Abstract
Phototropism allows plants to redirect their growth towards the light to optimize photosynthesis under reduced light conditions. Phototropin 1 (phot1) is the primary low blue light-sensing receptor triggering phototropism in Arabidopsis. Light-induced autophosphorylation of phot1, an AGC-class protein kinase, constitutes an essential step for phototropism. However, apart from the receptor itself, substrates of phot1 kinase activity are less clearly established. Phototropism is also influenced by the cryptochromes and phytochromes photoreceptors that do not provide directional information but influence the process through incompletely characterized mechanisms. Here, we show that Phytochrome Kinase Substrate 4 (PKS4), a known element of phot1 signalling, is a substrate of phot1 kinase activity in vitro that is phosphorylated in a phot1-dependent manner in vivo. PKS4 phosphorylation is transient and regulated by a type 2-protein phosphatase. Moreover, phytochromes repress the accumulation of the light-induced phosphorylated form of PKS4 showing a convergence of photoreceptor activity on this signalling element. Our physiological analyses suggest that PKS4 phosphorylation is not essential for phototropism but is part of a negative feedback mechanism.
Keywords: light-activated protein kinase, photoreceptor co-action, phototropin 1, phototropism, Phytochrome Kinase Substrate 4
Introduction
The survival of living organisms critically depends on accurate perception and responses to external stimuli. Light is a crucial environmental factor that elicits adaptive behaviours in many species, including in plants (Kami et al, 2010; Keuskamp et al, 2010). Being sessile and photoautotrophic, plants have evolved the ability for plastic growth and development in response to the ever-changing environmental light conditions. They possess sophisticated light-sensing systems allowing them to monitor light quality, quantity, duration and direction (Kami et al, 2010; Keuskamp et al, 2010). Integration of all these informational cues determines the timing of important developmental transitions (e.g., germination, flowering and senescence) (Mockler et al, 2003; Turck et al, 2008; Sellaro et al, 2009). The light environment also triggers adaptive responses influencing plant morphology in order to optimize light capture and the resulting photosynthetic efficiency (Inoue et al, 2008b). In Arabidopsis thaliana, five classes of photoreceptors have been identified so far: the recently identified UVR8 perceives Ultraviolet-B (Rizzini et al, 2011), phytochromes (phyA to phyE) absorb mainly red and far red light, but also contribute to the perception of blue light together with phototropins (phot1 and phot2), cryptochromes (cry1 and cry2) and members of the zeitlupe (ztl, lkp2 and fkf1) families (Kami et al, 2010).
Numerous light-regulated responses such as photoperiodic control of flowering time or de-etiolation, a developmental transition during which the seedling switches from using energy from its seed reserves to photoautotrophic growth are controlled by multiple photoreceptors (Mockler et al, 2003; Turck et al, 2008; Sellaro et al, 2009). This has also been shown for the control of phototropism, a physiological response allowing plants to optimally position their photosynthetic organs towards the light (Whippo and Hangarter, 2003, 2004; Lariguet and Fankhauser, 2004). This photomorphogenic response is important for seedling establishment because carbon availability is the main factor limiting leaf and plant growth in early developmental phases (Pantin et al, 2011). While phototropins are responsible for sensing blue light direction, phytochromes and cryptochromes act to modulate the physiological response. For instance, both inhibit gravity-induced vertical orientation of growth providing the seedling with increased flexibility (Lariguet and Fankhauser, 2004; Ohgishi et al, 2004). In addition, phytochromes and cryptochromes also affect the responsiveness to the phytohormone auxin, whose differential accumulation between the shaded and lit sides of the hypocotyl is important for the establishment of asymmetric growth (Stowe-Evans et al, 2001; Esmon et al, 2006; Nagashima et al, 2008). Finally, it has been proposed that activation of phytochromes and cryptochromes might participate more directly to the phototropic response by enhancing phototropin signalling, either by regulating the expression of components of the signalling pathway (Iino, 2006; Lariguet et al, 2006; Tsuchida-Mayama et al, 2010; Kami et al, 2012) or by affecting phot1 subcellular localization (Han et al, 2008).
Phototropins possess an amino-terminal light-sensing portion constituted of two Light Oxygen Voltage (LOV) domains (LOV1 and LOV2) and a carboxy-terminal protein kinase domain of the AGC class (Christie, 2007). LOV2 constitutes the major light-sensing domain of phot1, while it has been proposed that LOV1 is involved in modulating photosensitivity (Christie et al, 2002; Cho et al, 2007). An alpha helix (Jα) undergoing a light-regulated conformational change connects LOV2 to the kinase domain (Harper et al, 2003). This conformational change liberates the kinase domain from the inhibitory effect of the amino-terminus of the photoreceptor upon light treatment thus activating phototropin kinase activity (Harper et al, 2004; Matsuoka and Tokutomi, 2005; Tokutomi et al, 2008). In Arabidopsis, both phot1 and phot2 are phosphorylated upon blue light treatments and several phosphorylation sites have been identified on both light sensors (Inoue et al, 2008a, 2011; Sullivan et al, 2008). Of particular interest are sites in the activation loop of the kinase domain which when mutated to Ala prevent all tested phototropin response in vivo (Inoue et al, 2008a, 2011). This suggests that upon blue light perception phototropin autophosphorylation in the activation loop is an essential step for subsequent signalling events. Surprisingly, little is known about the substrates of this kinase activity. The auxin transporter ABCB19 was recently shown to be phosphorylated in vitro by phot1 but data demonstrating that this occurs in planta are currently not at hand (Christie et al, 2011). Phot1-dependent dephosphorylation of the early phototropin signalling component non-phototropic hypocotyl 3 (NPH3) has been reported but the steps leading from phot1 activation to NPH3 dephosphorylation are currently unknown (Pedmale and Liscum, 2007; Tsuchida-Mayama et al, 2008).
Members of the Phytochrome Kinase Substrate family (PKS1 to PKS4 in Arabidopsis) have also been proposed to act early in phot1 signalling given that they interact with the photoreceptor and NPH3 at the plasma membrane (Lariguet et al, 2006; Boccalandro et al, 2008; de Carbonnel et al, 2010). Moreover, PKS proteins control phototropin and phytochrome-mediated growth responses, suggesting that they may contribute to photoreceptor co-action during phototropism (Fankhauser et al, 1999; Lariguet et al, 2003, 2006; Boccalandro et al, 2008; Molas and Kiss, 2008; Schepens et al, 2008; de Carbonnel et al, 2010). In particular, PKS4 has a strong impact on phot1-mediated phototropism and phytochrome-mediated deviation from vertical growth (Lariguet et al, 2006; Schepens et al, 2008). These findings prompted us to investigate PKS4 protein regulation in response to light treatments eliciting photomorphogenic responses during seedling establishment. We analysed the phosphorylation state of PKS4 given that light-induced phosphorylation events are important early signalling events during photomorphogenesis in particular during phototropin-mediated light responses (Sullivan et al, 2008; Inoue et al, 2008a, 2011).
Results
PKS4 is rapidly and transiently phosphorylated upon light irradiation
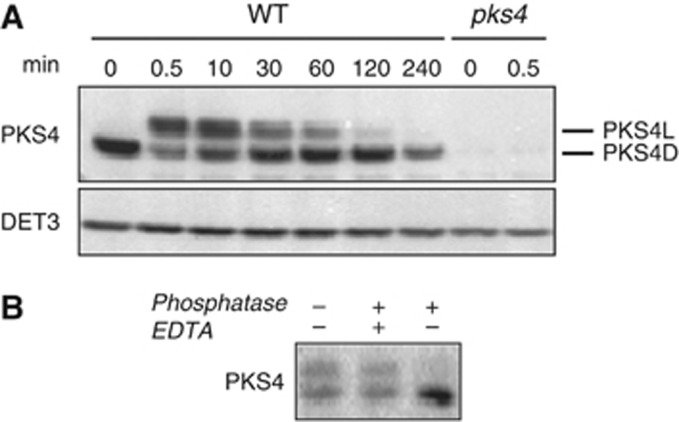
Previous studies have shown that PKS (Phytochrome Kinase Substrate) gene expression is light regulated and PKS1 protein is phosphorylated (Fankhauser et al, 1999; Lariguet et al, 2003; Schepens et al, 2008). To investigate the regulation of PKS4 at the protein level, we raised an anti-PKS4 antibody. The specificity of the antibody was confirmed by using pks4 null mutant extracts (Figure 1A). Since PKS4 is required for normal de-etiolation (Lariguet et al, 2006; Schepens et al, 2008), we analysed PKS4 protein levels in etiolated seedlings transferred to white light. Light treatment resulted in the rapid appearance of an additional slower migrating isoform (Figure 1A). Thus, two co-existing isoforms of PKS4 were detected in the light: a faster migrating form already present in darkness (PKS4D) and a slower migrating form that was only detected upon light treatment (PKS4L).
Figure 1.
