Abstract
AIM: To determine whether expression of certain enzymes related to 5-fluorouracil (5-FU) metabolism predicts 5-FU chemosensitivity in cholangiocarcinoma (CCA).
METHODS: The histoculture drug response assay (HDRA) was performed using surgically resected CCA tissues. Tumor cell viability was determined morphologically with hematoxylin and eosin- and terminal deoxynucleotide transferase-mediated dUTP nick-end labeling-stained tissues. The mRNA expression of thymidine phosphorylase (TP), orotate phosphoribosyl transferase (OPRT), thymidylate synthase (TS), and dihydropyrimidine dehydrogenase (DPD) was determined with real-time reverse transcriptase-polymerase chain reaction. The levels of gene expression and the sensitivity to 5-FU were evaluated.
RESULTS: Twenty-three CCA tissues were obtained from patients who had been diagnosed with intrahepatic CCA and who underwent surgical resection at Srinagarind Hospital, Khon Kaen University from 2007 to 2009. HDRA was used to determine the response of these CCA tissues to 5-FU. Based on the dose-response curve, 200 μg/mL 5-FU was selected as the test concentration. The percentage of inhibition index at the median point was selected as the cut-off point to differentiate the responding and non-responding tumors to 5-FU. When the relationship between TP, OPRT, TS and DPD mRNA expression levels and the sensitivity of CCA tissues to 5-FU was examined, only OPRT mRNA expression was significantly correlated with the response to 5-FU. The mean expression level of OPRT was significantly higher in the responder group compared to the non-responder group (0.41 ± 0.25 vs 0.22 ± 0.12, P < 0.05).
CONCLUSION: OPRT mRNA expression may be a useful predictor of 5-FU chemosensitivity of CCA. Whether OPRT mRNA could be used to predict the success of 5-FU chemotherapy in CCA patients requires confirmation in patients.
Keywords: Histoculture drug response assay, 5-fluorouracil, Cholangiocarcinoma, Orotate phosphoribosyl transferase, Chemosensitivity
INTRODUCTION
Cholangiocarcinoma (CCA), a bile duct epithelial tumor, has poor prognosis owing to the absence of an early diagnostic method and effective treatments. The only curative therapy is surgical resection, but most CCA patients are diagnosed at an unresectable stage. Therefore, chemotherapy is the only practical treatment[1].
5-fluorouracil (5-FU) is one of the most common anticancer agents and is used to treat a variety of solid tumors. 5-FU alone or 5-FU-based regimens are widely used to treat CCA patients. As reviewed by Thongprasert[2], the overall response rate and median survival time following treatment with 5-FU for CCA are 10% and 6.5 mo, respectively. In addition, a combination of 5-FU and leucovorin yields a response rate of 32% and a median survival time of 6 mo[2]. Combination therapy of 5-FU with cisplatin consistently yields response rates of 10%-40%, and median survival times are better than those observed with 5-FU alone[2]. Combinations of 5-FU with taxanes or etoposide, however, have not shown convincing superiority over 5-FU alone for CCA treatment[3,4]. Thus, data obtained from these clinical studies revealed relatively poor response rates of CCA to 5-FU-based regimens.
After entering cells, 5-FU is converted to 5-fluorodeoxyuridine monophosphate (FdUMP) through intermediary molecules by thymidine phosphorylase (TP) and orotate phosphoribosyl transferase (OPRT)[5-7]. FdUMP then forms a complex with thymidylate synthase (TS), leading to inhibition of DNA synthesis[5]. In addition, 5-FU can be phosphorylated by OPRT to form 5-fluorouridine monophosphate, and then to 5-fluorouridine triphosphate, which is subsequently incorporated into RNA, resulting in RNA dysfunction[8]. In the degradation pathway, 5-FU is metabolized by dihydropyrimidine dehydrogenase (DPD) to an inactive metabolite, 5-fluoro-dihydrouracil, and subsequently excreted in the urine[9]. The resistance of several cancer types to 5-FU may be due to alterations in the expression of several genes that are involved in the metabolism and action of this drug[10]. Moreover, the expression and activities of these enzymes have been proposed as markers to predict the response to 5-FU of several cancers such as gastric cancers and metastatic colorectal cancer[11-14].
Because 5-FU is the main chemotherapeutic agent for treatment of CCA, elucidating the molecular mechanism involved in the response to 5-FU may be useful for the treatment of CCA. In the present study, we examined the correlation between mRNA expression of target genes involved in 5-FU metabolism and the chemosensitivity of cancer tissues to 5-FU in 23 CCA patients.
