Abstract
EMBO J 31 16, 3398–3410 (2012)
The ATM/ATR-dependent checkpoint kinases restrain mitotic entry when cells accumulate unrepaired DNA double-strand breaks (DSBs) or when telomeres become uncapped. New work by Thanasoula et al (2012) reveals an intricate network of checkpoint interactions in the wake of telomere uncapping through removal of TRF2 or POT1, providing compelling evidence that the mechanism of G2/M checkpoint activation at chromosome end is distinct from the canonical DNA damage response to ionizing radiation (IRs).
DNA DSBs are genotoxic lesions that can initiate genomic instability through aberrant repair of DNA breaks, resulting in nonreciprocal chromosome translocations that can be cancer-promoting. DSBs can arise from exogenous sources such as IR, or endogenously as in the case of telomere uncapping following erosion of telomeric repeats or loss of telomere-binding proteins. Uncapped telomeres activate DNA damage checkpoints, as demonstrated in work using mouse models for TRF2 and POT1 deficiencies (Celli and de Lange, 2005; Hockemeyer et al, 2006; Wu et al, 2006; Guo et al, 2007); but their effect on the G2/M checkpoint that prevents entry into mitosis had not been examined. Cells entering mitosis with uncapped telomeres generate chromatids that are joined in G1 to initiate bridge-fusion-breakage cycles, leading to the accumulation of deleterious chromosomal rearrangements. The G2/M checkpoint response to uncapped telomeres is thus an essential mechanism for the maintenance of genome integrity.
TRF2 or POT1 deletion in mouse cells activates ATM- and ATR-dependent DNA damage checkpoints, respectively, through phosphorylation of checkpoint kinases CHK1 and CHK2 (Denchi and de Lange, 2007; Guo et al, 2007). This observation, together with the apparent similarity between artificially uncapped telomeres and IR-induced DSBs, makes it attractive to speculate that the checkpoints triggered by the two processes are mechanistically similar; yet, the contribution of downstream effectors of the G2/M transition has been only partly investigated so far. p53 inactivation is known to reverse the cell-cycle arrest phenotype induced by deletion of TRF2 or POT1 (Celli and de Lange, 2005; Wu et al, 2006). The new results of Thanasoula et al (2012) show that in response to telomere dysfunction, p53 is phosphorylated at Ser15 in an ATM/ATR-dependent manner, specifically during the G2/M transition, and abrogating this phosphorylation enables mitotic entry with damaged telomeres. The authors also show that in addition to p53, phosphorylation of CHK1 and CHK2 are key events in preventing mitotic entry of uncapped telomeres. Eliminating CHK1 and CHK2 functions in TRF2- or POT1-depleted cells resulted in progression into mitosis with a high frequency of damaged telomeres, further illustrating the critical roles these checkpoint kinases play in enforcing the G2/M checkpoint response.
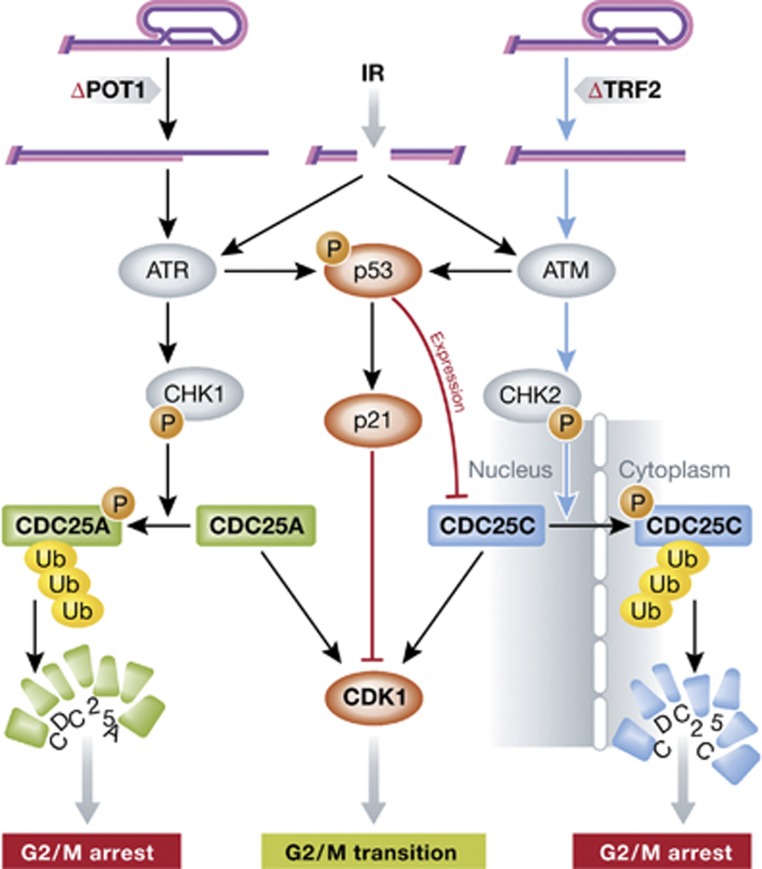
