Abstract
NF-kB is a critical transcription factor for the production of many inflammatory cytokines. It is activated in the airway epithelium of human asthmatics and in mice after allergic stimulation. To examine the role of NF-kB activation in allergic inflammation we generated transgenic mouse lines that allowed for the inducible stimulation of NF-kB in airway epithelial cells. After allergic sensitization with ovalbumin and alum, mice were challenged daily with ovalbumin aerosols and NF-kB was activated in airway epithelium by administration of doxycycline. Enhancement of airway epithelial NF-kB expression alone did not lead to increased airway responsiveness to methacholine. However induction of epithelial NF-kB during allergic inflammation caused airway hyperresponsiveness, increased airway neutrophilic and lymphocytic inflammation and goblet cell hyperplasia. Accompanying the exaggerated inflammation was an increase in the cytokines, G-CSF, IL-15, and KC. Interestingly, the counter regulatory interleukin, IL-10, was suppressed by NF-kB activation. The epithelial NF-kB dependent modulation of these cytokines provides a plausible explanation for the increased inflammation seen with overexpression of NF-kB. Modulation of airway epithelial NF-kB activation enhances the airway hyperresponsiveness and mucus secretion found in the mouse lung during allergic inflammation. NF-kB represents a potential target for pharmacologic intervention in human asthma.
Introduction
The ubiquitous transcription factor complex, nuclear factor kappa B (NF-kB), is necessary for directing high level transcription of many cytokines, adhesion molecules, and other proinflammatory proteins. Expression of NF-kB is increased in the airway epithelium of human asthmatics and in the lungs of allergically sensitized and challenged mice [1-4]. Inhibitors of NF-kB activation diminish the influx of inflammatory cells and reduce the airway responsiveness provoked by allergic inflammation [5-12]. Transgenic mice, with expression in Clara cells of a dominant negative IkB resistant to phosphorylation induced degradation, had less inflammation to allergic stimuli but retained increases in airway constrictor responses [13]. The effect of NF-kB activation, as opposed to inhibition, in airway epithelial cells during allergic inflammation is uncertain. To determine the effect of enhanced epithelial cell NF-kB signaling in allergic inflammation we utilized a tetracycline-inducible transgenic mouse with targeted expression of a constitutively active form of IκB kinase 2 in airway epithelium which allowed tissue specific conditional over expression of NF-kB [14]. Inducing NF-kB with doxycycline (dox) just prior to the initiation of sensitization and challenge with ovalbumin caused increased lung mechanics responses to intravenous methacholine and increased cellular inflammation in the lung. We conclude that NF-kB signaling in the epithelium alone is capable of modulating bronchial responsiveness and airway inflammation during allergen challenge. Inhibition of airway epithelial NF-kB could be a means of diminishing asthma severity.
Materials and Methods
Transgenic mice
The production of the inducible transgenic mouse line has been described previously [14]. In brief, a mutated constitutively activated human IKK2 (gift of Dr. F, Mercurio, Signal Pharmaceutical, San Diego CA) coupled to a tetracycline promoter and a CC10 promoter controlled transcriptional silencer were co-injected into FVB mice by the Vanderbilt transgenic core laboratory. These mice were then bred with FVVB mice bearing the CC 10-rTTA (gift of Dr. J. A. Whitsett, University of Cincinnati, Cincinnati, OH) to allow non leaky tetracycline inducible airway epithelial specific activation of NF-kB. These triple transgenic mice were called IKTA mice. All mice were treated humanely and in accordance with the federal and state government guidelines, and their use was approved by the Vanderbilt Institutional Animal Care Committee.
Protocol
Groups of 3-5 transgene positive mice and 3-5 control mice were studied in parallel. Control mice were either FVB mice or transgenic mice lacking the CC10-rTTA transgene. Mice were sensitized by intraperitoneal injections of ova and alum on days 0 and 7, and on day fourteen began daily aerosols of 1% ovalbumin (Sigma Chemicals, St. Louis, MO) as described previously [15]. Doxycyline 0.5mg/ml in 2% sucrose was administered to the mice in their drinking water beginning on the day of the first aerosol challenge. Lung cytokines were measured on days 5 and 7 of ova aerosol challenge, and airway mechanics were determined on day 9.
Lung Mechanics
Mice were anesthetized by intraperitoneal injection of 85mg/kg of sodium pentobarbital. A tracheostomy tube was placed and the internal jugular was canulated with a silastic catheter to administer methacholine. Mice were placed in a whole body plethysmography chamber (Buxco Electronics, Troy, NY) and mechanically ventilated with a Harvard rodent ventilator (model 683, Southnatick, MA). The ventilation rate was 200 breaths per minute with a tidal volume of 5-6ml/kg and an end expiratory pressure of 2cm H2O. Lung volume changes and pressure changes were measured with differential pressure transducers (Buxco Electronics). Total respiratory resistance and compliance were calculated using BioSystem XA software (Buxco Electronics). Acetyl-B-methacholine (Sigma-Aldrich Chemical Company, St. Louis, MO) was dissolved in normal saline and administered intravenously at a starting dose of 5 ug per kilogram of mouse weight. Three-fold increasing concentrations were administered at 2-3 minute intervals when the resistance had returned to baseline.