MATERIALS AND METHODS
Chemicals
Hank’s balanced salt solution (HBSS), RPMI 1640 medium, fetal bovine serum, penicillin, and streptomycin were purchased from GIBCO BRL (Grand Island, NY, United States). Collagen gel sponges were purchased from Pharmacia and Upjohn (Kalamazoo, MI, United States). 5-FU was provided by Fresenius Kabi Oncology Ltd. (Hayarna, India). The DeadEnd Colorimetric Terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) system was purchased from Promega (Madison, WI, United States). TRIzol reagent was purchased from Invitrogen (Carlsbad, CA, United States). The TaqMan® gene expression assay kit and TaqMan Universal polymerase chain reaction (PCR) master mix with AmpErase UNG were purchased from Applied Biosystems (Foster City, CA, United States).
CCA tissues
Twenty-three samples of CCA tissues were obtained from patients who had been diagnosed with intrahepatic CCA and who underwent surgical resection at the Department of Surgery, Srinagarind Hospital, Khon Kaen University between 2007 and 2009. The histological types of the CCA tissues were classified according to the World Health Organization classification. Of these patients, 69.6% were men. The median age of the CCA patients was 61 years (range: 42-70 years). Written informed consent was obtained from all patients before the collection of tumor tissues. The study protocol was approved by the Khon Kaen Ethics Committee for Human Research, Khon Kaen University, Thailand (HE500501).
Histoculture drug response assay
After surgery, the tumor tissues were immediately transferred to the laboratory in HBSS containing 100 IU/mL penicillin and 100 μg/mL streptomycin. Histoculture drug response assay (HDRA) was performed as described[15] with some modifications. Cubes of collagen gel sponge (1 cm3) were immersed in 1 mL RPMI 1640 containing 20% fetal bovine serum, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 5-FU at final concentrations of 100, 200, and 400 μg/mL in a 24-well plate. After washing six times in HBSS, the tumor tissues were aseptically cut into small pieces using biopsy punches (3 mm diameter), placed on the collagen gel sponges, and cultured at 37 °C for 4 d in a 5% CO2 atmosphere. Duplicate tissue cultures were performed for each drug concentration. Wells containing culture medium without 5-FU were used as controls.
After 4 d of culture, the viability of tumor cells in the cultured tissues was examined with histology. Hematoxylin and eosin (HE) and TUNEL staining were used to assess cell viability[15]. In brief, tissues were fixed in 4% formaldehyde and embedded in paraffin, and 4-μm tissue sections were cut. Deparaffinized sections were rehydrated, stained with HE and TUNEL, and examined under a microscope. In situ TUNEL was carried out according to the manufacturer’s instructions. TUNEL-positive cells were quantified in at least four high-power fields (× 40) of randomly selected tissue sections. The total live tumor cells showing anaplastic characteristics with hyperchromatic nuclei/cytoplasm were counted and scored as the percent of the total tumor cells. TUNEL-stained tumor cells were identified as dead cells. The efficacy of 5-FU was calculated and expressed as the inhibition index (% I.I.) using the following formula: % I.I. = (1 - % living tumor cells in 5-FU-treated tumor tissue/% living tumor cells in control tissue) × 100. The % I.I.s at various concentrations of 5-FU ranging from doses of 100-400 μg/mL were determined.
Determination of mRNA expression
The mRNA expression of target genes including those encoding TS, DPD, TP, OPRT and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in CCA tissues was determined by reverse transcription and quantitative real-time PCR. In brief, total RNA of each CCA tissue was isolated using TRIzol reagent according to the manufacturer’s protocol. Reverse transcription was performed as described[16].
The mRNA expression of the target gene was determined using the TaqMan® gene expression assay kit according to the manufacturer’s instructions. Real-time PCR was performed in 20-μL PCR reactions containing TaqMan Universal PCR master mix, target-specific primers, one TaqMan® MGB FAM™ dye-labeled probe, and 50 ng cDNA. Each PCR was carried out in duplicate. The PCR conditions were 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. All data were analyzed using the ABI PRISM 7500 Real-time PCR system sequence detection software v.1.4 (Applied Biosystems). The quantity of target cDNA or GAPDH PCR product was calculated using the corresponding standard curve, and the amount of target cDNA in a given sample was normalized to that of GAPDH cDNA.
Statistical analysis
The Student’s t-test was used to compare the % I.I. values between the responder and non-responder groups.
