Abstract
AIM: To assess the symptomatic efficacy of Lactobacillus plantarum 299v (L. plantarum 299v) (DSM 9843) for the relief of abdominal symptoms in a large subset of irritable bowel syndrome (IBS) patients fulfilling the Rome III criteria.
METHODS: In this double blind, placebo-controlled, parallel-designed study, subjects were randomized to daily receive either one capsule of L. plantarum 299v (DSM 9843) or placebo for 4 wk. Frequency and intensity of abdominal pain, bloating and feeling of incomplete rectal emptying were assessed weekly on a visual analogue scale while stool frequency was calculated.
RESULTS: Two hundred and fourteen IBS patients were recruited. After 4 wk, both pain severity (0.68 + 0.53 vs 0.92 + 0.57, P < 0.05) and daily frequency (1.01 + 0.77 vs 1.71 + 0.93, P < 0.05) were lower with L. plantarum 299v (DSM 9843) than with placebo. Similar results were obtained for bloating. At week 4, 78.1 % of the patients scored the L. plantarum 299v (DSM 9843) symptomatic effect as excellent or good vs only 8.1 % for placebo (P < 0.01).
CONCLUSION: A 4-wk treatment with L. plantarum 299v (DSM 9843) provided effective symptom relief, particularly of abdominal pain and bloating, in IBS patients fulfilling the Rome III criteria.
Keywords: Irritable bowel syndrome, Probiotics, Lactobacillus plantarum 299v, Clinical trial, Abdominal pain
INTRODUCTION
Irritable bowel syndrome (IBS) is one of the most frequent digestive tract disorders encountered by general practitioners and gastroenterologists. IBS is a functional bowel disorder characterized by chronic and relapsing abdominal pain or discomfort associated with altered bowel habits. The primary aim of any treatment is the relief of abdominal pain which can significantly impair the patient’s quality of life. According to published guidelines, the main treatment options for abdominal pain include anti-spasmodics or anti-depressants at low dose while anti-diarrheal or laxative drugs are given to improve transit disturbances[1,2]. However, in many cases, all these options remain disappointing for the relief of abdominal pain. The therapeutic efficacy in IBS is probably impacted by the heterogeneous pathogenesis of the disease which includes altered intestinal motility, visceral hypersensitivity, abnormal brain-gut interactions, food intolerance, altered intestinal permeability and post infectious and/or inflammatory changes[3].
Recently, the deleterious role of qualitative or quantitative alterations of gut microbiota at the onset of symptoms has been emphasized. Therefore, a rationale exists to discuss the therapeutic use of probiotics, which are live microorganisms conferring health benefits to the host when ingested in adequate amounts[4]. Clinical evidence regarding the efficacy of some probiotic strains to improve IBS symptoms has recently emerged[5,6], although the mechanism of action of probiotics on IBS symptoms is not completely understood. Some probiotics bind to small and large bowel epithelium and may produce substances with antibiotic properties, while others compete for attachment and thereby reduce invasion by pathogenic organisms[7]. Probiotics also modulate gastrointestinal luminal immunity by changing the cytokine and cellular milieu from a pro-inflammatory to anti-inflammatory state[8]. They may also convert undigested carbohydrates into short chain fatty acids, which act as nutrients for colonocytes and affect gut motility[4].
Lactobacillus plantarum 299v (L. plantarum 299v) (DSM 9843) is a probiotic strain able to reside in the human colonic mucosa in vivo due to a specific mechanism of mannose adhesion[7]. L. plantarum 299v (DSM 9843) also increases the amount of carboxylic acid, particularly acetic and propionic acids, in the stools of healthy volunteers[9]. The strain has shown antibacterial activity against several potential pathogenic agents such as Listeria monocytogenes, Escherischia coli, Yersinia enterolytica, Enterobacter cloacae and Enterococcus faecalis[10]. L. plantarum 299v (DSM 9843) also has beneficial immunomodulatory activity via an increased interleukin-10 synthesis and secretion in macrophages and T-cells derived from the inflamed colon. And recently, an experimental study reported that L. plantarum 299v (DSM 9843) increased the transcription and excretion of the mucins MUC2 and MUC3 in goblet cells[11,12].
