Abstract
BACKGROUND/OBJECTIVES:
Serum amyloid A (SAA) is an acute-phase protein that has been recently correlated with obesity and insulin resistance. Therefore, we first examined whether human recombinant SAA (rSAA) could affect the proliferation, differentiation and metabolism of 3T3-L1 preadipocytes.
DESIGN:
Preadipocytes were treated with rSAA and analyzed for changes in viability and [3H-methyl]-thymidine incorporation as well as cell cycle perturbations using flow cytometry analysis. The mRNA expression profiles of adipogenic factors during the differentiation protocol were also analyzed using real-time PCR. After differentiation, 2-deoxy-[1,2-3H]-glucose uptake and glycerol release were evaluated.
RESULTS:
rSAA treatment caused a 2.6-fold increase in cell proliferation, which was consistent with the results from flow cytometry showing that rSAA treatment augmented the percentage of cells in the S phase (60.9±0.54%) compared with the control cells (39.8±2.2%, *** P<0.001). The rSAA-induced cell proliferation was mediated by the ERK1/2 signaling pathway, which was assessed by pretreatment with the inhibitor PD98059. However, the exposure of 3T3-L1 cells to rSAA during the differentiation process resulted in attenuated adipogenesis and decreased expression of adipogenesis-related factors. During the first 72 h of differentiation, rSAA inhibited the differentiation process by altering the mRNA expression kinetics of adipogenic transcription factors and proteins, such as PPARγ2 (peroxisome proliferator-activated receptor γ 2), C/EBPβ (CCAAT/enhancer-binding protein β) and GLUT4. rSAA prevented the intracellular accumulation of lipids and, in fully differentiated cells, increased lipolysis and prevented 2-deoxy-[1,2-3H]-glucose uptake, which favors insulin resistance. Additionally, rSAA stimulated the secretion of proinflammatory cytokines interleukin 6 and tumor necrosis factor α, and upregulated SAA3 mRNA expression during adipogenesis.
CONCLUSIONS:
We showed that rSAA enhanced proliferation and inhibited differentiation in 3T3-L1 preadipocytes and altered insulin sensitivity in differentiated cells. These results highlight the complex role of SAA in the adipogenic process and support a direct link between obesity and its co-morbidities such as type II diabetes.
Keywords: Serum amyloid A, preadipocyte, proliferation, adipogenesis, insulin resistance, inflammation
Introduction
Obesity is a chronic metabolic disorder caused by an imbalance between energy intake and expenditure. This disorder occurs by hyperplastic growth caused by the mitotic activity in precursor cells (preadipocytes) and by hypertrophic growth due to intracellular lipid accumulation.1 This scenario is closely associated with a state of chronic low-grade inflammation characterized by abnormal production of cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin 6, and by the activation of inflammatory signaling pathways in adipose tissue.2, 3 The expression and secretion of inflammatory molecules is increased in obesity, and this increase negatively affects insulin sensitivity in adipose tissue.4, 5, 6
In obese individuals, a chronic and systemic elevation of serum amyloid A (SAA) protein was recently associated with the size of adipocytes.7 Until recently, it was thought that the expression and release of SAA occurred predominantly in the liver,8 however, it is now known that human adipose tissue is a major SAA expression site during the non-acute-phase reaction condition.9, 10, 11 In rodents, the main isoform found in adipocytes is SAA3,4, 12 and it is strongly upregulated in the adipose tissue of obese mice as compared with lean controls.13
In previous studies, we had demonstrated immunomodulatory activities of SAA in human leukocytes, such as the induction of expression and release of TNF-α, interleukin-1β, interleukin-8 and enhanced leukocyte migration and adhesion.14, 15, 16 Recently, we showed that SAA is an inducer of nitric oxide production in macrophages and may be an endogenous agonist for the TLR4 complex.17 Furthermore, we have demonstrated that SAA was triggered in chronic conditions, such as diabetes.18 Fibroblasts and endothelial cells are also responsive to SAA. In Swiss 3T3 fibroblasts, SAA increased proliferation that was completely abolished by the addition of antioxidants.19
3T3-L1 preadipocyte, a murine cell line, is commonly used as a model of adipogenesis, lipogenesis and lipolysis. The differentiation of growth-arrested 3T3-L1 cells into adipocytes is induced using hormonal stimuli that initiate the mitotic clonal expansion of preadipocytes leading to phenotype modifications.20, 21 Expression of C/EBPβ, a member of the CCAAT/enhancer-binding protein (C/EBP) family of transcription factors, is rapidly induced in response to these stimuli (dexamethasone, 3-isobutyl-1-methylxanthine, insulin) and regulates peroxisome proliferator-activated receptor γ 2 (PPARγ2), a spliced variant of PPARγ in adipocytes, and C/EBPα, which have crucial roles in adipocyte maturation.20, 22
Several roles of SAA in 3T3-L1 cell lines have already been investigated, including the expression of GLUT4, the serine phosphorylation rate of the IRS-1 (insulin receptor substrate 1) and glucose uptake in fully differentiated 3T3-L1 cells. It was demonstrated that SAA reduced insulin sensitivity, which was mediated by the JNK pathway.23 Although these conclusions were relevant to understand the complications of obesity and metabolic syndrome, the low-grade inflammation state that occurs during the early and advanced stages of adipogenesis (proliferation and differentiation) was not elucidated. Therefore, to fully address the role of SAA in adipogenesis, we performed assays in three different stages of 3T3-cells. Firstly, we treated preadipocytes with rSAA and proliferation was assessed by [3H-methyl]-thymidine incorporation and flow cytometry. Secondly, we verified the effect of rSAA on the differentiation of 3T3-L1 cells into adipocytes by lipid accumulation and the mRNA expression profiles of adipogenic factors. Finally, we observed the effect of rSAA on 2-deoxy-[1,2-3H]-glucose uptake and glycerol release in differentiated 3T3-cells.
Materials and methods
Reagents
rSAA was purchased from Peprotech Inc. (Rocky Hill, NJ, USA). PD98059, SB203580, pertussis toxin and wortmannin were purchased from Calbiochem (La Jolla, CA, USA). Insulin, dexamethasone, 3-isobutyl-1-methylxanthine, Oil Red O, annexin V-fluorescein isothiocyanate (V–FITC), phenylmethylsulfonyl fluoride and propidium iodide (PI) were supplied by Sigma Chemical Co. (St Louis, MO, USA). [3H-methyl]-thymidine and 2-deoxy-[1,2-3H]-glucose were acquired from Amersham Biosciences (São Paulo, Brazil). Dulbecco's modified Eagle's medium (DMEM), calf serum (CS), penicillin, streptomycin and fetal bovine serum were purchased from Invitrogen (Carlsbad, CA, USA). All other reagents used were purchased from Merck (Darmstadt, Germany) unless otherwise indicated.
