Abstract
The cellular and molecular mechanisms that control lung homeostasis and regeneration are still poorly understood. It has been proposed that a population of cells exists in the mouse lung with the potential to differentiate into all major lung bronchioalveolar epithelium cell types in homeostasis or in response to virus infection. A new population of E-Cad/Lgr6+ putative stem cells has been isolated, and indefinitely expanded from human lungs, harbouring both, self-renewal capacity and the potency to differentiate in vitro and in vivo. Recently, a putative population of human lung stem cells has been proposed as being c-Kit+. Unlike Integrin-α6+ or c-Kit+ cells, E-Cad/Lgr6+ single-cell injections in the kidney capsule produce differentiated bronchioalveolar tissue, while retaining self-renewal, as they can undergo serial transplantations under the kidney capsule or in the lung. In addition, a signalling network involving the p38α pathway, the activation of p53 and the regulation of the miR-17-92 cluster has been identified. Disruption of the proper cross-regulation of this signalling axis might be involved in the promotion of human lung diseases.
Keywords: human, lung, miR-17-92, p38, stem cells
Introduction
The existence of a population of lung cells that could be the source of all main cell types of the lung bronchioalveolar epithelium is controversial and has been the focus of many recent investigations. Unlike in other tissues, the understanding of the cellular and molecular mechanisms that maintain adult lung homeostasis and may be involved in regeneration is in its infancy (Morrisey and Hogan, 2010; Rock and Hogan, 2011; Weiss et al, 2011). Recently, a number of groups have reported the existence of certain populations of putative stem cells in mouse lungs (Giangreco et al, 2002; Kim et al, 2005; Ventura et al, 2007; Teisanu et al, 2009; Chapman et al, 2011). These cells have the potential to differentiate into the major bronchiolar (Clara) and Alveolar type-1 (AT1) or type-2 (AT2) cells. However, if the cellular hierarchy of the mouse alveolar epithelium is still poorly known, the human lung biology has been even more neglected. A putative population of human lung stem cells (HLSCs) has been proposed as being c-Kit+ (Kajstura et al, 2011). This population would have the potential to differentiate not only into lung epithelial cells but also into mesenchymal and endothelial tissues. Nevertheless, this proposed cell type has been the subject of a much greater controversy and its origin and defined profile remain unclear (Anversa et al, 2011). Other stem cell markers have been extensively used to detect and isolate stem cells from various epithelial tissues (Kumar et al, 2011). Among the most specific markers found to label epithelial stem cells are the members of the family of leucine-rich repeat-containing G-protein-coupled receptors (Lgr), and in particular Lgr5 and Lgr6 (Barker et al, 2007; Barker and Clevers, 2010; Snippert et al, 2010).
Several extracellular signals have been linked to the regulation of embryonic lung stem cells during development (Que et al, 2007; Lange et al, 2009; Morrisey and Hogan, 2010; Rock et al, 2011). However, the intracellular mechanisms involved in adult lung homeostasis and regeneration are still mostly unknown. One intracellular signal involved in mouse lung homeostasis is the MAPK p38α pathway (Ventura et al, 2007). The activity of this kinase is necessary to maintain the differentiation mechanisms and control the self-renewal of mouse lung stem cells (Hui et al, 2007; Ventura et al, 2007; Liu et al, 2008). p38α regulates transcription factors (e.g., C/EBPα) involved in lung differentiation (Efimova et al, 2002; Martis et al, 2006) and downregulates several factors involved in stem/progenitor cell proliferation (Sugahara et al, 2001; Ventura et al, 2007). Nevertheless, the mediators and cross-talking pathways that may be involved in maintaining HLSCs homeostasis are not yet known.
Besides kinase pathways, the role of some microRNAs in the regulation of lung development and cancer has been reported (Qian et al, 2008). The miR-17-92 cluster has been found to play roles in both processes (Mendell, 2008), with a relevant function in the lung. The miR-17-92 cluster is associated with a negative regulation of lung development (Lu et al, 2007; Ventura et al, 2008) and a promotion of cancer cell proliferation (Hayashita et al, 2005). One way to regulate the activity of this cluster is by controlling its levels, and miR-17-92 promoter activity can be suppressed by the transcription factor p53, downregulating pri-miR-17-92 expression (Yan et al, 2009).
Understanding the mechanisms involved in the proper function of HLSCs, and defining potential markers that could be used to detect and isolate a specific and homogenous population of lung stem cells are absolutely essential, prior to considering any possible cellular or molecular therapy involving stem cells in the human lung.
Results
Isolation and characterization of a human alveolar E-Cad/Lgr6 multipotent population
The lack of studies investigating adult lung homeostasis prompted us to focus on the identification and isolation of a population of putative HLSCs. Using a similar protocol as Kim et al (2005) but CD34− (Ventura et al, 2007), cells have been isolated from mouse lungs that could be indefinitely expanded in vitro while retaining their self-renewal and differentiation potential (Ventura et al, 2007). A similar, modified approach was used in an attempt to detect and isolate HLSCs.
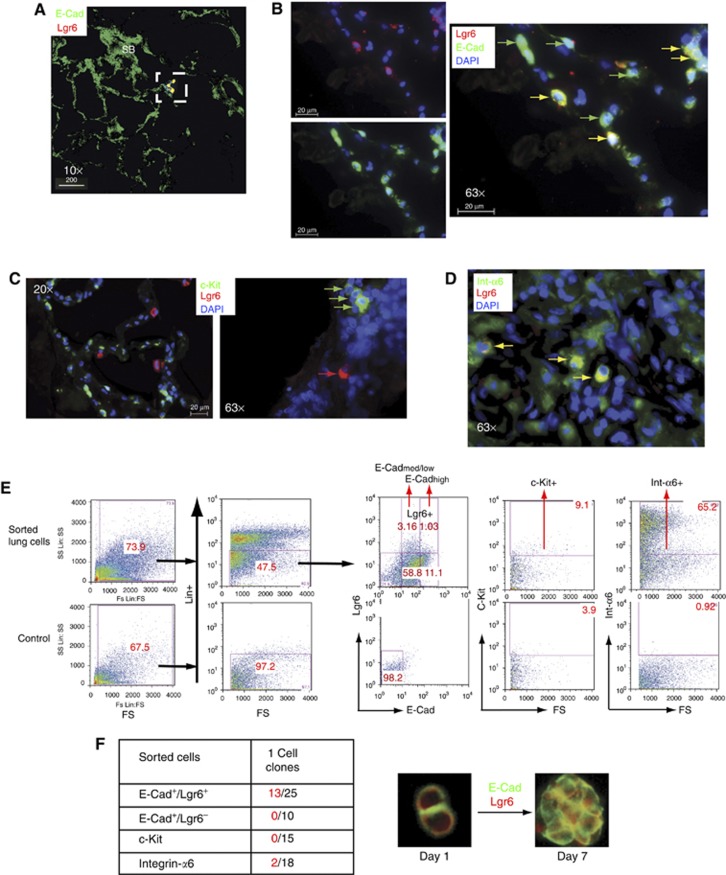
The stem cell marker Sca-1 was previously used to isolate mouse stem cells (Ventura et al, 2007). However, it is not present in humans, and other markers that might be specifically expressed in human lung cells were investigated (Holmes and Stanford, 2007). The expression, in human lungs, of published putative epithelial and stem cell markers, which may be suitable to be used as targets, such as E-Cadherin, c-Kit, Integrin-α6, Lgr5 and Lgr6, was tested. Only Lgr6 was found to be restricted to a discrete population of E-Cadherin-positive cells (Figure 1A and B; Supplementary Figure S1A and B) that did not express other lung differentiation markers. They localized mainly near small bronchioles (Figure 1A; Supplementary Figure S1E) and endothelium (Supplementary Figure S1A and D) and co-expressed Lgr5 (Supplementary Figure S1C). c-Kit (Figure 1C) and Integrin-α6 (Figure 1D) were expressed in a heterogeneous number of cell types, including haematopoietic and endothelial lineages, and Lgr5 was also expressed in Clara cells (Supplementary Figure S2D). Lgr6 and c-Kit did not co-express (Figure 1C; Supplementary Figure S2A and B) labelling distinct cells. Based on the previous results, several populations were sorted using a preliminary negative selection to avoid mesenchymal, endothelial or haematopoietic (Lin−) contaminants (Figure 1E). Single cells (from four human lung samples) from the different populations were used to test for clonal capacity in serial dilution assays (Supplementary Figure S2E). E-Cad+/Lgr6− and c-Kit+ single cells failed to grow clonally in vitro and only two clones (15%) of single Integrin-α6+ cells grew after four passages (Figure 1F). However, 13 of 25 (52%) E-Cad/Lgr6+ single-cell clones were successfully expanded for >15 passages (Figure 1F). E-Cad/Lgr6+ cells expressed Integrin-α6 (Figure 1D; Supplementary Figure S2C) and could be considered as a sub-population within the lung Integrin-α6 heterogeneous population.