Bronchoalveolar lavage
Saline (0.9ml) was instilled into anesthetized mice via a tracheotomy. The lungs were gently massaged, and the fluid was withdrawn. Cell number was determined, and slides were prepared by Cytospin (Thermo-Shandon). After air drying and staining with Hema 3 (modified Wright-Geimsa stain kit) differentials were enumerated.
Histology
Lungs were perfused through the right ventricle with saline, inflated with 4% paraformaldehyde and fixed overnight. The tissue was dehydrated through graded alcohol and embedded in paraffin. 5 μm paraffin sections of the lung tissue were stained with hematoxylin and eosin, or with PAS for detection of mucin. Slides were reviewed by a pathologist blinded to the investigation. Special attention was given to analysis of general signs of inflammation and airway epithelium alterations. Semi-quantitative scores of inflammation and goblet cell hyperplasia were performed. Inflammation was assessed by analysis of ten sequential non-overlapping tissue fields using x200 magnification. Each tissue field was scored using a 0 to 4 point system (0, normal lung parenchymal architecture; 1, increased thickness of single interalveolar septa with interstitial edema and accumulation of inflammatory cells; 2, increased thickness of 50% or more interalveolar septa with luminal accumulation of inflammatory cells; 3, thickening of interalveolar septa with luminal accumulation of inflammatory cells and formation of isolated inflammatory cell aggregates; 4, formation of inflammatory patches with local distortions of lung parenchymal tissue). Mean scores for all fields were calculated for each mouse. Goblet cell hyperplasia was measured by individual assessment of each airway in the tissue section. Each airway was scored using a 0 to 4 point system (0 - no goblet cells between lining airway epithelial cells, 1 - for < 5% goblet cells, 2 - for 5-10% goblet cells, 3 - for 10-25% and 4 - for more than 25%). Mean scores for all analyzed airways were calculated for each animal.
Cytokine Measurements
Whole lungs were perfused via the right ventricle till the effluent was clear. Lungs were then removed, ground (Tissuemizer, Tekmar), and the supernatant stored in aliquots for measurement of cytokines with a LINCOplex mouse cytokine/chemokine kit (MILLIPORE, St. Charles, MO) using fluorescently labeled microsphere beads and a Luminex reader (Luminex Corp., Austin, Texas) by multiplex techniques. Cytokines measured were MIP-1α, GM-CSF, MCP-1, mKC, RANTES, IFN-y, mIL-1ß, IL-1α, G-CSF, IP-10, mIL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12, TNF-α, IL-9, IL-13, IL-15, IL-17.
IgE
Total IgE was measured in serum by ELISA [15].
Statistics
Comparisons between groups were made by paired t test unless the data were non randomly distributed, when the non parametric Mann Whitney U test was used. A two way analysis of variance with repeated measures analysis was used to compare methacholine dose response curves, with a post hoc test used to examine differences at each dose. Data are expressed as mean ± SEM. Significance was accepted at P < 0.05. Graph Pad Prism (San Diego, CA) was the statistical software utilized.
Results
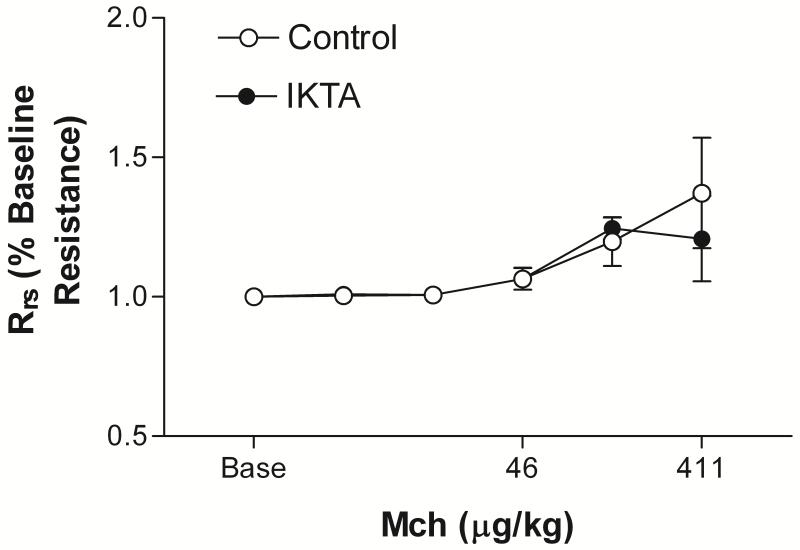
Induction of NF-kB alone does not cause increased responsiveness to methacholine Mice with induction of NF-kB in airway epithelial cells in the absence of allergic inflammation had slightly increased baseline resistance compared with controls (1.87 ± 0.16 cmH2O/ml/sec vs 1.31 ± 0.03, P < 0.02 by unpaired t test). Normalizing the methacholine response curves to baseline airway resistance revealed no difference in the airway reactivity of IKTA mice with induction of epithelial NF-kB compared to control (P=0.8 by two way analysis of variance with repeated measures). (Fig.1). As we have shown previously the induction of NF-kB resulted in an increase in the cellularity of bronchoalveolar lavage (BAL) fluid with a predominance of neutrophils and no eosinophils (data not shown) [14].
Figure 1.
