Abstract
DP1, a dimerization partner protein of the transcription factor E2F, is known to inhibit Wnt/β-catenin signalling along with E2F, although the function of DP1 itself was not well characterized. Here, we present a novel dual regulatory mechanism of Wnt/β-catenin signalling by DP1 independent from E2F. DP1 negatively regulates Wnt/β-catenin signalling by inhibiting Dvl–Axin interaction and by enhancing poly-ubiquitination of β-catenin. In contrast, DP1 positively modulates the signalling upon Wnt stimulation, via increasing cytosolic β-catenin and antagonizing the kinase activity of NLK. In Xenopus embryos, DP1 exerts both positive and negative roles in Wnt/β-catenin signalling during anteroposterior neural patterning. From subcellular localization analyses, we suggest that the dual roles of DP1 in Wnt/β-catenin signalling are endowed by differential nucleocytoplasmic localizations. We propose that these dual functions of DP1 can promote and stabilize biphasic Wnt-on and Wnt-off states in response to a gradual gradient of Wnt/β-catenin signalling to determine differential cell fates.
Keywords: DP1, neural patterning, NLK, Wnt, Xenopus
Introduction
Wnt/β-catenin signalling regulates cell proliferation, differentiation and fate decision during embryonic development and tissue homoeostasis in the adult (Wodarz and Nusse, 1998). The signalling is initiated by Wnts, a family of lipid-modified glycoproteins, which are secreted and bind to cell surface Frizzled and LRP5/6 receptors to stimulate an intracellular signalling pathway. In the absence of Wnt, β-catenin levels are kept low by the destruction complex, comprised of Axin, APC and GSK3β, which phosphorylates β-catenin to promote its ubiquitination and proteosomal degradation (MacDonald et al, 2009). Axin is a scaffolding protein and acts as a rate-limiting factor of Wnt/β-catenin signalling because its concentration remains much lower than those of the other signalling components; thus the signalling can be perturbed greatly with relatively slight changes in Axin's concentration or activity (Lee et al, 2003). In the presence of Wnt, Dishevelled (Dvl) binds to the cytosolic side of the Frizzled receptor, recruits the destruction complex via binding to Axin, stimulates phosphorylation of LRP6 through GSK3β, and promotes dissociation of the destruction complex. These sequential events result in accumulation of cytosolic β-catenin. The accumulated β-catenin enters into nucleus to initiate transcriptional responses with the help of TCF/LEF transcription factor (Clevers, 2006). It has been commonly accepted that the interaction of Dvl with Axin is one of the crucial steps to transmit Wnt/β-catenin signalling from Wnt receptors to the destruction complex (Malbon and Wang, 2006).
Dimerization Partner 1 (DP1) is known to function as a binding partner of the E2F transcription factor. DP1 forms heterodimers with E2F and enhances both DNA binding and transactivation activities of the latter. The E2F/DP1 heterodimer regulates cell-cycle progression, DNA replication and apoptosis (Hitchens and Robbins, 2003; van den Heuvel and Dyson, 2008). Both E2F and DP1 contain DNA binding and heterodimerization domains. However, DP1, unlike E2F, has neither transactivation nor regulatory domains for pocket proteins (Rowland and Bernards, 2006). Moreover, subcellular localization of DP1 is governed by E2F, as DP1 lacks functional NLS (nuclear localization sequence) and NES (nuclear export sequence) (Magae et al, 1999). Thus, most DP1 studies were focused on its regulation of E2F activity whereas much less attention was given to the E2F-independent functions of DP1. Recently, the involvement of DP1 in the regulation of Wnt/β-catenin signalling was addressed by two independent studies in Drosophila (DasGupta et al, 2005; Morris et al, 2008). However, the two studies supported opposite functions of DP1 in the control of the signalling. In one study using the Drosophila S2 cell line, DP1 was identified as a positive regulator of Wnt/β-catenin signalling (DasGupta et al, 2005). In the other study, DP1 was suggested to assist E2F1 in the transcriptional induction of two negative regulators of Wnt/β-catenin signalling, Axin2 and Siah1, to promote β-catenin degradation (Morris et al, 2008). Therefore, the exact molecular mechanisms by which DP1 regulates Wnt/β-catenin signalling continue to be controversial. In addition, it might be intriguing to explore whether DP1 has E2F-independent functions.
Here, we investigated the ability of DP1 to regulate Wnt/β-catenin signalling in vertebrate systems, using mammalian cell culture and Xenopus embryos as well as in invertebrate, Drosophila. Unexpectedly, we found that DP1 played both positive and negative roles in Wnt/β-catenin signalling in an E2F-independent manner. We also addressed the molecular mechanisms of the dual regulatory functions of DP1, and our results suggest that these dual roles of DP1 in the regulation of Wnt/β-catenin signalling could explain how Wnt-on and Wnt-off states are stabilized during anteroposterior neural patterning in Xenopus.
Results
DP1 plays dual roles in the Wnt/β-catenin signalling pathway
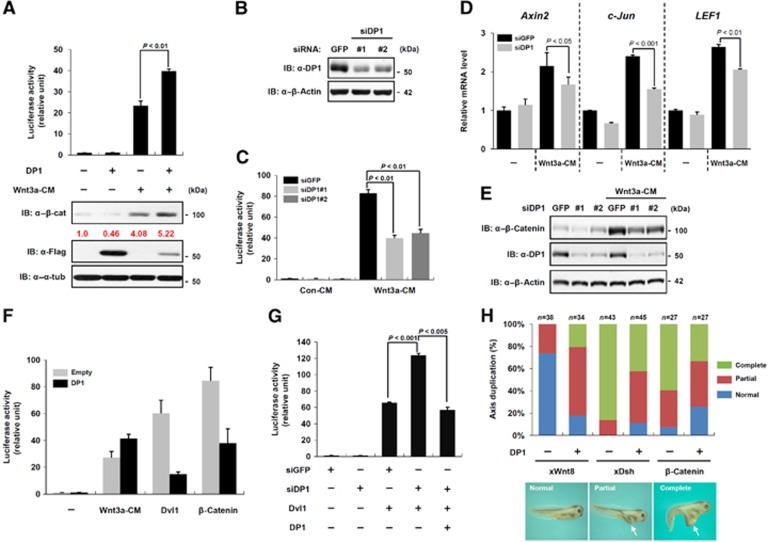
A recent study using genome-wide RNAi screening in a Drosophila cell line identified DP1 as one of novel positive regulators in the Wnt/β-catenin signalling pathway (DasGupta et al, 2005). However, in another study, DP1 was suggested to negatively regulate the signalling as a co-factor of E2F1 (Morris et al, 2008). In view of these discrepancies, we investigated molecular mechanisms of DP1 function in Wnt/β-catenin signalling. To examine the mechanisms by which DP1 affects Wnt/β-catenin signalling and thereby can modulate the signalling either positively or negatively, we first measured the Wnt-reporter (TOP-FLASH) activity in HEK293T cell. DP1 augmented the Wnt3a conditioned media (CM) or Wnt3a plasmid-mediated increases of TOP-FLASH activity and cytosolic β-catenin level (Figure 1A; Supplementary Figure S1A and B). We also assessed loss-of-function effects of DP1 on TOP-FLASH activity by using two small interference RNAs (siRNA) targeting different regions of human DP1. DP1 knockdown (Figure 1B) impeded Wnt3a-induced TOP-FLASH (Figure 1C), mRNA expression of Wnt-target genes such as Axin2, c-Jun and LEF1 (Fagotto et al, 1999; Hovanes et al, 2001; Jho et al, 2002) (Figure 1D) and the level of cytosolic β-catenin (Figure 1E) as well as E2F-dependent reporter activations in HEK293T cell (Supplementary Figure S1C). There were no off-target effects since co-expression of the siDP1-resistant mouse DP1 (mDP1) rescued DP1 knockdown effects on E2F-reporter activity (Supplementary Figure S1C). These results indicate that DP1 positively modulates Wnt/β-catenin signalling, in response to Wnt ligand stimulation. To further investigate the underlying mechanisms by which DP1 regulates Wnt/β-catenin signalling, we performed additional analysis of its function regarding different Wnt signalling components. Unexpectedly, DP1 suppressed Dvl- and β-catenin-induced TOP-FLASH activities (Figure 1F; Supplementary Figure S1B), while it had no influence on the reporter activation mediated by the constitutively active form of β-catenin (β-catS37A) (Supplementary Figure S1D). Analysis of reporter activities using the luciferase construct driven by endogenous Axin2 or Cyclin D1 promoter (Axin2-luc or Cyclin D1-luc) further suggested that DP1 negatively regulates the signalling activity induced by β-catenin (Supplementary Figure S1E). In addition, DP1 knockdown augmented Dvl-induced reporter activity and the siRNA-resistant mDP1 rescued this increase (Figure 1G). These findings provide evidence that DP1 may exert both positive and negative roles in Wnt/β-catenin signalling, depending on the presence of a Wnt ligand.
Figure 1.