Figure 1.
Isolation, clonal expansion and in vitro characterization of human lung stem cells. (A) Confocal section, of a 3D image, showing E-Cad/Lgr6+ cells nearby small bronchioles (SB) in the human lung. (B) E-Cad/Lgr6+ cells (yellow arrows) in the epithelium and E-Cad+ epithelial cells (green arrows). (C) Immunofluorescent staining of human lung tissue with c-Kit+ (green) and Lgr6+ (red) labelling different cells. (D) A small number of Integrin-α6+ cells (green) express Lgr6 (yellow) in the human lung bronchioalveolar epithelium. (E) Lung cells isolated from human lung tissue (from three different patients), and then negative sorted for CD45, CD31, CD73 and CD34 (Lin−) and positive for E-Cad and Lgr6, c-Kit or Integrin-α6 and were used for functional assays or clonal expansion. (F) Clonogenicity assay of E-Cad/Lgr6+, c-Kit+ or Integrin-α6+ single sorted cells. Cells from three different patients were used. Positive colonies (red) and total single cells seeded (black). Images of E-Cad/Lgr6+ single cell first division at day 1 and clonal aggregate at day 7 (see also Supplementary Figure S1).
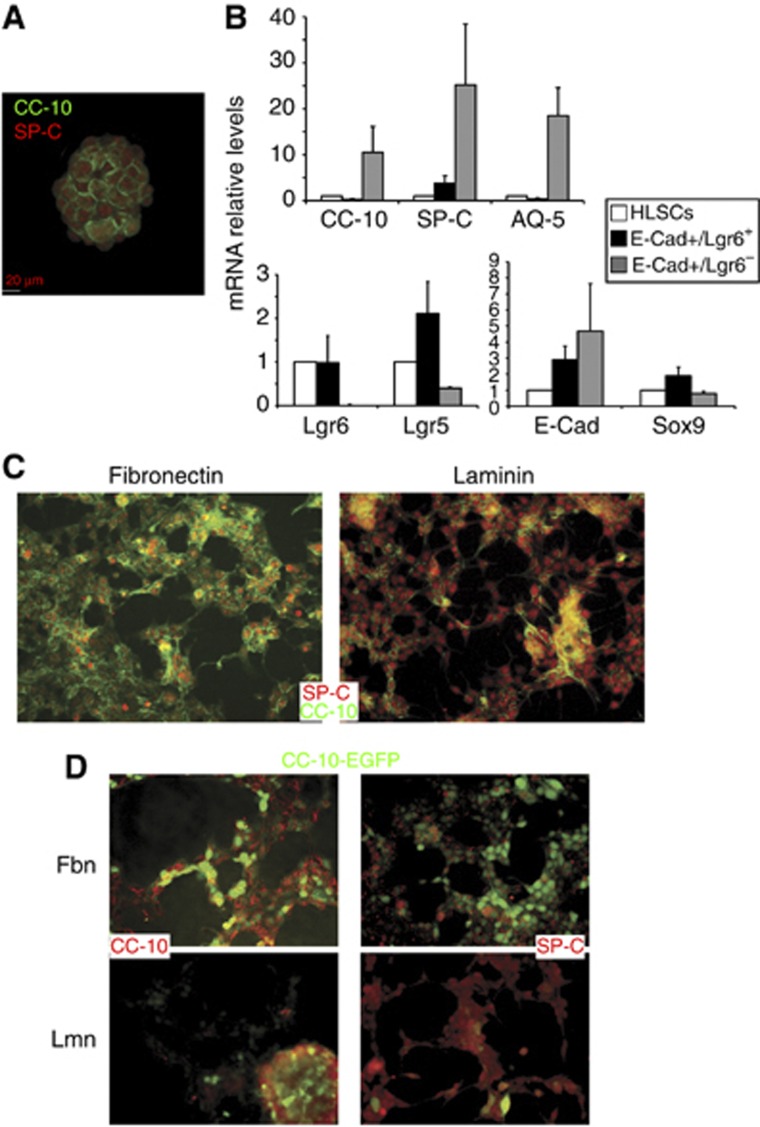
Clonally derived E-Cad/Lgr6+ cells (HLSCs) grew in vitro forming aggregates that could be expanded for >50 passages while expressing lung-specific (SP-C, CC-10, AQ-5), epithelial (E-Cad) and stem cell markers (Sox9, Lgr5/6, Integrin-α6) (Supplementary Figure S3). Although in-vivo E-Cad/Lgr6+ cells did not express the AT2 (SP-C) and Clara (CC-10) cell markers, the in-vitro aggregates were positive for these lung markers (Figure 2A). In general, there was a reduction of lung-specific and epithelial markers, and an increase in mRNA expression of stem cell markers in the clonally expanded (HLSCs) and the freshly isolated E-Cad+/Lgr6+ cells, compared to E-Cad+/Lgr6− (Figure 2B; Supplementary Figure S3). In vitro, HLSCs responded to matrices morphologically, forming monolayers, and molecularly, differentially expressing AT2 (SP-C) or Clara (CC-10) cell markers (Figure 2C). HLSCs carrying an EGFP reporter under the control of the CC-10 promoter maintained promoter activity and CC-10 protein levels in fibronectin but lost SP-C expression (Figure 2D, upper). However, on laminin HLSCs shutdown the CC-10 promoter but maintained the alveolar SP-C (Figure 2D, lower) and AQ5 (Supplementary Figure S2F) expression.
Figure 2.
In-vitro expansion and characterization of clonally grown HLSCs. (A) Immunostaining of in vitro expanded HLSC aggregates for the lung markers SP-C (red) and CC-10 (green). (B) mRNA expression of lung (upper) or stem and epithelial cell (lower) markers in clonal HLSCs (empty bars), or freshly isolated E-Cad+/Lgr6+ (black bars) or E-Cad+/Lgr6− (grey bars). A representative experiment of three replicates is depicted as the mean of five different values±s.d., from four different human samples. (C) Immunostaining for SP-C (red) and CC-10 (green) of HLSCs growing in fibronectin or laminin. (D) HLSCs expressing a CC-10-EGFP reporter growing in fibronectin or laminin and stained for SP-C or CC-10 (red). HLSCs, human lung stem cells (see also Supplementary Figure S2).
