Abstract
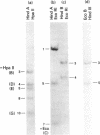
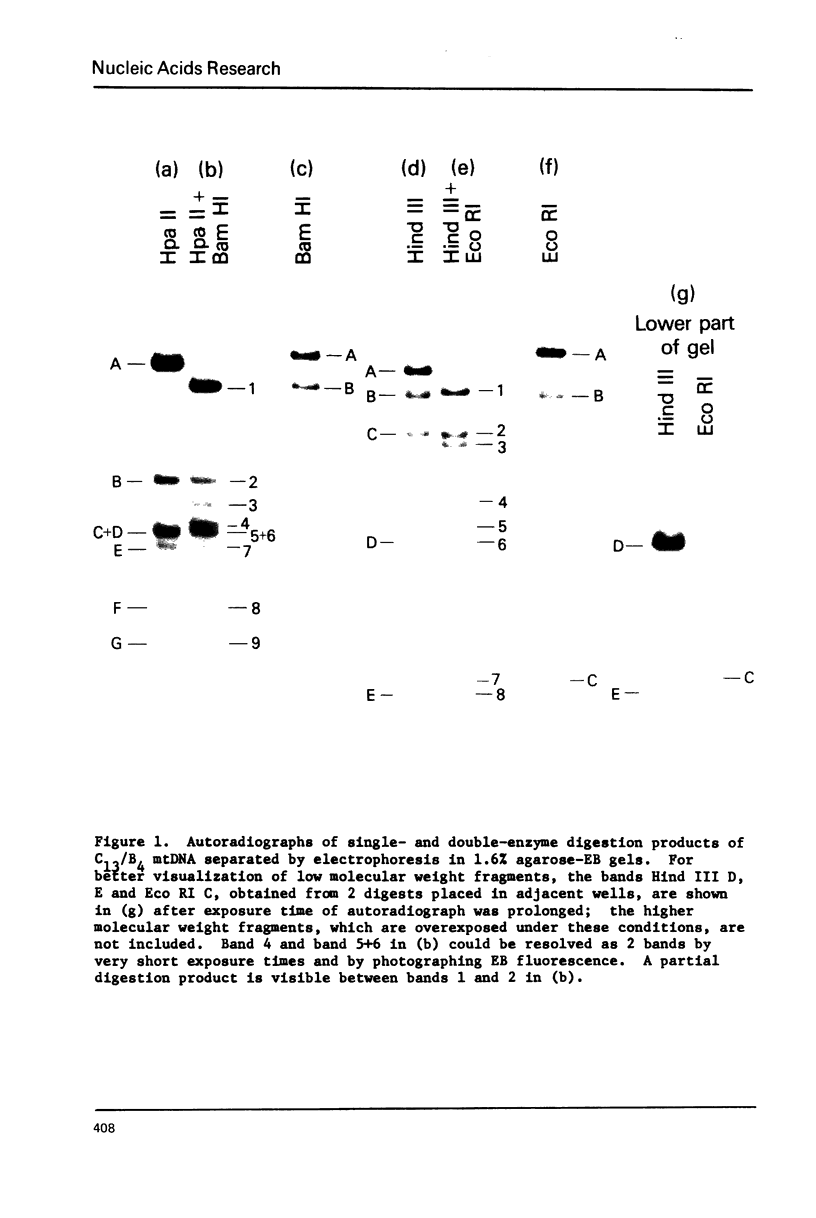
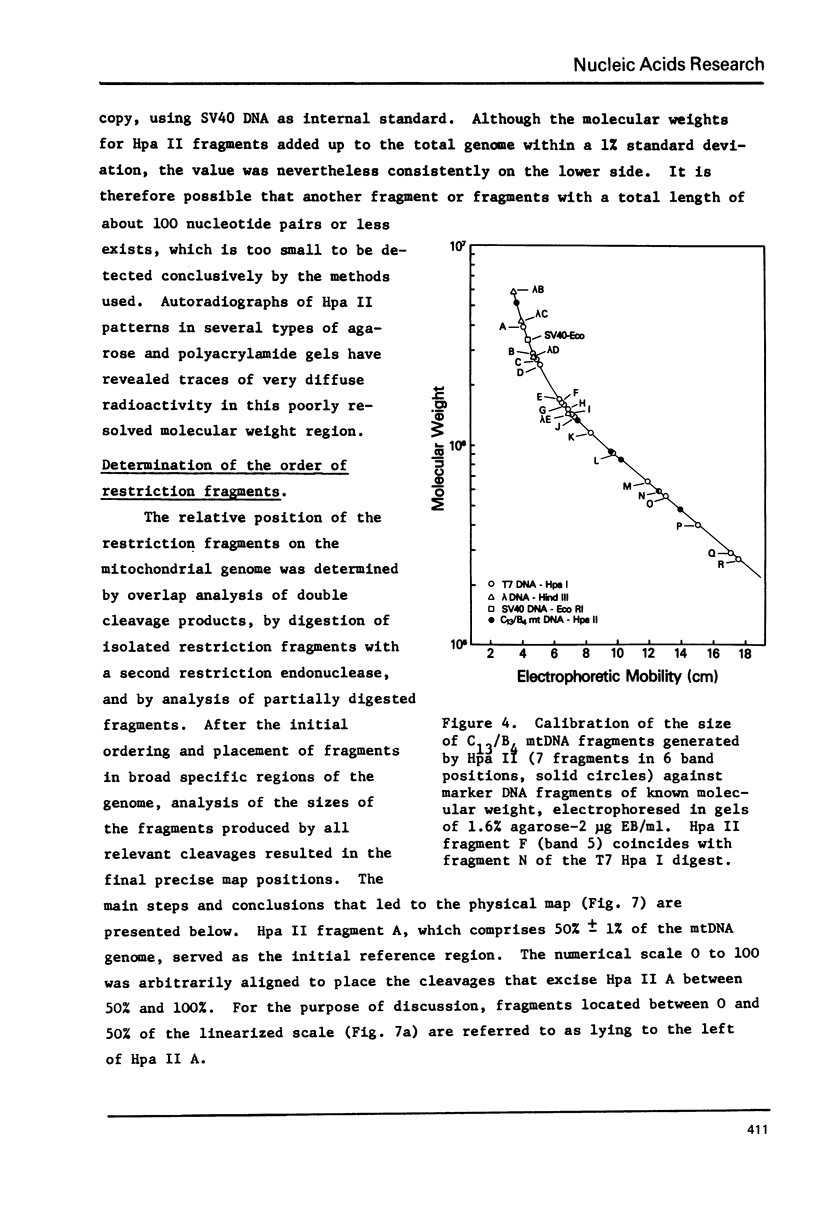
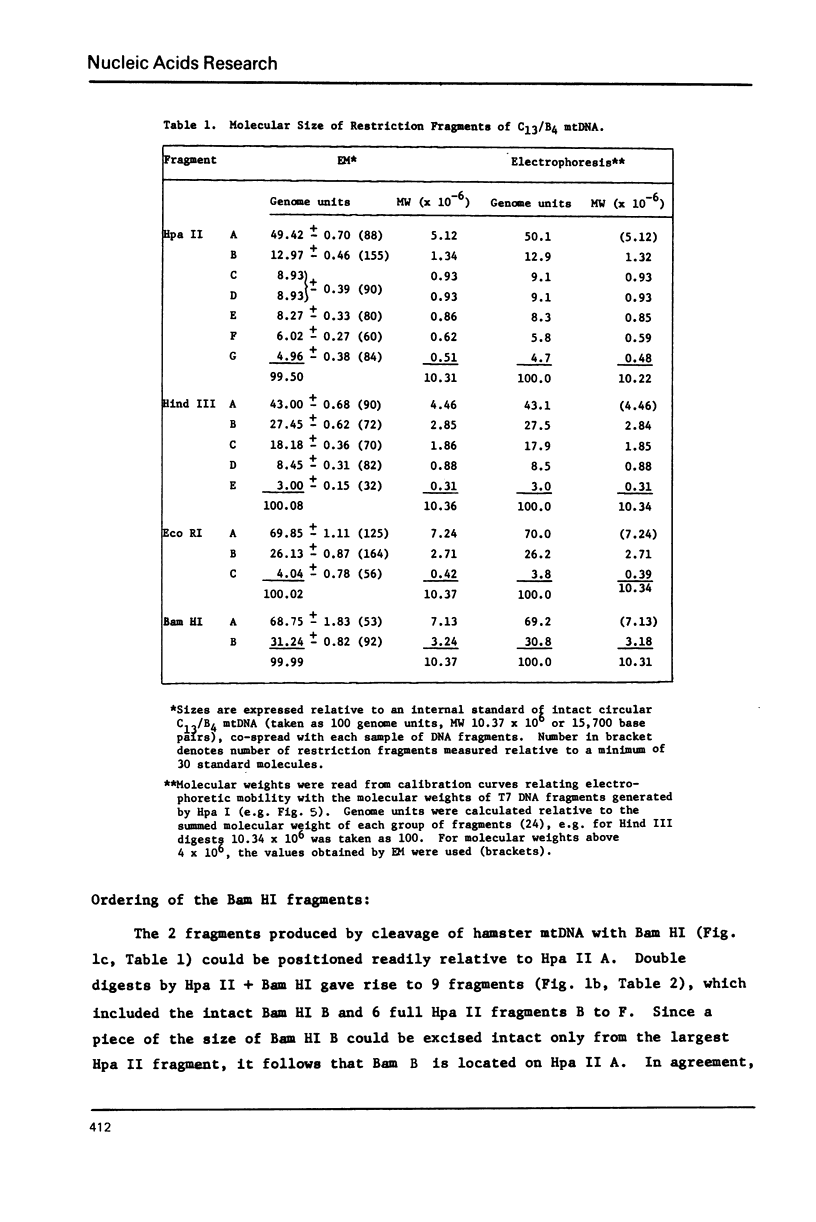
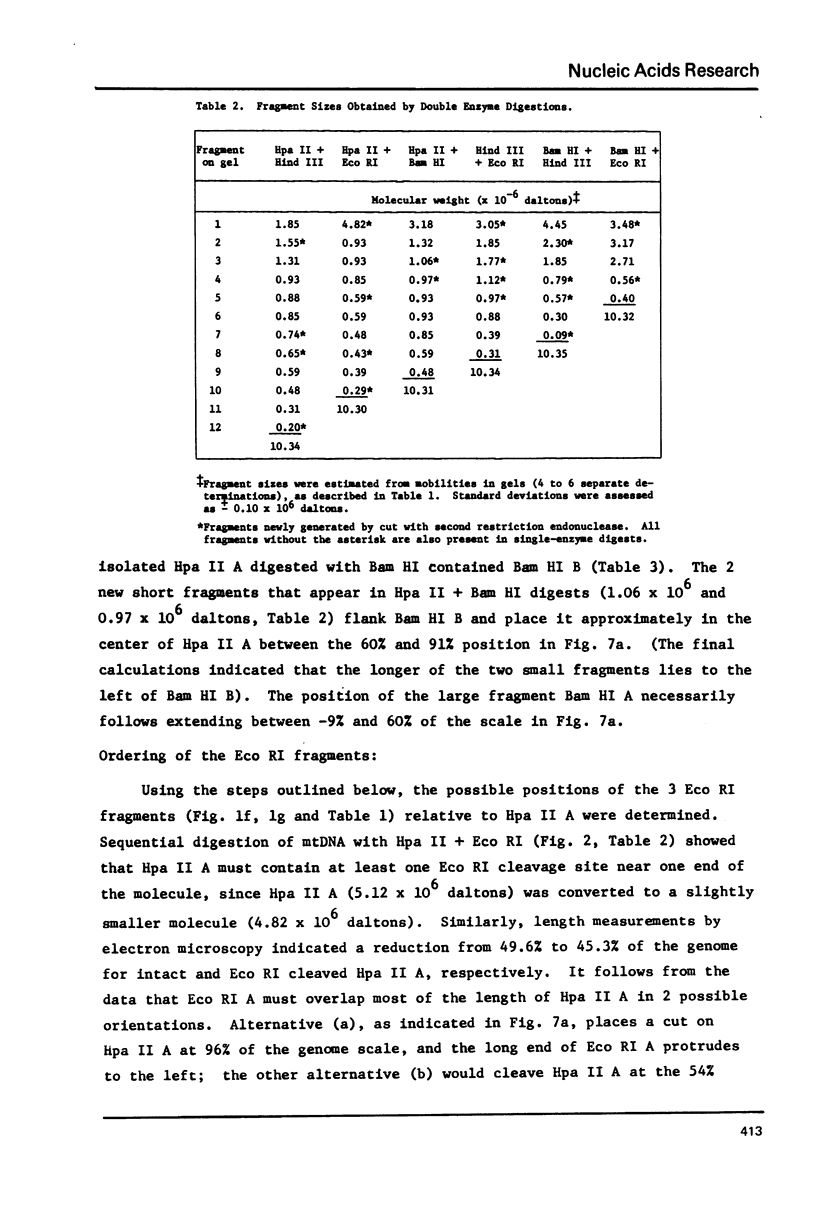
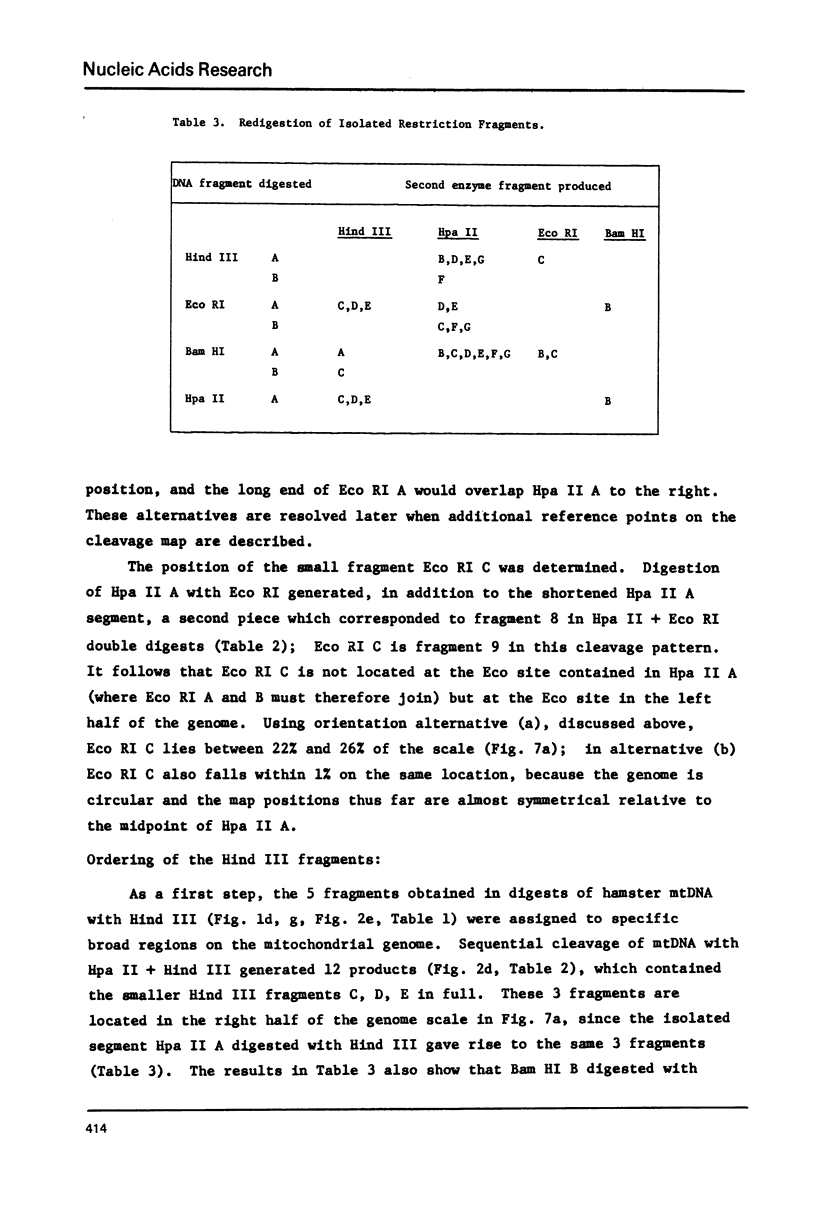
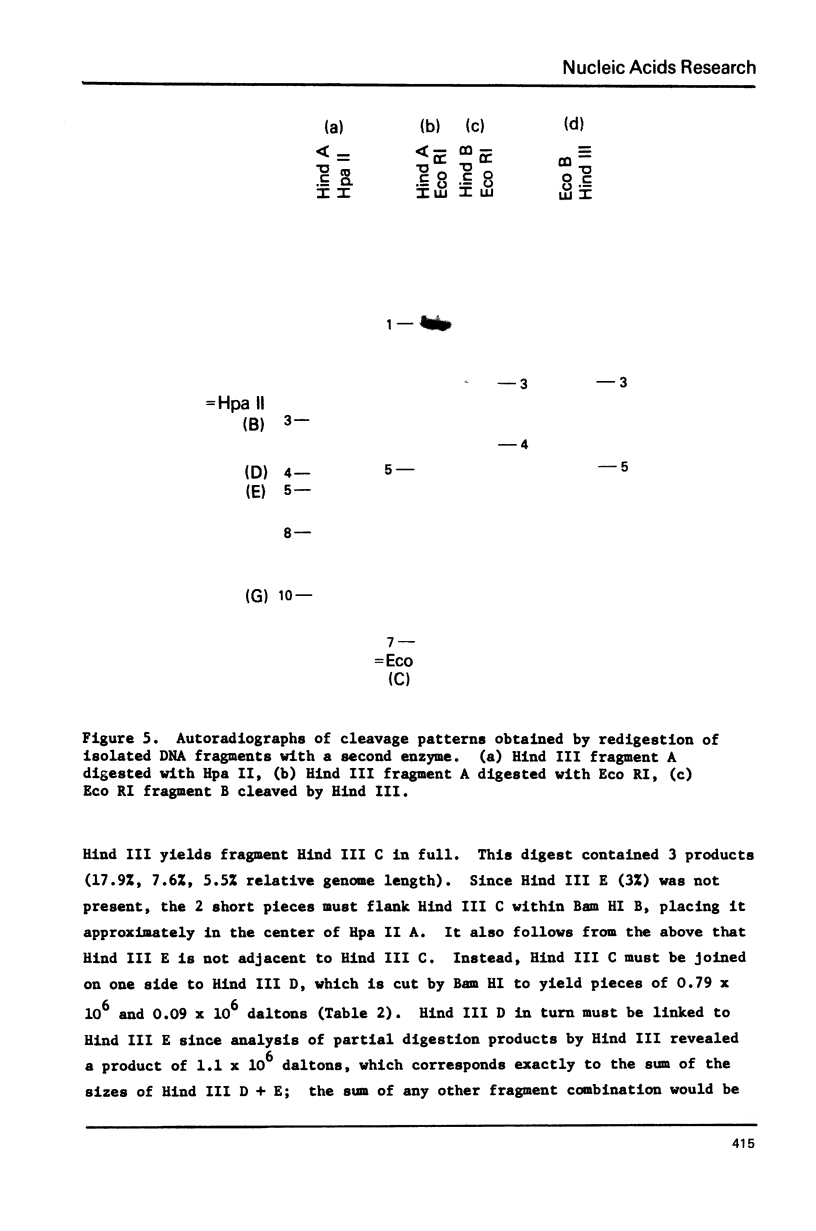
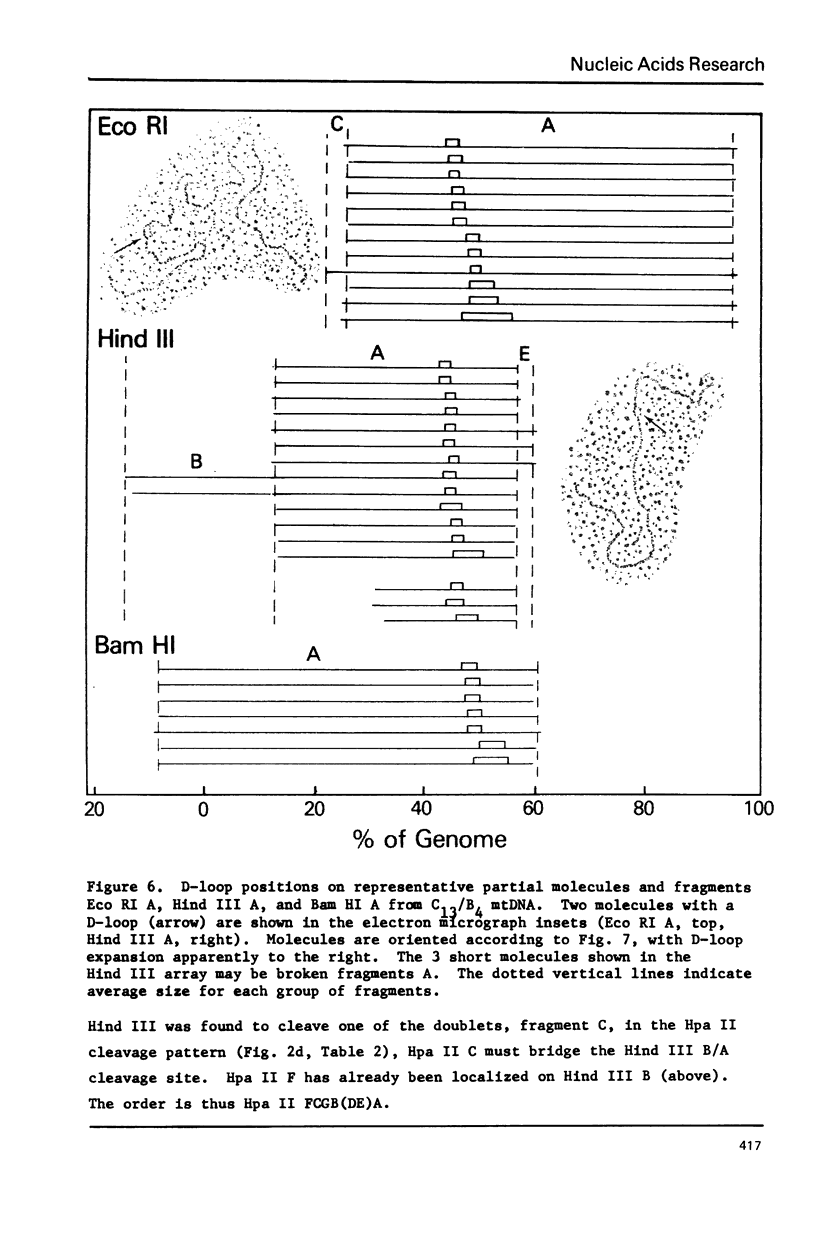
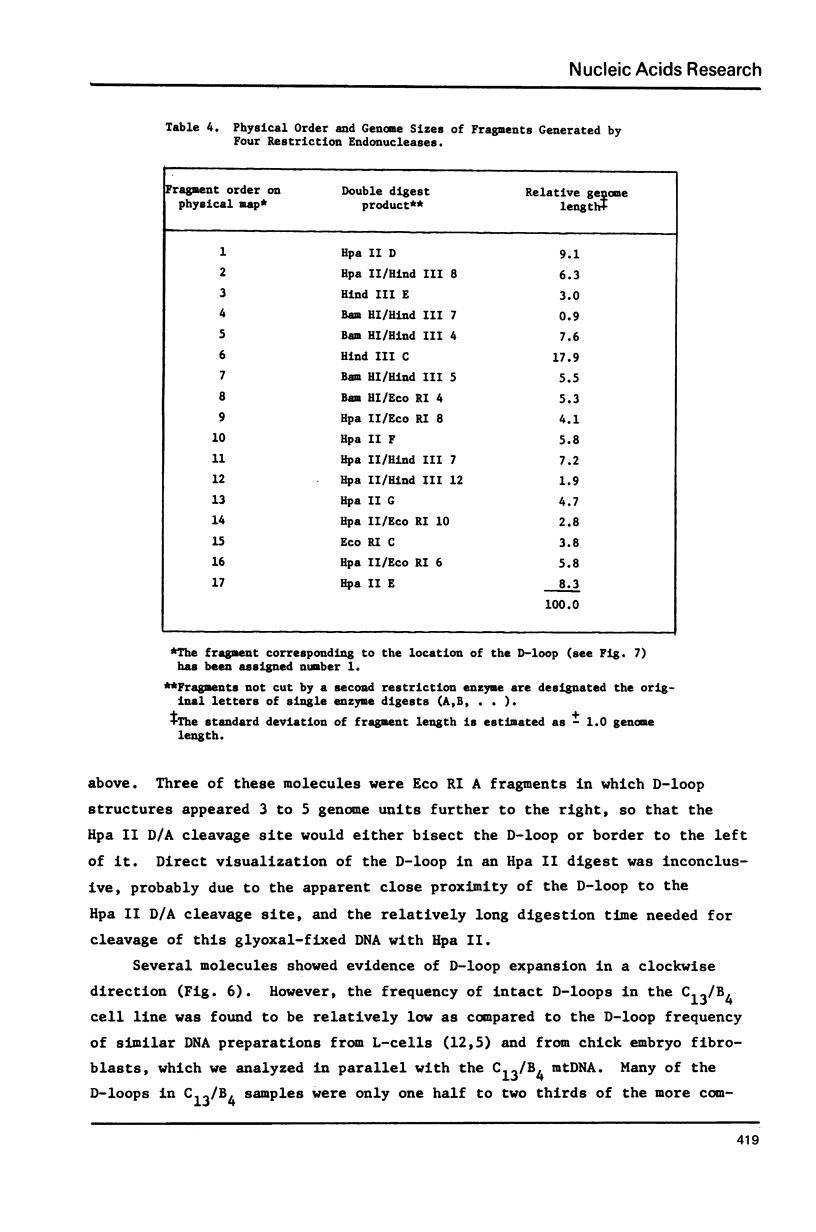
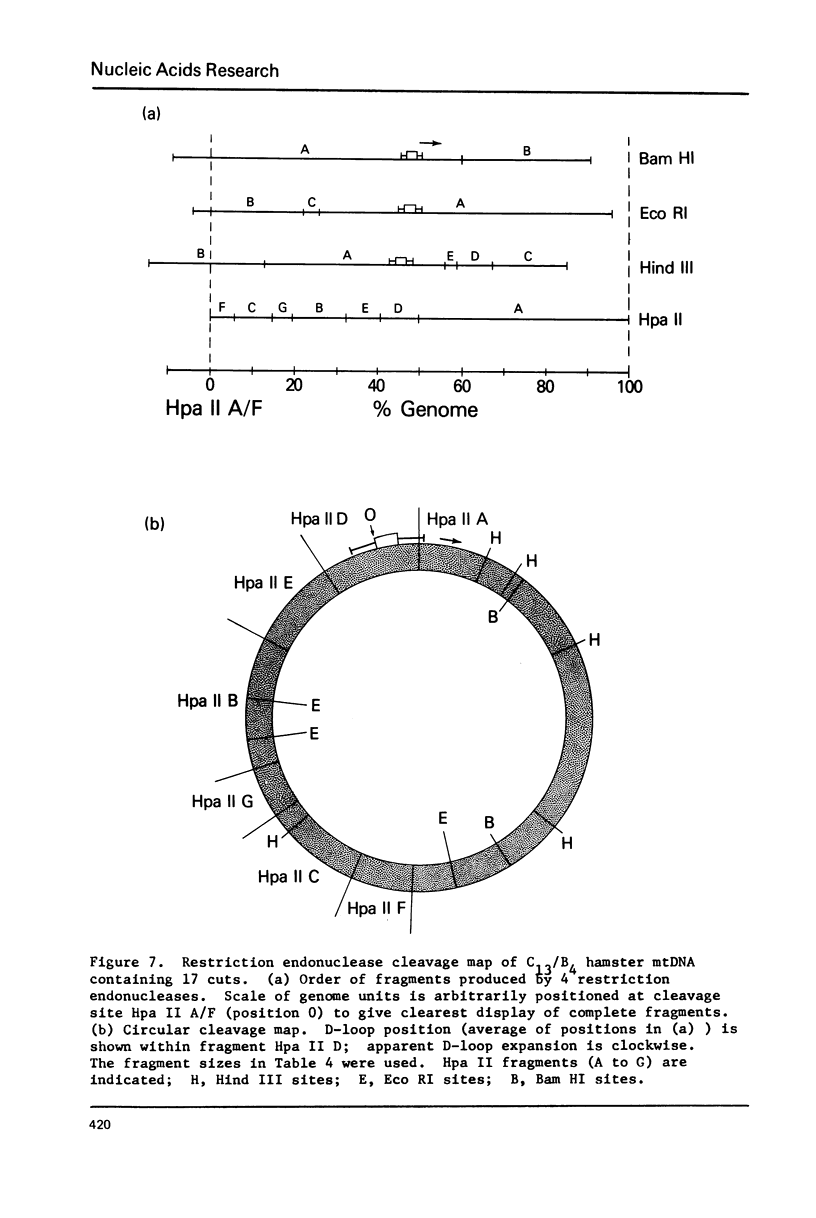
Mitochondrial DNA from cultured C13/B4 hamster cells was cleaved by the restriction endonucleases Hpa II, Hind III, Eco RI and Bam HI into 7, 5, 3 and 2 unique fragments, respectively. The summed molecular weights of fragments obtained from electrophoretic mobilities in agarose-ethidium bromide gels (with Hpa I-cleaved T7 DNA as standard) and electron microscopic analysis of fragment classes isolated from gels (with SV40 DNA as standard) were in good agreement with the size of 10.37 +/- 0.22 x 10(6) daltons (15,700 +/- 330 nucleotide pairs) determined for the intact circular mitochondrial genome. Cyclization of all Hind III, Eco RI and Bam HI fragments was observed. A