Abstract
Background:
Inducible activation of nuclear factor (NF)-κB is one of the principal mechanisms through which resistant prostate cancer cells are protected from radiotherapy. We hypothesised that inactivation of inducible NF-κB with a novel NF-κB inhibitor, DHMEQ, would increase the therapeutic effects of radiotherapy.
Methods:
PC-3 and LNCaP cells were exposed to irradiation and/or DHMEQ. Cell viability, cell cycle analysis, western blotting assay, and NF-κB activity were measured. The antitumour effect of irradiation combined with DHMEQ in vivo was also assessed.
Results:
The combination of DHMEQ with irradiation resulted in cell growth inhibition and G2/M arrest relative to treatment with irradiation alone. Inducible NF-κB activity by irradiation was inhibited by DHMEQ treatment. The expression of p53 and p21 in LNCaP, and of 14-3-3σ in PC-3 cells, was increased in the combination treatment. In the in vivo study, 64 days after the start of treatment, tumour size was 85.1%, 77.1%, and 64.7% smaller in the combination treatment group than that of the untreated control, DHMEQ-treated alone, and irradiation alone groups, respectively.
Conclusion:
Blockade of NF-κB activity induced by radiation with DHMEQ could overcome radio-resistant responses and may become a new therapeutic modality for treating prostate cancer.
Keywords: prostate cancer, radiation, nuclear factor-κB
Radiation therapy, such as external beam radiotherapy (EBRT) or brachytherapy, is one of the standard options for organ-confined or locally advanced prostate cancer. The therapeutic efficacies of radical prostatectomy, high dose of EBRT, and brachytherapy are similar (Kupelian et al, 2004), being approximately 75–80% for stage T1–T2 prostate cancer. Doses used in EBRT or brachytherapy are highly associated with therapeutic efficacy; however, the incidences of related side effects increase as the dose of radiation increases (Pollack et al, 2002). Therefore, a modality for improving the therapeutic efficacy of radiotherapy for locally confined or advanced prostate cancer is warranted both to enhance radiation-induced cytotoxicity and to reduce related side effects.
With respect to the technical aspects of radiation therapy, three-dimensional conformal radiation therapy and intensity-modulated radiation therapy for external therapy have been implemented (Zelefsky et al, 2001; Hanks et al, 2002). Meanwhile, the therapeutic mean, including manipulating the radio-adaptive response of prostate cancer cells, could be another tool for improving the therapeutic effects of radiotherapy and minimising related side effects. Several factors associated with radio-resistance, such as the expression of antiapoptotic proteins (Chin et al, 2005) and regulation of the cell cycle (Takagi et al, 1998) in cancer cells, have been reported. However, the mechanism of the radio-adaptive response has not yet been adequately elucidated.
It has been reported that in radio-adaptive resistance, the prosurvival network was initiated by nuclear factor (NF)-κB. Activated NF-κB signal produces various genes associated with antiapoptotic proteins and modulation of cell cycle regulation, which contribute to increased cell survival and cell radio-resistance. Among the transcription factors, NF-κB is one of the most decisive regulators of irradiation-induced gene expression.
Activation of NF-κB has also been reported to reduce the therapeutic effect of radiotherapy in cancer cells (Starenki et al, 2004). It has been reported that ionising radiation activates DNA binding of NF-κB (Brach et al, 1991; Sun et al, 2007). Furthermore, blocking NF-κB activation increases the apoptotic response and decreases the growth and clonogenic survival of several human cancer cells (Tang et al, 2001; Chen et al, 2002). In prostate cancer that includes the hormone-insensitive prostate tumour cell, DU145, PC-3 lines, and the hormone responsive LNCaP cell line, radiation exposure was found to lead to an increase in NF-κB activity (Palayoor et al, 1999; Wen et al, 2003; Raffoul et al, 2006; Sun et al, 2007). Inhibition of NF-κB by a dominant-negative IκB mutant enhanced apoptosis in DU145 cells (Flynn et al, 2003). Thus, these results strongly suggest that NF-κB plays a key role in radio-adaptive resistance under ionising radiation in prostate cancer.
We have previously synthesised a dehydroxymethyl derivative of epoxyquinomicin, DHMEQ, from a natural product and it is a novel and potent NF-κB inhibitor (Matsumoto et al, 2000). The mechanism by which DHMEQ inhibits activation of NF-κB is unique because DHMEQ inhibits NF-κB translocation from the cytoplasm to the nucleus (Ariga et al, 2002). Recently, it was shown to covalently bind to the specific cysteine of NF-κB components (Yamamoto et al, 2008). The antitumour effect of DHMEQ has been proven in various types of cancer, including breast cancer (Matsumoto et al, 2005), pancreatic cancer (Takatsuna and Umezawa, 2004), thyroid cancer (Starenki et al, 2004), multiple myeloma (Watanabe et al, 2005), and leukaemia (Ohsugi et al, 2005). In our laboratory, we have investigated the therapeutic effect of DHMEQ on hormone-insensitive prostate cancer cells (Kikuchi et al, 2003; Kuroda et al, 2005). We speculated that the NF-κB inhibitor would enhance the therapeutic effect of radiation in prostate cancer cells. Therefore, in this study, we examined whether DHMEQ had the potential to inhibit NF-κB activity induced by radiation and to enhance cytotoxicity in an in vitro study and animal model.