IKTA mice (n=5) given dox for 6-9 days prior to measurement of lung mechanics had increased baseline Rrs compared with control mice given dox (FVB wild type mice (n=2) or mice with the rTTA transgene but not bearing the IKK transgene (IKK+/rTTA-) (n=2) (1.87 ± 0.16 cmH2O/ml/sec vs 1.31 ± 0.03, P<0.02 by unpaired t test))). When normalized to this baseline increase, the responses of the two groups of mice to methacholine were not different (P=0.08 by two analysis of variance with repeated measures). The data from the control mice were combined.
Induction of NF-kB during ovalbumin aerosol challenge increases pulmonary inflammatory cell number and responsiveness to methacholine
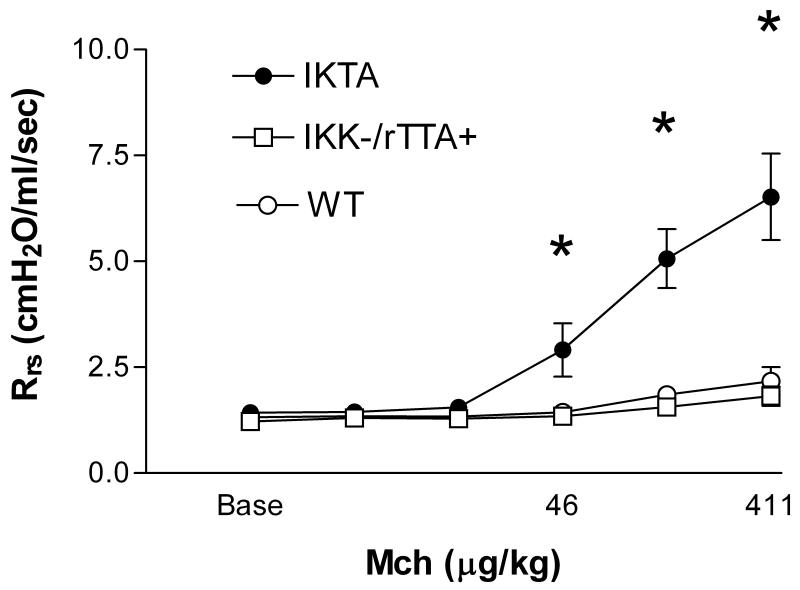
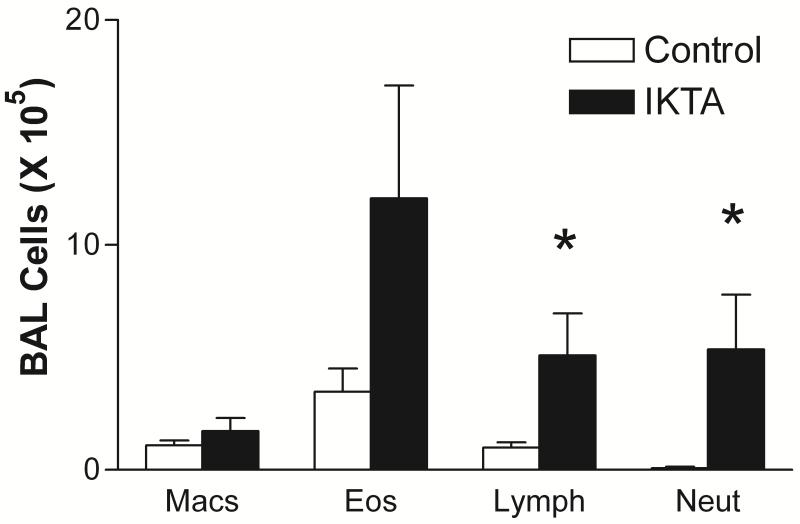
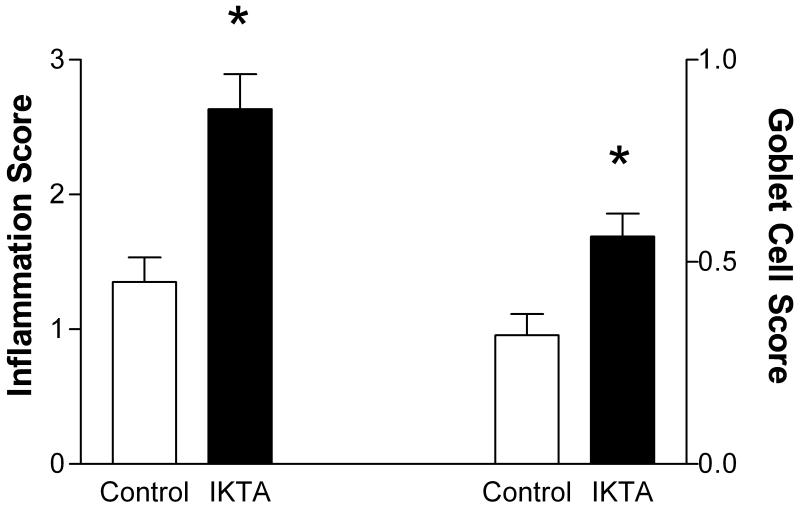
Doxcyline alone does not alter the lung mechanics responses or BAL cellularity of ova sensitized and challenged FVB mice (data not shown). Induction of NF-kB in epithelial cells in sensitized IKTA mice given dox during the ovalbumin aerosol challenge resulted in a significant increase in lung mechanics responsiveness to methacholine compared with mice sensitized and challenged with ovalbumin alone (Fig. 2). The number of inflammatory cells in bronchoalveolar lavage fluid was dramatically increased in the IKTA mice (24.2 ± 8.8 × 105/ml, n=5) vs control mice (1.1 ± 0.2 × 105/ml, n=8, P < 0.05) The absolute number of lymphocytes and neutrophils was markedly increased in IKTA mice (Fig. 3). There was no statistically significant increase in eosinophils. The increase in inflammatory cells in these ova challenged mice during NF-kB activation suggests an important enhancing effect of epithelial NF-kB on the development of allergic inflammation. Histological examination of hematoxylin and eosin stained tissue sections revealed more intense cell infiltration in the lung parenchyma of mice receiving both ovalbumin and dox compared to mice receiving only ovalbumin (Fig. 4). Goblet cell hyperplasia was more intense in mice receiving both ovalbumin and dox. Serum IgE at Day 9 was higher in the IKTA mice (4.4 ± 1.3 μg/ml, n=7 vs 8.0 ± 1.5, n=6), but this difference was not statistically significant (P = 0.09).