Regenerative potential of E-Cad/Lgr6+ cells
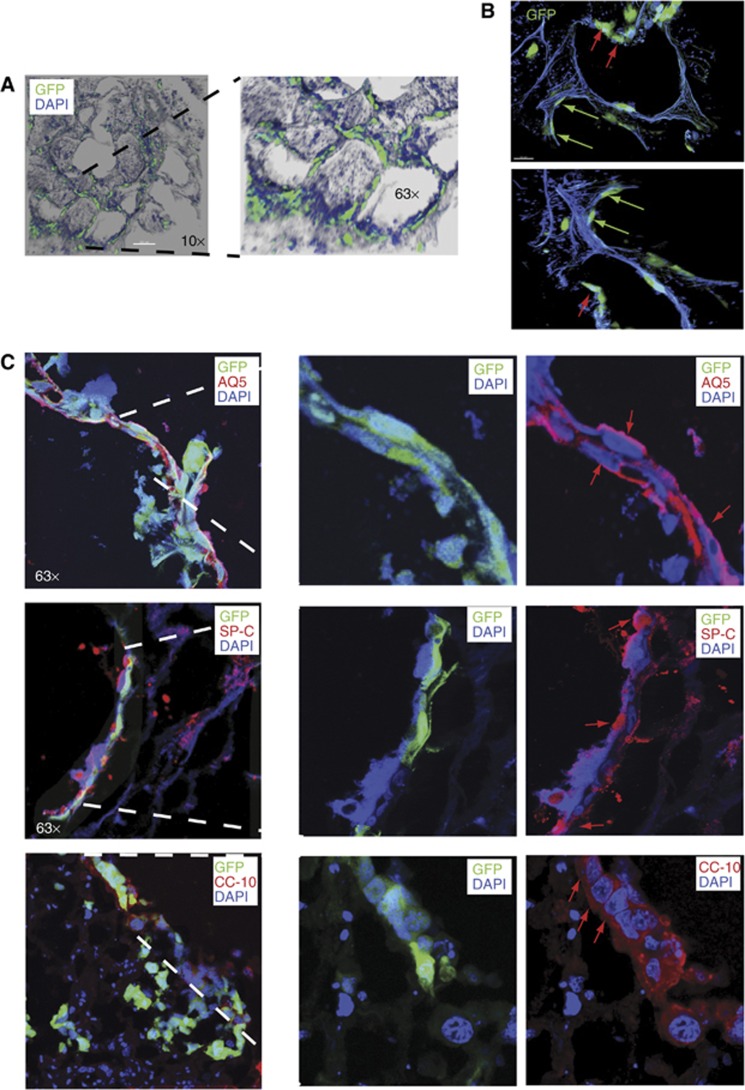
Ex-vivo and in-vivo approaches were used to functionally test the stem cell potential of E-Cad/Lgr6+ (double positive) cells using a bleomycin-induced lung injury model (Aso et al, 1976). E-Cad/Lgr6+ or HLSCs carrying a PGK-EGFP reporter were injected into human lung explants that had been treated with bleomycin in vitro (Supplementary Figures S4A and S5B). The injected cells migrated from the site of injection to the epithelium (Supplementary Figure S4B). HLSCs replenished the dead cells at the damaged alveoli (Figure 3A). The stem cells differentiated and acquired the morphology of AT1 or AT2 cells in the alveoli (Figure 3B). HLSCs were not only migrated but they also got integrated into the endogenous human tissue, mixed with the remaining surviving cells, and differentiated into polygonal AT2 (SP-C positive), elongated AT1 (AQ5), or cuboidal Clara (CC-10) cells, regenerating the bronchioalveolar tissue (Figure 3C; Supplementary Figures S4C and S5). Human lungs have a reduced proportion of bronchiolar tissue compared to mouse, so the contribution to Clara cell (CC-10 positive) differentiation was marginal.
Figure 3.
Assessment of stem cell regenerative potential of HLSCs using bleomycin-induced lung injury. (A) 3D images of HLSCs carrying a PGK-EGFP reporter injected into human lung explants treated with bleomycin in culture. After 7 days, cells integrated into the structure of the lung replenishing the alveoli. (B) EGFP+ cells showed morphologies of AT1 (green arrows) or AT2 (red arrows) cells in the alveoli. (C) Human explants were fixed and stained for lung markers expression. Injected cells mixed with endogenous cells and differentiated into AT1 (AQ5), AT2 (SP-C) or Clara (CC-10) cells (red arrows).
An in-vivo model of bleomycin-induced lung injury was further used to study the potential of E-Cad/Lgr6+ cells to regenerate bronchioalveolar tissue. Bleomycin was injected into the tail vein of nude mice prior to the injection of HLSCs. Mouse tail vein injection (TVI) has been extensively used to deliver cells to the lungs (Kennedy et al, 2003), allowing HLSCs to migrate into the damaged alveolar epithelium (Supplementary Figure S4D). After 10 days, the animals were sacrificed and the lungs were histologically examined by immunofluorescence or used to isolate the resident HLSCs. EGFP+ HLSCs contributed to regenerate the damaged tissue forming small bronchioles (Clara cells) or alveoli (AT1 and AT2 cells), and the engrafted cells expressed specific bronchiolar (CC-10) or alveolar (SP-C, AQ5) markers (Supplementary Figures S4E and S6). The human origin of the engrafted cells was confirmed with a specific anti-human mitochondrial antibody (Supplementary Figure S4F).
Single E-Cad/Lgr6+ cell ability to produce epithelium and recruit a niche
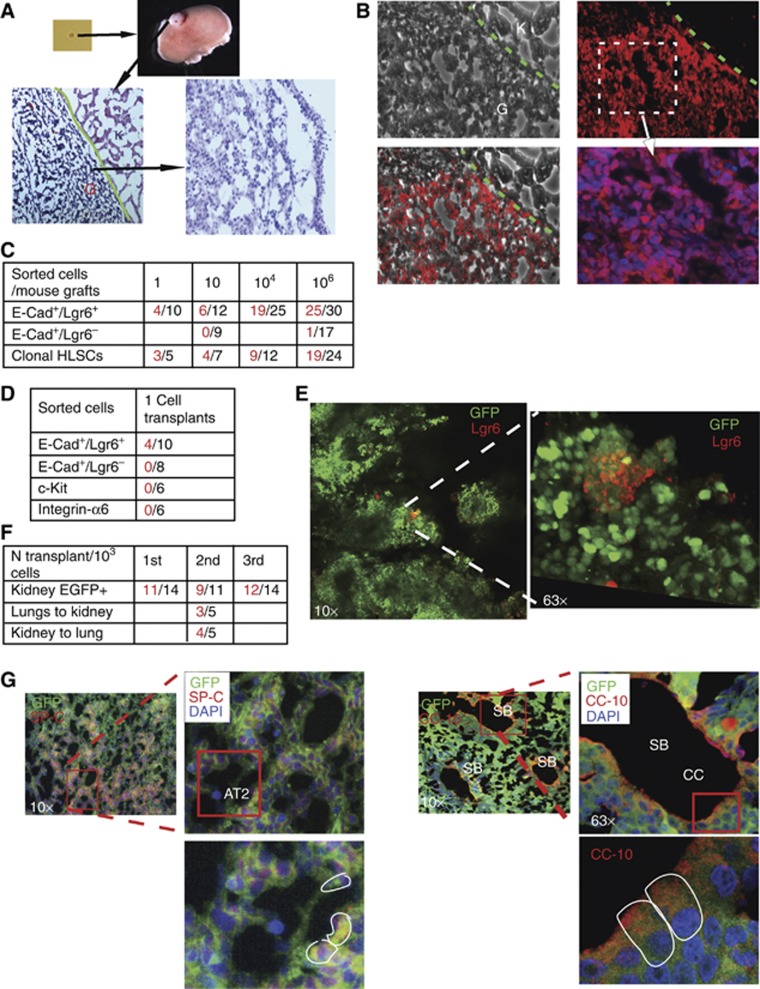
The in-vivo stem cell potential of E-Cad/Lgr6+ cells was further examined using kidney capsule engraftments, which have been used to test other tissue stem cells (Eirew et al, 2008). Different dilutions of sorted E-Cad+/Lgr6+, E-Cad+/Lgr6− or clonal HLSCs were injected under the kidney capsule, and the grafts were examined at different times, showing dose- and time-dependent growth (Figure 4A; Supplementary Figure S7A). The engrafted human E-Cad+/Lgr6+ cells were distinguishable from the mouse kidney using a commercial specific human nuclear antibody (Figure 4B). E-Cad+/Lgr6+ and clonal HLSCs cells engrafted in the kidney, even with single-cell injections, but E-Cad+/Lgr6− cells failed (Figure 4C). Only the single-cell injections were carried out in matrigel to avoid the spillage of the content. Injections of higher number of cells were performed with the cells in PBS solutions. As shown in previous clonal assays, comparative analysis showed a superior stem cell potential of E-Cad+/Lgr6+ cells over E-Cad+/Lgr6−, c-Kit+ or Integrin-α6+ cells in single-cell kidney grafts (Figure 4D).
