Abstract
In recent years, the Piwi pathway has been shown to regulate the silencing of mobile genetic elements. However, we know little about how Piwi pathways impose silencing and even less about trans-generational stability of Piwi-induced silencing. We demonstrate that the Caenorhabditis elegans Piwi protein PRG-1 can initiate an extremely stable form of gene silencing on a transgenic, single-copy target. This type of silencing is faithfully maintained over tens of generations in the absence of a functional Piwi pathway. Interestingly, RNAi can also trigger permanent gene silencing of a single-copy transgene and the phenomenon will be collectively referred to as RNA-induced epigenetic silencing (RNAe). RNAe can act in trans and is dependent on endogenous RNAi factors. The involvement of factors known to act in nuclear RNAi and the fact that RNAe is accompanied by repressive chromatin marks indicate that RNAe includes a transcriptional silencing component. Our results demonstrate that, at least in C. elegans, the Piwi pathway can impose a state of gene silencing that borders on ‘permanently silent’. Such a property may be more widely conserved among Piwi pathways in different animals.
Keywords: chromatin, elegans, piRNA, Piwi, RNAi
Introduction
Organisms need to protect themselves from the invasion of exogenous nucleic acids such as transposons and retro-viruses. A widely conserved defence mechanism is based on small RNA molecules that recognize the invading species and trigger a silencing response on that sequence (Malone and Hannon, 2009). Multiple small RNA-based mechanisms have been identified, each capable of recognizing and silencing potentially harmful sequences through unique mechanisms (Ghildiyal and Zamore, 2009). One of these mechanisms is known as the Piwi pathway, named after the Argonaute-family protein Piwi that was first identified in Drosophila (Cox et al, 1998). Piwi proteins directly bind small RNA cofactors that are better known as piRNAs (Aravin et al, 2006; Girard et al, 2006; Watanabe et al, 2006). Piwi–piRNA complexes have been studied in diverse organisms and in each case a role in the silencing of transposable elements could be demonstrated (Saito and Siomi, 2010; Ketting, 2011).
The Caenorhabditis elegans Piwi proteins are named PRG-1 and PRG-2, where PRG-1 is responsible for most, if not all, silencing activities (Batista et al, 2008; Das et al, 2008). Initially, only a few C. elegans piRNAs (also known as 21U RNAs) have been described to target a transposable element although many different piRNAs are produced and many transposons escape 21U-mediated recognition (Batista et al, 2008; Das et al, 2008). However, more recent studies show that imperfect base-pairing between C. elegans piRNAs (also known as 21U RNAs) and target RNAs actually impose gene-regulatory effects on many more targets (Bagijn et al, 2012; Lee et al, 2012). Bagijn et al (2012) also revealed that PRG-1–21U complexes trigger a downstream endogenous RNAi pathway that is mediated by the Argonaute protein WAGO-9 and other endogenous RNAi factors such as MUT-7 and RDE-3. This response is accompanied by the production of a class of small RNA molecules known as 22G RNAs. Interestingly, this is quite similar to how other C. elegans Argonaute proteins like ERGO-1, ALG-3/4 and RDE-1 trigger secondary RNAi pathways (Yigit et al, 2006; Gent et al, 2009, 2010; Conine et al, 2010; Vasale et al, 2010).
A major question for Piwi pathway biology in general remains how Piwi-targets are silenced. In mice, a link has been suggested between Piwi-pathway activity and DNA methylation (Aravin and Bourc’his, 2008; Aravin et al, 2008; Kuramochi-Miyagawa et al, 2008), suggesting that in mice the Piwi pathway is set-up to trigger the establishment of a heritable state of gene silencing. However, a causative link between Piwi-pathway activity and DNA methylation has been hard to demonstrate. Besides mammals, in flies it has also been suggested that piRNAs may trigger chromatin-related effects on their targets (Klenov et al, 2007; Yin and Lin, 2007; Riddle and Elgin, 2008; Wang and Elgin, 2011). Still, the interactions between the Piwi-pathway and chromatin are unclear at best, especially when target silencing is considered. We now show that the C. elegans Piwi (PRG-1) pathway can trigger the establishment of an extremely stable state of gene silencing. Once established, the silencing becomes independent of the initiating Piwi pathway itself. The maintenance of silencing requires previously characterized nuclear RNAi pathway components (Guang et al, 2008, 2010; Burkhart et al, 2011; Burton et al, 2011) and the Argonaute protein WAGO-9. Furthermore, the chromatin at the silenced locus is characterized by repressive histone marks. The silenced state is much more stable than previously reported heritable RNAi (Grishok et al, 2000; Vastenhouw et al, 2006; Alcazar et al, 2008; Burton et al, 2011), as it is inherited faithfully to 100% of the progeny and can last for tens of generations. Reactivation of the silenced state reveals a striking maternal effect, suggesting that during germline transmission, the silenced state is re-established through nuclear RNAi. In summary, we describe a tractable system that sheds new light on how Piwi proteins can trigger heritable gene silencing effects.