Materials and Methods
Cell lines and chemicals
LNCaP and PC-3 prostate cancer cells (American Type Culture Collection, Manassas, VA, USA) were grown in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 μg ml−1 streptomycin, and 100 IU ml−1 penicillin. Racemic DHMEQ was synthesised as described previously (Matsumoto et al, 2000), dissolved in dimethyl sulfoxide (DMSO), and subsequently diluted in culture medium to a final concentration of less than 0.1%.
Irradiation
Cells and tumours were irradiated using a MBR 1520 R (Hitachi, Tokyo, Japan) radiation source operating at 150 kV and 20 mA X-rays with 0.5 mm Al and 0.1 mm Cu filters at a radiation dose rate of 1.74 Gy min−1. In the in vivo study, irradiation was locally confined to the tumour by shielding the rest of the body with a lead block.
WST-1 assay
Cells were seeded in 96-well plates and incubated overnight at 37 °C under 5% CO2 in a humidified incubator. Cells were treated with various concentrations of DHMEQ for 24 h. Cells treated with the same concentrations of DMSO served as controls. After 24 h of incubation, cytotoxicity was determined by WST-1 reagent in accordance with the manufacturer’s instructions (Roche, Indianapolis, IN, USA). The quantity of formazan dye was determined with a photometer at 450 nm.
Clonogenic survival assay
LNCaP and PC-3 cells were plated at a density of 2000 to 10 000 cells per well and 100 to 500 cells per well into 6-well plates, respectively. Treatments with various doses of DHMEQ and irradiation were performed. After 14 days incubation, they were washed and stained with crystal violet, and colonies containing >50 cells were counted as clonogenic survivors. Survival fractions were obtained according to the standard protocol by comparing cell counts with the plating efficiency of untreated controls.
Flow cytometric analysis of cell cycle phase distribution
The vehicle control, 5.0 μg ml−1 of DHMEQ alone, 4 Gy of irradiation alone, and their combination treatments were analysed by flow cytometric analysis of cells labelled with BrdU assay in LNCaP and PC-3 cells. After incubation for 48 h, cells were labelled with BrdU assay using BrdU kits (Cell Signaling Technology, Beverly, MA, USA) according to the manufacturer’s protocol. Anti-BrdU was detected by flow cytometry (Beckman Coulter, Fullerton, CA, USA).
Electrophoresis mobility shift assay
NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL, USA) were used to prepare nuclear extracts. The binding reaction mixture contained nuclear extract (5 μg of protein), 2 μg poly (dI–dC), and 10 000 c.p.m. 32P-labelled probe in binding buffer (75 mℳ NaCl, 1.5 mℳ EDTA, 1.5 mℳ DTT, 7.5% glycerol, 1.5% NP-40, 15 mℳ Tris-HCl; pH 7.0). By using the recombinant protein, we incubated the reaction mixture containing 20 μℳ recombinant protein and 5% DMSO with or without DHMEQ in PBS at 4 °C. To determine the DNA binding activity of recombinant protein, we used 20 ng of recombinant protein from the reaction mixture for the electrophoresis mobility shift assay (EMSA). Samples were incubated for 20 min at room temperature in this mixture. DNA/protein complexes were separated from free DNA on a 4% native polyacrylamide gel in 0.25 mℳ TBE buffer. The following sequences were used as a C/EBPβ probe (Promega, Madison, WI, USA): 5′-TGCAGATTGCGCAATCTGCA-3′ and 5′-TGCAGATTGCGCAATCTGCA-3′. These oligonucleotides were labelled with [γ-32P]-ATP (3000 Ci mmol−1; GE Healthcare, Little Chalfont, Buckinghamshire, UK) using T4 polynucleotide kinase (Takara, Ohtsu, Japan) and purified by passage through a Nick column (GE Healthcare).
Western blot analysis
The expressions of p53, p21, and 14-3-3σ were determined by western blot analysis. Samples containing equal amounts of 20 μg protein were subjected to electrophoresis on a sodium dodecyl sulphate–polyacrylamide gel and transferred to a nitrocellulose filter. Filters were blocked with Tris-buffered saline containing 5% nonfat milk powder at 4°C overnight and then incubated for 1 h with an anti-p53 mouse monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), an anti-p21 Waf1/Cip1 mouse monoclonal antibody (Cell Signaling Technology), an anti-14-3-3σ goat polyclonal antibody (Santa Cruz Biotechnology), or an anti-β-actin mouse antibody (Sigma, St Louis, MO, USA). Filters were then incubated for 1 h with an anti-mouse secondary antibody (Dako A/S, Glostrup, Denmark), an anti-goat secondary antibody (Dako A/S), or an anti-rabbit secondary antibody (Dako A/S), and reactivity was detected using an enhanced chemiluminescence system (Amersham Life Science, Little Chalfont, Buckinghamshire, UK).