cleavage map containing the 17 restriction sites (+/- 1% s.d.) was constructed by electrophoretic analysis of 32P-labeled single- and double-enzyme digestion products and reciprocal redigestion of isolated fragments. The 7 Hpa II sites were located in one half of the genome. The total distribution of the 17 cleavages around the genome was relatively uniform. The position of the D-loop was determined from its location and expansion on 3 overlapping restriction fragments.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaij C., Borst P. The gel electrophoresis of DNA. Biochim Biophys Acta. 1972 May 10;269(2):192–200. doi: 10.1016/0005-2787(72)90426-1. [DOI] [PubMed] [Google Scholar]
- Angerer L., Davidson N., Murphy W., Lynch D., Attardi G. An electron microscope study of the relative positions of the 4S and ribosomal RNA genes in HeLa cells mitochondrial DNA. Cell. 1976 Sep;9(1):81–90. doi: 10.1016/0092-8674(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Avadhani N. G., Lewis F. S., Rutman R. J. Mitochondrial RNA and protein metabolism. Subcell Biochem. 1976 Jun;4(2):93–145. [PubMed] [Google Scholar]
- Berk A. J., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: asynchronous replication of strands, segregation of circular daughter molecules, aspects of topology and turnover of an initiation sequence. J Mol Biol. 1974 Jul 15;86(4):801–824. doi: 10.1016/0022-2836(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Blin N., von Gabain A., Bujard H. Isolation of large molecular weight DNA from agarose gels for further digestion by restriction enzymes. FEBS Lett. 1975 Apr 15;53(1):84–86. doi: 10.1016/0014-5793(75)80688-0. [DOI] [PubMed] [Google Scholar]
- Borst P. Mitochondrial nucleic acids. Annu Rev Biochem. 1972;41:333–376. doi: 10.1146/annurev.bi.41.070172.002001. [DOI] [PubMed] [Google Scholar]
- Brown W. M., Vinograd J. Restriction endonuclease cleavage maps of animal mitochondrial DNAs. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4617–4621. doi: 10.1073/pnas.71.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. A comparative study of glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry. 1970 Nov 10;9(23):4567–4576. doi: 10.1021/bi00825a016. [DOI] [PubMed] [Google Scholar]