Cell culture and differentiation induction
Mouse 3T3-L1 preadipocytes were generously provided by Dr. Mari Cleide Sogayar (Instituto de Química, Universidade de São Paulo, Brazil). Cells were maintained in DMEM supplemented with 10% CS containing 100 IU ml−1 of penicillin and 100 μg ml−1 of streptomycin. Cells were cultured at 37 °C in a humidified atmosphere at 5% CO2, and the medium was changed every 48 h. 3T3-L1 cells were induced to differentiate as described previously.24 Starting on day 1 of differentiation, cells were treated with 5 μg ml−1 rSAA and during each medium change, rSAA was restored. At the times indicated, cells were stained with Oil Red O to detect cytoplasmic triglycerides, extracted and read spectrophotometrically.25 In cell-free supernatants of the cultures, glycerol levels were determined using the Glycerol 3-phosphate Oxidase-Trinder Kit (Sigma). The cytokines interleukin 6 and TNF-α were measured using an enzyme-linked immunosorbent assay (ELISA) (DuoSet, R&D System, Minneapolis, MN, USA).
Cell proliferation
Mitogen-activated protein kinases (MAPK), especially ERK1/2 and p38MAPK, have been implicated in cell cycle control and differentiation. The phosphoinositide 3-kinase (PI3 K) pathway, an important signaling pathway in the mediation of cell survival, adipocyte differentiation and glucose transport was also evaluated. We used specific pharmacological inhibitors of ERK1/2 (PD98059), p38MAPK (SB203580) and PI3 K (wortmannin) for 3T3-L1 cell pretreatment that was followed by rSAA stimulation in a cell proliferation assay. For that, cells were deprived of serum (1% CS) for 48 h, pretreated with specific inhibitors including PD98059 (10 μM), SB203580 (10 μM), pertussis toxin (100 ng ml−1) or wortmannin (100 nM) and treated with rSAA (1, 5 and 10 μg ml−1) and/or insulin (100 nM) at the times indicated. Then, 0.5 μCi of [3H-methyl]-thymidine (specific activity 248 GBq mmol−1) was added for 24 h. Cells were washed twice with cold phosphate-buffered saline (PBS), fixed with ice-cold 10% trichloroacetic acid, lysed with 0.5 M NaOH and transferred to filters (10 × 5 mm). The lysates were placed in vials containing 2 ml of scintillation fluid and were counted using the liquid scintillation counter (Beckman Instruments, Palo Alto, CA, USA).
Flow cytometry analysis
For the cell cycle and viability assays, cells were serum-starved in DMEM containing 1% CS for 48 h and treated with rSAA (1 and 5 μg ml−1) for 24 h. Cells were harvested and washed twice with PBS. For cell cycle analysis, cells were gently resuspended in 300 μl of a solution containing 2 μg ml−1 PI, 0.1% sodium citrate and 0.1% Triton X-100. The proportion of cells in each phase of the cell cycle was determined using a flow cytometer set to 488 nm (excitation wavelength), and the data were analyzed using FlowJo software (Tree star, Inc., Ashland, OR, USA). For the viability assay using double staining (annexin V–FITC and PI), cells were resuspended in 100 μl of binding buffer (10 mM HEPES/NaOH, 140 mM NaCl and 2.5 mM CaCl2). Next, 5 μl of annexin V-FITC was added. The cells were gently agitated and incubated for 20 min at room temperature in the dark. Subsequently, 40 μl of PI solution (2 μg ml−1) and 400 μl of binding buffer were added, and the samples were analyzed using flow cytometry. The fluorescence of annexin V-FITC was measured in the FL1 channel (green fluorescence: 530/30 nm) and PI fluorescence was measured in the FL2 channel (orange-red fluorescence: 585/42 nm) using the FACSCanto flow cytometric equipment (Becton Dickinson, San Diego, CA, USA). The percentage of necrotic or apoptotic cells and the analysis of the cell cycle were calculated by Flowjo software (Tree star, Inc.).
RNA extraction and complementary DNA synthesis
Total RNA from 3T3-L1 cells was isolated at the indicated times using the Qiagen RNeasy Mini kit (Qiagen, Hilden, Germany). The complementary DNA was synthesized from 600 ng of RNA using the SuperScript First Strand kit (Invitrogen, Carlsbad, CA, USA).
Quantitative Real-Time PCR
The following primers were used: PPARγ2 (5′-CACAGAGATGCCATTCTGGC-3′ and 5′-GGCCTGTTGTAGAGCTGGGT-3′), perilipin (5′-CATGTCCCTATCCGATGCC-3′ and 5′-TCGGTTTTGTCGTCCAGG-3′), C/EBPα (5′-GTGTGCACGTCTATGCTAAACCA-3′ and 5′-GCCGTTAGTGAAGAGTCTCAGTTTG-3′), C/EBPβ (5′-GTTTCGGGACTTGATGCAATC-3′ and 5′-AACAACCCCGCAGGAACAT-3′), FABP4 (5′-CCAATGAGCAAGTGGCAAGA-3′ and 5′-GATGCCAGGCTCCAGGATAG-3′), GLUT4 (5-GCTGTGCCATCTTGATGACGG-3′ and 5-TGAAGAAGCCAAGCAGGAGGAC-3′) and 18S (5′-GTAACCCGTTGAACCCCATT-3′ and 5′-CCATCCAATCGGTAGTAGCG-3′), which was used as a constitutive control. BLAST searches were conducted on all primer sequences to ensure gene specificity. Each amplification reaction was performed in triplicate and included the addition of the SyBr Green Master Mix (Applied Biosystems, Mount Holly, NJ, USA). Each data set also included a negative control (no complementary DNA). Reaction conditions were as follows: 95 °C for 10 min, 40 cycles of 95 °C for 10 s (melting) and 60 °C for 1 min (annealing and elongation). Melting curve analyses from 76 to 84 °C were performed at the end of each run as a quality control step. The Ct (cycle threshold) for each run was set to 0.1, when amplification was observed in the log phase. Relative gene expression was determined using the ΔΔCt method, and the efficiency of each reaction was validated as previously described.26 The expression levels of SAA3 mRNAs were quantified using the TaqMan PCR reagent kit's detection system according to the protocols provided by the manufacturer (Applied Biosystems). The levels of mRNA were normalized to the amount of β-actin RNA detected in each sample. PCR reactions were performed in the Gene AMP 7500 Sequence Detection System (Applied Biosystems).
