (LD50 = 700-800 µg/kg in the knockout and 50-60 mg/kg in the wild-type mice) due to an increased accumulation in the brain [32,33]. These results were consistent with the suggested role of P-gp and the high expression in brain capillaries [16,17]. Application of radiolabelled ivermectin revealed that absolute brain concentrations were 87-fold higher in the brain of mdr1a(-/-) knockout mice compared with the wild-type mice (131 ± 16 ng/g vs. 1.5±1.2 ng/g), whereas the drug concentrations in most other tissues were only 3- to 4-fold higher. This general increase in tissue concentrations was likely due to an increased net uptake of ivermectin from the gastrointestinal tract combined with reduced elimination through the liver and kidney [32]. Even after intravenous and spot-on applications of 200 µg/kg ivermectin to mdr1a,b(-/-) knockout mice, where intestinal absorption does not affect the drug bioavailability, the absolute ivermectin concentrations in the brain were 59-fold (130 ng/g vs. 2 ng/g) and 49-fold (27 ng/g vs. 0.6 ng/g) higher in the knockout mice compared with the wild-type mice, respectively [35] Fig. (2).
Fig. (2).
Brain penetration of macrocyclic lactones in wild-type mice (black columns), as well as in P-gp deficient mice (white columns) and dogs (grey columns). Ivermectin (IVM), moxidectin (MOX), eprinomectin (EPM), doramectin (DOR) and selamectin (SEL) were experimentally given to mdr1a(-/-) knockout mice (Schinkel et al. 1995a [43], 1994 [32]), mdr1a,b(-/-) double knockout mice (Geyer et al. 2008 [35], Kiki-Mvouaka et al. 2010 [44]), drug-sensitive CF-1 mice (Kwei et al. 1999 [39]), and ivermectin-sensitive Collies (Pulliam et al. 1985 [42]) or were therapeutically applied to MDR1(-/-) dogs at the following dosages: 200 µg/kg orally [32,35,39,42 left column,43], 200 µg/kg subcutaneously [44], 600 µg/kg orally [42 right column] and 1 mg/kg doramectin subcutaneously. Absolute drug concentrations in brain tissue were determined by liquid scintillation counting using the respective radiolabeled drugs [32,35,39,43] or by HPLC analysis [42,44]. Generally, drug concentrations in the brain were marginal in the wild-type mice and dramatically increased in the absence of P-gp. The two MDR1(-/-) dogs, "Sunny" and "Jake", were therapeutically given 1 mg/kg doramectin and developed severe neurotoxicosis. Both dogs died 5-6 days after treatment and were subjected to necropsy within 18 hours of death.
Apart from the genetically engineered mdr1 knockout mice, researchers at the Merck Research Laboratories identified in the CF-1 mouse strain a subpopulation of mice which were much more sensitive to avermectins compared to other mice [36]. Further analysis revealed that these drug sensitive mice did not express the mdr1a P-gp in the brain capillary endothelial cells lining the blood-brain barrier due to an insertion of a solo long terminal repeat of the ecotropic murine leukemia virus, resulting in abnormal splicing of the mdr1a transcript and thereby leading to the translation of a non-functional P-gp [37,38]. As a consequence, high levels of ivermectin accumulated in the brain of the CF-1 mice, up to 70-fold, after oral drug application of 200 µg/kg [36,39].
Based on these data, it became absolutely clear that P-gp expression at the blood-brain barrier is the major and critical determinant for the safety margin of ivermectin and other macrocyclic lactones in mammals. These findings shed new light on clinical data from veterinary medicine that identified a subpopulation of Collie dogs as extremely sensitive to ivermectin in the early 1980s [40,41]. However, it has to be noted that P-gp at that time had not yet been localised in the blood-brain barrier and P-gp transport of macrocyclic lactones was completely unknown. Most interesting was a study by Pulliam et al. [42], demonstrating that ivermectin-sensitive Collie dogs showed highly increased ivermectin accumulation in the brain, suggesting that in ivermectin-sensitive dogs the protective barrier function of the blood-brain barrier is defective and ivermectin can penetrate into the brain unhindered Fig. (2). Referring to this study, Schinkel et al. [32] and other experts in the field hypothesized that ivermectin-sensitive Collies, analogous to the mdr1 knockout mice, must have a genetic deficiency in the canine MDR1 gene resulting in the expression of a non-functional P-gp. From that point researchers began to clone and sequence the canine MDR1 cDNA in order to identify the proposed genetic defect in ivermectin-sensitive Collies.
IVERMECTIN-SENSITIVE COLLIES AND THE NT230(DEL4) MDR1 MUTATION
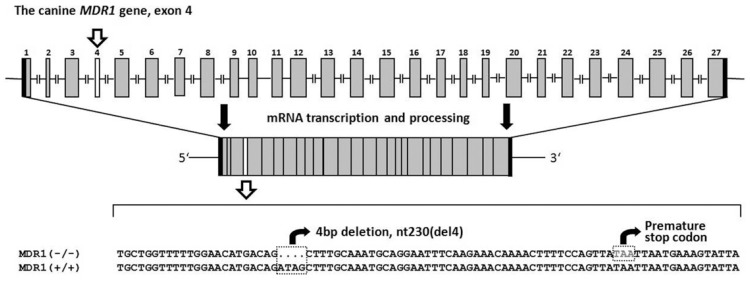
When ivermectin-sensitive Collies are exposed to 100-120 µg/kg ivermectin they develop mild neurological toxicity including mydriasis, ataxia and depression [40,41], whereas much higher doses of up to 2 mg/kg are well tolerated by Beagle dogs or ivermectin non-sensitive Collies [29]. Ivermectin susceptibility, though, is not present in all individuals of the Collie breed and it is not related to sex, collie-eye anomaly, or hair coat type. Nevertheless, this was regarded as a genetically determined drug susceptibility [40,42,45]. In 2001, Mealey et al. [46] were the first to identify a 4-bp deletion mutation in the MDR1 gene of an ivermectin-sensitive Collie. This nt230(del4) MDR1 deletion produces a frame shift at amino acid position 75 followed by a premature stop codon at amino acid position 91 Fig. (3). This severely truncated protein is non-functional and was undetectable by Western blotting [47]. Based on microsatellite analyses it has been proposed that all dogs carrying this mutant nt230(del4) MDR1 allele are descendants of a dog that lived in the United Kingdom in the 1800s, predating the emergence of formal breed lines [57].
Fig. (3).
The nt230(del4) MDR1 mutation in dogs. The canine MDR1 gene is organised in 27 exons, located on chromosome 14. The transcribed MDR1 coding sequence comprises 3846 bp and codes for the 1281 amino acids canine P-gp. The nt230(del4) mutation was localised to exon 4 and involves a 4-bp deletion followed by a premature stop codon. The truncated P-gp expressed from the mutant MDR1(-) allele is non-functional. Dogs with a homozygous nt230(del4) mutation are extremely sensitive to macrocyclic lactones.
Because of the predominant role of P-gp in drug disposition, mutation of the MDR1 gene alters the pharmacokinetic properties of P-gp transported drugs, leading to enhanced oral bioavailability and reduced drug elimination through the liver, kidney and gut. Moreover, the brain penetration of P-gp transported drugs is increased and in many cases provokes neurological toxicity [8-10,48,49] Fig. (1). Clinical observations have already indicated that apart from macrocyclic lactones, the antidiarrheal drug loperamide, which is normally excluded from the brain by P-gp, causes neurological toxicosis in MDR1(-/-) dogs at normal therapeutic doses [50,51]. Although not explicitly analysed, loperamide toxicosis in these dogs was most likely due to increased brain penetration in the absence of P-gp [52,53]. An increased brain penetration of many further drugs was experimentally demonstrated in mdr1 knockout mice including vinblastine, doxorubicin, paclitaxel, quinidine, ondansetron, cyclosporine and verapamil (see Table 1). Although drug transport was not investigated with the canine P-gp for most of these drugs, increased brain penetration and central adverse effects also have to be assumed in MDR1(-/-) mutant dogs. The plasma pharmacokinetics of other drugs are altered in MDR1 mutant dogs and so may provoke increased adverse effects in these dogs. For example, digoxin toxicity was documented in a MDR1(-/-) Collie dog which developed an unusually high serum digoxin concentration leading to digoxin toxicosis [54]. Furthermore, increased sensitivity to acepromazine and butorphanol was observed in MDR1(-/-) dogs that experienced a more pronounced and protracted central nervous system (CNS) depression compared to MDR1 normal dogs [55]. A recent clinical study showed that the MDR1 genotype is also highly relevant for veterinary oncology. Mealey et al. [56] analysed 34 dogs diagnosed with lymphoma that were to be treated with vincristine including four MDR1(-/-) and four MDR1(+/-) dogs. This study showed that MDR1 mutant dogs are extremely susceptible to myelosuppression and gastrointestinal toxicosis induced by the vincristine treatment, resulting in higher morbidity and mortality rates and treatment delays [56].
Table 1.
Drugs Transported by P-gp which Show Enhanced Brain Concentrations in mdr1a(-/-) or mdr1a,b(-/-) Knockout Mice Compared to Wild-Type Mice after Intravenous Application (if not Otherwise Stated). Macrocyclic Lactones are Depicted in Bold Face
| Drug | Time after Application | Brain Concentration Ratio [Knockout / Wild-Type] | Reference |
|---|---|---|---|
| Ivermectin | 24 h | 87a,c | [32] |
| Ivermectin | 24 h | 60b,c | [35] |
| Ivermectin | 4 h | 46a,c | [43] |
| Ivermectin | 8 h | 36b | [35] |
| Nelfinavir | 4 h | 36a | [63] |
| Digoxin | 4 h | 35a | [64] |
| Tacrolimus | 5 h | 33a | [65] |
| Quinidine | 0.5-5 h | 33b,d | [53] |
| Quinidine | 10 min | 28a,e | [66] |
| Ivermectin | 24 h | 27b,d | [44] |
| Flesinoxan | 3 h | 27a | [67] |
| Vinblastine | 4 h | 22a | [32] |
| Verapamil | 0.5-5 h | 21b,d | [53] |
| Amiodarone | 0.5-5 h | 19b,d | [53] |
| Cyclosporin A | 4 h | 17a | [64] |
| Loperamide | 0.5-5 h | 17b,d | [53] |
| Loperamide | 4 h | 14a,c | [52] |
| Paclitaxel | 24 h | 12b | [68] |
| Moxidectin | 24 h | 11b,d | [44] |
| Indinavir | 4 h | 11a | [63] |
| Verapamil | 1 h | 9.5a | [69] |
| Asimadoline | 1 h | 9.1b | [70] |
| Metoclopramide | 0.5-5 h | 7.6b,d | [53] |
| Saquinavir | 4 h | 7.4a | [63] |
| Docetaxel | 24 h | 6.2b | [71] |
| Selamectin | 24 h | 5.0b,c | [35] |
| Doxorubicin | 24 h | 5.0a | [72] |
| Cortisol | 2 h | 4.6b,d | [73] |
| Ondansetron | 30 min | 4.0a | [52] |
| Sparfloxacin | 2 h | 3.9b,e | [74] |
| Doxorubicin | 1 h | 3.2a,e | [75] |
| Grepafloxacin | 2 h | 2.9b,e | [74] |
| Dexamethasone | 4 h | 2.5a | [64] |
| Morphine | 4 h | 1.7a | [64] |
mdr1a(-/-) knockout mice were used
mdr1a,b(-/-) double knockout mice were used
oral application
subcutaneous injection
data represent brain-to-plasma partition coefficient (Kp,brain,ko / Kp,brain,wt).