- Garfin D. E., Goodman H. M. Nucleotide sequences at the cleavage sites of two restriction endonucleases from Hemophilus parainfluenzae. Biochem Biophys Res Commun. 1974 Jul 10;59(1):108–116. doi: 10.1016/s0006-291x(74)80181-6. [DOI] [PubMed] [Google Scholar]
- Hedgpeth J., Goodman H. M., Boyer H. W. DNA nucleotide sequence restricted by the RI endonuclease. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3448–3452. doi: 10.1073/pnas.69.11.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Robberson D. L., Vinograd J. A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klietmann W., Sato N., Nass M. M. Establishment and characterization of ethidium bromide resistance in simian virus 40-transformed hamster cells. Effects on mitochondria in vivo. J Cell Biol. 1973 Jul;58(1):11–26. doi: 10.1083/jcb.58.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klukas C. K., Dawid I. B. Characterization and mapping of mitochondrial ribosomal RNA and mitochondrial DNA in Drosophila melanogaster. Cell. 1976 Dec;9(4 Pt 1):615–625. doi: 10.1016/0092-8674(76)90044-1. [DOI] [PubMed] [Google Scholar]
- Kroon A. M., Bakker H., Holtrop M., Terpstra P. The restriction endonuclease cleavage map of rat liver mitochondrial DNA. Biochim Biophys Acta. 1977 Jan 3;474(1):61–68. doi: 10.1016/0005-2787(77)90214-3. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Davis R. W., Davidson N. A physical study by electron microscopy of the terminally reptitious, circularly permuted DNA from the coliphage particles of Escherichia coli 15. J Mol Biol. 1970 Feb 28;48(1):1–22. doi: 10.1016/0022-2836(70)90215-9. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L., Andreone T. L., Donelson J. E. Translation in Escherichia coli mini-cells containing hamster mitochondrial DNA-Co1E1 - Ampr recombinant plasmids. Biochim Biophys Acta. 1977 Aug 16;477(4):323–333. doi: 10.1016/0005-2787(77)90251-9. [DOI] [PubMed] [Google Scholar]
- Moore K. H., Johnson P. H., Chandler S. E., Grossman L. I. A restriction endonuclease cleavage map of mouse mitochondrial DNA. Nucleic Acids Res. 1977;4(5):1273–1289. doi: 10.1093/nar/4.