2-deoxy-[1,2-3H]-glucose uptake
Glucose uptake by differentiated 3T3-L1 cells was measured as previously described27 with modifications. Briefly, 3T3-L1 cells were differentiated into adipocytes and incubated with or without rSAA (1, 5 and 10 μg ml−1) in serum-free DMEM for 24 h. The adipocytes were washed twice with 37 °C Krebs–Ringer phosphate buffer (pH 7.4) (128 mM NaCl, 4.7 mM KCl, 1.65 mM CaCl2, 2.5 mM MgSO4 and 5 mM Na2HPO4). Adipocytes were either untreated (basal, insulin-independent) or treated with insulin (100 nM) for 10 min in Krebs–Ringer phosphate buffer. Without changing the buffer, glucose uptake was initiated by adding 1.0 μCi per well of 2-deoxy-[1,2-3H]-glucose (specific activity 740 GBq mmol−1) for 10 min at 37 °C. The cells were gently washed three times with ice-cold PBS and lysed in 800 μl of solution containing 0.5 M NaOH and 0.1% sodium dodecyl sulfate. Samples were assayed for glucose uptake using a liquid scintillation counter. The level of glucose uptake induced by insulin (100 nM) was set at 100%.
Measurement of SAA3 protein levels in cell lysates
Cells were washed with PBS, harvested using sonication buffer (PBS pH 7.4 and 2 mM phenylmethylsulfonyl fluoride), centrifuged at 300 × g for 5 min and lysed by sonication (Branson Ultrasonics Corporation, Danbury, CT, USA) for 10 s at 40 W. The supernatants were cleared by centrifugation at 15 000 × g for 15 min. SAA3 was measured using the ELISA kit (Invitrogen).
Statistical analysis
Results were shown as the mean±s.e. of 6–9 determinations from 2–3 experiments. Statistical analyses were performed with Graph Pad Prism4 (Graph Pad Software Inc., San Diego, CA, USA). When multiple samples were compared with one independent variable, one-way analysis of variance with Newman–Keuls post hoc test was performed. Data with two independent variables were tested by two-way analysis of variance, and tested with Bonferroni post hoc test, as indicated in figure legends. The level of significance was set at P<0.05.
Results
rSAA prevented the necrosis induced by serum starvation
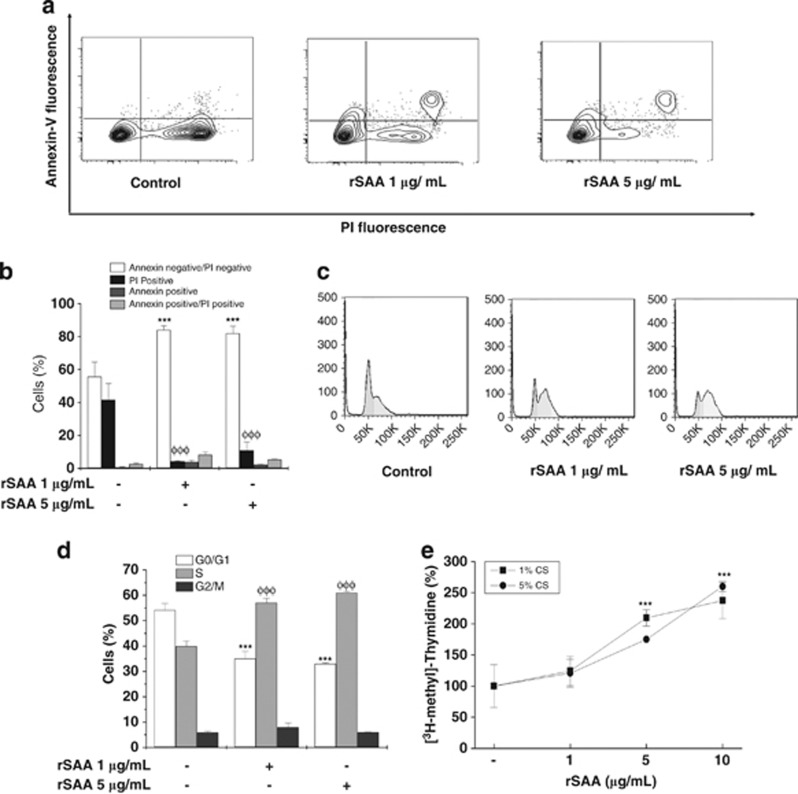
After 48 h of serum starvation, preadipocytes were treated with rSAA for 24 h before performing the flow cytometry analysis using simultaneous staining with annexin V–FITC and PI. Double-staining analysis demonstrated that rSAA reduced serum starvation-induced necrosis, which was assessed by PI-positive cells. rSAA at 1 and 5 μg ml−1 enhanced cell viability (81.9±4.4% and 83.9±2.5%, respectively) compared with unstimulated cells (55.5±9%, ***P<0.001) (Figures 1a and b). However, a small fraction of rSAA-treated cells underwent apoptosis (annexin-positive cells), whereas the prevention of necrosis was markedly decreased.
Figure 1.
Enhanced cell viability and increased proliferation are induced by rSAA in 3T3-L1 preadipocytes. (a) Dot plots show the intensity of Annexin V fluorescence plotted on the Y-axis and PI fluorescence plotted on the X-axis. (b) The percentage of live cells (annexin-negative/PI-negative), necrotic cells (PI-positive), apoptotic cells (annexin-positive) and late apoptotic cells (annexin-positive/PI-positive) after analysis by flow cytometry. (c) Representative flow cytometry histograms showing cell cycle distributions. (d) The percentage of cells in each phase of the cell cycle stained with PI solution (2 μg ml−1). (e) 3T3-L1 cells were treated with increasing concentrations of rSAA (1–10 μg ml−1) in serum-deprived medium for 24 h. During treatment, the cells were labeled with [3H-methyl]-thymidine. Data are the mean±s.e. of three independent experiments, which were performed in triplicate and two-way analysis of variance were performed (***, φφφP<0.001 vs control groups).
rSAA stimulated preadipocyte proliferation
Subconfluent 3T3-L1 preadipocytes were stimulated with rSAA (1, 5 and 10 μg ml−1) and cultured in serum-deprived medium (1% or 5% CS). The incorporation of radiolabeled thymidine into newly replicated DNA was used to assess the effects of rSAA on cell proliferation. As shown in Figure 1e, treatment of 3T3-L1 preadipocytes with rSAA promoted an increase in [3H-methyl]-thymidine incorporation in a dose-dependent manner; that increase reached 260% of the control with 10 μg ml−1 rSAA at 24 h (***P<0.001). When we analyzed the cell cycle, rSAA also stimulated cell cycle progression from the G1 to S phase (Figures 1c and d), which was demonstrated by a higher percentage of cells in S phase (60.9±0.54%, rSAA-treated cells) compared with the control cells (39.8±2.2%) (***P<0.001).