RESULTS
Microscopic examination of CCA tissues after HDRA
HE- and TUNEL-stained sections of control and 5-FU treated CCA tissues are shown in Figure 1. After 4 d of culture on the collagen gel sponge, the CCA tissue architecture including cell-to-cell contact was well maintained. Most of the tumor cells were alive and showed anaplastic characteristics with hyperchromatic nuclei/cytoplasm (short arrows). On the other hand, cells with eosinophilic cytoplasm, shrunken (condensed) nuclei (pyknotic nuclei), and fragmented nuclei (karyorrhexis, indicated by long arrows) were found in 5-FU-treated cells. TUNEL-stained tumor cells were identified as dead cells. The mean percentage of living tumor cells in control tissues at day 4 was 83.3% ± 14.2% of those observed at day 0. In addition, the proportion of viable tumor cells in control condition gradually decreased on days 5, 6 and 7 of culture; therefore HDRA of CCA tissues was performed for 4 d.
Figure 1.
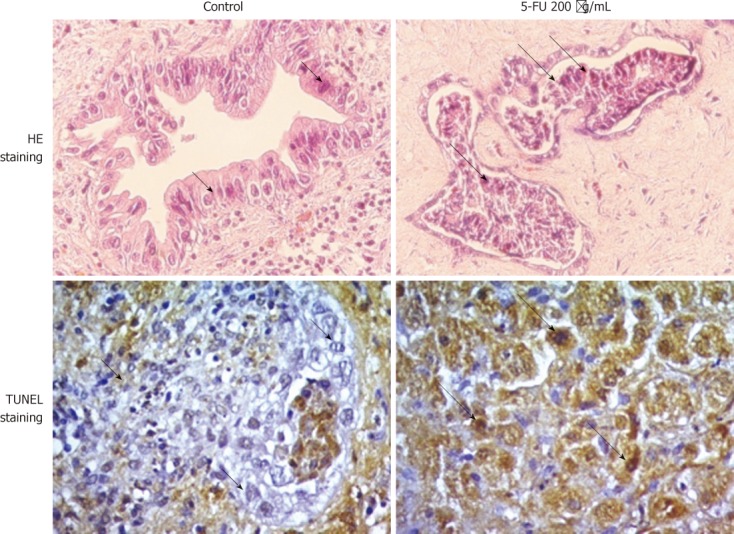
Photomicrographs (40 ×) of hematoxylin and eosin-stained and terminal deoxynucleotide transferase-mediated dUTP nick-end label-stained sections of cholangiocarcinoma tissues cultured on collagen gel sponges for 4 d in the absence or presence of 5-fluorouracil. Tumor cells with anaplastic characteristics showing hyperchromatic nuclei/cytoplasm are indicated with short arrows. Cells with eosinophilic cytoplasm with shrunken (condensed) nuclei (pyknotic nuclei) and fragmented nuclei (karyorrhexis) are indicated with long arrows. 5-FU: 5-fluorouracil; HE: Hematoxylin and eosin; TUNEL: Terminal deoxynucleotide transferase-mediated dUTP nick-end label
Response of CCA tissues to 5-FU
The responses of CCA tissues to various concentrations of 5-FU ranging from 100-400 μg/mL were determined using CCA tissues obtained from five patients. Dose-dependent responses of CCA tissues to 5-FU were observed (Figure 2). From these results, a 5-FU concentration of 200 μg/mL was selected as the test concentration. The % I.I. values for 5-FU (200 μg/mL) treatment of 23 CCA tissues are shown in Figure 3. The median % I.I. value was selected as the cut-off to classify CCA tissues as responders or non-responders (Figure 3).
Figure 2.
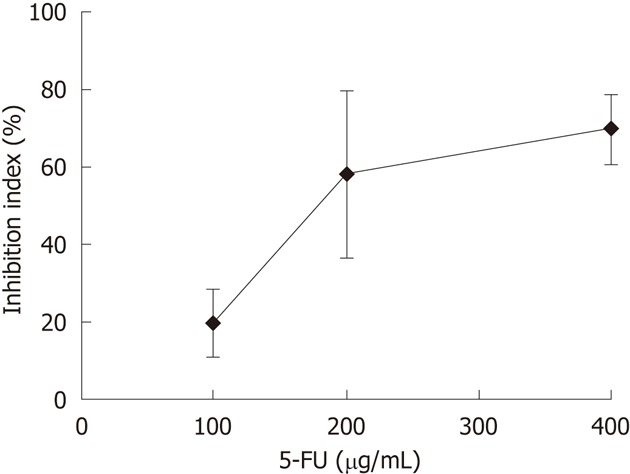
Dose-response-curve of cholangiocarcinoma tissues to 5-fluorouracil. Sensitivities of five cholangiocarcinoma tissues to 5-fluorouracil (5-FU) are shown as the percent of inhibition index (% I.I.), which was evaluated at doses of 100 μg/mL, 200 μg/mL, and 400 μg/mL using histoculture drug response assay. Data are the mean ± SD of two independent experiments.
Figure 3.