Figure 4.
In-vivo assessment of the stem cell potential of HLSCs using kidney capsule engraftments. (A) Different dilutions (1–106 cells) of freshly sorted E-Cad/Lgr6+, E-Cad/Lgr6+ or clonal HLSCs cells were injected under the kidney capsule of nude mice. After 4–8 weeks, the kidneys were removed and the grafts stained for H&E. (B) A human nuclear DNA-specific cell marker labelled the engrafted human cells (G) but not the mouse kidney (K). (C) The table shows numbers of mice (right) and positive grafts (left) from different dilutions of different types of injected cells. One-way ANOVA test P<0.05. (D) Single-cell transplantations under the kidney capsule of different types of freshly sorted cells (positive grafts left, total mice right). (E) Eight weeks after injection of a single EGFP+ HLSC, small pools of undifferentiated Lgr6+ stem cells remained in the kidney grafts. (F) Number of mice injected in serial transplantations (right) and positive engraftments (left) and cross-transplantations of sorted EGFP+ cells between lungs and kidney. One-way ANOVA test P<0.05. (G) Fluorescence staining of AT2 (SP-C encircled) or Clara (CC-10 encircled) cells in alveoli or bronchiole structures, respectively. A representative image of 3–5 independent experiments is shown (see also Supplementary Figure S5).
Clonal EGFP expressing cells were used for further characterization of the kidney grafts, allowing better tracking of HLSCs differentiation (Supplementary Figure S7B). After 8 weeks, grafts from single EGFP+ HLSCs injections still harboured small pools of Lgr6+ undifferentiated cells (Figure 4E). Retention of self-renewal potential was demonstrated with serial transplantation of sorted EGFP+ cells from kidney engraftments or cross-transplantation with cells from lung or kidney grafts (Figure 4F).
EGFP+ HLSCs remained epithelial in the kidney grafts, but were able to recruit connective and endothelial tissues to the graft to generate a microenvironment (Supplementary Figure S7C). The engrafted tissue resembled a bronchioalveolar epithelium (Supplementary Figure S7D), with alveolar-like (AT1 and AT2 cells) and bronchiolar-like (Clara cells) structures formed by cells with AT1 and AT2 or Clara cell morphologies (Supplementary Figure S7E). Alveolar and bronchiolar (AT2, AT1 or Clara) cells expressed the specific markers SP-C, AQ5 and CC-10, respectively (Figure 4G; Supplementary Figures S7F and S8A). The human origin of the engrafted epithelium was confirmed with a specific human nuclear DNA antibody (Supplementary Figure S8B).
p38α activity in HLSCs regulates miR-17-92 levels to maintain homeostasis
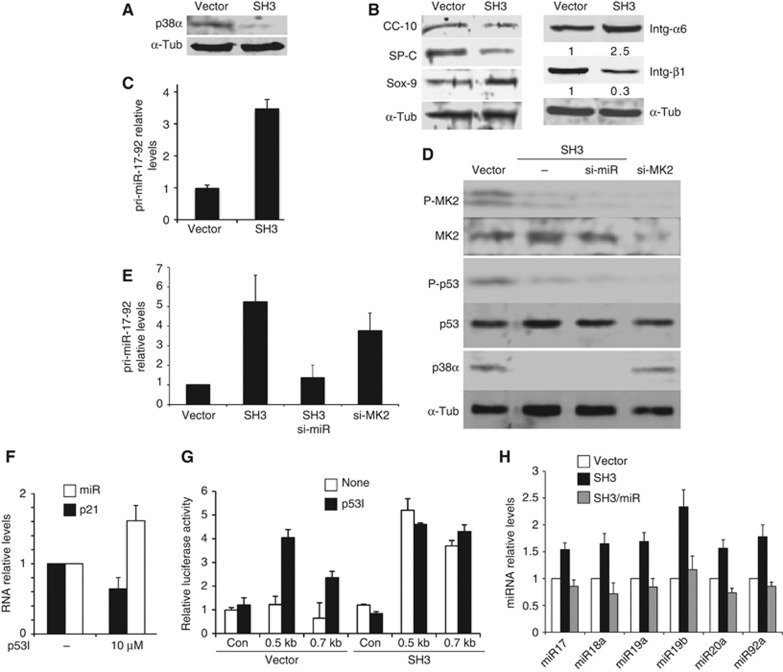
p38αMAPK has been previously shown to regulate the homeostasis of mouse lung stem cells (Ventura et al, 2007). However, the intermediate molecules and cross-talking pathways involved in this physiological control remain elusive. As it was not possible to use the same genetic deletion strategy to study human cells, RNAi was employed to dissect the mechanisms involved in p38α regulation of HLSCs (WT HLSCs carried a scrambled siRNA in all RNAi experiments). Four siRNAs against p38α were tested in HLSCs (Supplementary Figure S9A). Two siRNAs to generate shp38α lentiviral constructs capable of stable p38α knockdown in clonal HLSCs (SH2/SH3) (Figure 5A). These cells had increased proliferation similar to p38α−/− mouse lung cells (data not shown). Protein analysis showed that lack of p38α in HLSCs disrupted the expression of both lung markers and integrins (Figure 5B; Supplementary Figure S9C–E). However, the stem cell marker Sox9 was expressed at higher levels.
Figure 5.
Mechanism of regulation of HLSC homeostasis by p38α involving the MK2-p53-dependent downregulation of pri-miR-17-92 expression. (A) HLSCs stably expressing a p38α short hairpin lentiviral vector (shp38α) (SH3) knocked down p38α protein levels. (B) Western blot analysis of lung-specific and stem markers and integrins in total lysates of WT or SH3 cell in vitro. SH3 cells had disrupted levels of lung and stem cell markers. (C) HLSCs lacking p38α (SH3) showed overexpression of pri-miR-17-92. Values are depicted as mean±standard error of the mean (s.e.m.) from four different experiments. (D) Protein blots of WT, SH3, SH3+simiR or WT+si-MK2 cells. SH3 (lacking P-MK2 activation) and HLSCs deficient in the p38α downstream target MK2, and p53 Serine 20 phosphorylation. (E) HLSCs lacking MK2 overexpressed pri-miR-17-92. A representative experiment of five replicates is depicted as the mean of five different values±s.d. (F) Chemical inhibition of p53 (p53I) increased pri-miR-17-92 levels but reduced the p53 target gene p21. A representative experiment of three replicates is depicted as the mean of five different values±s.d. (G) p53 chemical inhibition or p38α knockdown (SH3) induced pri-miR-17-92 promoter activity. Data are mean of three independent experiments±s.e.m. (H) miR-17-92 components were overexpressed in SH3 cells but the levels were restored to normal by compound knockdown with a pri-miR-17-92 siRNA.