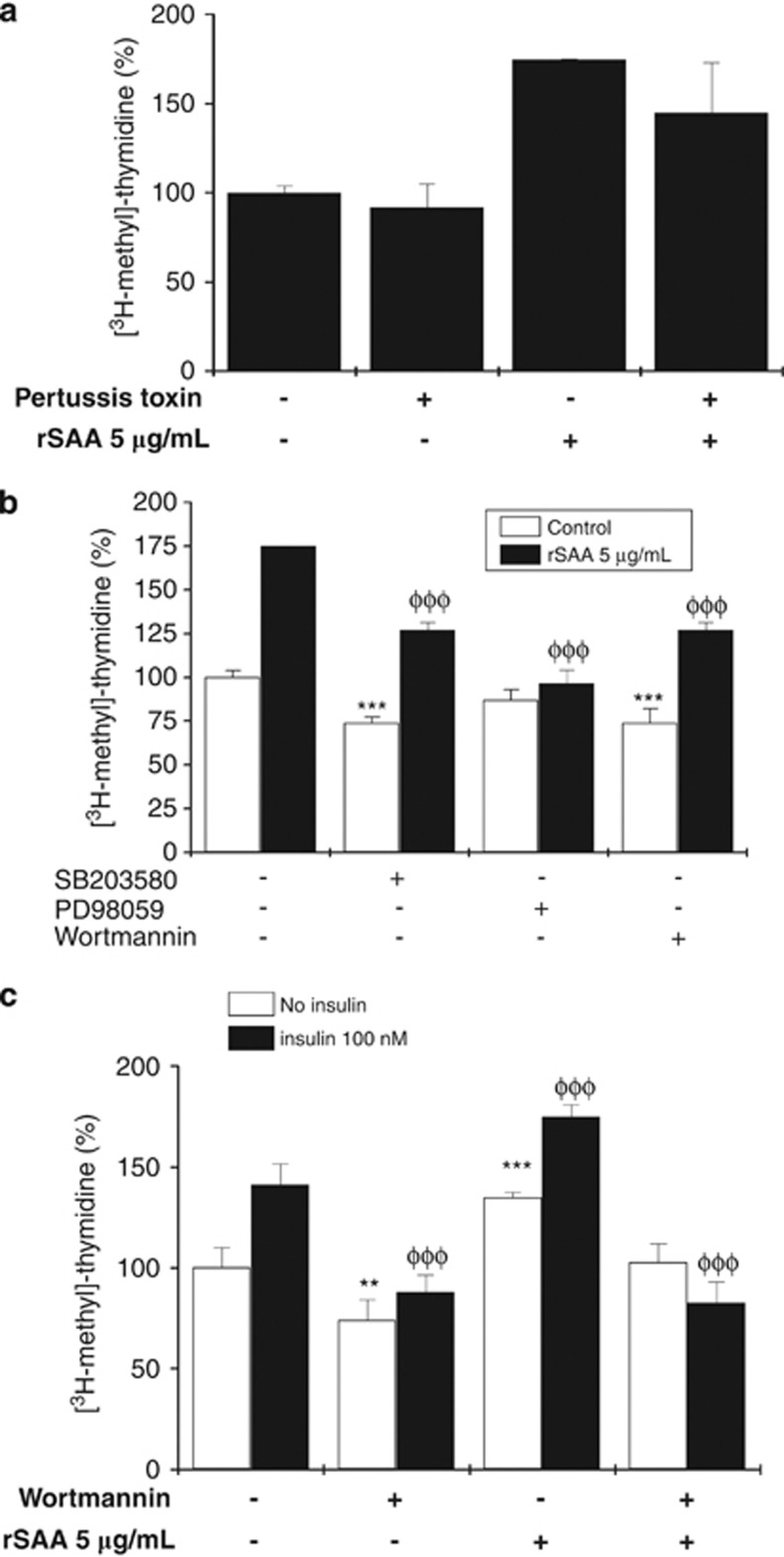
rSAA induced proliferation through the ERK1/2 pathway
Because the mitogenic effects of rSAA in preadipocytes were pronounced, we explored the involvement of several signaling pathways that could be involved in the proliferative effects of rSAA. We hypothesized that rSAA-induced proliferation was mediated by the FPR2 receptor because the pertussis-sensitive G-proteins have been reported to be involved in SAA biological effects. To test this hypothesis, cells were pretreated with the pertussis toxin, and [3H-methyl]-thymidine incorporation was determined in the absence and presence of rSAA. As shown in Figure 2a, no changes in proliferation were observed. However, PD98059, an ERK1/2 inhibitor, prevented the rSAA-induced increase in [3H-methyl]-thymidine incorporation, which suggests that the effects of rSAA on preadipocyte proliferation were mediated through the ERK1/2 signaling pathway. A 24-h exposure to rSAA in the absence or presence of the PI3 K inhibitor wortmannin or the p38MAPK inhibitor SB203580 revealed that the inhibitors affected proliferation in control cells and in rSAA-treated cells (Figure 2b). This result precludes the evaluation of the p38MAPK and PI3 K signaling pathways in the rSAA-induced cell survival.
Figure 2.
The involvement of proteins in different signaling pathways on the preadipocyte proliferation induced by rSAA. (a) Pretreatment of 3T3-L1 cells with pertussis toxin under the conditions described in the methods. (b) 3T3-L1 preadipocytes were pretreated with SB 203580, PD98059 and wortmannin and then stimulated with rSAA. (c) The influence of insulin (100 nM) and rSAA (5 μg ml−1) on activation of the PI3 K pathway. Data are the mean±s.e. of three independent experiments, which were each performed in triplicate and one-way analysis of variance was performed in A and two-way analysis of variance was performed in B and C (**P<0.01, ***, φφφP<0.001 vs control groups).
In 3T3-L1 preadipocytes, insulin activates two major signaling cascades, the PI3 K and MAPK pathways. The PI3K pathway was activated in cells using insulin, a well-known preadipocyte mitogen, and these cells were compared with cells stimulated with rSAA alone. As observed in Figure 2c, insulin increased the proliferation of preadipocytes, which was inhibited using wortmannin. Although the cells treated with both rSAA and insulin showed a higher rate of proliferation (174.7±5.9%) compared with insulin-treated cells (141.1±10.2%), the presence of wortmannin in both treatments significantly attenuated this effect (82.6±10.1%).