In vivo treatment
All procedures involving animals and their care in this study were approved by the Animal Care Committee of Keio University in accordance with institutional and Japanese government guidelines for animal experiments. The antitumour effect of irradiation combined with DHMEQ was evaluated in an animal model. Male BALB/c-nu/nu mice were obtained from Sankyo Lab Service (Tokyo, Japan). PC-3 cells (5 × 105) were implanted subcutaneously into the flank of each nude mouse. When an animal in DHMEQ-treated groups developed a palpable tumour, it was given a once daily intraperitoneal injection of DHMEQ at a concentration of 4 mg kg−1 in 0.5 ml of PBS for 14 consecutive days. As a control, 0.5 ml of vehicle alone was administered. In irradiation groups, irradiation consisted of 8 Gy in 2 fractions on the 1st and 8th days. Tumour volume (V) was calculated using the formula V=A × B2/2, where A is the greatest diameter and B is the diameter at a perpendicular to A. Tumour volume was monitored every 4 days.
Statistical analysis
All data are expressed as the mean±s.e. from three or more independent experiments. Differences between groups were examined for significance with ANOVA and/or the Student’s t-test where appropriate. A P<0.05 indicated a significant difference.
Results
DHMEQ enhanced the growth inhibition of PC-3 cells by irradiation
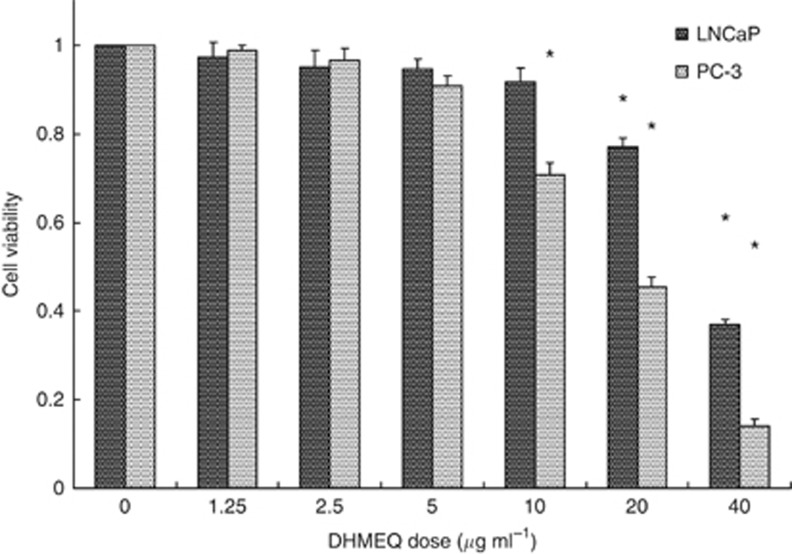
The cell growth rate in LNCaP and PC-3 cells at various doses of 24-h exposure of DHMEQ was evaluated (Figure 1). Significant cell growth inhibitory effects were observed at a 10 μg ml−1 or higher dose of DHMEQ and 20 μg ml−1 or higher dose of DHMEQ in PC-3 and LNCaP cells, respectively. The IC50 values were 18.2 and 33.5 μg ml−1 in PC-3 and LNCaP cells, respectively.
Figure 1.
Cell growth inhibitory effects of DHMEQ in LNCaP and PC-3 prostate cancer cells. Cells were treated with various concentrations of 24-h exposure of DHMEQ. *Significantly different from the vehicle control, P<0.05.
The combination of irradiation with various doses of DHMEQ treatment was then evaluated by clonogenic survival assay (Figures 2A and B). As shown in Figure 2A, at a radiation dose of 4 Gy, the inhibitory effect on colony formation in LNCap cells was significantly higher after treatment with the 2.5 μg ml−1 or higher dose of DHMEQ than that with the vehicle control. At a radiation dose of 4 Gy, the inhibitory effect of colony formation in PC-3 cells was significantly higher after treatment with the 5 μg ml−1 or higher dose of DHMEQ than that with the vehicle control (Figure 2B).
Figure 2.
Clonogenic survival in LNCaP (A) and PC-3 (B) prostate cancer cells after irradiation with various doses of DHMEQ treatment. Cells were treated with various doses of irradiation (2, 4, and 6 Gy, at a radiation dose rate of 1.0 Gy min−1) after 6-h exposure to various concentrations of DHMEQ (1.25, 2.5, 5.0, and 10 μg ml−1). Cells treated with the same concentrations of DMSO served as controls. After 14 days incubation, they were stained with crystal violet and colonies containing >50 cells were counted as clonogenic survivors. *Statistically significant difference compared to control (DMSO treatment alone), P<0.05.