Figure 2.
IKTA mice (n=6) sensitized and challenged with ova during NFKB activation had significantly greater respiratory system resistance to intravenous methacholine than identically treated control mice with the rTTA transgene not bearing the IKK transgene (open squares, n=4) and FVB mice (open circles, n=4). * indicates P < 0.05 by repeated measures ANOVA with post hoc Bonferroni test.
Figure 3.
Absolute cell numbers in bronchoalveolar lavage fluid in ovalbumin sensitized and challenged mice given doxycycline (n+5) and FVB wild type mice (n=4).There was no difference in the results of the IKK+/fTTA- mice and those of the FVB wild type mice. The responses of these two control groups were combined for analysis by unpaired t test. The IKTA mice had greater numbers of lymphocytes and eosinophils than control mice. * indicates P < 0.05 Although the IKTA mice had increased numbers of eosinophils this difference was not statistically significant. (P=0.058)
Figure 4.
Histology of lungs from IKTA mice (n=3) and control mice (n=4) sensitized and challenged with ovalbumin and treated with doxycline at Day 9 revealed increased inflammation and goblet cell hyperplasia in IKTA mice. * indicates P < 0.05
NF-kB activation during ovalbumin challenge modulates lung cytokines
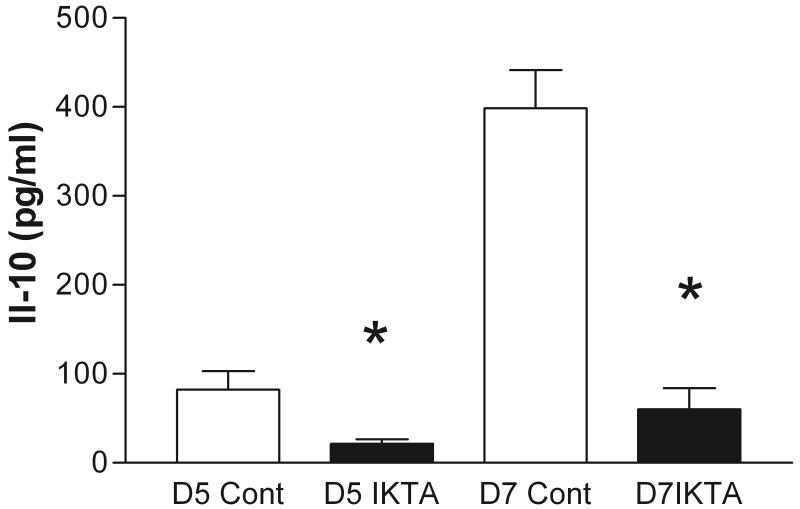
To explore the possible mechanism of NF-kB enhancement of allergic inflammation and lung responsiveness to methacholine, lung supernatants were analyzed by multiplex technology for cytokines. Interestingly the cytokine IL-10 which has been associated with down regulation of allergic inflammation was suppressed on days 5 and 7 by activation of NF-kB in airway epithelial cells (Fig. 5). IL-9 was less than that of control mice on Day 5 (146 ± 27 pg/ml vs 288 ± 42 (P<0.05)) but did not differ on Day 7 (Table 1). Other cytokines which were significantly different on either day 5 or 7 from dox treated mice are given in Table 1. Neither IL-13 nor IL-4 differed between the dox treated and control mice
Figure 5.
Interleukin 10 levels measured by multiplex techniques in lung homogenates from control (n=4) and IKTA (n=3) mice at Day 5 or 7 of ovalbumin challenge with dox treatment. IL-10 levels were significantly lower at both days in IKTA mice. * indicates P < 0.05
Table 1.