Three single-centre studies have tested the clinical efficacy of L. plantarum 299v (DSM 9843) in IBS patients[13-15]. Two trials have demonstrated significant benefits in comparison with placebo on improvement of flatulence scores[13] and a reduction of abdominal pain[14] while the results of the third trial, based on only 12 patients, were not conclusive. The aim of the present randomized, double-blind, placebo controlled clinical trial was to assess the symptomatic efficacy of L. plantarum 299v (DSM 9843) in a larger subset of IBS patients fulfilling the Rome III criteria.
MATERIALS AND METHODS
Patients
Participants (n = 214) were recruited by general practitioners in four clinical centres in India: one in Mumbai, two in Chennai and one in Bangalore. Subjects between 18-70 years of age with IBS according to the Rome III criteria were eligible for inclusion. All subjects had a colonic examination at baseline to exclude any organic disease while an intestinal infection was excluded by stool cultures in any patient in whom this diagnosis was suspected. Subjects with severe chronic medical disease including colorectal and other gastrointestinal diseases were excluded. Pregnant and breast-feeding women and patients with dietary habits which might interfere with the assessment of the study product or patients with known allergy to the study product components were also excluded. Throughout the study, the subjects were not allowed to consume any other probiotic and were encouraged not to change their usual dietary and physical exercise habits.
Study design
This study was designed as a multicentre double blind, placebo-controlled study with parallel groups to assess the beneficial effects of a daily consumption of L. plantarum 299v (DSM 9843) on IBS symptoms. Treatment duration was 4 wk with 3 follow-up visits at weekly intervals. The study protocol was conducted in accordance with the Declaration of Helsinki and approved by the local Ethics Committee. All volunteers gave written informed consent prior to participation in the study.
Study products
The test product was a probiotic preparation containing a mixture of freeze-dried lactic acid bacteria and excipients. The lactic acid bacteria strain was L. plantarum 299v (DSM 9843). It is deposited at the DSM collection (Deutsche Sammlung von Mikrooorganismen und Zellkulturen GmbH) under number DSM 9843. The test product contained 10 billion colony-forming units (cfu) per capsule in a potato starch and magnesium stearate base. The control product contained potato starch (97%) and magnesium stearate (3%). Both the test and control products had a similar appearance, texture and taste. Both products were specifically prepared for the study and provided by the Rosell-Lallemand Institute (Blagnac, France).
Assessments and study endpoints
The primary endpoint was the improvement of the frequency of abdominal pain episodes. Secondary endpoints were changes in severity of abdominal pain, changes in frequency and severity of abdominal bloating and in feeling of incomplete rectal emptying. Both frequency of abdominal pain and feeling of incomplete rectal emptying were assessed weekly using a four-point scale ranging from 1 (only occasional symptom) to 4 (daily symptom). Symptom severity (abdominal pain, abdominal bloating and feeling of incomplete rectal emptying) was rated on a visual analogue scale (VAS 1-10) and converted to a 4 point scale ranging from 0 (No pain , VAS = 0) to 3 (Severe, VAS = 8 to 10 ).
The daily number of stools and bloating episodes were calculated and registered at each visit. At the end of the 4-wk treatment period, both the patient’s and the practitioner’s opinion about the overall efficacy of the treatment were recorded using a 4-point scale, from “poor” to “excellent”.
Regarding safety assessment, blood samples were taken at baseline and week 4 in each patient for the assessment of blood cell counts, glycaemia, blood urea nitrogen and liver function tests. Physical examinations and verification of any adverse events were performed at each visit.
Sample size and randomization
The sample-size calculation was based on the main outcome, the frequency of abdominal pain episodes. The trial sample size required to detect a significant difference of 20% between the two groups with an 80% power and 5% statistical significance level was calculated to be at least seventy-nine patients per group. Taking into account that all subjects who withdrew prematurely were not replaced, 214 subjects were randomised according to a computer-generated randomization list in the ratio 1:1. For each site, randomization charts were provided to investigators keeping a 1:1 ratio. All investigators, patients and monitors were blinded throughout the study. To ensure allocation concealment, packaging and labelling were performed by a third party, and the randomization code was kept in a secure place during the study.