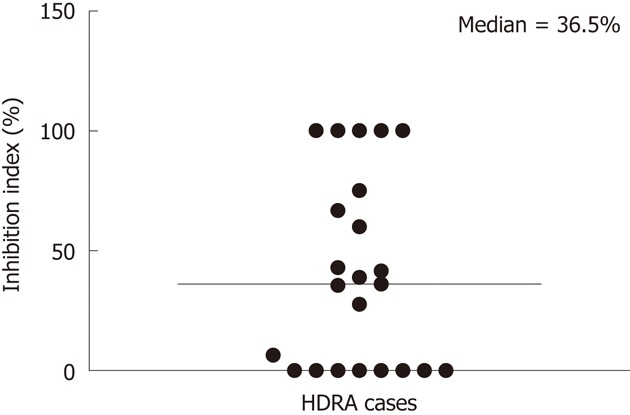
Distribution of percent of inhibition index of 23 cholangiocarcinoma tissues to 200 μg/mL 5-fluorouracil. Sensitivities of 23 cholangiocarcinoma (CCA) tissues to 5-fluorouracil (5-FU) were evaluated at 200 μg/mL 5-FU using histoculture drug response assay (HDRA). The median value of % I.I. (36.5%) was selected as the cut-off for classifying these tissues as responders and non-responders. Each circle represents one CCA sample. The median is shown by the long, thin horizontal line.
Relationship between the expression of target genes and the sensitivity to 5-FU
For each CCA tissue, mRNA expression was quantified with real-time PCR using specific TaqMan probes for genes encoding enzymes involved in the 5-FU metabolic pathway, including TS, DPD, TP and OPRT. Moderate variability in the expression levels of TS, TP and OPRT mRNA normalized to GAPDH expression among individual samples was observed (24-fold, range: 0.05-1.22; 33-fold, range: 0.13-4.27, and 17-fold, range: 0.06-1.02, respectively), whereas high variability was observed for DPD expression (135-fold, range: 0.05-6.76).
Scattered, overlapping expression levels of these genes were observed in the responder and non-responder groups. However, the mean expression level of OPRT was significantly higher in the responder group compared to the non-responder group (0.41 ± 0.25 vs 0.22 ± 0.12, P < 0.05, Figure 4). The mean expression levels of TS, DPD, and TP appeared higher in the responder group compared to the non-responder group, but the differences were not statistically significant (0.26 ± 0.32 vs 0.18 ± 0.12, P = 0.43; 1.73 ± 1.96 vs 0.74 ± 0.50, P = 0.12; and 1.60 ± 1.16 vs 1.02 ± 0.72, P = 0.16, respectively; Figure 4).
Figure 4.
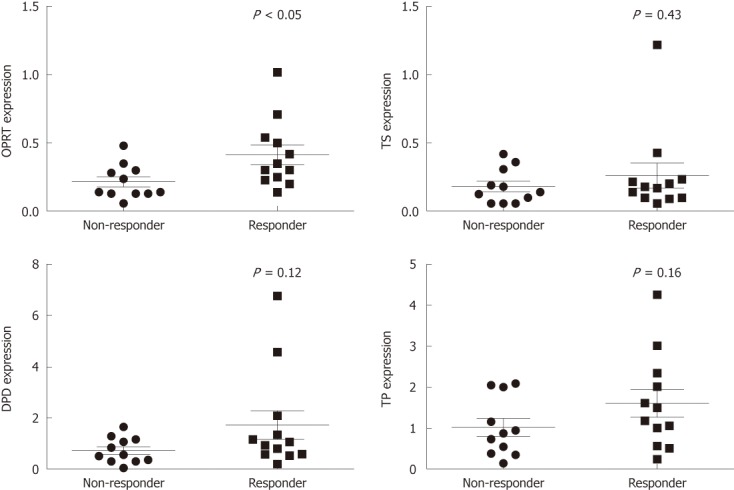
Relationship between chemosensitivity to 5-fluorouracil and mRNA expression of thymidine phosphorylase, orotate phosphoribosyl transferase, thymidylate synthase, and dihydropyrimidine dehydrogenase in 23 cholangiocarcinoma tissues. Chemosensitivity to 5-fluorouracil (5-FU) and mRNA expression were analyzed for 23 cholangiocarcinoma (CCA) tissues using histoculture drug response assay and quantitative real-time reverse transcription-polymerase chain reaction. The responders and non-responders were classified using the median value of the % I.I. as the cut-off point. The mRNA expression of orotate phosphoribosyl transferase (OPRT) was significantly higher in the responder (P < 0.05) group. The mRNA levels of thymidylate synthase (TS), and dihydropyrimidine dehydrogenase (DPD) and thymidine phosphorylase (TP) in the non-responder and responder groups, however, were not significantly different. The long, thin lines represent the mean, and the error bars represent the SD values. Each symbol (circles and squares) represents one CCA tissue sample.