Damaged DNA is known to induce CHK1/CHK2-dependent phosphorylation of CDC25 phosphatases, which in turn prevents mitotic CDK activation and thus causes cell-cycle arrest (Bartek and Lukas, 2003). Thanasoula et al (2012) indeed found effects on CDC25 phosphatases, but with unexpected twists (Figure 1). Depletion of TRF2 resulted in CHK2-mediated phosphorylation of CDC25C on Ser216, which in turn leads to CDC25C export into the cytoplasm and its subsequent proteasome-mediated degradation. Expression of a non-phosphorylatable Ser216Ala mutant resulted in both CDC25C stabilization and mitotic entry with uncapped telomeres. Additionally, p53 further contributed to TRF2-specific G2/M arrest through downregulation of CDC25C at the transcriptional level, independent of its phosphorylation status. On the other hand, depletion of POT1 potently activates CHK1 and subsequent destruction of CDC25A, but also had an additional impact on CDC25C stability. One plausible explanation for the latter finding is that deletion of POT1 in MEFs results in the formation of dicentric chromosomes, which when resolved generate DSBs that potently activate ATM/CHK2 signalling (Wu et al, 2006).
Figure 1.
Telomere damage signalling at the G2/M transition. Telomeres uncapped through POT1 or TRF2 depletion activate ATR or ATM signalling, respectively, leading to CHK1- or CHK2-dependent CDC25A or CDC25C phosphorylation. This targets CDC25 phosphatases for ubiquitin-proteasome-mediated degradation either directly (CDC25A) or following export into the cytoplasm (CDC25C). As a consequence, inhibitory CDK1 Tyr15 phosphorylation persists, blocking progression into mitosis. IR-induced DSBs activate both ATM and ATR, but selectively affect CDC25A stability.
The CDC25C-dependent checkpoint response to telomere dysfunction reported here is fundamentally different from the well-studied IR-induced G2/M arrest, which involves proteasome-mediated destruction of CDC25A (Sorensen et al, 2003; Melixetian et al, 2009). Conversely, the authors show that CDC25C degradation does not occur in response to treatments that induce genomic DSBs, but it is specific to telomere damage inflicted by TRF2 loss. One possibility is that TRF2-depleted telomeres primarily activate ATM/CHK2, while IR-induced DSBs activate ATR/CHK1. Supporting this concept is the fact that TRF2 depletion causes loss of the telomeric single-stranded DNA (ssDNA) overhang (Celli and de Lange, 2005), resulting in the activation of ATM in vitro (Shiotani and Zou, 2009). CHK1 targets CDC25A for degradation in response to IR, while existing evidence argues against a role for CHK2 in this process (Jin et al, 2008). Whether CHK2 is similarly required to target CDC25C for destruction in response to telomere dysfunction is a possibility worthy of future investigation. The data presented by Thanasoula et al (2012) support this concept, as CDC25C levels are efficiently restored in TRF2-deficient cells upon CHK2 co-depletion, but less so upon CHK1 inhibition.
