Abstract
Across evolution, dolichols and polyprenols serve as sugar carriers in biosynthetic processes that include protein glycosylation and lipopolysaccharide biogenesis. Liquid chromatography coupled with electrospray ionization mass spectrometry offers a powerful tool for studying dolichols and polyprenols in their alcohol or glycan-modified forms in members of all three domains of life. In the following, recent examples of the how different versions of this analytical approach, namely reverse phase liquid chromatography-multiple reaction monitoring, normal phase liquid chromatography/tandem mass spectrometry and normal phase liquid chromatography-precursor ion scan detection have respectively served to address novel aspects of dolichol or polyprenol biology in Eukarya, Archaea and Bacteria. This article is part of a Special Issue entitled Lipodomics and Imaging Mass Spectrometry.
Keywords: Liquid chromatography/tandem mass, spectrometry, Multiple reaction monitoring, Precursor ion scan, Dolichol, Polyprenol
1. Introduction
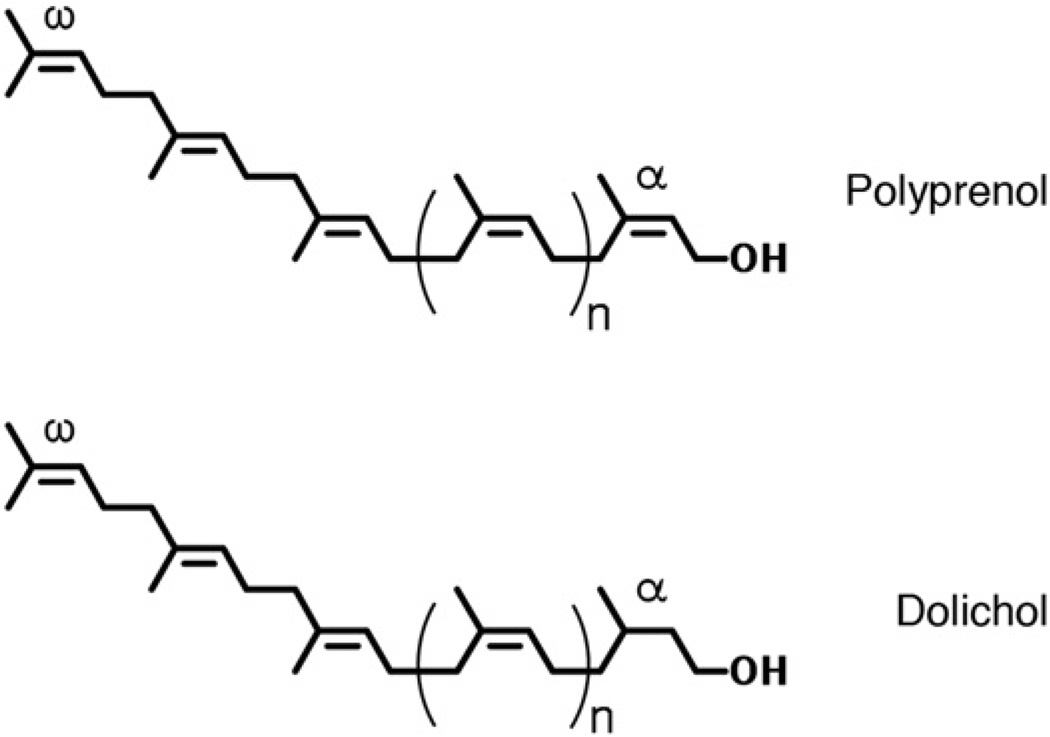
Dolichols and polyprenols are specific examples of polyisoprenoid alcohols, a family of hydrophobic polymers containing linearly linked isoprene subunits found in all living organisms [1,2]. Comprising up to 25 isoprene subunits and bearing a hydroxyl group at their α ends, dolichols can be distinguished from polyprenols on the basis of the saturated α isoprene subunit found in the former but not in the latter (Fig. 1).
Fig. 1.
Chemical structures of polyprenol and dolichol. The positions of the α and ω isoprene units are indicated.
The main and still best defined role played by dolichols and polyprenols is as a sugar carrier in various biosynthetic processes. In the N-glycosylation pathways of Eukarya and Archaea, the glycans eventually transferred to select Asn residues of target proteins are first assembled on phosphorylated dolichols. In each case, both simple sugars and complex oligosaccharides are attached to these dolichol carriers, with various organisms employing dolichols of different lengths [3,4]. Eukaryal organisms contain families of dolichol species consisting of six to eight members, with C80, C90 and C95 dolichols predominating in yeast, rat and man, respectively [1]. Multiple dolichol species are also involved in archaeal N-glycosylation [5]. By contrast, the bacterial N-glycosylation pathway predominantly relies on a single polyprenol species, C55 undecaprenol, as glycan carrier [6]. The same polyprenol carrier is employed in the assembly of peptidoglycans [7,8], and covalent modification of lipid A (endotoxin), the hydrophobic anchor of lipopolysaccharide (LPS1) in Gram-negative bacteria [9].
Despite the wealth of information on N-glycosylation and LPS biosynthesis available, outstanding questions remain. Often, these unknowns are centered on those pathway steps involving the biosynthesis and assembly of dolichol or polyprenol, as well as their glycan-charged derivatives. Liquid chromatography (LC) coupled with electrospray ionization tandem mass spectrometry (ESI-MS/MS) has proven to be a highly sensitive and specific tool for studying dolichols and polyprenols in their alcohol or glycan-modified forms in members of all three domains of life [10–17].
In the following, recent examples of the use of LC-ESI/MS to provide novel insight in dolichol and polyprenol biology are reviewed. Specifically, we describe: 1) reverse phase LC-multiple reaction monitoring (MRM) detection of polyprenols and dolichols in mouse embryos; 2) normal phase LC ESI-MS/MS of dolichol phosphate-linked glycans in Archaea; 3) normal phase LC-precursor ion scan detection of undecaprenyl phosphate-linked monosaccharide donors in the covalent modification of lipid A in Bacteria.