Possible mediators and pathways potentially involved in p38α function were then investigated. One focus of interest was the miR-17-92 cluster of microRNAs, as it has been reported to have an opposing role to p38α, acting as a suppressor of lung differentiation while promoting lung cell proliferation (Hayashita et al, 2005; Qian et al, 2008; Ventura et al, 2008). Analysis of RNA showed upregulation of this cluster in HLSCs lacking p38α (Figure 5C). A potential cross-talk between these two signals was then investigated. Interestingly, it has been reported that p53 may repress miR-17-92 expression (Yan et al, 2009). Furthermore, it is also known that the downstream p38α target MK2 (MAPKAPK-2) (Stokoe et al, 1992) can phosphorylate p53 (Ser20), switching on p53 transcriptional activity (She et al, 2002; Hsu et al, 2011). A similar RNAi strategy was used to study MK2, and its expression was downregulated by specific siRNAs (Supplementary Figure S9B). Knockdown of MK2 in HLSCs resulted in the loss of p53 Ser20 phosphorylation (Figure 5D). Either lack of MK2 (Figure 5E) or inhibition of p53 transcriptional activity (Figure 5F) correlated with increased pri-miR-17-92 levels. Direct repression by p53 was confirmed using a luciferase reporter vector controlled by the human miR-17-92 promoter containing a p53-binding site that has been linked to miR-17-92 repression (Yan et al, 2009). Chemical inhibition of p53 transcriptional activity in HLSCs induced miR-17-92 promoter activity, which was already constitutively active in SH3 (Figure 5G). All mature miR-17-92 components were upregulated in SH3 cells (Figure 5H). This demonstrated a cross-talk between the p38α pathway and a microRNA cluster in HLSCs.
miR-17-92 regulates lung transcription factors and HLSCs differentiation
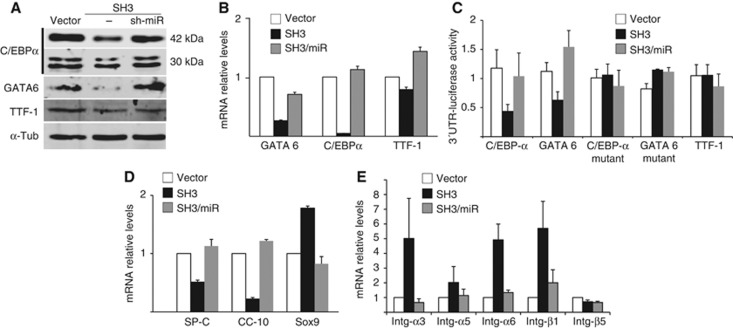
Having demonstrated a negative regulation of miR-17-92 expression by p38α, the cellular and physiological consequences of that cross-talk were investigated. RNAi was used to study the downstream mediators of miR-17-92. Downregulation of this microRNA cluster and p38α with shRNAs allowed further examination of the role of miR-17-92 in HLSCs (Figure 5E and H). As has been previously shown in mouse lung, p38α deficiency is accompanied by reduced expression of lung differentiation factors, although the mechanism supporting this correlation was still unknown (Ventura et al, 2007). Interestingly, among the predicted targets for miR-17-92 found in public databases (TargetScan Human5.1, PicTar) are several lung differentiation transcription factors, including C/EBPα and GATA6. C/EBPα is downregulated in the lungs of p38α−/− mice (Ventura et al, 2007). C/EBPα and GATA6 were found to be downregulated in SH3 cells in a miR-17-92-dependent manner, as normal protein and mRNA levels could be rescued following the concurrent knockdown of miR-17-92 (Figure 6A and B). miR-17-92 did not regulate other non-target lung differentiation transcription factors, such as TTF-1 (Figure 6A and B). Direct repression of C/EBPα and GATA6 by miR-17-92 was confirmed using luciferase reporters carrying the 3′-UTR of each gene containing the putative target site for miR-92, a member of the miR-17-92 cluster. Luciferase activity was downregulated in SH3 cells, but not in a 3′-UTR carrying a non-binding mutated target sequence, and that activity was restored by concomitant knocking down of miR-17-92 (Figure 6C). Conversely, the TTF-1 3′-UTR was not sensitive to miR-17-92 levels (Figure 6C). Downregulation and rescue of these transcription factors levels correlated with changes in the expression of lung-specific markers and stem cell markers (Figure 6D), together with misexpression of integrins (Figure 6E; Supplementary Figure S9F).
Figure 6.
Regulation of lung-specific transcriptional programme in HLSCs by the p38α-miR-17-92 axis. Regulation of pri-miR-17-92 levels by p38α modulates HLSCs self-renewal and differentiation. (A) Simultaneous downregulation of miR-17-92 in p38α-deficient HLSCs rescued the protein levels of lung differentiation transcription factors C/EBPα and GATA6. (B) Defective mRNA expression of lung transcription factors was restored by concomitant knockdown of mir-17-92 in SH3 cells. A representative experiment (±s.d.) of four independent replicates is shown. (C) Direct regulation of the 3′-UTR of predicted targets for miR-17-92 (GATA6 and C/EBPα) but not of the non-target TTF-1 or the 3′-UTRs with mutated target sequences that avoid binding. (D) Misexpression of lung and stem cell markers was rescued by concurrent knockdown of miR-17-92. A representative experiment (±s.d.) of four independent replicates is shown. (E) Rescue of normal integrin mRNA levels by compound downregulation of miR-17-92 levels in SH3 cells. A representative experiment (±s.d.) of five independent replicates is shown (see also Supplementary Figure S7).
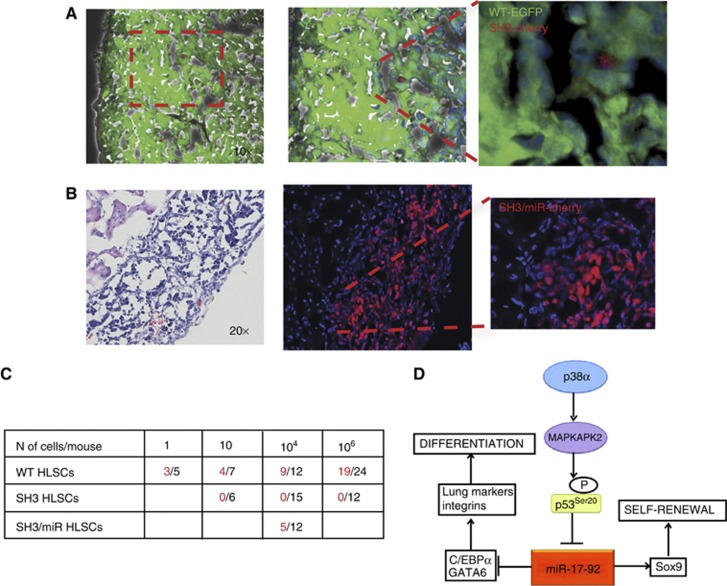
To assess the functional role of the p38α/miR-17-92 network in HLSCs, their in-vivo stem cell potential was examined using kidney capsule engraftments. SH3 cell injections failed to produce kidney engraftments (Figure 7C). The inability of SH3 to form kidney grafts was further confirmed by co-injecting a mix of HLSCs, WT (EGFP) and SH3 (H2B-cherry) cells (Figure 7A). Only WT, but not SH3 cells differentiated and produced alveolar-like tissue (Figure 7A). The involvement of the miR-17-92 cluster mediating the functional role of p38α was examined using p38α/miR-17-92 concomitant knockdown cells (SH3/miR). Restoration of miR-17-92 levels to normal in SH3 cells rescued the potential to produce lung-like epithelium in kidney grafts (Figure 7B). The stem cell potential of HLSCs to differentiate in vivo was controlled by p38α and miR-17-92 in a coordinated way (Figure 7C). It could be concluded that p38α repressed miR-17-92, via p53 activation, in order to maintain the proper balance between differentiation and self-renewal potential in HLSCs (Figure 7D).