Results
Piwi-induced stable gene silencing
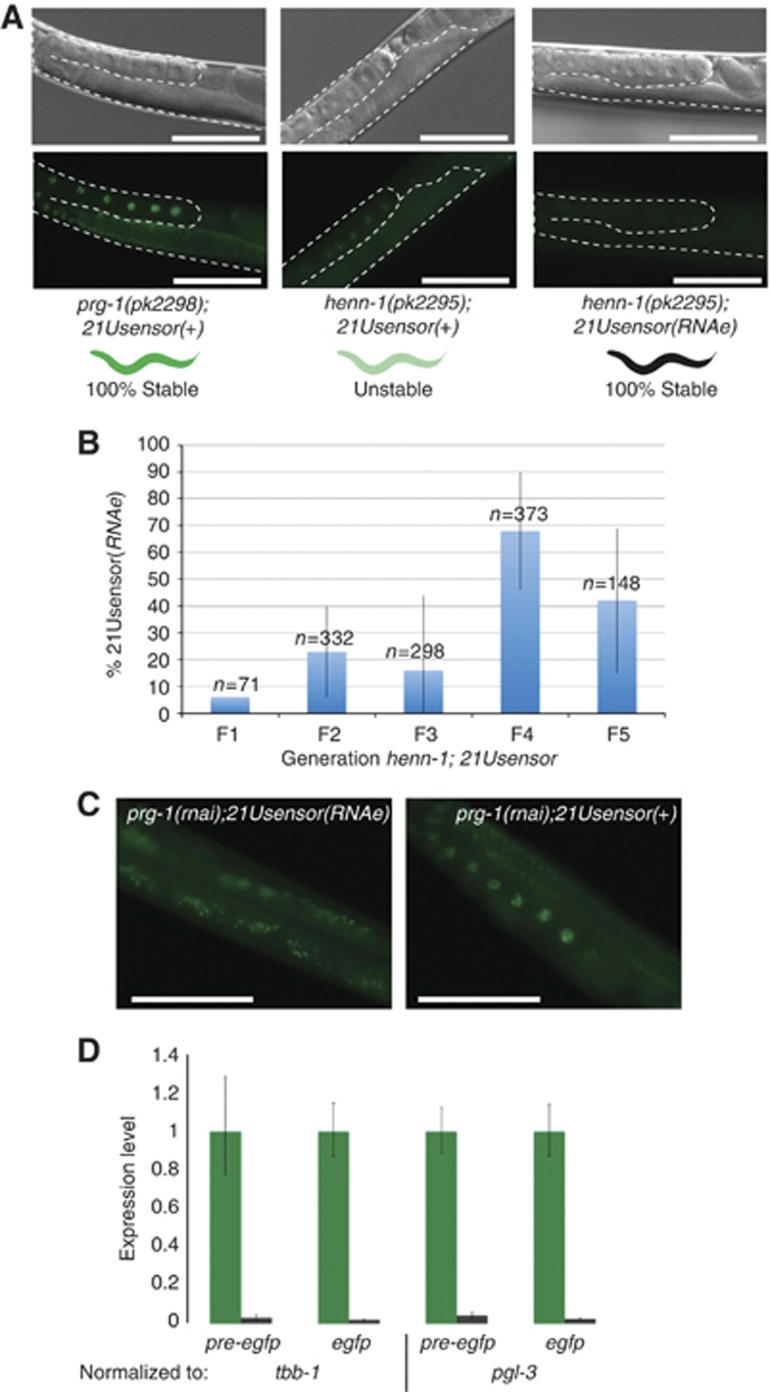
Recent work has described a transgenic system in which a specific 21U RNA, named 21UR1, targets the silencing of a single-copy GFP–HIS-58 expressing transgene (Bagijn et al, 2012). This transgene will be referred to as ‘21Usensor’ throughout this manuscript. Subsequently, we described (Kamminga et al, 2012) that mutations in HENN-1, an enzyme involved in the methylation of 21U RNAs, can re-activate this sensor, despite the fact that 21U RNA levels remain virtually unchanged. Interestingly, significant 21U RNA-mediated repression is still present in henn-1 mutants, as the observed GFP levels in a henn-1 mutant background are far below those in a prg-1 mutant background. Upon culturing of these henn-1 mutant animals, we noticed that the 21U sensor transgene had a strong tendency to become silenced, a phenomenon that we will refer to as RNA-induced epigenetic silencing (RNAe) throughout this manuscript (Figure 1A). Active transgenes will be marked with ‘+’. We determined the dynamics of RNAe in a henn-1 mutant background by scoring the number of animals that lost GFP expression in henn-1 mutant animals over the course of five generations. This revealed that RNAe is initiated at every generation in a significant number of the progeny (Figure 1B). In all cases tested, when an animal became silenced, 100% of its brood was silenced and remained silent for many generations. We checked whether the 21U sensor has an inherent tendency to become silenced, independent of PRG-1. However, we have never observed spontaneous silencing of an active 21Usensor(+) in a prg-1 mutant background, nor did we observe spontaneous silencing of a similar reporter transgene that lacks a 21U RNA-binding site (non21Usensor; not shown). Thus, in a henn-1 mutant background, PRG-1 can initiate an extremely stable form of gene silencing. RNAe can also be initiated in henn-1 wild-type animals, as we can identify animals carrying the 21U sensor in a henn-1 wild-type background that fail to activate the 21U sensor transgene upon RNAi against prg-1 (Figure 1C). Interestingly, we have observed that dsRNA-induced silencing of a 21Usensor(+) transgene can also initiate a form of silencing that resembles RNAe (not shown). We have not observed any spontaneous reactivation of either Piwi-induced or RNAi-induced silenced 21Usensor(RNAe) transgenes during day-to-day culturing and manipulation for a period of more than 6 months. We conclude that RNAe is much more stable than any previously described RNAi-related heritable silencing effects in C. elegans (Grishok et al, 2000; Vastenhouw et al, 2006; Alcazar et al, 2008; Burton et al, 2011), although we note that antiviral responses in C. elegans have been reported to be similarly stable (Rechavi et al, 2011). In all further studies described in this manuscript we made use of PRG-1-initiated RNAe.