rSAA inhibited the expression of adipogenic transcriptional regulators and adipocyte differentiation
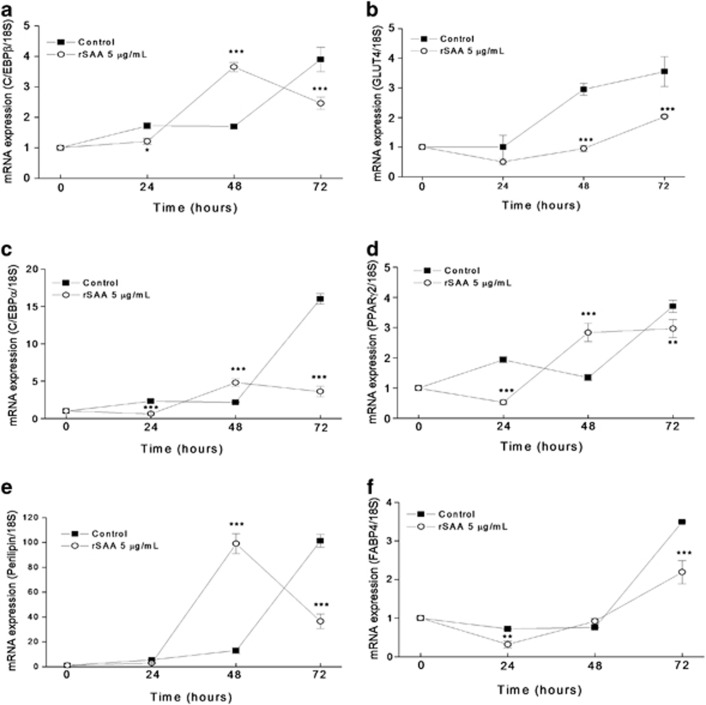
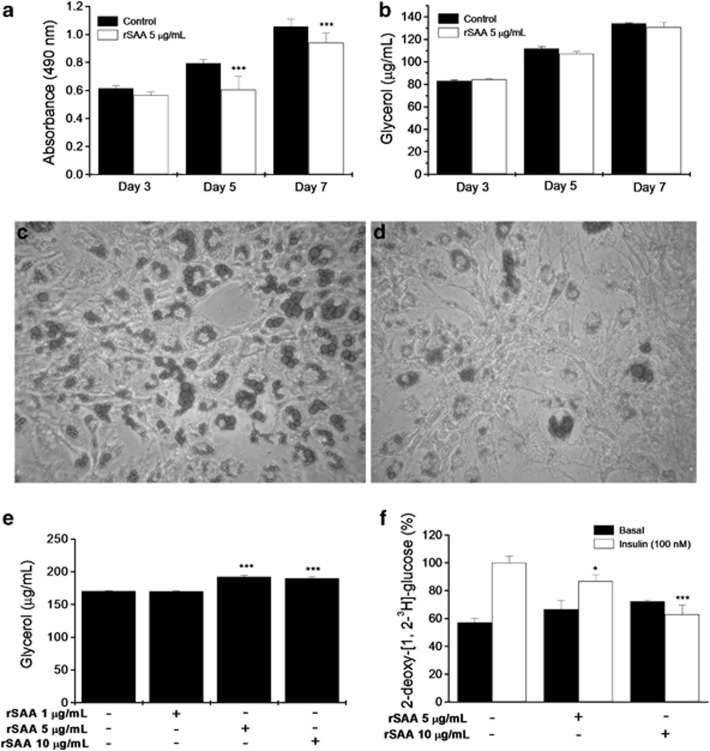
To determine whether rSAA affects adipocyte differentiation, we maintained 3T3-L1 cells in DMEM containing a hormonal stimulus with or without rSAA during the entire process of differentiation. At the indicated times, we analyzed the effect of rSAA on the mRNA expression of transcription factors and adipocyte-specific genes during the first 72 h of the differentiation program by quantitative real-time PCR. Changes in the mRNA expression profiles of C/EBPβ, C/EBPα, PPARγ2, GLUT4, FABP4 and perilipin were observed in the cultures maintained with rSAA. At 48 h of differentiation, rSAA induced an increase in the mRNA expression of PPARγ2, perilipin and C/EBPα and β (Figure 3). However, at 72 h, rSAA significantly downregulated mRNA expression of PPARγ2, C/EBPα and C/EBPβ, which are the critical genes for the adipocyte phenotype. Interestingly, cultures maintained with rSAA showed a significant decrease in GLUT4 mRNA expression at 48 and 72 h after the hormonal stimulus compared with those cultures without rSAA. Oil red O elution after cell staining, which was conducted during the differentiation process (Figure 4a), revealed that the formation of lipid droplets, a marker for adipocyte differentiation, was significantly decreased in rSAA-treated cells (0.9395±0.07) compared with control cells (1.056±0.054). However, the glycerol measurements from the supernatants of cell cultures were not altered under either conditions (control and rSAA-treated cells), which suggests that the decreased lipid deposition in cells treated with rSAA was caused by the inhibition of differentiation and not subsequent lipolysis (Figure 4b). These data were consistent with those shown in the microscopic analysis of lipid droplet deposition at day 7 of differentiation in control cells (Figure 4c) and rSAA-treated cells (Figure 4d).
Figure 3.
The effect of rSAA on 3T3-L1 preadipocyte differentiation. Quantitative real-time PCR was performed to assess the mRNA expression of (a) C/EBPβ, (b) GLUT4, (c) C/EBPα, (d) PPARγ2, (e) perilipin and (f) FABP4. Cells were treated with a hormonal stimulus in the presence (○) or absence (▪) of rSAA. Data are the mean±s.e. of three independent experiments and two-way analysis of variance was performed (**P<0.01, ***P<0.001 vs control groups).
Figure 4.
The effect of rSAA on adipocyte differentiation and metabolism. (a) Oil red O elution in 3T3-L1 cells treated with a hormonal stimulus in the presence (white bars) or absence (black bars) of rSAA at the indicated times. (b) Glycerol release into the culture medium under the same conditions that are described above. Oil red O staining at day 7 of differentiation (c) in the absence or (d) presence of rSAA (5 μg ml−1). (e) Glycerol release after a 24-h treatment with rSAA in fully differentiated adipocytes. (f) 2-deoxy-[1,2-3H]-glucose uptake under basal (black bars) and insulin-stimulated (white bars) conditions. Data are the mean±s.e. of three independent experiments, and two-way analysis of variance was performed in a, b and f and one-way analysis of variance was performed in e (*P<0.05, ***P<0.001 vs control).
rSAA increased lipolysis and decreased glucose transport
The differentiated 3T3-L1 cells were incubated with different concentrations of rSAA (1, 5 and 10 μg ml−1) for 24 h. After the 24 h of rSAA stimulation at the indicated concentrations, glycerol release was measured. Figure 4e showed that rSAA increased glycerol release in the cell-free supernatant of cultures. Furthermore, glucose uptake was determined under basal conditions (without insulin) and stimulated conditions (100 nM insulin). The 24-h incubation with rSAA at 5 and 10 μg ml−1 decreased glucose uptake in insulin-stimulated 3T3-L1 cells (Figure 4f).