Cytokine levels in lung at Day 5 and Day 7 of ova challenge in Control and Dox treated IKTA mice. (mean ± SEM, picograms per milliliter)
| n=3 | mKC | G-CSF | IL-15 | IL-4 | IL-13 |
|---|---|---|---|---|---|
| Control D5 | 552 ± 266 | 435±314 | 12 ± 4 | 4.5 ± 1 | 316 ± 66 |
| IKTA D5 | 640 ± 246 | 2680± 1530 | 46 ± 6 | 7.7 ± 1.7 | 383 ± 126 |
| P | NS | NS | 0.02 | NS | NS |
| n=4 | |||||
| Control D7 | 338 ± 57 | 83 ± 4 | 12 ± 2 | 7.3 ± 1.5 | 393 ± 53 |
| IKTA D7 | 1152±386 | 1361±938 | 17 ± 8 | 6.7 ± 2 | 399± 107 |
| P | 0.03 | 0.03 | NS | NS | NS |
Discussion
Activation of NF-kB in airway epithelial cells in mice not undergoing allergen challenge resulted in a small increase in baseline respiratory system resistance but no increased responsiveness to methacholine (Fig. 1). We have previously shown that induction of NF-kB in murine epithelial cells results in the influx of inflammatory cells such as neutrophils which are typically associated with innate immune responses [14]. Neutrophilia is also seen in the setting of ozone exposure where airway responsiveness to non specific agonists such as methacholine is increased, and airway epithelial cell NF-kB is activated [16-19]. Likewise there is increasing evidence that human severe asthma is accompanied by airway neutrophilia, perhaps mediated by epithelial cell damage and activation of NF-kB [20].
We have shown previously that activation of NF-kB in airway epithelial cells of mice ex vivo leads to increased expression of IL-6, G-CSF, GM-CSF, MIP-2, Keratinocyte-derived chemokine (KC), and RANTES [14]. In vivo activation resulted in elevation in bronchoalveolar lavage fluid of a further group of cytokines, IL-1α, IL-1β, and IL-12p40. This latter group of cytokines may have been produced indirectly through recruitment or activation of inflammatory cells in the lungs [14]. During ova induced allergic inflammation, NF-kB activation results in an increase in airway inflammation, goblet cell hyperplasia and airway hyperresponsiveness to methacholine. This was accompanied by increases in lung KC, G-CSF as well as increased IL-15 and lower amounts of IL-10 and IL-9. Because the last three cytokines were not found with NF-kB induction alone, we speculate that interaction of NF-kB pathways and others activated during allergic inflammation interact to yield this cytokine milieu. We suggest that these cytokines and the subsequent neutrophilia resulted in the airway hyperresponsiveness during allergic inflammation and NF-kB activation.
Allergic inflammation in the mouse lung results in the induction of a number of cytokines including canonical Th 2 cytokines, IL-4, IL-5 and IL-13. As reported here, the overexpression of NF-kB in airway epithelial cells during allergen challenge results in modulation of several potentially important cytokines. Similar to the findings in NF-kB overexpression alone, we found increases in murine KC and G-CSF after allergen challenge in the NF-kB induced mice compared with control mice. However no significant differences in IL-6, GM-CSF, MIP-2 or RANTES were found. On the other hand IL-15 was elevated on Day 5. Of the three cytokines found to be increased by transgene activation of NF-kB, each could plausibly contribute to the documented increase in allergic inflammation.
Keratinocyte-derived chemokine (KC, CXCL1) is a major chemoattractant for neutrophils and is similar to human IL-8 in its functions. Antibody blockade of KC in conjunction with blockade of MIP-2 reduced the neutrophilia and IgE production caused by allergic sensitization and challenge in mice [21, 22]. The human counterpart of KC, IL-8, was increased in the sputum from asthmatics with predominant neutrophilia [23]. Likewise G-CSF is important for neutrophil influx and is elevated together with IL-8 in epithelial cell supernatants from patients with asthma [24]. Although traditionally the eosinophil has been associated with asthma, the neutrophil is now thought to be involved in more severe cases of asthma [20]. Airway neutrophils have been found in certain subtypes of severe asthma and in those with status asthmaticus [25, 26].
IL-15 is a pleiotropic cytokine that activates mast cells, eosinophils, and T and B lymphocytes. It is produced by epithelial cells, macrophages, and dendritic cells and appears to be under the control of NF-kB [27]. Treatment of mice with the soluble receptor for IL-15 blocks allergic inflammation and Th1 mediated collagen induced arthritis [28, 29]. Thus the increase we discovered in IL-15 in the IKTA ova challenged mice may have contributed to the increased inflammation. IL-4 and IL-13 are critical type 2 cytokines that have been shown to be important for the regulation of allergic inflammation in mice, yet the levels of these cytokines did not differ between the NF-kB overexpressing and control mice. Thus it appears that other mechanisms, such as the reduction of IL-10, are involved.
IL-10 is produced by a number of cells, including T regulatory cells, dendritic cells, macrophages and epithelial cells. Although IL-10 is not thought to be under the direct control of NFκB, the level of this important cytokine was diminished by NF-kB activation on both day 5 and day 7 of ovalbumin inhalation challenge. IL-10 is of particular relevance for allergic inflammation as it appears to be capable of downregulating inflammation in mouse models [30-32]. Human regulatory T lymphocytes may suppress allergic inflammation by secreting IL-10 [33]. A decrease in IL-10 secreting peripheral blood monocytes has been found in severe asthma [34]. Thus the decrease in Il-10 found in the IKTA dox treated mice may have reduced the restraint normally exerted by IL-10 on allergic inflammation.