2. Reverse phase liquid chromatography-multiple reaction monitoring (LC-MRM) detection of polyprenols and dolichols in mouse embryos
Although the role of phosphorylated forms of dolichol as glycosyl carrier lipids in eukaryal N-glycosylation was described over 40 years ago [18], the enzymes responsible for several biosynthetic steps remain unknown. Recently, the gene responsible for catalyzing reduction of the α isoprene of polyisoprenol and giving rise to dolichol was described. Humans bearing mutations in the steroid 5α-reductase 3 (SRD5A3) gene suffer from a congenital disorder of glycosylation, characterized by mental retardation, and eye and cerebellar defects [11]. Since yeast cells lacking DFG10, encoding the ortholog of human SRD5A3, fail to fully reduce the α isoprene, as required to convert polyprenol to dolichol, it was proposed that SRD5A3 corresponds to the long-elusive dolichol reductase in mammals. To confirm this hypothesis, as well as to unequivocally identify the last step of dolichol biosynthesis, Srd5α3 knock-out mice were generated and reverse phase LC-MS was used to analyze polyprenols in wild type and SRD5A3-lacking mice embryos at the 11-day stage, representing the last day of viability of the mutant mice. However, the small size of the embryos presented an obstacle for the detection of polyprenols by MS in the full scan mode using a QSTAR XL quadrupole time-of-flight tandem mass spectrometer (Applied Biosystems, Foster City, CA), as previously described [10]. Accordingly, LC coupled with MRM, offering maximum sensitivity for the detection of dolichols and polyprenols, and especially valuable when only small amounts of cells or tissues are available, was employed.
MRM is primarily performed on triple quadrupole mass spectrometers, where the first quadrupole (Q1) isolates the precursor (parent) ion, Q2 acts as a collision cell and the third quadrupole (Q3) selects a specific fragment of the precursor ion. The two mass filters, Q1 and Q3, produce a very specific and sensitive response for the selected analyte that can subsequently be used to detect and integrate a peak in a simple one-dimensional chromatographic separation of the sample. Of all LC/MS/MS approaches, MRM offers the highest sensitivity for the detection and quantification of specific analytes.
In our study [11], LC was performed using a Shimadzu LC system (comprising a solvent degasser, two LC-10A pumps and a SCL-10A system controller) coupled to a 4000 Q-Trap hybrid triple quadrupole linear ion-trap mass spectrometer equipped with a Turbo V ion source (Applied Biosystems Inc, Foster City, CA) for the detection of C90 and C95 dolichols and polyprenols. LC was operated at a flow rate of 200 µl/min with a linear gradient as follows: 100% of mobile phase A was held isocratically for 2 min and then linearly increased to 100% mobile phase B over 14 min and held at 100% B for 4 min. Mobile phase A consisted of methanol/acetonitrile/aqueous 1 mM ammonium acetate (60/20/20, v/v/v), while mobile phase B consisted of 100% ethanol containing 1 mM ammonium acetate. A Zorbax SB-C8 reverse-phase column (5 µm, 2.1 × 50 mm) was obtained from Agilent. MRM was performed in the negative ion mode with MS settings as follows: Curtain gas (CUR) = 20 psi; Ion source gas 1 (GS1) = 20 psi; Ion source gas 2 (GS2) = 30 psi; Ion spray voltage (IS) = −4500 V; Heater temperature (TEM) = 350 °C; Interface heater = ON; De-clustering potential (DP) = −40 V; Entrance potential (EP) = −10 V; Collision cell exit potential (CXP) = −5 V. The voltage used for collision-induced dissociation was −40 V. The MRM pairs for C90 and C95 dolichols and polyprenols are 1304.2/59, 1372.2/59, 1302.2/59 and 1370.2/59, respectively. In these MRM pairs, the precursor ions are the [M+ acetate]− adduct ions, with the product ions being acetate ions (m/z 59).
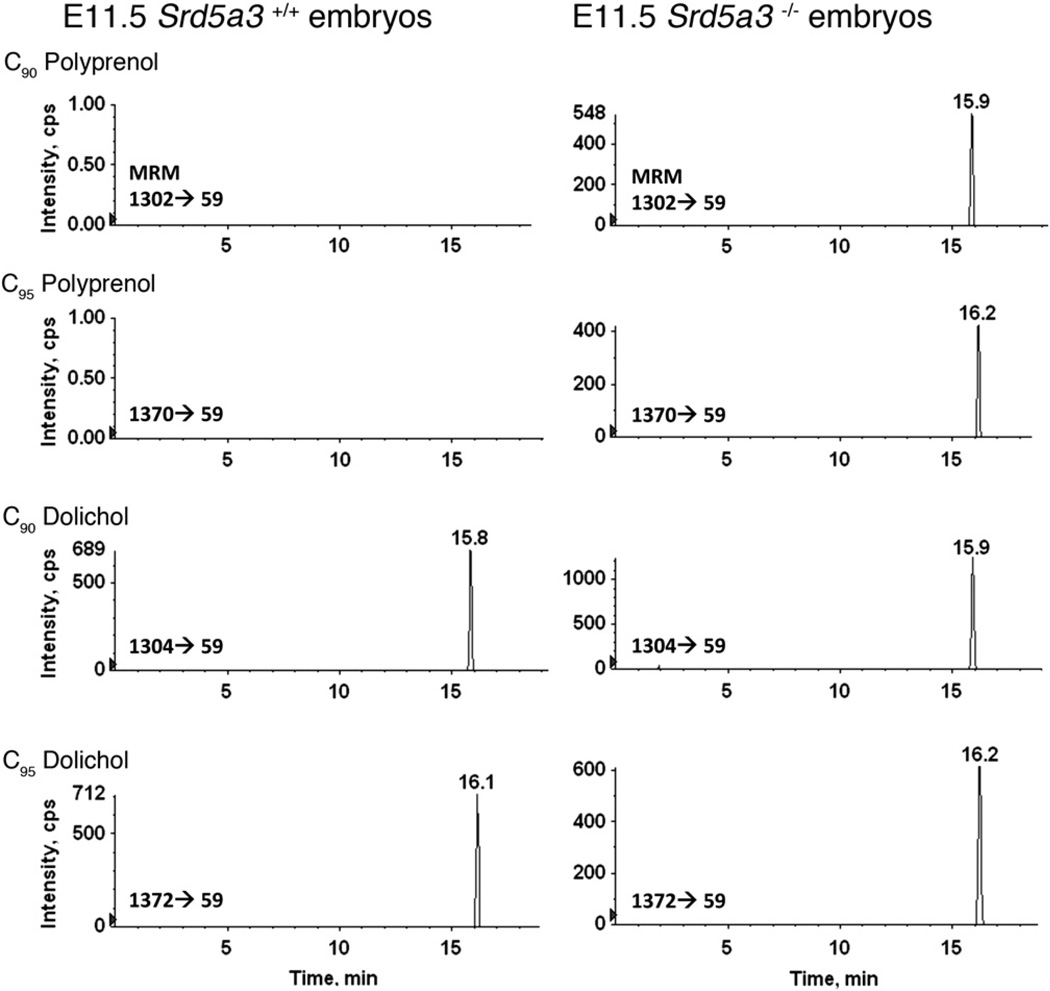
Such analysis revealed the absence of C90 and C95 polyprenols and the presence of C90 and C95 dolichols in 11-day old (E11.5) Srd5α3+/+ wild type embryos (Fig. 2). When, however, E11.5 Srd5α3−/− mutant mice were similarly examined, significant accumulations of C90 and C95 polyprenols were observed. Moreover, considerable levels of C90 and C95 dolichols were also detected. The results show that while SRD5A3 is indeed required for the reduction of polyprenol to dolichol, it is apparently not unique.