DHMEQ inhibited NF-κB activation induced by irradiation
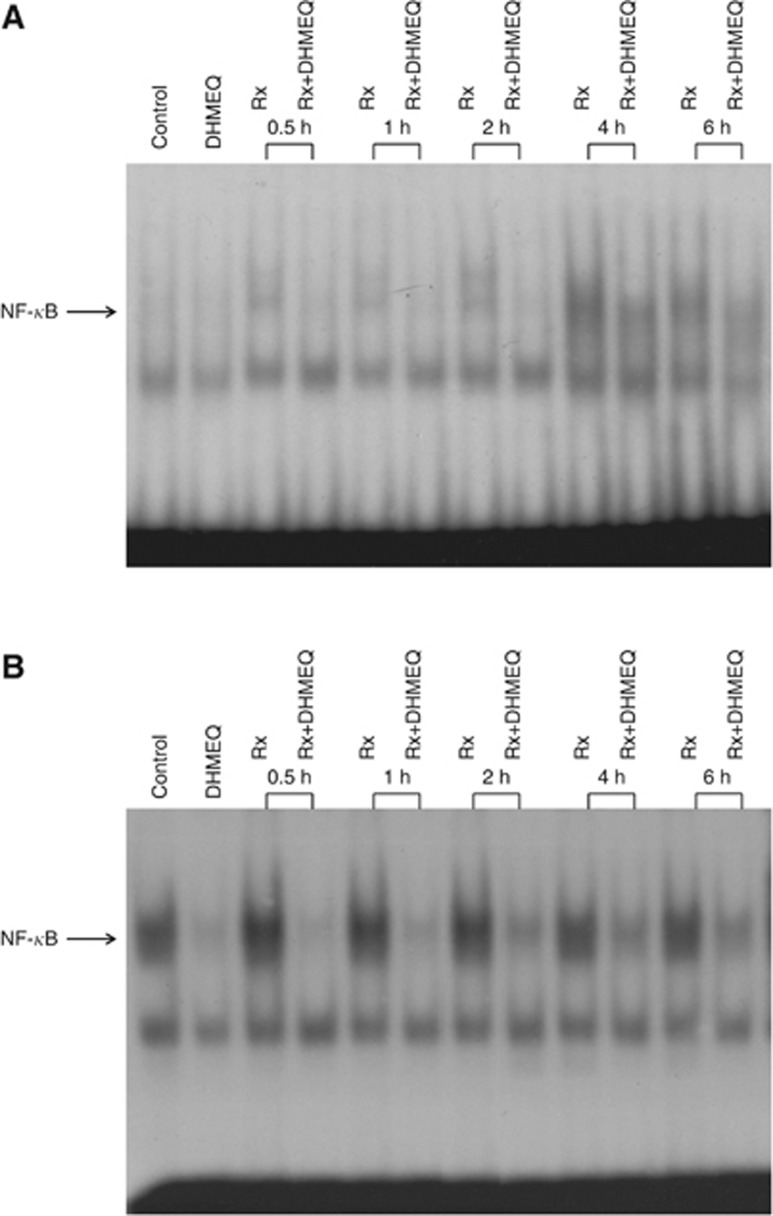
In the experiment of EMSA assay, the DNA activity of NF-κB was induced 4 h after irradiation in LNCaP cells and this increase was inhibited by 5.0 μg ml−1 of DHMEQ treatment (Figure 3A). In PC-3 cells, the DNA activity of NF-κB was completely inhibited by 6-h exposure of 5.0 μg ml−1 of DHMEQ treatment (Figure 3B). The DNA binding activity of NF-κB was increased after irradiation (especially 0.5, 1, and 2 h after irradiation) and this increase was inhibited by 5.0 μg ml−1 of DHMEQ treatment. These data confirm that DHMEQ could inhibit the activation of NF-κB induced by irradiation.
Figure 3.
Effects of DHMEQ on NF-κB binding activity after irradiation. LNCaP (A) and PC-3 (B) cells were treated with or without 5.0 μg ml−1 of DHMEQ for 6 h and then 4 Gy of irradiation was performed. At various time points after irradiation, nuclear extracts were prepared and EMSA was performed.
Cell cycle analysis
The percentage of G2/M arrest in LNCaP cells treated with 4 Gy of irradiation (38.0±1.4%) was higher than that of non-treated cells (26.7±4.2%) and of 5.0 μg ml−1 of DHMEQ treatment alone (24.8±1.8% P<0.05 in each, Table 1). Furthermore, the percentage of G2/M arrest in LNCaP cells treated with the combination treatment of DHMEQ and 4 Gy of irradiation (44.8±2.5%) was higher than that of irradiation alone (P<0.05). In PC-3 cells, the percentage of G2/M arrest in the combination treatment of DHMEQ with irradiation group (56.1±2.1%) was significantly higher than that in the non-treated control (30.8±3.6%), DHMEQ treatment alone (42.5±3.3%), and irradiation alone (44.6±2.4%) groups (P<0.05, in each).