Statistical analysis
All the analyses of efficacy were performed with full analysis set (FAS) population. The FAS population corresponds to all randomised subjects who took at least one dose of the study drug and who had at least one post-baseline efficacy assessment. Overall assessment of symptoms were analysed using a repeated-measures analysis of variance (ANOVA) with time, treatment group, interaction time x product and baseline score as fixed factors for each period.
RESULTS
The flow chart of the study is given in Figure 1. A total of 214 patients were randomized and 108 subjects assigned to receive L. plantarum 299v (DSM 9843) group and 106 patients the placebo. Among these 214 subjects, 10 were excluded, either because they did not complete the entire 4-wk double-blind period, or because they did not provide any available data about the treatment period. A majority of patients were IBS-D patients, 63.89% and 60.3% in L. plantarum 299v (DSM 9843) and placebo groups, respectively. Baseline characteristics of the two groups are given in Table 1.
Figure 1.
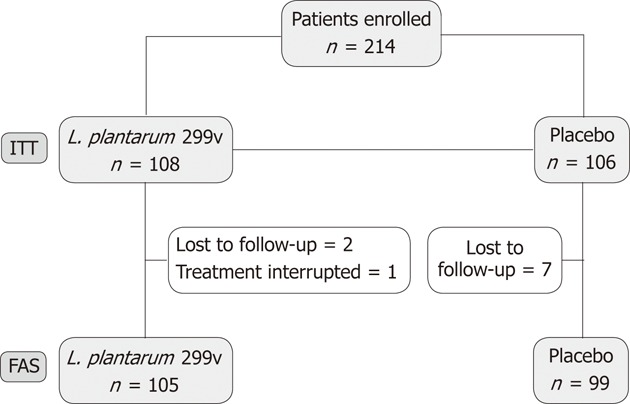
Flow-chart of the study. L. plantarum 299v: Lactobacillus plantarum 299v; FAS: Full analysis set; ITT: Intention to treat.
Table 1.
Baseline characteristics of the subjects between the two groups (mean ± SD)
| L. plantarum 299v (DSM 9843) (n =108) | Placebo (n =106) | P | |
| Age (yr) | 36.53 ± 12.08 | 38.40 ± 13.13 | NS |
| Men/women | 70/38 | 81/25 | NS |
| IBS duration (yr) | 3.4 | 4.6 | NS |
| Abdominal pain frequency | 2.1 ±1.01 | 1.98±0.91 | NS |
| Abdominal pain severity | 1.24 ± 0.60 | 1.20 ± 0.63 | NS |
| Bloating severity | 1.07 ± 0.62 | 1.14 ±0.64 | NS |
| Stool frequency | 3.94 ± 1.51 | 3.69 ± 1.34 | NS |
| Pure vegetarians (%) | 30.5 | 20.2 | NS |
| Daily yoghurt intake (%) | 46.7 | 42.1 | NS |
Frequency of digestive symptoms
The mean changes over this 4-wk period of the frequency of each digestive symptom are shown in Figure 2. The decrease of abdominal pain frequency was significantly higher in the L. plantarum 299v (DSM 9843) group than in the placebo group at weeks 3 and 4. At the end of week 4 the mean frequency was reduced significantly by 51.9% in the L. plantarum 299v group in comparison with the 13.6% reduction in the placebo group. Overall reductions in stool frequency, bloating and feeling of incomplete emptying frequency were also significantly greater in the L. plantarum 299v (DSM 9843) group when compared with the placebo group over the 4-wk period (P < 0.05). The effects of both treatments on stool frequency are shown in Figure 3. A significant reduction of the daily number of stools was observed with L. plantarum 299v (DSM 9843) after the second week of treatment.
Figure 2.