Fig. 2.
Reverse phase LC-MRM of dolichols and polyprenols in mouse embryos. Homozygous SDR5A3 mutation in mice causes the death of embryos and accumulation of polyprenols. The C90 polyprenol and C95 polyprenol are detected in the 11-day old (E11.5) Srd5α3−/− mutant embryos, but not in the E11.5 Srd5α3+/+ wild type embryos. C90 polyprenol and C95 polyprenol are detected through the MRM pairs of 1302/59 and 1370/59, respectively. C90 dolichol and C95 dolichol are detected through the MRM pairs of 1304/59 and 1372/59, respectively. C90 dolichol and C95 dolichol are known to be retained at 15.5–16.5 min [28].
Finally, it should be noted that an alternative approach using lithiated (+ Li+) adducts for the MRM detection of dolichol and polyprenol in human plasma has been described [11,19,20].
3. Normal phase liquid chromatography/tandem mass spectrometry of glycan-charged dolichol phosphates in Archaea
In Archaea, dolichol phosphates have been implicated as glycan carriers in the N-glycosylation pathway, much like their eukaryal counterparts (for review, see Ref. [4]). Still, much concerning the role of these glycan carriers and their participation in archaeal N-glycosylation remains unknown. Specifically, one can ask whether the glycans found on archaeal dolichol phosphate carriers are derived from soluble, activated monosaccharides, from monosaccharides transferred from individual dolichol phosphate carriers or from both, as in Eukarya. Likewise, it is not known whether the protein-targeted oligosaccharide is fully pre-assembled on a single phosphodolichol carrier or whether multiple glycan-charged lipid carriers are required. To address these and related questions, dolichol phosphate species of the halophilic archaeon, Haloferax volcanii, were analyzed by normal phase LC-ESI/MS [5]. Normal phase LC allows one to separate polyiosprenoids based on charge and polarity, whereas reverse phase LC is used to separate polyiosprenoids based on hydrophobicity, reflecting differences in isoprenoid chain length.
Normal phase LC-ESI/MS of the haloarchaeal lipids was performed using an Agilent 1200 Quaternary LC system coupled to a QSTAR XL quadrupole time-of-flight tandem mass spectrometer (Applied Biosystems, Foster City, CA). An Ascentis Si HPLC column (5 µm, 25 cm × 2.1 mm) was used for liquid chromatography. Mobile phase A consisted of chloroform/methanol/aqueous ammonium hydroxide (800:195:5, v/v/v), mobile phase B consisted of chloroform/methanol/water/ aqueous ammonium hydroxide (600:340:50:5, v/v/v/v) and mobile phase C consisted of chloroform/methanol/water/aqueous ammonium hydroxide (450:450:95:5, v/v/v/v). The elution program consisted of the following: 100% mobile phase A was held isocratically for 2 min and then linearly increased to 100% mobile phase B over 14 min and held at 100% B for 11 min. The LC gradient was then changed to 100% mobile phase C over 3 min and held at 100% C for 3 min, and finally returned to 100% A over 0.5 min and held at 100% A for 5 min. The total LC flow rate was 300 µl/min. The post-column splitter diverted ~ 10% of the LC flow to the ESI source of the Q-Star XL mass spectrometer, with MS settings as follows: Ion spray voltage (IS) = −4500 V; Curtain gas (CUR) = 20 psi; Ion source gas 1 (GS1)= 20 psi; De-clustering potential (DP) = −55 V; Focusing potential (FP) = −150 V. For MS/MS, collision-induced dissociation was performed with collision energy ranging from 40 V to 70 V (laboratory frame of energy) and with nitrogen as the collision gas. Data acquisition and analysis were performed using the instrument's Analyst QS software.
