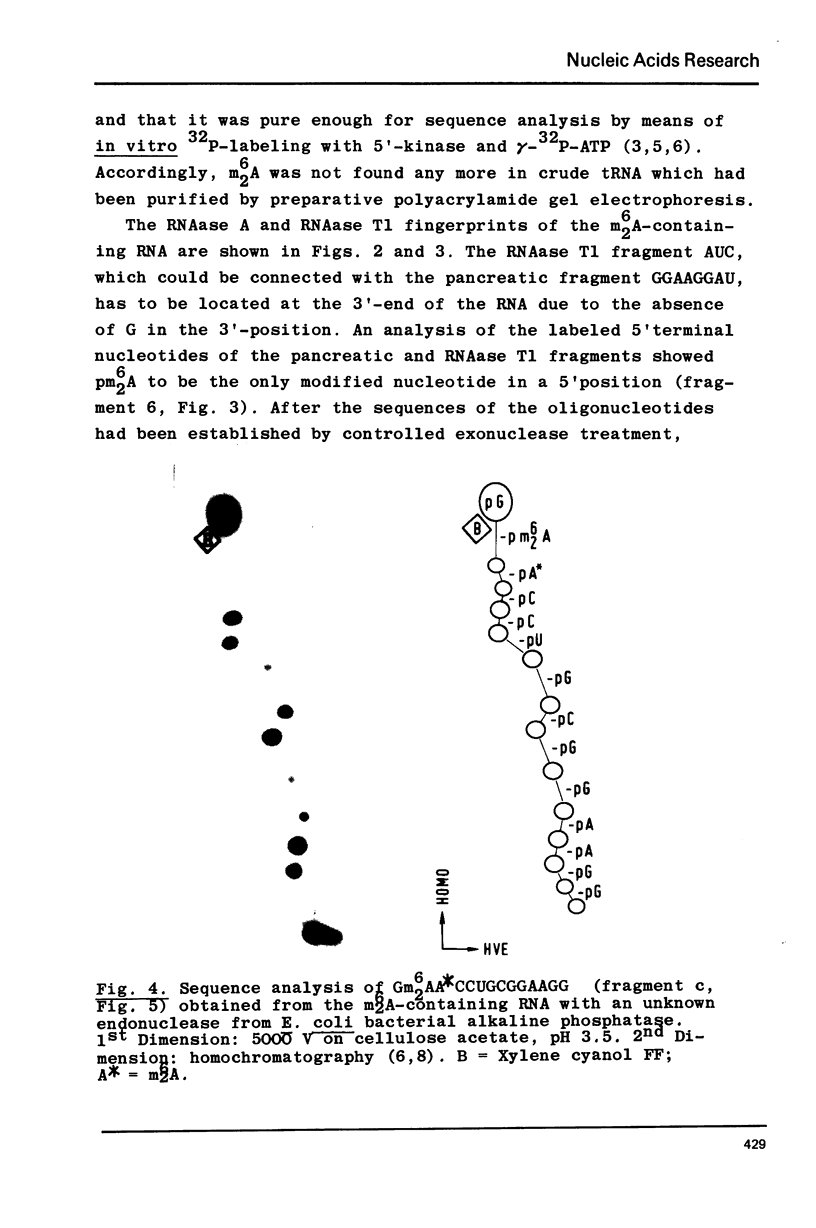
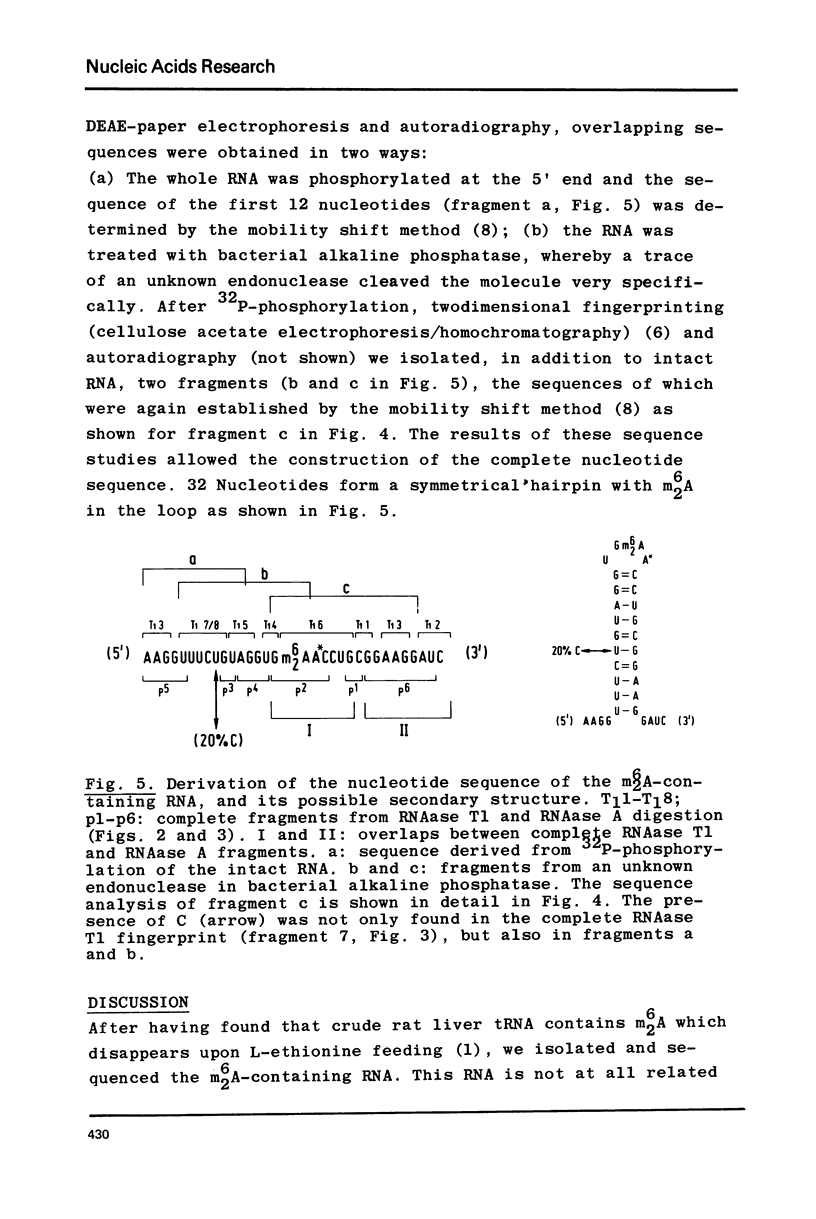
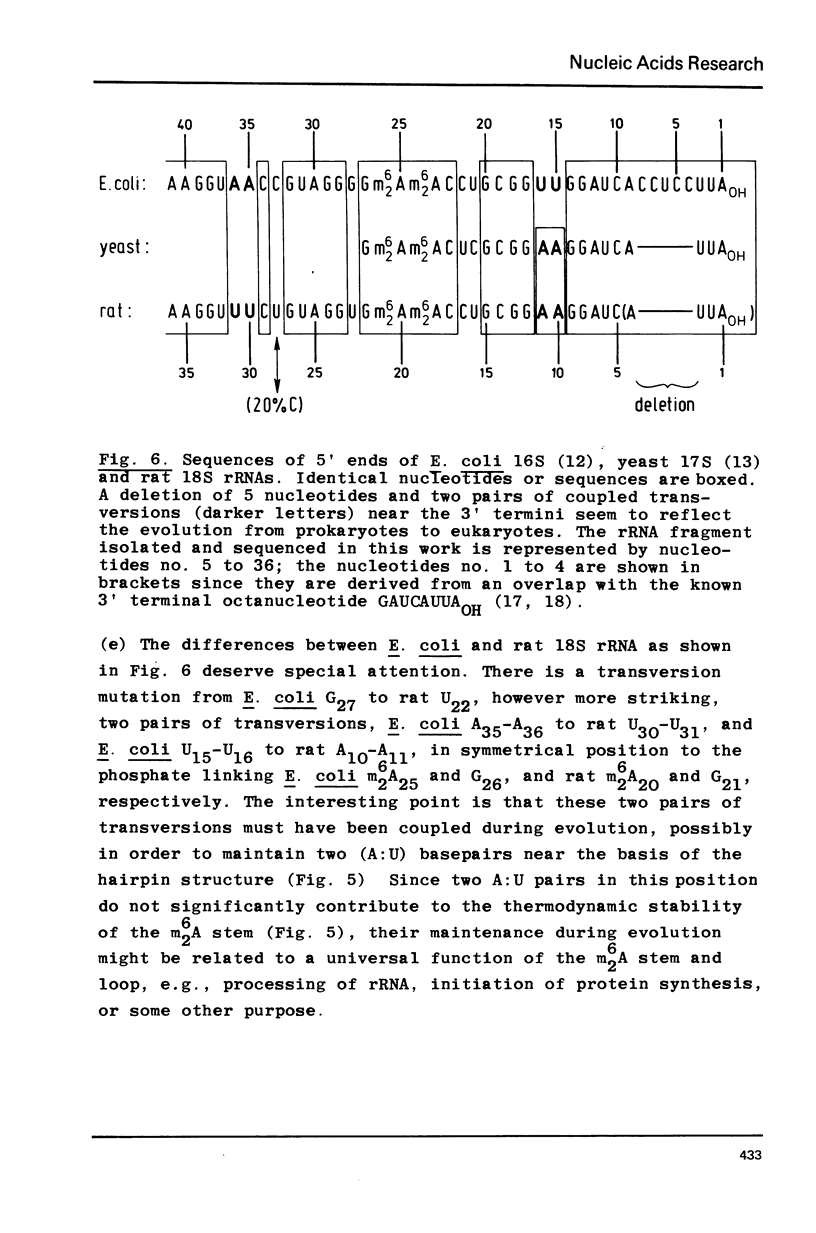
Abstract
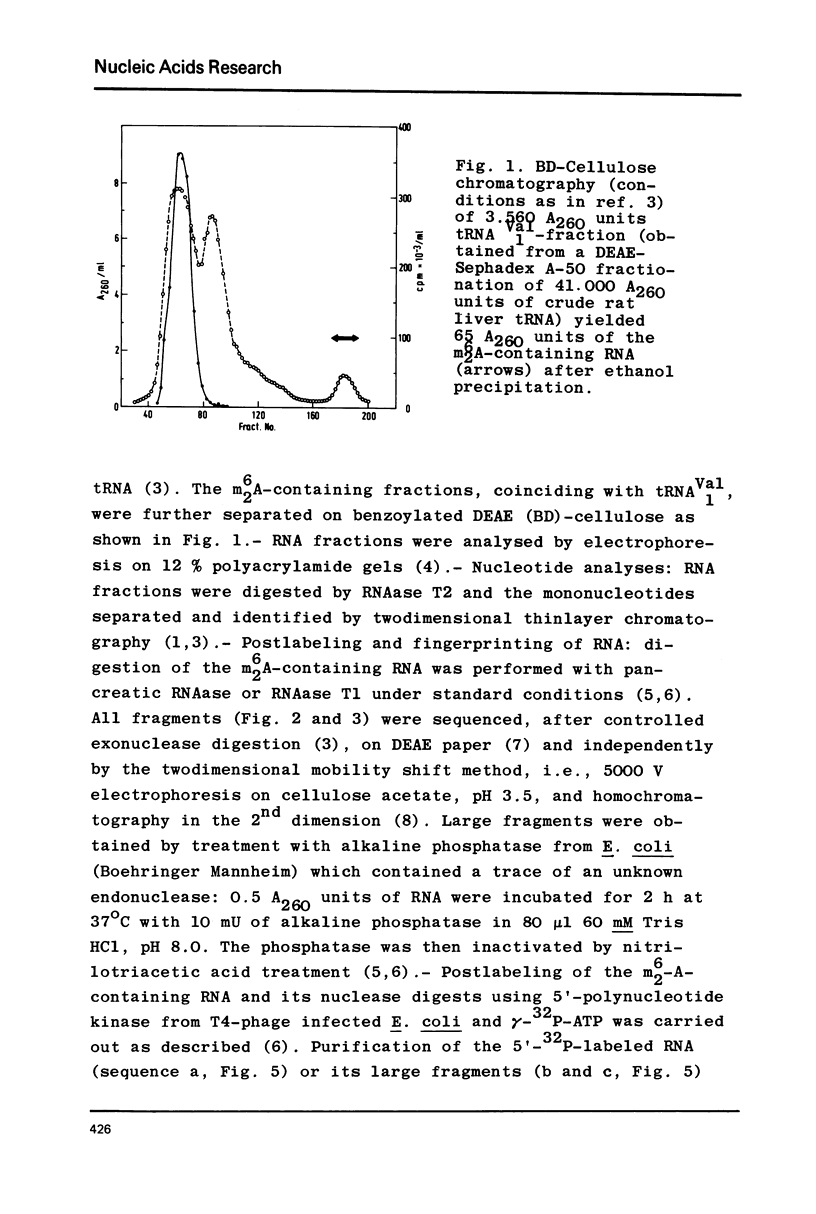
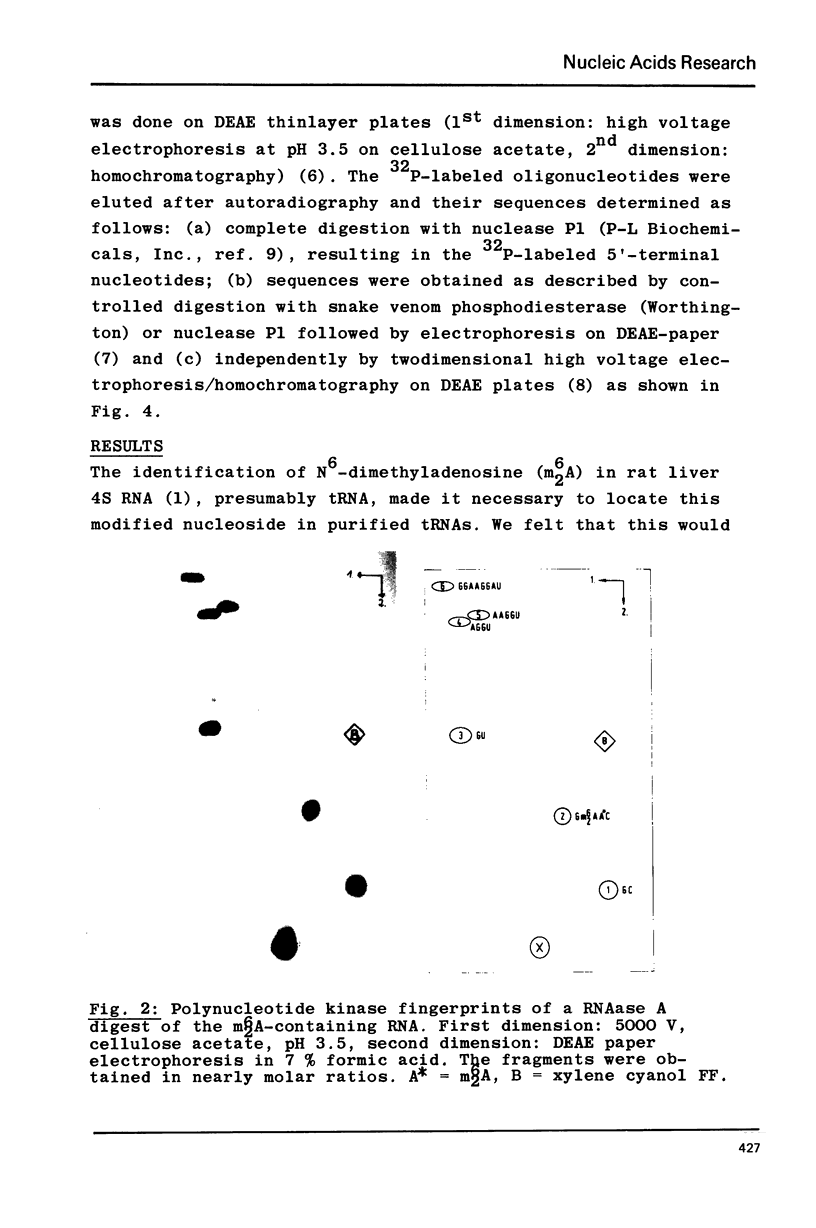
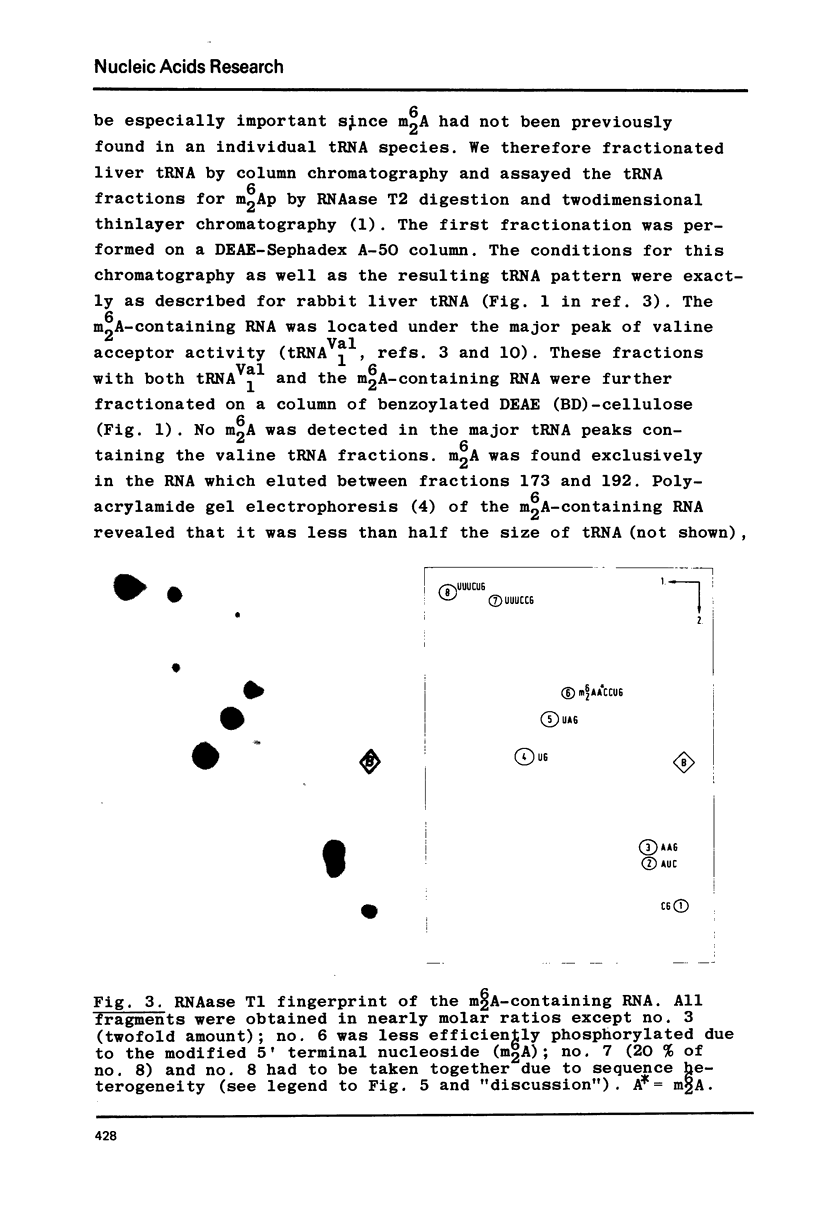
Crude tRNA isolated from rat liver by the method of Rogg et al. (Biochem. Biophys. Acta 195, 13-15 1969) contains N6-dimethyladenosine (m6-2A) and was therefore fractionated in order to identify the m6-2A-containing RNAs. A unique species of RNA was purified which contained all the m62A present in the crude tRNA. Sequence analysis by postlabeling with gamma-32p-ATP and polynucleotide kinase revealed that this RNA represents the 32 nucleotides AAGGUUUC(C)U GUAGGUGm62Am62ACCUGCGGAAGGAUC from position 5 to 36 of the 3' terminus of ribosomal 18S RNA. The 36 nucleotide long sequence from the 3' end of rat liver 18S rRNA exhibits extensive homology with the corresponding sequence of E. coli 16S