Figure 7.
Functional control of HLSCs by the p38α-miR-17-92 axis. (A) Co-injection of WT (EGFP) and SH3 cells (H2B-cherry) resulted in kidney engraftments with alveolar tissue of WT (green) cells and a marginal population of SH3 cells (red). (B) miR-17-92 downregulation rescued the potential of p38α-deficient HLSCs to form lung-like epithelium in kidney engraftments. (C) SH3 cells failed to produce kidney engraftments but their potential is restored by compound knockdown of miR-17-92. Total injections (right) or positive engraftments (left) after 8 weeks. One-way ANOVA test P<0.04. (D) Model of HLSC regulation by p38α-mediated repression of miR-17-92 expression.
Discussion
There have been extensive efforts and reports in the search for common stem/progenitors of the adult lung bronchioalveolar epithelium. However, adult lung stem cell research is still in its infancy and most previous studies have focused on the proximal airways (Johnson and Hubbs, 1990; Engelhardt et al, 1995; Hong et al, 2004) or isolated cells of an uncertain origin and controversial stem cell potential (Fujino et al, 2011; Kajstura et al, 2011). In particular, the knowledge about cell hierarchy in human lungs is still very limited. Here, we show the existence of a distinct population of human alveolar stem cells, with a defined signature, that can be isolated and clonally expanded in culture while maintaining their potential to differentiate. This population could be considered as a sub-population of the number of Integrin-α6+ cells in the human lung. They harbour self-renewal, shown by in-vitro indefinite expansion and in-vivo serial transplantation experiments and differentiation capacity, as would be expected from a stem cell. The in-vitro and in-vivo stem cell potential of this population has been demonstrated with some of the most commonly used techniques to test tissue stem cells, including kidney capsule or lung injury engraftments. Unlike other reported putative HLSCs (Kajstura et al, 2011), our HLSC population has an epithelial origin and does not differentiate into mesenchymal or endothelial cells. E-Cad/Lgr6+ but not c-Kit+, single-cell injections produce lung epithelium in the kidney, and they did not require an extra-stromal compartment like the reported Integrin-α6/β4 mouse lung cells (Chapman et al, 2011). This comparative study has shown the superior stem cell potential of human lung E-Cad/Lgr6+ cells. Indefinite expansion of lung E-Cad/Lgr6+ cells brings new possibilities, such as their use in pharmacological screenings, generation of disease models or genetic manipulation to repair defective mutations.
In addition, it could be demonstrated that regulation of HLSCs relies on an integrated network involving p38α and the miR-17-92 cluster. Both signals have been previously related to lung differentiation and proliferation, but the molecular insights of this function remained unknown. A negative regulation of miR-17-92 expression has been discovered through the transcriptional activation of p53 by the p38α pathway. Defects in the fine-tuning between these two signalling pathways may result in disease (e.g., cancer) or defective regeneration (e.g., lung fibrosis) (Hayashita et al, 2005; Mendell, 2008). p38α deficiency produces an unbalanced increase of miR17-92 levels that downregulate lung-specific transcription factors and results in misexpression of integrins. Integrins are involved in the response of stem cells to extracellular matrix-directed terminal differentiation (Watt, 2002). Thus, the p38α/miR-17-92 axis regulates the intracellular machinery and the response to extracellular signals involved in lung stem cell fate decision. Disruption of this network in p38α-deficient cells causes loss of the potential of HLSCs to engraft and differentiate in the kidney capsule. HLSCs differentiate into epithelium, but they can also recruit endothelium and connective tissue to create a proper microenvironment. The contribution of differentiation factors and/or integrins in establishing a lung stem cell niche it is still unclear. Delineation of the molecular mediators and functional effectors in this network may contribute to a better understanding of the regulation of HLSCs in homeostasis and disease.
The E-Cad/Lgr6+ population adds pieces to the cellular puzzle regulating lung homeostasis and the regenerative response to injury. This population appears to have a larger stem cell potential, ranking higher in the lung bronchioalveolar hierarchy than other of the previously reported cell types, although the existence of cells of a higher stemness in the bronchioalveolar epithelium cannot be discounted (as it is in the haematopoietic system).
It highlights new molecular targets that may help to easy detection and isolation of human lung bronchioalveolar multipotent cells. Overall, our work provides a reliable model for in-vitro studies, a more complete understanding of the cellular mechanisms regulating lung cell fate decision, and a leap forward in the search for potential lung cellular and molecular targets for regenerative therapies.
Materials and methods
Isolation and culture of human lung progenitor cells
Human lung tissue was obtained from patients undergoing lung resection (Papworth Hospital, UK). This study was approved by the Ethics Committee at Cambridge University and the Papworth Hospital. All subjects gave informed consent. All samples collected were healthy lung tissue biopsies from cancer patients, used in the clinic as a control. No samples from patients with COPD or other inflammatory diseases were used to isolate stem cells.
Normal lung was used for cell isolation and histological evaluation. After pleura was separated bluntly, lung specimens were finely minced and resuspended in collagenase (0.5–3 mg/ml, Whorthington)/dispase (1 mg/ml, Invitrogen) containing DMEM (Invitrogen) and incubated for 30–45 min at 37°C in a shaking incubator. The suspension was spun for 5 min at 1200, r.p.m. and the supernatant removed. The pellet was resuspended in fresh DMEM containing 0.1 mg/ml DNase (optional) and incubated for further 5–10 min. The suspension was washed with PBS, filtered through cell strainers (100, 70 μm, BD) and treated with red blood cell lysis buffer (Roche Applied Science). Following further filtration (40 μm mesh) and centrifugation (5 min at 1200, r.p.m.), the isolated cells were cultured in RH-B (Stem Cell Science) medium containing 2% FCS, with additional insulin (5 μg/ml, Pepro Tech), EGF (10 ng/ml, Pepro Tech) and FGF2 (20 ng/ml, Pepro Tech) for 2 days. This was then was replaced with fresh, serum-free medium containing growth factors (37°C in a 7% humidified CO2 incubator).
Lentiviral vector preparation
The lentiviral vector pSINPGKEGFP was used to generate pSINhCC10EGFP, containing the human CC10 promoter. Lentiviral particles were produced by co-transfecting 293T cells with pSINPGKEGFP or pSINhCC10EGFP, pCMVΔ8.9 and pMD.G (encoding VSV-G), as previously described (Naldini et al, 1996; Capowski et al, 2007).
Four siRNAs (Thermo Scientific) were tested to knockdown p38α shRNA n.3 was cloned into the PLKO.1-TRC (Sigma) lentiviral vector to generate a p38α knockdown construct (SH3). Infectious virus was added to cells in the presence of 8 mg/ml polybrene (hexadimethrine bromide, Sigma) and incubated for 6 h.
A commercial (Applied Biosystems) siRNA was used to knockdown the pri-miR-17-92: sense-GGAGAGCUCAAUCUGCACAtt.