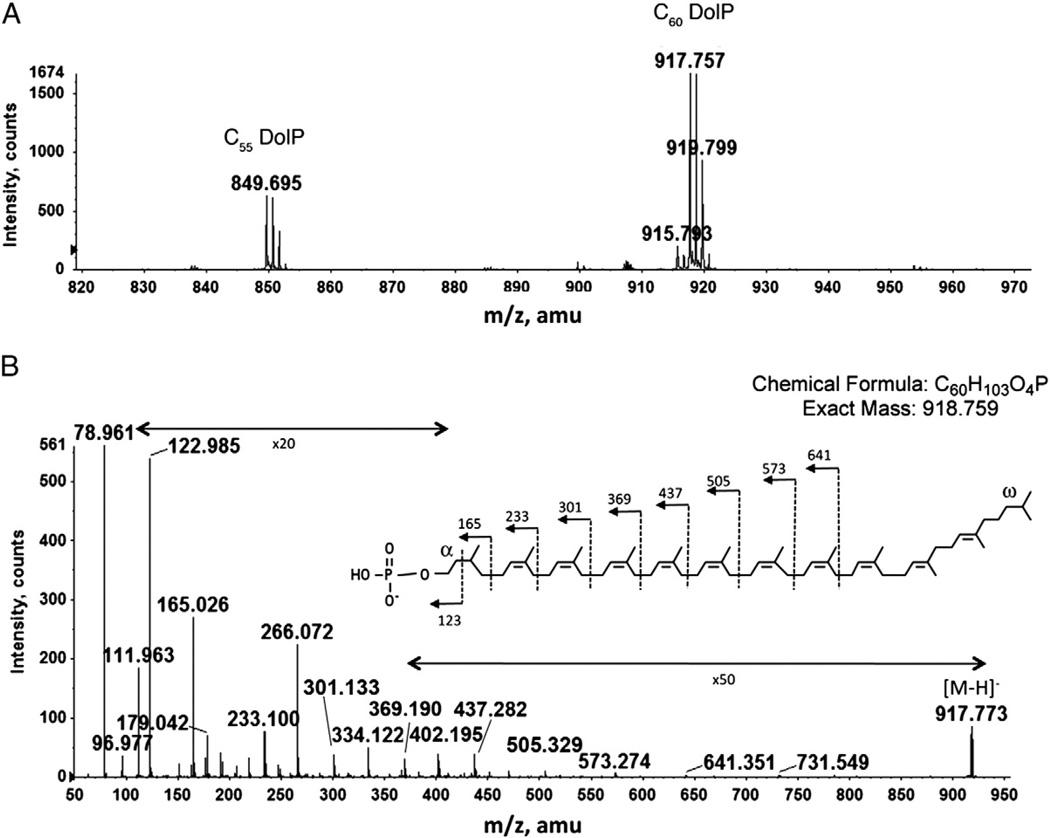
A total Hfx. volcanii lipid extract was subjected to normal phase LC-ESI/MS. The mass spectrum averaged from those acquired during the retention time of 20–21 min shows prominent ion peaks (m/z 849.695 and m/z 917.757) corresponding to the [M-H]− ions of C55 and C60 dolichol phosphates (Fig. 3A). MS/MS performed on C60 dolichol phosphate yielded a fragmentation pattern consistent with a previously described chemical structure [21], presenting saturated isoprene units at both the α and the ω positions (Fig. 3B). This structure differs from the C55 undecaprenol involved in bacterial N-glycosylation, where the α isoprene is unsaturated, and from the longer C70–C110 dolichols involved in eukaryal N-glycosylation, where the α position is saturated [2]. The presence of two saturated isoprene units in the archaeal dolichol phosphate is striking, considering the unsaturated nature of bacterial polyprenol phosphate and that only the α isoprenes are saturated in eukaryal dolichols [3].
Fig. 3.
Normal phase LC/MS identification of dolichol phosphate from the total lipid extract of Hfx. volcanii. A. The [M-H]− ions of C55 and C60 dolichol phosphate detected at m/z 849.695 and 917.757, indicated by C55 DolP and C60 DolP, respectively. B. MS/MS of the [M-H]− ion of C60 dolichol phosphate. The inset shows the predicted chemical structure of dolichol phosphate (according to [10]) and the MS/MS fragmentation scheme.
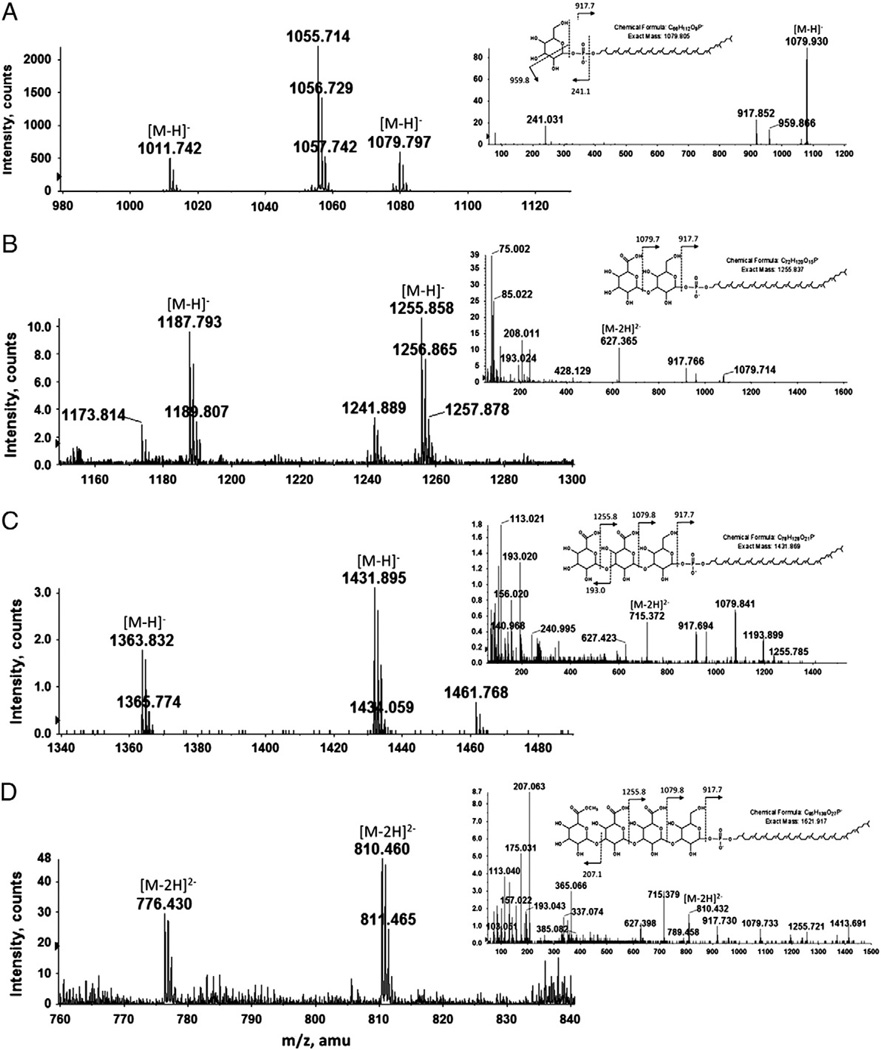
It is known that the Hfx. volcanii surface (S)-layer glycoprotein is modified by an N-linked pentasaccharide comprising a hexose, two hexuronic acids, a methyl ester of hexuronic acid and a final hexose [22,23]. To determine whether Hfx. volcanii dolichol phosphate is modified by similar glycans, glycan-charged dolichol phosphates were assessed by normal phase LC/MS in a Hfx. volcanii total lipid extract. Such analysis detected dolichol phosphate species modified by one to four saccharides (Figs. 4A–D), while MS/MS analysis confirmed the nature of these sugar residues (insets). Specifically, the fraction eluting at the retention time of 16–16.5 min (Fig. 4A) contains hexose-modified C55 and C60 dolichol phosphate (peaks at m/z 1011.724 and 1079.797, respectively). A major peak at m/z 1055.714, corresponding to a previously described sulfoglycolipid [24] was also observed. The fraction eluting at the retention time of 20.8–21.3 min (Fig. 4B) contains C55 and C60 dolichol phosphates modified by a hexose and a hexuronic acid (peaks at m/z 1187.793 and 1255.858, respectively), while the fraction eluting at a retention time of 26–27 min (Fig. 4C) contains C55 and C60 dolichol phosphates modified by a hexose and two hexuronic acids (peaks at m/z 1363.832 and 1431.895, respectively). Lastly, the fraction eluting at the retention time of 35.5–36 min (Fig. 4D) contains C55 and C60 dolichol phosphates modified by a tetrasaccharide comprising a hexose, two hexuronic acids and a methyl ester of hexuronic acid (their doubly charged ions [M-2H]2− are observed at m/z 766.43 and 810.46, respectively). The structure of the C60 phosphodolichol-linked tetrasaccharide was verified by MS/MS (Fig. 4D, inset). The most prominent ion at m/z 207 in the MS/MS spectrum derived from the [M-2H]2− ion at m/z 810.46 is derived from the fourth tetrasaccharide residue. Its methanol-less (32Da) ion, shown at m/z 175, supports the earlier assignment of this residue as a methyl ester of hexuronic acid [23].