DISCUSSION
5-FU is phosphorylated in cells to become an active metabolite that inhibits DNA synthesis and induces RNA dysfunction[5,8]. Intra-tumoral gene expression and activities of several enzymes related to 5-FU metabolism correlate with sensitivity to this drug for the treatment of several cancers[11-14]. Of the genes we studied, OPRT seems to have predictive power and may be a promising marker. High enzymatic activity of OPRT in tumor tissues is associated with high sensitivity of urinary bladder cancer[17] and colorectal cancer[18] to 5-FU. In addition, OPRT mRNA or the ratio of OPRT/DPD mRNA is associated with prolonged survival of metastatic colon cancer patients receiving oral tegafur-uracil and leucovorin[11] and colorectal liver metastasis patients receiving intra-arterial chemotherapy with 5-FU[19]. In addition, combined expression of OPRT and TS in pre-chemotherapeutic fresh-frozen samples obtained from primary tumors may predict the response to S-1, an oral DPD-inhibiting fluoropyrimidine, in metastatic gastric cancer patients[20]. Consistent with the previous studies, we observed here that intratumoral expression of OPRT mRNA in the responder group was significantly higher than expression in the non-responder group. These results suggest that the OPRT mRNA level in CCA tissues may be a promising predictor of an in vitro sensitivity to 5-FU using the HDRA technique. It should be noted that we observed overlapping expression levels of each gene among responder and non-responder tumors.
We observed moderate variability in the expression levels of TS, TP, and OPRT mRNA among individual samples (24-fold, 33-fold and 17-fold, respectively). The moderate differences in OPRT and TS expression levels observed in the present study were similar to those previously observed in gastric carcinoma and colorectal tissues[21,22]. Similar to the variability reported in colorectal[22] and esophageal carcinoma[23], we observed high variability in the expression of DPD among CCA tissues. Genetic polymorphisms of genes encoding TP, DPD, TS and OPRT are well known[24-27]. Variable expression of DPD, TP, TS and OPRT may explained by genetic polymorphisms in these genes.
TP is the first enzyme involved in the metabolic activation pathway of 5-FU to 5-fluorodeoxyuridine (FUdR), which can be phosphorylated by thymidine kinase to form FdUMP[28]. In this study, no relationship was found between the intratumoral expression of TP mRNA and the sensitivity to 5-FU. In addition to TP, that the rate of conversion of 5-FU to FUdR could be influenced by the availability of the TP cofactor, deoxyribose-1-phosphate (dRib-1-P) in cells[29]. It has been previously demonstrated that the addition of dRib-1-P greatly increases the incorporation of thymidine into DNA and increases the potency of the growth-inhibitory actions of 5-FU[30]. Thus, it may be possible that the amount of dRib-1-P in CCA tissues may be limited. Similar observations have been reported in colorectal tumor that there was no relationship between TP expression and 5-FU sensitivity[31,32].
TS is a key enzyme that catalyzes the methylation of deoxyuridine monophosphate to deoxythymidine monophosphate, an important step in DNA synthesis[33]. Colon cancer patients with high TS expression are reportedly un-responsive to 5-FU and show poor prognosis[34]. In contrast, tumoral expression of TS mRNA is associated with response to protracted infusions of 5-FU-based chemotherapy and survival in patients with disseminated colorectal cancer[35]. Consistent with a report on colorectal tumors[36], no relationship between TS expression and sensitivity to 5-FU in CCA in vitro was observed in our present study.
DPD is a major enzyme in the metabolism of 5-FU to an inactive metabolite. Some reports have suggested a negative correlation between 5-FU sensitivity and DPD activity in human stomach cancer cells[37]. In patients with advanced colorectal cancer treated with 5-FU/leucovorin, patients who responded to the treatment exhibited low levels and a narrow distribution range of DPD mRNA expression compared to the non-responder group[32]. In the present study, DPD mRNA expression was apparently higher in the responders than in the non-responders. However, no clear relationship was found between DPD mRNA expression and 5-FU sensitivity.
In conclusion, we developed HDRA as an in vitro screening of the response of CCA tissues to 5-FU. We found that the level of OPRT mRNA may be a promising predictor of CCA sensitivity to 5-FU. Whether the OPRT mRNA level could be used as a predictor of the success of 5-FU chemotherapy in CCA patients needs to be confirmed further in patients.
ACKNOWLEDGMENTS
The authors sincerely thank Professor Will J, University of Wisconsin, United States and Professor Nawa Y, Visiting Professor, Faculty of Medicine, Khon Kaen University for reviewing the manuscript.