PKS4 is phosphorylated in response to light. (A) PKS4 exists as two isoforms in the light. Three-day-old etiolated seedlings (WT) were exposed to constant white light (140 μmol m−2 s−1). Total proteins were extracted at the indicated times, separated by SDS–PAGE and transferred onto nitrocellulose membrane. PKS4 accumulation was analysed by immunoblotting using anti-PKS4 antibodies, and DET3 accumulation was used as loading control. Null pks4 mutant extracts (pks4) were used to check anti-PKS4 antibodies specificity. The isoform present in the dark is marked as PKS4D and the one appearing in the light as PKS4L. (B) PKS4L formation results from PKS4D phosphorylation. Total proteins extracted from 3-day-old etiolated seedlings illuminated with white light (30 s, 140 μmol m−2 s−1) were subjected (+) or not (−) to λ-phosphatase treatment (phosphatase) in presence (+) or absence (−) of inhibitor (EDTA).
PKS4L appeared very rapidly upon light treatment, reaching a maximum accumulation within 30 s to 10 min. Accumulation of PKS4L was transient, as it progressively disappeared until being no longer detectable after 4 h in white light (Figure 1A). In general, PKS4D protein levels mirrored those of PKS4L; they decreased upon irradiation and increased following longer irradiation (Figure 1A). These results suggest that a portion of PKS4 is post-translationally modified in a light-dependent manner. Given that another member of the PKS family, PKS1, is phosphorylated upon light treatment, we tested whether generation of PKS4L resulted from protein phosphorylation by subjecting extracts to phosphatase treatments (Fankhauser et al, 1999). A reduction of PKS4L levels coupled to an increase in PKS4D accumulation upon phosphatase treatment indicated that PKS4 undergoes light-dependent phosphorylation (Figure 1B).
PKS4 is a phot1 kinase substrate
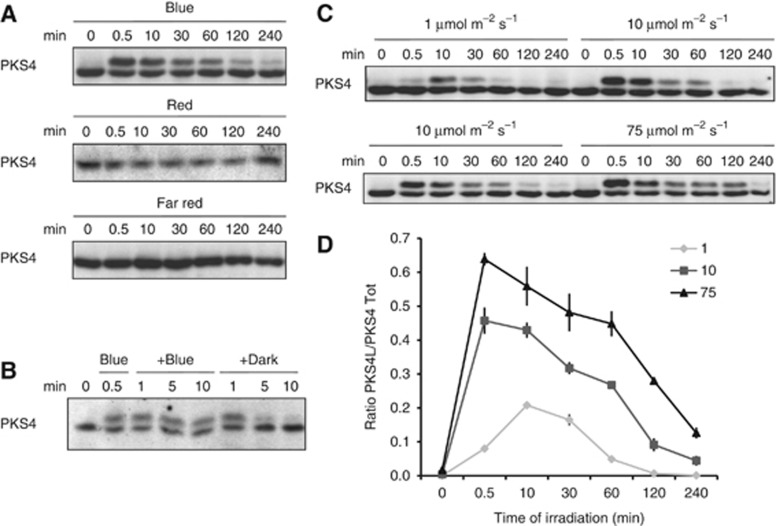
We next examined the effect of blue, red and far-red light to determine which wavelengths lead to PKS4 phosphorylation. PKS4L was detected only in response to blue light treatment, but not with red or far-red light irradiation (Figure 2A). When seedlings were treated with a pulse of blue light and returned to darkness PKS4L quickly disappeared (Figure 2B). PKS4L accumulation increased in response to higher fluence rates of blue light (Figure 2C). Similarly, the peak of PKS4L accumulation occurred earlier with higher fluence rates (Figure 2C). The influence of the light intensity on PKS4L accumulation was confirmed by quantification of the portion of PKS4 present as PKS4L (PKS4L/PKS4tot) along the time of irradiation (Figure 2D).
Figure 2.
PKS4L accumulates upon blue light perception, specifically. (A) Blue light-dependent PKS4 phosphorylation. Three-day-old etiolated seedlings (WT) were exposed to constant blue light (10 μmol m−2 s−1), red light (30 μmol m−2 s−1) or far-red light (15 μmol m−2 s−1). (B) PKS4 phosphorylation is reversible. Three-day-old etiolated seedlings were exposed to 10 μmol m−2 s−1 blue light for 30 s and then kept in blue light or transferred to darkness for the indicated time. (C) Fluence rate-dependent PKS4 phosphorylation. Three-day-old etiolated seedlings were exposed to 1, 10 and 75 μmol m−2 s−1 blue light. Samples treated with 10 μmol m−2 s−1 blue light are shown on two blots to allow direct comparisons with those treated with 1 and 75 μmol m−2 s−1 blue light. Proteins were analysed as described in Figure 1. (D) Quantification of PKS4L accumulation upon light treatment. Three-day-old etiolated seedlings were exposed to 1, 10 and 75 μmol m−2 s−1 blue light. Quantifications of PKS4L signal relative to the PKS4D+PKS4L total signal were performed on three biological replicates. Values are means, and error bars represent standard errors.
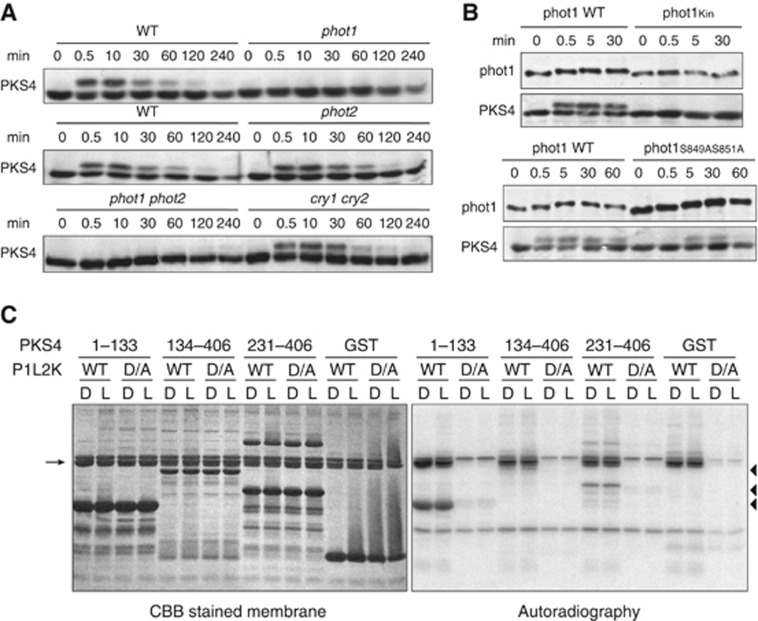
To determine which blue light receptor was responsible for these effects, we examined blue light induced PKS4L phosphorylation in phototropin or cryptochrome-deficient mutants. PKS4 phosphorylation was still detected in the cryptochrome double mutant (cry1cry2) showing that cryptochromes are dispensable for PKS4L formation (Figure 3A). In contrast, no accumulation of PKS4L was detected in the phot1phot2 double mutant (Figure 3A). Further genetic analysis revealed that phot1 is the photoreceptor required for PKS4 phosphorylation, as PKS4L accumulation was still detected in phot2, but not in phot1 single mutant (Figure 3A).
Figure 3.
PKS4 is a target of phot1 kinase activity in vivo and in vitro. (A, B) PKS4 phosphorylation depends on phot1 kinase activity. Three-day-old etiolated seedlings of Col-0 (WT), cry1-cry2, phot1-phot2 double mutants, phot1 and phot2 single mutants (A), or phot1-phot2 mutant expressing wild-type or mutated versions of phot1 kinase domain (B), were exposed to constant blue light (10 μmol m−2 s−1 blue light). Total proteins were extracted at the indicated times, separated by SDS–PAGE and transferred onto nitrocellulose membrane. PKS4 and phot1 accumulations were analysed by immunoblotting using anti-PKS4 and anti-phot1 antibodies. (C) Phot1 phosphorylates PKS4 fragments in vitro. GST-fused PKS4 polypeptides (PKS4 amino acids 1–133, 134–406 or 231–406; position marked by dark triangles) or GST were used as substrates for in-vitro phosphorylation assay in presence of GST-fused LOV2-Kinase domain polypeptides of phot1 either wild-type version or D788A kinase inactive mutated version (P1L2K WT and D/A, position marked by arrow). The reaction was performed under blue light (L) or mock irradiation (D). Proteins were separated on 15% acrylamide gel and transferred onto PVDF membrane. Left panel corresponds to Coomassie blue staining of the membrane. Right panel corresponds to autoradiography.