Fig. 4.
Normal phase LC/MS/MS identification of mono-, di-, tri-, and tetrasacchride-charged dolichol phosphate from the total Hfx. volcanii lipid extract. Shown are the [M-H]− ions of (A) hexose-, (B) hexuronic acid-hexose- and (C) dihexuronic acid-hexose-modified C55 and C60 phosphodolichol, as well as (D) the doubly charged [M-2H]2− ions of methyl ester of hexuronic acid-dihexuronic acid-hexose-modified C55 and C60 phosphodolichol, respectively detected at m/z 766.43 and 810.46. The insets show MS/MS spectra of the [M-H]− ion of (A) hexose-, (B) hexuronic acid-hexose- or (C) dihexuronic acid-hexose-modified C60 phosphodolichol or (D) of the doubly charged [M-2H]2− ions of methyl ester of hexuronic acid-dihexuronic acid-hexose-modified C60 phosphodolichol. The chemical structure of the glycan-charged C60 phosphodolichol is shown in each case.
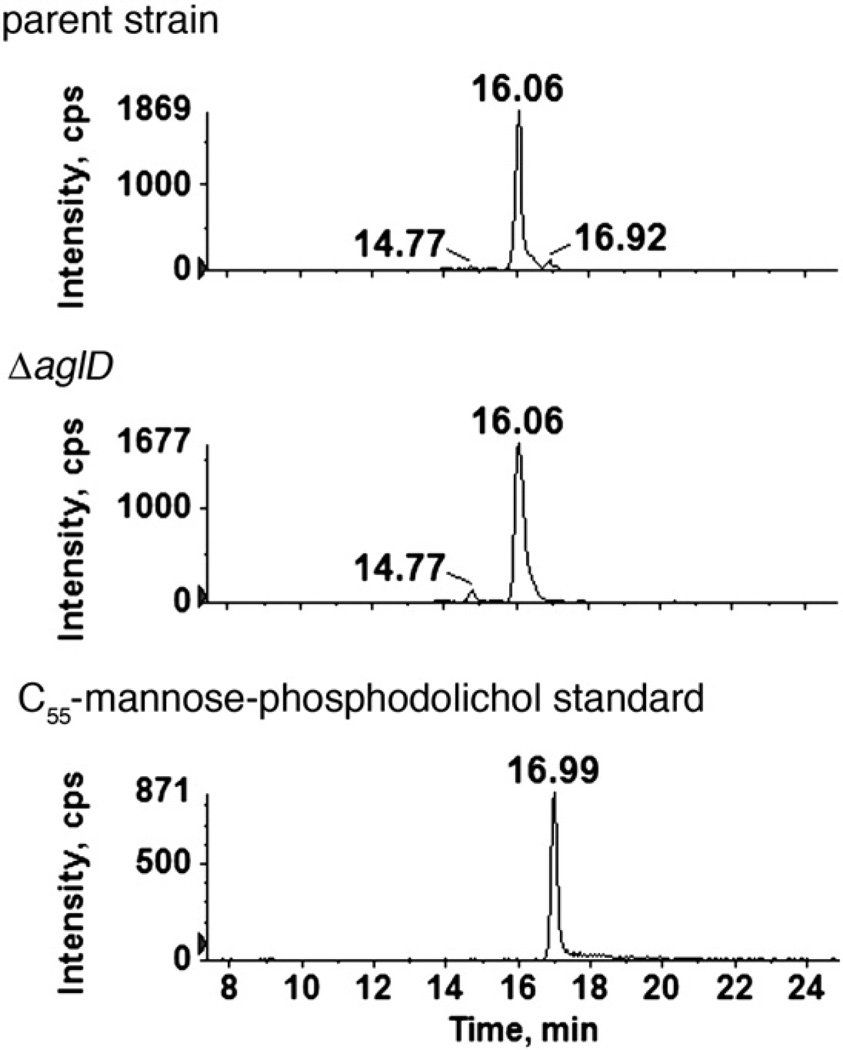
Despite the fact that the Hfx. volcanii S-layer glycoprotein is modified by a N-linked pentasaccharide, the longest oligosaccharide linked to dolichol phosphate corresponds to a tetrasaccharide comprising pentasaccharide residues one through four. To assess whether the final pentasaccharide residue is derived from its own dolichol phosphate carrier, the monosaccharide-charged peak obtained by normal phase LC-ESI/MS was analyzed. Analysis of the extracted ion chromatograms revealed the presence of three hexose-modified phosphodolichol species, retained at 14.77, 16.06 and 16.92 min, respectively. Of these, only the hexose-modified phosphodolichol with a retention time of 16.92 min was eliminated in Hfx. volcanii cells deleted of aglD, previously implicated in the addition of the final pentasaccharide subunit [22] (Fig. 5). In a parallel experiment, a mannose-charged C55 phosphodolichol standard examined as above eluted at 16.99 min.