Figure 1.
Piwi-initiated silencing of a single-copy transgene. (A) Images of animals with the indicated genotypes. The gonads are outlined with a dashed line. GFP expression from the 21Usensor is nuclear and chromosome bound due to the HIS-58 fusion. ‘100% stable’ indicates no observed spontaneous changes in GFP expression during months of culturing. Scale bars are 100 μm. (B) Quantification of spontaneous 21Usensor silencing in a henn-1 mutant background. Y-axis shows percentage of silenced animals in the broods of up to 10 individuals. X-axis displays the generation following the isolation of a single GFP-positive henn-1;21Usensor founder animal. Error bars reflect standard deviation of between 5 and 10 replicates. The F1 was scored on only one plate. (C) Animals wild-type for henn-1 but carrying the 21Usensor were treated with RNAi against prg-1. Ten activated the sensor (right panel), 26 did not (left panel). Further culturing showed that the transgene is indeed stably silenced in animals that failed to activate (not shown). Note that these numbers not necessarily reflect the initiation rate of silencing (RNAe) but rather may reflect the fraction of RNAe animals present in the strain at the time of the experiment. Scale bars are 100 μm. (D) q-RT–PCR on mature mRNA and pre-mRNA isolated from 21Usensor(RNAe) (black bars) and 21Usensor(+) (green bars; set to 1) animals, both in a prg-1 mutant background. The qPCR was normalized to the two different reference genes indicated. Error bars reflect standard error based on three replicates.
We quantified the silencing effect imposed by RNAe through q-RT–PCR on both mature and primary transcripts. This revealed that both mRNA and pre-mRNA levels are strongly reduced in backgrounds showing RNAe (Figure 1D). These data indicate that RNAe may act at both the transcriptional and post-transcriptional levels.
Genetics of RNAe
Next we looked into the genetics of the RNAe silenced transgene. First, we determined whether the silenced state was dependent on the presence of PRG-1. Interestingly, while PRG-1 is absolutely required to initiate RNAe, it is dispensable during maintenance of RNAe (Supplementary Figure S1; Table I). This reveals that PRG-1 purely acts as an initiator of RNAe. Furthermore, established RNAe silencing is inherited via both the male and the female germline, since animals carrying just one 21Usensor(RNAe) transgene copy do not express GFP, independent of whether the transgene comes from the father or the mother (not shown).
Table 1. Genetic requirements for RNAe.

aFirst generation homozygous mutant animals did not show GFP expression.
bFirst generation homozygous mutant animals was not analysed.
To probe if RNAe can act in trans, we performed crosses in which an active, germline-expressed transgene lacking 21U RNA-binding sites (non21Usensor(+)) was put into animals carrying 21Usensor(RNAe) transgenes. The non21Usensor transgene is integrated at the same site in the C. elegans genome as the 21Usensor transgene. First, we crossed prg-1 mutant males carrying a 21Usensor(RNAe) transgene with hermaphrodites containing the non21Usensor(+) transgene and analysed F2 offspring carrying non21Usensor transgenes only. These experiments revealed that, although transmission is not complete, the RNAe-state of the 21Usensor can be imposed in trans (Figure 2A). The original 21Usensor (RNAe) transgene remained silent during this experiment (not shown). We note that for some unexplained reason, PRG-1 seems to be able to counteract RNAe spreading in trans, since we consistently observe that in prg-1 mutant backgrounds significantly more animals harbour non21Usensor (RNAe) transgenes (Figure 2A). This may indicate that PRG-1 could be involved in setting up anti-silencing responses as well. Nevertheless, this cross illustrates that RNAe can act in trans.
Figure 2.
Genetics of RNAe. (A) Crossing scheme illustrating the trans-acting capabilities of an RNAe silenced transgene. (B) Crossing scheme illustrating in trans silencing activities of a 21Usensor(RNAe) transgene in the female, but not in the male germline. Scale bars are 100 μm. (C) Crossing scheme displaying in trans silencing activity in oocytes and sperm that have lost the 21Usensor(RNAe) transgene. In the F2 two possible genotypes can be found, but a (nearly) homogeneous GFP expression status for was observed. Scale bars are 100 μm.
To separate the in trans effect from potential de novo RNAe initiation events in the F1 we repeated the cross, but now in a completely prg-1 mutant background, analysing GFP expression in the F1 (Figure 2B). This confirmed that an RNAe silenced transgene can act in trans, but only via the female germline. A 21Usensor(RNAe) transgene brought in via the sperm is not able to efficiently induce silencing upon a non21Usensor(+) transgene (Figure 2B). This illustrates that the female germline carries a dominant factor that sets the expression status of the transgene. Such a factor may involve a diffusible agent and/or direct allelic interactions. To examine this further, we performed an experiment in which we introduced a non21Usensor(+) transgene into an oocyte that has just lost a 21Usensor(RNAe) transgene. In this scenario, no allelic interactions between the two transgenes are possible. Still, such oocytes are capable to impose silencing upon the active transgene brought in via the sperm (Figure 2C). Again, sperm does not contain such a dominant activity (Figure 2C). These data indicate that the in trans activity of RNAe includes a diffusible, cytoplasmic agent in the female germline.