Table 1. Cell cycle analysis after combination treatment with 5 μg ml−1 of DHMEQ and 4 Gy of irradiation in LNCaP and PC-3 cells.
| Cell line | |||
|---|---|---|---|
| Cell cycle | Treatments | LNCaP | PC-3 |
| % G1 | Untreated | 58.8±2.4 | 55.2±4.2 |
| DHMEQ alone | 63.9±3.4 | 46.2±3.8 | |
| Irradiation alone | 54.4±2.3 | 42.9±1.7 | |
| DHMEQ+irradiation | 46.9±3.9 | 35.5±2.2 | |
| % S | Untreated | 14.5±3.0 | 14.0±5.1 |
| DHMEQ alone | 11.3±1.8 | 11.3±1.0 | |
| Irradiation alone | 7.6±2.3 | 10.5±1.1 | |
| DHMEQ+irradiation | 8.3±2.6 | 8.4±5.1 | |
| % G2/M | Untreated | 26.7±4.2 | 30.8±3.6 |
| DHMEQ alone | 24.8±1.8 | 42.5±3.3 | |
| Irradiation alone | 38.0±1.4 | 46.6±2.4 | |
| DHMEQ+irradiation | 44.8±2.5 | 56.1±2.1 | |
Analysis of cell cycle–related protein expression, p53, p21, and 14-3-3σ
In LNCaP cells, the expression of p53 and p21was increased after 4 Gy of irradiation (Figure 4). Furthermore, expressions of p53 and p21were enhanced in cells treated with the combination of 5.0 μg ml−1 of DHMEQ and 4 Gy of irradiation. In PC-3 cells, no p53 expression was observed and their expressions did not change after DHMEQ and/or irradiation treatment. On the contrary, the expression of 14-3-3σ was increased after 4 Gy of irradiation. The increase in 14-3-3σ expression after irradiation was enhanced in cells treated with the combination of 5.0 μg ml−1 of DHMEQ and 4 Gy of irradiation.
Figure 4.
p53, p21, and 14-3-3σ protein expressions after DHMEQ and irradiation treatment in LNCaP and PC-3 cells. Western blot analysis was performed using antibodies specific for p53, p21, and 14-3-3σ. Protein was prepared after 4-Gy irradiation and/or 5.0 μg ml−1 DHMEQ pretreatment in LNCaP and PC-3 cells.
The antitumour effect of DHMRQ and irradiation treatment in vivo
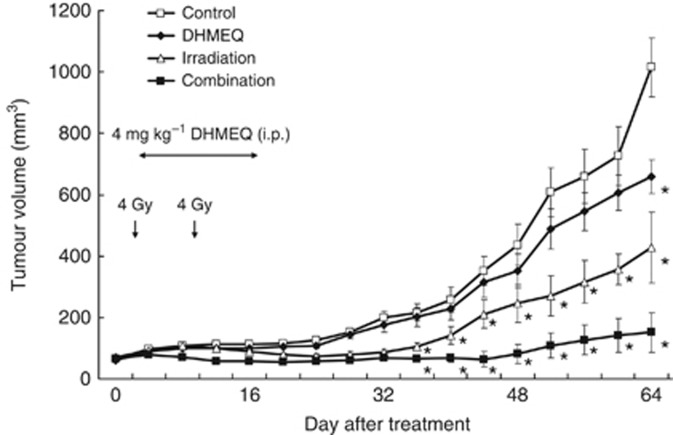
Mean tumour volume in mice treated with irradiation and DHMEQ (66.8±9.5 mm3) was significantly lower than that in untreated controls (215.7±27.3 mm3), DHMEQ treated alone (200.9±29.5 mm3), and irradiation alone (105.2±13.4 mm3) 36 days after the start of treatment (P<0.05 for each; Figure 5). No body weight loss or skin reactions were observed in the combination treatment or other groups. Sixty-four days after the start of treatment, tumour size was 85.1%, 77.1%, and 64.7% smaller in the combination treatment group than that of the untreated control, DHMEQ treated alone, and irradiation alone groups, respectively. In another set of experiments, we evaluated the toxic and side effects of DHMEQ itself in mice. No abnormal data, including liver and renal function, were demonstrated in mice treated with any dose of DHMEQ up to 16 mg kg−1 (data no shown). We also analysed the histological appearance of major organs, including the heart, lung, liver, kidney, bladder, and prostate. Haematoxylin and eosin staining did not reveal significant differences in histological appearance and damage between control and DHMEQ-treated mice.
Figure 5.