Activation of epithelial NFκB during ova challenge caused a non significant increase in IgE and eosinophilia without altering the levels of the classic Th2 cytokines, IL-4 and IL-13, which are known to be critical for allergic inflammation in the mouse. This finding implies a cooperation between the Th2 pathways and those typically associated with innate immunity to produce heightened allergic inflammation. For example the inflammatory response to inhaled endotoxin is reduced by inhibition of NFκB [35]. Recently Kim and colleagues have reported that endotoxin enhanced the airway responsiveness and IgE production caused by ova, and increased the numbers of neutrophils in bronchoalveolar lavage fluid [36]. This suggests that endotoxin working via airway epithelial NFκB activation increases allergic responsiveness. However they found that IL-12, IFN-γ and TNF-α were elevated in association with endotoxin and we found no differences in these cytokines with induction of NFκB. Thus there are other mechanisms, perhaps via KC, G-CSF, IL-15 and IL-10, by which NF-kB activation in epithelial cells can influence allergic inflammation. Similar findings in man have been reported by Peden and colleagues, who demonstrated heightened responses to inhaled allergen in atopic asthmatics inhaling low doses of endotoxin [37, 38]. Endotoxin does appear to activate human airway epithelial cell NFκB [39]. Thus these results and others demonstrate that inflammation driven by non Th2 mechanisms can enhance allergic inflammation. Others have demonstrated that induction of inflammation by non Th2 mechanisms can inhibit allergic inflammation [40]. This surely suggests that genetic background, timing and severity of exposure must play a role in determining the outcome.
Since preparation of this manuscript, Janssen-Heininger and colleagues have reported on their findings in a similar transgenic mouse system [41]. They found that activation of NF-kB alone caused inflammation and increased responsiveness to methacholine aerosol challenge. Likewise activation of NF-kB in ova challenged mice resulted in increases in KC and methacholine responsiveness. However we did not find the increases in IL-17, IL-4 or MIP-1β which they report. Unlike their findings we found an increased cellular inflammation and goblet cell hyperplasia in the NF-kB activated mice. The differences in our results, which are otherwise congruent, could have resulted from the background of the mice (FVB vs C57/BL6J) and the intensity of the transgene expression.
Our data demonstrate that NF-kB induction in airway epithelial cells profoundly increases inflammation in the mouse lung during allergen challenge. In concert with previous work, these findings implicate NF-kB signaling in the airway epithelium as a critical pathway for asthma and airway hyperresponsiveness. NF-kB is a central transcription factor for the production of numerous inflammatory proteins. The airway epithelial cell is strategically placed to function as part of the immune system response to inhaled agents. Here we show that working via NF-kB activation, the epithelial cell produces proteins that enhance the non specific airway reactivity and goblet cell hyperplasia characteristic of asthma. Using pharmacologic agents or genetic approaches several investigators have shown that inhibition of NF-kB reduces allergic inflammation in mice. These data all point to epithelial NF-kB regulation as an important modulator of allergic inflammation. Thus agents targeting epithelial NF-kB may have a role in the therapy of human asthma.
Acknowledgements
We wish to thank Shaoquan Ji, D.V.M., Ph.D., for help with cytokine measurements. Supported in part by a grant from the Sandler Program for Asthma Research, and grants from the National Institutes of Health (NIH) R01AI054660 and R01HL061419.
Reference List
- 1.Bureau F, Delhalle S, Bonizzi G, Fievez L, Dogne S, Kirschvink N, Vanderplasschen A, Merville MP, Bours V, Lekeux P. Mechanisms of persistent NF-kappa B activity in the bronchi of an animal model of asthma. J Immunol. 2000;165(10):5822–5830. doi: 10.4049/jimmunol.165.10.5822. [DOI] [PubMed] [Google Scholar]
- 2.Gagliardo R, Chanez P, Mathieu M, Bruno A, Costanzo G, Gougat C, Vachier I, Bousquet J, Bonsignore G, Vignola AM. Persistent activation of nuclear factor-kappaB signaling pathway in severe uncontrolled asthma. Am J Respir Crit Care Med. 2003;168(10):1190–1198. doi: 10.1164/rccm.200205-479OC. [DOI] [PubMed] [Google Scholar]
- 3.Lilly CM, Tateno H, Oguma T, Israel E, Sonna LA. Effects of allergen challenge on airway epithelial cell gene expression. Am J Respir Crit Care Med. 2005;171(6):579–586. doi: 10.1164/rccm.200404-532OC. [DOI] [PubMed] [Google Scholar]