RNAe-associated small RNAs
Next, we tried to detect small RNAs derived from the (non)21Usensor(RNAe) transgenes in different genetic backgrounds. As published (Bagijn et al, 2012), we could easily detect 22G RNAs upon 21U RNA-induced silencing of the 21Usensor(+) transgene (Figure 3A). Similar small RNAs were also observed, although at reduced levels, in strains carrying transgenes silenced through RNAe and placed in a prg-1 mutant background. Since 22G RNAs that are directly triggered through PRG-1 are absent in these backgrounds, these results demonstrate that a 22G-like population can be maintained independently of Piwi-pathway activity. To analyse these small RNAs in further detail we cloned small RNAs from strains carrying a 21Usensor(RNAe) or 21Usensor(+) transgene. This confirmed that GFP-derived 22G-like RNAs are present specifically in the RNAe displaying background (Figure 3B; Supplementary Tables S1 and S2). Like previously reported 22G-RNAs, the majority of GFP-matching small RNA reads have a G-nucleotide at their 5′ end (Figure 3C) and show a size preference of 22 nucleotides (Figure 3D). We note that the cloned 22G-like RNAs are located quite far upstream from the 21UR1 recognition site that initiates the silencing, and seem to be further upstream compared with the 22G RNA sequences that were described by Bagijn et al (2012). This may reflect spreading of 22Gs to more distant, upstream regions during establishment or maintenance of RNAe, compared with 22G RNA production that is triggered directly by PRG-1.
Figure 3.
RNAe-associated small RNAs. (A) Northern blot, probed for 21UR1 (top panel), 21Usensor-derived 22G RNA (middle panel; probe just upstream of the 21UR1 recognition site), and let-7 (bottom panel). WT: wild-type animals. (B) Cloning frequencies (normalized to miRNAs and weighted to correct for non-unique reads) of small RNA reads from the two indicated genetic backgrounds (both wild-type for prg-1). Note that only egfp is specific for the transgene. The arrow indicates 21UR1 sequences for which the transgene has an engineered recognition site (dark green box). (C) Frequencies of base identities found at the 5′ end of cloned small RNAs that are anti-sense to egfp. Virtually no sense-RNA sequences matching to egfp were recovered. (D) Length distribution of small RNAs anti-sense to egfp (blue) and of small RNAs anti-sense to endogenous genes (red).
Genetic requirements of RNAe
We proceeded to identify genes that are required for RNAe. First, we checked the involvement of MUT-7, a well-known endogenous RNAi factor that recently has been shown to be required for PRG-1-mediated silencing (Bagijn et al, 2012). We found that RNAe also depends on MUT-7 (Table I; Supplementary Figure S2). Interestingly, mut-7 behaves as a maternal effect gene in these crosses: loss of MUT-7 only reactivates the sensor in the second generation of homozygosity. This result indicates that RNAe may be established in the germline of the parents. It also indicates that MUT-7 is not required for the maintenance of established RNAe within an individual. Two other genes previously implicated in the biogenesis of some 22G RNAs are smg-2 and smg-5 (Gu et al, 2009). Mutation of neither of these genes restored GFP expression of the RNAe silenced transgene (Table I). The RNA-dependent RNA polymerase producing the RNAe-related 22G RNAs still has to be identified.
Next we set out to identify Argonaute proteins involved in RNAe. We first tested wago-9, also known as hrde-1 (Buckley et al, 2012), since it was recently shown to function in the Piwi pathway (Bagijn et al, 2012). Disruption of WAGO-9 function reactivated the RNAe-silenced transgene, again only in animals coming from a wago-9 homozygous mutant hermaphrodite (Table I; Supplementary Figure S2). We note, however, that the GFP levels in wago-9 animals are notably lower than those observed in other mutant backgrounds, including mut-7, suggesting redundancy. Based on protein sequence, WAGO-9 is related WAGO-10, WAGO-11 and NRDE-3. We crossed mutant alleles of wago-10, wago-11 and nrde-3 into RNAe backgrounds but detected no reactivation of GFP expression (Table I; Supplementary Figure S2). Thus, the activity mediating the remaining silencing activity in wago-9 mutants remains to be identified.
Finally, we tested whether nrde-1 and nrde-2 are required for maintenance of RNAe. NRDE-1 and NRDE-2 are factors known to interact with nascent RNA and the nuclear Argonaute protein NRDE-3 and play a role in the establishment of repressive chromatin and the inhibition of transcription elongation at target loci following RNAi (Guang et al, 2008, 2010; Burkhart et al, 2011; Gu et al, 2012). Interestingly, disruption of both nrde-1 and nrde-2 results in restoration of GFP expression (Table I; Supplementary Figure S2), suggesting that RNAe at least partially acts at the chromatin level.
Interestingly, both nrde-1 and wago-9 mutants display enhanced de-silencing activity in prg-1 mutant backgrounds compared with prg-1 wild-type backgrounds, while the prg-1 mutation alone does not reactivate the RNAe sensor at all (Table I; Supplementary Figure S2). Thus, upon disruption of these two genes, PRG-1 re-engages in silencing of the 21U sensor, independent of WAGO-9 and NRDE-1, again suggesting redundancy. This effect is not observed in mut-7 mutant animals (Table I; Supplementary Figure S2), suggesting that MUT-7 acts at an early step during PRG-1-mediated silencing, while WAGO-9 and NRDE-1 act downstream of a branching point in the pathway.