In vivo therapeutic effect of DHMEQ in combination with irradiation. PC-3 cells (5 × 105) were implanted subcutaneously into the flank of nude mice. When an animal developed a palpable tumour, it was given an intraperitoneal injection of DHMEQ daily at a concentration of 4 mg kg−1 in 0.5 ml of PBS for 14 consecutive days in the DHMEQ alone group and the combination group. As a control, 0.5 ml of vehicle alone was administered. In the irradiation and combination groups, irradiation consisted of 8 Gy in 2 fractions on the 1st day and 8th day. Tumour volume was monitored every 4 days for 9 weeks. *Significantly different from controls, P<0.05.
Discussion
We first investigated the cytotoxic effect of DHMEQ treatment in both LNCaP and PC-3 cells. No significant cytotoxic effects of DHMEQ itself were observed at doses of up to 5.0 μg ml−1 in either LNCaP or PC-3 cells. At a radiation dose of 4 Gy, a significant inhibitory effect of colony formation in LNCaP and PC-3 cells was observed after treatment with the 2.5 μg ml−1 or higher dose and 5 μg ml−1 or higher dose of DHMEQ, respectively. Next, we evaluated if the combined treatment of DHMEQ with irradiation could affect NF-κB binding activity. NF-κB binding activity was induced by radiation alone in both PC-3 and LNCaP cells. DHMEQ also inhibited NF-κB activation induced by irradiation, as confirmed by EMSA assay. These results indicate that DHMEQ could inhibit NF-κB activation induced by irradiation, thereby reducing the growth of prostate cancer cell lines.
We then evaluated cell cycle analysis, which showed that G2/M arrest was enhanced by the combined treatment of irradiation with DHMEQ in both LNCaP and PC-3 cells. This result showed that the combination of irradiation with DHMEQ induced strong G2/M arrest in prostate cancer cells.
According to western blot results, the appearance of p53 increased after irradiation and p53 expression was enhanced by the combination treatment of DHMEQ with irradiation in LNCaP cells. Previous reports showed that p53 is also responsible for cell cycle arrest upon DNA damage and is a key regulator of apoptosis (Vousden, 2002). Similar results were observed in p21 expression after the combination treatment in LNCaP cells. In PC-3 cells, no p53 expression was observed and did not change after DHMEQ and/or irradiation. In G2/M arrest, cdc2, the kinase required for entry into mitosis, is inhibited by various mechanisms. p21 and 14-3-3σ, which are transcriptional targets of p53, play key roles in inhibiting cdc2 activity (Taylor and Stark, 2001; Dellinger et al, 2003). 14-3-3σ has been reported to be a critical regulator of the G2/M checkpoint in both p53 wild-type and mutant PC-3 cancer cells (Han et al, 2006). In this study, 14-3-3σ protein expression was increased after irradiation, especially after the combination treatment of DHMEQ with irradiation in PC-3 cells.
Because of these encouraging in vitro findings, we evaluated the inhibitory effect of the combination therapy in PC-3 subcutaneous tumours inoculated into nude mice to see if the radiosensitivity of PC-3 tumours was enhanced by the combined use of the 4 mg kg−1 dose of DHMEQ, even at a concentration that had a modest therapeutic impact on tumour growth. Tumour growth was dramatically lower in mice treated with both irradiation and DHMEQ than that with each individual treatment alone and the vehicle control. DHMEQ treatment (up to 16 mg kg−1) was well tolerated, leading to no body weight losses, skin problems, or abnormal clinical laboratory data, including liver and renal function and histological appearance. These findings suggest that radiotherapy with DHMEQ treatment could have strong antitumour effects and may reduce related side effects for organ-confined and locally advanced prostate cancer.
Several studies have examined the effects of combined treatment consisting of radiation therapy and NF-κB inhibitors in prostate cancer (Pajonk et al, 1999; Palayoor et al, 1999; Wen et al, 2003; Chendil et al, 2004; Raffoul et al, 2006). Nonsteroidal anti-inflammatory drugs, such as ibuprofen, inhibit NF-κB activation by direct inhibition of IκB-α kinase, which ubiquitinates IκB-α (Palayoor et al, 1999). The combined treatment of NS398, which is a COX-2 inhibitor, with irradiation had a stronger antitumour effect than when used individually in the human prostate cancer cell line DU145 (Wen et al, 2003). However, as these agents are not specific inhibitors of only NF-κB, side effects may occur. Gene therapy aimed at inhibiting NF-κB activity in prostate cancer cells has also been investigated (Pajonk et al, 1999; Flynn et al, 2003). When a recombinant adenovirus vector that contains a gene encoding a form of IκB was transfected to PC-3 cells, the constitutive DNA activity of NF-κB activity induced by irradiation was dramatically decreased (Pajonk et al, 1999). An important problem is that the clinical feasibility of gene therapy for inhibiting NF-κB is quite limited by requisite intratumoural delivery of a vector inhibiting NF-κB in a clinical setting (Kikuchi et al, 2003). Curcumin (Chendil et al, 2004) and genistein (Davis et al, 1999; Raffoul et al, 2006) are natural products that have been reported to inhibit activation of NF-κB induced by irradiation; however, their antitumour effects were not confirmed in in vivo studies, and the precise mechanisms by which these products inactivate NF-κB are still not known.