Fig. 5.
The absence of AglD eliminates mannose-modified phosphodolichol. Normal phase LC extracted ion chromatograms of the dolichylphosphate-hexose [M-H]− ion at m/z 1079.8 from the parent (upper panel) and ΔaglD strains (middle panel) are shown. As the 16.92 min peak is eliminated in the mutant, AglD is apparently responsible for the formation of this monosaccharide-modified phosphodolichol species, retained at the position of a mannose-modified phosphodolichol standard (16.99 min; lower panel).
Thus, the first four saccharide subunits of the pentasaccharide N-linked to the Hfx. volcanii S-layer glycoprotein are sequentially added to a common dolichol phosphate carrier, while mannose, the fifth and final S-layer glycoprotein N-linked pentasaccharide subunit, is added to its own dolichol phosphate carrier.
4. Normal phase liquid chromatography-precursor ion scan detection of undecaprenol phosphate-linked monosaccharide donors for lipid A modification in Bacteria
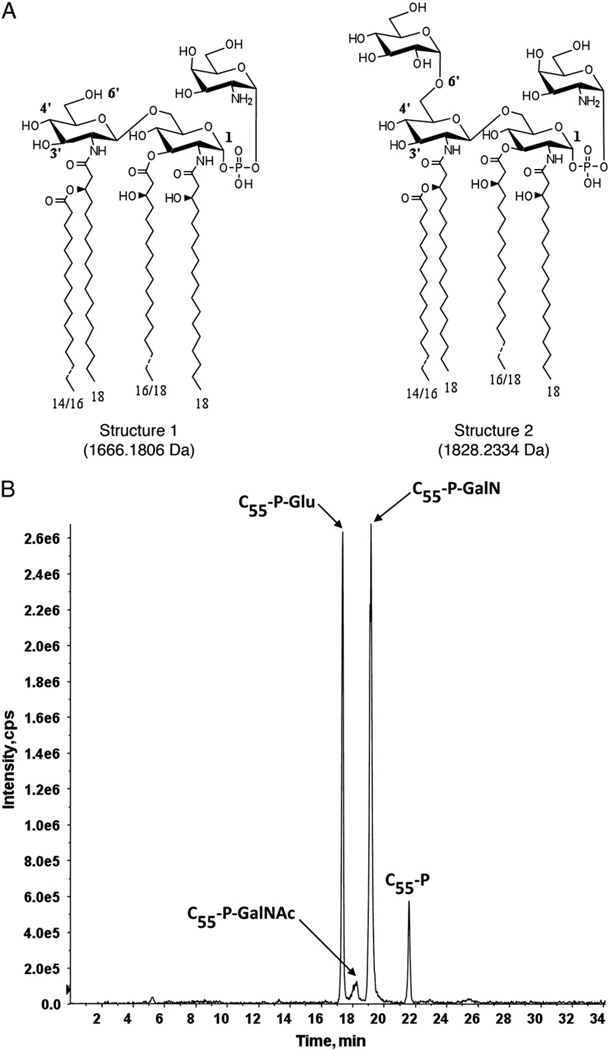
In contrast to Eukarya and Archaea, the bacterial N-glycosylation pathway relies on a polyprenol species as glycan carrier, usually C55 undecaprenol, in which the α isoprene is not saturated. The same polyprenol carrier participates in the biosynthesis of LPS, the major component of the outermost layer of the Gram-negative bacterial cell envelope. LPS consists of the hydrophobic lipid A moiety, a non-repeating core oligosaccharide and a distal repeating oligosaccharide, termed the O-antigen [9]. In Francisella novicida, a mouse pathogen, the major lipid A species is glycosylated at the 1-position with a galactosamine (GalN) residue, whereas a minor lipid A species is modified with an additional glucose unit at the 6′-position [25] (Fig. 6A, structures 1 and 2, respectively). Undecaprenol phosphate-GalN and undecaprenol phosphate-glucose serve as the donors of the GalN and glucose residues, respectively. While the identification and structural elucidation of these lipid carriers have been described [26,27], more rapid and sensitive techniques to detect these and other undecaprenol phosphate-linked sugar donors in a F. novicida total crude lipid extract (i.e. without first performing additional fractionation/separation steps) are highly desirable. For this purpose, normal phase LC/MS/MS in the precursor ion scan mode proved to be particularly advantageous.
Fig. 6.
LC-precursor ion scan chromatogram of m/z 845.6, showing the identification of undecaprenol phosphate-linked sugars that are involved in the modification of lipid A in F. novicida. A. The major lipid A species (structure 1) in F. novicida is glycosylated at the 1-position with a galactosamine (GalN) residue; a minor lipid A species (structure 2) is modified with an additional glucose unit at the 6′-position. B. C55-P-GalN, C55-P-GalNAc, and C55-P-Glu are the major undecaprenyl phosphate linked sugars detected in the total Bligh-Dyer lipid extract of F. novicida.
Like MRM, precursor ion scan analysis is also primarily performed on triple quadrupole mass spectrometers. Here, the third quadrupole (Q3) is set to scan for a specific m/z ratio (e.g. m/z 845.6). Upstream, the first quadrupole (Q1) is set to scan the mass range that includes all those precursor ions whose fragmentation (occurring in the second quadrupole, Q2) would result in appearance of the selected product ion (i.e. m/z 845.6). Since the mass spectrum obtained is derived from only precursor ions whose fragmentation results in a common daughter ion (i.e. m/z 845.6), this approach significantly increases the sensitivity of tandem mass spectrometry by filtering out chemical noise and other irrelevant ions from the spectrum. Moreover, precursor ion scan analysis possesses the unique advantage of rapid detection of a class of molecular species containing a given common substructure (e.g. undecaprenyl) in complex mixtures.