Phototropins are composed of an N-terminal photosensory domain that represses the activity of the C-terminal Ser/Thr kinase domain in the dark. Phototropins are activated upon blue light absorption owing to conformational changes that alleviate the repression of kinase activity (Harper et al, 2003; Matsuoka and Tokutomi, 2005; Tokutomi et al, 2008). A primary step of phot1 signalling is the autophosphorylation of one or two serines (Ser 849 and 851) located within the activation loop of the kinase domain (Inoue et al, 2008a). We hypothesized that if light-dependent phosphorylation of PKS4 depends on phot1 kinase activity, then mutations in the kinase domain of phot1 should affect PKS4L formation. We thus examined PKS4L formation in phot1phot2 double mutants expressing either kinase mutants of phot1 or wild-type phot1 as a control. Phot1 kinase activity is abolished in the kinase-inactivated line (phot1Kin) (Christie et al, 2002; Cho et al, 2007), and diminished in phot1S849AS851A line (Inoue et al, 2008a). PKS4L accumulation was no longer detectable in the phot1Kin line and was decreased in the phot1S849AS851A line (Figure 3B). Importantly, altered PKS4L formation in these lines was not due to reduced levels of phot1 expression (Figure 3B). Together, these results indicate that PKS4L accumulation is dependent on phot1 kinase activity.
Both phot1 and PKS4 are strongly expressed in the elongation zone of the hypocotyl (Schepens et al, 2008; Wan et al, 2008). Moreover similar to PKS1 and PKS2, PKS4 co-immunoprecipitated with phot1-GFP in vivo suggesting that phot1 may directly phosphorylate PKS4 (Supplementary Figure S1; Lariguet et al, 2006; Boccalandro et al, 2008; de Carbonnel et al, 2010). We thus tested the ability of phot1 to directly phosphorylate PKS4 in vitro. GST-fused recombinant proteins were purified from bacterial extracts. Due to poor solubility and stability of full-length proteins, phot1 and PKS4 were produced as truncated versions (Christie et al, 2011; Okajima et al, 2011). For PKS4 we tested three different fragments, the N-terminus (amino acids 1–133) and the two C-terminal fragments (amino acids 134–406 and 231–406). The largest PKS4 fragment (amino acids 134–406) was most difficult to express and purify and we were unable to produce fractions as concentrated as for the other two. Phosphorylation assays were performed either in darkness or in presence of light. Even though no light regulation was observed, phosphorylation of the N-terminal PKS4 fragment and the most C-terminal PKS4 fragment were clearly detected in presence of active phot1 kinase but not in presence of a kinase inactivated version (Figure 3C). Phosphorylation of the third PKS4 fragment (amino acids 134–406) is detected as a faint band just below phot1 (P1L2K). The apparently weaker phosphorylation of this fragment of PKS4 might simply result from the lower concentration of substrate in these reactions (see Coomassie stained membrane) (Figure 3C). As observed previously autophosphorylation of phot1 also occurred independently of the light treatment (Christie et al, 2011; Okajima et al, 2011). We conclude that PKS4 is a substrate of phot1 kinase in vitro.
PKS4L accumulation is repressed by phytochromes under low blue light intensity
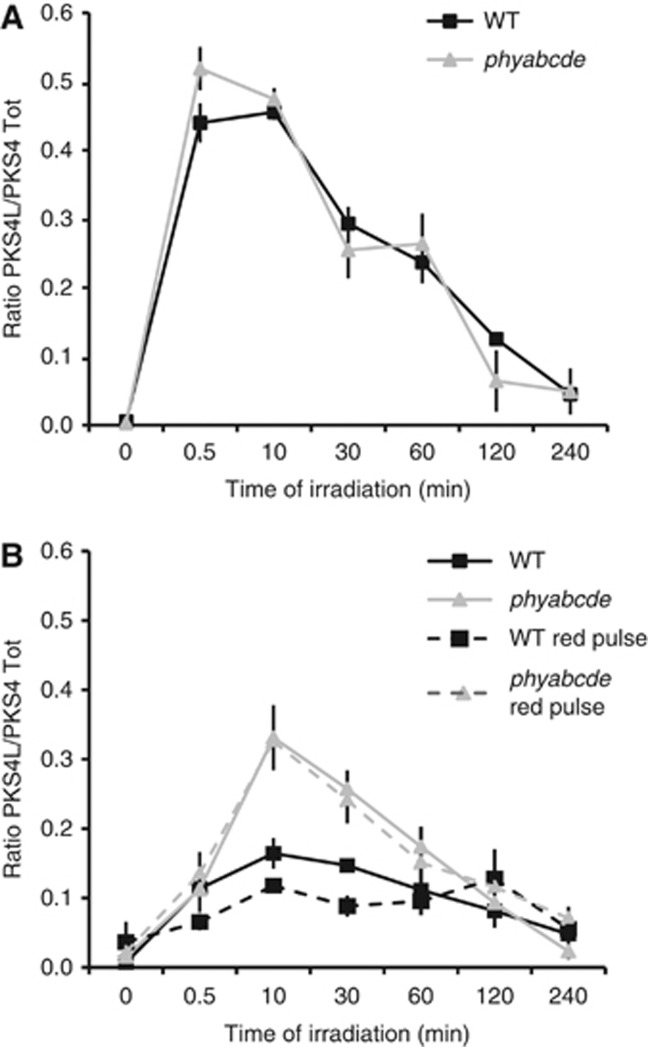
Phytochromes are red/far-red photoreceptors, but are also known to mediate blue light responses (Kami et al, 2010). We thus analysed their involvement in PKS4L accumulation. PKS4L still accumulated in the quintuple phytochrome mutant (Figure 4). The PKS4L/PKS4tot ratio was not strikingly affected by the absence of phytochrome activity in high blue light condition where this mutant still shows phototropism (Figure 4A; Strasser et al, 2010). However, PKS4L accumulation was strongly enhanced in the quintuple phytochrome mutant when seedlings were irradiated with low fluence rates of blue light where phytochromes are known to promote phototropism (Figure 4B; Lariguet et al, 2006; Tsuchida-Mayama et al, 2010; Kami et al, 2012). This result shows that phytochromes negatively regulate PKS4L accumulation in low blue light conditions.
Figure 4.
Phytochromes modulate PKS4 phosphorylation. (A) Phytochromes have a very weak effect on PKS4L accumulation in high blue light intensity. Three-day-old etiolated seedlings of wild-type (WT) and the quintuple phytochrome mutant (phyabcde) were irradiated with 10 μmol m−2 s−1 blue light. Quantifications of PKS4L signal relative to the PKS4D+PKS4L total signal were performed on three biological replicates. Values are means, and error bars represent standard errors. (B) Phytochromes decrease PKS4L accumulation in low blue light intensity and upon red light pre-irradiation. Three-day-old etiolated seedlings of WT and the quintuple phytochrome mutant (phyabcde) were irradiated with 30 μmol m−2 s−1 red light for 1 min or mock irradiated, kept in darkness for 1 h, then irradiated with 1 μmol m−2 s−1 blue light for the indicated time. Quantifications of PKS4L signal relative to the PKS4D+PKS4L total signal were performed on four biological replicates. Values are means, and error bars represent standard errors.
A striking example of concerted action between phot1 and the phytochromes is that phot1-mediated phototropism is enhanced by phytochrome photoactivation (Liscum and Briggs, 1996; Parks et al, 1996). Irradiation of seedlings with a pulse of red light prior to blue light irradiation accelerates the phototropic response. In the wild type, red light pre-treatment slightly but significantly decreased the PKS4L/PKS4tot ratio. However, this effect was not observed in the quintuple phytochrome mutant (Figure 4B). This result confirms that phytochromes repress the accumulation of PKS4L and shows that PKS4D to PKS4L inter-conversion is controlled by the antagonistic action of two photoreceptors: phot1 promotes PKS4 phosphorylation while phytochromes repress the accumulation of the phosphorylated form.
PKS4L formation occurs upstream of auxin signalling/redistribution
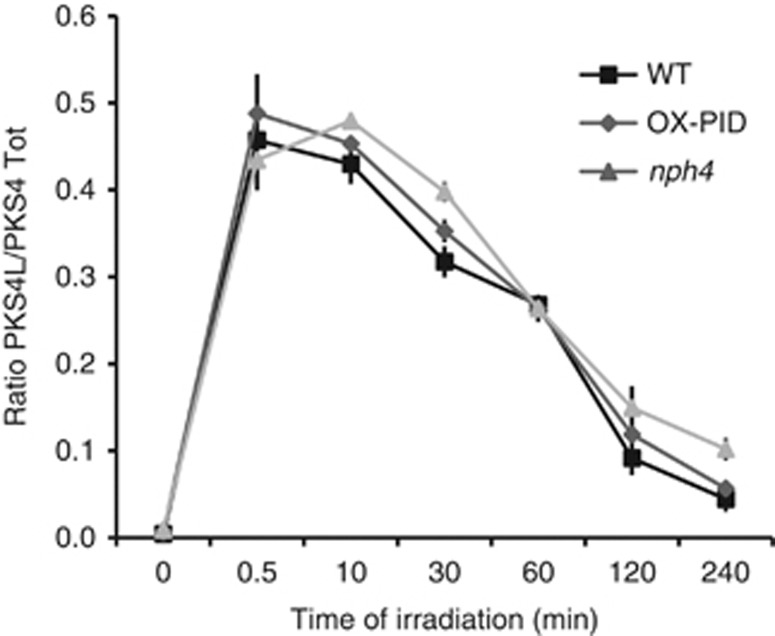
In etiolated seedlings, phosphorylation of PKS4 depends on phot1 and is modulated by the phytochromes (Figures 3 and 4). Following phototropin activation, a gradient of auxin forms in the hypocotyl that precedes asymmetric growth of the embryonic stem (Esmon et al, 2006). PKS4 phosphorylation is very rapid and phot1 dependent (Figure 3) and we wished to determine whether PKS4L appearance is affected by auxin transport or signalling. Tropic growth and auxin transport are altered in seedlings overexpressing PINOID (OX-PID; Ding et al, 2011), but the appearance of PKS4L upon light stimulation was normal in this genetic background (Figure 5). Similarly, PKS4 phosphorylation was not affected in the phototropism-deficient auxin-signalling mutant nph4 (Figure 5; Harper et al, 2000). Collectively, these data indicate that the phot1-dependent PKS4 phosphorylation is an early response occurring upstream of auxin redistribution and the NPH4-mediated transcriptional response.