rRNA and with the 21 nucleotide long 3' terminal sequence so far known from Saccharomyces carlsbergensis 17S rRNA. A heterogeneity in this sequence provides the first evidence on the molecular level for the existence of (at least) two sets of redundant ribosomal 18S RNA genes in the rat.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheim N., Southern E. M. Heterogeneity of the ribosomal genes in mice and men. Cell. 1977 Jun;11(2):363–370. doi: 10.1016/0092-8674(77)90053-8. [DOI] [PubMed] [Google Scholar]
- Baralle F. E. Complete nucleotide sequence of the 5' noncoding region of rabbit beta-globin mRNA. Cell. 1977 Apr;10(4):549–558. doi: 10.1016/0092-8674(77)90088-5. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M. A very large repeating unit of mouse DNA containing the 18S, 28S and 5.8S rRNA genes. Cell. 1977 Aug;11(4):795–805. doi: 10.1016/0092-8674(77)90292-6. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Dahlberg J. E. Binding of ribosomal protein S1 of Escherichia coli to the 3' end of 16S rRNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2940–2944. doi: 10.1073/pnas.72.8.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgarno L., Shine J. Conserved terminal sequence in 18SrRNA may represent terminator anticodons. Nat New Biol. 1973 Oct 31;245(148):261–262. doi: 10.1038/newbio245261a0. [DOI] [PubMed] [Google Scholar]
- Dasgupta R., Shih D. S., Saris C., Kaesberg P. Nucleotide sequence of a viral RNA fragment that binds to eukaryotic ribosomes. Nature. 1975 Aug 21;256(5519):624–628. doi: 10.1038/256624a0. [DOI] [PubMed] [Google Scholar]
- De Jonge P., Klootwijk J., Planta R. J. Sequence of the 3'-terminal 21 nucleotides of yeast 17S ribosomal RNA. Nucleic Acids Res. 1977 Oct;4(10):3655–3663. doi: 10.1093/nar/4.10.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Mackie G. A., Zimmermann R. A., Ebel J. P., Fellner P. Primary sequence of the 16S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1975 Feb;2(2):265–278. doi: 10.1093/nar/2.