COMMENTS
Background
Cholangiocarcinoma (CCA) has a poor prognosis owing to the absence of effective treatments. Data from clinical studies have revealed that the response rate of CCA to 5-fluorouracil (5-FU) or 5-FU-based regimens is relatively poor. Therefore, a method to identify patients who may benefit from 5-FU is required.
Research frontiers
The histoculture drug response assay (HDRA) was used to determine the response of 23 CCA tissues to 5-FU. By determining the relative expression levels of several genes involved in the action and metabolism of 5-FU, they found that the orotate phosphoribosyl transferase (OPRT) mRNA level was significantly correlated with the response of CCA to 5-FU.
Innovations and breakthroughs
This is the first report to show a relationship between OPRT mRNA expression and the sensitivity of CCA tissues to 5-FU.
Applications
The HDRA may be useful as an in vitro test for determining the sensitivity of CCA tissues to anticancer agents. Based on the results from this study, OPRT mRNA expression may be a useful predictor of the chemosensitivity of CCA to 5-FU.
Peer review
This is a good descriptive study in which authors analyze whether expression of certain enzymes related to 5-FU metabolism predicts 5-FU chemosensitivity in CCA. This is an interesting paper of the basic research data of CCA using HDRA.
Footnotes
Supported by The Research Team Strengthening Grant, National Genetic Engineering and Biotechnology Center, National Science and Technology Development Agency, Thailand; The Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Thailand (to Chaiyagool J)
Peer reviewer: Zenichi Morise, Professor, Department of Surgery, School of Medicine, Fujita Health University, Banbuntane Houtokukai Hospital, 3-6-10 Otobashi Nakagawa-ku, Nagoya 454-8509, Japan
S- Editor Gou SX L- Editor A E- Editor Zheng XM
References
- 1.Gatto M, Alvaro D. New insights on cholangiocarcinoma. World J Gastrointest Oncol. 2010;2:136–145. doi: 10.4251/wjgo.v2.i3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thongprasert S. The role of chemotherapy in cholangiocarcinoma. Ann Oncol. 2005;16 Suppl 2:ii93–ii96. doi: 10.1093/annonc/mdi712. [DOI] [PubMed] [Google Scholar]
- 3.Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13:415–423. doi: 10.1634/theoncologist.2007-0252. [DOI] [PubMed] [Google Scholar]
- 4.Mosconi S, Beretta GD, Labianca R, Zampino MG, Gatta G, Heinemann V. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2009;69:259–270. doi: 10.1016/j.critrevonc.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Pinedo HM, Peters GF. Fluorouracil: biochemistry and pharmacology. J Clin Oncol. 1988;6:1653–1664. doi: 10.1200/JCO.1988.6.10.1653. [DOI] [PubMed] [Google Scholar]
- 6.Peters GJ, Laurensse E, Leyva A, Lankelma J, Pinedo HM. Sensitivity of human, murine, and rat cells to 5-fluorouracil and 5’-deoxy-5-fluorouridine in relation to drug-metabolizing enzymes. Cancer Res. 1986;46:20–28. [PubMed] [Google Scholar]
- 7.Peters GJ, van Groeningen CJ, Laurensse EJ, Pinedo HM. A comparison of 5-fluorouracil metabolism in human colorectal cancer and colon mucosa. Cancer. 1991;68:1903–1909. doi: 10.1002/1097-0142(19911101)68:9<1903::aid-cncr2820680910>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Peters GJ, Backus HH, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J, Calvert AH, Marsh S, et al. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta. 2002;1587:194–205. doi: 10.1016/s0925-4439(02)00082-0. [DOI] [PubMed] [Google Scholar]
- 9.Allegra CJ. Dihydropyrimidine dehydrogenase activity: prognostic partner of 5-fluorouracil? Clin Cancer Res. 1999;5:1947–1949. [PubMed] [Google Scholar]