Figure 5.
PKS4 phosphorylation occurs upstream of auxin signalling. Three-day-old etiolated seedlings (WT, OX-PID, nph4) were irradiated with 10 μmol m−2 s−1 blue light. Quantifications of PKS4L signal relative to the PKS4D+PKS4L total signal were performed on three biological replicates. Values are means, and error bars represent standard errors.
RCN1-dependent PKS4 dephosphorylation
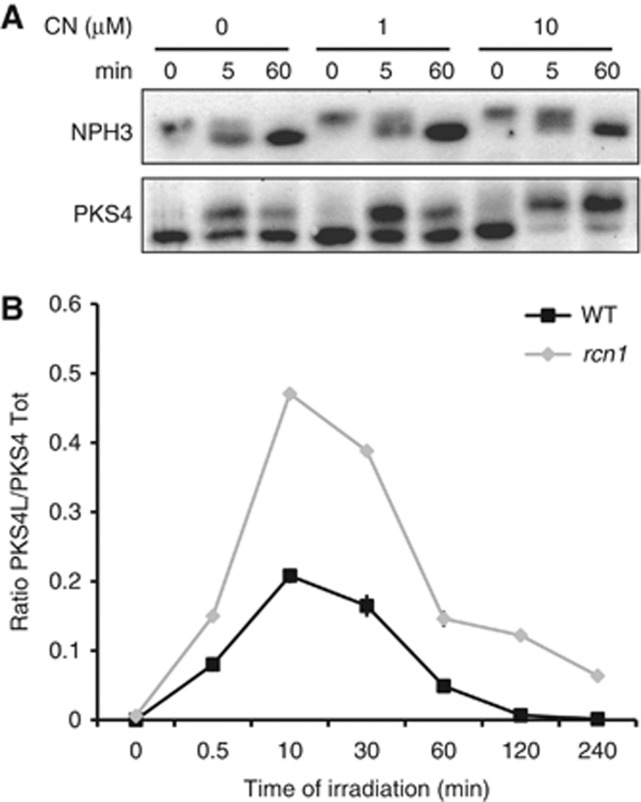
The conversion of PKS4D to PKS4L is transient and reversible as a decrease in the PKS4L isoform is paralleled by an increase in PKS4D (Figures 1A and 2B). Given that PKS4L is a phosphorylated isoform its disappearance upon prolonged blue light treatment could be due to dephosphorylation. To test this hypothesis, we treated etiolated seedlings with the phosphatase inhibitor cantharidin (CN) 1 h before blue light irradiation (Li et al, 1993). We observed that PKS4L accumulation was higher when the seedlings were subjected to CN treatment (Figure 6A), supporting the hypothesis that the disappearance of PKS4L involves phosphatase activity. In the dark, PKS4 exists as a single isoform. The absence of PKS4L in the dark could result either from the absence of phosphorylation or from a high phosphatase activity that counteracts phosphorylation. The fact that PKS4L was not detected in darkness in the presence of CN excluded the latter possibility and confirms that PKS4L formation is due to light-induced phosphorylation that correlates with the phot1 activity.
Figure 6.
PP2A activity modulates PKS4 phosphorylation. (A) PKS4L accumulation is enhanced upon phosphatase inhibitor treatment (CN=cantharidin). Three-day-old etiolated seedlings were transferred onto medium containing 0, 1 and 10 μM CN 1 h before light exposure. Proteins were extracted after 0, 5 and 60 min of 15 μmol m−2 s−1 blue light irradiation and analysed as described in Figure 2. (B) PKS4L accumulation is enhanced in rcn1 mutant. Three-day-old etiolated seedlings (WT and rcn1) were irradiated with 1 μmol m−2 s−1 blue light for the indicated time. Quantifications of PKS4L signal relative to the PKS4D+PKS4L total signal were performed on three biological replicates. Values are means, and error bars represent standard errors.
CN is a selective inhibitor of PP1 and PP2A phosphatases, but has a higher affinity for PP2A than PP1 (Li et al, 1993). The phot1-interacting protein NPH3 is reported to be a PP1 target (Pedmale and Liscum, 2007). NPH3 is dephosphorylated in response to blue light in a process that is prevented in the presence of 50 μM CN (Pedmale and Liscum, 2007). Our studies indicate that PKS4 phosphorylation has a higher sensitivity to CN than NPH3. Indeed, 10 μM CN was sufficient to increase strongly PKS4L accumulation but had no obvious effect on NPH3 dephosphorylation (Figure 6A). The higher sensitivity of PKS4 to CN compared to NPH3 suggests that PKS4 dephosphorylation could be mediated by PP2A activity rather than PP1. To test this hypothesis, we examined PKS4L/PKS4tot ratio in a mutant affected for PP2A activity. PP2As are heterotrimeric Ser/Thr-specific protein phosphatases, composed of one catalytic C subunit and two regulatory subunits, A and B. RCN1 has been identified as one of the three regulatory subunits A in Arabidopsis. Loss of RCN1 function strongly impairs global PP2A activity in Arabidopsis (Deruere et al, 1999). Consistent with our pharmacological studies, the PKS4L/PKS4D ratio was higher in rcn1 mutant as compared to the wild type at each time point of our analysis (Figure 6B). This result indicates that PKS4D to PKS4L inter-conversion is regulated by PP2A activity.
Phosphorylation of PKS4 negatively regulates phototropism
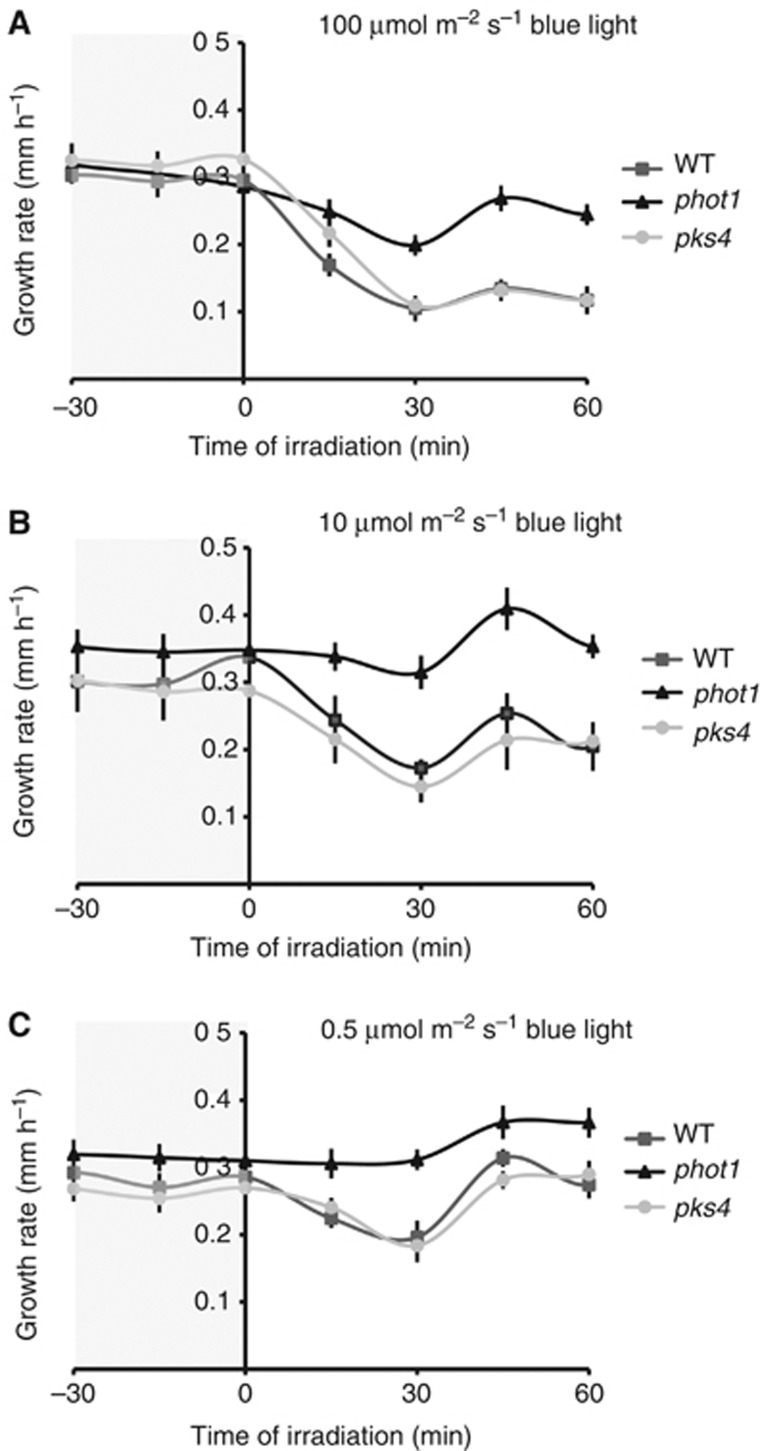
Upon blue light perception, rapid inhibition of hypocotyl growth precedes the onset of curvature. This process is rapid, phot1 and fluence rate dependent, reminiscent of the formation of PKS4L (Figures 2 and 3; Folta and Spalding, 2001). Given the similarities between these two events, we examined the role of PKS4 in the rapid inhibition of hypocotyl elongation by blue light. Our results confirmed the fluence-rate dependent feature of this response and the essential role of phot1, however, the pks4 mutant displayed a response similar to the wild type (Figure 7).