- 4.Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor-kappaB in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol. 2002;160(4):1325–1334. doi: 10.1016/s0002-9440(10)62559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birrell MA, Hardaker E, Wong S, McCluskie K, Catley M, De AJ, Newton R, Haj-Yahia S, Pun KT, Watts CJ, Shaw RJ, Savage TJ, Belvisi MG. Ikappa-B kinase-2 inhibitor blocks inflammation in human airway smooth muscle and a rat model of asthma. Am J Respir Crit Care Med. 2005;172(8):962–971. doi: 10.1164/rccm.200412-1647OC. [DOI] [PubMed] [Google Scholar]
- 6.Catley MC, Chivers JE, Holden NS, Barnes PJ, Newton R. Validation of IKK beta as therapeutic target in airway inflammatory disease by adenoviral-mediated delivery of dominant-negative IKK beta to pulmonary epithelial cells. Br J Pharmacol. 2005;145(1):114–122. doi: 10.1038/sj.bjp.0706170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi IW, Kim DK, Ko HM, Lee HK. Administration of antisense phosphorothioate oligonucleotide to the p65 subunit of NF-kappaB inhibits established asthmatic reaction in mice. Int Immunopharmacol. 2004;4(14):1817–1828. doi: 10.1016/j.intimp.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 8.Henderson WR, Jr., Chi EY, Teo JL, Nguyen C, Kahn M. A small molecule inhibitor of redox-regulated NF-kappa B and activator protein-1 transcription blocks allergic airway inflammation in a mouse asthma model. J Immunol. 2002;169(9):5294–5299. doi: 10.4049/jimmunol.169.9.5294. [DOI] [PubMed] [Google Scholar]
- 9.Huang TJ, Adcock IM, Chung KF. A novel transcription factor inhibitor, SP100030, inhibits cytokine gene expression, but not airway eosinophilia or hyperresponsiveness in sensitized and allergen-exposed rat. Br J Pharmacol. 2001;134(5):1029–1036. doi: 10.1038/sj.bjp.0704344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong DW, Yoo MH, Kim TS, Kim JH, Kim IY. Protection of mice from allergen-induced asthma by selenite: prevention of eosinophil infiltration by inhibition of NF-kappa B activation. J Biol Chem. 2002;277(20):17871–17876. doi: 10.1074/jbc.M200808200. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor kappaB in the induction of eosinophilia in allergic airway inflammation. J Exp Med. 1998;188(9):1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegelbauer K, Gantner F, Lukacs NW, Berlin A, Fuchikami K, Niki T, Sakai K, Inbe H, Takeshita K, Ishimori M, Komura H, Murata T, Lowinger T, Bacon KB. A selective novel low-molecular-weight inhibitor of IkappaB kinase-beta (IKK-beta) prevents pulmonary inflammation and shows broad anti-inflammatory activity. Br J Pharmacol. 2005;145(2):178–192. doi: 10.1038/sj.bjp.0706176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poynter ME, Cloots R, van WT, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM. NF-kappa B activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol. 2004;173(11):7003–7009. doi: 10.4049/jimmunol.173.11.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, Blackwell TS. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J Immunol. 2007;178(10):6504–6513. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- 15.Peebles RS, Jr., Dworski R, Collins RD, Jarzecka K, Mitchell DB, Graham BS, Sheller JR. Cyclooxygenase inhibition increases interleukin 5 and interleukin 13 production and airway hyperresponsiveness in allergic mice. Am J Respir Crit Care Med. 2000;162(2 Pt 1):676–681. doi: 10.1164/ajrccm.162.2.9911063. [DOI] [PubMed] [Google Scholar]
- 16.Cho HY, Morgan DL, Bauer AK, Kleeberger SR. Signal transduction pathways of tumor necrosis factor--mediated lung injury induced by ozone in mice. Am J Respir Crit Care Med. 2007;175(8):829–839. doi: 10.1164/rccm.200509-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtzman MJ, Cunningham JH, Sheller JR, Irsigler GB, Nadel JA, Boushey HA. Effect of ozone on bronchial reactivity in atopic and nonatopic subjects. Am Rev Respir Dis. 1979;120(5):1059–1067. doi: 10.1164/arrd.1979.120.5.1059. [DOI] [PubMed] [Google Scholar]
- 18.Holtzman MJ, Fabbri LM, O’Byrne PM, Gold BD, Aizawa H, Walters EH, Alpert SE, Nadel JA. Importance of airway inflammation for hyperresponsiveness induced by ozone. Am Rev Respir Dis. 1983;127(6):686–690. doi: 10.1164/arrd.1983.127.6.686. [DOI] [PubMed] [Google Scholar]
- 19.Kafoury RM, Hernandez JM, Lasky JA, Toscano WA, Jr., Friedman M. Activation of transcription factor IL-6 (NF-IL-6) and nuclear factor-kappaB (NF-kappaB) by lipid ozonation products is crucial to interleukin-8 gene expression in human airway epithelial cells. Environ Toxicol. 2007;22(2):159–168. doi: 10.1002/tox.20246. [DOI] [PubMed] [Google Scholar]
- 20.Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997;156(3 Pt 1):737–743. doi: 10.1164/ajrccm.156.3.9610046. [DOI] [PubMed] [Google Scholar]
- 21.Knott PG, Gater PR, Dunford PJ, Fuentes ME, Bertrand CP. Rapid up-regulation of CXC chemokines in the airways after Ag-specific CD4+ T cell activation. J Immunol. 2001;166(2):1233–1240. doi: 10.4049/jimmunol.166.2.1233. [DOI] [PubMed] [Google Scholar]