rSAA induced secretion of cytokines and expression of SAA3
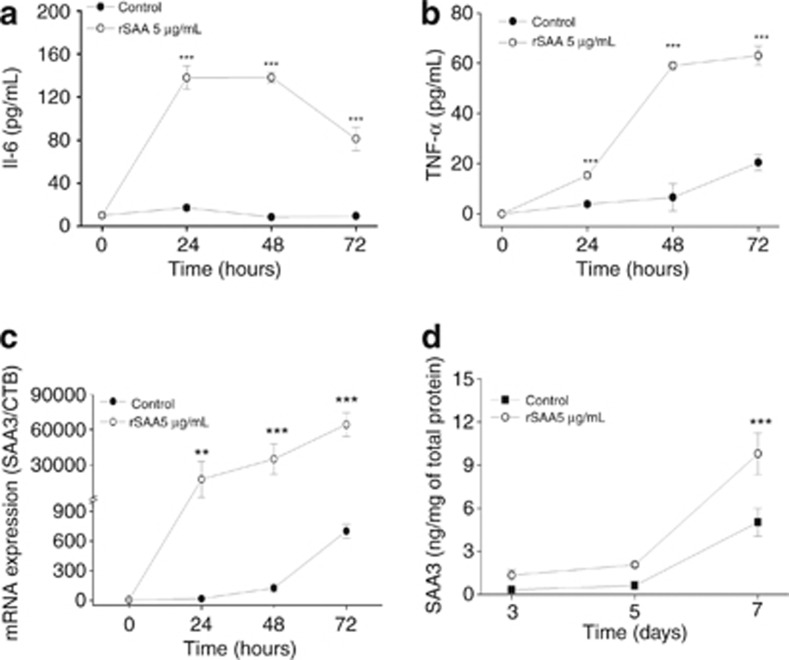
When the hormonal stimulus to induce differentiation was added, 3T3-L1 cells released cytokines, especially TNF-α. This release was determined by testing the supernatant of cultures using an ELISA (Figures 5a and b). The addition of rSAA induced a nine-fold and three-fold increase in interleukin 6 and TNF-α release, respectively, in 3T3-L1 cells at day 3 of differentiation. The SAA3 mRNA expression and protein synthesis were quantified during 3T3-L1 differentiation in the presence or absence of rSAA. SAA3 is usually expressed and produced during differentiation. The presence of rSAA caused a significant increase in SAA3 gene expression (almost 90-fold) (Figure 5c) and a modest increase in SAA3 protein synthesis (almost 2-fold) (Figure 5d).
Figure 5.
Cytokine release and SAA3 production during the course of differentiation. Using an ELISA assay, (a) interleukin 6 (IL-6) and (b) TNF-α release were assessed using the supernatant of cultured 3T3-L1 cells that underwent differentiation in the absence (•) or presence (○) of rSAA (5 μg ml−1). (c) The effect of rSAA on the expression of SAA3 in cells treated with hormonal induction (▪) or with rSAA (5 μg ml−1) and hormonal induction (○). (d) SAA3 protein production assessed by ELISA assay in the absence (•) or presence (○) of rSAA (5 μg/ml). Data are the mean±s.e. of three independent experiments and two-way analysis of variance was performed (**P<0.01, ***P<0.001 vs control).
Discussion
In the current study, we reported that SAA had a strong influence on 3T3-L1 proliferation, differentiation and metabolism. SAA effectively increased the proliferation of 3T3-L1 preadipocytes in a dose-dependent manner. The cell cycle analysis showed an increased number of cells in the S phase, which is consistent with the results of the thymidine incorporation assay. Moreover, SAA increased cell viability that was verified by a marked inhibition of necrosis under the same conditions. A similar biological effect of SAA on cell proliferation was previously observed in Swiss 3T3 fibroblasts19 and two glioma cell lines;28 these results support a growth factor-like activity of SAA. SAA activity is dependent on its concentration and its intended cell. We reported here that SAA induced 3T3-L1 cell proliferation and inhibited cell death. This is an entirely unique contribution on the role of SAA in adiposity.
The signaling involved in SAA-induced proliferation is not well-known, and may involve multiple pathways. Although a signaling study was not our focus, we generated data showing the involvement of ERK1/2 on SAA-induced proliferation by using pharmacological inhibitors of the main signaling pathways involved in cell survival and proliferation.29, 30 However, we were unable to elucidate the roles of the PI3 K and p38MAPK pathways in our system. Although pretreatment with their specific inhibitors (wortmannin and SB203580, respectively) attenuated SAA-induced proliferation, the inhibitors also decreased the proliferation in control cells.
3T3-L1 cells were exposed to insulin (100 mM) and then subjected to a proliferation assay that included the SAA and wortmannin conditions described above. It is known that insulin-induced proliferation is mediated by the PI3K signaling pathway and uses the IGF-I receptor.29, 31, 32 Our current study using wortmannin was noteworthy because the inhibitory effect of wortmannin on SAA-induced proliferation was equivalent to its inhibitory effect on insulin-treated cells. SAA and insulin had an additive effect on 3T3-L1 proliferation, which suggests that insulin and SAA exert mitogenic effects on 3T3-L1 cells that are mediated by both the ERK1/2 and PI3K signaling pathways.
FPR2 is a putative receptor for SAA present in adipocytes and is involved in the activation of MAPK.33 However, our results imply that FPR2 is not involved in SAA-induced proliferation because the effects elicited by SAA on 3T3-L1 cells were not sensitive to pertussis toxin. Given the myriad effects triggered by SAA, it is likely that multiple receptors are involved in the biological effects described here. Adipocytes contain many of the SAA-sensitive receptors, for example, CD36,34 selenoprotein S (SELS)35, TLR4,17 and FPR2.33
Although SAA appeared to be a potent mitogenic factor for adipocytes, SAA inhibited differentiation. The differentiation process is regulated by a network of transcription factors and adipocyte marker genes.36, 37 The exposure of 3T3-L1 cells to a hormonal stimulus caused differentiation. The induction of gene expression peaked at around 2 to 3 days and was followed by intracellular lipid accumulation, which was easily observed after 5 days of treatment. The addition of SAA caused a remarkable change in the expression profile of the adipogenic genes C/EBPβ, C/EBPα, PPARγ2, perilipin, FABP4, GLUT-4 and C/EBPα. These genes are the essential transcriptional regulators of adipogenesis and are targets of other adipogenic inhibitors.21 Additionally, SAA prevented intracellular lipid deposition, although the glycerol release from 3T3-L1 cells during differentiation was not altered. The decrease in lipid accumulation may have been caused by the loss of adipogenic capacity or decreased glucose uptake rather than lipolysis.
Furthermore, production of the cytokines TNF-α and interleukin 6 during the differentiation of 3T3-L1 cells was demonstrated in the current study. The presence of SAA caused the pronounced release of these cytokines. We have previously demonstrated that SAA is a potent stimulus for the release of several cytokines from immune cells14, 16, 38 and that this protein is a potent inflammatory stimulus. The current study presents the first evidence that SAA induces cytokines in cells other than immune cells.