DP1 exerts both positive and negative roles in Wnt/β-catenin signalling. (A) TOP-FLASH activities and cytosolic β-catenin level were measured in HEK293T cells. Flag–DP1 was transfected and cells were treated with Wnt3a conditioned medium (Wnt3a-CM) for 20 h. Error bars indicate the standard deviations of three independent analyses. P<0.01. (B) Knockdown efficiencies of two siDP1 were measured by immunoblotting with anti-DP1 antibody. (C) TOP-FLASH activities were measured in cells transfected with siDP1 and treated with Wnt3a-CM. siGFP was transfected as a control. Data represent average values from one representative experiment performed in triplicate. Error bars indicate standard deviations of triplicate. (D) Quantitative real-time PCR analysis for the expression of Wnt-target genes (Axin2, c-Jun and LEF1) after knockdown of DP1 by siRNA in HEK293 cells treated with or without Wnt3a-CM. The quantities of indicated mRNA were normalized by β-actin. Data represent average values from one representative experiment performed in triplicate. Error bars indicate standard deviations of triplicate. (E) Knockdown of DP1 hampered Wnt3a-mediated stabilization of cytosolic β-catenin in HEK293 cells. (F) TOP-FLASH activities were measured in cells treated with Wnt3a-CM or transfected with plasmids as indicated. Data represent average values from one representative experiment performed in triplicate. Error bars indicate standard deviations of triplicate. (G) TOP-FLASH activities were measured in cells transfected with indicated plasmids. Data represent average values from one representative experiment performed in triplicate. Error bars indicate standard deviations of triplicate. Significant differences were evaluated either by P<0.001 or by P<0.005. (H) Axis duplication was assessed in Xenopus tadpole embryo. mRNAs for xWnt8 (4 pg), xDsh (500 pg), β-catenin (500 pg) and DP1 (1 ng with β-catenin or 2 ng each with xWnt8 and xDsh) were injected as indicated. Arrows indicate duplicated axis. Each number of analysed embryos (n) is indicated.
To further confirm these differential activities of DP1 in Wnt/β-catenin signalling, we used a Xenopus axis duplication assay. In accordance with the reporter assay results, co-injection of mouse DP1 and Xenopus Wnt8 (xWnt8) mRNAs into one ventrovegetal blastomere of Xenopus embryos induced a secondary axis, which is indicative of the signalling activation, more frequently and strongly than injection of xWnt8 alone. In contrast, mDP1 significantly inhibited Xenopus Dishevelled (xDsh) or β-catenin-induced axis duplication (Figure 1H). Thus, DP1 seemed to modulate Wnt/β-catenin signalling activity both positively and negatively, also in Xenopus. The role of DP1 was specific to the Wnt/β-catenin signalling pathway because DP1 knockdown did not affect BMP4, Nodal, FGF-ERK or TNF-α signalling (Supplementary Figure S1F–J) (Ohkawara et al, 2004; Coulombe and Meloche, 2007). Collectively, these results suggest that DP1 is a bimodal regulator having both positive and negative functions in Wnt/β-catenin signalling.
Mechanisms for the negative role of DP1
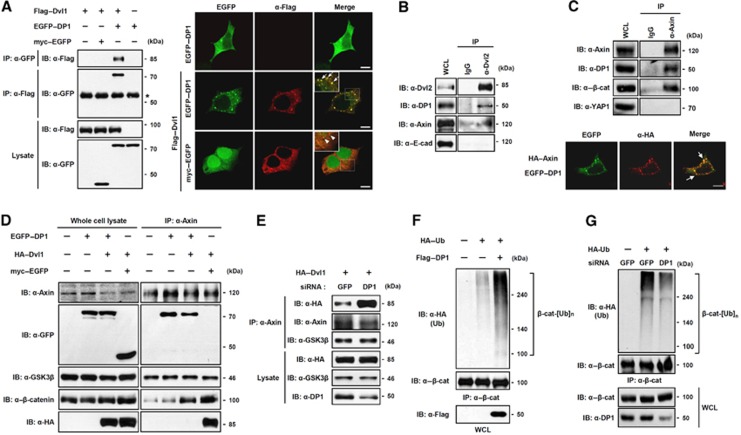
Next, we asked which component(s) of the Wnt/β-catenin signalling pathway DP1 interacts with. Since Dvl and β-catenin are key components of Wnt/β-catenin signalling and DP1 inhibited Dvl- or β-catenin-mediated activation of the signalling (Figure 1), we first tested interaction of DP1 with Dvl or β-catenin. By co-immunoprecipitation and in-vitro binding assays, we found that DP1 directly associated with Dvl1 but not with β-catenin (Figure 2A; Supplementary Figure S2A and B). We then analysed whether DP1 co-localized with Dvl. Ectopically expressed DP1 mainly localized in the cytoplasm diffusively without Dvl co-expression. However, co-expression of Dvl1 led to a punctate pattern of DP1 localization, coinciding with Dvl1 (Figure 2A) (Schwarz-Romond et al, 2007). Moreover, both ectopically expressed and endogenous Dvl were associated and co-localized with endogenous DP1, suggesting that Dvl interacts with DP1 in cell culture experiment (Figure 2B; Supplementary Figure S2C).
Figure 2.
DP1 inhibits Dvl–Axin interaction and enhances poly-ubiquitination of β-catenin. (A) DP1 associated with Dvl. Cells were transfected with plasmids as indicated and subjected to immunoprecipitation (IP) and immunofluorescence (IF). Asterisk indicates IgG heavy chain. Arrows indicate co-localized puncta between EGFP–DP1 and Flag–Dvl1. Scale bar, 10 μm. (B) Interaction between endogenous DP1 and Dvl. (C) IP was performed to show the interaction between endogenous DP1 and Axin (top). IF was performed in cells transfected with EGFP–DP1 and HA–Axin. Arrows indicate co-localized puncta (bottom). Scale bar, 10 μm. (D) HA–Dvl1 was co-transfected with either myc–EGFP or EGFP–DP1. IP was performed with anti-Axin antibody. (E) Cells expressing siGFP or siDP1 were transfected with HA–Dvl1. IP was performed with anti-Axin antibody. (F, G) HA–Ub was transfected with or without Flag–DP1 (F). HA–Ub was transfected with siGFP or siDP1 RNA (G). Cell lysates were immunoprecipitated with anti-β-catenin antibody and analysed by Western blotting using antibodies indicated.
Because the subcellular localization of DP1 has been known to depend on E2F (Magae et al, 1999), we examined whether Dvl interacted and co-localized with E2F proteins, through which it might affect DP1 localization. However, Dvl1 associated and co-localized only with DP1 but not with E2F proteins Supplementary Figure S2D and E), suggesting that DP1 interacted with Dvl independently of E2F.
We next tried to determine the interaction domains in DP1 and Dvl. Domain mapping studies with various Dvl mutants revealed that the C-terminal region of Dvl1 is necessary in order to interact with DP1 (Supplementary Figure S3A–C). We also found that both the N-terminal (aa 1–112) and heterodimerization (HD, 197–268) regions of DP1 were sufficient to associate with Dvl1 (Supplementary Figure S3D and E). Concordantly, only DP1 truncation mutants, having the ability to interact with Dvl1, showed clear co-localization with Dvl1 (Supplementary Figure S3F). Therefore, it can be concluded that DP1 binds to the C-terminal part of Dvl1 with its N-terminal and HD regions. The HD region of DP1 has been previously known to be required for its heterodimerization with E2F (Qiao et al, 2007). Thus, we suspected that Dvl might compete with E2F for binding to DP1. As expected, the interaction between E2F1 and DP1 was significantly inhibited by Dvl1 (Supplementary Figure S3G). Collectively, these results suggest that the HD region of DP1 was involved in the interaction with Dvl.
We next examined whether DP1 associated with Axin, because Axin forms a destruction complex with Dvl and β-catenin to negatively regulate Wnt/β-catenin signalling. As shown in Figure 2C, Axin was able to associate with DP1. Like Dvl, Axin could also drive localization of DP1 to cytoplasmic puncta (Figure 2C). Additionally, domain mapping analysis indicated that the HD domain of DP1 and the aa 531–723 region of Axin were required for their interaction (Supplementary Figure S4A–D). Together, these results suggest that DP1 and Axin interact in cells.
It has been reported that Dvl interacts with the C-terminal region of Axin containing the DIX domain (Schwarz-Romond et al, 2007) and we showed here that DP1 associated with the aa 531–723 region of Axin, indicating that the Dvl and DP1 interaction regions of Axin overlapped. We postulated that DP1 might compete with Dvl for Axin binding and thus might block the interaction between Dvl and Axin. Consistent with our hypothesis, co-expression of DP1 severely inhibited the interaction between Dvl1 and Axin (Figure 2D). This inhibitory effect was specific to the Axin–Dvl interaction since the GSK3β–Axin and β-catenin–Axin interactions were not affected (Figure 2D). Furthermore, addition of in vitro translated DP1 reduced the interaction between Axin and GST–Dvl in vitro (Supplementary Figure S4E). Consistently, reduction of DP1 levels by siDP1 significantly increased the interaction of Axin with Dvl but not with GSK3β (Figure 2E).
It has been commonly accepted that Wnt-induced association of Dvl and Axin is essential for the disruption of the β-catenin destruction complex, and thus for the stabilization of β-catenin by inhibiting its phosphorylation, ubiquitination and subsequent degradation. Our results prompted us to examine whether DP1 promoted poly-ubiqutination of β-catenin by inhibiting the interaction between Dvl and Axin. As expected, DP1 enhanced poly-ubiquitination of β-catenin (Figure 2F). Consistently, reduction of DP1 levels by siDP1 significantly reduced poly-ubiquitination of β-catenin (Figure 2G). To exclude the possible interpretation that β-catenin-associated proteins were ubiquitinated, we performed two-step immunoprecipitation experiment as described in our previous study (Kim and Jho, 2010). As shown in Supplementary Figure S4F, DP1 significantly elevated poly-ubiquitination of β-catenin but not of β-catenin-associated proteins.
Taken together, our data suggest that DP1 negatively modulates Wnt/β-catenin signalling via disrupting the interaction between Dvl and Axin, thereby enhancing poly-ubiquitination of β-catenin. This provides a possible underlying mechanism by which DP1 impeded Dvl- or β-catenin-mediated TOP-FLASH and axis-duplicating activities in the absence of Wnt ligand stimulation (Figure 1F–H; Supplementary Figure S1B and E).