Luciferase assays
To test miR-17-92 promoter activity and transcription factors 3′-UTRs processing, pGL3 reporter vectors containing the firefly luciferase were used (with a pGL3 basic for the promoter and the pGL3-Vector control for the 3′-UTR as activity controls). As a control of the transfection efficiency a vector expressing the Renilla luciferase was used.
The 0.5 and 0.7 kb fragments of the miR-17-92 promoter were obtained by PCR using specific primers (see Supplementary data), and cloned in the promoterless pGL3 basic vector after NheI/XhoI restriction digestion. The 3′-UTRs of the C/EBPα, GATA6 and TTF1 genes were obtained by PCR using specific primers (see Supplementary data) and cloned in the pGL3-Vector control after XbaI digestion.
Mutant 3′-UTRs were created introducing a mutation in the miR-92a target sequence of the C/EBPα and GATA 6 3′-UTRs using the appropriate oligos (see Supplementary data).
Flow cytometry
Single-cell FACS was performed (MoFlo, Dako) following incubation with anti-Lgr6 (Santa Cruz), anti-E-cadherin (Biolegend), anti-Integrinα6 (CD49f, Biolegend), anti-αSMA (Sigma), anti-CD31 (Biolegend), anti-CD34 (Biolegend), anti-C-Kit (CD117, DAKO), anti-CD73 (Abcam) and anti-CD45 (Biolegend) antibodies.
In-vitro assessment of differentiation potential
Cells were grown to low confluence on 10 μg/ml fibronectin- (Millipore) or laminin- (in PBS, Sigma) coated tissue culture dishes (24- or 6-well plates). All differentiation cultures were maintained for 10–15 days, medium (containing 2–5% FCS) being renewed every 72 h.
Immunofluorescent staining
Cultured cytospin (800 g, 3 min) cells were fixed with 4% paraformaldehyde (PFA) for 15–20 min at RT. The remaining human tissues were also fixed with 4% PFA for 24 h and embedded in paraffin or OCT compound (Sakura, UK) after 30% sucrose treatment (at 4°C for 24 h). Samples were blocked and permeabilized with 0.1% Triton-X/4% goat serum/PBS for 60 min at RT. Cells were incubated with primary antibodies overnight at 4°C and then washed three times with PBS at room temperature (5 min per wash). Secondary antibody (Alexa Fluor 488 and/or Alexa Fluor 594 secondary antibodies, Invitrogen) incubation took place for 1 h at RT. Cells were visualized following DAPI (4',6-Diamidino-2-phenylindole) counterstaining. Images were collected using a Leica SP5 confocal microscope.
Mouse experiments
All mouse experiments were performed according to UK Home Office Regulations. CD-1® nude mice (Charles River) were maintained under standard pathogen-free conditions.
Kidney capsule engraftments
Six- to eight-week-old mice were anaesthetized with isofluorane (0.5–2%). Cells were disassociated with accutase to generate a single-cell suspension (1–1 × 106 cells in 20 μl PBS) and this suspension was injected under the kidney capsule. Mice were killed 2, 4, 6 and 8 weeks later and the kidneys harvested to examine in-vivo differentiation of the injected cells. The engraftments were removed and prepared for immunofluorescent microscopy.
For transplantations experiments, the GFP engrafted cells were sorted prior to be injected into kidney capsule or use for TVI for lung co-transplantations.
Bleomycin treatment of mice and lung stem cell transplantation
Six- to eight-week-old mice were given one tail vein (t.v.) injection of 5 U/kg of bleomycin in 100 μl PBS. Control animals were given an equivalent volume of PBS. Control groups received PBS and experimental groups received bleomycin. Forty-eight hours later, the experimental group received the stem cells (t.v.). Each group had six mice (repeated three times), which were analysed 10 days post bleomycin/progenitor cell injection. Lungs were fixed overnight with buffered neutral formalin 10% (VWR) or 4% PFA at room temperature. Tissues were then processed for paraffin embedding or for cryosectioning. Slides were stained in Mason’s Trichrome stain (Fisher Scientific) and H&E (Dako), according to manufacturer’s instructions. Cryosections were analysed for the presence of human lung cells by microscopy. For co-transplantation experiments, the GFP cells engaged in mouse lungs were sorted prior to be used for further injections.
Bleomycin treatment of mice and human lung explants ex vivo
Human or adult mouse lungs were cultured as slices (200–800 μm thickness) and exposed to bleomycin (3 days) in vitro. After injury, EGFP-labelled HLSCs were microinjected into the lungs and cultured for 7–10 days. Lungs were fixed overnight with buffered neutral formalin 10% (VWR) or 4% PFA at room temperature. Tissues were then processed for paraffin embedding or for cryosectioning for the presence and differentiation of the HLSCs.
Colony assays
To isolate and expand clonogenic cells, cells were dissociated with Accutase (PAA). Single cells that were seeded into 96-well plates by limited dilutions. The cells were maintained in stem cell restricted medium RH-B (Stem Cell Science) containing 2% FCS, with additional insulin (5 μg/ml, Pepro Tech), EGF (10 ng/ml, Pepro Tech) and FGF2 (20 ng/ml, Pepro Tech) for 2 days. This was then was replaced with fresh, serum-free medium containing growth factors (37°C in a 7% humidified CO2 incubator). After 14 days, the number of wells with colonies was counted. Every assay was repeated four times.
Total RNA isolation and quantitative RT–PCR
Total RNA was extracted using TRIzol (Invitrogen) and was DNAse I (Promega) treated. In all, 1 μg RNA was reverse transcribed per sample (Bio-Rad), according to manufacturer’s instructions. Quantitative Real-Time PCR (qPCR) was used to determine the expression levels of the different genes using human-specific primer pairs (Eppendorf, Realplex2). Reaction conditions for amplification were as follows: first step of 95°C 20 s, then 40 cycles of three-step 95°C 3 s, 60°C 30 s and 68°C 20 s with 2 μl of cDNA per reaction in 10 μl SYBR Green PCR Master Mix (Applied Biosystems). Specificity of PCR products was tested by dissociation curves. Threshold cycles of primer probes were normalized to a housekeeping gene (GAPDH or HPRT) and relative values calculated (Livak and Schmittgen, 2001).
Pri-miR-17-92 expression was quantified by TaqMan qPCR and normalized to GAPDH-expression (see Supplementary data). In all, 2 μl of cDNA was used per 10 μl of Taqman Fast Universal PCR Master Mix (2 ×). The cycles were first step of 95°C 20 s, then 40 cycles two-step 95°C for 1 s and 60°C 20 s (default set-up in StepOne machine). VIC-labelled human GAPDH from Applied Biosystems was used as internal control.
Immunoblot analysis
Proteins were extracted and analysed as previously described (Ventura et al, 2007), to confirm that observed changes in mRNA expression were reflected in the amount of protein present.
Antibodies
Antibodies used were anti-human CC-10 (Santa Cruz, SC-25554), anti-human SPC (Santa Cruz, SC-7705), anti-human AQP-5 (Santa Cruz, SC-28628), anti-human LGR6 (Santa Cruz, SC-48236), anti-human LGR5 (Santa Cruz, SC-68580), anti-human Integrinα6 (Santa Cruz, SC-10730), anti-human E-cadherin (AbCam, Ab53033), anti-human C-Kit (Dako), anti-human Sox-9 (Millipore, AB5535), anti-human mitochondrial Ab (Thermo, MAS-12017).
Supplementary Material
Acknowledgments
We thank D Sanchez-Tatay, Peter Humphreys, W Mansfield, M McLeish and N Miller for technical help. A Smith, M Frye, D Winder and E Hoste for critically reading the manuscript. Grants from the Medical Research Council (MRC) (RG51968/RG57589) and Cancer Research UK (RG52191) funded this work. AG is supported by a Herchel Smith PhD scholarship.