- 22.McKinley L, Kim J, Bolgos GL, Siddiqui J, Remick DG. CXC chemokines modulate IgE secretion and pulmonary inflammation in a model of allergic asthma. Cytokine. 2005;32(3-4):178–185. doi: 10.1016/j.cyto.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Simpson JL, Grissell TV, Douwes J, Scott RJ, Boyle MJ, Gibson PG. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax. 2007;62(3):211–218. doi: 10.1136/thx.2006.061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattoli S, Marini M, Fasoli A. Expression of the potent inflammatory cytokines, GM-CSF, IL6, and IL8, in bronchial epithelial cells of asthmatic patients. Chest. 1992;101(3 Suppl):27S–29S. doi: 10.1378/chest.101.3_supplement.27s. [DOI] [PubMed] [Google Scholar]
- 25.Tillie-Leblond I, Pugin J, Marquette CH, Lamblin C, Saulnier F, Brichet A, Wallaert B, Tonnel AB, Gosset P. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am J Respir Crit Care Med. 1999;159(2):487–494. doi: 10.1164/ajrccm.159.2.9805115. [DOI] [PubMed] [Google Scholar]
- 26.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160(3):1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 27.Mariner JM, Lantz V, Waldmann TA, Azimi N. Human T cell lymphotropic virus type I Tax activates IL-15R alpha gene expression through an NF-kappa B site. J Immunol. 2001;166(4):2602–2609. doi: 10.4049/jimmunol.166.4.2602. [DOI] [PubMed] [Google Scholar]
- 28.Ruchatz H, Leung BP, Wei XQ, McInnes IB, Liew FY. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J Immunol. 1998;160(11):5654–5660. [PubMed] [Google Scholar]
- 29.Ruckert R, Brandt K, Braun A, Hoymann HG, Herz U, Budagian V, Durkop H, Renz H, Bulfone-Paus S. Blocking IL-15 prevents the induction of allergen-specific T cells and allergic inflammation in vivo. J Immunol. 2005;174(9):5507–5515. doi: 10.4049/jimmunol.174.9.5507. [DOI] [PubMed] [Google Scholar]
- 30.Akbari O, Dekruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2(8):725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 31.Oh JW, Seroogy CM, Meyer EH, Akbari O, Berry G, Fathman CG, Dekruyff RH, Umetsu DT. CD4 T-helper cells engineered to produce IL-10 prevent allergen-induced airway hyperreactivity and inflammation. J Allergy Clin Immunol. 2002;110(3):460–468. doi: 10.1067/mai.2002.127512. [DOI] [PubMed] [Google Scholar]
- 32.Vissers JL, van Esch BC, Hofman GA, Kapsenberg ML, Weller FR, van Oosterhout AJ. Allergen immunotherapy induces a suppressive memory response mediated by IL-10 in a mouse asthma model. J Allergy Clin Immunol. 2004;113(6):1204–1210. doi: 10.1016/j.jaci.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 33.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, Loke TK, Robinson DS, Barrat FJ, O’Garra A, Lavender P, Lee TH, Corrigan C, Hawrylowicz CM. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116(1):146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto K, Inoue H, Fukuyama S, Tsuda M, Ikegami T, Kibe A, Yoshiura Y, Komori M, Hamasaki N, Aizawa H, Nakanishi Y. Decrease of interleukin-10-producing T cells in the peripheral blood of severe unstable atopic asthmatics. Int Arch Allergy Immunol. 2004;134(4):295–302. doi: 10.1159/000079167. [DOI] [PubMed] [Google Scholar]
- 35.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J Immunol. 2003;170(12):6257–6265. doi: 10.4049/jimmunol.170.12.6257. [DOI] [PubMed] [Google Scholar]
- 36.Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, Bang B, Lee HS, Oh MH, Kim YS, Kim JH, Gho YS, Cho SH, Min KU, Kim YY, Zhu Z. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J Immunol. 2007;178(8):5375–5382. doi: 10.4049/jimmunol.178.8.5375. [DOI] [PubMed] [Google Scholar]
- 37.Alexis NE, Lay JC, Almond M, Peden DB. Inhalation of low-dose endotoxin favors local T(H)2 response and primes airway phagocytes in vivo. J Allergy Clin Immunol. 2004;114(6):1325–1331. doi: 10.1016/j.jaci.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Boehlecke B, Hazucha M, Alexis NE, Jacobs R, Reist P, Bromberg PA, Peden DB. Low-dose airborne endotoxin exposure enhances bronchial responsiveness to inhaled allergen in atopic asthmatics. J Allergy Clin Immunol. 2003;112(6):1241–1243. doi: 10.1016/j.jaci.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 39.Schroeder TH, Lee MM, Yacono PW, Cannon CL, Gerceker AA, Golan DE, Pier GB. CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF-kappa B translocation. Proc Natl Acad Sci U S A. 2002;99(10):6907–6912. doi: 10.1073/pnas.092160899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuipers H, Hijdra D, De V, Hammad H, Prins JB, Coyle AJ, Hoogsteden HC, Lambrecht BN. Lipopolysaccharide-induced suppression of airway Th2 responses does not require IL-12 production by dendritic cells. J Immunol. 2003;171(7):3645–3654. doi: 10.4049/jimmunol.171.7.3645. [DOI] [PubMed] [Google Scholar]
- 41.Pantano C, Ather JL, Alcorn JF, Poynter ME, Brown AL, Guala AS, Beuschel SL, Allen GB, Whittaker LA, Bevelander M, Irvin CG, Janssen-Heininger YM. Nuclear factor-kappaB activation in airway epithelium induces inflammation and hyperresponsiveness. Am J Respir Crit Care Med. 2008;177(9):959–969. doi: 10.1164/rccm.200707-1096OC. [DOI] [PMC free article] [PubMed] [Google Scholar]