Normal phase LC/MS/MS in the precursor ion scan mode is performed using an Agilent 1200 system coupled to a 4000 Q-Trap hybrid triple quadrupole linear ion-trap mass spectrometer equipped with a Turbo V ion source (as above). The normal phase LC column, mobile phase compositions, and gradient as described above were used. The precursor ion scan of m/z 845 (corresponding the [M-H]− ion of the undecaprenol phosphate, C55-P) was run in the negative ion mode. The precursor ion scan ranges from m/z 800 to 1100, with the scanning conditions were set as follows: Scan mode = profile; Step size = 0.1 Da; Scan rate = 1 s; Pause between mass ranges = 5 ms. Resolution Q1 = Low; Resolution Q3=Unit. The TurboIonSpray ion source conditions and MS settings were optimized as the following: CUR = 25 psi; Collision gas (CAD) = medium; IS = −4500 V; TEM = 350 °C; GS1 = 25 psi; GS2 = 30 psi; Interface heater = on; DP = −60 V; EP = −10 V; CXP = −5 V. The voltage used for collision-induced dissociation was −65 V.
Fig. 6B shows the normal phase LC-coupled precursor ion scan chromatogram of m/z 845.6, showing the detection of several undecaprenol phosphate-linked monosaccharides in the total lipid extract of F. novicida, confirming the existence of hexose- and hexosamine-modified C55 dolichol phosphates. Also observed is an N-acetylhexosamine-modified C55 undecaprenol phosphate (C55-P-GalNAc). It has been proposed that C55-P-GalNAc can be deacetylated to produce C55-P-GalN through the action of a putative deacetylase (the product of the Ftn_0544 gene). However, no endogenous C55-P-GalNAc species was detected in a previous MS analysis of a F. novicida lipid extract. Hence, the precursor ion scan protocol employed allowed for undecaprenol phosphate-derived sugars in the total lipid extract of F. novicida to be identified quickly with more enhanced sensitivity than before. The relatively straightforward precursor ion scan protocol can thus be carried out with a non-fractionated total Bligh–Dyer lipid extract and can, moreover, be considered as complementary to more elaborate and time-consuming procedures required for structural analysis of lipid A.
5. Conclusions
In the past, efforts to provide detailed insight into various dolichol- or polyprenol-dependent glycosylation events were hampered by challenges associated with either accumulating sufficient material for biochemical characterization or by difficulties associated with the structural elucidation of these molecular species. Today, liquid chromatography coupled with electrospray ionization mass spectrometry offers a powerful tool to surmount both of these obstacles, as reflected in the three examples presented here. Indeed, the combination of LC-ESI/MS/MS with manipulations at the gene level promises to provide further insight into a variety of polyisoprenoid-based processes, including the assembly of various glycans on dolichol or polyprenol carriers.
Acknowledgments
The mass spectrometry facility in the Department of Biochemistry of the Duke University Medical Center and Z.G. are supported by the LIPID MAPS Large Scale Collaborative Grant number GM-069338 from NIH. J.E. is supported by the Israel Science Foundation (grant 30/07). We thank Dr. Christian R.H. Ratez for advice and support, Dr. Robert Murphy for advice on the LC/MS analysis of lipids, Mr. Reza Kordestani for early development of the normal phase LC/MS method, and our collaborators from the laboratories of Dr. Joseph Gleeson and Dr. Hudson Freeze on the SDR5A3 polyprenol reductase project.
Abbreviations
- ESI-MS/MS
electrospray ionization tandem mass spectrometry
- GalN
galactosamine
- GalNAc
N-acetyl galactosamine
- Glu
glucose
- LC
liquid chromatography
- LPS
lipopolysaccharide
- MRM
multiple reaction monitoring
- SRD5A3
steroid 5α-reductase 3
- S-layer
surface layer
Footnotes
This article is part of a Special Issue entitled Lipodomics and Imaging Mass Spectrometry.
References
- 1.Swiezewska E, Danikiewicz W. Polyisoprenoids: structure, biosynthesis and function. Prog. Lipid Res. 2005;44:235–258. doi: 10.1016/j.plipres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Jones MB, Rosenberg JN, Betenbaugh MJ, Krag SS. Structure and synthesis of polyisoprenoids used in N-glycosylation across the three domains of life. Biochim. Biophys. Acta. 2009;1790:485–494. doi: 10.1016/j.bbagen.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burda P, Aebi M. The dolichol pathway of N-linked glycosylation. Biochim. Biophys. Acta. 1999;1426:239–257. doi: 10.1016/s0304-4165(98)00127-5. [DOI] [PubMed] [Google Scholar]
- 4.Calo D, Kaminski L, Eichler J. Protein glycosylation in Archaea: sweet and extreme. Glycobiology. 2010;20:1065–1079. doi: 10.1093/glycob/cwq055. [DOI] [PubMed] [Google Scholar]
- 5.Guan Z, Naparstek S, Kaminski L, Konrad Z, Eichler J. Distinct glycan-charged phosphodolichol carriers are required for the assembly of the pentasaccharide N-linked to the Haloferax volcanii S-layer glycoprotein. Mol. Microbiol. 2010;78:1294–1303. doi: 10.1111/j.1365-2958.2010.07405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weerapana E, Imperiali B. Asparagine-linked protein glycosylation: from eukaryotic to prokaryotic systems. Glycobiology. 2006;16:91R–101R. doi: 10.1093/glycob/cwj099. [DOI] [PubMed] [Google Scholar]
- 7.Higashi Y, Strominger JL, Sweeley CC. Biosynthesis of the peptidoglycan of bacterial cell walls. XXI. Isolation of free C55-isoprenoid alcohol and of lipid intermediates in peptidoglycan synthesis from Staphylococcus aureus. J. Biol. Chem. 1970;245:3697–3702. [PubMed] [Google Scholar]