RNAe induces repressive chromatin
To follow-up on the suggestion that RNAe induces changes at the chromatin level, we compared the chromatin states of the 21Usensor(+) and 21Usensor(RNAe) transgenes using ChIP-qPCR. In order to focus our experiments on the maintenance phase of RNAe, we performed these experiments in a prg-1 mutant background. To control for our ChIP efficiency, we first checked whether transposon Tc1 was enriched in our ChIP-qPCR experiments. This was indeed the case (Supplementary Figure S3). We then probed three distinct regions throughout the transgene. Consistent with the above results, we find H3K9 tri-methylation to be significantly enriched in all tested regions of the 21Usensor(RNAe) transgene (Figure 4A), while the 21Usensor(+) transgene did not show this enrichment. Among the tested regions is one upstream promoter fragment, indicating that repressive chromatin formation is not restricted to transcribed areas. H3K9 tri-methylation was lost upon disruption of nrde-1 or wago-9 (Figure 4A), just like RNAe-associated 22G RNAs (Figure 4B).
Figure 4.
RNAe affects chromatin. (A) ChIP-qPCR on animals carrying 21Usensor(+), 21Usensor(RNAe) and 21Usensor(RNAe) transgenes reactivated by nrde-1 and wago-9 mutations. All strains also carried the prg-1(pk2298) allele. H3K9 tri-methylation was probed in the indicated regions of the transgene. ChIP-qPCRs were normalized against the gph-1 promoter. Error bars reflect standard error, based on three replicates. (B) Northern blot for 22G and 21U RNAs in genetic backgrounds in which the 21Usensor(RNAe) transgene has been reactivated. As loading control let-7 is shown. *: non-specific signal. WT: wild-type. Note that the 21UR1 probe also visualizes 22G RNAs.
Discussion
In conclusion, we describe a phenomenon, RNAe, in which the C. elegans Piwi pathway can initiate a state of gene silencing that is extremely stable across generations. Similar findings were recently reported by others as well (Ashe et al, 2012; Lee et al, 2012; Shirayama et al, 2012). Although the mechanism behind RNAe has to be further elucidated, we present a model for RNAe based on the data presented in this manuscript (Figure 4B). RNAe taps into a nuclear RNAi pathway that has been suggested to interfere with transcriptional elongation (Guang et al, 2010). The transitivity of the silencing effect suggests that the involved factors are not stably bound to chromatin, but can diffuse through the nucleus and/or the cytoplasm. RNAe can be clearly separated into two phases: initiation and maintenance (Figure 5). Initiation can be through different input signals, as both PRG-1 and double stranded RNA can trigger RNAe. This initiation phase is accompanied by the production of 22G RNAs. In case of PRG-1 triggered silencing, these initiating 22G RNAs depend on the presence of PRG-1 and the response appears to be a relatively local event as these 22G RNAs are found close to the 21U recognition site on the targeted mRNA (Bagijn et al, 2012).
Figure 5.
Model for RNAe in C. elegans. PRG-1, but also long dsRNA, can induce a form a stably inherited gene silencing, named RNAe. The maintenance of RNAe across generations depends on the exo-ribonuclease MUT-7, on the previously identified nuclear RNAi factors NRDE-1 and NRDE-2 and on the Argonaute WAGO-9. In analogy with published work on NRDE-1/NRDE-2 and the nuclear Argonaute NRDE-3, we hypothesize that NRDE-2 and NRDE-1 bind to WAGO-9, while NRDE-1 may be bound to RNAe chromatin directly as well. The silencing pathway branches downstream of MUT-7, but upstream of WAGO-9. In addition to silencing, our results suggest that PRG-1 may also act to prevent silencing. See main text for further discussion.
Maintenance of RNAe is also accompanied by 22G RNA biogenesis. Interestingly, these maintenance-related 22G RNAs are distinct from the initiating 22G RNAs, as they are independent of PRG-1 and appear more upstream on the targeted mRNA. Like all 22G RNA studied so far, these small RNAs are most likely made by one of the RNA-dependent RNA polymerases RRF-1 and/or EGO-1. They are dependent on MUT-7 and WAGO-9, the latter being a likely acceptor for these 22G RNAs. Furthermore, the maintenance associated 22G RNAs are lost upon disruption of nrde-1, consistent with the previously proposed role for nrde-1 in inheritance of 22G RNAs following RNAi (Burton et al, 2011). It will be interesting to analyse the requirements and the dynamics of these two phases of 22G RNA production in further detail.
Given our finding that RNAe can be maintained within one generation in the absence of MUT-7 or WAGO-9, maintenance of RNAe seems to involve two distinct steps: a MUT-7/WAGO-9-dependent and a MUT-7/WAGO-9-independent step. The MUT-7/WAGO-9-independent phase may represent the heterochromatic nature of the silenced locus. The observed H3K9 tri-methylation of the transgene under RNAe conditions may keep it silent independent of ongoing RNAi. Following the parallels from the RNAi-chromatin pathway in Schizosaccharomyces pombe, the MUT-7/WAGO-9-dependent phase may reflect a requirement to re-initiate heterochromatin formation at each germline transmission by a nuclear RNAi pathway. Consistent with this notion, H3K9 tri-methylation and 22G RNAs are lost from RNAe silenced transgenes upon disruption of wago-9 or nrde-1. These data would suggest that WAGO-9, like its sequence-related paralog NRDE-3, is a nuclear Argonaute. Indeed, recently published papers confirm this idea (Ashe et al, 2012; Buckley et al, 2012; Shirayama et al, 2012). Since MUT-7 has also been described to be in the nucleus (Tops et al, 2005), we have to consider the possibility that RNAe-related 22G RNA biogenesis may be (partly) nuclear.
The just described mechanisms are characterized by redundancies that are revealed by differences in expression levels upon reactivation of an RNAe affected transgene through different mutations. One source of redundancy may be found in different types of RNAi-like pathways acting in parallel; for example, nuclear and cytoplasmic pathways. A second source of redundancy may be found between Argonaute proteins acting redundantly within similar, or identical pathways. Further experiments are required to resolve these issues.