DHMEQ is a unique and potent NF-κB inhibitor that works at the level of nuclear translocation of NF-κB (Ariga et al, 2002). It has recently been demonstrated that DHMEQ can inhibit constitutive NF-κB activity consisting of p50/p65, although its effect is less potent against the p50 homodimer. This may explain why DHMEQ can effectively induce apoptosis in cancer cells with constitutive NF-κB activity consisting of p50/p65, but does not affect normal-resting lymphocytes whose NF-κB activity consists mainly of the p50 homodimer.
Conclusions
DHMEQ inhibits NF-κB binding activity induced by irradiation and causes an increase in the inhibitory effect on colony formation and in the enhancement of cell cycle arrest in prostate cancer cells. Blockade of NF-κB function induced by radiation with DHMEQ could overcome radio-resistant responses and may become a new therapeutic modality for treating organ-confined and locally advanced prostate cancer.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Ariga A, Namekawa J, Matsumoto N, Inoue J, Umezawa K (2002) Inhibition of tumor necrosis factor-alpha -induced nuclear translocation and activation of NF-kappa B by dehydroxymethylepoxyquinomicin. J Biol Chem 277: 24625–24630 [DOI] [PubMed] [Google Scholar]
- Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D (1991) Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest 88: 691–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shen B, Xia L, Khaletzkiy A, Chu D, Wong JY, Li JJ (2002) Activation of nuclear factor kappaB in radioresistance of TP53-inactive human keratinocytes. Cancer Res 62: 1213–1221 [PubMed] [Google Scholar]
- Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM (2004) Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene 23: 1599–1607 [DOI] [PubMed] [Google Scholar]
- Chin C, Bae JH, Kim MJ, Hwang JY, Kim SJ, Yoon MS, Lee MK, Kim DW, Chung BS, Kang CD, Kim SH (2005) Radiosensitization by targeting radioresistance-related genes with protein kinase A inhibitor in radioresistant cancer cells. Exp Mol Med 37: 608–618 [DOI] [PubMed] [Google Scholar]
- Davis JN, Kucuk O, Sarkar FH (1999) Genistein inhibits NF-kappa B activation in prostate cancer cells. Nutr Cancer 35: 167–174 [DOI] [PubMed] [Google Scholar]
- Dellinger RW, Karjian PL, Neuteboom ST (2003) NB1011 induces Ser15 phosphorylation of p53 and activates the G2/M checkpoint. Anticancer Drugs 14: 449–455 [DOI] [PubMed] [Google Scholar]
- Flynn V, Ramanitharan A, Moparty K, Davis R, Sikka S, Agrawal KC, Abdel-Mageed AB (2003) Adenovirus-mediated inhibition of NF-kappaB confers chemo-sensitization and apoptosis in prostate cancer cells. Int J Oncol 23: 317–323 [PubMed] [Google Scholar]
- Han B, Xie H, Chen Q, Zhang JT (2006) Sensitizing hormone-refractory prostate cancer cells to drug treatment by targeting 14-3-3sigma. Mol Cancer Ther 5: 903–912 [DOI] [PubMed] [Google Scholar]
- Hanks GE, Hanlon AL, Epstein B, Horwitz EM (2002) Dose response in prostate cancer with 8-12 years' follow-up. Int J Radiat Oncol Biol Phys 54: 427–435 [DOI] [PubMed] [Google Scholar]
- Kikuchi E, Horiguchi Y, Nakashima J, Kuroda K, Oya M, Ohigashi T, Takahashi N, Shima Y, Umezawa K, Murai M (2003) Suppression of hormone-refractory prostate cancer by a novel nuclear factor kappaB inhibitor in nude mice. Cancer Res 63: 107–110 [PubMed] [Google Scholar]
- Kupelian PA, Potters L, Khuntia D, Ciezki JP, Reddy CA, Reuther AM, Carlson TP, Klein EA (2004) Radical prostatectomy, external beam radiotherapy <72 Gy, external beam radiotherapy >or =72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1-T2 prostate cancer. Int J Radiat Oncol Biol Phys 58: 25–33 [DOI] [PubMed] [Google Scholar]
- Kuroda K, Horiguchi Y, Nakashima J, Kikuchi E, Kanao K, Miyajima A, Ohigashi T, Umezawa K, Murai M (2005) Prevention of cancer cachexia by a novel nuclear factor {kappa}B inhibitor in prostate cancer. Clin Cancer Res 11: 5590–5594 [DOI] [PubMed] [Google Scholar]
