Abstract
EMBO J 31 16, 3384–3397 (2012)
Wnt signalling has been under intense scrutiny now for many years, and this effort has led to the identification and characterization of many components, and to a quite satisfactory general picture of the pathway. Yet, as components were discovered at a fast pace and started to crowd around a few scaffold proteins (Axin, APC, Dishevelled, and the main protagonist, β-catenin), the complexity of the system soon became apparent. Thus, behind the simple model presented in all introductions, how the pathway actually works is still far from being fully understood (see, for example, Taelman et al, 2010 and Li et al, 2012). In a context where every molecule seems to be able to interact with every other molecule and single components can perform simultaneously several different reactions, how can one dissect the role of any of them? If everything is so intricate, is there any hope to obtain a satisfactory view of the process? Jho and colleagues’ study of one of these molecules, Dimerization Partner 1 (DP1), presented in this issue of The EMBO Journal (Kim et al, 2012), is a beautiful demonstration that this is indeed a reachable goal, although the path is not for the faint of heart! The final result of what probably represents the most comprehensive single study ever published in the field is a coherent description of the dual role of DP1 in Wnt signalling, coupled to the discovery of a new regulatory mechanism involved in refining embryonic patterning.
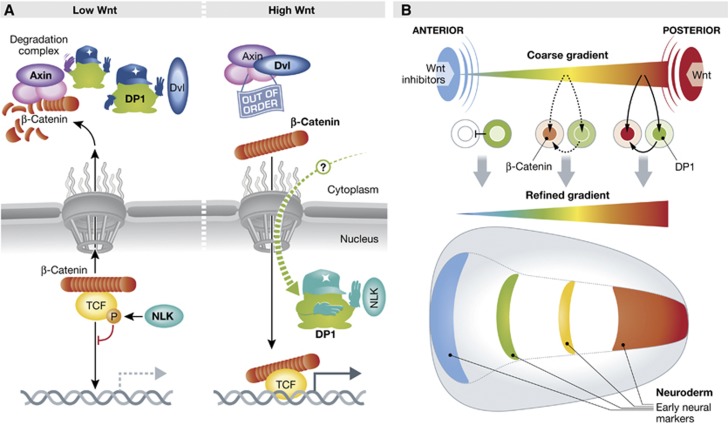
Two genetic studies in Drosophila had reported a role for Dimerization Partner 1 (DP1) in Wnt signalling, but in one case the role was negative, in the other case positive (DasGupta et al, 2005; Morris et al, 2008). Such contradictory results are usually indicative of multiple molecular interactions and cellular functions. Jho and colleagues undertook to tackle this puzzle using a combination of functional and biochemical experiments in mammalian culture cells and in Xenopus embryos. They first observed that DP1 tends to act negatively when the pathway’s activity is low, and positively when the activity is high. Thus, DP1 reinforces both the ‘off’ or the ‘on’ states. They then demonstrated that these two effects were due to completely distinct interactions (Figure 1A). Under low signal activity, DP1 maintains the Axin complex fully active by directly competing with the Axin–Dvl interaction. DP1 can do so by binding either to Axin or to Dvl. The authors propose that this mechanism prevents ‘noise’ inactivation of the complex under conditions of weak or no Wnt signal. The opposite positive action of DP1 is detectable only under Wnt stimulation, and concerns TCF, the nuclear partner of β-catenin itself: TCF is known to be phosphorylated by the kinase NLK, which weakens its association with DNA (Ishitani et al, 1999). Here, DP1 binds directly to NLK and inhibits TCF phosphorylation. In doing so, DP1 insures that NLK does not hinder the transcriptional activity of the β-catenin–TCF pair. In a series of elegant experiments, the authors rigorously demonstrated that the negative and positive functions of DP1 are strictly compartmentalized.
Figure 1.
(A) DP1 regulates the Wnt pathway both negatively and positively. Under conditions of low Wnt signalling, cytoplasmic DP1 prevents Dvl to bind Axin and thus keeps the β-catenin degradation complex maximally active. When Wnt signalling is high, DP1 shuttles by an yet undefined mechanism into the nucleus, where it sequesters the kinase NLK away from TCF, thus preventing TCF phosphorylation and inactivation. This ensures efficient DNA binding and transcriptional activity of the β-catenin–TCF complex. (B) DP1 dual activity refines patterning of the neuroderm during early Xenopus development: a coarse gradient is established by Wnt factors produced in the posterior part of the embryo, counteracted in the anterior region by secreted Wnt inhibitors. Cytoplasmic DP1 further represses the weak Wnt-β-catenin activity in the front of the future nervous system. In the trunk/posterior region, DP1, which has accumulated in the nucleus under the influence of high Wnt signalling, boosts β-catenin accumulation and β-catenin–TCF transcriptional activity. This results in stabilization of the extreme conditions, as well as a steeper effective gradient of activity in the median region and thus a sharpening of the pattern.