- 10.Oguri T, Achiwa H, Bessho Y, Muramatsu H, Maeda H, Niimi T, Sato S, Ueda R. The role of thymidylate synthase and dihydropyrimidine dehydrogenase in resistance to 5-fluorouracil in human lung cancer cells. Lung Cancer. 2005;49:345–351. doi: 10.1016/j.lungcan.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Ichikawa W, Uetake H, Shirota Y, Yamada H, Takahashi T, Nihei Z, Sugihara K, Sasaki Y, Hirayama R. Both gene expression for orotate phosphoribosyltransferase and its ratio to dihydropyrimidine dehydrogenase influence outcome following fluoropyrimidine-based chemotherapy for metastatic colorectal cancer. Br J Cancer. 2003;89:1486–1492. doi: 10.1038/sj.bjc.6601335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochiai T, Nishimura K, Noguchi H, Kitajima M, Tsukada A, Watanabe E, Nagaoka I, Futagawa S. Prognostic impact of orotate phosphoribosyl transferase among 5-fluorouracil metabolic enzymes in resectable colorectal cancers treated by oral 5-fluorouracil-based adjuvant chemotherapy. Int J Cancer. 2006;118:3084–3088. doi: 10.1002/ijc.21779. [DOI] [PubMed] [Google Scholar]
- 13.Ma T, Zhu ZG, Ji YB, Zhang Y, Yu YY, Liu BY, Yin HR, Lin YZ. Correlation of thymidylate synthase, thymidine phosphorylase and dihydropyrimidine dehydrogenase with sensitivity of gastrointestinal cancer cells to 5-fluorouracil and 5-fluoro-2’-deoxyuridine. World J Gastroenterol. 2004;10:172–176. doi: 10.3748/wjg.v10.i2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toriumi F, Kubota T, Saikawa Y, Yoshida M, Otani Y, Watanabe M, Kumai K, Kitajima M. Thymidylate synthetase (TS) genotype and TS/dihydropyrimidine dehydrogenase mRNA level as an indicator in determining chemosensitivity to 5-fluorouracil in advanced gastric carcinoma. Anticancer Res. 2004;24:2455–2463. [PubMed] [Google Scholar]
- 15.Seubwai W, Wongkham C, Puapairoj A, Okada S, Wongkham S. 22-oxa-1,25-dihydroxyvitamin D3 efficiently inhibits tumor growth in inoculated mice and primary histoculture of cholangiocarcinoma. Cancer. 2010;116:5535–5543. doi: 10.1002/cncr.25478. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa Y, Kubota T, Otani Y, Watanabe M, Teramoto T, Kumai K, Kitajima M, Takechi T, Okabe H, Fukushima M. Dihydropyrimidine dehydrogenase activity and messenger RNA level may be related to the antitumor effect of 5-fluorouracil on human tumor xenografts in nude mice. Clin Cancer Res. 1999;5:883–889. [PubMed] [Google Scholar]
- 17.Mizutani Y, Wada H, Fukushima M, Yoshida O, Nakanishi H, Li YN, Miki T. Prognostic significance of orotate phosphoribosyltransferase activity in bladder carcinoma. Cancer. 2004;100:723–731. doi: 10.1002/cncr.11955. [DOI] [PubMed] [Google Scholar]
- 18.Isshi K, Sakuyama T, Gen T, Nakamura Y, Kuroda T, Katuyama T, Maekawa Y. Predicting 5-FU sensitivity using human colorectal cancer specimens: comparison of tumor dihydropyrimidine dehydrogenase and orotate phosphoribosyl transferase activities with in vitro chemosensitivity to 5-FU. Int J Clin Oncol. 2002;7:335–342. doi: 10.1007/s101470200051. [DOI] [PubMed] [Google Scholar]
- 19.Matsuyama R, Togo S, Shimizu D, Momiyama N, Ishikawa T, Ichikawa Y, Endo I, Kunisaki C, Suzuki H, Hayasizaki Y, et al. Predicting 5-fluorouracil chemosensitivity of liver metastases from colorectal cancer using primary tumor specimens: three-gene expression model predicts clinical response. Int J Cancer. 2006;119:406–413. doi: 10.1002/ijc.21843. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa W, Takahashi T, Suto K, Shirota Y, Nihei Z, Shimizu M, Sasaki Y, Hirayama R. Simple combinations of 5-FU pathway genes predict the outcome of metastatic gastric cancer patients treated by S-1. Int J Cancer. 2006;119:1927–1933. doi: 10.1002/ijc.22080. [DOI] [PubMed] [Google Scholar]
- 21.Sakurai Y, Sakamoto K, Sugimoto Y, Yoshida I, Masui T, Tonomura S, Inaba K, Shoji M, Nakamura Y, Uyama I, et al. Orotate phosphoribosyltransferase levels measured by a newly established enzyme-linked immunosorbent assay in gastric carcinoma. Cancer Sci. 2006;97:492–498. doi: 10.1111/j.1349-7006.2006.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue T, Hibi K, Nakayama G, Komatsu Y, Fukuoka T, Kodera Y, Ito K, Akiyama S, Nakao A. Expression level of thymidylate synthase is a good predictor of chemosensitivity to 5-fluorouracil in colorectal cancer. J Gastroenterol. 2005;40:143–147. doi: 10.1007/s00535-004-1512-9. [DOI] [PubMed] [Google Scholar]