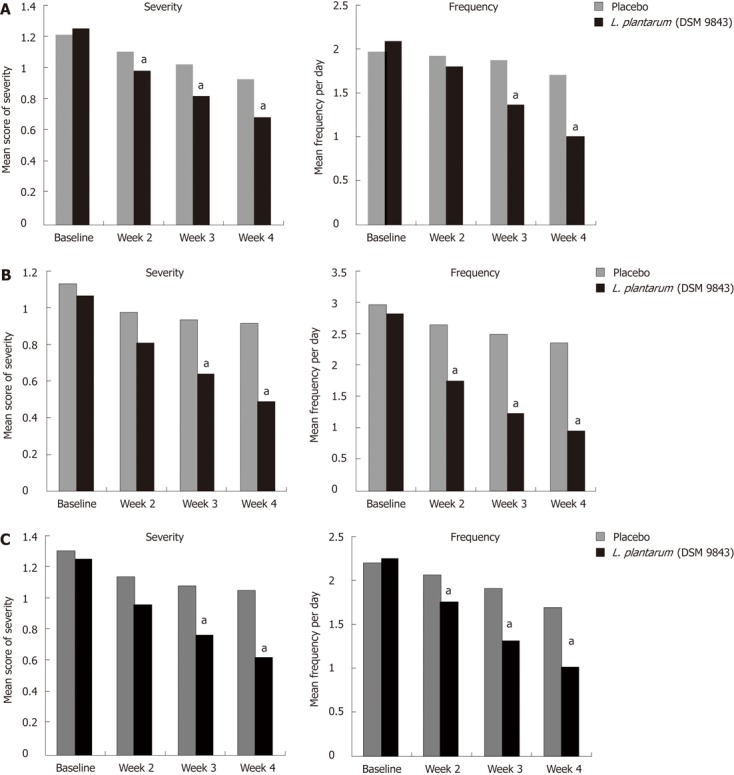
Changes in frequency and severity of symptoms in both groups. A: Abdominal pain; B: Bloating; C: Feeling of incomplete evacuation. L. plantarum (DSM 9843): Lactobacillus plantarum 299v. aP < 0.05 vs baseline group.
Figure 3.
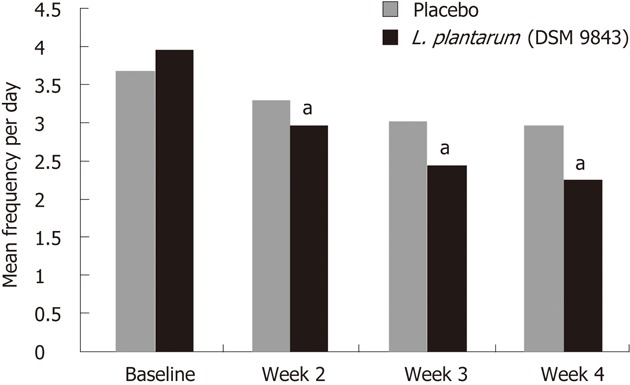
Changes in stool frequency in both groups. L. plantarum (DSM 9843): Lactobacillus plantarum 299v. aP < 0.05 vs baseline group.
Severity of digestive symptoms
The change in mean severity of abdominal pain over the 4-wk period was analysed on the VAS. At the end of the 4th week, the mean score was reduced by 45.2% in the L. plantarum 299v (DSM 9843) group and reduced by only 23.3% in the placebo group (Figure 2A). The weekly analysis of this score showed significantly lower scores at weeks 2, 3 and 4 in the L. plantarum 299v (DSM 9843) group in comparison with placebo. The decrease of the mean scores of severity of abdominal bloating and feeling of incomplete emptying were also statistically higher in the L. plantarum 299v (DSM 9843) group when compared to the placebo group at weeks 3 and 4 (Figure 2B and C).
Overall assessment
The percentage of patients who considered the efficacy of the treatment they received as good or excellent was significantly higher in the L. plantarum 299v (DSM 9843) group than in the placebo group (78.1% vs 8.1%) (Figure 4). Similar results were observed when the efficacy was estimated by the investigators (82.8% vs 11.1%) (Figure 4).