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass M. K. Studies on the synthesis and structure of mitochondrial DNA in cells infected by Rous sarcoma viruses and on the occurrence of intramitochondrial virus-like particles in certain RSV-induced tumor cells. Mol Cell Biochem. 1977 Feb 4;14(1-3):121–128. doi: 10.1007/BF01734175. [DOI] [PubMed] [Google Scholar]
- Nass M. M. Differential effects of ethidium bromide on mitochondrial and nuclear DNA synthesis in vivo in cultured mammalian cells. Exp Cell Res. 1972 May;72(1):211–222. doi: 10.1016/0014-4827(72)90583-6. [DOI] [PubMed] [Google Scholar]
- Nass M. M. Differential methylation of mitochondrial and nuclear DNA in cultured mouse, hamster and virus-transformed hamster cells. In vivo and in vitro methylation. J Mol Biol. 1973 Oct 15;80(1):155–175. doi: 10.1016/0022-2836(73)90239-8. [DOI] [PubMed] [Google Scholar]
- Old R., Murray K., Boizes G. Recognition sequence of restriction endonuclease III from Hemophilus influenzae. J Mol Biol. 1975 Feb 25;92(2):331–339. doi: 10.1016/0022-2836(75)90232-6. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M. Restriction endonuclease cleavage maps of rat and mouse mitochondrial DNAs. Nucleic Acids Res. 1977;4(5):1291–1299. doi: 10.1093/nar/4.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M., Vinograd J. Mapping of closed circular DNAs by cleavage with restriction endonucleases and calibration by agarose gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):851–855. doi: 10.1073/pnas.74.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S. S., Newbold J. E., Hutchison C. A., 3rd, Edgell M. H. Specific cleavage analysis of mammalian mitochondrial DNA. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4496–4500. doi: 10.1073/pnas.72.11.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunell A., Bernardi G. The mitochondrial genome of wild-type yeast cells. VI. Genome organization. J Mol Biol. 1977 Feb 15;110(1):53–74. doi: 10.1016/s0022-2836(77)80098-3. [DOI] [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A., Morrow J. F. Cleavage of replicating forms of mitochondrial DNA by EcoRI endonuclease. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4447–4451. doi: 10.1073/pnas.71.11.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J., Wilson G. A., Young F. E. Recognition sequence of specific endonuclease BamH.I from Bacillus amyloliquefaciens H. Nature. 1977 Jan 6;265(5589):82–84. doi: 10.1038/265082a0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Soslau G., Fuhrer J. P., Nass M. M., Warren L. The effect of ethidium bromide on the membrane glycopeptides in control and virus-transformed cells. J Biol Chem. 1974 May 25;249(10):3014–3020. [PubMed] [Google Scholar]
- Subramanian K. N., Pan J., Zain S., Weissman S. M. The mapping and ordering of fragments of SV40 DNA produced by restriction endonucleases. Nucleic Acids Res. 1974 Jun;1(6):727–752. doi: 10.1093/nar/1.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Davidson N., Attardi G., Aloni Y. Expression of the mitochondrial genome in HeLa cells. XIV. The relative positions of the 4 S RNA genes and of the ribosomal RNA genes in mitochondrial DNA. J Mol Biol. 1972 Oct 28;71(1):81–93. doi: 10.1016/0022-2836(72)90402-0. [DOI] [PubMed] [Google Scholar]