In previous studies using fully differentiated adipocytes, secreted TNF-α stimulated lipolysis and inhibited lipogenesis, which elevated the high free fatty acid concentration in the culture medium.6, 39, 40 Our data suggest that SAA by itself or SAA-induced TNF-α promoted lipolysis. The possible involvement of TNF-α in insulin resistance has been suggested in a number of studies. TNF-α increases plasma triglycerides and very low-density lipoprotein concentrations,41, 42 as well as lipolysis in mouse, rat and human adipocytes.40, 43, 44, 45
Although the mechanism by which SAA promotes lipolysis is unknown, one reasonable possibility is the decrease in perilipin expression, a lipid droplet-associated protein that acts as a protective coating against lipases. Additionally, analysis of GLUT4 gene expression revealed that SAA caused a decrease in the mRNA levels observed at 48 and 72 h of differentiation. This finding was consistent with results from the glucose uptake assay, which was performed in fully differentiated adipocytes and showed that a decrease in glucose uptake occurred under insulin-stimulated conditions. These results show that SAA may contribute to insulin resistance in adipose tissue during the inflammatory state. In previous study using fully differentiated adipocytes, SAA attenuated cellular insulin sensitivity, upregulated the level of phosphor-JNK, and downregulated the level of phosphotyrosine-IRS-1.23 Our data support these observations and also demonstrate the role of SAA in different stages of the adipogenic process, including the final step of differentiation with reduced glucose-uptake capacity.
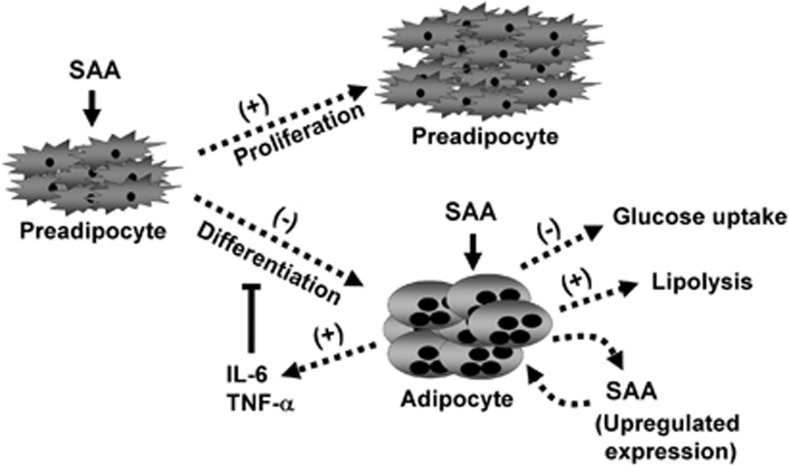
Data from the current study support a role for SAA in the pathogenesis of obesity through the increase in preadipocyte proliferation and inhibition of the differentiation process. In differentiated adipocytes, SAA also impairs glucose metabolism, which may contribute to insulin resistance. Curiously, cells treated with SAA displayed an increase in the expression of SAA3 by almost two orders of magnitude. These results may represent a positive feedback loop that maintains the inflammatory condition (Figure 6).
Figure 6.
The influence of SAA on adipose cells. Adipocytes may be affected by SAA, which is produced by the liver during inflammatory processes and/or synthesized by adipose tissue. SAA alters the proliferation, differentiation and metabolism of adipocytes and contributes to the inflammatory state of adipose tissue.
Acknowledgments
We thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Brazil), CAPES and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Brazil) for the financial support.
The authors declare no conflict of interest.
References
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose-tissue expression of tumor-necrosis-factor-alpha in human obesity and insulin-resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- Scheja L, Heese B, Zitzer H, Michael MD, Siesky AM, Pospisil H, et al. Acute-phase serum amyloid a as a marker of insulin resistance in mice. Exp Diabetes Res. 2008;2008:230837. doi: 10.1155/2008/230837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkamaki A, Ueki K, Kahn CR. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest. 1999;103:931–943. doi: 10.1172/JCI6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, et al. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. Plos Medicine. 2006;3:884–894. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoholm K, Lundgren M, Olsson M, Eriksson JW. Association of serum amyloid A levels with adipocyte size and serum levels of adipokines: differences between men and women. Cytokine. 2009;48:260–266. doi: 10.1016/j.cyto.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Urieli-Shoval S, Linke RP, Matzner Y. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. 2000;7:64–69. doi: 10.1097/00062752-200001000-00012. [DOI] [PubMed] [Google Scholar]
- Jernas M, Palming J, Sjoholm K, Jennische E, Svensson PA, Gabrielsson BG, et al. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. Faseb Journal. 2006;20:1540. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- Poitou C, Viguerie N, Cancello R, De Matteis R, Cinti S, Stich V, et al. Serum amyloid A: production by human white adipocyte and regulation by obesity and nutrition. Diabetologia. 2005;48:519–528. doi: 10.1007/s00125-004-1654-6. [DOI] [PubMed] [Google Scholar]
- Poitou C, Coussieu C, Rouault C, Coupaye M, Cancello R, Bedel JF, et al. Serum amyloid A: a marker of adiposity-induced low-grade inflammation but not of metabolic status. Obesity. 2006;14:309–318. doi: 10.1038/oby.2006.40. [DOI] [PubMed] [Google Scholar]
- Sommer G, Weise S, Kralisch S, Scherer PE, Lossner U, Bluher M, et al. The adipokine SAA3 is induced by interleukin-1 beta in mouse adipocytes. J Cell Biochem. 2008;104:2241–2247. doi: 10.1002/jcb.21782. [DOI] [PubMed] [Google Scholar]
- Lin Y, Rajala MW, Berger JP, Moller DE, Barzilai N, Scherer PE. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J Biol Chem. 2001;276:42077–42083. doi: 10.1074/jbc.M107101200. [DOI] [PubMed] [Google Scholar]
- Furlaneto CJ, Campa A. A novel function of serum amyloid A: a potent stimulus for the release of tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 by human blood neutrophil. Biochem Biophys Res Commun. 2000;268:405–408. doi: 10.1006/bbrc.2000.2143. [DOI] [PubMed] [Google Scholar]
- Hatanaka E, Furlaneto CJ, Ribeiro FP, Souza GM, Campa A. Serum amyloid A-induced mRNA expression and release of tumor necrosis factor-alpha (TNF-alpha) in human neutrophils. Immunol Lett. 2004;91:33–37. doi: 10.1016/j.imlet.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Ribeiro FP, Furlaneto CJ, Hatanaka E, Ribeiro WB, Souza GM, Cassatella MA, et al. mRNA expression and release of interleukin-8 induced by serum amyloid A in neutrophils and monocytes. Mediators Inflamm. 2003;12:173–178. doi: 10.1080/0962935031000134897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri S, Rodriguez D, Gomes E, Monteiro HP, Russo M, Campa A. Is serum amyloid A an endogenous TLR4 agonist. J Leukoc Biol. 2008;83:1174–1180. doi: 10.1189/jlb.0407203. [DOI] [PubMed] [Google Scholar]