Figure 4.
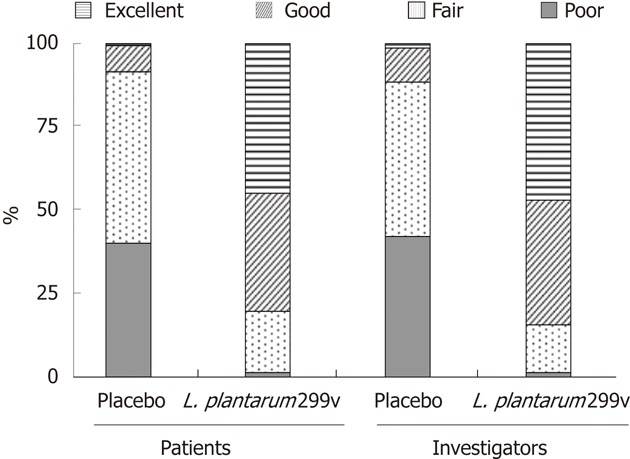
Overall assessment of the treatment efficacy by the patients and by the investigators.
Comparative efficacy according to dietary habits
Yoghurt consumption did not affect the results and did not induce any difference between the two arms of treatment (data not shown). The frequency of abdominal pain was also not different between the two arms when the vegetarian or non vegetarian status was considered. However, the severity of the abdominal pain with L. plantarum 299v (DSM 9843) was lower in the vegetarians than in the non-vegetarians at weeks 2, 3 and 4 (P < 0.05).
Safety
No significant side-effect was reported in any group during the 4 wk of treatment. The only adverse event reported was a transient vertigo onset by one of the patients who received L. plantarum 299v (DSM 9843). No change in blood parameters was detected throughout the study.
DISCUSSION
The present placebo-controlled trial demonstrated that in an Indian population L. plantarum 299v (DSM 9843) is a probiotic strain able to relieve IBS symptoms, particularly abdominal pain and bloating, in IBS patients fulfilling the Rome III criteria. Abdominal pain was chosen as the primary end point because it is the major symptom leading to the seeking of medical advice by IBS patients. This trial was designed for a group of IBS patients of any subtype, complaining of moderate IBS symptoms and recruited by general practitioners. Several trials with probiotics have involved mainly IBS-D patients but microbiological studies have emphasized that qualitative changes of the microbiota exist in all IBS sub-types[16]. Therefore, we considered that any IBS patient, whatever the subtype, could be eligible to participate. In the present study, the majority of recruited participants were males as compared to previous trials where approximately two-thirds of study subjects were females. The female predominance in IBS patients reported in the West has not been observed in Asian populations, particularly in India. Two major recent community studies reported higher prevalence of IBS in the male population. In Mumbai, male prevalence was 7.9% vs female prevalence of 6.9%, and in a pan-Indian study male prevalence was 4.3% vs female prevalence of 4.0%[17]. However, other population surveys in the Indian subcontinent have reported an IBS prevalence of 8.5% using the Rome I criteria and demonstrated a female predominance similar to Western countries[18]. The notable gender difference between the population of this study and that of previously published trials can also be explained by the fact that, in the Indian subcontinent but not in other parts of Asia, men seem to have a greater access to healthcare[19]. However, data about the consultation behaviour of the community groups are not all in agreement. In the recent large survey conducted by the Indian Society of Gastroenterology Task Force (3000 IBS patients and 4500 community subjects in 18 centres), 33% of men and 38% of women had consulted a doctor in the preceding 12 mo[20]. Eating behaviours of the patients enrolled in this trial were also somewhat different from that of Western IBS patients with a high percentage of pure vegetarians and with daily yoghurt consumption in almost half of the cases. Due to the possible interactions between nutrients and bacteria, we cannot exclude that this regimen might have influenced the therapeutic results even if eating behaviours were not different between the two groups. We have even observed that L. plantarum results on abdominal pain intensity were better in vegetarian than in non vegetarian IBS patients. This suggests that the symptomatic effect of the strain could be, at least partly, related to interactions between the luminal content and L. plantarum or that the strain affects the luminal metabolism of nutrients. However, the design of our study does not allow us to conclude that this is indeed the case.