- Matsumoto G, Namekawa J, Muta M, Nakamura T, Bando H, Tohyama K, Toi M, Umezawa K (2005) Targeting of nuclear factor kappaB Pathways by dehydroxymethylepoxyquinomicin, a novel inhibitor of breast carcinomas: antitumor and antiangiogenic potential in vivo. Clin Cancer Res 11: 1287–1293 [PubMed] [Google Scholar]
- Matsumoto N, Ariga A, To-e S, Nakamura H, Agata N, Hirano S, Inoue J, Umezawa K (2000) Synthesis of NF-kappaB activation inhibitors derived from epoxyquinomicin C. Bioorg Med Chem Lett 10: 865–869 [DOI] [PubMed] [Google Scholar]
- Ohsugi T, Horie R, Kumasaka T, Ishida A, Ishida T, Yamaguchi K, Watanabe T, Umezawa K, Urano T (2005) In vivo antitumor activity of the NF-kappaB inhibitor dehydroxymethylepoxyquinomicin in a mouse model of adult T-cell leukemia. Carcinogenesis 26: 1382–1388 [DOI] [PubMed] [Google Scholar]
- Pajonk F, Pajonk K, McBride WH (1999) Inhibition of NF-kappaB, clonogenicity, and radiosensitivity of human cancer cells. J Natl Cancer Inst 91: 1956–1960 [DOI] [PubMed] [Google Scholar]
- Palayoor ST, Youmell MY, Calderwood SK, Coleman CN, Price BD (1999) Constitutive activation of IkappaB kinase alpha and NF-kappaB in prostate cancer cells is inhibited by ibuprofen. Oncogene 18: 7389–7394 [DOI] [PubMed] [Google Scholar]
- Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, Huang E, von Eschenbach AC, Kuban DA, Rosen I (2002) Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 53: 1097–1105 [DOI] [PubMed] [Google Scholar]
- Raffoul JJ, Wang Y, Kucuk O, Forman JD, Sarkar FH, Hillman GG (2006) Genistein inhibits radiation-induced activation of NF-kappaB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. BMC Cancer 6: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starenki DV, Namba H, Saenko VA, Ohtsuru A, Maeda S, Umezawa K, Yamashita S (2004) Induction of thyroid cancer cell apoptosis by a novel nuclear factor kappaB inhibitor, dehydroxymethylepoxyquinomicin. Clin Cancer Res 10: 6821–6829 [DOI] [PubMed] [Google Scholar]
- Sun Y, Clair DK, Fang F, Warren GW, Rangnekar VM, Crooks PA, Clair WH (2007) The radiosensitization effect of parthenolide in prostate cancer cells is mediated by nuclear factor-kappaB inhibition and enhanced by the presence of PTEN. Mol Cancer Ther 6: 2477–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M, Delia D, Chessa L, Iwata S, Shigeta T, Kanke Y, Goi K, Asada M, Eguchi M, Kodama C, Mizutani S (1998) Defective control of apoptosis, radiosensitivity, and spindle checkpoint in ataxia telangiectasia. Cancer Res 58: 4923–4929 [PubMed] [Google Scholar]
- Takatsuna H, Umezawa K (2004) Screening of bioactive metabolites for pancreatic regeneration chemotherapy. Biomed Pharmacother 58: 610–613 [DOI] [PubMed] [Google Scholar]
- Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A (2001) Inhibition of JNK activation through NF-kappaB target genes. Nature 414: 313–317 [DOI] [PubMed] [Google Scholar]
- Taylor WR, Stark GR (2001) Regulation of the G2/M transition by p53. Oncogene 20: 1803–1815 [DOI] [PubMed] [Google Scholar]
- Vousden KH (2002) Activation of the p53 tumor suppressor protein. Biochim Biophys Acta 1602: 47–59 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Dewan MZ, Okamura T, Sasaki M, Itoh K, Higashihara M, Mizoguchi H, Honda M, Sata T, Watanabe T, Yamamoto N, Umezawa K, Horie R (2005) A novel NF-kappaB inhibitor DHMEQ selectively targets constitutive NF-kappaB activity and induces apoptosis of multiple myeloma cells in vitro and in vivo. Int J Cancer 114: 32–38 [DOI] [PubMed] [Google Scholar]
- Wen B, Deutsch E, Eschwege P, De Crevoisier R, Nasr E, Eschwege F, Bourhis J (2003) Cyclooxygenase-2 inhibitor NS398 enhances antitumor effect of irradiation on hormone refractory human prostate carcinoma cells. J Urol 170: 2036–2039 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Horie R, Takeiri M, Kozawa I, Umezawa K (2008) Inactivation of NF-kappaB components by covalent binding of (-)-dehydroxymethylepoxyquinomicin to specific cysteine residues. J Med Chem 51: 5780–5788 [DOI] [PubMed] [Google Scholar]
- Zelefsky MJ, Fuks Z, Hunt M, Lee HJ, Lombardi D, Ling CC, Reuter VE, Venkatraman ES, Leibel SA (2001) High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol 166: 876–881 [PubMed] [Google Scholar]