- Hatanaka E, Monteagudo PT, Marrocos MSM, Campa A. Interaction between serum amyloid A and leukocytes - A possible role in the progression of vascular complications in diabetes. Immunol Lett. 2007;108:160–166. doi: 10.1016/j.imlet.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Hatanaka E, Dermargos A, Armelin HA, Curi R, Campa A. Serum amyloid A induces reactive oxygen species (ROS) production and proliferation of fibroblast. Clin Exp Immunol. 2011;163:362–367. doi: 10.1111/j.1365-2249.2010.04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SR. Regulation of PPAR gamma activity during adipogenesis. Int J Obes. 2005;29:S13–S16. doi: 10.1038/sj.ijo.0802907. [DOI] [PubMed] [Google Scholar]
- Hamm JK, Park BH, Farmer SR. A role for C/EBP beta in regulating peroxisome proliferator-activated receptor gamma activity during adipogenesis in 3T3-L1 preadipocytes. J Biol Chem. 2001;276:18464–18471. doi: 10.1074/jbc.M100797200. [DOI] [PubMed] [Google Scholar]
- Kawai M, Sousa KM, MacDougald OA, Rosen CJ. The many facets of PPAR gamma: novel insights for the skeleton. Am J Physiol Endocrinol Metab. 2010;299:E3–E9. doi: 10.1152/ajpendo.00157.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye XY, Xue YM, Sha JP, Li CZ, Zhen ZJ. Serum amyloid A attenuates cellular insulin sensitivity by increasing JNK activity in 3T3-L1 adipocytes. J Endocrinol Invest. 2009;32:568–575. doi: 10.1007/BF03346510. [DOI] [PubMed] [Google Scholar]
- Moon HS, Chung CS, Lee HG, Kim TG, Choi YJ, Cho CS. Inhibitory effect of (-)-epigallocatechin-3-gallate on lipid accumulation of 3T3-L1 cells. Obesity. 2007;15:2571–2582. doi: 10.1038/oby.2007.309. [DOI] [PubMed] [Google Scholar]
- Zou C, Shen Z. One-step intracellular triglycerides extraction and quantitative measurement in vitro. J Pharmacol Toxicol Methods. 2007;56:63–66. doi: 10.1016/j.vascn.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Hsu HF, Tsou TC, Chao HR, Shy CG, Kuo YT, Tsai FY, et al. Effects of arecoline on adipogenesis, lipolysis, and glucose uptake of adipocytes-A possible role of betel-quid chewing in metabolic syndrome. Toxicol Appl Pharmacol. 2010;245:370–377. doi: 10.1016/j.taap.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Knebel FH, Albuquerque RC, Maria-Engler SS, Campa A.Dual effect of serum amyloid A protein on gliomas Inflamm Res 201160195–195.20924638 [Google Scholar]
- Cleveland-Donovan K, Maile LA, Tsiaras WG, Tchkonia T, Kirkland JL, Boney CM. IGF-I activation of the AKT pathway is impaired in visceral but not subcutaneous preadipocytes from obese subjects. Endocrinology. 2010;151:3752–3763. doi: 10.1210/en.2010-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews LC, Taggart MJ, Westwood M. Modulation of caveolin-1 expression can affect signalling through the phosphatidylinositol 3-kinase/Akt pathway and cellular proliferation in response to insulin-like growth factor I. Endocrinology. 2008;149:5199–5208. doi: 10.1210/en.2007-1211. [DOI] [PubMed] [Google Scholar]
- Benito M, Valverde AM, Lorenzo M. IGF-I: A mitogen also involved in differentiation processes in mammalian cells. Int J Biochem Cell Biol. 1996;28:499–510. doi: 10.1016/1357-2725(95)00168-9. [DOI] [PubMed] [Google Scholar]
- Gagnon A, Dods P, Roustan-Delatour N, Chen CS, Sorisky A. Phosphatidylinositol-3,4,5-trisphosphate is required for insulin-like growth factor 1-mediated survival of 3T3-L1 preadipocytes. Endocrinology. 2001;142:205–212. doi: 10.1210/endo.142.1.7902. [DOI] [PubMed] [Google Scholar]
- Lee HY, Kim SD, Shim JW, Kim HJ, Yun J, Baek SH, et al. A pertussis toxin sensitive G-protein-independent pathway is involved in serum amyloid A-induced formyl peptide receptor 2-mediated CCL2 production. ExpMol Med. 2010;42:302–309. doi: 10.3858/emm.2010.42.4.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranova IN, Vishnyakova TG, Bocharov AV, Kurlander R, Chen ZG, Kimelman ML, et al. Serum amyloid a binding to CLA-1 (CD36 and LIMPII analogous-1) mediates serum amyloid A protein-induced activation of ERK1/2 and p38 mitogen-activated protein kinases. J Biol Chem. 2005;280:8031–8040. doi: 10.1074/jbc.M405009200. [DOI] [PubMed] [Google Scholar]
- Du JL, Sun CK, Lü B, Men LL, Yao JJ, An LJ, et al. Association of SelS mRNA expression in omental adipose tissue with Homa-IR and serum amyloid A in patients with type 2 diabetes mellitus. Chin Med J. 2008;121:1165–1168. [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nature Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- Sandri S, Hatanaka E, Franco AG, Pedrosa AMC, Monteiro HP, Campa A. Serum amyloid A induces CCL20 secretion in mononuclear cells through MAPK (p38 and ERK1/2) signaling pathways. Immunol Lett. 2008;121:22–26. doi: 10.1016/j.imlet.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Guilherme A, Tesz GJ, Guntur KVP, Czech MP. Tumor necrosis factor-alpha induces caspase-mediated cleavage of peroxisome proliferator-activated receptor gamma in adipocytes. J Biol Chem. 2009;284:17082–17091. doi: 10.1074/jbc.M809042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza SC, de Vargas LM, Yamamoto MT, Lien P, Franciosa MD, Moss LG, et al. Overexpression of perilipin A and B blocks the ability of tumor necrosis factor alpha to increase lipolysis in 3T3-L1 adipocytes. J Biol Chem. 1998;273:24665–24669. doi: 10.1074/jbc.273.38.24665. [DOI] [PubMed] [Google Scholar]
- Lang CH, Dobrescu C, Bagby GJ. Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology. 1992;130:43–52. doi: 10.1210/endo.130.1.1727716. [DOI] [PubMed] [Google Scholar]
- Peraldi P, Spiegelman B. TNF-alpha and insulin resistance: summary and future prospects. Mol Cell Biochem. 1998;182:169–175. [PubMed] [Google Scholar]
- Langin D, Arner P. Importance of TNF alpha and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol Metabol. 2006;17:314–320. doi: 10.1016/j.tem.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Arner P, Caro JF, Atkinson RL, Hotamisligil GS. Elevation of adipose-tissue expression of TNF-alpha and TNF receptors in human obesity and insulin-resistance. Diabetes. 1995;44:A45–A45. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassaux G, Negrel R, Ailhaud G, Gaillard D. Proliferation and differentiation of rat adipose precursor cells in chemically-defined medium - differential action of anti-adipogenic agents. J Cell Physiol. 1994;161:249–256. doi: 10.1002/jcp.1041610209. [DOI] [PubMed] [Google Scholar]