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Sänger H. L. Comparative oligonucleotide fingerprints of three plant viroids. Nucleic Acids Res. 1977 Jun;4(6):2021–2028. doi: 10.1093/nar/4.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood S. M., Kennedy D. S., Bester A. J. Separation of specific initiation factors involved in the translation of myosin and myoglobin messenger RNAs and the isolation of a new RNA involved in translation. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2428–2431. doi: 10.1073/pnas.71.6.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jank P., Shindo-Okada N., Nishimura S., Gross H. J. Rabbit liver tRNA1Val:I. Primary structure and unusual codon recognition. Nucleic Acids Res. 1977 Jun;4(6):1999–2008. doi: 10.1093/nar/4.6.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B. R. '2S' RNA, a new ribosomal RNA component in cultured Drosophila cells. FEBS Lett. 1974 Aug 15;44(1):39–42. doi: 10.1016/0014-5793(74)80301-7. [DOI] [PubMed] [Google Scholar]
- Legon S. Characterization of the ribosome-protected regions of 125I-labelled rabbit globin messenger RNA. J Mol Biol. 1976 Sep 5;106(1):37–53. doi: 10.1016/0022-2836(76)90299-0. [DOI] [PubMed] [Google Scholar]
- Mayr U., Bermayer H. P., Weidinger G., Jungwirth C. Release of interferon-induced translation inhibition by tRNA in cell-free extracts from mouse erythroleukemia cells. Eur J Biochem. 1977 Jun 15;76(2):541–551. doi: 10.1111/j.1432-1033.1977.tb11624.x. [DOI] [PubMed] [Google Scholar]
- Philipps G. R. Analysis of purified tRNA species by polyacrylamide gel electrophoresis. Anal Biochem. 1971 Dec;44(2):345–357. doi: 10.1016/0003-2697(71)90220-x. [DOI] [PubMed] [Google Scholar]