- 8.Guan Z, Breazeale SD, Raetz CRH. Extraction and identification by mass spectrometry of undecaprenyl diphosphate-MurNAc-pentapeptide-GlcNAc from Escherichia coli. Anal. Biochem. 2005;345:336–339. doi: 10.1016/j.ab.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Raetz CRH, Whitfield C. LPS endotoxins. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrett TA, Guan Z, Raetz CRH. Analysis of ubiquinones, dolichols, and dolichol diphosphate-oligosaccharides by liquid chromatography-electrospray ionization-mass spectrometry. Methods Enzymol. 2007;432:117–143. doi: 10.1016/S0076-6879(07)32005-3. [DOI] [PubMed] [Google Scholar]
- 11.Cantagrel V, Lefeber DJ, Ng BG, Guan Z, Silhavy JL, Bielas SL, Lehle L, Hombauer H, Adamowicz M, Swiezewska E, De Brouwer A, Bluemel P, Cegielska J, Houliston SR, Swistun D, Ali BR, Babovic-Vuksanovic D, van Bokhoven H, Wevers RA, Raetz CRH, Freeze HH, Morava E, Al-Gazali L, Gleeson JG. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. 2010;142:1–15. doi: 10.1016/j.cell.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreyev AY, Fahy E, Guan Z, Kelly S, Li X, McDonald JG, Milne S, Myers D, Park H, Ryan A, Thompson BM, Wang E, Zhao Y, Brown HA, Merrill AH, Raetz CRH, Russell DW, Subramaniam S, Dennis EA. Subcellular organelle lipidomics in TLR-4-activated macrophages. J. Lipid Res. 2010;51:2785–2797. doi: 10.1194/jlr.M008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grabinska KA, Cui J, Chatterjee A, Guan Z, Raetz CRH, Robbins PW, Samuelson J. Molecular characterization of the cis-prenyltransferase of Giardia lamblia. Glycobiology. 2010;20:824–832. doi: 10.1093/glycob/cwq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rush JS, Matveev S, Guan Z, Raetz CRH, Waechter CJ. Expression of functional bacterial undecaprenyl pyrophosphate synthase in the yeast rer2{Delta} mutant and CHO cells. Glycobiology. 2010;20:1585–1593. doi: 10.1093/glycob/cwq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skorupinska-Tudek K, Bienkowski T, Olszowska O, Furmanowa M, Chojnacki T, Danikiewicz W, Swiezewska E. Divergent pattern of polyisoprenoid alcohols in the tissues of Coluria geoides: a new electrospray ionization MS approach. Lipids. 2003;38:981–990. doi: 10.1007/s11745-003-1152-3. [DOI] [PubMed] [Google Scholar]
- 16.Reid CW, Stupak J, Szymanski CM, Li J. Analysis of bacterial lipid-linked oligosaccharide intermediates using porous graphitic carbon liquid chromatography-electrospray ionization mass spectrometry: heterogeneity in the polyisoprenyl carrier revealed. Anal. Chem. 2009;81:8472–8478. doi: 10.1021/ac9013622. [DOI] [PubMed] [Google Scholar]
- 17.Haeuptle MA, Hulsmeier AJ, Hennet T. HPLC and mass spectrometry analysis of dolichol-phosphates at the cell culture scale. Anal. Biochem. 2010;396:133–138. doi: 10.1016/j.ab.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Behrens NH, Leloir LF. Dolichol monophosphate glucose: an intermediate in glucose transfer in liver. Proc. Natl. Acad. Sci. USA. 1970;66:153–159. doi: 10.1073/pnas.66.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Alexandri FL, Gozzo FC, Eberlin MN, Katzin AM. Electrospray ionization mass spectrometry analysis of polyisoprenoid alcohols via Li + cationization. Anal. Biochem. 2006;355:189–200. doi: 10.1016/j.ab.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Gutkowska M, Bienkowski T, Hung VS, Wanke M, Hertel J, Danikiewicz W, Swiezewska E. Proteins are polyisoprenylated in Arabidopsis thaliana. Biochem. Biophys. Res. Comm. 2004;322:998–1004. doi: 10.1016/j.bbrc.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Kuntz C, Sonnenbichler J, Sonnenbichler I, Sumper M, Zeitler R. Isolation and characterization of dolichol-linked oligosaccharides from Haloferax volcanii. Glycobiology. 1997;7:897–904. doi: 10.1093/glycob/7.7.897. [DOI] [PubMed] [Google Scholar]
- 22.Abu-Qarn M, Yurist-Doutsch S, Giordano A, Trauner A, Morris HR, Hitchen P, Dell A, Eichler J. Haloferax volcanii AglB and AglD are involved in N-glycosylation of the S-layer glycoprotein and proper assembly of the surface layer. J. Mol. Biol. 2007;14:1224–1236. doi: 10.1016/j.jmb.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 23.Magidovich H, Yurist-Doutsch S, Konrad Z, Ventura VV, Dell A, Hitchen PG, Eichler J. AglP is a S-adenosyl-l-methionine-dependent methyltransferase that participates in the N-glycosylation pathway of Haloferax volcanii. Mol. Microbiol. 2010;76:190–199. doi: 10.1111/j.1365-2958.2010.07090.x. [DOI] [PubMed] [Google Scholar]
- 24.Sprott GD, Larocque S, Cadotte N, Dicaire CJ, McGee M, Brisson JR. Novel polar lipids of halophilic eubacterium Planococcus H8 and archaeon Haloferax volcanii. Biochim. Biophys. Acta. 2003;1633:179–188. doi: 10.1016/j.bbalip.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Ribeiro AA, Guan Z, McGrath SC, Cotter RJ, Raetz CRH. Structure and biosynthesis of free lipid A molecules that replace LPS in Francisella tularensis subsp, Novicida. Biochemistry. 2006;45:14427–14440. doi: 10.1021/bi061767s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Ribeiro AA, Guan Z, Raetz CRH. Identification of undecaprenyl phosphate-beta-d-galactosamine in Francisella novicida and its function in lipid A modification. Biochemistry. 2009;48:1162–1172. doi: 10.1021/bi802211k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song F, Guan Z, Raetz CR. Biosynthesis of undecaprenyl phosphate-galactosamine and undecaprenyl phosphate-glucose in Francisella novicida. Biochemistry. 2009;48:1173–1182. doi: 10.1021/bi802212t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward WC, Guan Z, Zucca FA, Fariello RG, Kordestani R, Zecca L, Raetz CR, Simon JD. Identification and quantification of dolichol and dolichoic acid in neuromelanin from substantia nigra of the human brain. J. Lipid Res. 2007;248:1457–1462. doi: 10.1194/jlr.C700008-JLR200. [DOI] [PubMed] [Google Scholar]