What is then the physiological impact of DP1’s double function in vivo? The authors chose to examine the anterior–posterior patterning of the neuroderm in Xenopus embryos. This fundamental process, which defines the future regions of the brain and the spinal cord, is controlled by a gradient of Wnt activity (Kiecker and Niehrs, 2001), generated by a posterior source of Wnts counterbalanced in the front by secreted Wnt inhibitors (Figure 1B). The subcellular localization of endogenous DP1 was found to vary exactly as predicted from its relationship to Wnt activity. DP1 was cytoplasmic in the anterior cells, but concentrated in the nucleus towards the posterior end of the embryo, thus matching the Wnt gradient. While loss-of-function showed that DP1 was globally required for patterning the neuroderm, careful analysis revealed that the needs were distinct in different regions. Targeted manipulations of the Wnt activity, DP1 levels and DP1 subcellular localization, conclusively demonstrated that cytoplasmic DP1 is necessary in the future brain region to keep Wnt-β-catenin signalling low, while nuclear DP1 is essential in the trunk-tail region to antagonize NLK and thus maintain high β-catenin/TCF signalling. Altogether, the biochemical data on protein–protein interactions, the localization of the protein, and the functional experiments resulted in a remarkably coherent model of DP1 dual function. DP1 thus represents a novel type of intracellular ‘morphogen’, a single ubiquitously expressed protein that can be set to produce two opposite and mechanistically unrelated feedbacks in response to an activity gradient, thus stabilizing the extreme states, and increasing the ‘contrast’ of the gradient and the sharpness of the resulting pattern (Figure 1B).
The name DP1, for ‘Dimerization Partner 1’, originally referring to its ability to bind another transcription factor, E2F, turns out to be particularly well chosen. Indeed, DP1 seems to exert various functions simply by binding selected components, Axin and Dvl in the cytoplasm, NLK in the nucleosol, E2F on DNA. In all cases, the interaction is due to the same ‘heterodimerization domain’. It is fascinating to observe how this simple binding interface has conferred DP1 with polyvalent properties, and how these properties have been exploited to control a pathway in such a refined way.
The DP1 story is certainly not yet closed. Competing with other protein–protein interactions is surely not the only vocation of DP1. Could then DP1-Dvl and DP1-NLK dimers serve other functions? DP1 is also efficiently phosphorylated by NLK: Is this a merely a way to compete with other NLK substrates, or does this modification confer new properties to DP1? Another key issue is the regulation of its subcellular localization. What mediates its release from Axin and Dvl and its nuclear translocation (DP1 lacks a classical nuclear localization sequence)? How is DP1 sent back to the cytoplasm as the Wnt signal fades? There is a good chance that answering such questions may collaterally yield new insights into the general regulation of the pathway.
For developmental biologists keen at conceptualizing patterning mechanisms, a regulator with two opposite functions is a rather intriguing phenomenon. One question that comes immediately to mind is what happens at intermediate signal intensities, that is in the steep slope of the gradient, which is precisely the range where activity must be most precisely regulated: Will cells contain simultaneously two pools of DP1 working antagonistically, one in the cytoplasm, and the second one in the nucleus? Or will on the contrary both mechanisms be turned off? Or is there rather an abrupt switch from one mechanism to the other? It will be highly interesting to unravel how the system behaves as Wnt activity varies, and it is tempting to speculate about whether what physicists call ‘boundary conditions’ will be observed. Jho and colleagues have found a powerful and versatile system and set the stage for further exploration of the multiple aspects of DP1. Their tour de force will also certainly stimulate revisiting other parts of the Wnt signalling machinery: Complexity is within our reach!
Footnotes
The author declares that he has no conflict of interest.
References
- DasGupta R, Kaykas A, Moon RT, Perrimon N (2005) Functional genomic analysis of the Wntwingless signaling pathway. Science 308: 826–833 [DOI] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K (1999) The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature 399: 798–802 [DOI] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C (2001) A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128: 4189–4201 [DOI] [PubMed] [Google Scholar]
- Kim W-t, Kim H, Katanaev VL, Lee SJ, Ishitani T, Cha B, Han J-K, Jho E-h (2012) Dual functions of DP1 promote biphasic Wnt-on and Wnt-off states during anteroposterior neural patterning. EMBO J 31: 3384–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, Mohammed S, Heck AJ, Maurice MM, Mahmoudi T, Clevers H (2012) Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell 149: 1245–1256 [DOI] [PubMed] [Google Scholar]
- Morris EJ, Ji JY, Yang F, Di Stefano L, Herr A, Moon NS, Kwon EJ, Haigis KM, Naar AM, Dyson NJ (2008) E2F1 represses beta-catenin transcription and is antagonized by both pRB and CDK8. Nature 455: 552–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM (2010) Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143: 1136–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]