Mechanisms for the positive role of DP1
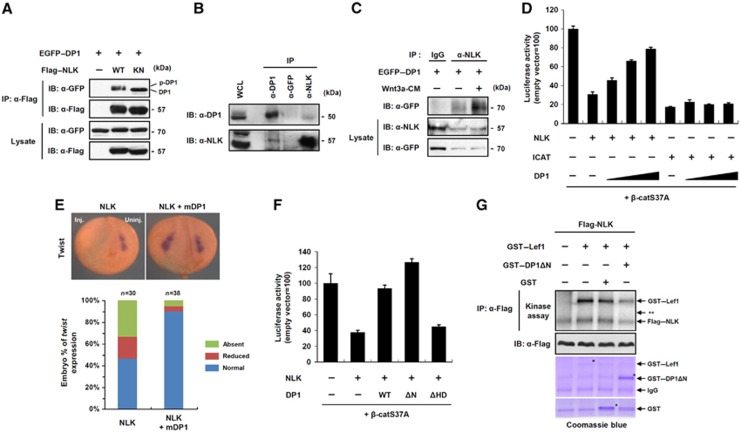
We showed that DP1 could also positively regulate Wnt/β-catenin signalling (Figure 1; Supplementary Figure S1). While ectopically expressed DP1 was mainly localized to the cytoplasm, endogenous DP1 was localized to both the nucleus and cytoplasm (Figure 2A; Supplementary Figure S2C). Interestingly, DP1 was mainly localized in the nucleus of trunk region of Xenopus embryos, whereas it was distributed throughout the cytoplasm in the cranial region (see for details in Figure 6A). Since DP1 negatively regulated the signalling in the cytoplasm (Figure 2), we hypothesized that DP1 might positively modulate Wnt/β-catenin signalling in the nucleus upon Wnt ligand stimulation. Because DP1 could not bind to β-catenin in an in-vitro system (Supplementary Figure S2B), we reasoned that the positive function of DP1 in the nucleus was not exerted directly on the β-catenin/TCF complex. An alternative way in which nuclear DP1 may execute its function would be to antagonize inhibitors operating on the β-catenin/TCF complex. NLK is a conserved serine-threonine protein kinase, which inhibits Wnt/β-catenin signalling by phosphorylating TCF/LEFs, resulting in dissociation of the β-catenin/TCF complex from the promoters of Wnt-target genes (Ishitani et al, 1999). Consistent with these findings, knockdown of NLK enhanced basal TOP-FLASH reporter activity (Supplementary Figure S5A). We found that NLK interacted with DP1 independently of its kinase activity in cell culture experiment (Figure 3A and B), and Wnt stimulation increased this interaction (Figure 3C). Moreover NLK, but not kinase-inactive NLK, led to the mobility shift of DP1 (Figure 3A). An in-vitro kinase assay and elimination of the mobility shift by treatment with λ phosphatase confirmed that DP1 was phosphorylated directly by NLK (Supplementary Figure S5B and C). DP1 contains five evolutionarily conserved putative NLK phosphorylation sites in its N-terminal region (aa 1–112) (Supplementary Figure S5D) and deletion of the region impaired the mobility shift of DP1 by NLK (Supplementary Figure S5C). We next asked whether DP1 antagonized the inhibitory functions of NLK in Wnt/β-catenin signalling. We used ICAT, another inhibitor of the β-catenin/TCF complex (Tago et al, 2000) as a control. Consistent with the previous reports, both ICAT and NLK inhibited TOP-FLASH activation induced by β-catS37A (Figure 3D). However, co-expression of DP1 rescued only the NLK-mediated suppression of TOP-FLASH activity but not that mediated by ICAT, in a dose-dependent manner, indicating that DP1 specifically acted against NLK to modulate Wnt/β-catenin signalling (Figure 3D). Next, we examined whether or not knockdown of DP1 affects TOP-FLASH activity induced by stable β-catenin (β-catS37A). Knockdown of DP1 reduced β-catS37A-mediated reporter activity (Supplementary Figure S5E).
Figure 3.
Nuclear DP1 antagonizes kinase activity of NLK thereby enhancing Wnt/β-catenin signalling. (A) Cells were transfected with EGFP–DP1 and co-transfected with either Flag–NLK WT or kinase-inactive mutant (KN). IP and immunoblotting were performed with indicated antibodies. DP1 and phospho-DP1 bands are indicated. (B) Endogenous interaction of DP1 with NLK. (C) Cells expressing EGFP–DP1 were stimulated with either control-CM (marked as —) or Wnt3a-CM for 6 h. IP was performed with anti-NLK antibody or IgG. (D) TOP-FLASH activities were measured from cells transfected with indicated plasmids. Data represent average values from one representative experiment performed in triplicate. Error bars indicate standard deviations of triplicate. (E) mRNAs for NLK (4 ng), mDP1 (1 ng) and β-galactosidase (200 pg), a lineage tracer, were unilaterally injected. Inj.: injected side; Uninj.: uninjected side. At neurula stage, embryos were subjected to in situ hybridization for Twist. (F) TOP-FLASH activities were measured in cells transfected with indicated plasmids. Data represent average values from one representative experiment performed in triplicate. Error bars indicate standard deviations of triplicate. (G) In-vitro kinase assay of immunoprecipitated Flag–NLK toward purified GST-fusion proteins in the presence of 10μCi of [γ32-ATP]. ** indicates the expected size of DP1Δ N.
To confirm the inhibitory role of DP1 against NLK in vivo, we utilized the Xenopus embryo assay. Injection of NLK mRNA into Xenopus embryos inhibited Twist expression, which is known as a Wnt-target gene during neural crest development (Borchers et al, 2001), and this inhibition was significantly rescued by co-injection of DP1 (Figure 3E). We next examined whether NLK-mediated phosphorylation of DP1 is necessary for its function to antagonize the NLK activity. We performed reporter assays with the phosphorylation-deficient mutant (DP1ΔN) and the NLK-interaction defective mutant (DP1ΔHD) (Figure 3F; Supplementary Figure S5F). While DP1ΔHD failed to restore the NLK-mediated suppression of TOP-FLASH activity, it was rescued significantly by both wild-type (WT) DP1 and DP1ΔN (Figure 3F). In addition, Wnt3a-mediated TOP-FLASH activity was enhanced by DP1ΔN, but not by DP1ΔHD (Supplementary Figure S5G). These findings suggest that only the interaction of DP1 with NLK, but not the phosphorylation of DP1 by NLK, is critical to antagonize the NLK-mediated inhibition of Wnt/β-catenin signalling. Because NLK inhibits Wnt/β-catenin signalling by phosphorylating TCF/LEFs, we therefore reasoned that DP1 might inhibit the kinase activity of NLK. NLK can auto-phosphorylate itself which is required for its kinase activity (Ishitani et al, 2011). We observed that both LEF1 phosphorylation and NLK auto-phosphorylation by the immunoprecipitated Flag–NLK protein were significantly hampered by the presence of purified GST–DP1ΔN (Figure 3G). Moreover, DP1 inhibited the NLK-induced mobility shift of TCF4 (Supplementary Figure S5H). Taken together, these results indicate that DP1 enhances Wnt/β-catenin signalling in the nucleus at least in part via antagonizing the kinase activity of NLK.
DP1 is required for Xenopus neural crest development
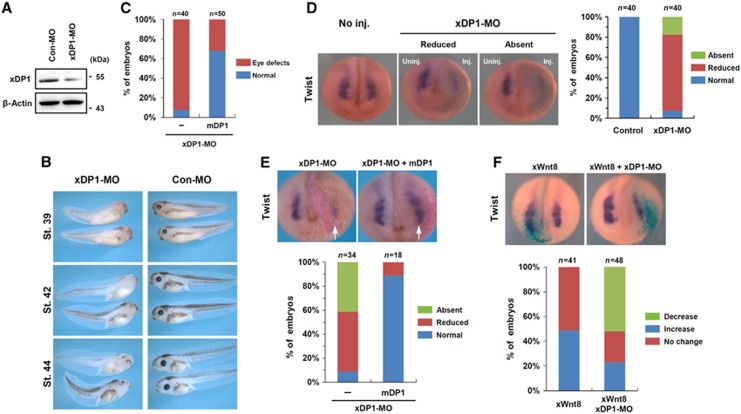
We next tried to elucidate the physiological relevance of the DP1 function in the regulation of Wnt/β-catenin signalling in Xenopus laevis embryos. Xenopus DP1 (xDP1) was ubiquitously expressed throughout embryonic development and its expression was not spatially restricted, as revealed by in situ hybridization and RT–PCR (Supplementary Figure S6A and B). We used a morpholino antisense oligonucleotide (MO) targeting xDP1 to address its endogenous functions during Xenopus embryogenesis (Figure 4A). Injection of xDP1-MO in dorsal animal blastomeres led to eye and pigment defects when observed at tailbud stages and these were rescued by co-injection of the morpholino-insensitive mouse DP1, confirming the specificity of xDP1-MO (Figure 4B and C). The eye and pigment defects were reminiscent of those elicited by perturbation of neural crest development (Nakata et al, 1997). Therefore, we investigated the effect of xDP1 knockdown on the expression of the Twist gene, which is a neural crest marker and a Wnt-target gene (Borchers et al, 2001). Injection of xDP1-MO led to a significant reduction in Twist expression and co-injection of mouse DP1 efficiently rescued Twist expression, suggesting that xDP1-MO is specific and the DP1 function is evolutionally conserved (Figure 4D and E). We further examined whether DP1 regulated Twist expression via modulating Wnt/β-catenin signalling. Wnt8 injection expanded Twist expression and co-injection of xDP1-MO significantly suppressed this increase (Figure 4F). Notably, Wnt/β-catenin signalling during early gastrulation was not affected by xDP1-MO injection, as revealed by Xbra and Gsc expression (Supplementary Figure S6C). Moreover, cell death was not appreciably increased or decreased by xDP1-MO at neurula stage, excluding non-specific effects (Supplementary Figure S6D). From these results, we conclude that DP1 is required for the modulation of Wnt/β-catenin signalling during Xenopus neural crest development.