Following identification of the nt230(del4) MDR1 gene deletion mutation several MDR1 genotyping methods were developed. Most of them use PCR amplification of the nt230 flanking region on exon 4 of the canine MDR1 gene, followed by length polymorphism analysis using polyacrylamide gel electrophoresis [57-61]. More recently, a fluorogenic 5' nuclease TaqMan allelic discrimination method was described which allows MDR1 genotyping without post-PCR processing and is useful for routine diagnostics [62]. To date, MDR1 genotyping is also commercially available in many countries so that veterinarians can find the MDR1 genotype in a canine patient before treatment is started with a P-gp transported drug.
Breed Predisposition of the MDR1 Mutation
Over the last few years, systematic genotyping analyses of the distribution of the nt230(del4) MDR1 mutation among different breeds were performed on more than 15,000 dogs worldwide [76]. These studies revealed, that apart from the Collie, 12 further dog breeds are affected by this gene deletion mutation: Longhaired Whippet, Shetland Sheepdog, Miniature Australian Shepherd, Silken Windhound, McNab, Australian Shepherd, Wäller, White Swiss Shepherd, Old English Sheepdog, English Shepherd, German Shepherd and Border Collie (Table 2) [57,61,76,77]. Among all of these dog breeds, the allelic frequency for the mutant MDR1(-) allele is highest in the Collie with similar frequency values worldwide: 51%-56% in the USA [57,61,78], 55%-59% in Germany [76,77], 60% in the United Kingdom [57] and 56% in Australia [79]. Apart from these purebred dogs, surprisingly high frequency values were also found in herding breed mixes as well as in many unclassified mixed breed dogs [61,76]. In contrast, several other dog breeds that also show close genetic relationships or share a breeding history with one of these predisposed dog breeds are presumed to be free of this mutation including the Bearded Collie, Anatolian Shepherd Dog, Greyhound, Belgian Tervuren, Kelpie, Borzoi, Australian Cattle Dog and Irish Wolfhound [57, 61,76].
Table 2.
Breed Distribution of the nt230(del4) MDR1 Mutation in Dogs Worldwide
| Dog Breed | Range of Allelic Frequency (%) MDR1(-) | References |
|---|---|---|
| Collie | 55 – 57 | [57,61,76-79] |
| Longhaired Whippet | 42 | [57] |
| Shetland Sheepdog | 7– 35 | [57,61,76,77,80] |
| Miniature Australian Shepherd | 20 – 26 | [57,61] |
| Silken Windhound | 18 | [57] |
| McNab | 17 | [57] |
| Australian Shepherd | 17 – 46 | [57,61,76,77] |
| Wäller | 17 – 19 | [76,77] |
| White Swiss Shepherd | 14 | [76] |
| Old English Sheepdog | 1 – 11 | [57,61,76,80] |
| English Shepherd | 7 | [57] |
| German Shepherd | 6 | [61] |
| Border Collie | 1 – 2 | [61,76,77,80] |
| Herding-breed mix | 6 – 7 | [61,76] |
| Mixed breed | 2 – 7 | [61,76] |
Note: Data from the referenced studies were included when at least 30 dogs were analysed per breed.
Despite plenty of MDR1 genotyping data, on a practical basis it is difficult for veterinarians and dog owners to recognise whether this MDR1 gene deletion mutation is relevant for an individual canine patient/dog. Nevertheless, MDR1 genotyping is essential for all of the purebred dogs listed in Table 2 and also for mixed breed dogs prior to extra-label use of higher doses of macrocyclic lactones, e.g. for the treatment of canine generalised demodicosis (see Table 3), if severe and life-threatening adverse drug reactions are to be avoided. On the other hand, MDR1 genotyping is not absolutely necessary in other purebred dog breeds not listed in Table 2.
Table 3.
Treatment Safety of Ectoparasitic and Endoparasitic Infections with Macrocyclic Lactones in MDR1(+/+) Normal and MDR1(-/-) Mutant Dogs
| Indication | Drug | Dosage | Label | MDR1(+/+) | MDR(-/-) |
|---|---|---|---|---|---|
| Heartworm prevention | Ivermectin | 6-12 µg/kg PO once monthly | Heartgarda | + | + |
| Moxidectin | 170 µg/kg SC every six months | ProHearta | + | + | |
| Moxidectin | 2.5 mg/kg moxidectin + 10 mg/kg imidacloprid spot-on monthly | Advocateb, Advantage multia | + | + | |
| Selamectin | 6 mg/kg spot-on monthly | Strongholdb, Revolutiona | + | + | |
| Milbemycin oxime | 500 µg/kg milbemycin oxime + 5 mg/kg praziquantel PO monthly | Milbemaxb | + | + | |
| Milbemycin oxime | 500-990 µg/kg milbemycin oxime PO monthly | Interceptora | + | + | |
| Generalised demodicosis | Moxidectin | 2.5 mg/kg moxidectin + 10 mg/kg imidacloprid spot-on monthly | Advocateb, Advantage multia | + | + |
| Moxidectin | 200-400 µg/kg PO daily | Extra-label | + | - | |
| Ivermectin | 400-600 µg/kg PO daily | Extra-label | + | - | |
| Doramectin | 600 µg/kg SC weekly | Extra-label | + | - | |
| Milbemycin oxime | 0.5-2.0 mg/kg PO daily | Extra-label | + | - | |
| Other ectoparasitic and endoparasitic infections | Ivermectin | 50-200 µg/kg PO once | Extra-label | + | +/-c |
| Ivermectin | 300-400 µg/kg PO or SC weekly | Extra-label | + | - | |
| Moxidectin | 250 µg/kg SC weekly | Extra-label | + | ? | |
| Moxidectin | 400 µg/kg PO every 3-4 days for 3-6 weeks | Extra-label | + | - | |
| Moxidectin | 2.5 mg/kg moxidectin + 10 mg/kg imidacloprid spot-on monthly | Advocateb, Advantage multia | + | + | |
| Selamectin | 6 mg/kg spot-on monthly | Strongholdb, Revolutiona | + | + | |
| Milbemycin oxime | 500 µg/kg milbemycin oxime + 5 mg/kg praziquantel PO monthly | Milbemaxb | + | + | |
| Milbemycin oxime | 500-990 µg/kg milbemycin oxime PO monthly | Interceptora | + | + |
FDA approved
EMEA approved
toxic at > 100 µg/kg; PO, oral application, SC, subcutaneous application; "+", tolerated, "-", not tolerated, may induce neurotoxicosis.
TREATMENT OF MDR1 MUTANT DOGS WITH MACROCYCLIC LACTONES
Macrocyclic lactones have potent anthelmintic and ectoparasitic properties and are widely used in veterinary medicine for the treatment of parasitic diseases [81]. Commercially available products include the avermectins ivermectin, doramectin and selamectin as well as the milbemycins moxidectin and milbemycin oxime [82]. In vertebrates, all macrocyclic lactones are considered to have the same mechanism-based toxicity by binding to neuronal GABA-gated chloride channels [26-28]. Therefore, MDR1 mutant dogs which do not express P-gp at the blood-brain barrier in general are prone to neurotoxicity by macrocyclic lactones due to the increased brain penetration. Nevertheless, treatment of these dogs with macrocyclic lactones does not inevitably result in neurological symptoms, since, the safety of treatment depends on the following four factors:
Dosage/Treatment Indication
Neurotoxicosis is induced in MDR1(-/-) dogs after oral application of ( 100 µg/kg ivermectin or doramectin [40-42,60,83], ( 400 µg/kg moxidectin [58] or ( 5 mg/kg milbemycin oxime [84]. Treatment below these dosages, e.g. for heartworm prevention, is tolerated even by MDR1 mutant dogs. The dosage of macrocyclic lactones is also crucial for the outcome from intoxication, irrespective of whether the macrocyclic lactone was therapeutically applied or accidentally ingested. For example, after the subcutaneous application of doramectin at 600 µg/kg two MDR mutant White Swiss Shepherd dogs showed severe neurotoxicosis which required intensive care, but both dogs fully recovered within 14 days [60]. In contrast, we attended two other clinical cases where an Australian Shepherd and a Collie dog, both with the MDR1(-/-) genotype, died within 5-6 days after application of a slightly higher dose of 1 mg/kg (unpublished cases). Both dogs showed extremely high drug concentrations in their brain see Fig. (2).
Route of Application
Spot-on applications of ivermectin and moxidectin did not induce neurological toxicity in MDR1(-/-) dogs at dosage of up to 1.0 mg/kg and 2.5 mg/kg, respectively [85,86]. In contrast, oral application of adequate doses for treatment purposes would be highly toxic for MDR1 mutant dogs. Therefore, the route of application is crucial for the safety of treatment with macrocyclic lactones [87]. This particularly applies to spot-on formulations of moxidectin and selamectin which are labelled for use in dogs against a number of endo- and ectoparasitic diseases and should not be applied orally to MDR1(-/-) dogs. Ivermectin, apart from the oral application, is often extra-label used subcutaneously at concentrations of 400 µg/kg for the treatment of mange disease. This route of application in MDR1(-/-) dogs generally results in less severe neurotoxicosis which is, however, longer lasting compared with the oral route of application [60,88,89].
Individual Compound
In dogs with homozygous MDR1(-/-) mutation, ivermectin and doramectin seem to have a similar neurotoxicological potential and induce severe neurological toxicity at dosages of 200-600 µg/kg which often results in coma and death of the animals [40,42,60]. In contrast, other macrocyclic lactones, such as selamectin, moxidectin and milbemycin oxime, claim to be safer in the treatment of MDR1(-/-) dogs and seem to have a lower neurotoxicological potential. Milbemycin oxime and selamectin only showed mild neurological toxicity in MDR1 mutant dogs at oral doses of ≥ 5 mg/kg and >15 mg/kg, respectively [84,90,91]. Moxidectin seems to be intermediate in this respect and induced mild neurotoxicosis in MDR1 mutant dogs at doses of ≥ 400-1000 µg/kg [58,92]. Nevertheless, it has to be emphasised that for these compounds the safety margin is also dramatically reduced if P-gp is not expressed at the blood-brain barrier, so that treatment of MDR1 mutant dogs with macrocyclic lactones in general requires particular caution.
Heterozygous MDR1(+/-) or Homozygous MDR1(-/-) Genotype of the Dog
Although systematic studies on the application of macrocyclic lactones to MDR1(-/-) and MDR1(+/-) dogs have not been performed for any compound, many clinical cases of macrocyclic lactone intoxication in Collies or related dog breeds with unknown genetic MDR1 status resulted in two different kinds of toxic reactions: dogs with mild ataxia and CNS depression or even more severe neurotoxicosis which quickly recovered (presumably MDR1(+/-) dogs) and dogs with severe and long-lasting intoxications (presumably or post-case documented MDR1(-/-) dogs) [51,83,93]. Furthermore, it was shown that MDR1(+/+) as well as MDR1(+/-) dogs can tolerate oral doses of ivermectin at up to 600 µg/kg [40,46,94], whereas this dosage would induce life-threatening neurotoxicosis in MDR1(-/-) dogs [42,88]. Therefore, MDR1(+/-) dogs can be regarded as having an intermediate macrocyclic lactone sensitive phenotype which is relevant in cases of a high dose protocol, e.g. for the treatment of canine generalised demodicosis [94]. However, it is unlikely (and the authors were not aware of any clinical case) that MDR1(+/-) dogs would suffer from coma or death even under such high therapeutic dosage regimens, unlike MDR1(-/-) dogs (see Table 3).
HEARTWORM PREVENTION IN DOGS WITH MACROCYCLIC LACTONES
Among the available macrocyclic lactones, four compounds are currently used as heartworm preventatives in a variety of different formulations: ivermectin, selamectin, milbemycin oxime and moxidectin. These compounds interrupt larval development during the first two months after infection and, therefore, have a long application phase and are administered monthly or even less frequently [95].
Ivermectin
is marketed as a once monthly heartworm preventative at 6-12 µg/kg (Heartgard®) and also is microfilaricidal at this dosage [96]. Although MDR1(-/-) dogs are extremely sensitive to ivermectin, it has to be emphasised that no adverse drug reactions have been identified at these low preventive doses of ivermectin. Even after oral application of the 10-fold dose of 60 µg/kg [83] no adverse drug reactions were observed in ivermectin-sensitive Collies. This indicates that low dose ivermectin is a safe heartworm preventative even in MDR1 mutant dogs [83].
Compared with other macrocyclic lactone compounds selamectin is unique within its clinical spectrum and is available in a topical formulation (Stronghold®, Revolution®). Selamectin is applied at 6-12 mg/kg once per month and at this dosage is effective at preventing heartworm infections and is additionally indicated for flea infestation, sarcoptic mange, ear mites and even tick infestation [90,97]. The safety of selamectin treatment was specifically analysed in ivermectin-sensitive Collies and it produced no adverse drug reactions, even at supra-therapeutic doses [90,91].
Milbemycin oxime
is, apart from other indications, an effective heartworm preventative at a minimum recommended oral dose of 0.5 mg/kg at monthly intervals (Interceptor®, Milbemax®) [98]. When doses of 0.5-2.5 mg/kg milbemycin oxime were orally applied to ivermectin-sensitive Collies, no clinical signs of neurotoxicosis were observed [84]. Therefore, this compound can be safely used for heartworm prevention in MDR1(-/-) dogs. At higher concentrations of 5-10 mg/kg, as well as at daily dosing protocols of 0.5-2.8 mg/kg, which are normally well tolerated in dogs, milbemycin oxime provoked neurological toxicity in MDR1 mutant dogs including ataxia, salivation and depression [84,99].
Moxidectin
has been more recently marketed as a heartworm preventative (ProHeart®) and has been shown to be safe and effective at oral doses of 3 µg/kg given monthly [100]. A safety evaluation was specifically performed in ivermectin-sensitive Collies using an oral application of up to 90 µg/kg. Even at this 30-fold therapeutic dosage, moxidectin produced no neurotoxic adverse effects [92], indicating that moxidectin can be safely used as a heartworm preventative in MDR1(-/-)dogs. Apart from the oral formulation, moxidectin is also approved for heartworm prevention in a topical combination formulation of 10% w/v imidacloprid plus 2.5% w/v moxidectin (Advocate®, Advantage multi®) at a monthly application [101]. At the recommended therapeutic dosage, 2.5 mg/kg of moxidectin is applied per interval and this treatment has been shown to be well tolerated, even in ivermectin-sensitive Collies [86].
In summary, ivermectin, selamectin, moxidectin and milbemycin oxime are effective and safe drugs for heartworm prophylaxis with varying spectra and routes of application [95]. All of these drugs can be safely administered to MDR1(-/-) mutant dogs at the preventative dosage and by the correct application [102]. However, it has to be emphasised that at doses higher than those used for heartworm prevention, MDR1 mutant dogs will experience neurological toxicity with any of the macrocyclic lactones in this category (see Table 3).
MANGE TREATMENT IN DOGS WITH MACROCYCLIC LACTONES
Generalised demodicosis caused by Demodex canis mites is one of the most common skin diseases in dogs and is commonly regarded as difficult to treat successfully. Only a very few drugs are approved for the treatment of canine generalised demodicosis, including amitraz, an alpha-adrenergic receptor agonist used as a dip, and the topical combination formulation of 10% w/v imidacloprid plus 2.5% w/v moxidectin marketed as Advocate® or Advantage multi®. Apart from these medications, other macrocyclic lactones, although not approved for this indication, are commonly used in the management of this disease including ivermectin, doramectin and milbemycin oxime [103,104]. For example, ivermectin is administered at oral daily doses of 300-600 µg/kg, doramectin at 400-600 µg/kg and milbemycin oxime at 0.5-2.8 mg/kg for the treatment of canine generalised demodicosis [103,105-109]. Due to the hypersensitivity of MDR1 mutant dogs to macrocyclic lactones, these medications have not been officially approved for the treatment of canine generalised demodicosis. Nevertheless, several therapeutic protocols exist which are generally well tolerated in MDR1 normal dogs. These protocols usually use a gradual increase in dose over the first few days of treatment in order to recognise sensitive dogs before reaching a critical dosage that would induce life-threatening intoxication. This procedure was essential before the nt230(del4) MDR1 mutation was discovered, but nowadays it may be replaced by MDR1 genotyping. Nevertheless, due to the widespread distribution of the MDR1 mutation amongst different breeds, also including many mixed breed dogs, it is difficult to recognise whether an individual canine patient might be affected by this mutation or not. Therefore, the gradually increasing treatment protocol is still recommended. In detail, such protocols start with e.g. an oral ivermectin application at 50 µg/kg on the first day, followed by 100 µg/kg on the second day, 150 µg/kg on the third day, 200 µg/kg on the fourth day, and finally 300 µg/kg on the fifth and following days for at least 12 weeks [110]. During this dosage regimen it is very important to recognise the initial symptoms of ivermectin-induced neurological toxicity, such as ataxia, mydriasis and hypersalivation, as soon as possible and to immediately discontinue treatment in such a case.
Ivermectin
As already mentioned above, ivermectin orally applied at 50-60 µg/kg is well tolerated by ivermectin-sensitive dogs [42,83], but neurological toxicity is induced at higher doses of >100 µg/kg: after application of 100-120 µg/kg, mild depression and ataxia, as well as disorientation and mydriasis have been observed within 12 hours after application [40,41,92,111]; 125-170 µg/kg induced more severe ataxia, stupor, recumbency, head bobbing, apparent blindness, facial twitches, hypersalivation, episodes of hyperventilation and bradycardia [40,41,83,112,113]; still higher doses of 200-250 µg/kg caused severe neurotoxicosis, including depression, ataxia and apparent blindness as early onset symptoms, as well as vomiting, paddling movements, tremor and excessive salivation, followed by stupor, feeble attempts to crawl, recumbency, and finally non-responsiveness and coma within 30-50 hours after application, often resulting in death [40-42,88,113-115]; very high doses of ivermectin, of up to 600 µg/kg, accelerated the onset of symptoms and often resulted in death within 48 hours or euthanasia if the option of mechanical ventilation was rejected [40,42,88]. In conclusion, treatment protocols with 300-600 µg/kg ivermectin or even doramectin are unfeasible in MDR1 mutant dogs and would clearly result in life-threatening intoxication (see Tables 3 and 4).
Table 4.
Neurotoxic Potential of Macrocyclic Lactones and Treatment Outcome in MDR1 Mutant Dog
| Compound | Breed (Genotype or Phenotype)* | Dose (Application) | Clinical Signs of Neurotoxicosis and Outcome | Reference |
|---|---|---|---|---|
| Ivermectin | Collie (ISC) | 60 µg/kg (PO) | No | [83] |
| Collie (ISC) | 100-120 µg/kg (PO) | Mild depression, ataxia, disorientation, mydriasis, recovery | [40,41,92,111] | |
| Collie (ISC) | 125-170 µg/kg | Ataxia, recumbency, stupor, apparent blindness, hypersalivation, recovery | [83,112,113] | |
| Collie (ISC) | 200-250 µg/kg (PO) | Ataxia, depression, apparent blindness, paddling movements, tremor, excessive salivation, stupor, coma, death/recovery | [40,42,88,113-115] | |
| Collie (ISC), Australian Shepherd MDR1(-/-) | 200 µg/kg (SC) | Ataxia, loss of vision, hypersalivation, recumbency, stupor, recovery | [88,89] | |
| Collie MDR1(-/-) | 400 µg/kg (SC) | Ataxia, salivation, tremor, nonresponsiveness, stupor, coma, recovery | AUD | |
| Collie (ISC) | 1 mg/kg (spot-on) | No | [85] | |
| Doramectin | Collie | 200 µg/kg (SC) | Apparent blindness, ataxia, hypersalivation, recumbency, recovery | [129] |
| 2 White Swiss Shepherd dogs MDR1(-/-) | 700 µg/kg (SC) | Loss of vision, ataxia, depression, hypersalivation, hyperventilation, tremor, recumbency, recovery | [60] | |
| Collie MDR1(-/-), Australian Shepherd MDR1(-/-) | 1 mg/kg (SC) | Ataxia, tremor, stupor, coma, death | AUD | |
| Selamectin | Collie (ISC) | 40 mg/kg (spot-on) 15 mg/kg (PO) | No | [90,91] |
| Moxidectin | Collie (ISC) | 90 µg/kg (PO) | No | [92] |
| Australian Shepherd MDR1(-/-) | 100 µg/kg increased to 400 µg/kg (PO) | Ataxia, crawling, hyperexcitability, recovery | [58] | |
| Collie (ISC) | 32.5 mg/kg (plus 130 mg/kg imidacloprid) (spot-on) | No | [86] | |
| Milbemycin oxime | Collie MDR1(-/-) | 800 µg/kg/day incrementally increased to 1.5 mg/kg/day (PO) | Ataxia, recovery | [99] |
| 300 µg/kg/day incrementally increased to 1.6 mg/kg/day (PO) | Ataxia, recovery | |||
| Collie (ISC) | 1.25 mg/kg (PO) 2.5 mg/kg (PO) | No Ataxia, recovery |
[90] | |
| Collie (ISC) | 2.5 mg/kg (PO) 5 mg/kg (PO) | No Ataxia, salivation, depression, recovery |
[84] | |
Before the discovery of the nt230(del4) MDR1 mutation, ivermectin-sensitive Collies (ISC) were identified by test application of 120-200 µg/kg ivermectin orally followed by documentation of neurological toxicity including ataxia and CNS depression. PO, oral application; SC, subcutaneous application; AUD, author's unpublished data
In contrast, acute and subchronic ivermectin toxicity studies in MDR1 normal dogs demonstrated a large safety margin: ivermectin can be administered to Beagles, as well as to ivermectin non-sensitive Collies, at a single oral dose of 2 mg/kg or at a daily oral dose of 500 µg/kg over 14 days without any evidence of toxicosis [29]. Only at higher doses of 1 mg/kg ivermectin applied daily for 14 weeks or at a single dose of 2.5 mg/kg did these dogs show mydriasis as the initial symptom of drug-induced neurological toxicity [116,117]. Even higher doses of 5-20 mg/kg additionally caused ataxia and tremor, and 40 mg/kg proved to be fatal [93,115,116]. The oral LD50 for ivermectin in Beagle dogs was estimated to 80 mg/kg. Post-mortem, these ivermectin-poisoned dogs were pathologically normal and no specific lesions were observed in the brain [118].
Moxidectin
Several in vitro transport studies also confirmed that moxidectin is transported by P-gp, although it seems to be a weaker substrate and inhibitor of P-gp compared with ivermectin [30,119-121]. Closer analysis of the interaction between moxidectin and P-gp revealed that moxidectin can inhibit the P-gp efflux function with a similar efficiency compared to ivermectin, however, it requires concentrations 10 times higher to reach the same inhibitory effect. This would be consistent with a lower affinity binding of moxidectin to P-pg compared with other macrocyclic lactones [31]. Very recently, Kiki-Mvouaka et al. [44] analysed the in vivo pharmacokinetics of moxidectin in the mdr1a,b(-/-) knockout mouse model in comparison with ivermectin. After subcutaneous applications of 200 µg/kg for both drugs, equal drug concentrations of approximately 65 ng/g were found in the brain 24 hours after application, indicating that in the absence of P-gp in the blood-brain barrier both compounds show comparable brain penetration. However, in the wild-type mice absolute brain concentrations were more than 10-fold higher for moxidectin, demonstrating that P-gp in vivo transports moxidectin less effectively at the blood-brain barrier compared with ivermectin [44] see Fig. (2).
For the treatment of canine generalised demodicosis, 200-400 µg/kg moxidectin is commonly applied orally per day and this treatment is normally well tolerated in dogs [108]. Higher doses of 1 mg/kg were even tolerated in Beagle dogs with no clinical signs of neurological toxicity [122]. As already mentioned above, moxidectin is safely tolerated even by ivermectin-sensitive Collies, but only at low oral doses of 90 µg/kg [83,92]. However, as shown in an Australian Shepherd with the MDR1(-/-) genotype, which was treated with a gradually increasing dosage protocol, neurological toxicity was induced after reaching the target dose of 400 µg/kg. After discontinuing the treatment the dog fully recovered, indicating that the neurotoxicosis was induced by moxidectin [58] (Table 4). Therefore, a treatment protocol of 400 µg/kg moxidectin orally per day cannot be applied to MDR1(-/-) dogs for the treatment of canine generalised demodicosis. On the other hand and in contrast to this extra-label oral application, moxidectin is approved for the treatment of canine generalised demodicosis and a range of other endo- and extoparasites in a topical combination formulation of 10% w/v imidacloprid plus 2.5% w/v moxidectin [123]. To the best of our knowledge, this topical formulation currently represent the only macrocyclic lactone-containing treatment option which is licensed for the treatment of canine generalised demodicosis. The safety of this formulation was evaluated under field conditions in different dog breeds and was generally well tolerated at the therapeutic dosage [104,123]. During the safety evaluation this combination formulation was also specifically administered to ivermectin-sensitive Collies. At the approved topical application, this formulation was safely tolerated by MDR1 mutant Collies even at the 5-fold therapeutic dosage containing 32.5 mg/kg moxidectin [86]. However, after oral application of only 40% (1 mg/kg) of the recommended topical dose, neurological toxicity occurred in the drug-sensitive Collies [124], emphasising that oral ingestion of this formulation must definitely be precluded in MDR1(-/-) dogs.
Milbemycin Oxime
Milbemycin oxime has been used for the treatment of generalised demodicosis at doses ranging from 0.5-2.8 mg/kg [103,109]. Only one study is available which analysed the safety of milbemycin oxime in ivermectin-sensitive Collies. In this study, the characteristic neurological toxicity, albeit of short duration, was observed in individual Collies applied with 5 mg/kg milbemycin oxime orally, and included mild depression, excessive salivation and ataxia [84]. At the higher dosage of 10 mg/kg all sensitive Collies developed signs of mild depression and ataxia within 6 hours after treatment. Neurotoxicosis persisted for at least 24 hours, but all dogs fully recovered within 48 hours after treatment. In another study, milbemycin oxime was applied to two ivermectin-sensitive Collies as a sesame oil solution in gelatin capsules. Whereas at 1.25 mg/kg no adverse drug reactions were observed, both dogs became ataxic within 4 hours of treatment at 2.5 mg/kg [90]. In contrast, in an earlier study of the application of milbemycin oxime at dosages of up to 25 mg/kg in rough-coated Collies, no adverse drug reactions were induced [125]. However, it has to be assumed that these dogs, although of the Collie breed, were not affected by the MDR1 mutation. More recently, Barbet et al. [99] analysed the safety of treatment with milbemycin oxime in 22 dogs diagnosed with generalised demodicosis including two MDR1(-/-) and one MDR1(+/-) dog. All dogs received milbemycin oxime at a daily dose of 1-2.2 mg/kg, which normally is well tolerated in dogs [109]. None of the MDR1 normal dogs nor the heterozygous MDR1(+/-) dogs experienced any adverse drug reactions under treatment. In contrast, and despite the low dose initiation of treatment with 300-800 µg/kg/day, both MDR1(-/-) mutant dogs experienced ataxia following an increase of the dose to 1.5-1.6 mg/kg/day. When the treatment dose of milbemycin oxime then was decreased to a tolerable dose of 600 µg/kg, both dogs recovered [99] (Table 4). This study clearly indicates that the knowledge of the MDR1 genotype is critical in order to achieve a milbemycin oxime dosage regimen that is tolerated without causing adverse drug reactions. It further shows that milbemycin oxime may be the safer choice than ivermectin or doramectin for the treatment of generalised demodicosis in MDR1(-/-) dogs.
Selamectin
Selamectin is marketed as a spot-on formulation with a minimum therapeutic dosage of 6 mg/kg (Stronghold®, Revolution®). Although not effective for the treatment of canine generalised demodicosis, selamectin shows activity against both insect and arachnid classes of ectoparasites and is licensed for the control of canine sarcoptic mange [90,97,126]. In vitro transport studies showed that selamectin is transported by P-gp as equally as ivermectin [30,121]. Thus, it was assumed that MDR1 mutant dogs would also exhibit increased drug sensitivity against selamectin. Indeed, an in vivo study by our laboratory confirmed that selamectin at concentrations of 12 mg/kg (representing the maximum dose for the body weight range) shows significantly higher brain penetration in mdr1a,b(-/-) knockout mice compared with wild-type mice by any route of application (oral, intravenous, spot-on) [35]. However, the brain concentration ratios (knockout vs. wild-type mice) of 5-fold to 10-fold were much less pronounced than the respective ratios for ivermectin applied at 200 µg/kg (i.e. 36-fold to 60-fold) see Fig. (2). Furthermore, brain-to-plasma concentration ratios in the wild-type mice were much higher for selamectin than for ivermectin (0.32 vs. 0.09, respectively), which is consistent with a higher efflux rate at the blood-brain barrier for ivermectin than for selamectin [35]. These findings support more recent data from in vitro studies by Lespine et al. [31] which showed that ivermectin has much higher affinity for P-gp compared with selamectin (Ki values: 0.05 µM vs. 1.0 µM, respectively). Based on this data, it may be speculated that the dimensions of the sugar moiety on the macrocycle (disaccharide substitution in ivermectin, monosaccharide substitution in selamectin) account for these different affinities to P-gp and can thus determine the extent of brain penetration of the respective compounds.
During a safety evaluation of the topical selamectin formulation, application studies were specifically performed in ivermectin-sensitive Collies. Although increased brain penetration has to be assumed in these dogs, selamectin produced no adverse drug reactions at a dosage of 40 mg/kg topically or 15 mg/kg orally, which represent 3-7 times the minimum therapeutic dosage of 6 mg/kg [90,91] (Table 4). Thus, the neurotoxic potential of selamectin seems to be much lower compared to all of the other macrocyclic lactones which provoke neurological toxicity in MDR1 mutant dogs at much lower dosages and which reach much lower drug concentrations in the brain see Fig. (2). Therefore, we would anticipate that selamectin and ivermectin would exhibit different affinities or different intrinsic activities for vertebrate GABA-activated chloride channels. To prove this conjecture, it will be necessary to conduct comparative receptor binding assays in brain preparations. These should specifically address the role of the substitutions at positions C5 (NOH in selamectin and OH in ivermectin) and C25 (cyclohexyl in selamectin and sec-butyl/isopropyl in ivermectin B1) since these have previously been reported to significantly affect the antiparasitic activity of both compounds [81,127,128].
MDR1 SINGLE NUCLEOTIDE POLYMORPHISMS AND IVERMECTIN SENSITIVITY
Several clinical studies showed that ivermectin and even milbemycin oxime or moxidectin applied at a high dose protocol for the treatment of canine generalised demodicosis are generally well tolerated in MDR1 normal dogs [103]. However, signs of subchronic neurotoxicity are occasionally seen in individual dogs treated with a daily high dose protocol. In these dogs, the onset of toxicity signs is seen several days or weeks after initiation of the treatment, in particular with ivermectin at 400-600 µg/kg/day, but these normally resolve after discontinuing the treatment, indicating that the macrocyclic lactone causes this intoxication [94]. These subchronic neurotoxicity reactions most likely represent individual differences in the sensitivity to ivermectin based on genetic variants [110]. For example, Bissonnette et al. [94] described a MDR1(+/-) juvenile mixed breed dog which showed neurological toxicity including ataxia, tremor and depression after daily ivermectin application at 670 µg/kg after seven weeks of therapy, indicating that at least one intact MDR1 allele may protect the dog from acute neurotoxicity after high dose applications of ivermectin. On the other hand, dogs that actually have the intact MDR1(+/+) genotype, developed subchronic neurological toxicity at much shorter treatment intervals. It may be speculated that these dogs show lower expression of P-gp at the blood-brain barrier or that they may express a less active polymorphic P-gp. Currently, more than 30 single nucleotide polymorphisms are known in the canine MDR1 gene (see Table 5) which might affect the transport function of P-gp, such as the Gln532Arg amino acid substitution which is located in direct proximity to the highly conserved and functionally important ABC signature motif of P-gp. However, whether one of these polymorphisms indeed correlates with increased drug sensitivity under high dose treatment protocols with macrocyclic lactones has to be investigated further.
Table 5.
Single Nucleotide Polymorphisms in the Canine MDR1 cDNA Sequence
| Single Nucleotide Polymorphism | Exon | GenBank Accession No. | Amino Acid Substitution | PolyPhen Prediction | SIFT Prediction |
|---|---|---|---|---|---|
| A23G | 2 | AJ419568 | Silent | ||
| A51G | 2 | AJ419568 | Silent | ||
| A86G | 3 | AJ419568 | Silent | ||
| A265G | 4 | AF536758, FJ617477 | Thr89Ala | Benign | Tolerated |
| T564C | 7 | AJ419568 | Silent | ||
| A574G | 7 | AF045016 | Silent | ||
| A591C | 7 | AF536758 | Silent | ||
| G635C | 7 | AF045016 | Silent | ||
| A862G | 9 | AF536758 | Arg288Gly | Benign | Tolerated |
| T985A | 9 | AF045016 | Ser329Thr | Benign | Tolerated |
| A996G | 9 | AF045016 | Silent | ||
| T1232C | 12 | AJ419568 | Silent | ||
| A1595G | 14 | AB066299, AF045016 | Gln532Arg | Probably damaging | May affect protein function |
| G1863A | 15 | AF092810 | Silent | ||
| G1914C | 16 | AF092810 | Glu638Asp | Benign | Tolerated |
| A2082T | 17 | AF045016 | Silent | ||
| C2086T | 17 | AB066299, AF045016 | Pro696Ser | Benign | Tolerated |
| A2181G | 17 | AF092810 | Silent | ||
| A2258T | 18 | AF092810 | Asn753Ile | Benign | May affect protein function |
| C2322T | 18 | AF092810 | Silent | ||
| C2328T | 19 | AF092810 | Silent | ||
| G2349A | 19 | AF092810 | Silent | ||
| C2426T | 20 | AF092810 | Pro809Leu | Possibly damaging | Tolerated |
| A2451C | 20 | AF092810 | Silent | ||
| G2471T | 20 | AF092810 | Silent | ||
| A2601G | 21 | AY582533 | Silent | ||
| G2741A | 22 | AF092810 | Arg914Gln | Benign | Tolerated |
| A2781G | 22 | AF092810 | Silent | ||
| T2758C | 22 | AF092810 | Silent | ||
| G2907A | 23 | AJ419568 | Silent | ||
| A3442G | 26 | AY582533 | Met1148Val | Benign | Tolerated |
| T3792C | 28 | AF536758 | Silent | ||
| G3817A | 28 | AF045016 | Silent | ||
| G3840A | 28 | AJ419568 | Silent |
Note: The polymorphisms were identified based on sequence alignment of all MDR1 cDNA sequences available in the GenBank/EBI/DDBJ database with the following accession numbers: AB066299, AF045016, AF092810, AF403240, AF536758, AJ419568, AY582533, DQ068953 and FJ617477. Potential effects of the non-silent polymorphisms were evaluated by SIFT and PolyPhen algorithms. Both programs consistently predicted the Gln532Arg polymorphism located in proximity to the ABC signature motif to functionally affect the P-gp transport function.
INTOXICATIONS FROM HORSE DEWORMING MEDICATION
Ivermectin and moxidectin are commonly used in horses to treat parasitic diseases and are available as oral paste, oral liquid gel or tablet formulations, usually with 12-23 mg/g of the active drug. As these preparations deliver doses intended for the treatment of horses, they are highly concentrated and contain very high absolute amounts of drug (≥ 120 mg ivermectin or > 200 mg moxidectin per applicator or package) which result in severe intoxication when accidentally ingested by dogs [130-134], particularly when the dog has the MDR1(-/-) genotype [135-137]. The severity of ivermectin or moxidectin induced neurotoxicosis in such cases is generally a dose-dependent phenomenon. Therefore, prognosis and successful treatment can only be predicted on the basis of certain knowledge about the amount of drug ingested, although in most cases this cannot be reconstructed. In addition, the prognosis and eventual outcome depend on a number of further factors, including, first of all, the MDR1 genotype of the dog, the individual as well as breed-typical constitution of the dog (which is somewhat different e.g. between Collie and Longhaired Whippet dogs) and the history of the toxicosis development (how rapidly and with which clinical signs it appears). Generally, severe clinical CNS depression occurring within 1-2 hours after ingestion or rapidly worsening toxicosis indicate the presence of the MDR1 mutation and/or ingestion of very high doses of ivermectin. Mostly, these cases have a more critical progression and worse prognosis. On the other hand, MDR1 mutant dogs with a slow onset of clinical signs, typically mydriasis, ataxia and apparent blindness within 4-8 hours after ingestion, have normally ingested low amounts and can be given a good prognosis. Although the recovery of poisoned dogs may take several weeks, many cases with long comatose episodes have returned to complete health [40,41,88,113,137]. This, however, requires good nursing and supportive care, including fluid treatment, nutritional support and vigilant cardiopulmonary monitoring.
TREATMENT OF MACROCYCLIC LACTONE INDUCED NEUROTOXICOSIS
Currently, there is no specific and safe antidote available for the treatment of macrocyclic lactone-induced toxicosis. Therefore, treatment is solely based on symptomatic and supportive care [138]. Following oral ingestion, the initial therapy should focus on drug removal by inducing emesis as soon as possible, or by gastric lavage. Then the serial administration of adsorbents (e.g. activated charcoal) is indicated, as long as the dog shows responsiveness, in order to discourage enteral absorption of the parent compound and to enhance the elimination of ivermectin via the faeces, which is the main route of excretion [139]. In contrast, forced diuresis will not facilitate the excretion of ivermectin. During long lasting phases of nonresponsiveness and recumbency, supportive care, parenteral alimentation and prevention of decubital ulcers are of particular importance, while electrolytes, fluid balance, blood pressure, heart rate, body temperature, blood gases and respiratory function have to be monitored [40]. In order to reduce gastric irritation and acidification overshoot, substances which inhibit gastric acid secretion such as cimetidine have been applied [118]. However, as cimetidine is a substrate of P-gp, omeprazole is recommended for better medication in MDR1 mutant dogs. In severe cases with pronounced respiratory depression, mechanical ventilation may be required.
In some cases of severe neurotoxicosis where the dogs became unresponsive, physostigmine (a cholinesterase inhibitor) was administered slowly twice a day at a total dose of 40 µg/kg intravenously [41,89,113]. Physostigmine increases the synaptic concentration of acetylcholine in the central and peripheral nervous system. In the central nervous system acetylcholine acts as an excitatory neurotransmitter and induces central stimulation. In the periphery, acetylcholine acts parasympathomimetic and might be beneficial for gastrointestinal and motor stimulation. In the unresponsive dogs, physostigmine application resulted in a transient increase of responsiveness, muscle activity, heart and respiratory rate, and attempts to drink and eat, whereas in mild intoxication and responsive dogs it was of little benefit [41]. Nevertheless, in our experience physostigmine application is a helpful premedication in the recovery phase before food is made available or the dog is exercised by assisted walking. However, the duration of action for physostigmine lasted only 30-90 minutes. Physostigmine has no potency in accelerating the recovery of the dogs, or for general improvements of the outcome. Furthermore, in cases of overdose, physostigmine may be associated with the development of convulsions and bradycardia, and therefore must be handled with caution [41]. Treatment with glycopyrrolate before physostigmine administration may be warranted to avoid severe side effects, e.g. bradycardia. In summary, physostigmine is beneficial for transiently vitalizing a dog's constitution and for encouraging the owners that recovery might be possible. The major difficulty, however, involves the potential toxicity of physostigmine itself.
Picrotoxin has been used in a few cases of ivermectin-induced neurotoxicosis since it blocks GABA-activated chloride channels, and therefore was suggested as a possible antidote to ivermectin poisoning [140]. It was given by intravenous infusion at a dosage rate of 1 mg/min for 8 minutes and appeared to reverse severe CNS depression. However, picrotoxin infusion also induced violent clonic seizures 30 minutes after application which required treatment with thiopental. Because of this, and due to its narrow safety margin, picrotoxin cannot be recommended for routine use as an antidote for ivermectin intoxication [140].
In the case of ivermectin-induced tremor, which often occurs as a late-onset symptom (typically within 12 hours after oral drug ingestion), benzodiazepine drugs such as diazepam should be avoided as avermectins enhance binding of these drugs to the GABA receptor and further enhance the GABAergic activity, leading to a more pronounced CNS depression [139]. Although not approved in a large number of dogs, propofol as a short acting hypnotic drug might be an appropriate medication in this phase of neurotoxicosis [132, 133].
Although there has been intensive research on macrocyclic lactones over three decades, the search for an effective and direct antidote against macrocyclic lactone-induced neurotoxicosis has not yet been successful. Therapy of intoxication is still based on symptomatic relief, but it lacks a macrocyclic lactone receptor binding antagonist. Ideally, this antagonist should exhibit high binding affinity without or with much less intrinsic activity than the macrocyclic lactones used therapeutically.
CONCLUSIONS
Identification of the nt230(del4) MDR1 mutation in drug-sensitive dogs has clearly improved the safety of treatment of parasitic diseases with macrocyclic lactones. Pharmacogenetic diagnostics can determine the MDR1 genotype for an individual canine patient before macrocyclic lactone treatment is started which is particularly important for dog breeds highly predisposed towards this mutation. Depending on the MDR1 genotype predictions can be made on whether the treatment will be safe and beneficial or whether the dog may experience neurological toxicity following drug application.
ACKNOWLEDGEMENT
None declared.
ABBREVIATIONS
- ABC
= ATP-binding cassette
- CNS
= Central nervous system
- GABA
= Gamma-aminobutyric acid
- MDR1
= Multidrug resistance
- P-gp
= P-glycoprotein
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genomics Hum. Genet. 2005;6:123–142. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- 2.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta. 1976;455(1 ):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 3.Chen CJ, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM, Roninson IB. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47(3 ):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- 4.Ueda K, Clark DP, Chen CJ, Roninson IB, Gottesman MM, Pastan I. The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J. Biol. Chem. 1987;262(2 ):505–508. [PubMed] [Google Scholar]
- 5.Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 2001;42(7 ):1007–1017. [PubMed] [Google Scholar]
- 6.Pastan I, Gottesman MM. Multidrug resistance. Annu. Rev. Med. 1991;42:277–286. doi: 10.1146/annurev.me.42.020191.001425. [DOI] [PubMed] [Google Scholar]
- 7.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86(4 ):1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 8.Fromm MF. Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol. Sci. 2004;25(8 ):423–429. doi: 10.1016/j.tips.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Gerloff T. Impact of genetic polymorphisms in transmembrane carrier-systems on drug and xenobiotic distribution. Naunyn Schmiedebergs Arch. Pharmacol. 2004;369(1 ):69–77. doi: 10.1007/s00210-003-0813-5. [DOI] [PubMed] [Google Scholar]
- 10.Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin. Pharmacol. Ther. 2004;75(1 ):13–33. doi: 10.1016/j.clpt.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood-brain barrier as a supporting and protecting interface for the brain, importance for CNS drug discovery and development. Pharm. Res. 2007;24(9 ):1745–1758. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- 12.Raviv Y, Pollard HB, Bruggemann EP, Pastan I, Gottesman MM. Photosensitized labeling of a functional multidrug transporter in living drug-resistant tumor cells. J. Biol. Chem. 1990;265(7 ):3975–3980. [PubMed] [Google Scholar]
- 13.Sharom FJ, Lugo MR, Eckford PD. New insights into the drug binding, transport and lipid flippase activities of the p-glycoprotein multidrug transporter. J. Bioenerg. Biomembr. 2005;37(6 ):481–487. doi: 10.1007/s10863-005-9496-6. [DOI] [PubMed] [Google Scholar]
- 14.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA. 1987;84(21 ):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J. Histochem. Cytochem. 1989;37(2 ):159–164. doi: 10.1177/37.2.2463300. [DOI] [PubMed] [Google Scholar]
- 16.Cordon-Cardo C, O'Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc. Natl. Acad. Sci. USA. 1989;86(2 ):695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordon-Cardo C, O'Brien JP, Boccia J, Casals D, Bertino JR, Melamed MR. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J. Histochem. Cytochem. 1990;38(9 ):1277–1287. doi: 10.1177/38.9.1974900. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump in human hematopoietic stem cells. Cell. 1991;66(1 ):85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhary PM, Mechetner EB, Roninson IB. Expression and activity of the multidrug resistance P-glycoprotein in human peripheral blood lymphocytes. Blood. 1992;80(11 ):2735–2739. [PubMed] [Google Scholar]
- 20.Ginn PE. Immunohistochemical detection of P-glycoprotein in formalin-fixed and paraffin-embedded normal and neoplastic canine tissues. Vet. Pathol. 1996;33(5 ):533–541. doi: 10.1177/030098589603300508. [DOI] [PubMed] [Google Scholar]
- 21.Schinkel AH. The physiological function of drug-transporting P-glycoproteins. Semin. Cancer Biol. 1997;8(3 ):161–170. doi: 10.1006/scbi.1997.0068. [DOI] [PubMed] [Google Scholar]
- 22.Fromm MF. P-glycoprotein a defense mechanism limiting oral bioavailability and CNS accumulation of drugs. Int. J. Clin. Pharmacol. Ther. 2000;38(2 ):69–74. doi: 10.5414/cpp38069. [DOI] [PubMed] [Google Scholar]
- 23.Martinez M, Modric S, Sharkey M, Troutman L, Walker L, Mealey K. The pharmacogenomics of P-glycoprotein and its role in veterinary medicine. J. Vet. Pharmacol. Ther. 2008;31(4 ):285–300. doi: 10.1111/j.1365-2885.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- 24.Turner MJ, Schaeffer JM. In: Ivermectin and Abamectin. Campbell, editor. pp. New York: Springer-Verlag V; 1989. pp. 73–88. [Google Scholar]
- 25.Wolstenholme AJ, Rogers AT. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology. 2005;131(Suppl ):S85–S95. doi: 10.1017/S0031182005008218. [DOI] [PubMed] [Google Scholar]
- 26.Sigel E, Baur R. Effect of avermectin B1a on chick neuronal gamma-aminobutyrate receptor channels expressed in Xenopus oo-cytes. Mol. Pharmacol. 1987;32(6 ):749–752. [PubMed] [Google Scholar]
- 27.Huang J, Casida JE. Avermectin B1a binds to high- and low-affinity sites with dual effects on the gamma-aminobutyric acid-gated chloride channel of cultured cerebellar granule neurons. J. Pharmacol. Exp. Ther. 1997;281(1 ):261–266. [PubMed] [Google Scholar]
- 28.Dawson GR, Wafford KA, Smith A, Marshall GR, Bayley PJ, Schaeffer JM, Meinke PT, McKernan RM. Anticonvulsant and adverse effects of avermectin analogs in mice are mediated through the gamma-aminobutyric acid(A) receptor. J. Pharmacol. Exp. Ther. 2000;295(3 ):1051–1060. [PubMed] [Google Scholar]
- 29.Pulliam JD, Preston JM. In: Ivermectin and Abamectin. Campbell, editor. New York: Springer-Verlag V; 1989. pp. 149–161. [Google Scholar]
- 30.Griffin J, Fletcher N, Clemence R, Blanchflower S, Brayden DJ. Selamectin is a potent substrate and inhibitor of human and canine P-glycoprotein. J. Vet. Pharmacol. Ther. 2005;28(3 ):257–265. doi: 10.1111/j.1365-2885.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 31.Lespine A, Martin S, Dupuy J, Roulet A, Pineau T, Or-lowski S, Alvinerie M. Interaction of macrocyclic lactones with P-glycoprotein: structure-affinity relationship. Eur. J. Pharm. Sci. 2007;30(1 ):84–94. doi: 10.1016/j.ejps.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77(4 ):491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 33.Schinkel AH, Mayer U, Wagenaar E, Mol CA, van Deemter L, Smit JJ, van der Valk MA, Voordouw AC, Spits H, van Tellingen O, Zijlmans JM, Fibbe WE, Borst P. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc. Natl. Acad. Sci. USA. 1997;94(8 ):4028–4033. doi: 10.1073/pnas.94.8.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panwalla CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene mdr1a spontaneously develop colitis. J. Immunol. 1998;161:5733–44. [PubMed] [Google Scholar]
- 35.Geyer J, Gavrilova O, Petzinger E. Brain penetration of ivermectin and selamectin in mdr1a,b P-glycoprotein- and bcrp- deficient knockout mice. J. Vet. Pharmacol. Ther. 2009;32(1 ):87–96. doi: 10.1111/j.1365-2885.2008.01007.x. [DOI] [PubMed] [Google Scholar]
- 36.Lankas GR, Cartwright ME, Umbenhauer D. P-glycoprotein deficiency in a subpopulation of CF-1 mice enhances avermectin-induced neurotoxicity. Toxicol. Appl. Pharmacol. 1997;143(2 ):357–365. doi: 10.1006/taap.1996.8086. [DOI] [PubMed] [Google Scholar]
- 37.Umbenhauer DR, Lankas GR, Pippert TR, Wise LD, Cart-wright ME, Hall SJ, Beare CM. Identification of a P-glycoprotein-deficient subpopulation in the CF-1 mouse strain using a restriction fragment length polymorphism. Toxicol. Appl. Pharmacol. 1997;146(1 ):88–94. doi: 10.1006/taap.1997.8225. [DOI] [PubMed] [Google Scholar]
- 38.Jun K, Lee SB, Shin HS. Insertion of a retroviral solo long terminal repeat in mdr-3 locus disrupts mRNA splicing in mice. Mamm. Genome. 2000;11(10 ):843–848. doi: 10.1007/s003350010176. [DOI] [PubMed] [Google Scholar]
- 39.Kwei GY, Alvaro RF, Chen Q, Jenkins HJ, Hop CE, Keohane CA, Ly VT, Strauss JR, Wang RW, Wang Z, Pippert TR, Umbenhauer DR. Disposition of ivermectin and cyclosporin A in CF-1 mice deficient in mdr1a P-glycoprotein. Drug Metab Dispos. 1999;27(5 ):581–587. [PubMed] [Google Scholar]
- 40.Paul AJ, Tranquilli WJ, Seward RL, Todd KS , Jr, DiPietro JA. Clinical observations in collies given ivermectin orally. Am. J. Vet. Res. 1987;48(4 ):684–685. [PubMed] [Google Scholar]
- 41.Tranquilli WJ, Paul AJ, Seward RL, Todd KS, DiPietro JA. Response to physostigmine administration in collie dogs exhibiting ivermectin toxicosis. J. Vet. Pharmacol. Ther. 1987;10(1 ):96–100. doi: 10.1111/j.1365-2885.1987.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 42.Pulliam JD, Seward RL, Henry RT, Steinberg SA. Investigating ivermectin toxicity in collies. Veterinary Medicine. 1985;80:33–40. [Google Scholar]
- 43.Schinkel AH, Mol CA, Wagenaar E, van Deemter L, Smit JJ, Borst P. Multidrug resistance and the role of P-glycoprotein knockout mice. Eur. J. Cancer. 1995a;31A(7-8):1295–1298. doi: 10.1016/0959-8049(95)00130-b. [DOI] [PubMed] [Google Scholar]
- 44.Kiki-Mvouaka S, Menez C, Borin C, Lyazrhi F, Foucaud-Vignault M, Dupuy J, Collet X, Alvinerie M, Lespine A. Role of P-glycoprotein in the disposition of macrocyclic lactones: a comparison between ivermectin eprinomectin and moxidectin in mice. Drug Metab Dispos. 2010;38(4 ):573–580. doi: 10.1124/dmd.109.030700. [DOI] [PubMed] [Google Scholar]
- 45.Seward RL. Reactions in dogs given ivermectin. J. Am. Vet. Med. Assoc. 1983;183(4 ):493. [PubMed] [Google Scholar]
- 46.Mealey KL, Bentjen SA, Gay JM, Cantor GH. Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics. 2001;11(8 ):727–733. doi: 10.1097/00008571-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Roulet A, Puel O, Gesta S, Lepage JF, Drag M, Soll M, Alvinerie M, Pineau T. MDR1-deficient genotype in Collie dogs hypersensitive to the P-glycoprotein substrate ivermectin. Eur. J. Pharmacol. 2003;460(2-3 ):85–91. doi: 10.1016/s0014-2999(02)02955-2. [DOI] [PubMed] [Google Scholar]
- 48.Tamai I, Tsuji A. Transporter-mediated permeation of drugs across the blood-brain barrier. J. Pharm. Sci. 2000;89(11 ):1371–1388. doi: 10.1002/1520-6017(200011)89:11<1371::aid-jps1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 49.Mealey KL. Therapeutic implications of the MDR-1 gene. J. Vet. Pharmacol. Ther. 2004;27(5 ):257–264. doi: 10.1111/j.1365-2885.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- 50.Hugnet C, Cadore JL, Buronfosse F, Pineau X, Mathet T, Berny PJ. Loperamide poisoning in the dog. Vet. Hum. Toxicol. 1996;38(1 ):31–33. [PubMed] [Google Scholar]
- 51.Sartor LL, Bentjen SA, Trepanier L, Mealey KL. Loperamide toxicity in a collie with the MDR1 mutation associated with ivermectin sensitivity. J. Vet. Intern. Med. 2004;18(1 ):117–118. doi: 10.1892/0891-6640(2004)18<117:ltiacw>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 52.Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J. Clin. Invest. 1996;97(11 ):2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doran A, Obach RS, Smith BJ, Hosea NA, Becker S, Callegari E, Chen C, Chen X, Choo E, Cianfrogna J, Cox LM, Gibbs JP, Gibbs MA, Hatch H, Hop CE, Kasman IN, Laperle J, Liu J, Liu X, Logman M, Maclin D, Nedza FM, Nelson F, Olson E, Rahematpura S, Raunig D, Rogers S, Schmidt K, Spracklin DK, Szewc M, Troutman M, Tseng E, Tu M, Van Deusen JW, Venkatakrishnan K, Walens G, Wang EQ, Wong D, Yasgar AS, Zhang C. The impact of P-glycoprotein on the disposition of drugs targeted for indications of the central nervous system: evaluation using the MDR1A/1B knockout mouse model. Drug Metab. Dispos. 2005;33(1 ):165–174. doi: 10.1124/dmd.104.001230. [DOI] [PubMed] [Google Scholar]
- 54.Henik RA, Kellum HB, Bentjen SA, Mealey KL. Digoxin and mexiletine sensitivity in a Collie with the MDR1 mutation. J. Vet. Intern. Med. 2006;20(2 ):415–417. doi: 10.1892/0891-6640(2006)20[415:damsia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 55.Mealey KL. Adverse drug reactions in herding-breed dogs: the role of P-glycoprotein. Comp. Contin. Educ. Pract. Vet. 2006;28(1 ):23–33. [Google Scholar]
- 56.Mealey KL, Fidel J, Gay JM, Impellizeri JA, Clifford CA, Bergman PJ. ABCB1-1Delta polymorphism can predict hematologic toxicity in dogs treated with vincristine. J. Vet. Intern. Med. 2008;22(4 ):996–1000. doi: 10.1111/j.1939-1676.2008.0122.x. [DOI] [PubMed] [Google Scholar]
- 57.Neff MW, Robertson KR, Wong AK, Safra N, Broman KW, Slatkin M, Mealey KL, Pedersen NC. Breed distribution and history of canine mdr1-1D a pharmacogenetic mutation that marks the emergence of breeds from the collie lineage. Proc. Natl. Acad. Sci. USA. 2004;101(32 ):11725–11730. doi: 10.1073/pnas.0402374101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geyer J, Döring B, Godoy JR, Moritz A, Petzinger E. Development of a PCR-based diagnostic test detecting a nt230(del4) MDR1 mutation in dogs: verification in a moxidectin-sensitive Australian Shepherd. J. Vet. Pharmacol. Ther. 2005;28(1 ):95–99. doi: 10.1111/j.1365-2885.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- 59.Kawabata A, Momoi Y, Inoue-Murayama M, Iwasaki T. Canine mdr1 gene mutation in Japan. J. Vet. Med. Sci. 2005;67(11 ):1103–1107. doi: 10.1292/jvms.67.1103. [DOI] [PubMed] [Google Scholar]
- 60.Geyer J, Klintzsch S, Meerkamp K, Wohlke A, Distl O, Moritz A, Petzinger E. Detection of the nt230(del4) MDR1 mutation in White Swiss Shepherd dogs: case reports of doramectin toxicosis, breed predisposition and microsatellite analysis. J. Vet. Pharmacol. Ther. 2007;30(5 ):482–485. doi: 10.1111/j.1365-2885.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 61.Mealey KL, Meurs KM. Breed distribution of the ABCB1-1Delta (multidrug sensitivity) polymorphism among dogs undergoing ABCB1 genotyping. J. Am. Vet. Med. Assoc. 2008;233(6 ):921–924. doi: 10.2460/javma.233.6.921. [DOI] [PubMed] [Google Scholar]
- 62.Klintzsch S, Meerkamp K, Döring B, Geyer J. Detection of the nt230[del4] MDR1 mutation in dogs by a fluorogenic 5' nuclease TaqMan allelic discrimination method. Vet. J. 2009;185:272–277. doi: 10.1016/j.tvjl.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 63.Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J. Clin. Invest. 1998;101(2 ):289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone digoxin and cyclosporin A. J. Clin. Invest. 1995b;96(4 ):1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yokogawa K, Takahashi M, Tamai I, Konishi H, Nomura M, Moritani S, Miyamoto K, Tsuji A. P-glycoprotein-dependent disposition kinetics of tacrolimus: studies in mdr1a knockout mice. Pharm. Res. 1999;16(8 ):1213–1218. doi: 10.1023/a:1018993312773. [DOI] [PubMed] [Google Scholar]
- 66.Kusuhara H, Suzuki H, Terasaki T, Kakee A, Lemaire M, Sugiyama Y. P-Glycoprotein mediates the efflux of quinidine across the blood-brain barrier. J. Pharmacol. Exp. Ther. 1997;283(2 ):574–580. [PubMed] [Google Scholar]
- 67.van d, Sandt I, Smolders R, Nabulsi L, Zuideveld KP, de Boer AG, Breimer DD. Active efflux of the 5-HT(1A) receptor agonist flesinoxan via P-glycoprotein at the blood-brain barrier. Eur. J. Pharm. Sci. 2001;14(1 ):81–86. doi: 10.1016/s0928-0987(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 68.Kemper EM, Cleypool C, Boogerd W, Beijnen JH, van Tellingen O. The influence of the P-glycoprotein inhibitor zosuquidar trihydrochloride (LY335979) on the brain penetration of paclitaxel in mice. Cancer Chemother. Pharmacol. 2004;53(2 ):173–178. doi: 10.1007/s00280-003-0720-y. [DOI] [PubMed] [Google Scholar]
- 69.Hendrikse NH, Schinkel AH, de Vries EG, Fluks E, Van der Graaf WT, Willemsen AT, Vaalburg W, Franssen EJ. Com-plete in vivo reversal of P-glycoprotein pump function in the blood-brain barrier visualized with positron emission tomography. Br. J. Pharmacol. 1998;124(7 ):1413–1418. doi: 10.1038/sj.bjp.0701979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jonker JW, Wagenaar E, van Deemter L, Gottschlich R, Bender HM, Dasenbrock J, Schinkel AH. Role of blood-brain barrier P-glycoprotein in limiting brain accumulation and sedative side-effects of asimadoline a peripherally acting analgaesic drug. Br. J. Pharmacol. 1999;127(1 ):43–50. doi: 10.1038/sj.bjp.0702497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kemper EM, Verheij M, Boogerd W, Beijnen JH, van Tellingen O. Improved penetration of docetaxel into the brain by co-administration of inhibitors of P-glycoprotein. Eur. J. Cancer. 2004;40(8 ):1269–1274. doi: 10.1016/j.ejca.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 72.Zhang ZJ, Saito T, Kimura Y, Sugimoto C, Ohtsubo T, Saito H. Disruption of mdr1a p-glycoprotein gene results in dysfunction of blood-inner ear barrier in mice. Brain Res. 2000;852(1 ):116–126. doi: 10.1016/s0006-8993(99)02223-4. [DOI] [PubMed] [Google Scholar]
- 73.Uhr M, Holsboer F, Muller MB. Penetration of endogenous steroid hormones corticosterone, cortisol aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J. Neuroendocrinol. 2002;14(9 ):753–759. doi: 10.1046/j.1365-2826.2002.00836.x. [DOI] [PubMed] [Google Scholar]
- 74.Tamai I, Yamashita J, Kido Y, Ohnari A, Sai Y, Shima Y, Naruhashi K, Koizumi S, Tsuji A. Limited distribution of new quinolone antibacterial agents into brain caused by multiple efflux transporters at the blood-brain barrier. J. Pharmacol. Exp. Ther. 2000;295(1 ):146–152. [PubMed] [Google Scholar]
- 75.Kusuhara H, Sugiyama Y. Efflux transport systems for drugs at the blood-brain barrier and blood-cerebrospinal fluid barrier (Part 2) Drug Discov. Today. 2001;6(4 ):206–212. doi: 10.1016/s1359-6446(00)01643-3. [DOI] [PubMed] [Google Scholar]
- 76.Gramer I, Leidolf R, Döring B, Klintzsch S, Krämer EM, Yalcin E, Petzinger E, Geyer J. Breed distribution of the nt230(del4) MDR1 mutation in dogs. Vet. J. 2010;189(1 ):67–71. doi: 10.1016/j.tvjl.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 77.Geyer J, Döring B, Godoy J R, Leidolf R, Moritz A, Petzinger E. Frequency of the nt230 (del4) MDR1 mutation in Collies and related dog breeds in Germany. J. Vet. Pharmacol. Ther. 2005;28(6 ):545–551. doi: 10.1111/j.1365-2885.2005.00692.x. [DOI] [PubMed] [Google Scholar]
- 78.Mealey KL, Bentjen SA, Waiting DK. Frequency of the mutant MDR1 allele associated with ivermectin sensitivity in a sample population of collies from the northwestern United States. Am. J. Vet. Res. 2002;63(4 ):479–481. doi: 10.2460/ajvr.2002.63.479. [DOI] [PubMed] [Google Scholar]
- 79.Mealey KL, Munyard KA, Bentjen SA. Frequency of the mutant MDR1 allele associated with multidrug sensitivity in a sample of herding breed dogs living in Australia. Vet. Parasitol. 2005;131(3-4 ):193–196. doi: 10.1016/j.vetpar.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 80.Tappin SW, Goodfellow MR, Peters IR, Day MJ, Hall EJ, Bentjen SA, Mealey KL. Frequency of the mutant MDR1 allele associated with multidrug sensitivity in dogs in the United Kingdom. BSAVA Congress Scientific Proceedings. 2008;83/49 [Google Scholar]
- 81.Shoop WL, Mrozik H, Fisher MH. Structure and activity of avermectins and milbemycins in animal health. Vet. Parasitol. 1995;59(2 ):139–156. doi: 10.1016/0304-4017(94)00743-v. [DOI] [PubMed] [Google Scholar]
- 82.Plumb CD. Veterinary Drug Handbook. 5th. Iowa: lackwell Publishing Professional; 2005. [Google Scholar]
- 83.Fassler PE, Tranquilli WJ, Paul AJ, Soll MD, DiPietro JA, Todd KS. Evaluation of the safety of ivermectin administered in a beef-based formulation to ivermectin-sensitive Collies. J. Am. Vet. Med. Assoc. 1991;199(4 ):457–460. [PubMed] [Google Scholar]
- 84.Tranquilli WJ, Paul AJ, Todd KS. Assessment of toxicosis induced by high-dose administration of milbemycin oxime in collies. Am. J. Vet. Res. 1991;52(7 ):1170–1172. [PubMed] [Google Scholar]
- 85.Paul AJ, Hutchens DE, Firkins LD, Keehan CM. Effects of dermal application of 10.0% imidacloprid-0.08% ivermectin in ivermectin-sensitive Collies. Am. J. Vet. Res. 2004a;65(3 ):277–278. doi: 10.2460/ajvr.2004.65.277. [DOI] [PubMed] [Google Scholar]
- 86.Paul AJ, Hutchens DE, Firkins LD, Borgstrom M. Dermal safety study with imidacloprid/moxidectin topical solution in the ivermectin-sensitive collie. Vet. Parasitol. 2004b;121(3-4 ):285–291. doi: 10.1016/j.vetpar.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 87.See AM, McGill SE, Raisis AL, Swindells KL. Toxicity in three dogs from accidental oral administration of a topical endectocide containing moxidectin and imidacloprid. Aust. Vet. J. 2009;87(8 ):334–337. doi: 10.1111/j.1751-0813.2009.00448.x. [DOI] [PubMed] [Google Scholar]
- 88.Hopper K, Aldrich J, Haskins SC. Ivermectin toxicity in 17 collies. J. Vet. Intern. Med. 2002;16(1 ):89–94. doi: 10.1892/0891-6640(2002)016<0089:itic>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 89.Nelson OL, Carsten E, Bentjen SA, Mealey KL. Ivermectin toxicity in an Australian Shepherd dog with the MDR1 mutation associated with ivermectin sensitivity in Collies. J. Vet. Intern. Med. 2003;17(3 ):354–356. [PubMed] [Google Scholar]
- 90.Bishop BF, Bruce CI, Evans NA, Goudie AC, Gration KA, Gibson SP, Pacey MS, Perry DA, Walshe ND, Witty MJ. Selamectin: a novel broad-spectrum endectocide for dogs and cats. Vet. Parasitol. 2000;91(3-4 ):163–176. doi: 10.1016/s0304-4017(00)00289-2. [DOI] [PubMed] [Google Scholar]
- 91.Novotny MJ, Krautmann MJ, Ehrhart JC, Godin CS, Evans EI, McCall JW, Sun F, Rowan TG, Jernigan AD. Safety of selamectin in dogs. Vet. Parasitol. 2000;91(3-4 ):377–391. doi: 10.1016/s0304-4017(00)00306-x. [DOI] [PubMed] [Google Scholar]
- 92.Paul AJ, Tranquilli WJ, Hutchens DE. Safety of moxidectin in avermectin-sensitive collies. Am. J. Vet. Res. 2000;61(5 ):482–483. doi: 10.2460/ajvr.2000.61.482. [DOI] [PubMed] [Google Scholar]
- 93.Lovell R A. Ivermectin and piperazine toxicoses in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 1990;20(2 ):453–468. doi: 10.1016/s0195-5616(90)50038-8. [DOI] [PubMed] [Google Scholar]
- 94.Bissonnette S, Paradis M, Daneau I, Silversides DW. The ABCB1-1Delta mutation is not responsible for subchronic neurotoxicity seen in dogs of non-collie breeds following macrocyclic lactone treatment for generalized demodicosis. Vet. Dermatol. 2009;20(1 ):60–66. doi: 10.1111/j.1365-3164.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 95.McCall JW. The safety-net story about macrocyclic lactone heartworm preventives: a review, an update, and recommendations. Vet. Parasitol. 2005;133(2-3 ):197–206. doi: 10.1016/j.vetpar.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 96.McCall JW, Genchi C, Kramer L, Guerrero J, Dzimianski MT, Supakorndej P, Mansour AM, McCall SD, Supakorndej N, Grandi G, Carson B. Heartworm and Wolbachia: therapeutic implications. Vet. Parasitol. 2008;158(3 ):204–214. doi: 10.1016/j.vetpar.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 97.Fisher MA, Shanks DJ. A review of the off-label use of selamectin (Stronghold/Revolution) in dogs and cats. Acta Vet. Scand. 2008;50(1 ):46. doi: 10.1186/1751-0147-50-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McCall JW, McTier TL, Ryan WG, Gross SJ, Soll MD. Evaluation of ivermectin and milbemycin oxime efficacy against Dirofilaria immitis infections of three and four months' duration in dogs. Am. J. Vet. Res. 1996;57(8 ):1189–1192. [PubMed] [Google Scholar]
- 99.Barbet JL, Snook T, Gay JM, Mealey KL. ABCB1-1 Delta (MDR1-1 Delta) genotype is associated with adverse reactions in dogs treated with milbemycin oxime for generalized demodicosis. Vet. Dermatol. 2009;20(2 ):111–114. doi: 10.1111/j.1365-3164.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 100.Genchi C, Poglayen G, Kramer LH, Venco L, Agostini A. Efficacy of moxidectin for the prevention of adult heartworm (Dirofilaria immitis) infection in dogs. Parassitologia. 2001;43(3 ):139–141. [PubMed] [Google Scholar]
- 101.Arther RG, Bowman DD, Slone RL, Travis LE. Imidacloprid plus moxidectin topical solution for the prevention of heartworm disease (Dirofiloria immitis) in dogs. Parasitol. Res. 2005;97(Suppl 1 ):S76–S80. doi: 10.1007/s00436-005-1448-x. [DOI] [PubMed] [Google Scholar]
- 102.Mealey KL. Canine ABCB1 and macrocyclic lactones: heartworm prevention and pharmacogenetics. Vet. Parasitol. 2008;158(3 ):215–222. doi: 10.1016/j.vetpar.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 103.Mueller RS. Treatment protocols for demodicosis an evidence-based review. Vet. Dermatol. 2004;15(2 ):75–89. doi: 10.1111/j.1365-3164.2004.00344.x. [DOI] [PubMed] [Google Scholar]
- 104.Heine J, Krieger K, Dumont P, Hellmann K. Evaluation of the efficacy and safety of imidacloprid 10% plus moxidectin 2.5% spot-on in the treatment of generalized demodicosis in dogs: results of a European field study. Parasitol. Res. 2005;97(Suppl 1 ):S89–S96. doi: 10.1007/s00436-005-1450-3. [DOI] [PubMed] [Google Scholar]
- 105.Ristic Z, Medleau L, Paradis M, White-Weithers NE. Ivermectin for treatment of generalized demodicosis in dogs. J. Am. Vet. Med. Assoc. 1995;207(10 ):1308–1310. [PubMed] [Google Scholar]
- 106.Fondati A. Efficacy of daily oral ivermectin in the treatment of 10 cases of generalized demodicosis in adult dogs. Vet. Dermatol. 1996;7(2 ):99–104. doi: 10.1111/j.1365-3164.1996.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 107.Medleau L, Ristic Z, McElveen DR. Daily ivermectin for treatment of generalized demodicosis in dogs. Vet. Dermatol. 1996;7(4 ):209–212. doi: 10.1111/j.1365-3164.1996.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 108.Wagner R, Wendlberger U. Field efficacy of moxidectin in dogs and rabbits naturally infested with Sarcoptes spp., Demodex spp. and Psoroptes spp. mites. Vet. Parasitol. 2000;93(2 ):149–158. doi: 10.1016/s0304-4017(00)00357-5. [DOI] [PubMed] [Google Scholar]
- 109.Holm BR. Efficacy of milbemycin oxime in the treatment of canine generalized demodicosis: a retrospective study of 99 dogs (1995-2000) Vet. Dermatol. 2003;14(4 ):189–195. doi: 10.1046/j.1365-3164.2003.00339.x. [DOI] [PubMed] [Google Scholar]
- 110.Müller RS, Bettenay SV. A proposed new therapeutic protocol for the treatment of canine mange with ivermectin. J. Am. Anim. Hosp. Assoc. 1999;35(1 ):77–80. doi: 10.5326/15473317-35-1-77. [DOI] [PubMed] [Google Scholar]
- 111.Tranquilli WJ, Paul AJ, Seward RL. Ivermectin plasma concentrations in collies sensitive to ivermectin-induced toxicosis. Am. J. Vet. Res. 1989;50(5 ):769–770. [PubMed] [Google Scholar]
- 112.Houston DM, Parent J, Matushek KJ. Ivermectin toxicosis in a dog. J. Am. Vet. Med. Assoc. 1987;191(1 ):78–80. [PubMed] [Google Scholar]
- 113.Hadrick MK, Bunch SE, Kornegay JN. Ivermectin toxicosis in two Australian shepherds. J. Am. Vet. Med. Assoc. 1995;206(8 ):1147–1150. [PubMed] [Google Scholar]
- 114.Vaughn DM, Simpson ST, Blagburn BL, Whitmer WL, Heddens-Mysinger R, Hendrix CM. Determination of homovanillic acid, 5-hydroxyindoleacetic acid and pressure in the cerebrospinal fluid of collie dogs following administration of ivermectin. Vet. Res. Commun. 1989;13(1 ):47–55. doi: 10.1007/BF00366852. [DOI] [PubMed] [Google Scholar]
- 115.Merola V, Khan S, Gwaltney-Brant S. Ivermectin toxicosis in dogs: a retrospective study. J. Am. Anim Hosp. Assoc. 2009;45(3 ):106–111. doi: 10.5326/0450106. [DOI] [PubMed] [Google Scholar]
- 116.Campbell WC, Benz GW. Ivermectin: a review of efficacy and safety. J. Vet. Pharmacol. Ther. 1984;7(1 ):1–16. doi: 10.1111/j.1365-2885.1984.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 117.Lankas GR, Gordon LR. Ivermectin and Abamectin. New York: Springer-Verlag V; 1989. pp. 89–112. [Google Scholar]
- 118.Campbell A, Chapman M. Handbook of poisoning in dogs and cats. 1st. London: Blackwell Science Ltd; 2000. [Google Scholar]
- 119.Dupuy J, Larrieu G, Sutra JF, Eeckhoutte C, Alvinerie M. Influence of verapamil on the efflux and metabolism of 14C moxidectin in cultured rat hepatocytes. J. Vet. Pharmacol. Ther. 2001;24(3 ):171–177. doi: 10.1046/j.1365-2885.2001.00335.x. [DOI] [PubMed] [Google Scholar]
- 120.Lespine A, Roulet A, Dupuy J, Pineau T, Alvinerie M. Role of the P-glycoprotein in the cellular efflux of macrocyclic lactones: influence of interfering agents. J. Vet. Pharmacol. Ther. 2003;26(Suppl. 1 ):161–162. [Google Scholar]
- 121.Brayden DJ, Griffin J. Avermectin transepithelial transport in MDR1- and MRP-transfected canine kidney monolayers. Vet. Res. Commun. 2008;32(1 ):93–106. doi: 10.1007/s11259-007-9007-9. [DOI] [PubMed] [Google Scholar]
- 122.Vanapalli SR, Hung YP, Fleckenstein L, Dzimianski MT, McCall JW. Pharmacokinetics and dose proportionality of oral moxidectin in beagle dogs. Biopharm. Drug Dispos. 2002;23(7 ):263–272. doi: 10.1002/bdd.313. [DOI] [PubMed] [Google Scholar]
- 123.Krieger K, Heine J, Dumont P, Hellmann K. Efficacy and safety of imidacloprid10% plus moxidectin 2.5% spot-on in the treatment of sarcoptic mange and otoacariosis in dogs: results af a European field study. Parasitol. Res. 2005;97(Suppl 1 ):S81–S88. doi: 10.1007/s00436-005-1449-9. [DOI] [PubMed] [Google Scholar]
- 124.Advocate: Scientific discussion. EMEA. 2009. (CVMP/0297/03).
- 125.Sasaki Y, Kitagawa H, Murase S, Ishihara K. Susceptibility of rough-coated collies to milbemycin oxime. Nippon Juigaku. Zasshi. 1990;52(6 ):1269–1271. doi: 10.1292/jvms1939.52.1269. [DOI] [PubMed] [Google Scholar]
- 126.Curtis CF. Current trends in the treatment of Sarcoptes, Cheyletiella and Otodectes mite infestations in dogs and cats. Vet. Dermatol. 2004;15(2 ):108–114. doi: 10.1111/j.1365-3164.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 127.Banks BJ, Bishop BF, Evans NA, Gibson SP, Goudie AC, Gration KA, Pacey MS, Perry DA, Witty MJ. Avermectins and flea control: structure-activity relationships and the selection of selamectin for development as an endectocide for companion animals. Bioorg. Med. Chem. 2000;8(8 ):2017–2025. doi: 10.1016/s0968-0896(00)00120-6. [DOI] [PubMed] [Google Scholar]
- 128.Michael B, Meinke PT, Shoop W. Comparison of ivermectin, doramectin, selamectin, and eleven intermediates in a nematode larval development assay. J. Parasitol. 2001;87(3 ):692–696. doi: 10.1645/0022-3395(2001)087[0692:COIDSA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 129.Yas-Natan E, Shamir M, Kleinbart S, Aroch I. Doramectin toxicity in a collie. Vet. Rec. 2003;153(23 ):718–720. [PubMed] [Google Scholar]
- 130.Hopkins KD, Marcella KL, Strecker AE. Ivermectin toxicosis in a dog. J. Am. Vet. Med. Assoc. 1990;197(1 ):93–94. [PubMed] [Google Scholar]
- 131.Hollins JD, Marlow BP, Hatherell PJ. Ingestion of equine moxidectin by dogs. Vet. Rec. 2000;147(8 ):227–228. [PubMed] [Google Scholar]
- 132.Snowden NJ, Helyar CV, Platt SR, Penderis J. Clinical presentation and management of moxidectin toxicity in two dogs. J. Small Anim. Pract. 2006;47(10 ):620–624. doi: 10.1111/j.1748-5827.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- 133.Gallagher AE. Coma and respiratory failure due to moxidectin intoxication in a dog. J. Vet. Emerg. Crit. Care. 2010;18(1 ):81–85. [Google Scholar]
- 134.Crandell DE, Weinberg GL. Moxidectin toxicosis in a puppy successfully treated with intravenous lipids. J. Vet. Emerg. Crit. Care. 2009;19(2 ):181–186. doi: 10.1111/j.1476-4431.2009.00402.x. [DOI] [PubMed] [Google Scholar]
- 135.Smith RA, Stronski EJ, Beck BE, Wolper GG. Alberta. Death of a Rough Collie exposed to an ivermectin-based paste. Can. Vet. J. 1990;31(3 ):221. [PMC free article] [PubMed] [Google Scholar]
- 136.Beal MW, Poppenga RH, Birdsall WJ, Hughes D. Respiratory failure attributable to moxidectin intoxication in a dog. J. Am. Vet. Med. Assoc. 1999;215(12 ):1813–71806. [PubMed] [Google Scholar]
- 137.Linek J, Spiess C, Dallmeyer J, Geyer J. Ivermectin-Intoxikation bei drei Hunden mit und ohne MDR1-Gen-Defekt durch ein für Pferde zugelassenes orales Antiparasitikum. Tierärztl. Prax. 2007;35(K) [Google Scholar]
- 138.Roder JD, Stair EL. An overview of ivermectin toxicosis. Vet. Hum. Toxicol. 1998;40(6 ):369–370. [PubMed] [Google Scholar]
- 139.Campbell WC, Fisher MH, Stapley EO, Albers-Schonberg G, Jacob TA. Ivermectin: a potent new antiparasitic agent. Science. 1983;221(4613 ):823–828. doi: 10.1126/science.6308762. [DOI] [PubMed] [Google Scholar]
- 140.Sivine F, Plume C, Ansay M. Picrotoxin the antidote to ivermectin in dogs? Vet. Rec. 1985;116(7 ):195–196. doi: 10.1136/vr.116.7.195-a. [DOI] [PubMed] [Google Scholar]