This trial was performed according to the Rome III guidelines on design of trials for functional GI disorders[21] in order to demonstrate statistical superiority to placebo with a double-blind, placebo-controlled parallel design and outcome measures including both the effect of the treatment on the main symptom, i.e., abdominal pain, and a global assessment of the treatment efficacy to obtain adequate relief. Several clinical trials testing the symptomatic efficacy of probiotics in IBS were longer than this trial. However, a duration of treatment of 4 weeks follows not only the Rome III guidelines but is also the recommendation of international agencies[22]. One potential weakness of this study was the choice of a four-point Likert scale to analyze the frequency of symptoms instead of a score such as the IBS symptom severity scale that has been shown to be responsive to treatment effect[23].
We enrolled patients with moderate abdominal pain. Some studies have suggested that the achievement of a satisfactory relief end-point was significantly influenced by baseline symptom severity[23,24]. However, the concern that baseline severity compromises the achievement of an end point, such as satisfactory relief, does not appear to affect the current design of clinical trials. For example, trials with 5-HT3 antagonists[25] or antidepressant at low dose[26] or even with a non pharmacological approach[27] have not confirmed the impact of baseline severity on the achievement of an adequate relief as a trial end point[17,28].
In accordance with previous findings in many trials, IBS patients who received placebo exhibited a significant improvement with time. However this improvement was lower than in the L. plantarum group and the overall number of patients in the placebo group who considered themselves as improved was low. Furthermore, in the present study, the placebo results were lower than that calculated in a recent meta-analysis of 73 randomized controlled trials (RCTs) reporting a pooled placebo response of 37.5%. But the same meta-analysis of the factors affecting placebo response rate outlined that rates were significantly higher in European RCTs[29]. The percentage of patients who considered the efficacy of the treatment as good or excellent was very high (78.1%) in the L. plantarum 299v (DSM 9843) group and low in the placebo group (8.1%). This result cannot be explained only by the greater effects of L. plantarum 299v (DSM 9843) vs placebo on each IBS symptom. This satisfaction rate could also be explained by a possible efficacy of the strain on upper abdominal symptoms that are very frequent in Indian IBS patients. Indeed, the Indian Society of Gastroenterology Task Force have outlined that 49% of Indian IBS patients reported epigastric pain, and that 70% complained of upper abdominal fullness or bloating rather than pain[20].
Three studies using L. plantarum 299v (DSM 9843) have been published prior to this trial. In the first study, Nobaek et al[13] enrolled 60 IBS patients and compared L. plantarum 299v to placebo to determine whether endogenous colonic flora could be altered by probiotic consumption. Multiple secondary symptom-based end-points were also evaluated. The active treatment period lasted 4 wk after a 2-wk observation period. Compared with placebo, a statistically significant decrease in flatulence was observed during the second half of the treatment period but only 52/60 patients were included in the analysis of this secondary endpoint. In another study, Niedzielin et al[14] enrolled 40 IBS patients and assessed abdominal pain and global IBS symptoms as primary and secondary outcomes, respectively. At 4 wk, 20/20 patients in the L. plantarum 299v group compared to 11/20 in the control group had complete resolution of their pain (P = 0.0012). Moreover, 19/20 patients in the L. plantarum 299v group compared to 3/20 patients in the control group also experienced improvement in their global IBS symptoms (P < 0.0001). In both trials, no adverse effects were identified. The final study, performed by Sen et al[15], showed no significant improvement but it was a pseudo-randomized study with only 12 patients with a cross-over design and evaluated changes of a composite score of IBS symptoms. At 8 wk, no significant differences were identified between groups[15]. Given the significant differences in the enrolled populations, study designs, outcome variables, and statistical analyses, it is difficult to make comparisons across the studies and all three previous studies suffered from multiple design flaws.
In conclusion, the present study shows the potential benefit of a particular strain L. plantarum 299v (DSM 9843), in the management of IBS. Further studies are warranted in order to identify the mechanism of the probiotic’s potential beneficial effect.