Figure 7.
PKS4 is not essential for phot1-dependent hypocotyl growth inhibition. Two-day-old etiolated seedlings (WT, pks4, phot1) were irradiated with 100 μmol m−2 s−1 (A), 10 μmol m−2 s−1 (B) or 0.5 μmol m−2 s−1 (C) blue light from above. Data represent means of hypocotyl growth rates in darkness (−30 to 0 min) or upon light treatment (0–60 min). Bars indicate two standard errors (n>20).
Since PKS4 plays a positive role in phototropism (Lariguet et al, 2006), we hypothesized that PKS4 phosphorylation may modulate the phototropic response. Two experiments suggested that phosphorylation of PKS4 was not required for phototropism. First in phot1phot2 plants expressing a truncated version of phot1 (L2K) that is sufficient to enable phototropism (Sullivan et al, 2008) we saw no light-induced phosphorylation of PKS4 suggesting that the appearance of PKS4L is not a prerequisite for phototropism. In addition, robust appearance of PKS4L was most efficient at fluence rates that are much higher than those required to induce phototropism (Figure 2). We thus envisaged the possibility that PKS4 phosphorylation may rather inhibit phototropism, which is known to occur in response to high fluences of light in particular when phototropism is triggered by pulses of light (Konjevic et al, 1989; Janoudi and Poff, 1991).
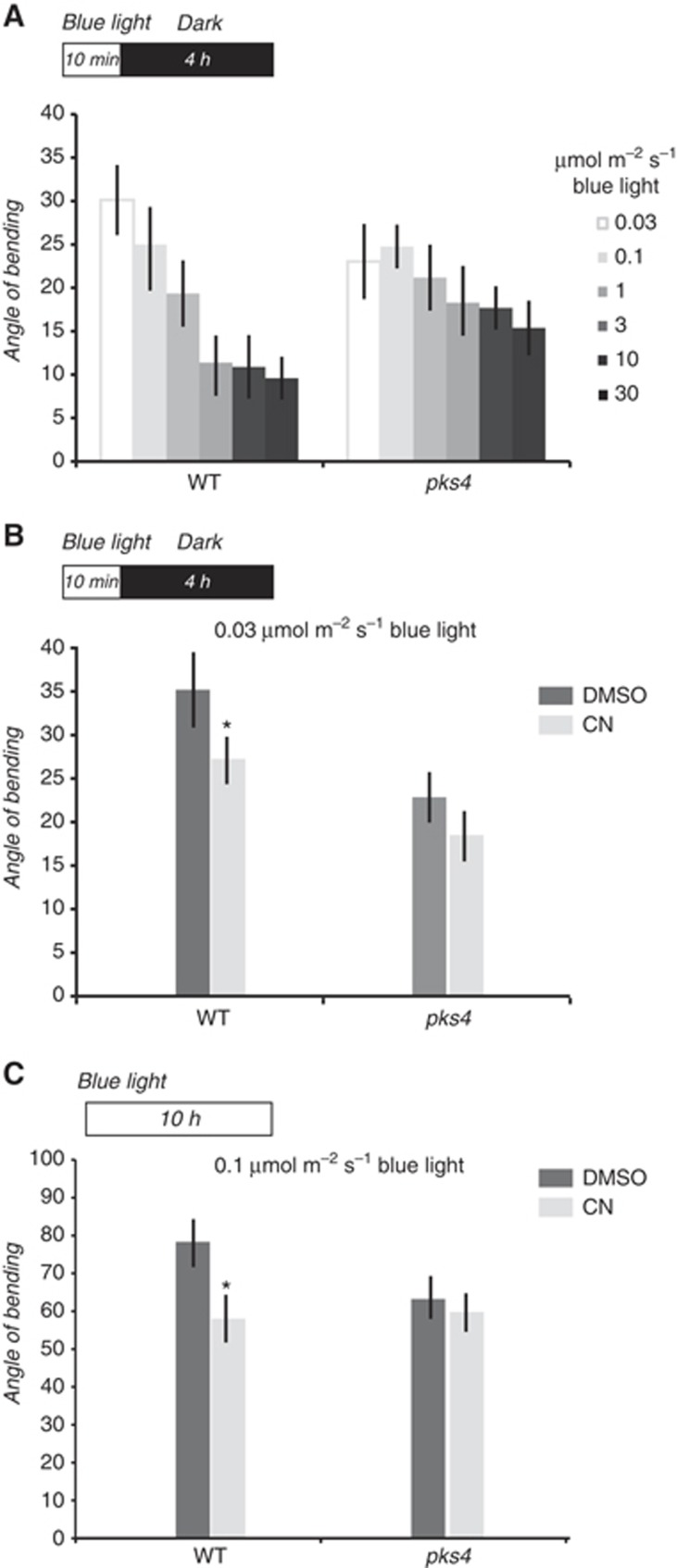
The relationship between the total fluence of light and the phototropic response is complex with an early phase where the two are proportional followed by a phase where increasing blue light fluence inhibits phototropism and finally time-dependent phototropism that is most often studied (Konjevic et al, 1989; Iino, 2006). The mechanisms leading to an inhibition of phototropism in response to increasing fluences of blue light are unknown. In order to test whether PKS4 and its phosphorylation were involved in this process wild-type and pks4 seedlings were irradiated with unilateral blue light for 10 min with variable fluence rates (leading to different PKS4L/PKS4tot ratios; Figure 2) and phototropism measured after returning them into darkness for 4 h (Figure 8A). Curvature of wild-type seedlings decreased by increasing the fluence rate of irradiation (significant difference with P-value <0.05 between 0.03 and fluence rate higher than 0.1 μmol m−2 s−1). pks4 mutant displayed reduced phototropism at the lowest fluence rate tested (0.03 μmol m−2 s−1), confirming the positive role of PKS4 for phototropism reported previously (Lariguet et al, 2006). Increasing the fluence rate decreased curvature of pks4 mutant. However, pks4 was less sensitive than the wild type to the light intensity effect (significant difference with P-value <0.05 between 0.03 and fluence rate higher than 3 μmol m−2 s−1). Consequently, pks4 showed stronger phototropism than wild type for the highest fluence rates tested. Since increasing the fluence rates increased PKS4L accumulation (Figure 2D) while inhibiting phototropism (Figure 8A), we hypothesized that PKS4L may act as a negative regulator of phototropism.
Figure 8.
PKS4L accumulation negatively regulates phototropism. (A) Enhanced PKS4L accumulation by increasing blue light intensity inhibits pulse-induced phototropism. Three-day-old etiolated seedlings (WT and pks4) were irradiated for 10 min with unilateral blue light (0.03, 0.1, 1, 3, 10 or 30 μmol m−2 s−1) returned to darkness for 4 h before measuring the phototropic curvature. (B, C) Enhanced PKS4L accumulation by cantharidin (CN) treatment inhibits phototropism. Three-day-old etiolated seedlings (WT and pks4) were transferred onto agar plates containing 10 μM cantharidin (CN) or mock treated (DMSO) and returned to darkness for 1 h before the light treatment. (B) Seedlings were treated for 10 min with 0.03 μmol m−2 s−1 blue light, returned to darkness for 4 h before measuring phototropic curvature. (C) Seedlings were treated for 4 h with 0.1 μmol m−2 s−1 unilateral blue light before measuring phototropic curvature. Data are means±two standard errors (n>20). *Indicates significant differences between means of CN versus DMSO (P-value<0.05).