Figure 4.
DP1 is required for Wnt/β-catenin signalling during Xenopus neural crest development. (A) Xenopus DP1 morpholino (xDP1-MO) working test. Animal caps injected with control (Con-MO, 60 ng) or xDP1-MO (60 ng) were lysed and examined by western blot with anti-mouse DP1 antibody or anti-β-actin antibody. (B) Bilateral injection of xDP1-MO (60 ng) produced eye and pigment defects. The phenotypes were assessed at stages 39, 42 and 44. (C) Morpholino-insensitive mouse DP1 rescued (68% normal, n=50) xDP1-MO induced eye defects (92.5% defective, n=40). Injection doses: xDP1-MO: 40 ng, Flag–mDP1 mRNA: 2 ng. (D) Unilateral injection of xDP1-MO (45 ng) inhibited Twist expression at neurula stage and (E) this was rescued by co-injection of mDP1 (1 ng). Arrows indicate the injected sides. (F) Injection of Wnt8 mRNA (10 pg) expanded Twist expression and this was reversed by co-injection of xDP1-MO (45 ng).
DP1 plays dual roles in anteroposterior neural patterning
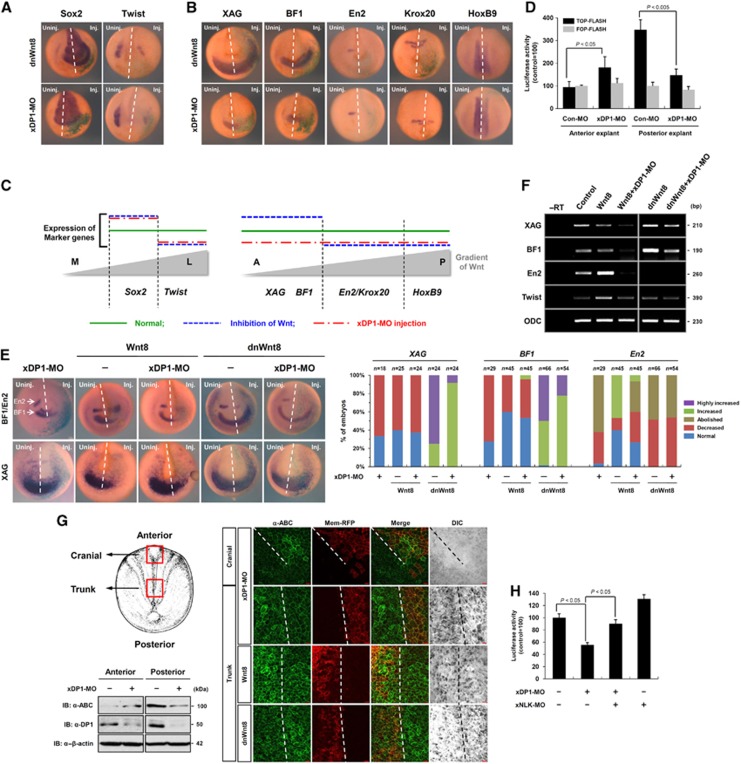
Although we found evidence that DP1 was a bimodal regulator having both positive and negative functions in Wnt/β-catenin signalling (Figures 1, 2, 3), depletion of DP1 in Xenopus embryos suggested that DP1 exerted only positive effects on the signalling during neural crest development (Figure 4). Therefore, we further investigated functions of DP1 in anteroposterior (A-P) neural patterning during which cells differentially respond to the Wnt/β-catenin signalling gradient depending on their relative positions along the A-P axis (Kiecker and Niehrs, 2001). Because DP1 enhanced and suppressed Wnt/β-catenin signalling when Wnt ligand was present or absent, respectively (Figures 1, 2, 3), we postulated that DP1 boosted the Wnt/β-catenin signalling activity in the Wnt-high posterior neural plate whereas it impeded the signalling activity in the Wnt-low (or Wnt-free) anterior neural plate. To test this hypothesis, we examined the effects of xDP1 depletion on the expression of anteroposterior neural markers such as XAG, BF1, En2, Krox20 and HoxB9. Inactivation of Wnt/β-catenin signalling by dnWnt8 injection led to the inhibition of the neural crest (Twist) and concomitant expansion of the neural plate (Sox2), as previously demonstrated (Heeg-Truesdell and LaBonne, 2006). Injection of xDP1-MO produced similar changes of Sox2 and Twist, reaffirming the positive functions of DP1 in Wnt/β-catenin signalling during Xenopus neural/neural crest development (Figure 5A). However, interestingly, xDP1-MO injection decreased expression of anterior neural markers, such as XAG and BF1, which were expanded by dnWnt8 injection (Figure 5B and C). Notably, the eye and head defects described in Figure 4B and C could be considered as anterior defects as well as neural crest defects. These findings suggest that DP1 negatively regulates Wnt/β-catenin signalling in the anterior neural plate where the signalling activity should be kept low for proper forebrain development (Lagutin et al, 2003). On the other hand, for posterior neural markers whose expression requires active Wnt/β-catenin signalling (Kiecker and Niehrs, 2001), xDP1-MO injection exerted similar effects as the dnWnt8 injection, such as inhibition of En2, Krox20 and HoxB9 (Figure 5B and C). Consistently, when the xDP1-MO was targeted to a posterior region (i.e., dorsal marginal injection of xDP1-MO), a significant portion of the MO-injected embryos showed posterior defects with shortened tail and body axes (Supplementary Figure S6E). Moreover, individual cell shape and overall structure of neural tube were disorganized along the spinal cord on the xDP1-MO injected side without changes in cell number (Supplementary Figure S6F). These results suggest that DP1 acts as a positive regulator of Wnt/β-catenin signalling in the posterior neural plate. In order to rule out the possibility that the observed phenotypes of DP1 morphants (especially the anterior defects) were due to weak perturbation of the maternal Wnt signalling, we tested whether DP1 DNA can rescue xDP1-MO-induced defects in both the anterior and the posterior tissues. Because transcription starts from mid-blastula stage, DP1 DNA should be unable to rescue DP1 morphants phenotypes if they were related to the maternal Wnt signalling. However, DP1 DNA rescued DP1-MO-induced anterior and posterior defects (Supplementary Figure S6G and H), reaffirming that the observed phenotypes of DP1 morphants were derived from the dual regulatory effects of DP1 on the zygotic anteroposterior Wnt signalling.
Figure 5.
DP1 exerts dual regulatory functions in Wnt/β-catenin signalling during Xenopus anteroposterior neural patterning. (A, B) Comparison between dominant-negative Wnt8 (dnWnt8) mRNA and xDP1-MO injected neurula embryos. Injected side (Inj.) was visualized by X-gal staining (green). Dashed lines indicate midlines. (A) Sox2, Twist; (B) XAG, BF1, En2, Krox20 and HoxB9 expression were assessed by in situ hybridization. (C) Summary of the results in (A, B). M stands for middle, L for lateral, A for anterior and P for posterior. (D) TOP-FLASH and FOP-FLASH activities were assessed from anterior or posterior dorsal explants at stage 13. Samples were separated into three pools of 10 explants each for analysis in triplicate (see Materials and methods, Xenopus embryos manipulation). Error bars indicate standard deviations. (E) Combinatorial effects of Wnt8 (10 pg) or dnWnt8 (1 ng) with xDP1-MO (45 ng) on BF1, En2 and XAG expression. To categorize as ‘Highly increased’ in case of XAG and BF1, both increased level of expression and posteriorward shift were considered. Similar results were obtained from three independent experiments. (F) Effects of xDP1-MO (60 ng) on Wnt8 (10 pg) or dnWnt8 (2 ng) mediated changes of XAG, BF1, En2 and Twist expressions analysed by semi-quantitative RT–PCR. ODC was used as a loading control. The data are representative of three independent experiments. (G) ABC levels were assessed by western blot and immunostaining of dorsal explants with anti-ABC antibody (1:250, Millipore) (see Supplementary data for detail). Dashed lines indicate supposed midlines. Mem-RFP was used as a lineage tracer. Scale bar, 20 μm. (H) TOP-FLASH activities were assessed from posterior dorsal explants at stage 13. xDP1-MO (60 ng) and xNLK-MO (10 ng) were injected as indicated. Samples were separated into three pools of ten explants each for statistical analysis (see Materials and methods, Xenopus embryos manipulation). Error bars indicate standard deviations.
To determine whether these effects of DP1 knockdown were directly related to the modulation of Wnt/β-catenin signalling, we further examined TOP-FLASH and reporter activities of a Wnt-target gene, siamois (Sia-luc). Consistent with the changes of the anteroposterior neural markers, depletion of DP1 activated TOP-FLASH and Sia-luc in anterior dorsal explants but inhibited the activities of reporters in posterior dorsal explants (Figure 5D; Supplementary Figure S6I), suggesting dual roles of DP1 in the regulation of Wnt/β-catenin signalling during anteroposterior neural patterning.