Our findings indicate that PRG-1 may also be involved in generating an anti-silencing response, since the in trans effect of RNAe seems to be more effective in prg-1 mutants. Furthermore, we detected a dominant activity in the female germline that is capable of preventing RNAe establishment on an active transgene. In relation to these findings, it is interesting to note that we find 22G RNA derived from the endogenous part of the 21U sensor transgene (his-58), and that these apparently do not trigger RNAe. Moreover, these his-58 22G RNAs are reduced when RNAe has been triggered, while the GFP 22G RNAs increase in abundance. We speculate that the his-58 derived small RNA may reflect an anti-silencing pathway that is also guided by small RNA molecules, potentially triggered by PRG-1 as well. Such a model, in which repressing and activating activities compete has recently also been suggested by others (Ashe et al, 2012; Shirayama et al, 2012).
Finally, we note that RNAe is reminiscent of stable gene silencing effects known as paramutation (Erhard and Hollick, 2011; Pilu, 2011). These effects were originally described in maize but were later detected in mammals as well. It will be interesting to further decipher RNAe and learn what is responsible for the extreme stability across generations and whether other Piwi pathways can initiate paramutation-like effects as well. In this light, the recently reported piRNA-mediated trans-generational effects in Drosophila (Grentzinger et al, 2012) may be of particular interest.
Materials and methods
Worm culture
C. elegans was grown on OP50 bacteria according to standard laboratory conditions. The alleles used in this study are the following: prg-1(pk2298), henn-1(pk2295), wago-9(tm1200), wago-10(tm1332), wago-11(tm1127), C04F12.1(tm1637), nrde-1(gg088), nrde-2(gg091), nrde-3(gg066), mut-7(pk204), smg-2(e2008), smg-5(r860).
RNAi
RNAi was performed as described (Kamath et al, 2003), using bacterial strains expressing dsRNA for the indicated target genes.
Transgenics
The transgene alleles were previously described in Bagijn et al (2012). 21Usensor: mjIS144. non21Uensor: mjIS145.
Microscopy
GFP fluorescence was scored on a Leica DM6000 microscope and on a Zeiss M2Bio microscope. Images were taken on the DM6000 with fixed exposure times and illumination.
Northern blotting analysis
Total RNA was isolated using RNA lysis buffer and Trizol. Subsequently, small RNA was isolated with the Mirvana kit. Northern blotting was done as described previously (Kamminga et al, 2010). In all, 20 μg of small RNA was loaded on a 12% polyacrylamide gel and blotted according to standard procedures. Probe sequences:
21U-R1: GCACGGTTAACGTACGTACCA
let-7: AACTATACAACCTACTACCTCA.
22G-1: AAAGTGGTCAAGCACGGTTAAC
22G-2: AGTAAACCCAGCTTTCTTGTAC
22G-1 and 22G-2 probes were mixed before hybridization in Ambion hybridization buffer (ULTRAhyb-Oligo). Blots were exposed to phosphor-imager screens that were scanned on a BAS-2500 imager.
ChIP qPCR
ChIP was performed as described before (Mukhopadhyay et al, 2008). Antibodies: H3K9me3 (Abcam, Cat# ab8898). The qPCR was performed in triplicate and revealed almost identical results in two biological duplicates.
Primers:
- TC1 F1
aaccgttaagcatggaggtg
- TC1 R1
cacacgacgacgttgaaacc
- gph-1_promoter F3
gcgcaagtttctgctgtttt
- gph-1_promoter R3
cggaagattcacaagaagcaa
- 21Usensor mex_1 F1
gaccatgattacgccaagcta
- 21Usensor mex1 R1
TTTAATTCGGTGCGCCTTTA
- 21Usensor mex2 F1
ACTTTCCCCAAAATCCTGCT
- 21Usensor mex2 R1
CCTTCACCCTCTCCACTGAC
- 21Usensor egfp F1
GTCAGTGGAGAGGGTGAAGG
- 21Usensor egfp R1
TCGAGAAGCATTGAACACCA
- 21Usensor his58 F2
ACCGCTGTCCGTTTGATTCT
- 21Usensor his58 R2
GAAGAAGGGAATGCTTGAAAGG
q-RT–PCR
Primers used for qPCR:
- RT_egfp_intron_F
CATATTTAAATTTTCAGGTGCTGAAGTCAAG
- RT_egfp_intron_R
GTTGTGTCTAATTTTGAAGTTCTGAAAATTTAAATCAG
- RT_tbb-1_F
GAGGCCAACAATGGCAAATACGTTCC
- RT_tbb-1_R
CCACCTCCAAGAGAGTGTGTGAGC
- RT_pgl-3_F1
CCCACTGCTCCCTCAAAGCG
- RT_pgl-3_R1
CAGTCCTTGGGCGAACTTTTTGAAG
- RT_egfp_F
CTACCTGTTCCATGGCCAAC
- RT_egfp_R
AGTTAACTTTGATTCCATTCTT
Deep sequencing
Libraries for deep sequencing were prepared as described in and sequenced on an Illumina platform (Kamminga et al, 2012). RNA samples were not treated to remove 5′-tri-phosphate groups, hence 22G cloning frequencies are relatively low. Bioinformatic analysis was essentially performed as previously described (Kamminga et al, 2012), using custom scripts to map reads to transgenic DNA. Small RNA sequences have been deposited at GEO, accession number GSE39226.