To further test whether PKS4L inhibits phototropism, we treated seedlings with CN leading to increased PKS4L formation in low light (Figure 6) and subjected those seedlings to unilateral blue light to test their phototropic response (Figure 8B). Treatment with CN impaired hypocotyl curvature in wild-type seedlings (Figure 8B). However, CN treatment had no significant effect on the curvature response of pks4 seedlings. This suggests that CN effect on WT phototropic responsiveness is at least partially due to enhanced accumulation of PKS4L. We also examined the effect of CN on phototropism under prolonged light irradiation (Figure 8C). In this condition, phototropic responsiveness was also decreased in WT but not in pks4. This result again indicates that stabilization of PKS4L during the time of irradiation correlates with reduced phototropism. Altogether our physiological results suggest that PKS4 has a dual role in phototropism: PKS4D is a positive regulator whereas PKS4L is a negative regulator of phototropism (Figure 8).
Discussion
PKS4 is a substrate of phot1 signalling
In this study, we show that PKS4 undergoes blue light-dependent phosphorylation that is mediated by phot1 kinase activity. Transphosphorylation catalysed by phot1 was previously demonstrated in vitro using the N-terminal region of phot1 or the auxin transporter ABCB19 as substrates (Christie et al, 2011; Okajima et al, 2011). Here, we present evidence that PKS4 is a target of phot1 kinase activity both in vivo and in vitro (Figure 3; Supplementary Figure S1). Phot1 LOV2-linker-kinase (P1L2K) phosphorylates both the N-terminal (amino acids 1–233) and the C-terminal (amino acids 231–406) regions of PKS4 indicating the existence of multiple phosphorylation sites. Light regulation of phot1-dependent PKS4 phosphorylation was clearly observed in vivo but not in vitro (Figure 3). The P1L2K phosphorylates an N-terminal portion of phot1 in a light-dependent manner, indicating that P1L2K can act as a light-regulated kinase (Okajima et al, 2011). However, PKS4 polypeptides did not show marked light-inducible phosphorylation. Several reasons may explain the absence of light regulation in vitro. First, autophosphorylation of P1L2K itself is not light regulated (Okajima et al, 2011; Figure 3). A longer fragment of the phot1 polypeptide, for example, including the N-terminal flanking region of LOV2 that is necessary for Chlamydomonas phototropin light regulation, may be necessary for light regulation of PKS4 phosphorylation in vitro (Aihara et al, 2012). In addition, full-length PKS4 may present a particular conformation potentially lost in the fragments, which allows its light-regulated phosphorylation. Finally, in contrast to the in-vivo situation where both the kinase and the substrate are associated with the plasma membrane (Supplementary Figure S1; Lariguet et al, 2006; Wan et al, 2008), these assays are performed with soluble components which may compromise light regulation of the reaction.
In planta PKS4 phosphorylation occurs very early following phot1 activation and does not depend upon auxin redistribution that precedes phototropism (Figures 3 and 5). PKS4L accumulation and autophosphorylation of phot1 share common features: they are both fluence rate dependent and reversible (Salomon and Knieb, 2003; Inoue et al, 2008a; Sullivan et al, 2008; Figure 2). Moreover, phot1-dependent PKS4 phosphorylation and phot1 autophosphorylation within the activation loop (at Ser 851) exhibit similar kinetics of dephosphorylation upon treatment with a blue light pulse (Inoue et al, 2008a; Figure 3B). The level of PKS4L is also influenced by the degree of phot1 kinase activity (Figure 3C). While PKS4L is absent in seedlings expressing a kinase-inactive version of phot1, expression of phot1S849AS851A restores light-dependent PKS4 phosphorylation albeit at a lower levels as compared to the wild type (Figure 3C). The observation that PKS4 phosphorylation still occurs in this line can be explained by the fact that phosphorylation within the activation loop of kinases typically enhances their activity, but is not always necessary for substrate recognition (Adams, 2003). Together with previous studies, our data reinforce the notion that early steps in phot1 signalling primarily involve changes in protein phosphorylation status (Ueno et al, 2005; Pedmale and Liscum, 2007; Sullivan et al, 2009).
PKS4 is an integration point between phototropin and phytochrome signalling
PKS4 plays a role in phytochrome signalling in red and far-red light (Schepens et al, 2008), as well as in phototropin signalling in blue light (Lariguet et al, 2006). How phytochromes modulate phototropism is not yet fully understood. It has been proposed that phyA exerts its role through regulation of nuclear gene expression such as PKS1 and RPT2 that are both positive regulators of phototropin signalling (Lariguet et al, 2006; Tsuchida-Mayama et al, 2010; Kami et al, 2012). Here, we report that PKS4L accumulation decreases with phytochrome activity (Figure 4). The phosphorylation status of PKS4 is thus controlled by the combined activities of phot1 and phytochromes, suggesting that PKS4 constitutes a molecular link between phot1 and phytochrome signalling in blue light. However, the mechanism by which the phytochromes control PKS4 phosphorylation remains to be determined. Phytochromes may control PKS4 phosphorylation by modulating the activity of a phosphatase and/or kinase in the cytosol. Alternatively, phytochromes may control the expression of genes coding for enzymes that modulate PKS4 phosphorylation.
PKS4L formation negatively regulates phototropism
It was previously shown that strong blue light treatments can negatively regulate phototropism, however, the underlying molecular mechanisms are unknown (Janoudi and Poff, 1991). Our data suggest that light-dependent PKS4 phosphorylation may contribute to this mechanism with PKS4L playing a negative role in phototropism. The increase in PKS4L accumulation observed in phytochrome-deficient mutants occurs at low blue light intensities where phytochromes are known to promote phototropic curvature (Parks et al, 1996; Janoudi et al, 1997; Lariguet and Fankhauser, 2004). In addition, the decrease in PKS4L accumulation following a red light pre-treatment also coincides with the phytochrome-mediated enhancement of phototropism (Liscum and Briggs, 1996; Parks et al, 1996; Figure 4B). More importantly support for a model where PKS4L functions to negatively regulate phototropism comes from our physiological experiments. Enhancement of the accumulation of PKS4L by increasing the intensity of blue light perceived by the plants or by pharmacological treatment involving the protein phosphatase inhibitor CN (Figure 8) leads to reduced phototropic curvature in WT but not in the pks4 mutant. Cantharidin treatment enhances phot2-mediated phototropism, but inhibits phot1-mediated phototropism (Pedmale and Liscum, 2007; Tseng and Briggs, 2010) (this study), implying that phosphatase activity has an antagonistic role in phot1 and phot2 signalling. PKS4 phosphorylation status is controlled by a PP2A (Figure 4) while phot1-dependent NPH3 dephosphorylation involves PP1 activity (Pedmale and Liscum, 2007). Phot1 signalling is thus regulated by multiple phosphatases to coordinate phototropic signalling. NPH3 dephosphorylation is proposed to be essential for phototropism (Pedmale and Liscum, 2007). On the other hand, PKS4 phosphorylation may constitute part of a negative feedback loop to prevent excessive phototropism or serve as a delay mechanism. This situation would then be similar to NPH3 dephosphorylation except that PKS4 is first phosphorylated by phot1 in response to light. Understanding how these early phosphorylation events impact those associated with controlling polar auxin transport are important questions for the future.
The role of phot-mediated phosphorylation during plant growth
Phototropins control a variety of physiological and growth responses throughout the life cycle of plants that can all be linked to the maximization of their photosynthetic potential (Christie, 2007). Such responses are particularly important during the early phases of plant development when carbon is a major factor limiting plant growth (Pantin et al, 2011). Among the phot-mediated responses members of the PKS family are specifically required for root and hypocotyl phototropism, leaf flattening and positioning (de Carbonnel et al, 2010). Phototropin-mediated phosphorylation is essential for all tested phot responses in Arabidopsis (Inoue et al, 2008a, 2011). Here, we studied the role of PKS4 phosphorylation during young seedling development, as our previous investigation showed that PKS4 has a selective role in the control of hypocotyl tropic responses (Lariguet et al, 2006; Schepens et al, 2008; de Carbonnel et al, 2010). Similarly a role for ABCB19, another recently identified substrate of phot1, is currently not known for phot-mediated responses beyond phototropism (Christie et al, 2011). It will be interesting to determine how phot-mediated phosphorylation events control plant growth later in development.
Materials and methods
Plant material and growth conditions
The Columbia (Col-O) ecotype of A. thaliana was used as the WT. All of the following mutant alleles were in the Col-O background: pks4-1 (Lariguet et al, 2006), phot1-5 (Huala et al, 1997), phot2-1 (Kagawa et al, 2001), cry1cry2 (Duek and Fankhauser, 2003) and nph4 (Liscum and Briggs, 1996). The mutant phyabcde was in ft background (Strasser et al, 2010). Transgenic lines overexpressing PINOID, or expressing wild-type or mutated versions of phot1 were described elsewhere (Geldner et al, 2003; Cho et al, 2007; Inoue et al, 2008a). Seeds were surface-sterilized, plated and treated as described (Lariguet and Fankhauser, 2004). For protein extraction, 50 seeds were sown for each time point. For CN and hypocotyl curvature measurement, seeds were sown on nylon mesh (160 μm, Micropore) disposed on the surface of the plate. Seedlings were grown for 3 days in darkness at 22°C before the indicated treatment. Light intensities were determined with an International Light IL1400A photometer (Newburyport, MA) equipped with an SEL033 probe with appropriate light filters.
