Correspondence to: mr@purdue.edu
Correction to: The EMBO Journal (2009) 28, 821–829. doi: 10.1038/emboj.2009.36
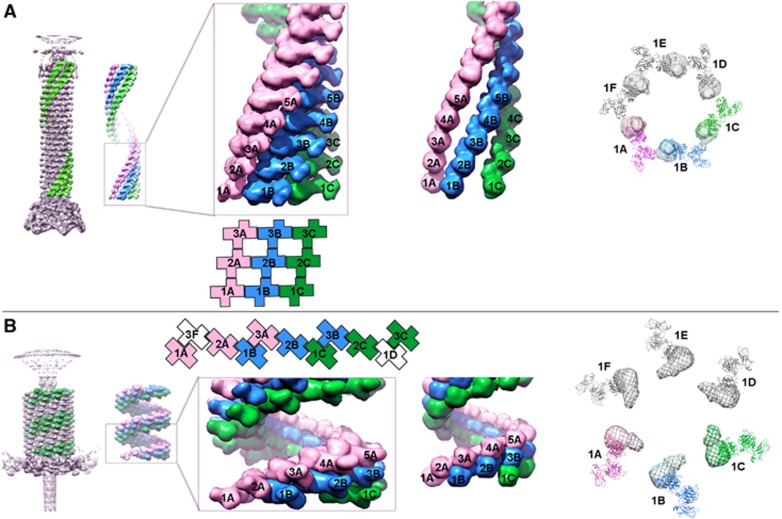
Since the publication of this article the authors have discovered a small mistake in Figure 4. The last column in Figure 4A should be exchanged with the last column in Figure 4B. Note that the figure legend is still correct.
The correct figure is shown here.
Figure 4.
Connectivity between subunits in the extended and contracted tail sheath. The subunits that form three of the six neighbouring helices (pink, A; blue, B; and green, C) within the sheath are shown as surface representations of (A) the extended and (B) the contracted sheath. The successive hexameric disks are numbered 1, 2, 3, 4 and 5, with the disk being closest to the baseplate numbered 1. On the left is shown a surface representation side view of the tail. Immediately next to it is shown the whole view with a closer view of the three neighbouring helices (in pink, blue and green). In the same column as the closer view of the helical arrangement is shown, the schematic diagram of the arrangement of the three outer domains using the same colour scheme with each gp18 molecule is being represented by a cross. Right next to this is shown the arrangement of the inner helices consisting only of domains IV. This domain retains the connectivity between neighbouring subunits within each helix in both (A) the extended and (B) the contracted sheath. At the right of each panel is shown the top view of one disk of the tail sheath in the (A) extended and (B) contracted conformations. The three outer domains that correspond to the atomic structure are shown in ribbon representation. Domain IV, whose structure has not yet been determined, is shown as density segmented from the cryo-EM map.
The authors apologise for any inconvenience caused.