ACKNOWLEDGMENTS
We thank the investigators and Soham consultancy for the management of the study; We thank also Dr. Bhavesh Kotak, clinical project coordinator for Ranbaxy Laboratories Ltd, India and Dr. Manish Maladkar for his involvement as the clinical project coordinator for Aristo Pharmaceuticals Pvt Ltd, India.
COMMENTS
Background
Lactobacillus plantarum 299v (L. plantarum 299v) (DSM 9843) is a probiotic strain able to reside in the human colonic mucosa in vivo, with an antibacterial activity against several potential pathogenic agents and an immunomodulatory activity via an increased interleukin-10 synthesis and secretion in colonic macrophages and T-cells.
Research frontiers
Recent studies have highlighted disturbances of the relationship between the complex community of the gut microbiota and their host in irritable bowel syndrome. The potential to correct this using probiotics has been suggested but the effective strains need to be determined.
Innovations and breakthroughs
After a treatment of 4 wk, the relief or improvement of irritable bowel syndrome (IBS) symptoms was greater with the L. plantarum 299v group than with placebo (P < 0.05) leading to greater patient satisfaction.
Applications
L. plantarum 299v (DSM 9843) is a suitable candidate for the relief of moderate symptoms in any IBS patient.
Peer review
Overall this is a well written paper reporting a trial of reasonable methodology. It should be published if authors can revise it in a satisfactory manner.
Footnotes
Supported by Rosell-Lallemand Institute, France and Probi AB, Sweden
Peer reviewer: John K Marshall, MD, Associate Professor of Medicine, Division of Gastroenterology, McMaster University Medical Centre, 1200 Main Street West, Hamilton, Ontario L8N 3Z5, Canada
S- Editor Cheng JX L- Editor A E- Editor Xiong L
No significant differences were found between the groups for all the variables tested. NS: No significant; L. plantarum 299v: Lactobacillus plantarum 299v.
References
- 1.Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1–35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 2.Jones J, Boorman J, Cann P, Forbes A, Gomborone J, Heaton K, Hungin P, Kumar D, Libby G, Spiller R, et al. British Society of Gastroenterology guidelines for the management of the irritable bowel syndrome. Gut. 2000;47 Suppl 2:ii1–i19. doi: 10.1136/gut.47.suppl_2.ii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertz HR. Irritable bowel syndrome. N Engl J Med. 2003;349:2136–2146. doi: 10.1056/NEJMra035579. [DOI] [PubMed] [Google Scholar]
- 4.Quigley EM, Flourie B. Probiotics and irritable bowel syndrome: a rationale for their use and an assessment of the evidence to date. Neurogastroenterol Motil. 2007;19:166–172. doi: 10.1111/j.1365-2982.2006.00879.x. [DOI] [PubMed] [Google Scholar]
- 5.McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14:2650–2661. doi: 10.3748/wjg.14.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325–332. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 7.Johansson ML, Molin G, Jeppsson B, Nobaek S, Ahrné S, Bengmark S. Administration of different Lactobacillus strains in fermented oatmeal soup: in vivo colonization of human intestinal mucosa and effect on the indigenous flora. Appl Environ Microbiol. 1993;59:15–20. doi: 10.1128/aem.59.1.15-20.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ménard S, Candalh C, Bambou JC, Terpend K, Cerf-Bensussan N, Heyman M. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut. 2004;53:821–828. doi: 10.1136/gut.2003.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobsen CN, Rosenfeldt Nielsen V, Hayford AE, Møller PL, Michaelsen KF, Paerregaard A, Sandström B, Tvede M, Jakobsen M. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol. 1999;65:4949–4956. doi: 10.1128/aem.65.11.4949-4956.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson ML, Nobaek S, Berggren A, Nyman M, Björck I, Ahrné S, Jeppsson B, Molin G. Survival of Lactobacillus plantarum DSM 9843 (299v), and effect on the short-chain fatty acid content of faeces after ingestion of a rose-hip drink with fermented oats. Int J Food Microbiol. 1998;42:29–38. doi: 10.1016/s0168-1605(98)00055-5. [DOI] [PubMed] [Google Scholar]