CN and λ-phosphatase treatments
Nylon meshes with 3-day-old etiolated seedlings were transferred onto freshly prepared plates supplemented by 0, 0.1, 1 or 10 μM cantharidin (Sigma) and 1% DMSO, 1 h before indicated light treatment. λ-Phosphatase treatment was performed as described in Lorrain et al (2008).
SDS–PAGE and immunoblot analysis
Total proteins (100 μl 2X Laemmli buffer for 20 mg fresh weight; 10 μg per lane) were separated on 8% SDS/PAGE gels and transferred onto nitrocellulose with 100 mM CAPS pH 11+10% (v/v) ethanol. The blots were probed with anti-DET3, anti-phot1 (Lariguet et al, 2006). Polyclonal anti-PKS4 antibodies were raised as follow: PKS4 full cDNA sequence was cloned into pHIS8-3 in frame with N-terminal 8-histidine (His) tag (to generate pIS25). His-PKS4 recombinant proteins were produced in E. coli by inducing gene expression with 0.2 mM IPTG for 4 h at 37°C. PKS4 proteins were purified from inclusion bodies and used to immunize rabbits (Eurogentec). After three boosts, the serum of one rabbit was retrieved and polyclonal antibodies specific to PKS4 were obtained by affinity purification (using purified GST-PKS4 proteins). Anti-PKS4 antibodies were used at a 1/500 dilution in PBS, 0.1% Tween-20, and 5% non-fat milk. Chemiluminescence signals were generated using Immobilon Western HRP Substrate (Millipore). Signals were captured with a Fujifilm ImageQuant LAS 4000 mini CCD camera system and quantifications were performed with ImageQuant TL software (GE Healthcare).
In-vitro phosphorylation assay
P1L2K polypeptides of the wild-type and the D788A mutant were prepared after the procedures reported previously (Okajima et al, 2011). The phot1 polypeptides were incubated with each PKS4 polypeptide or GST tag at 20°C for 30 min in a kinase reaction buffer containing 20 mM Tris–HCl (pH 7.8), 100 mM NaCl, 1 mM Na2EGTA, 10% (w/v) glycerol, 20 μM ATP and 74 kBq of (γ-32P) ATP in the presence of 10 mM MnCl2. The effect of blue light on phosphorylation was measured by irradiation with a blue LED illuminator using mock irradiation as a control. Reaction was stopped by adding an SDS–PAGE sample buffer followed by boiling for 3 min. The samples were run on SDS–PAGE and then the proteins were blotted onto PVDF membrane. After Coomassie staining, phosphorylation of the proteins was visualized with imaging plate (Fujifilm) and a STORM scanner (GE Healthcare).
Hypocotyl growth measurement
Two-day-old etiolated seedlings (WT, pks4, phot1) were irradiated with 0.5, 10 or 100 μmol m−2 s−1 blue light from above. Pictures were taken every 15 min, during 90 min before and after light treatment, using an Infra Red CCD Camera system. Hypocotyl growth rates were deduced from hypocotyl tips coordinates that were determined using HypoPhen software (http://www2.unil.ch/cbg/index.php?title=HypoPhen; Kami et al, 2012). Mean, standard error and Student’s t-test were performed on 20 seedlings.
Hypocotyl curvature
Seedlings were grown on vertically orientated plates for 3 days in darkness at 22°C. For continuous light treatment, seedlings were irradiated with 0.1 μmol m−2 s−1 unilateral blue light for 10 h. For pulse-light irradiation, seedlings were first sensitized by irradiation with 1800 μmol red light and kept in darkness for 90 min, and irradiated with a 10-min pulse of blue light at the indicated fluence rate and then kept in darkness at 22°C for 4 h. Seedlings with cotyledons in the opposite side of the incident light source were selected for measurement. Angles formed by the hypocotyl relative to vertical were measured with the NIH image software. Means, standard errors and Student’s t-test were performed on 20 seedlings minimum.
Supplementary Material
Acknowledgments
We thank Martine Trevisan and Laure Allenbach for technical support and Chitose Kami and Séverine Lorrain for discussions. This work was supported by the University of Lausanne, a grant from the Swiss National Science Foundation (3100A0-112638) to CF and the SystemsX.ch grant ‘plant growth in a changing environment’ to CF and SB, by Grants-in-Aid from the MEXT of Japan, No. 22120005 to ST and IS was supported by an EMBO LT post-doctoral fellowship.
Author contributions: ED designed the research; performed research; analysed data; wrote the paper. IS designed the research; performed research; analysed data. KO designed the research; performed research; analysed data. MH contributed new analytic/computational tool. SB contributed new analytic/computational tool. JC designed the research; performed research; analysed data. KIS contributed new analytic tool; analysed data. ST designed the research; analysed data. CF designed the research; analysed data; wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adams JA (2003) Activation loop phosphorylation and catalysis in protein kinases: is there functional evidence for the autoinhibitor model? Biochemistry 42: 601–607 [DOI] [PubMed] [Google Scholar]
- Aihara Y, Yamamoto T, Okajima K, Yamamoto K, Suzuki T, Tokutomi S, Tanaka K, Nagatani A (2012) Mutations in the N-terminal flanking region of the blue-light sensing domain LOV2 disrupt its repressive activity on the kinase domain in the Chlamydomonas phototropin. J Biol Chem 287: 9901–9909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccalandro HE, De Simone SN, Bergmann-Honsberger A, Schepens I, Fankhauser C, Casal JJ (2008) PHYTOCHROME KINASE SUBSTRATE1 regulates root phototropism and gravitropism. Plant Physiol 146: 108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Tseng TS, Kaiserli E, Sullivan S, Christie JM, Briggs WR (2007) Physiological roles of the light, oxygen, or voltage domains of phototropin 1 and phototropin 2 in Arabidopsis. Plant Physiol 143: 517–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM (2007) Phototropin blue-light receptors. Annu Rev Plant Biol 58: 21–45 [DOI] [PubMed] [Google Scholar]
- Christie JM, Swartz TE, Bogomolni RA, Briggs WR (2002) Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J 32: 205–219 [DOI] [PubMed] [Google Scholar]
- Christie JM, Yang H, Richter GL, Sullivan S, Thomson CE, Lin J, Titapiwatanakun B, Ennis M, Kaiserli E, Lee OR, Adamec J, Peer WA, Murphy AS (2011) phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol 9: e1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carbonnel M, Davis P, Roelfsema MR, Inoue S, Schepens I, Lariguet P, Geisler M, Shimazaki K, Hangarter R, Fankhauser C (2010) The Arabidopsis PHYTOCHROME KINASE SUBSTRATE2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol 152: 1391–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deruere J, Jackson K, Garbers C, Soll D, Delong A (1999) The RCN1-encoded A subunit of protein phosphatase 2A increases phosphatase activity in vivo. Plant J 20: 389–399 [DOI] [PubMed] [Google Scholar]
- Ding Z, Galvan-Ampudia CS, Demarsy E, Langowski L, Kleine-Vehn J, Fan Y, Morita MT, Tasaka M, Fankhauser C, Offringa R, Friml J (2011) Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat Cell Biol 13: 447–452 [DOI] [PubMed] [Google Scholar]
- Duek PD, Fankhauser C (2003) HFR1, a putative bHLH transcription factor, mediates both phytochrome A and cryptochrome signalling. Plant J 34: 827–836 [DOI] [PubMed] [Google Scholar]
- Esmon CA, Tinsley AG, Ljung K, Sandberg G, Hearne LB, Liscum E (2006) A gradient of auxin and auxin-dependent transcription precedes tropic growth responses. Proc Natl Acad Sci USA 103: 236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Yeh KC, Lagarias JC, Zhang H, Elich TD, Chory J (1999) PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 284: 1539–1541 [DOI] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jurgens G (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Han IS, Tseng TS, Eisinger W, Briggs WR (2008) Phytochrome A regulates the intracellular distribution of phototropin 1-green fluorescent protein in Arabidopsis thaliana. Plant Cell 20: 2835–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E (2000) The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12: 757–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper SM, Christie JM, Gardner KH (2004) Disruption of the LOV-Jalpha helix interaction activates phototropin kinase activity. Biochemistry 43: 16184–16192 [DOI] [PubMed] [Google Scholar]
- Harper SM, Neil LC, Gardner KH (2003) Structural basis of a phototropin light switch. Science 301: 1541–1544 [DOI] [PubMed] [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han IS, Larsen E, Briggs WR (1997) Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science 278: 2120–2123 [DOI] [PubMed] [Google Scholar]
- Iino M (2006) Toward understanding the ecological functions of tropisms: interactions among and effects of light on tropisms. Curr Opin Plant Biol 9: 89–93 [DOI] [PubMed] [Google Scholar]