- Randerath E., Yu C. T., Randerath K. Base analysis of ribopolynucleotides by chemical tritium labeling: a methodological study with model nucleosides and purified tRNA species. Anal Biochem. 1972 Jul;48(1):172–198. doi: 10.1016/0003-2697(72)90181-9. [DOI] [PubMed] [Google Scholar]
- Raymondjean M., Bogdanovsky D., Bachner L., Kneip B., Schapira G. Regulation of messenger RNA by a ribonucleic factor in the presence of polyamines. FEBS Lett. 1977 Apr 15;76(2):311–315. doi: 10.1016/0014-5793(77)80175-0. [DOI] [PubMed] [Google Scholar]
- Rogg H., Wehrli W., Staehelin M. Isolation of mammalian transfer RNA. Biochim Biophys Acta. 1969 Nov 19;195(1):13–15. doi: 10.1016/0005-2787(69)90597-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Identical 3'-terminal octanucleotide sequence in 18S ribosomal ribonucleic acid from different eukaryotes. A proposed role for this sequence in the recognition of terminator codons. Biochem J. 1974 Sep;141(3):609–615. doi: 10.1042/bj1410609a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steege D. A. 5'-Terminal nucleotide sequence of Escherichia coli lactose repressor mRNA: features of translational initiation and reinitiation sites. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4163–4167. doi: 10.1073/pnas.74.10.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Steege D. A. Characterization of two mRNA-rRNA complexes implicated in the initiation of protein biosynthesis. J Mol Biol. 1977 Aug 25;114(4):545–558. doi: 10.1016/0022-2836(77)90177-2. [DOI] [PubMed] [Google Scholar]
- Van de Voorde A., Contreras R., Rogiers R., Fiers W. The initiation region of the SV40 VP1 gene. Cell. 1976 Sep;9(1):117–120. doi: 10.1016/0092-8674(76)90057-x. [DOI] [PubMed] [Google Scholar]
- Wildenauer D., Gross H. J. Methyldeficient mammalian 4s RNA: evidence for L-ethionine-induced inhibition of N6-dimethyladenosine synthesis in rat liver tRNA. Nucleic Acids Res. 1974 Feb;1(2):279–288. doi: 10.1093/nar/1.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]