Supplementary Material
Acknowledgments
The research here described was funded by the following grants to RFK: an ERC Starting Grant from the Ideas Programme of the European Union Seventh Framework Programme (202819), the European Union Sixth Framework Programme Integrated Project SIROCCO (LSHG-CT-2006-037900) and two grants from NWO (ECHO 700.57.006 and Vici 724.011.001). MVA was supported by an Erasmus grant and is a student of the Masters in Evolutionary and Developmental Biology, University of Lisbon, Portugal. We thank Bruno Albuquerque for assistance in qPCR design and Josien van Wolfswinkel for discussions. Some of the strains used in this study were provided by the NRBP, Japan.
Author contributions: ML, PvB and LJTK designed, performed and analysed the experiments. MVA and EFR performed and analysed the experiments. EB performed bioinformatics analysis. RFK designed the study, analysed data and wrote the paper with input from all the authors.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alcazar RM, Lin R, Fire AZ (2008) Transmission dynamics of heritable silencing induced by double-stranded RNA in Caenorhabditis elegans. Genetics 180: 1275–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi-Miyagawa S, Nakano T, Chien M, Russo JJ, Ju J, Sheridan R, Sander C, Zavolan M, Tuschl T (2006) A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442: 203–207 [DOI] [PubMed] [Google Scholar]
- Aravin AA, Bourc’his D (2008) Small RNA guides for de novo DNA methylation in mammalian germ cells. Genes Dev 22: 970–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravin AA, Sachidanandam R, Bourc’his D, Schaefer C, Pezic D, Toth KF, Bestor T, Hannon GJ (2008) A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol Cell 31: 785–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe A, Sapetschnig A, Weick EM, Mitchell J, Bagijn MP, Cording AC, Doebley AL, Goldstein LD, Lehrbach NJ, Le Pen J, Pintacuda G, Sakaguchi A, Sarkies P, Ahmed S, Miska EA (2012) piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell 150: 88–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagijn MP, Goldstein LD, Sapetschnig A, Weick EM, Bouasker S, Lehrbach NJ, Simard MJ, Miska EA (2012) Function, targets and evolution of Caenorhabditis elegans piRNAs. Science advance online publication, 14 June 2012 doi:; DOI: 10.1126/science.1220952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista PJ, Ruby JG, Claycomb JM, Chiang R, Fahlgren N, Kasschau KD, Chaves DA, Gu W, Vasale JJ, Duan S, Conte D Jr., Luo S, Schroth GP, Carrington JC, Bartel DP, Mello CC (2008) PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol Cell 31: 67–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley BA, Burkhart KB, Gu SG, Spracklin G, Kerschner A, Fritz H, Kimble J, Fire A, Kennedy S (2012) A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature advance online publication, 18 June 2012 doi:; DOI: 10.1038/nature11352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhart KB, Guang S, Buckley BA, Wong L, Bochner AF, Kennedy S (2011) A pre-mRNA-associating factor links endogenous siRNAs to chromatin regulation. PLoS Genetics 7: e1002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton NO, Burkhart KB, Kennedy S (2011) Nuclear RNAi maintains heritable gene silencing in Caenorhabditis elegans. Proc Natl Acad Sci USA 108: 19683–19688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conine CC, Batista PJ, Gu W, Claycomb JM, Chaves DA, Shirayama M, Mello CC (2010) Argonautes ALG-3 and ALG-4 are required for spermatogenesis-specific 26G-RNAs and thermotolerant sperm in Caenorhabditis elegans. Proc Natl Acad Sci USA 107: 3588–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H (1998) A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev 12: 3715–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PP, Bagijn MP, Goldstein LD, Woolford JR, Lehrbach NJ, Sapetschnig A, Buhecha HR, Gilchrist MJ, Howe KL, Stark R, Matthews N, Berezikov E, Ketting RF, Tavare S, Miska EA (2008) Piwi and piRNAs act upstream of an endogenous siRNA pathway to suppress Tc3 transposon mobility in the Caenorhabditis elegans germline. Mol Cell 31: 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhard KF Jr., Hollick JB (2011) Paramutation: a process for acquiring trans-generational regulatory states. Curr Opin Plant Biol 14: 210–216 [DOI] [PubMed] [Google Scholar]
- Gent JI, Lamm AT, Pavelec DM, Maniar JM, Parameswaran P, Tao L, Kennedy S, Fire AZ (2010) Distinct phases of siRNA synthesis in an endogenous RNAi pathway in C. elegans soma. Mol Cell 37: 679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent JI, Schvarzstein M, Villeneuve AM, Gu SG, Jantsch V, Fire AZ, Baudrimont A (2009) A Caenorhabditis elegans RNA-directed RNA polymerase in sperm development and endogenous RNA interference. Genetics 183: 1297–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildiyal M, Zamore PD (2009) Small silencing RNAs: an expanding universe. Nat Rev Genet 10: 94–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard A, Sachidanandam R, Hannon GJ, Carmell MA (2006) A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442: 199–202 [DOI] [PubMed] [Google Scholar]
- Grentzinger T, Armenise C, Brun C, Mugat B, Serrano V, Pelisson A, Chambeyron S (2012) piRNA-mediated transgenerational inheritance of an acquired trait. Genome Res (advance online publication, 3 May 2012; doi:10.1101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishok A, Tabara H, Mello CC (2000) Genetic requirements for inheritance of RNAi in C. elegans. Science 287: 2494–2497 [DOI] [PubMed] [Google Scholar]