One possible remaining concern is that the chronic DNA damage response elicited by uncapped telomeres may well differ from the acute response to IR exposure. Thanasoula et al (2012) addressed this point by comparing G2/M arrest triggered by telomere dysfunction also with the response to chronic accumulation of unrepaired DSBs upon depletion of the key recombinase RAD51, finding that CDC25C degradation is really specific to dysfunctional telomeres. Thus, the nature of the DNA damage signal emanating from uncapped telomeres and possibly their downstream nucleolytic processing is fundamentally distinct from that of IR-induced DSBs. Although the exact nature of this signal remains elusive, one likely possibility is that it involves the 3′ ssDNA telomeric overhang unique to uncapped telomeres but not found in IR-induced DSBs.
The study by Thanasoula et al, 2012 brings new insights into how telomere integrity at the G2/M transition impacts upon cell-cycle progression and genome stability, and may have clinical implications. Many p53-deficient cancers rely on Chk1 to arrest cell-cycle progression in response to genotoxic stress. Chk1 inhibitors that are currently in clinical trials abrogate the G2/M checkpoint, resulting in massive mitotic catastrophe when coupled with DNA damaging therapeutics (Ma et al, 2010). While there are currently no drugs that can selectively induce POT1 or TRF2 loss at cancer telomeres, one can imagine that telomere uncapping could potentiate the effects of CHK1 inhibition. The recent observation that POT1 somatic mutations may be prevalent in chronic lymphocytic leukaemia (Quesada et al, 2011), the most common leukaemia in Western countries, suggests that CHK1 inhibitors might be especially efficacious in this disease setting.
Footnotes
The author declares that he has no conflict of interest.
References
- Bartek J, Lukas J (2003) Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3: 421–429 [DOI] [PubMed] [Google Scholar]
- Celli GB, de Lange T (2005) DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol 7: 712–718 [DOI] [PubMed] [Google Scholar]
- Denchi EL, de Lange T (2007) Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448: 1068–1071 [DOI] [PubMed] [Google Scholar]
- Guo X, Deng Y, Lin Y, Cosme-Blanco W, Chan S, He H, Yuan G, Brown EJ, Chang S (2007) Dysfunctional telomeres activate an ATM-ATR-dependent DNA damage response to suppress tumorigenesis. EMBO J 26: 4709–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Daniels JP, Takai H, de Lange T (2006) Recent expansion of the telomeric complex in rodents: Two distinct POT1 proteins protect mouse telomeres. Cell 126: 63–77 [DOI] [PubMed] [Google Scholar]
- Jin J, Ang XL, Ye X, Livingstone M, Harper JW (2008) Differential roles for checkpoint kinases in DNA damage-dependent degradation of the Cdc25A protein phosphatase. J Biol Chem 283: 19322–19328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CX, Janerka JW, Piwnica-Worms H. (2010) Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics. Trends Mol Med 17: 88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melixetian M, Klein DK, Sørensen CS, Helin K (2009) NEK11 regulates CDC25A degradation and the IR-induced G2/M checkpoint. Nat Cell Biol 11: 1247–1253 [DOI] [PubMed] [Google Scholar]
- Quesada V, Conde L, Villamor N, Ordóñez GR, Jares P, Bassaganyas L, Ramsay AJ, Beà S, Pinyol M, Martínez-Trillos A, López-Guerra M, Colomer D, Navarro A, Baumann T, Aymerich M, Rozman M, Delgado J, Giné E, Hernández JM, González-Díaz M et al. (2011) Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet 44: 47–52 [DOI] [PubMed] [Google Scholar]
- Shiotani B, Zou L (2009) Single-stranded DNA orchestrates and ATM-to-ATR switch at DNA breaks. Mol Cell 33: 547–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen CS, Syljuasen RG, Falck J, Schroeder T, Ronnstrand L, Khanna KK, Zhou BB, Bartek J, Lukas J (2003) CHK1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell 3: 247–258 [DOI] [PubMed] [Google Scholar]
- Thanasoula M, Escandell JM, Suwaki N, Tarsounas M (2012) ATM/ATR checkpoint activation downregulates CDC25C to prevent mitotic entry with uncapped telomeres. EMBO J 31: 3398–3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Multani AS, He H, Cosme-Blanco W, Deng Y, Deng JM, Bachilo O, Pathak S, Tahara H, Bailey SM, Deng Y, Behringer RR, Chang S (2006) Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell 126: 49–62 [DOI] [PubMed] [Google Scholar]