- 23.Ando T, Ishiguro H, Kuwabara Y, Kimura M, Mitsui A, Sugito N, Mori R, Ogawa R, Katada T, Fujii Y. Relationship between expression of 5-fluorouracil metabolic enzymes and 5-fluorouracil sensitivity in esophageal carcinoma cell lines. Dis Esophagus. 2008;21:15–20. doi: 10.1111/j.1442-2050.2007.00700.x. [DOI] [PubMed] [Google Scholar]
- 24.Maring JG, Groen HJ, Wachters FM, Uges DR, de Vries EG. Genetic factors influencing pyrimidine-antagonist chemotherapy. Pharmacogenomics J. 2005;5:226–243. doi: 10.1038/sj.tpj.6500320. [DOI] [PubMed] [Google Scholar]
- 25.Kindblom LG, Stenman G, Angervall L. Morphological and cytogenetic studies of angiosarcoma in Stewart-Treves syndrome. Virchows Arch A Pathol Anat Histopathol. 1991;419:439–445. doi: 10.1007/BF01605079. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi K, Arai Y, Kanda Y, Akagi K. Germline mutation of dihydropyrimidine dehydrogenese gene among a Japanese population in relation to toxicity to 5-Fluorouracil. Jpn J Cancer Res. 2001;92:337–342. doi: 10.1111/j.1349-7006.2001.tb01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh KW, Kim JH, Kim YB, Kim J, Jeong S. Thymidylate synthase gene polymorphism as a prognostic factor for colon cancer. J Gastrointest Surg. 2005;9:336–342. doi: 10.1016/j.gassur.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 29.Barankiewicz J, Henderson JF. Ribose 1-phosphate metabolism in Ehrlich ascites tumor cells in vitro. Biochim Biophys Acta. 1977;479:371–377. doi: 10.1016/0005-2787(77)90030-2. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz EL, Baptiste N, Megati S, Wadler S, Otter BA. 5-Ethoxy-2’-deoxyuridine, a novel substrate for thymidine phosphorylase, potentiates the antitumor activity of 5-fluorouracil when used in combination with interferon, an inducer of thymidine phosphorylase expression. Cancer Res. 1995;55:3543–3550. [PubMed] [Google Scholar]
- 31.Metzger R, Danenberg K, Leichman CG, Salonga D, Schwartz EL, Wadler S, Lenz HJ, Groshen S, Leichman L, Danenberg PV. High basal level gene expression of thymidine phosphorylase (platelet-derived endothelial cell growth factor) in colorectal tumors is associated with nonresponse to 5-fluorouracil. Clin Cancer Res. 1998;4:2371–2376. [PubMed] [Google Scholar]
- 32.Salonga D, Danenberg KD, Johnson M, Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman L, Diasio RB, et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6:1322–1327. [PubMed] [Google Scholar]
- 33.Johnston PG, Drake JC, Trepel J, Allegra CJ. Immunological quantitation of thymidylate synthase using the monoclonal antibody TS 106 in 5-fluorouracil-sensitive and -resistant human cancer cell lines. Cancer Res. 1992;52:4306–4312. [PubMed] [Google Scholar]
- 34.Allegra CJ, Parr AL, Wold LE, Mahoney MR, Sargent DJ, Johnston P, Klein P, Behan K, O’Connell MJ, Levitt R, et al. Investigation of the prognostic and predictive value of thymidylate synthase, p53, and Ki-67 in patients with locally advanced colon cancer. J Clin Oncol. 2002;20:1735–1743. doi: 10.1200/JCO.2002.07.080. [DOI] [PubMed] [Google Scholar]
- 35.Leichman CG, Lenz HJ, Leichman L, Danenberg K, Baranda J, Groshen S, Boswell W, Metzger R, Tan M, Danenberg PV. Quantitation of intratumoral thymidylate synthase expression predicts for disseminated colorectal cancer response and resistance to protracted-infusion fluorouracil and weekly leucovorin. J Clin Oncol. 1997;15:3223–3229. doi: 10.1200/JCO.1997.15.10.3223. [DOI] [PubMed] [Google Scholar]
- 36.Findlay MP, Cunningham D, Morgan G, Clinton S, Hardcastle A, Aherne GW. Lack of correlation between thymidylate synthase levels in primary colorectal tumours and subsequent response to chemotherapy. Br J Cancer. 1997;75:903–909. doi: 10.1038/bjc.1997.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inaba M, Mitsuhashi J, Sawada H, Miike N, Naoe Y, Daimon A, Koizumi K, Tsujimoto H, Fukushima M. Reduced activity of anabolizing enzymes in 5-fluorouracil-resistant human stomach cancer cells. Jpn J Cancer Res. 1996;87:212–220. doi: 10.1111/j.1349-7006.1996.tb03161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