To further gain insight into the dual functions of DP1, we analysed combinatorial effects of xDP1-MO with Wnt8 or dnWnt8. Loss of DP1 considerably suppressed both the Wnt8-mediated expansion of En2 and the dnWnt8-mediated XAG and BF1 expansions (Figure 5E and F), suggesting the requirements of DP1 both in the activation and the inhibition of Wnt/β-catenin signalling during Xenopus A-P neural patterning. As shown in Figure 1E, loss of DP1 suppressed Wnt3a-induced augmentation of cytosolic β-catenin level in HEK293T cells. Concordantly, we found that activated β-catenin (ABC) was increased in the anterior (cranial) region and concomitantly decreased in the posterior (trunk) region by xDP1-MO injection (Figure 5G). Furthermore, xDP1-MO-mediated suppression of TOP-FLASH in the posterior explants was rescued significantly by xNLK-MO co-injection (Figure 5H). Although changes of anteroposterior neural markers upon xNLK knockdown were not reversely correlated with those by xDP1-MO (Supplementary Figure S6J), we speculate that these were because NLK has pleiotrophic effects on various signalling pathways, including MAPK, Notch, FOXO and TGF-β signalling pathways (Ohkawara et al, 2004; Coulombe and Meloche, 2007; Ishitani et al, 2010; Kim et al, 2010). However, suppression of xNLK phosphorylation by Wnt was rescued by xDP1-MO in posterior neural plate (Supplementary Figure S6K), suggesting that Wnt-DP1 signalling still suppresses NLK activity in vivo. Overall, these results demonstrate that DP1 plays dual roles in the regulation of Wnt/β-catenin signalling during Xenopus anteroposterior neural patterning partly via controlling cytosolic or ABC levels and suppressing the kinase activity of NLK.
Differential nucleocytoplasmic localization of DP1
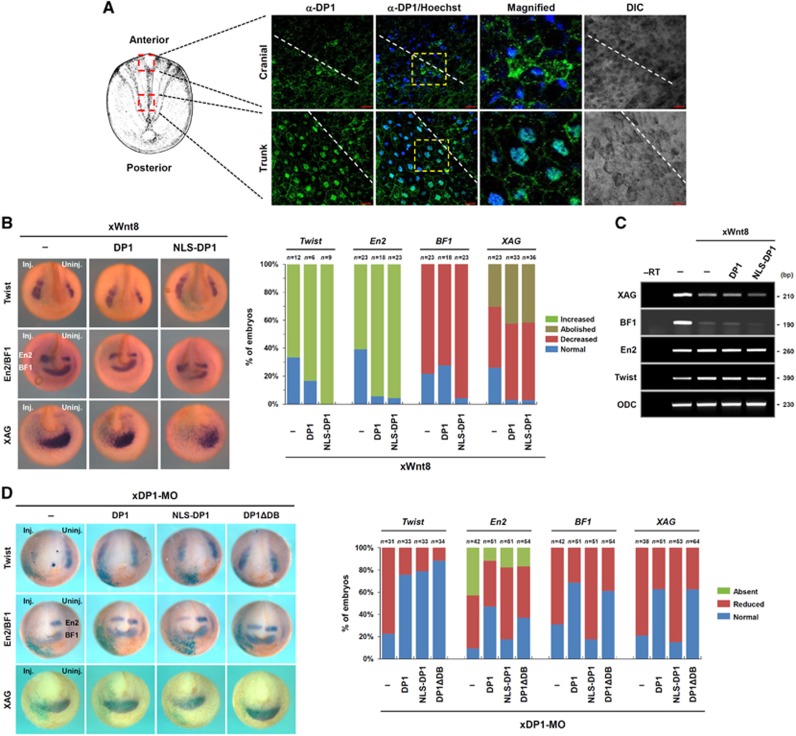
Since the results described in Figures 1, 2, 3 suggest that DP1 bimodally enhances or represses Wnt/β-catenin signalling via its distinct localizations, we therefore generated artificial NLS- and NES-fused DP1 to further explore the functions of cytosolic and nuclear DP1 (Supplementary Figure S7A). NLS-DP1 did not interact with Dvl1 or Axin unlike WT DP1. We also observed that NES-DP1 co-localized with Dvl1 in similar to WT DP1, whereas it has no influence on the expression pattern of NLK (Supplementary Figure S7B). We observed that NLS-DP1 had a stronger ability to augment Wnt3a-mediated TOP-FLASH activation than that of WT DP1 whereas NES-DP1 did not enhance the reporter activity (Supplementary Figure S7C). Moreover, NES-DP1 still impeded the β-catenin-induced reporter activation (Supplementary Figure S7D). Consistent with our hypothesis NLS-DP1 did not induce the poly-ubiquitination of β-catenin (Supplementary Figure S7E). We next asked whether NLS-DP1 and WT DP1 differentially regulates Dvl- or β-catenin-induced reporter activity. Surprisingly, NLS-DP1, unlike WT DP1, enhanced Dvl- or β-catenin-induced reporter activity (Supplementary Figure S7F). To further confirm these results, we injected suboptimal doses of xWnt8 or xDsh mRNA into the ventrovegetal region of the early Xenopus embryo. Although DP1 or NLS-DP1 alone did not induce the secondary axis, NLS-DP1 exerted a stronger positive effect on xWnt8- or xDsh-mediated axis duplication than WT DP1 did (Supplementary Figure S7G). Overall, these findings suggest that the dual roles of in Wnt/β-catenin signalling are determined by differential nucleocytoplasmic localizations of DP1.
We next examined subcellular localizations of DP1 in dorsal explants of the early neurula Xenopus embryo by confocal microscopy (see Supplementary Figure S7H for antibody specificity). Strikingly, DP1 predominantly displayed nuclear localization in the trunk region of dorsal explants, whereas it was distributed throughout the cell cytoplasm in the cranial region (Figure 6A). To further confirm the physiological significances of nuclear/cytoplasmic DP1s in the control of Wnt/β-catenin signalling, we tested effects of WT DP1 and NLS-DP1 on the expression of various neural markers in Wnt8 injected embryos. We found that NLS-DP1 had a tendency to boost forced Wnt8 signalling more strongly than WT DP1 (Figure 6B and C). More importantly, we found that NLS-DP1 could not restore anterior neural markers decreased by xDP1-MO while WT DP1 rescued those expressions significantly (Figure 6D). These results suggest that nuclear DP1 is only sufficient to the activation of Wnt/β-catenin signalling during posterior neural development whereas cytoplasmic DP1 is important for the inhibition of the signalling during anterior neural development.
Figure 6.
Differential nucleocytoplasmic localizations of DP1 confer its dual roles in Wnt/β-catenin signalling during Xenopus anteroposterior neural patterning. (A) Subcellular localization of DP1 was assessed from early neurula (stages 13–14) Xenopus dorsal explants. Dashed lines indicate midlines of the explants. Scale bar, 20 μm. (B, C) Combinatorial effects of Wnt8 with WT DP1 (DP1) or NLS-DP1 on Twist, En2, BF1 and XAG expression analysed by in situ hybridization (B) and RT–PCR (C). Similar results were obtained from three independent experiments. (D) Rescuing effects of Flag-tagged forms of WT DP1, NLS-DP1 and DNA-binding domain deficient DP1 (ΔDB) on xDP1-MO induced changes of markers (Twist, En2, BF1 and XAG). Injection doses: Flag–DP1, Flag–NLS-DP1, Flag–DP1ΔDB: 2 ng, xDP1-MO: 40 ng.
We also investigated whether the dual roles of DP1 depended on E2F during anteroposterior neural patterning. Several reports have shown that the DNA-binding domain of DP1 is required to enhance the transcriptional activity of E2F (Rowland and Bernards, 2006). However, the DP1 mutant with deleted DNA-binding domain (ΔDB) was still able to restore expression of neural markers decreased by xDP1-MO and enhance Wnt3a-mediated TOP-FLASH activity as appreciably as was WT DP1 (Figure 6D; Supplementary Figure S5G). Moreover, DP1 depleted embryos showed no change in apoptosis (Supplementary Figure S6D), the process which DP1-E2F has been demonstrated to be involved in (Hitchens and Robbins, 2003). Overall, these findings suggest that differential nucleocytoplasmic localizations of DP1 contribute to its dual roles in Wnt/β-catenin signalling during Xenopus anteroposterior neural patterning in an E2F-independent manner. However, it cannot be excluded that some, although marginal, activity of E2F-dependent DP1 function contribute to the posterior development (Supplementary Figure S6E). But for anterior development, DP1 must work E2F independently, because anterior DP1 function is dependent on its cytosolic localization (Figure 6A) and NLS-DP1 cannot rescue anterior defective phenotypes of DP1 morphants (Figure 6D).
Functions of DP1 in Wg signalling during Drosophila wing development
While the present study suggests DP1 as a bimodal regulator of Wnt/β-catenin signalling, having both positive and negative functions, two previous studies proposed DP1 as either a positive (DasGupta et al, 2005) or a negative (Morris et al, 2008) regulator of Wg signalling (Drosophila counterpart of the vertebrate Wnt/β-catenin signalling). In view of these discrepancies, we additionally assessed functions of DP1 in Wg signalling during Drosophila wing development. Interestingly, we found that both gain and loss of dDp1/dE2F1, but not dE2F2, led to the same results: suppression of Wg-target genes, Sens and Dll, in wing imaginal discs (although the reduction of Dll was not severe) accompanied by developmental defects of wing morphology (Supplementary Figure S8). Thus, the seemingly controversial conclusions between the two previous studies might be derived from different experimental approaches, either the gain-of-function or loss-of-function strategy. Nevertheless, there is a critical difference between vertebrate DP1 (Figure 7) and the Drosophila counterpart (Supplementary Figure S8). In HEK293T cells and Xenopus embryos, we showed that DP1 functioned independently of E2F in Wnt/β-catenin signalling (Figure 6D; Supplementary Figure S5G). However, in Drosophila, dDp1 seems to act together with dE2F1 in Wg signalling because gain and loss of dE2F1 produced phenotypes similar to those caused by dDp1 overexpression and knockdown (Supplementary Figure S8).
Figure 7.
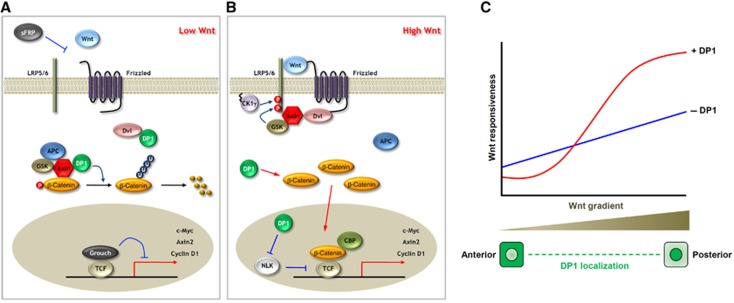
Model for dual regulatory functions of DP1. (A) In the absence or below the activation threshold of Wnt, DP1 prevents from aberrant (noisy) activation, by interfering Dvl–Axin interaction. Therefore, the destruction complex remains intact and it facilitates β-catenin degradation. This negative function of DP1 stabilizes Wnt-off state. (B) In the presence or above the activation threshold of Wnt, DP1 further enhances the signalling via increasing cytosolic β-catenin level and antagonizing kinase activity of NLK. The mechanism how and the location where DP1 increases β-catenin level are uncertain. Nevertheless, this positive function stabilizes Wnt-on state. (C) Morphogen gradient of Wnt is translated into biphasic on and off states by the proposed dual regulatory functions of DP1. In general term, morphogen gradient can be translated into biphasic states by the action of on/off states stabilizer. This would in turn make sharp boundary between cells and allow them to take different cell fates.
Discussion
The present study reveals novel dual regulatory mechanisms of DP1 in Wnt/β-catenin signalling. In the absence of a Wnt ligand, DP1 further decreases Wnt/β-catenin signalling activity by inhibiting Dvl–Axin interaction and enhancing poly-ubiquitination of β-catenin. However, in the presence of a Wnt, DP1 enhances the signalling by increasing cytosolic β-catenin levels and antagonizing nuclear NLK activity (Figure 7A and B). We suggest that such a dual regulatory mechanism can explain how Wnt activity gradient is efficiently translated into biphasic on/off states and how Wnt/β-catenin signalling regulates differential cell fate decision during development (Figure 7C).
E2F is known to regulate A-P patterning of Xenopus embryo (Suzuki and Hemmati-Brivanlou, 2000). However, there are several differences between E2F-dependent A-P patterning and DP1-regulated A-P patterning. First, E2F was previously known to negatively regulate Wnt signalling pathway through the transcriptional activation of Axin2. However, dnE2F overexpression does not affect anterior development of Xenopus embryo (Suzuki and Hemmati-Brivanlou, 2000), suggesting that E2F does not negatively regulate Wnt signalling during anterior development. In this study, we provided compelling evidence that DP1 negatively regulate Wnt signalling both in Xenopus anterior neural plate (Figures 5 and 6) and in HEK293T cells (Figures 1 and 2). Moreover, the negative regulatory activity of DP1 against Wnt activation relies on its cytoplasmic or membranous localization (Figure 6A and D), which excludes its function as a transcriptional co-factor of E2F in the nucleus. Second, E2F promotes ventral and posterior development possibly through FGF and/or TGF-β signalling pathways (Suzuki and Hemmati-Brivanlou, 2000). However, in this study, we provided evidence that DP1 is required for the posterior development through Wnt signalling pathway (Figures 4, 5, 6) but not through FGF (Supplementary Figure S1I) or TGF-β (Supplementary Figure S1F–H) pathway. In this study, we provided evidence that DP1 regulates Wnt/β-catenin signalling in an E2F-independent manner (Figure 6D; Supplementary Figures S5G and S6D). Previous reports also suggested that DP1 could function independently of E2F in certain cases. Jooss et al (1995) showed that activated Ha-ras can co-operate with DP1 or DP2 in the transformation of rat embryo fibroblast. Further analysis of a dominant-negative and mutant DP1 proteins suggest that the DP1-mediated oncogenic activity is mediated independent from E2F. Masuhiro et al (2008) showed that interaction between DP1 and SOCS-3 in the cytoplasm eliminates the inhibitory action of SOCS-3 on LIF-stimulated JAK-STAT signalling. It is unlike that E2F/DP1 heterodimers blocks SOCS-3 activity in cytoplasm, although authors did not tested, since E2F1 is mainly localized in the nuclei. Given that DP1 can function independently from E2F1, the seemingly different results of E2Fs functions in Xenopus shown by Suzuki and Hemmati-Brivanlou (2000) and what we found about DP1 functions are not contradictory anymore.
Dp1-deficient mice die by embryonic day 12.5 owing to defects in the extra-embryonic, trophectoderm-derived compartments, whereas loss of E2F family members results in much less severe adult and neonatal phenotypes (Kohn et al, 2003). It also has been shown that endometrial Wnts regulate conceptus development and Wnt7A increases trophectoderm cell proliferation (Hayashi et al, 2007). Since we showed that DP1 regulates Wnt/β-catenin signalling independent from E2F, the phenotypes shown in Dp1-deficient mice might be partly due to perturbation of that signalling during implantation periods or during the proliferation of trophectoderm cells.
In the present study, we have provided several lines of evidence to support the hypothesis that differential nucleocytoplasmic localization of DP1 dictates its dual function. However, we also noticed that the differential localization dependent dual functions of a protein is not unique to DP1 but to several signalling proteins as well. One of them is GSK3β in Wnt/β-catenin signalling. GSK3β is a kinase that phosphorylates β-catenin in the cytosol, leading to the degradation and the inhibition of Wnt/β-catenin signalling in a resting state. However, when Wnt ligand is present, GSK3β translocates to the plasma membrane and phosphorylates cytoplasmic tail of LRP5/6. This phosphorylation is required for the initiation and maintenance of the signalling pathway (Fagotto et al, 1999; Zeng et al, 2008). Therefore, DP1 and GSK3β share a quite similar perspective; having both positive and negative functions in a same signalling pathway depending on their differential localization when the signalling is on-state and off-state, respectively. Besides these similarities between the dual functions of DP1 and GSK3β, there are critical differences as well. In GSK3β-mode of dual regulatory mechanisms of Wnt signalling, the positive function of GSK3β via phosphorylating LRP5/6 leads to the inhibition of its negative cytoplasmic function possibly by sequestering it inside the multivesicular bodies (Taelman et al, 2010). However, in DP1-mode of dual roles on Wnt signalling, we suggest that the positive and negative functions are separated (Figure 7). These critical differences would elicit very different outcomes in response to the mutations of these factors during development or in pathological conditions. For example, loss-of-function mutation in GSK3β would result in only the elevated Wnt signalling activity, because the positive function of GSK3β is only to inhibit its negative function on the signalling. Supporting this, it was reported that pharmacological inhibition of the kinase activity of GSK3β in early Xenopus embryo resulted in hyperdorsalization (Brummelkamp et al, 2002), which is a hallmark of Wnt/β-catenin signalling activation. Moreover, GSK3β has only negative functions on Wnt signalling in Xenopus anterior-specific neural patterning (Onai et al, 2004). However, the loss of DP1 would result in more complex outcomes compared with the loss of GSK3β, as it would elevate Wnt signalling in Wnt-low region and suppress signalling in Wnt-high region and thus would dampen the signalling gradient and/or make the boundary between regions of Wnt-high and -low to be vague. In this study, we demonstrated evidence to support this hypothesis (Figures 4, 5, 6). In conclusion, we distinguish DP1-mode of dual regulatory mechanisms from GSK3β-mode and place it as unique mechanism to stabilize both Wnt-on and Wnt-off states and to regulate differential cell fate decision during development.
Some other regulatory factors also contribute to the fine regulation of Wnt/β-catenin signalling activity, stabilizing on/off states and establishing the activity gradient. For example, during Xenopus neural patterning, the extracellular Wnt antagonist Dickkopf (Dkk) is expressed in the anterior endomesoderm where it counteracts against the Wnt gradient to sharpen the Wnt activity gradient along the A-P axis of the neural plate (Davidson et al, 2002). Moreover, a context-dependent dual regulator Kremen could stabilize the Wnt-on/-off states depending on Dkk availability. Kremen negatively regulates Wnt/β-catenin signalling in the anterior-most neural plate where Dkk is present (Davidson et al, 2002). However, Kremen enhances the signalling in the neural crest territory where Dkk is absent (Hassler et al, 2007). Thus, these context-dependent dual functions of Kremen as well as the counter gradient of Dkk help to distinguish differential cell fates between the Wnt-free anterior neural and the Wnt-active neural crest fates.
In this regard, an intriguing question is raised. Are there other dual role factors involved in differential cell fate decision in general? Interesting evidence came from the BMP signalling pathway. Twisted Gastrulation (Tsg) was first identified as a secreted protein in Drosophila (Mason et al, 1994). In following studies, Tsg was characterized as a context-dependent dual regulatory factor of BMP signalling during dorsoventral (DV) mesoderm patterning (Oelgeschlager et al, 2000; Larrain et al, 2001). In the dorsal side of the gastrula embryo, Tsg facilitates Chordin/BMP complex formation, leading to the inhibition of BMP signalling (Larrain et al, 2001). However, in the ventral side, Tsg binds to proteolytic fragments of Chordin produced by Xolloid, enhancing BMP signalling (Larrain et al, 2001). Thus, the positive and negative roles of Tsg in BMP signalling are dependent on the presence of Xolloid. These Xolloid-dependent dual regulatory functions of Tsg could stabilize both the on and off states of BMP signalling and could explain how the signalling regulates differential cell fate decision during DV patterning (Larrain et al, 2001). Taken together, given the dual regulatory functions of DP1 in the Wnt/β-catenin and those of Tsg in the BMP signalling pathways, we suggest that dual role factors, which can promote and stabilize the on/off states of a signalling pathway, might generally play essential roles in determining differential cell fates in response to the shallow signalling gradients.
Materials and methods
DNA, RNA, Morpholino, primary antibody
Full-length DP1 was PCR-amplified from cDNAs of E13.5 mouse embryos and inserted into pCMV4–Flag (Sigma), pEGFP–C1 (Clontech) and pGEX-4T (GE Healthcare) expression vectors. Deletion mutants of DP1 were generated by PCR using WT DP1. The NLS (nuclear localization signal) sequence (PKKKRKVD) presented within SV40-T and NES (nuclear localization signal) sequence (LALKLAGLDI) presented within SV40-T and PKI, respectively, were generated by oligonucleotide synthesis (Bioneer). Three copies of the NLS sequences and two copies of the NES sequences were fused to the N-terminus of DP1. HA-tagged human Dvl1 and its mutant constructs were kindly provided from Dr Kikuchi (Hiroshima University, Japan). Myc-tagged mouse Axin and mouse Dvl1–Flag were constructed as previously described (Fagotto et al, 1999). pCMV5–HA–Axin was a gift from Dr Lin (Xiamen University, China). HA-tagged E2F1 and E2F4 were gifted from Dr Helin (European Institute of Oncology, Italy) and E2F coding sequences obtained by PCR were subcloned into pEGFP–C1. Flag-tagged NLK and NLK-KN (K155M) constructs were generated as described previously (Ishitani et al, 1999). All constructs were confirmed by DNA sequencing. pSuper-8XTOP, and -FOP were kindly gifted from Dr Moon (University of Washington, USA). The lucifease construct driven by Cyclin D protmoter was kindly provided from Dr Baek (Seoul National University, Korea)
Oligonucleotides harbouring 19-mer shRNA sequences for human DP1 (5′-GGAGACTTGAAAGAATAAA-3′) were inserted into the pSUPER vector. Oligonucleotide of siDP1 duplexes was purchased by Samchully and its sequence is follow: siDP1#1, 5′-GGAGACTTGAAAGAATAAA-3′; siDP1#2, 5′-GGACCACTTCCTACAACGA-3′. siRNA oligonucleotide sequences for GFP was used as negative control (Tiscornia et al, 2003). shNLK expression vector was kindly provided from Dr Kato (University of Tokyo, Japan).
mRNAs, used for injection into Xenopus embryos, were synthesized using mMessage mMachine kit (Ambion) following the manufacturer's protocol. Antisense morpholino oligonucleotides were obtained from Gene Tools. The morpholino sequence of Xenopus DP1 (xDP1-MO) was generated as 5′-AGACCAGCATCTTTTGCCATGTTAC-3′. The Xenopus NLK morpholino (xNLK-MO) was generated as 5′-GCCCTTCCCTACACGGATGTCCCCC-3′ and its specificity was confirmed previously (Satoh et al, 2007).
Rabbit polyclonal anti-DP1 (K-20), mouse monoclonal anti-α-tubulin (TU-02), anti-GFP (B-2), anti-HA (F-7) and goat polyclonal anti-Lamin B (M-20) antibodies were obtained from Santa Cruz. Rabbit polyclonal anti-HA (Cat # A190-108A) antibody was purchased from Bethyl Laboratories. Mouse monoclonal anti-Flag (M-2) and anti-β-actin (Cat # A5441) antibodies were from Sigma. Mouse monoclonal anti-β-catenin (Cat # 610154) and anti-GSK3 (Cat # 610201) antibodies were purchased from BD Transduction Laboratories. Rabbit polyclonal anti-Axin were kindly provided from Dr Virshup (University of Utah, USA). Rabbit polyclonal anti-NLK antibody was described previously (Kanei-Ishii et al, 2004). Goat polyclonal anti-Axin (Cat # AF3287) and mouse monoclonal anti-DP1 (Cat # ab17807) antibody were purchased from R&D System and Abcam, respectively.
Cell culture and in-vitro assay
HEK293T and HEK293 cells were maintained in DMEM supplemented with 10% FBS. Cells were transfected with the indicated plasmids using the calcium phosphate or lipofectamine (WelGene) methods. For reporter assay, cells were transiently transfected with the indicated plasmids. Luciferase activity was measured with the Dual Luciferase Reporter Assay System (Promega). Results are presented as mean±s.d. from at least three independent experiments. For immunoprecipitation assays, transfected cells were lysed and the lysates were incubated with the indicated antibodies. Protein A/G plus agarose beads (Santa Cruz) were added and precipitated. For in-vitro binding assays, GST-fused proteins were expressed in BL21 bacterial cells and purified using glutathione-agarose beads (GE Healthcare). Proteins harbouring [S35]-labelled methonine (Met) were generated by an in-vitro translation system (Invitrogen). For in-vitro kinase assays, cells were lysed and the immunoprecipitated lysates were incubated with GST-fused proteins and 10 μCi of [γ32-ATP] for 3 min at 25°C. For detailed methods and reagents used, see Supplementary data.
Real-time quantitative and semi-quantitative RT–PCR analysis
For both quantitative and semi-quantitative RT–PCR analysis, total RNA was isolated using TRIZOL reagent (Invitrogen) according to the manufacturer's protocol. cDNA was synthesized from total RNA using ImProm-II Reverse Transcriptase (Promega) with random primer. For quantitative real-time PCR, each cDNA was analysed by ABI prism 7000 Sequence Detector (Applied Biosystem) with SYBR Green PCR Master Mix (Applied Biosystem). PCR products were designed to amplify specific mRNA fragment, and displayed unique dissociation (melting) curve. PCR conditions were 95°C (10 min) and 40 cycles of 95°C (30 s) and 60°C (1 min). The threshold cycle (Ct) value for each gene was normalized to the Ct value for β-actin. The relative mRNA expression was calculated using ΔΔCt method.
Primers used were: Axin2, 5′-TTATGCTTTGCACTACGTCCCTCCA-3′ and 5′-CGCAACATGGTCAACCCTCAGAC-3′ (Yan et al, 2001); c-Jun, 5′-GTCCACGGCCAACATGCTCA-3′ and 5′-TGTTTGCAACTGCTGCGTTAG-3′; LEF1, 5′-CATCCTCCAGCTCCTGATATC-3′ and 5′-CTGACCTTGCCAGCCAAGAG-3′ (Lee et al, 2010); β-actin, 5′-GCGGGAAATCGTGCGTGACATT-3′ and 5′-GATGGAGTTGAAGGTAGTTTCGTG-3′; XAG, 5′-CTGACTGTCCGATCAGAC-3′ and 5′-GAGTTGCTTCTCTGGCAT-3′; BF1, 5′-CTGGGCAACAACCATTCTTT-3′ and 5′-TTTGTCCAGCCAAAAGGTTC-3′; En2, 5′-ATGAGCAGAATAACAGGGAAGTGGA-3′ and 5′-CCTCGGGGACATTGACTCGGTGGTG-3′; Twist, 5′-AGTCCGATCTCAGTGAAGGGCA-3′ and 5′-TGTGTGTGGCCTGAGCTGTAG-3′; COD, 5′-CAGCTAGCTGTGGTGTGG-3′ and 5′-CAACATGGAAACTCACACC-3′.
Xenopus embryos manipulation
Eggs were obtained from X. laevis primed with 800 units of human chorionic gonadotropin (Sigma) and fertilized in vitro using macerated testis (Kim and Han, 2007). Nieuwkoop and Faber stages were considered for developmental staging (Nieuwkoop and Faber, 1967). Reagents were introduced into the fertilized embryos by microinjection. For axis duplication assay, mRNAs were introduced into the ventral-vegetal one blastomere of eight-cell stage embryo. Axis duplication was monitored at stage 37, and embryos were classified as complete axis duplication, partial axis duplication or normal (Seo et al, 2009). In situ hybridizations were carried out as described previously (Harland, 1991). Briefly, digoxigenin (DIG)-labelled antisense RNA probes were hybridized and the hybridizations were detected using alkaline phosphatase-conjugated anti-DIG antibody (Roche), stained with BM purple (Roche) as a substrate and photographed.
Immunofluorescence in Xenopus dorsal explants
ABC level and subcellular localization of xDP1 were assessed in dorsal explants of early neurula stage Xenopus embryos by immunostaining as previously described (Choi and Han, 2005). Two-cell stage embryos were injected with morpholino or mRNAs as indicated. Mem-RFP mRNA was used for lineage tracing and Hoechst 33342 (Sigma) was used to stain nucleus. At stages 13–14, dorsal explants were dissected, fixed in 4% paraformaldehyde and incubated in PBSTB (0.1% Triton X-100, 2% BSA in PBS) to block non-specific binding, followed by standard immunostaining procedures. The antibodies used were polyclonal anti-ABC antibody (1:250, Millipore), polyclonal anti-DP1 antibody (1:200, Santa Cruz) and Alexa488-conjugated anti-mouse and anti-rabbit secondary antibodies (1:150, Invitrogen). Using confocal laser-scanning microscopes (Zeiss and Olympus), images were obtained and analysed. For statistical analysis used in the luciferase assays with Xenopus embryonic tissues, injected embryos with each indicated material were divided into three pools by random sampling to represent a population of the same manipulation.
Supplementary Material
Acknowledgments
We thank Dr Frank Costantini and Inchul Yeo for critical discussion and Natalya Katanayeva for technical assistance. This work was supported by NRF grants funded by the MEST to E Jho (No. 2011-0019353 and 2010-0021106), to JK Han (No. 2009-0092829), TR-SFB11 (Deutsche Forschungsgemeinschaft) to VL Katanaev and MEXT of Japan to T Ishitani. W Kim and B Cha were supported by the Brain Korea 21 program and the Seoul Science Fellowship.
Author contributions: Experimental design: W Kim, H Kim, VL Katanaev, T Ishitani, JK Han and E Jho. Experimental work: W Kim, H Kim, VL Katanaev, SJ Lee, T Ishitani and B Cha. Manuscript writing: W Kim, H Kim, VL Katanaev, JK Han and E Jho
Footnotes
The authors declare that they have no conflict of interest.
References
- Borchers A, David R, Wedlich D (2001) Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development 128: 3049–3060 [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553 [DOI] [PubMed] [Google Scholar]
- Choi SC, Han JK (2005) Rap2 is required for Wnt/beta-catenin signaling pathway in Xenopus early development. EMBO J 24: 985–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- Coulombe P, Meloche S (2007) Atypical mitogen-activated protein kinases: structure, regulation and functions. Biochim Biophys Acta 1773: 1376–1387 [DOI] [PubMed] [Google Scholar]
- DasGupta R, Kaykas A, Moon RT, Perrimon N (2005) Functional genomic analysis of the Wnt-wingless signaling pathway. Science 308: 826–833 [DOI] [PubMed] [Google Scholar]
- Davidson G, Mao B, del Barco Barrantes I, Niehrs C (2002) Kremen proteins interact with Dickkopf1 to regulate anteroposterior CNS patterning. Development 129: 5587–5596 [DOI] [PubMed] [Google Scholar]
- Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F (1999) Domains of axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol 145: 741–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland RM (1991) In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol 36: 685–695 [DOI] [PubMed] [Google Scholar]
- Hassler C, Cruciat CM, Huang YL, Kuriyama S, Mayor R, Niehrs C (2007) Kremen is required for neural crest induction in Xenopus and promotes LRP6-mediated Wnt signaling. Development 134: 4255–4263 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Burghardt RC, Bazer FW, Spencer TE (2007) WNTs in the ovine uterus: potential regulation of periimplantation ovine conceptus development. Endocrinology 148: 3496–3506 [DOI] [PubMed] [Google Scholar]
- Heeg-Truesdell E, LaBonne C (2006) Neural induction in Xenopus requires inhibition of Wnt-beta-catenin signaling. Dev Biol 298: 71–86 [DOI] [PubMed] [Google Scholar]
- Hitchens MR, Robbins PD (2003) The role of the transcription factor DP in apoptosis. Apoptosis 8: 461–468 [DOI] [PubMed] [Google Scholar]
- Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J, Holcombe RF, Waterman ML (2001) Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet 28: 53–57 [DOI] [PubMed] [Google Scholar]
- Ishitani S, Inaba K, Matsumoto K, Ishitani T (2011) Homodimerization of Nemo-like kinase is essential for activation and nuclear localization. Mol Biol Cell 22: 266–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Hirao T, Suzuki M, Isoda M, Ishitani S, Harigaya K, Kitagawa M, Matsumoto K, Itoh M (2010) Nemo-like kinase suppresses Notch signalling by interfering with formation of the Notch active transcriptional complex. Nat Cell Biol 12: 278–285 [DOI] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K (1999) The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature 399: 798–802 [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F (2002) Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22: 1172–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jooss K, Lam EW, Bybee A, Girling R, Muller R, La Thangue NB (1995) Proto-oncogenic properties of the DP family of proteins. Oncogene 10: 1529–1536 [PubMed] [Google Scholar]
- Kanei-Ishii C, Ninomiya-Tsuji J, Tanikawa J, Nomura T, Ishitani T, Kishida S, Kokura K, Kurahashi T, Ichikawa-Iwata E, Kim Y, Matsumoto K, Ishii S (2004) Wnt-1 signal induces phosphorylation and degradation of c-Myb protein via TAK1, HIPK2, and NLK. Genes Dev 18: 816–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C (2001) A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128: 4189–4201 [DOI] [PubMed] [Google Scholar]
- Kim GH, Han JK (2007) Essential role for beta-arrestin 2 in the regulation of Xenopus convergent extension movements. EMBO J 26: 2513–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Jho EH (2010) The protein stability of Axin, a negative regulator of Wnt signaling, is regulated by Smad ubiquitination regulatory factor 2 (Smurf2). J Biol Chem 285: 36420–36426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim Y, Lee J, Chung J (2010) Regulation of FOXO1 by TAK1-Nemo-like kinase pathway. J Biol Chem 285: 8122–8129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn MJ, Bronson RT, Harlow E, Dyson NJ, Yamasaki L (2003) Dp1 is required for extra-embryonic development. Development 130: 1295–1305 [DOI] [PubMed] [Google Scholar]
- Lagutin OV, Zhu CC, Kobayashi D, Topczewski J, Shimamura K, Puelles L, Russell HR, McKinnon PJ, Solnica-Krezel L, Oliver G (2003) Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev 17: 368–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrain J, Oelgeschlager M, Ketpura NI, Reversade B, Zakin L, De Robertis EM (2001) Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development 128: 4439–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW (2003) The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol 1: E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Kim IS, Kim H, Lee JS, Kim K, Yim HY, Jeong J, Kim JH, Kim JY, Lee H, Seo SB, Rosenfeld MG, Kim KI, Baek SH (2010) RORalpha attenuates Wnt/beta-catenin signaling by PKCalpha-dependent phosphorylation in colon cancer. Mol Cell 37: 183–195 [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magae J, Illenye S, Chang YC, Mitsui Y, Heintz NH (1999) Association with E2F-1 governs intracellular trafficking and polyubiquitination of DP-1. Oncogene 18: 593–605 [DOI] [PubMed] [Google Scholar]
- Malbon CC, Wang HY (2006) Dishevelled: a mobile scaffold catalyzing development. Curr Top Dev Biol 72: 153–166 [DOI] [PubMed] [Google Scholar]
- Mason ED, Konrad KD, Webb CD, Marsh JL (1994) Dorsal midline fate in Drosophila embryos requires twisted gastrulation, a gene encoding a secreted protein related to human connective tissue growth factor. Genes Dev 8: 1489–1501 [DOI] [PubMed] [Google Scholar]
- Masuhiro Y, Kayama K, Fukushima A, Baba K, Soutsu M, Kamiya Y, Gotoh M, Yamaguchi N, Hanazawa S (2008) SOCS-3 inhibits E2F/DP-1 transcriptional activity and cell cycle progression via interaction with DP-1. J Biol Chem 283: 31575–31583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EJ, Ji JY, Yang F, Di Stefano L, Herr A, Moon NS, Kwon EJ, Haigis KM, Naar AM, Dyson NJ (2008) E2F1 represses beta-catenin transcription and is antagonized by both pRB and CDK8. Nature 455: 552–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Nagai T, Aruga J, Mikoshiba K (1997) Xenopus Zic3, a primary regulator both in neural and neural crest development. Proc Natl Acad Sci USA 94: 11980–11985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J (1967) Normal Table of Xenopus Laevis (Daudin) Amsterdam: North Holland Publishing Co [Google Scholar]
- Oelgeschlager M, Larrain J, Geissert D, De Robertis EM (2000) The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature 405: 757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawara B, Shirakabe K, Hyodo-Miura J, Matsuo R, Ueno N, Matsumoto K, Shibuya H (2004) Role of the TAK1-NLK-STAT3 pathway in TGF-beta-mediated mesoderm induction. Genes Dev 18: 381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai T, Sasai N, Matsui M, Sasai Y (2004) Xenopus XsalF: anterior neuroectodermal specification by attenuating cellular responsiveness to Wnt signaling. Dev Cell 7: 95–106 [DOI] [PubMed] [Google Scholar]
- Qiao H, Di Stefano L, Tian C, Li YY, Yin YH, Qian XP, Pang XW, Li Y, McNutt MA, Helin K, Zhang Y, Chen WF (2007) Human TFDP3, a novel DP protein, inhibits DNA binding and transactivation by E2F. J Biol Chem 282: 454–466 [DOI] [PubMed] [Google Scholar]
- Rowland BD, Bernards R (2006) Re-evaluating cell-cycle regulation by E2Fs. Cell 127: 871–874 [DOI] [PubMed] [Google Scholar]
- Satoh K, Ohnishi J, Sato A, Takeyama M, Iemura S, Natsume T, Shibuya H (2007) Nemo-like kinase-myocyte enhancer factor 2A signaling regulates anterior formation in Xenopus development. Mol Cell Biol 27: 7623–7630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond T, Metcalfe C, Bienz M (2007) Dynamic recruitment of axin by Dishevelled protein assemblies. J Cell Sci 120: 2402–2412 [DOI] [PubMed] [Google Scholar]
- Seo E, Kim H, Kim R, Yun S, Kim M, Han JK, Costantini F, Jho EH (2009) Multiple isoforms of beta-TrCP display differential activities in the regulation of Wnt signaling. Cell Signal 21: 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Hemmati-Brivanlou A (2000) Xenopus embryonic E2F is required for the formation of ventral and posterior cell fates during early embryogenesis. Mol Cell 5: 217–229 [DOI] [PubMed] [Google Scholar]
- Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM (2010) Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143: 1136–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tago K, Nakamura T, Nishita M, Hyodo J, Nagai S, Murata Y, Adachi S, Ohwada S, Morishita Y, Shibuya H, Akiyama T (2000) Inhibition of Wnt signalling by ICAT, a novel beta-catenin-interacting protein. Genes Dev 14: 1741–1749 [PMC free article] [PubMed] [Google Scholar]
- Tiscornia G, Singer O, Ikawa M, Verma IM (2003) A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci USA 100: 1844–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel S, Dyson NJ (2008) Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol 9: 713–724 [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R (1998) Mechanisms of Wnt signalling in development. Annu Rev Cell Dev Biol 14: 59–88 [DOI] [PubMed] [Google Scholar]
- Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, Sakamoto D, Caothien RH, Fuller JH, Reinhard C, Garcia PD, Randazzo FM, Escobedo J, Fantl WJ, Williams LT (2001) Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signalling is activated in human colon tumors. Proc Natl Acad Sci USA 98: 14973–14978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, Wynshaw-Boris A, Hsieh JC, He X (2008) Initiation of Wnt signalling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135: 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.