Authors contribution: FO-W performed experiments and designed experiments, and analysed results. AG performed experiments and analysed results. AO performed experiments. J-JV performed and designed experiments, analysed results and wrote the manuscript.
The authors declare that they have no conflict of interest.
08/15/2012
Since Advance Online Publication, this article has been corrected and a corrigendum is also printed in this issue
References
- Anversa P, Kajstura J, Leri A, Loscalzo J (2011) Tissue-specific adult stem cells in the human lung. Nat Med 17: 1038–1039 [DOI] [PubMed] [Google Scholar]
- Aso Y, Yoneda K, Kikkawa Y (1976) Morphologic and biochemical study of pulmonary changes induced by bleomycin in mice. Lab Invest 35: 558–568 [PubMed] [Google Scholar]
- Barker N, Clevers H (2010) Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 138: 1681–1696 [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Capowski EE, Schneider BL, Ebert AD, Seehus CR, Szulc J, Zufferey R, Aebischer P, Svendsen CN (2007) Lentiviral vector-mediated genetic modification of human neural progenitor cells for ex vivo gene therapy. J Neurosci Methods 163: 338–349 [DOI] [PubMed] [Google Scholar]
- Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH (2011) Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest 121: 2855–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova T, Deucher A, Kuroki T, Ohba M, Eckert RL (2002) Novel protein kinase C isoforms regulate human keratinocyte differentiation by activating a p38 delta mitogen-activated protein kinase cascade that targets CCAAT/enhancer-binding protein alpha. J Biol Chem 277: 31753–31760 [DOI] [PubMed] [Google Scholar]
- Eirew P, Stingl J, Raouf A, Turashvili G, Aparicio S, Emerman JT, Eaves CJ (2008) A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat Med 14: 1384–1389 [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Schlossberg H, Yankaskas JR, Dudus L (1995) Progenitor cells of the adult human airway involved in submucosal gland development. Development 121: 2031–2046 [DOI] [PubMed] [Google Scholar]
- Fujino N, Kubo H, Suzuki T, Ota C, Hegab AE, He M, Suzuki S, Yamada M, Kondo T, Kato H, Yamaya M (2011) Isolation of alveolar epithelial type II progenitor cells from adult human lungs. Lab Invest 91: 363–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A, Reynolds SD, Stripp BR (2002) Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol 161: 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T (2005) A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res 65: 9628–9632 [DOI] [PubMed] [Google Scholar]
- Holmes C, Stanford WL (2007) Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells 25: 1339–1347 [DOI] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR (2004) Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol 164: 577–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YF, Sheu JR, Hsiao G, Lin CH, Chang TH, Chiu PT, Wang CY, Hsu MJ (2011) p53 in trichostatin A induced C6 glioma cell death. Biochim Biophys Acta 1810: 504–513 [DOI] [PubMed] [Google Scholar]
- Hui L, Bakiri L, Mairhorfer A, Schweifer N, Haslinger C, Kenner L, Komnenovic V, Scheuch H, Beug H, Wagner EF (2007) p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat Genet 39: 741–749 [DOI] [PubMed] [Google Scholar]
- Johnson NF, Hubbs AF (1990) Epithelial progenitor cells in the rat trachea. Am J Respir Cell Mol Biol 3: 579–585 [DOI] [PubMed] [Google Scholar]
- Kajstura J, Rota M, Hall SR, Hosoda T, D’Amario D, Sanada F, Zheng H, Ogorek B, Rondon-Clavo C, Ferreira-Martins J, Matsuda A, Arranto C, Goichberg P, Giordano G, Haley KJ, Bardelli S, Rayatzadeh H, Liu X, Quaini F, Liao R, Leri A, Perrella MA, Loscalzo J, Anversa P (2011) Evidence for human lung stem cells. N Engl J Med 364: 1795–1806 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kennedy NJ, Sluss HK, Jones SN, Bar-Sagi D, Flavell RA, Davis RJ (2003) Suppression of Ras-stimulated transformation by the JNK signal transduction pathway. Genes Dev 17: 629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T (2005) Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121: 823–835 [DOI] [PubMed] [Google Scholar]
- Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, Wang de Y, Lim B, Chow VT, Crum CP, Xian W, McKeon F (2011) Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147: 525–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange AW, Keiser AR, Wells JM, Zorn AM, Whitsett JA (2009) Sox17 promotes cell cycle progression and inhibits TGF-beta/Smad3 signaling to initiate progenitor cell behavior in the respiratory epithelium. PLoS ONE 4: e5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Martinez L, Ebine K, Abe MK (2008) Role for mitogen-activated protein kinase p38 alpha in lung epithelial branching morphogenesis. Dev Biol 314: 224–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lu Y, Thomson JM, Wong HY, Hammond SM, Hogan BL (2007) Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol 310: 442–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martis PC, Whitsett JA, Xu Y, Perl AK, Wan H, Ikegami M (2006) C/EBPalpha is required for lung maturation at birth. Development 133: 1155–1164 [DOI] [PubMed] [Google Scholar]
- Mendell JT (2008) miRiad roles for the miR-17-92 cluster in development and disease. Cell 133: 217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL (2010) Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 18: 8–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D (1996) In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272: 263–267 [DOI] [PubMed] [Google Scholar]
- Qian S, Ding JY, Xie R, An JH, Ao XJ, Zhao ZG, Sun JG, Duan YZ, Chen ZT, Zhu B (2008) MicroRNA expression profile of bronchioalveolar stem cells from mouse lung. Biochem Biophys Res Commun 377: 668–673 [DOI] [PubMed] [Google Scholar]
- Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL (2007) Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134: 2521–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL (2011) Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 8: 639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Hogan BL (2011) Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol 27: 493–512 [DOI] [PubMed] [Google Scholar]
- She QB, Ma WY, Dong Z (2002) Role of MAP kinases in UVB-induced phosphorylation of p53 at serine 20. Oncogene 21: 1580–1589 [DOI] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, Stange DE, Toftgard R, Clevers H (2010) Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327: 1385–1389 [DOI] [PubMed] [Google Scholar]
- Stokoe D, Campbell DG, Nakielny S, Hidaka H, Leevers SJ, Marshall C, Cohen P (1992) MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. EMBO J 11: 3985–3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara K, Iyama KI, Kimura T, Sano K, Darlington GJ, Akiba T, Takiguchi M (2001) Mice lacking CCAAt/enhancer-binding protein-alpha show hyperproliferation of alveolar type II cells and increased surfactant protein mRNAs. Cell Tissue Res 306: 57–63 [DOI] [PubMed] [Google Scholar]
- Teisanu RM, Lagasse E, Whitesides JF, Stripp BR (2009) Prospective isolation of bronchiolar stem cells based upon immunophenotypic and autofluorescence characteristics. Stem Cells 27: 612–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, Jaenisch R, Sharp PA, Jacks T (2008) Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 132: 875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura JJ, Tenbaum S, Perdiguero E, Huth M, Guerra C, Barbacid M, Pasparakis M, Nebreda AR (2007) p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet 39: 750–758 [DOI] [PubMed] [Google Scholar]
- Watt FM (2002) Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J 21: 3919–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DJ, Bertoncello I, Borok Z, Kim C, Panoskaltsis-Mortari A, Reynolds S, Rojas M, Stripp B, Warburton D, Prockop DJ (2011) Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc 8: 223–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HL, Xue G, Mei Q, Wang YZ, Ding FX, Liu MF, Lu MH, Tang Y, Yu HY, Sun SH (2009) Repression of the miR-17-92 cluster by p53 has an important function in hypoxia-induced apoptosis. EMBO J 28: 2719–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.