- Inoue S, Kinoshita T, Matsumoto M, Nakayama KI, Doi M, Shimazaki K (2008a) Blue light-induced autophosphorylation of phototropin is a primary step for signaling. Proc Natl Acad Sci USA 105: 5626–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Kinoshita T, Takemiya A, Doi M, Shimazaki K (2008b) Leaf positioning of Arabidopsis in response to blue light. Mol Plant 1: 15–26 [DOI] [PubMed] [Google Scholar]
- Inoue S, Matsushita T, Tomokiyo Y, Matsumoto M, Nakayama KI, Kinoshita T, Shimazaki K (2011) Functional analyses of the activation loop of phototropin2 in Arabidopsis. Plant Physiol 156: 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi AK, Poff KL (1991) Characterization of adaptation in phototropism of Arabidopsis thaliana. Plant Physiol 95: 517–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi AK, Whitelam GC, Gordon WR, Poff KL (1997) Both phytochrome A and phytochrome B are required for the normal expression of phototropism in Arabidopsis thaliana seedlings. Physiol Plant 101: 278–282 [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kami C, Hersch M, Trevisan M, Genoud T, Hiltbrunner A, Bergmann S, Fankhauser C (2012) Nuclear Phytochrome A Signaling Promotes Phototropism in Arabidopsis. Plant Cell 24: 566–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kami C, Lorrain S, Hornitschek P, Fankhauser C (2010) Light-regulated plant growth and development. Curr Topics Dev Biol 91: 29–66 [DOI] [PubMed] [Google Scholar]
- Keuskamp DH, Sasidharan R, Pierik R (2010) Physiological regulation and functional significance of shade avoidance responses to neighbors. Plant Signal Behav 5: 655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konjevic R, Steinitz B, Poff KL (1989) Dependence of the phototropic response of Arabidopsis thaliana on fluence rate and wavelength. Proc Natl Acad Sci USA 86: 9876–9880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariguet P, Boccalandro HE, Alonso JM, Ecker JR, Chory J, Casal JJ, Fankhauser C (2003) A growth regulatory loop that provides homeostasis to phytochrome a signaling. Plant Cell 15: 2966–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariguet P, Fankhauser C (2004) Hypocotyl growth orientation in blue light is determined by phytochrome A inhibition of gravitropism and phototropin promotion of phototropism. Plant J 40: 826–834 [DOI] [PubMed] [Google Scholar]
- Lariguet P, Schepens I, Hodgson D, Pedmale UV, Trevisan M, Kami C, de Carbonnel M, Alonso JM, Ecker JR, Liscum E, Fankhauser C (2006) PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc Natl Acad Sci USA 103: 10134–10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Mackintosh C, Casida JE (1993) Protein phosphatase 2A and its [3H]cantharidin/[3H]endothall thioanhydride binding site. Inhibitor specificity of cantharidin and ATP analogues. Biochem Pharmacol 46: 1435–1443 [DOI] [PubMed] [Google Scholar]
- Liscum E, Briggs WR (1996) Mutations of Arabidopsis in potential transduction and response components of the phototropic signaling pathway. Plant Physiol 112: 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C (2008) Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Matsuoka D, Tokutomi S (2005) Blue light-regulated molecular switch of Ser/Thr kinase in phototropin. Proc Natl Acad Sci USA 102: 13337–13342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockler T, Yang H, Yu X, Parikh D, Cheng YC, Dolan S, Lin C (2003) Regulation of photoperiodic flowering by Arabidopsis photoreceptors. Proc Natl Acad Sci USA 100: 2140–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molas ML, Kiss JZ (2008) PKS1 plays a role in red-light-based positive phototropism in roots. Plant Cell Environ 31: 842–849 [DOI] [PubMed] [Google Scholar]
- Nagashima A, Suzuki G, Uehara Y, Saji K, Furukawa T, Koshiba T, Sekimoto M, Fujioka S, Kuroha T, Kojima M, Sakakibara H, Fujisawa N, Okada K, Sakai T (2008) Phytochromes and cryptochromes regulate the differential growth of Arabidopsis hypocotyls in both a PGP19-dependent and a PGP19-independent manner. Plant J 53: 516–529 [DOI] [PubMed] [Google Scholar]
- Ohgishi M, Saji K, Okada K, Sakai T (2004) Functional analysis of each blue light receptor, cry1, cry2, phot1, and phot2, by using combinatorial multiple mutants in Arabidopsis. Proc Natl Acad Sci USA 101: 2223–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima K, Matsuoka D, Tokutomi S (2011) LOV2-linker-kinase phosphorylates LOV1-containing N-terminal polypeptide substrate via photoreaction of LOV2 in Arabidopsis phototropin1. FEBS Lett 585: 3391–3395 [DOI] [PubMed] [Google Scholar]
- Pantin F, Simonneau T, Rolland G, Dauzat M, Muller B (2011) Control of leaf expansion: a developmental switch from metabolics to hydraulics. Plant Physiol 156: 803–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Quail PH, Hangarter RP (1996) Phytochrome A regulates red-light induction of phototropic enhancement in Arabidopsis. Plant Physiol 110: 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedmale UV, Liscum E (2007) Regulation of phototropic signaling in Arabidopsis via phosphorylation state changes in the phototropin 1-interacting protein NPH3. J Biol Chem 282: 19992–20001 [DOI] [PubMed] [Google Scholar]
- Rizzini L, Favory JJ, Cloix C, Faggionato D, O'Hara A, Kaiserli E, Baumeister R, Schafer E, Nagy F, Jenkins GI, Ulm R (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332: 103–106 [DOI] [PubMed] [Google Scholar]
- Salomon M, Knieb E, von Zeppelin T, Rudiger W (2003) Mapping of low- and high-fluence autophosphorylation sites in phototropin 1. Biochemistry 42: 4217–4225 [DOI] [PubMed] [Google Scholar]
- Schepens I, Boccalandro HE, Kami C, Casal JJ, Fankhauser C (2008) PHYTOCHROME KINASE SUBSTRATE4 modulates phytochrome-mediated control of hypocotyl growth orientation. Plant Physiol 147: 661–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellaro R, Hoecker U, Yanovsky M, Chory J, Casal JJ (2009) Synergism of red and blue light in the control of Arabidopsis gene expression and development. Curr Biol 19: 1216–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe-Evans EL, Luesse DR, Liscum E (2001) The enhancement of phototropin-induced phototropic curvature in Arabidopsis occurs via a photoreversible phytochrome A-dependent modulation of auxin responsiveness. Plant Physiol 126: 826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser B, Sanchez-Lamas M, Yanovsky MJ, Casal JJ, Cerdan PD (2010) Arabidopsis thaliana life without phytochromes. Proc Natl Acad Sci USA 107: 4776–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S, Thomson CE, Kaiserli E, Christie JM (2009) Interaction specificity of Arabidopsis 14-3-3 proteins with phototropin receptor kinases. FEBS Lett 583: 2187–2193 [DOI] [PubMed] [Google Scholar]
- Sullivan S, Thomson CE, Lamont DJ, Jones MA, Christie JM (2008) In vivo phosphorylation site mapping and functional characterization of Arabidopsis phototropin 1. Mol Plant 1: 178–194 [DOI] [PubMed] [Google Scholar]
- Tokutomi S, Matsuoka D, Zikihara K (2008) Molecular structure and regulation of phototropin kinase by blue light. Biochim Biophys Acta 1784: 133–142 [DOI] [PubMed] [Google Scholar]
- Tseng TS, Briggs WR (2010) The Arabidopsis rcn1-1 mutation impairs dephosphorylation of Phot2, resulting in enhanced blue light responses. Plant Cell 22: 392–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida-Mayama T, Nakano M, Uehara Y, Sano M, Fujisawa N, Okada K, Sakai T (2008) Mapping of the phosphorylation sites on the phototropic transducer, NPH3. Plant Sci 174: 626–633 [Google Scholar]
- Tsuchida-Mayama T, Sakai T, Hanada A, Uehara Y, Asami T, Yamaguchi S (2010) Role of the phytochrome and cryptochrome signaling pathways in hypocotyl phototropism. Plant J 62: 653–662 [DOI] [PubMed] [Google Scholar]
- Turck F, Fornara F, Coupland G (2008) Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59: 573–594 [DOI] [PubMed] [Google Scholar]
- Ueno K, Kinoshita T, Inoue S, Emi T, Shimazaki K (2005) Biochemical characterization of plasma membrane H+-ATPase activation in guard cell protoplasts of Arabidopsis thaliana in response to blue light. Plant Cell Physiol 46: 955–963 [DOI] [PubMed] [Google Scholar]
- Wan YL, Eisinger W, Ehrhardt D, Kubitscheck U, Baluska F, Briggs W (2008) The subcellular localization and blue-light-induced movement of phototropin 1-GFP in etiolated seedlings of Arabidopsis thaliana. Mol Plant 1: 103–117 [DOI] [PubMed] [Google Scholar]
- Whippo CW, Hangarter RP (2003) Second positive phototropism results from coordinated co-action of the phototropins and cryptochromes. Plant Physiol 132: 1499–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whippo CW, Hangarter RP (2004) Phytochrome modulation of blue-light-induced phototropism. Plant Cell Environ 27: 1223–1228 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.