- 11.Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52:827–833. doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol. 1999;276:G941–G950. doi: 10.1152/ajpgi.1999.276.4.G941. [DOI] [PubMed] [Google Scholar]
- 13.Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231–1238. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 14.Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001;13:1143–1147. doi: 10.1097/00042737-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Sen S, Mullan MM, Parker TJ, Woolner JT, Tarry SA, Hunter JO. Effect of Lactobacillus plantarum 299v on colonic fermentation and symptoms of irritable bowel syndrome. Dig Dis Sci. 2002;47:2615–2620. doi: 10.1023/a:1020597001460. [DOI] [PubMed] [Google Scholar]
- 16.Rajilić-Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 17.Gwee KA, Lu CL, Ghoshal UC. Epidemiology of irritable bowel syndrome in Asia: something old, something new, something borrowed. J Gastroenterol Hepatol. 2009;24:1601–1607. doi: 10.1111/j.1440-1746.2009.05984.x. [DOI] [PubMed] [Google Scholar]
- 18.Mendis BL, Wijesiriwardena BC, Sheriff MH, Dharmadasa K. Irritable bowel syndrome. Ceylon Med J. 1982;27:171–181. [PubMed] [Google Scholar]
- 19.Masud MA, Hasan M, Khan AK. Irritable bowel syndrome in a rural community in Bangladesh: prevalence, symptoms pattern, and health care seeking behavior. Am J Gastroenterol. 2001;96:1547–1552. doi: 10.1111/j.1572-0241.2001.03760.x. [DOI] [PubMed] [Google Scholar]
- 20.Ghoshal UC, Abraham P, Bhatt C, Choudhuri G, Bhatia SJ, Shenoy KT, Banka NH, Bose K, Bohidar NP, Chakravartty K, et al. Epidemiological and clinical profile of irritable bowel syndrome in India: report of the Indian Society of Gastroenterology Task Force. Indian J Gastroenterol. 2008;27:22–28. [PubMed] [Google Scholar]
- 21.Irvine EJ, Whitehead WE, Chey WD, Matsueda K, Shaw M, Talley NJ, Veldhuyzen van Zanten SJ. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2006;130:1538–1551. doi: 10.1053/j.gastro.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 22.Committee for Proprietary Medicinal Products (CPMP) Points to consider on the evaluation of medicinal products for the treatment of irritable bowel syndrome. London: CPMP; 2003. Available from: http://www.emea.europa.eu/pdfs/human/ewp/078597en. [Google Scholar]
- 23.Camilleri M, Chang L. Challenges to the therapeutic pipeline for irritable bowel syndrome: end points and regulatory hurdles. Gastroenterology. 2008;135:1877–1891. doi: 10.1053/j.gastro.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitehead WE, Palsson OS, Levy RL, Feld AD, VonKorff M, Turner M. Reports of “satisfactory relief” by IBS patients receiving usual medical care are confounded by baseline symptom severity and do not accurately reflect symptom improvement. Am J Gastroenterol. 2006;101:1057–1065. doi: 10.1111/j.1572-0241.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- 25.Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545–555. doi: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leventer SM, Raudibaugh K, Frissora CL, Kassem N, Keogh JC, Phillips J, Mangel AW. Clinical trial: dextofisopam in the treatment of patients with diarrhoea-predominant or alternating irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27:197–206. doi: 10.1111/j.1365-2036.2007.03566.x. [DOI] [PubMed] [Google Scholar]
- 27.Lackner JM, Jaccard J, Krasner SS, Katz LA, Gudleski GD, Holroyd K. Self-administered cognitive behavior therapy for moderate to severe irritable bowel syndrome: clinical efficacy, tolerability, feasibility. Clin Gastroenterol Hepatol. 2008;6:899–906. doi: 10.1016/j.cgh.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ameen VZ, Heat AT, McSorley D, Spiegel BM, Chang L. