Abstract
BACKGROUND AND PURPOSE
Sphingosine kinase 1 catalyses formation of the bioactive lipid, sphingosine 1-phosphate, which protects cancer cells from apoptosis. Therefore, sphingosine kinase 1 is a novel target for intervention with anti-cancer agents. We have assessed the effect of the anti-cancer agent, resveratrol and its dimers (ampelopsin A and balanocarpol) on sphingosine kinase 1 activity and on survival of MCF-7 breast cancer cells.
EXPERIMENTAL APPROACH
Ampelopsin A and balanocarpol were purified from Hopea dryobalanoides and their effect on sphingosine kinase 1 activity and expression, [3H] thymidine incorporation, ERK-1/2 phosphorylation and PARP activity assessed in MCF-7 cells.
KEY RESULTS
Resveratrol, ampelopsin A and balanocarpol were novel inhibitors of sphingosine kinase 1 activity. Balanocarpol was a mixed inhibitor (with sphingosine) of sphingosine kinase 1 with a Kic= 90 ± 10 µM and a Kiu of ∼500 µM. Balanocarpol and ampelopsin A also induced down-regulation of sphingosine kinase 1 expression and reduced DNA synthesis, while balanocarpol stimulated PARP cleavage in MCF-7 breast cancer cells. Resveratrol was a competitive inhibitor (with sphingosine) of sphingosine kinase 1 with a Kic= 160 ± 40 µM, reduced sphingosine kinase 1 expression and induced PARP cleavage in MCF-7 cells.
CONCLUSIONS AND IMPLICATIONS
Each molecule of balanocarpol may bind at least two sphingosine kinase 1 catalytic molecules to reduce the activity of each simultaneously. These findings suggest that resveratrol, ampelopsin A and balanocarpol could perturb sphingosine kinase 1-mediated signalling and this might explain their activity against MCF-7 breast cancer cells.
LINKED ARTICLE
This article is commented on by Hergst and Yun, pp. 1603–1604 of this issue. To view this commentary visit http://dx.doi.org/10.1111/j.1476-5381.2012.01898.x
Keywords: sphingosine 1-phosphate, sphingosine kinase 1, apoptosis, cancer, proliferation, resveratrol, extracellular signal-regulated kinase, polyADP ribose polymerase, inhibitor kinetics, balanocarpol, ampelopsin A
Introduction
Resveratrol (3,4′,5-trihydroxy-trans-stilbene), isolated as an antifungal agent (Langcake and Pryce, 1977), has several beneficial effects on health including activities against cancer, inflammation, cardiovascular- and age-related diseases (Baur and Sinclair, 2006). Resveratrol exhibits antioxidant activity, which can be attributed to the phenol rings, which are strong scavengers of reactive oxygen species (Leonard et al., 2003). However, many biological effects of resveratrol cannot be explained by its antioxidant properties. For example, the anti-inflammatory effects of resveratrol involve inhibition of COX-2 in human mammary cells (Subbaramaiah et al., 1998). Resveratrol also reduces breast and skin cancer in mice models by blocking COX-1 and COX-1–associated hydroxyperoxidase activity (Jang et al., 1997). Resveratrol has also gained wide attention because of the ‘French paradox’ where consumption of red wine is associated with a lower incidence of cardiovascular-related deaths, despite patients consuming a high-fat diet (Frémont, 2000). It has been suggested that polyphenols, such as piceatannol and resveratrol may activate human deacetylase (SIRT 1) in vitro and sirtuin (SIR 2) in vivo to prolong the lifespan of Saccharomyces cerevisiae, which may be attributed to the stabilization of rDNA repeats (Howitz et al., 2003). Furthermore, resveratrol has been found to extend the lifespan of Caenorhabditis elegans (Wood et al., 2004) and mice fed on a high-calorie diet (Baur et al., 2006), indicating an evolutionary conserved mechanism of SIR2 in regulating metabolism and aging.
Resveratrol has also been shown to be therapeutically useful in reducing the growth and progression of skin, lung and breast cancers (Athar et al., 2007). Resveratrol induces apoptosis in MDA-MB-231 breast cancer cells via a PKCδ-dependent activation of serine palmitoyltransferase and neutral sphingomyelinase, which results in increased de novo synthesis of the pro-apoptotic sphingolipid, ceramide (Scarlatti et al., 2003). Resveratrol has also been shown to inhibit oxidative burst and sphingosine kinase 1 (SK1)-dependent degranulation in human neutrophils (Issuree et al., 2009).
Sphingosine kinase is an enzyme (two isoforms called SK1 and SK2), catalysing the formation of the bioactive lipid, sphingosine 1-phosphate (S1P) and has a central role in cancer progression. For instance, there is increased expression of SK1 mRNA transcript and/or SK1 protein in stomach, lung, brain, colon, kidney and breast cancers and non-Hodgkins lymphoma (Pyne and Pyne, 2010). Moreover, high tumour expression of SK1 is associated with reduced mean survival time and earlier recurrence of tamoxifen resistance in oestrogen receptor positive breast cancers (Long et al., 2010; Watson et al., 2010). Interestingly, resveratrol also reduces SK1 activity by inhibiting the activation of phospholipase D, which is an upstream regulator of SK1 (Issuree et al., 2009) and promotes the down-regulation of SK1 in PC-3 cells (Brizuela et al., 2010).
Plants in the Dipterocarpaceae family such as Hopea dryobalanoides are known to produce resveratrol oligomers (Sahidin et al., 2005). Even though these secondary metabolites exhibit high biological activities, they have been ignored, largely because of challenges in achieving their isolation in sufficient quantity from natural sources, coupled with an inability to chemically synthesize these molecules. Hopeaphenol (a resveratrol tetramer) was first isolated from H. odorata (Coggon et al., 1965; 1970). This compound is highly active against several cancer cell lines including human epidermoid nasopharynx carcinoma (KB cells), lung carcinoma (A549 cells) and breast cancer (MCF-7 cells) (Ohyama et al., 1999; Muhtadi et al., 2006). Another tetrameric resveratrol, known as vaticanol B was isolated from H. dryobalanoides and exhibited moderate cytotoxic activity against P-388 cells (Sahidin et al., 2005; Muhtadi et al., 2006). Therefore, most of the compounds isolated from H. dryobalanoides and related species are cytotoxic against several cancer cell lines. However, the exact mechanisms of action and possible molecular targets are unknown.
During our drug discovery programme to identify novel chemical scaffolds, which inhibit SK1 activity, we found that an extract of H. dryobalanoides reduced SK1 activity. Therefore, we sought to investigate the biological effects of resveratrol as a novel SK1 inhibitor, and to purify other compounds produced by H. dryobalanoides that inhibit SK1 activity, using bioassay-guided fractionation. We present evidence that resveratrol and its dimers, such as ampelopsin A and balanocarpol induce apoptosis of cancer cells, and this is associated with inhibition and down-regulation of SK1 activity and expression.
Methods
Extraction and isolation of ampelopsin A and balanocarpol
Dried and ground H. dryobalanoides leaf (50g) was placed in a brown jar with 500 ml of methanol and 5g of polyvinylpyrrolidone (PVP). The jar was sealed and left at room temperature for at least 48 h. The jar was then shaken twice daily for 1–2 min. The extract was filtered and dried by rotary evaporation to produce a gummy dark greenish extract (2 g). A sample (0.5 g) of this extract was fractionated by Flash chromatography using a 20 g ISOLUTE® Flash Si II cartridge in Biotage Flash Master. The flow rate was set at 20 ml min−1 for gradient elution using hexane, dichloromethane, butan-2-ol and methanol. The volumes of the fractions were then reduced by rotary evaporation. Subsequently, all fractions were freeze-dried. 12 major fractions were obtained by monitoring their UV-VIS spectra (F1: 2 mg; F2: 5 mg; F3: 3 mg; F4: 3 mg; F4A: 15 mg; F5: 11 mg; F 5B: 2.5 mg; F6: 7.7 mg; F7: 46 mg F8: 68 mg; F9: 3.4 mg; F10: 2 mg). Ampelopsin A was obtained from the methanol phase after extracting F5 with 2,2,4-trimethylpentane:methanol (1:1). The yield of ampelopsin A (5 mg) was 0.01% (based on dried weight of plant material). Additionally, 700 g of dried and ground stem bark of H. dryobalanoides was successively extracted with 3.5–5 L of hexane, ethyl acetate and methanol at their respective boiling points for 48–72 h. The solvent was removed from the ethyl acetate extract by rotary evaporation to yield 5 g of residue after freeze-drying; this was further processed by vacuum liquid chromatography (Coll and Bowden, 1986). Balanocarpol was then purified from this residue on a Sephadex LH-20 column (5 g) using methanol as the only eluent. Typically, 20 fractions were collected per column bed volumes and monitored by TLC, NMR and MS. The yield of balanocarpol (300 mg) was 0.043%, based on dried weight of plant material.
Structure elucidation using NMR and MS
All NMR experiments were performed with a JEOL (JNM LA400) operating at 400 (1H) and 100 (13C) MHz using deuterated and residual solvent peaks as internal reference. 1H-NMR was performed on all samples to establish identity and yield of compounds present in the sample. Further structure elucidation was performed using 2-D NMR experiments such as correlation spectroscopy (COSY), heteronuclear multiple quantum coherence (HMQC) and heteronuclear multiple bond coherence (HMBC). 13C and distortionless enhancement through polarization transfer (DEPT) NMR experiments were performed when samples were sufficiently pure. Spatial structural information was obtained with nuclear overhauser enhancement spectroscopy (NOESY). MS was used to establish the molecular weights and molecular formulae of selected samples. The sample (1 mg) was dissolved in appropriate solvents and separated by HPLC before being ionized in a ThermoFinnigan LCQ-Decaiontrap or Orbitrap HRESI mass spectrometer. Negative or positive mode electrospray ionization (ESI) analysis was used dependent on the nature of the compounds. Samples were also run in Agilent 6130, LC/MS (Agilent Technologies, Palo Alto, CA, USA) using atmospheric pressure chemical ionization (APCI).
Cell culture
MCF-7 breast cancer cells (either Neo cells expressing a vector containing a neomycin resistance gene or parental cells) were grown in a monolayer culture in high glucose Dulbecco's modified Eagle's medium (DMEM) with 10% European fetal calf serum (EFCS) and 100 U·mL−1 penicillin, 100 µg·mL−1 streptomycin, 0.4% Geneticin (for MCF-7 Neo cells), and 15 µg·mL−1 insulin at 37°C with 5% CO2. HEK 293 cells stably over-expressing SK1 were cultured in DMEM supplemented with 10% EFCS, 100 U·mL−1 penicillin, 100 µg·mL−1 streptomycin, 1% non-essential amino acids and 0.8% Geneticin at 37°C in 5% CO2.
Western blotting
Cell lysates were prepared in sample buffer containing 125 mM Tris, pH 6.7, 0.5 mM Na4P2O7, 1.25 mM EDTA, 0.5% w/v SDS containing 1.25% (v/v) glycerol, 0.06% (w/v) bromophenol blue and 50 mM dithiothreitol. Equal amount of proteins were subjected to SDS-PAGE and Western blotting. Resolved proteins were immunoblotted with anti-SK1, anti-ERK2, anti-phospho ERK-1/2, anti-PARP or anti-actin antibodies.
Sphingosine kinase (SK) activity assay
SK1 activity was assayed as described previously (Lim et al., 2011). Briefly, sphingosine was solubilized in Triton X-100 (final concentration 0.063% w/v) and combined with buffer 1 containing 20 mM Tris (pH 7.4), 1 mM EDTA, 1 mM Na3VO4, 40 mM β-glycerophosphate, 1 mM NaF, 0.007% (v/v) β-mercaptoethanol, 20% (v/v) glycerol, 10 µg·mL−1 aprotinin, 10 µg·mL−1 soybean trypsin inhibitor, 1 mM PMSF and 0.5 mM 4-deoxypyridoxine. For SK2 assays, the sphingosine was complexed with bovine serum albumin (final concentration 0.2 mg·mL−1) in buffer 1 supplemented with 400 mM KCl. SK activity was determined by incubating purified SK1 or SK2 (15 ng) or cell lysate (15 µg protein, determined by Bradford protein assay) from HEK 293 cells containing stably over-expressed recombinant SK1, for 15–20 min at 30°C, in the presence of sphingosine (0.5 to 20 µM), [32P]ATP (250 µM, 4.4 × 104 cpm·nmol−1 in 10 mM MgCl2), and varying concentrations of inhibitors or control vehicle (5% (v/v) DMSO final concentration). The total assay volume per sample was 200µl. For ATP kinetics, 20 µM sphingosine was combined with 25–1000 µM [γ32P]ATP (2.2 × 106 cpm/assay in 100 mM MgCl2), and varying concentrations of inhibitors or control vehicle (5% (v/v) DMSO final concentration). Reactions were stopped by the addition of 500 µL 1-butanol and mixed with 1 mL of 2 M KCl. [32P]-S1P was extracted from the organic phase by washing twice with 1 mL of 2 M KCl before quantification by Cerenkov counting. Kinetic parameters were obtained using the graph plotting and curve fitting programs Biograph (University of Strathclyde, Glasgow UK) and Prism 4.03 (GraphPad). Substrate kinetics were analysed according to the Michaelis-Menten equation and the inhibition constants (Kic and Kiu) were determined using Dixon and Cornish–Bowden plots (Cortés et al., 2001).
[3H] thymidine assay
MCF-7 cells were plated in 24-well plates (2.0–4.0 × 104 cells per well) and maintained in complete medium. After 24 h, cells were treated with varying concentrations of inhibitor or vehicle control for a further 15–72 h, as indicated. [3H]-thymidine (0.5 µCi·mL−1) was added for the final 5 h. Samples were terminated by washing 3 times with 1 mL ice cold 10% (w/v) trichloroacetic acid. [3H]-thymidine incorporated into DNA were harvested with 0.25 mL 0.1% (w/v) SDS, 0.3 M NaOH and quantified by liquid scintillation counting with 2 mL scintillation cocktail.
Materials
All general biochemicals and anti-actin antibody were from Sigma (Poole, UK). High glucose (DMEM), minimum essential medium (MEM), penicillin-streptomycin (10 000 U·mL−1 penicillin and 10 000 µg·mL−1 streptomycin) and Lipofectamine 2000™ were from Invitrogen (Paisley, UK). MCF-7 cells were a gift from R. Schiff (Baylor College of Medicine, Houston, TX, USA). Anti-ERK2 antibody was from BD Transduction Laboratories (Oxford, UK), and anti-SK1 antibody was a gift from A. Huwiler (University of Bern, Switzerland). Sphingosine and S1P were from Avanti Polar Lipids (Alabaster, AL, USA). Purified SK1 and SK2 were from Enzo Life Sciences (Exeter, UK). The SK inhibitor, 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole (SKi) was from Merck Biosciences (Nottingham, UK). Dried and ground leaves of H. dryobalanoides were originally collected by the Forest Institute of Malaysia (FRIM) and stored and extracted at Strathclyde Innovations in Drug Research. 1–2 kg of dried and ground leaves, bark and twigs of H. dryobalanoides were obtained.
Results
Isolation of ampelopsin A and balanocarpol as bioactive SK1 inhibitors
We previously demonstrated that MCF-7 (parental) and MCF-7 Neo breast cancer cells express SK1 (GenBank™ number: NM_001142601), which has a molecular mass of 42 kDa (Loveridge et al., 2010; Lim et al., 2011). MCF-7 Neo cells were used because the action of SK1 inhibitors on SK1 and apoptosis have been extensively characterized in these cancer cells (Loveridge et al., 2010; Lim et al., 2011). Initial screening of plant extracts for bioactivity against SK1 demonstrated that the H. dryobalanoides leaf extract reduced [3H]thymidine incorporation into DNA (Figure 1A), abolished S1P- and EGF-stimulated activation of ERK-1/2, promoted PARP cleavage (Figure 1B) and inhibited SK1 activity [SK1 stably over-expressed in HEK 293 cells 10–30-fold increase (dependent on passage number) of SK1 activity vs. lysate from vector-transfected cells] (Figure 1C). These effects were reproduced with the SK1 inhibitors, SKi and N,N-dimethylsphingosine (Figure 1A and B).
Figure 1.
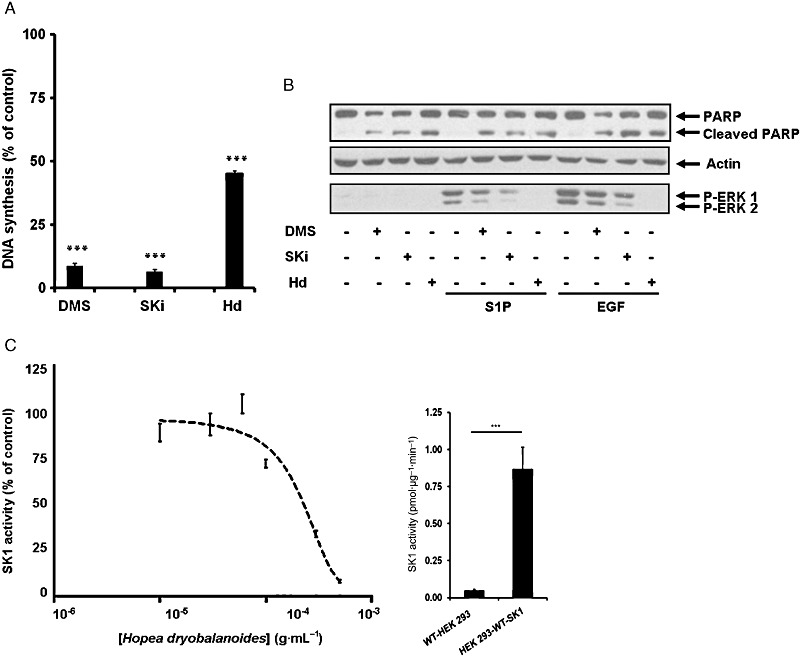
Biological activities of Hopea dryobalanoides extract. (A) Bar graph showing the effect of SK1 inhibitors and H. dryobalanoides leaf extract on [3H]-thymidine incorporation in MCF-7 Neo cells. Quiescent MCF-7 Neo cells were treated with 10 µM N,N-dimethylsphingosine (DMS), 10 µM SKi, 5 µg·mL−1H. dryobalanoides leaf (Hd) extract or control (0.05% v/v DMSO final concentration). Cells were then incubated for a further 15 h and then with [3H]-thymidine (0.5 µCi·mL−1) for 5 h. Data are expressed as percentage of control and represent means and SDs of triplicate determinations, ***P < 0.001, significantly different from control. (B) Western blot showing the effect of SK1 inhibitors and Hd extract on ERK-1/2 activation and PARP cleavage. Quiescent MCF-7 Neo cells were incubated with control (0.1% (v/v) DMSO final concentration) or indicated inhibitors (10 µM DMS, 10 µM SKi and 500 µg·mL−1H. dryobalanoides leaf extract (Hd)) for 48 h before being stimulated by 25 ng·mL−1 EGF or 1 µM S1P for 5 min. Cell lysates were separated by SDS-PAGE and immunoblotted with anti-PARP or anti-phospho-ERK1/2 antibodies. Blots were then stripped and reprobed with anti-actin antibody to ensure comparable protein loading. Results are representative of three independent experiments. (C) Graph showing the concentration-dependent inhibitory effect of Hd extract on SK1 activity. Enzyme activity was measured using 10 µM sphingosine and 250 µM [32P]-ATP as the substrates and using lysates of HEK 293 cells over-expressing recombinant SK1. Data are expressed as means ± SD of triplicate determinations. Also shown is a histogram comparing SK1 activity, using 10 µM sphingosine and 250 µM [32P]-ATP, in lysates of HEK293 cells stably transfected with vector (WT-HEK293) or from HEK293 cells stably transfected with SK1 (HEK293-WT-SK1). P < 0.001.
Therefore, the H. dryobalanoides extract was fractionated using Flash chromatography to isolate compounds with SK1 inhibitor activity. Fractions 4, 5 and 9 were the most active fractions in reducing [3H] thymidine incorporation into DNA in MCF-7 cells (Figure 2A), while fraction 5 was the most active fraction in inhibiting SK1 activity (∼90% inhibition) (Figure 2B). We have previously shown that the SK1 inhibitor, SKi reduces SK1 expression in cancer cells via an inhibitor-induced ubiquitin-proteasomal degradation pathway (Loveridge et al., 2010; Lim et al., 2011). This effect was reproduced here, along with the finding that SKi also reduced EGF-stimulated ERK-1/2 phosphorylation (Figure 2C). Similarly, the treatment of MCF-7 Neo cells with fraction 5 reduced the expression of SK1 and inhibited EGF-stimulated ERK-1/2 activation (Figure 2C). This was also observed using parental MCF-7 cells (data not shown). Interestingly, other fractions exhibited different effects. For instance, Fraction 4 and 5B inhibited SK1 activity (Figure 2B), but did not induce down-regulation of SK1 expression (Figure 2C). Fraction 9 reduced ERK1/2 activation but did not affect SK1 expression, whereas Fraction 10 reduced SK1 activity (Figure 2B) and expression, but had less effect on ERK1/2 activation (Figure 2C). The total plant extract also reduced SK1 expression, used as a positive control (Figure 2C). These data show that Fraction 5 exhibits properties typical of an SK1 inhibitor and this fraction was therefore subjected to NMR and MS analysis. These analyses demonstrated that Fraction 5 contained one major compound, ampelopsin A which is a resveratrol dimer (Supporting Information Figure S1, Table S1). The structure of ampelopsin A was consistent with that isolated from H. parviflora (Tanaka et al., 2000).
Figure 2.
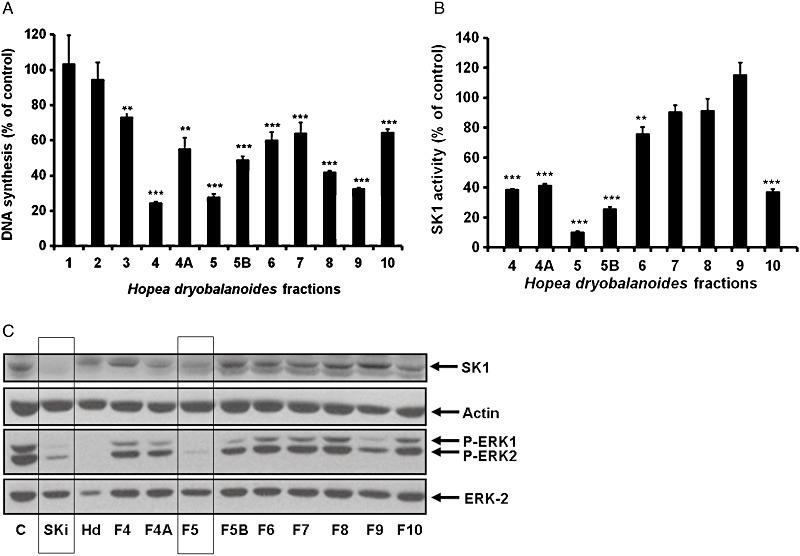
Biological activities of Hopea dryobalanoides fractions from Flash chromatography. (A) Bar graph showing the effect of purified H. dryobalanoides fractions on [3H]-thymidine incorporation in MCF-7 Neo cells. Quiescent MCF-7 Neo cells were treated with 5 µg·mL−1 of the solid residue from each H. dryobalanoides fractions (1–10) or control (0.05% v/v DMSO final concentration). The cells were then incubated for 15 h and then with [3H]-thymidine (0.5 µCi·mL−1) added for 5 h. Data are expressed as percentage of control and represent means ± SD of triplicate determinations, **P < 0.01, ***P < 0.001, significantly different from control. (B) Bar graph showing the effect of purified H. dryobalanoides fractions (500 µg·mL−1) on recombinant SK1 activity. Data are expressed as percentage of control and represent means ± SD of triplicate determinations, **P < 0.01, ***P < 0.001, significantly different from control. (C) Western blots showing the effect of purified H. dryobalanoides fractions on SK1 and phosphorylated ERK-1/2 levels. MCF-7 Neo cells were treated with control (0.2% v/v DMSO), C; 10 µM SKi, 200 µg·mL−1H. dryobalanoides leaf extract (Hd), 20 µg·mL−1 Fractions 4–10 (F4-F10) for 48 h (inhibitors were replenished after 24 h). EGF 25 ng·mL−1 was added for 5 min before harvesting the cells. Cells lysates were analysed by Western blotting using anti-SK1 antibody. Blots were also probed with anti-phospho ERK1/2 antibody and then stripped and reprobed with anti-actin or anti-ERK-2 antibodies to ensure comparable protein loading. Results are representative of three independent experiments.
The aromatic region (δ 6–8) of 1H-NMR of the bark extract of H. dryobalanoides indicated the presence of phenolic compounds (data not shown). Upon fractionation of the bark extract and subsequent purification using Sephadex LH-20, balanocarpol was isolated. Structural elucidation with NMR revealed that balanocarpol is an isomer (7a-epimer) of ampelopsin A (Supporting Information Figure S1, Table S1). The structure of balanocarpol is consistent with that isolated from H. parviflora (Tanaka et al., 2000). The molecular weights of ampelopsin A and balanocarpol were established by MS; both compounds have the same molecular formula of C28H22O7.
Resveratrol is an SK1 inhibitor
Because the isomers ampelopsin A and balanocarpol are resveratrol dimers formed by the fusion of cis- and trans-isomers of resveratrol (Supporting Information Figure S2), we investigated whether resveratrol itself is an SK1 inhibitor. In this regard, both cis- and trans-resveratrol inhibited purified SK1 but not purified SK2 activity (Figure 3A). Trans-resveratrol inhibited purified SK1 activity and SK1 stably over-expressed in HEK 293 cells to a similar extent, for example ∼30–40% (Figure 3A and B). Calculation of the % inhibition of SK1 activity using the Michaelis–Menten equation, 10 µM sphingosine, the kinetic constants determined using lysates from HEK 293 cells stably over-expressing SK1 (see later) and 500 µM reseveratrol yielded a 25% inhibition of SK1 activity, which is also close to the level of inhibition determined experimentally for the purified enzyme (∼30%, Figure 3A).
Figure 3.
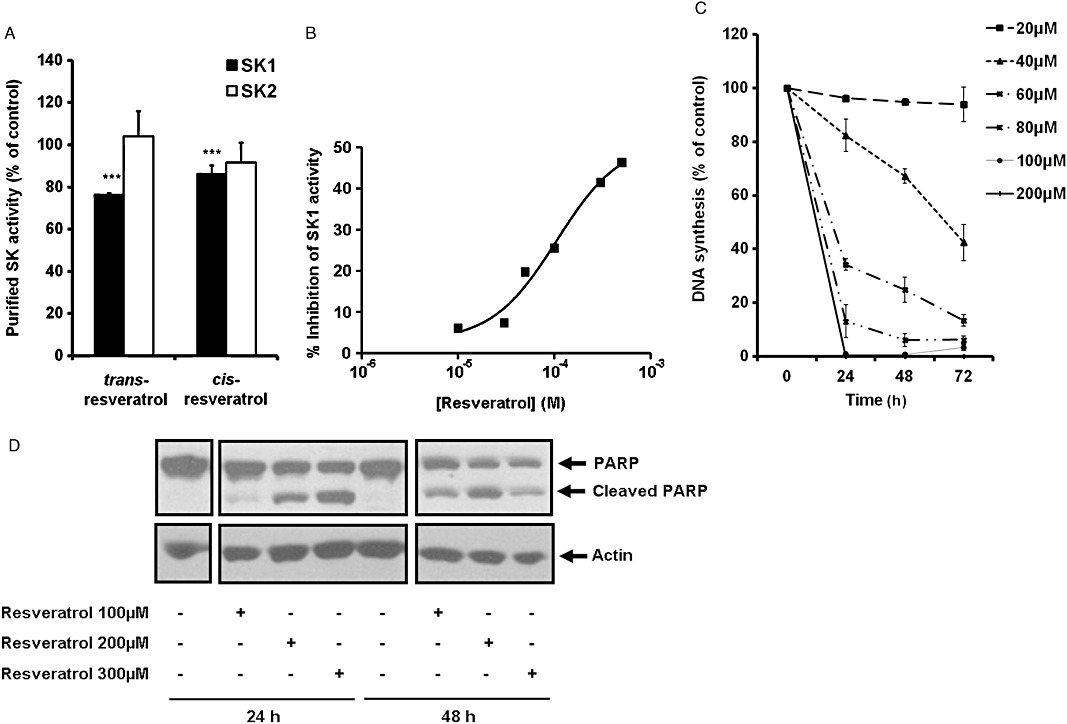
Biological activities of resveratrol. (A) Bar graph showing the effect of trans- and cis-resveratrol (both at 500 µM) on purified SK1 or SK2 activity. SK1/2 was assayed using 10 µM sphingosine and 250 µM [32P]-ATP as the substrates. Data are expressed as percentage of control and represent mean and SDs of six determinations, ***P < 0.001, significantly different from control. (B) Graph showing the concentration-dependent inhibitory effect of trans-resveratrol on SK1 activity in lysates from SK1 stably over-expressed HEK 293 cells. (C) Graph showing the effect of trans-resveratrol on [3H]-thymidine incorporation in MCF-7 cells. MCF-7 cells were treated with trans-resveratrol at indicated concentrations or control (0.05% v/v DMSO final concentration) for 24–72 h and then with 0.5 µCi·mL−1 of [3H]-thymidine added for 5 h. Data are expressed as percentage of control and represent means and SDs of triplicate determinations. (D) Western blot showing the effect of trans-resveratrol on PARP cleavage. MCF-7 cells were treated with trans-resveratrol (100, 200, 300 µM) or control (0.3% v/v DMSO final concentration) for 24 and 48 h. Cell lysates were prepared and analysed by Western blotting using anti-PARP antibody and then stripped and reprobed with anti-actin antibody to ensure comparable protein loading. The images shown were taken from the same Western blot. Results are representative of three independent experiments.
Trans-resveratrol inhibited SK1 activity in a concentration-dependent manner in lysates from cells stably over-expressing SK1 (Figure 3B). The treatment of MCF-7 cells with trans-resveratrol also reduced [3H] thymidine incorporation into DNA in a time and concentration-dependent manner (Figure 3C) and induced PARP cleavage in MCF-7 cells (Figure 3D). Kinetic analysis of lysates from HEK 293 cells stably over-expressing SK1 demonstrated that SK1 obeyed Michaelis–Menten kinetics and that trans-resveratrol (thereafter referred to as resveratrol) was a competitive inhibitor (with sphingosine) of SK1 with a Kic= 160 ± 40 µM (Figure 4A–C). When ATP was varied and the sphingosine concentration kept constant (20 µM), resveratrol reduced the Vmax without affecting the Km for ATP indicating that it is non-competitive with ATP (control, Km= 42.3 µM, Vmax= 0.25 pmol·µg−1·min−1; 100 µM resveratrol, Km= 36.1 µM, Vmax= 0.21 pmol·µg−1·min−1; 300 µM resveratrol, Km= 41 µM, Vmax= 0.18 pmol·µg−1·min−1; 500 µM resveratrol, Km= 34.5 µM, Vmax= 0.15 pmol·µg−1·min−1, n= 4).
Figure 4.
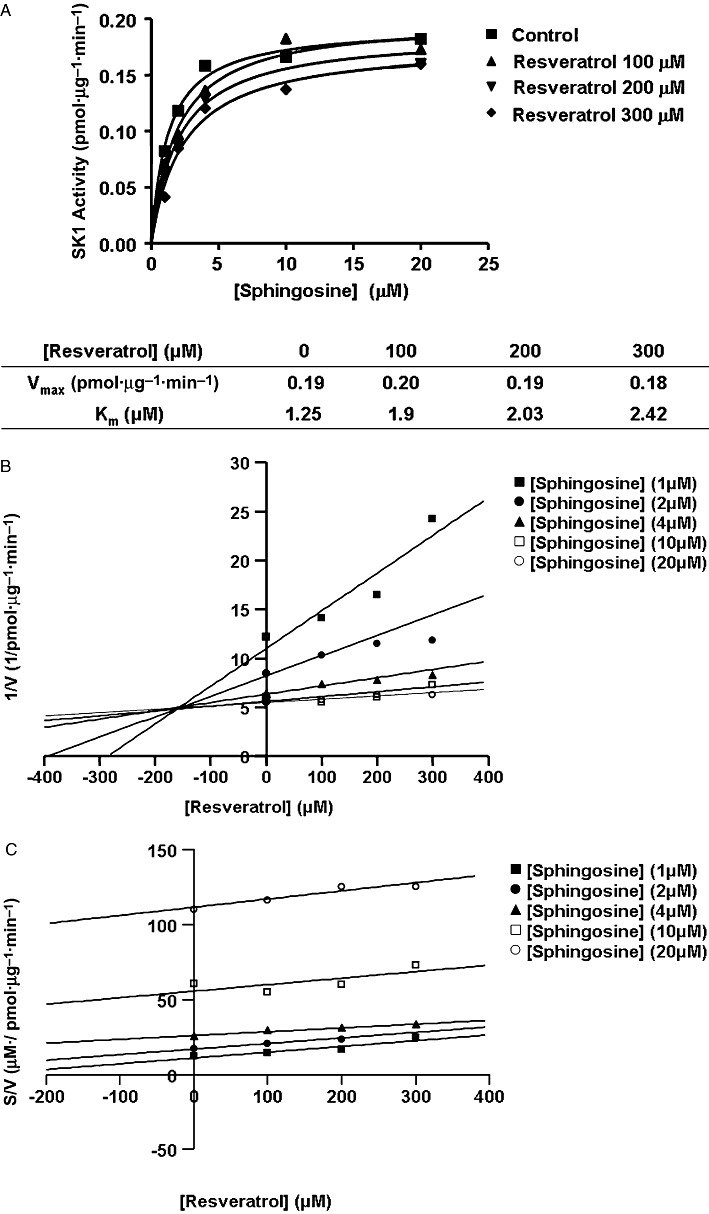
Inhibitor kinetic analysis of resveratrol for stably over-expressed recombinant SK1 in lysates from HEK 293 cells. (A) Non-linear regression analysis. (B) Dixon plot. (C) S/V versus resveratrol concentration plot. Results are representative of three independent experiments.
Kinetic and functional studies of balanocarpol
In contrast with resveratrol, balanocarpol is a mixed competitive inhibitor (with sphingosine) of SK1 with a Kic= 90 ± 10 µM and a Kiu∼500 µM (Figure 5A–C). These findings demonstrate that balanocarpol is more potent (approximately twofold) compared with resveratrol in inhibiting SK1 activity. When ATP was varied and the sphingosine concentration kept constant (20 µM), balanocarpol reduced the Vmax without affecting the Km for ATP indicating that it is non-competitive with ATP (control, Km= 36.6 µM, Vmax= 0.24 pmol·µg−1·min−1; 300 µM balanocarpol, Km= 31.1 µM, Vmax= 0.18 pmol·µg−1·min−1, n= 4). Moreover, balanocarpol is more potent in inhibiting [3H] thymidine incorporation into DNA in MCF-7 cells; the IC50 for inhibition of DNA synthesis is ∼50 µM for resveratrol (Figure 3C) and ∼10 µM for balanocarpol (Figure 6A). In common with resveratrol and SKi, balanocarpol also induced PARP cleavage indicating that it is an inducer of apoptosis (Figure 6B). Resveratrol and balanocarpol also exhibited properties typical of SK1 inhibitors by inducing the down-regulation of SK1 expression in MCF-7 Neo cells (Figure 6C).
Figure 5.
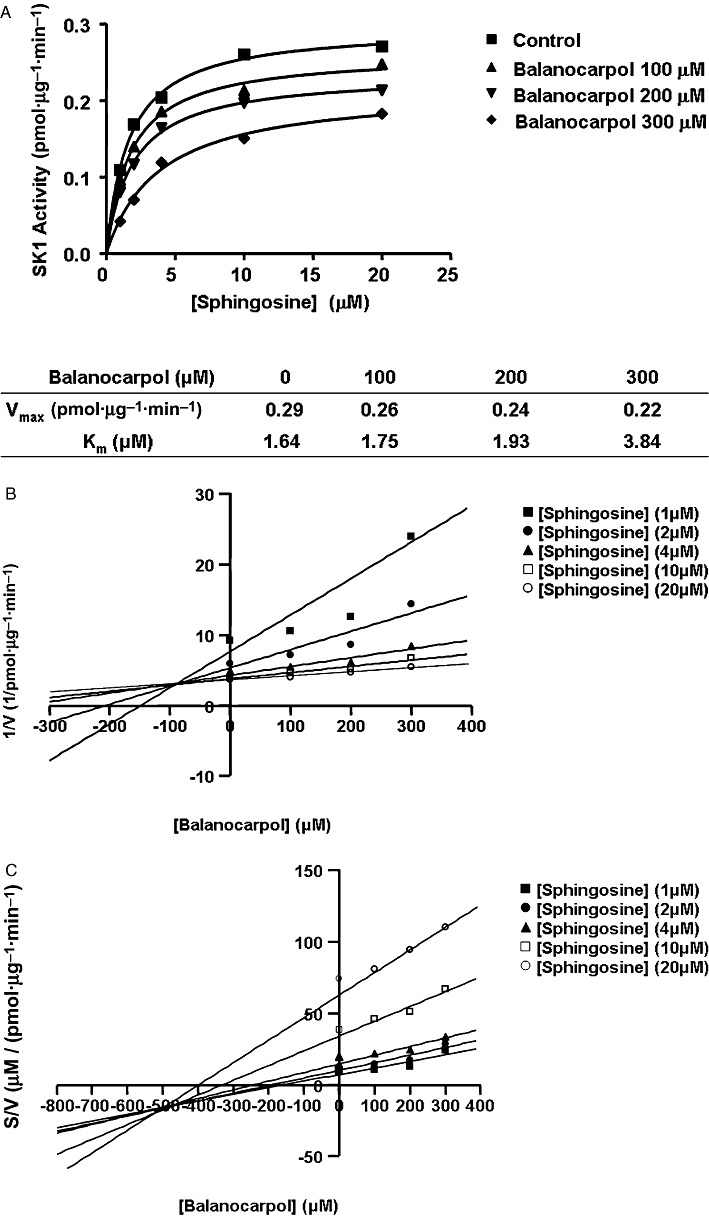
Inhibitor kinetic analysis of balanocarpol for stably over-expressed recombinant SK1 in lysates from HEK 293 cells. (A) Non-linear regression analysis. (B) Dixon plot. (C) S/V versus balanocarpol concentration plot. Results are representative of three independent experiments.
Figure 6.
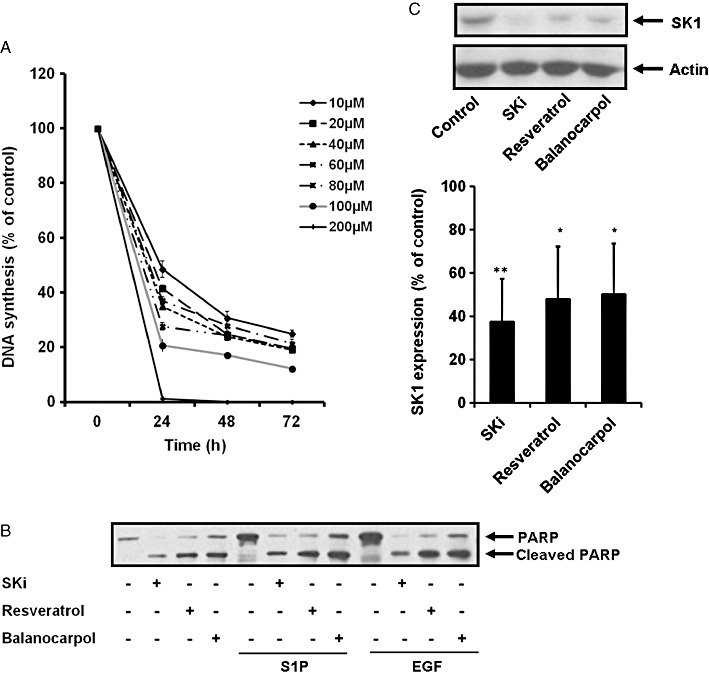
Biological activities of balanocarpol. (A) Graphs showing the effect of balanocarpol on [3H]-thymidine incorporation in MCF-7 cells. MCF-7 cells were treated with balanocarpol at indicated concentrations or control (0.05% v/v DMSO final concentration) for 24–72 h and then with 0.5 µCi·mL−1 of [3H]-thymidine added for 5 h. Data are expressed as percentage of control and represent means and SDs of triplicate determinations. (B) Western blots showing the effect of balanocarpol, SKi or resveratrol on PARP cleavage. Quiescent MCF-7 Neo cells were incubated with control (0.1% v/v DMSO final concentration) or indicated inhibitors (10 µM SKi, 200 µM resveratrol or 200 µM balanocarpol for 48 h before being stimulated with 25 ng·mL−1 EGF or 1 µM S1P for 5 min. Cell lysates were separated by SDS-PAGE and immunoblotted with anti-PARP antibody. Results are representative of three independent experiments. (C) Western blots showing the effect of balanocarpol, SKi or resveratrol on SK1 expression. Quiescent MCF-7 Neo cells were incubated with control (0.1% v/v DMSO final concentration), 10 µM SKi, 200 µM resveratrol or 200 µM balanocarpol for 48 h. Cells were harvested and analysed by Western blotting using anti-SK1 antibody. Blots were then stripped and reprobed with anti-actin antibody to ensure comparable protein loading. The bar graph represents densitometric quantification of the effects of inhibitors on SK1 expression (SK1: actin ratio). Data are expressed as a percentage of control (*P < 0.05, **P < 0.01, significantly different from control, n= 3). Results are representative of three independent experiments.
Discussion
The major finding of our study is that resveratrol and its dimers, ampelopsin A and balanocarpol are SK1 inhibitors. This conclusion is based on the finding that these compounds inhibited SK1 catalytic activity and induced the down-regulation of SK1 expression, properties similar to that observed for the known inhibitor, SKi. Moreover, these compounds also inhibit proliferation and induce apoptosis of MCF-7 breast cancer cells. Indeed, the effect of these compounds on apoptosis can be reproduced by siRNA knock-down of SK1 in MCF-7 breast cancer cells; this involving caspase activation associated with increased ceramide formation and Bax oligomerization (Taha et al., 2006). In addition, our findings concerning the effect of resveratrol, ampelopsin A and balanocarpol on SK1 expression are supported by studies that demonstrate that dietary polyphenols including resveratrol induce down-regulation of SK1 activity and expression in prostate cancer cells (Brizuela et al., 2010). The mechanism of down-regulation of SK1 expression might involve changes in SK1 protein turnover, as demonstrated for other SK1 inhibitors that induce the ubiquitin-proteasomal degradation of SK1 (Tonelli et al., 2010; Loveridge et al., 2010) or lysosomal-cathepsin B catalysed proteolysis (Ren et al., 2010) or changes in gene promoter activity. Further studies are required to establish the precise mechanism by which resveratrol and balanocarpol reduce the expression of SK1.
Previous studies using red grape skin polyphenolic extracts (SGE) to study the ‘French paradox’, linked the anti-angiogenic effects of resveratrol (which is present in SGE) to effects on S1P and VEGF signalling (Barthomeuf et al., 2006). Thus, pretreatment of endothelial cells with SGE reduced S1P-induced migration, ERK1/2 activation and platelet activating factor synthesis (Barthomeuf et al., 2006). However, SGE contains other polyphenolic compounds including significant quantities of resveratrol oligomers. Based on the finding that balanocarpol is a more potent inhibitor of SK1 compared with resveratrol, the attribution of the anti-angiogenic effect of SGE to resveratrol alone should be exercised with caution. Our findings also raise the question of whether the anti-cancer effect of resveratrol might be due to direct inhibition of SK1 and/or down-regulation of SK1 expression leading to alterations in sphingolipid metabolism and signalling. Indeed, perturbation of sphingolipids has been demonstrated before by resveratrol, which induces an increase in the synthesis of ceramide and which, in turn, stimulates the apoptosis of MDA-MB-231 cells (Scarlatti et al., 2003). Our findings also suggest that some of the effects of resveratrol on the inflammatory response to C5a, previously described by Issuree et al. (2009), and on prostate cancer survival (Brizuela et al., 2010) might be a consequence of the direct inhibition of SK1 activity and down-regulation of SK1 expression.
In the current study, we also provide a novel insight into the enzymatic mechanism by which resveratrol inhibits SK1 activity. Kinetic analysis established that resveratrol is a competitive inhibitor (with sphingosine) of SK1. The Kic for inhibition of SK1 activity is 160 ± 40 µM. Although this represents a relatively low potency, it is consistent with the concentration of resveratrol required for induction of apoptosis of cancer cells (Nakagawa et al., 2001; Pozo-Guisado et al., 2002; Scarlatti et al., 2003). The maximum serum concentration of resveratrol is 2 µM using relevant dietary concentrations in humans (Walle et al., 2004). Although this concentration is considerably lower than the Ki for inhibition of SK1 activity, it is possible that the intracellular concentration obtained from a serum concentration of 2 µM is considerably higher. Low serum concentrations of resveratrol might also be increased by higher dose administration. Interestingly, resveratrol is also an oestrogen receptor antagonist and can therefore reduce oestrogen-induced MCF-7 cell growth (Lu and Serrero, 1999). In this regard, SKi is also an oestrogen receptor antagonist and exhibits structural similarity with resveratrol (Antoon et al., 2011). Therefore, both resveratrol and SKi could be usefully exploited to launch a two-pronged attack on cancer.
High concentrations (usually >50 µM) of resveratrol induce apoptosis whereas low concentrations (10–40 µM) suppress cancer cell growth (Nakagawa et al., 2001; Pozo-Guisado et al., 2002; Scarlatti et al., 2003). This is in line with our findings, where apoptosis was induced by high concentrations of resveratrol (100–300 µM) and this was correlated with inhibition/down-regulation of SK1 expression. In contrast, the IC50 for inhibition of MCF-7 cell growth is ∼50 µM and might therefore, be SK1-independent although, as the intracellular concentration of inhibitor attained is unknown, the inhibition of growth by targeting SK1 cannot be entirely excluded. This is relevant if, for instance, different levels of SK1 inhibition are required to observe effects on growth, as distinct from apoptosis. Alternatively, the mechanism for growth inhibition might be via a different target where reported IC50 values are closer to those determined here, such as COX-1 (15 µM), COX-2 (32.2 µM), antioxidant/free radical scavenging (27 µM) and oestrogen receptors (5 µM) (Jang et al., 1997; Subbaramaiah et al., 1998; Lu and Serrero, 1999; Frémont, 2000).
The fact that secondary metabolites are produced via the biogenesis of resveratrol monomers raises the question whether oligomerization produces compounds which exhibit better potency as anti-cancer agents. Biosynthesis of resveratrol oligomers might therefore be regarded as a diversity-oriented approach. This is supported by the finding that balanocarpol is about twice as potent as resveratrol with respect to inhibition of SK1 activity. Therefore, it is possible that a single molecule of balanocarpol might bind at least two SK1 catalytic molecules to inhibit the activity of each simultaneously. Indeed, we have previously demonstrated that SK1 is a minimal dimer in which the catalytic sites are non-cooperative and therefore function independently of each other (Lim et al., 2011). Therefore, each catalytic site in the SK1 dimer might bind balanocarpol simultaneously, while resveratrol might bind to and inhibit only one catalytic site in the SK1 dimer. Indeed, this possible generic mechanism of action, where potency is augmented by dimerization of resveratrol is supported by the finding that the resveratrol tetramer, dibalanocarpol is more potent than resveratrol and its dimers in inhibiting the proliferation of P-388 cells (Sahidin et al., 2005). The proposed action of balanocarpol as an SK1 inhibitor bears some similarity with rapamycin, which inhibits two different proteins (FKBP-12 and mTOR) in a complex concomitantly (Choi et al., 1996).
In summary, we have provided the first evidence that resveratrol and balanocarpol directly inhibit SK1 activity and induce down-regulation of the enzyme in cancer cells. We also speculate that oligomerization of resveratrol might provide a means to improving inhibitor potency against SK1. The study of resveratrol oligomers has been hampered by difficulty in isolation from natural sources and intractable chemical synthesis. Nevertheless, a recent study reported a programmable, controlled and potentially scalable synthesis of the resveratrol family via a three-stage design (Snyder et al., 2011). It is anticipated that this will accelerate the development of novel therapeutics and translation to the clinic.
Acknowledgments
This work was supported by a Strathclyde University Scholarship to K. G. Lim. We would like to thank Carol Clements for assistance with preparation of extracts and Flash chromatography. We also thank the Forestry Research Institute of Malaysia for the supply of H. dryobalanoides.
Glossary
- COSY
correlation spectroscopy
- DEPT
distortionless enhancement through polarization transfer
- DMS
N,N-dimethylsphingosine
- HMBC
heteronuclear multiple bond coherence
- HMQC
heteronuclear multiple quantum coherence
- Kic
competitive inhibitor constant
- Kiu
uncompetitive inhibitor constant
- NOESY
nuclear overhauser enhancement spectroscopy
- SKi
2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole
- SK1
sphingosine kinase 1
- S1P
sphingosine 1-phosphate
Conflict of interest
The authors declare that there are no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Molecular structures of ampelopsin A and balanocarpol.
Figure S2 Proposed biosynthetic route of ampelopsin A or balanocarpol. Adapted from Sotheeswaran et al. (1993).
Table S1 Chemical shifts of ampelopsin A and balanocarpol
Please note: Wiley–Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Antoon JW, Meacham WD, Bratton MR, Slaughter EM, Rhodes LV, Ashe HB, et al. Pharmacological inhibition of sphingosine kinase isoforms alters estrogen receptor signalling in human breast cancer. J Mol Endocrinol. 2011;46:205–216. doi: 10.1530/JME-10-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athar M, Back JH, Tang X, Kim KM, Kopelovich L, Bickers DR, et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthomeuf C, Lamy S, Blanchette M, Boivin D, Gingras D, Béliveau R. Inhibition of sphingosine-1-phosphate- and vascular endothelial growth factor-induced endothelial cell chemotaxis by red grape skin polyphenols correlates with a decrease in early platelet-activating factor synthesis. Free Radic Biol Med. 2006;40:581–590. doi: 10.1016/j.freeradbiomed.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizuela L, Dayon A, Doumerc N, Ader I, Golzio M, Izard JC, et al. The sphingosine kinase-1 survival pathway is a molecular target for the tumor-suppressive tea and wine polyphenols in prostate cancer. FASEB J. 2010;24:3882–3894. doi: 10.1096/fj.10-160838. [DOI] [PubMed] [Google Scholar]
- Choi J, Chen J, Schreiber SL, Clardy J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science. 1996;273:239–242. doi: 10.1126/science.273.5272.239. [DOI] [PubMed] [Google Scholar]
- Coggon P, Janes NF, King FE, Kin TJ, Molyneux RJ, Morgan JWW, et al. Hopeaphenol an extractive of heartwood of Hopea odorata and Balanocarpus heimii. J Chem Soc. 1965:406–409. [Google Scholar]
- Coggon P, McPhail AT, Wallwork SC. Structure of hopeaphenol – X-ray analysis of benzene solvate of dibromodeca-o-methylhopeaphenol. J Chem Soc B. 1970:884–897. [Google Scholar]
- Coll JC, Bowden BF. The application of vacuum liquid-chromatography to the separation of terpene mixtures. J Nat Prod. 1986;49:934–936. [Google Scholar]
- Cortés A, Cascante M, Cárdenas ML, Cornish-Bowden A. Relationships between inhibition constants, inhibitor concentrations for 50% inhibition and types of inhibition: new ways of analysing data. Biochem J. 2001;357:263–268. doi: 10.1042/0264-6021:3570263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frémont L. Minireview – biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Issuree PD, Pushparaj PN, Pervaiz S, Melendez AJ. Resveratrol attenuates C5a-induced inflammatory responses in vitro and in vivo by inhibiting phospholipase D and sphingosine kinase activities. FASEB J. 2009;23:2412–2424. doi: 10.1096/fj.09-130542. [DOI] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Langcake P, Pryce RJ. New class of phytoalexins from grapevines. Experientia. 1977;33:151–152. doi: 10.1007/BF02124034. [DOI] [PubMed] [Google Scholar]
- Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, et al. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 2003;309:1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- Lim KG, Tonelli F, Li Z, Lu X, Bittman R, Pyne S, et al. FTY720 analogues as sphingosine kinase 1 inhibitors: enzyme inhibition kinetics, allosterism, proteasomal degradation and actin rearrangement in MCF-7 breast cancer cells. J Biol Chem. 2011;286:18633–18640. doi: 10.1074/jbc.M111.220756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JS, Edwards J, Watson C, Tovey S, Mair KM, Schiff R, et al. Sphingosine kinase 1 induces tolerance to human epidermal growth factor receptor 2 and prevents formation of a migratory phenotype in response to sphingosine 1-phosphate in estrogen receptor-positive breast cancer cells. Mol Cell Biol. 2010;30:3827–3841. doi: 10.1128/MCB.01133-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveridge C, Tonelli F, Leclercq T, Lim KG, Long JS, Berdyshev E, et al. The sphingosine kinase 1 inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole induces proteasomal degradation of sphingosine kinase 1 in mammalian cells. J Biol Chem. 2010;285:38841–38852. doi: 10.1074/jbc.M110.127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Serrero G. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J Cell Physiol. 1999;179:297–304. doi: 10.1002/(SICI)1097-4652(199906)179:3<297::AID-JCP7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Muhtadi, Hakim EH, Juliawaty LD, Syah YM, Achmad SA, Latip J, et al. Cytotoxic resveratrol oligomers from the tree bark of Dipterocarpus hasseltii. Fitoterapia. 2006;77:550–555. doi: 10.1016/j.fitote.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Kiyozuka Y, Uemura Y, Senzaki H, Shikata N, Hioki K, et al. Resveratrol inhibits human breast cancer cell growth and may mitigate the effect of linoleic acid, a potent breast cancer cell stimulator. J Cancer Res Clin Oncol. 2001;127:258–264. doi: 10.1007/s004320000190. [DOI] [PubMed] [Google Scholar]
- Ohyama M, Tanaka T, Ito T, Iinuma M, Bastow KF, Lee KH. Antitumor agents 200. Cytotoxicity of naturally occurring resveratrol oligomers and their acetate derivatives. Bioorg Med Chem Lett. 1999;9:3057–3060. doi: 10.1016/s0960-894x(99)00520-x. [DOI] [PubMed] [Google Scholar]
- Pozo-Guisado E, Alvarez-Barrientos A, Mulero-Navarro S, Santiago-Josefat B, Fernandez-Salguero PM. The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: cell-specific alteration of the cell cycle. Biochem Pharmacol. 2002;64:1375–1386. doi: 10.1016/s0006-2952(02)01296-0. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Ren S, Xin C, Pfeilschifter J, Huwiler A. A novel mode of action of the putative sphingosine kinase inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole (SKI II): induction of lysosomal sphingosine kinase 1 degradation. Cell Physiol Biochem. 2010;26:97–104. doi: 10.1159/000315110. [DOI] [PubMed] [Google Scholar]
- Sahidin, Hakim EH, Juliawaty LD, Syah YM, bin Din L, Ghisalberti EL, et al. Cytotoxic properties of oligostilbenoids from the tree barks of Hopea dryobalanoides. Z Naturforsch C. 2005;60:723–727. doi: 10.1515/znc-2005-9-1011. [DOI] [PubMed] [Google Scholar]
- Scarlatti F, Sala G, Somenzi G, Signorelli P, Sacchi N, Ghidoni R. Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo ceramide signalling. FASEB J. 2003;17:2339–2341. doi: 10.1096/fj.03-0292fje. [DOI] [PubMed] [Google Scholar]
- Snyder SA, Gollner A, Chiriac MI. Regioselective reactions for programmable resveratrol oligomer synthesis. Nature. 2011;474:461–466. doi: 10.1038/nature10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotheeswaran S, Pasupathy V. Distribution of resveratol oligomers in plants. Phytochemistry. 1993;32:1083–1092. [Google Scholar]
- Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, Inoue H, et al. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem. 1998;273:21875–21882. doi: 10.1074/jbc.273.34.21875. [DOI] [PubMed] [Google Scholar]
- Taha TA, Kitatani K, El-Alwani M, Bielawski J, Hannun YA, Obeid LM. Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: modulation of sphingolipid levels and the induction of apoptosis. FASEB J. 2006;20:482–484. doi: 10.1096/fj.05-4412fje. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Ito T, Ido Y, Son TK, Nakaya K, Iinuma M, et al. Stilbenoids in the stem bark of Hopea parviflora. Phytochemistry. 2000;53:1015–1019. doi: 10.1016/s0031-9422(00)00019-4. [DOI] [PubMed] [Google Scholar]
- Tonelli F, Lim KG, Loveridge C, Long J, Pitson SM, Tigyi G, et al. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal. 2010;22:1536–1542. doi: 10.1016/j.cellsig.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle T, Hsieh F, DeLegge MH, Otis JE, Jr, Walle UK. High adsorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- Watson C, Long JS, Orange C, Tannahill CL, Mallon E, McGlynn LM, et al. High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am J Pathol. 2010;177:2205–2215. doi: 10.2353/ajpath.2010.100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