- Gu SG, Pak J, Guang S, Maniar JM, Kennedy S, Fire A (2012) Amplification of siRNA in Caenorhabditis elegans generates a transgenerational sequence-targeted histone H3 lysine 9 methylation footprint. Nat Genet 44: 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Shirayama M, Conte D Jr, Vasale J, Batista PJ, Claycomb JM, Moresco JJ, Youngman EM, Keys J, Stoltz MJ, Chen CC, Chaves DA, Duan S, Kasschau KD, Fahlgren N, Yates JR III, Mitani S, Carrington JC, Mello CC (2009) Distinct argonaute-mediated 22G-RNA pathways direct genome surveillance in the C. elegans germline. Mol Cell 36: 231–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guang S, Bochner AF, Burkhart KB, Burton N, Pavelec DM, Kennedy S (2010) Small regulatory RNAs inhibit RNA polymerase II during the elongation phase of transcription. Nature 465: 1097–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guang S, Bochner AF, Pavelec DM, Burkhart KB, Harding S, Lachowiec J, Kennedy S (2008) An Argonaute transports siRNAs from the cytoplasm to the nucleus. Science 321: 537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J (2003) Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237 [DOI] [PubMed] [Google Scholar]
- Kamminga LM, Luteijn MJ, den Broeder MJ, Redl S, Kaaij LJ, Roovers EF, Ladurner P, Berezikov E, Ketting RF (2010) Hen1 is required for oocyte development and piRNA stability in zebrafish. EMBO J 29: 3688–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamminga LM, van Wolfswinkel JC, Luteijn MJ, Kaaij LJ, Bagijn MP, Sapetschnig A, Miska EA, Berezikov E, Ketting RF (2012) Differential impact of the HEN1 homolog HENN-1 on 21U and 26G RNAs in the germline of Caenorhabditis elegans. PLoS Genet 8: e1002702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketting RF (2011) The many faces of RNAi. Dev Cell 20: 148–161 [DOI] [PubMed] [Google Scholar]
- Klenov MS, Lavrov SA, Stolyarenko AD, Ryazansky SS, Aravin AA, Tuschl T, Gvozdev VA (2007) Repeat-associated siRNAs cause chromatin silencing of retrotransposons in the Drosophila melanogaster germline. Nucleic Acids Res 35: 5430–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Totoki Y, Toyoda A, Ikawa M, Asada N, Kojima K, Yamaguchi Y, Ijiri TW, Hata K, Li E, Matsuda Y, Kimura T, Okabe M, Sakaki Y, Sasaki H, Nakano T (2008) DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev 22: 908–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Gu W, Shirayama M, Youngman E, Conte D Jr, Mello CC (2012) C. elegans piRNAs Mediate the Genome-wide Surveillance of Germline Transcripts. Cell 150: 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone CD, Hannon GJ (2009) Small RNAs as guardians of the genome. Cell 136: 656–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Deplancke B, Walhout AJ, Tissenbaum HA (2008) Chromatin immunoprecipitation (ChIP) coupled to detection by quantitative real-time PCR to study transcription factor binding to DNA in Caenorhabditis elegans. Nature Protocols 3 698–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilu R (2011) Paramutation: just a curiosity or fine tuning of gene expression in the next generation? Curr Genomics 12: 298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi O, Minevich G, Hobert O (2011) Transgenerational inheritance of an acquired small RNA-based antiviral response in C. elegans. Cell 147: 1248–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle NC, Elgin SC (2008) A role for RNAi in heterochromatin formation in Drosophila. Curr Topics Microbiol Immunol 320: 185–209 [DOI] [PubMed] [Google Scholar]
- Saito K, Siomi MC (2010) Small RNA-mediated quiescence of transposable elements in animals. Dev Cell 19: 687–697 [DOI] [PubMed] [Google Scholar]
- Shirayama M, Seth M, Lee HC, Gu W, Ishidate T, Conte D Jr, Mello CC (2012) piRNAs initiate an epigenetic memory of nonself RNA in the C. elegans germline. Cell 150: 65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops BB, Tabara H, Sijen T, Simmer F, Mello CC, Plasterk RH, Ketting RF (2005) RDE-2 interacts with MUT-7 to mediate RNA interference in Caenorhabditis elegans. Nucleic Acids Res 33: 347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasale JJ, Gu W, Thivierge C, Batista PJ, Claycomb JM, Youngman EM, Duchaine TF, Mello CC, Conte D Jr (2010) Sequential rounds of RNA-dependent RNA transcription drive endogenous small-RNA biogenesis in the ERGO-1/Argonaute pathway. Proc Natl Acad Sci USA 107: 3582–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastenhouw NL, Brunschwig K, Okihara KL, Muller F, Tijsterman M, Plasterk RH (2006) Gene expression: long-term gene silencing by RNAi. Nature 442: 882. [DOI] [PubMed] [Google Scholar]
- Wang SH, Elgin SC (2011) Drosophila Piwi functions downstream of piRNA production mediating a chromatin-based transposon silencing mechanism in female germ line. Proc Natl Acad Sci USA 108: 21164–21169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Takeda A, Tsukiyama T, Mise K, Okuno T, Sasaki H, Minami N, Imai H (2006) Identification and characterization of two novel classes of small RNAs in the mouse germline: retrotransposon-derived siRNAs in oocytes and germline small RNAs in testes. Genes Dev 20: 1732–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yigit E, Batista PJ, Bei Y, Pang KM, Chen CC, Tolia NH, Joshua-Tor L, Mitani S, Simard MJ, Mello CC (2006) Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell 127: 747–757 [DOI] [PubMed] [Google Scholar]
- Yin H, Lin H (2007) An epigenetic activation role of Piwi and a Piwi-associated piRNA in Drosophila melanogaster. Nature 450: 304–308 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.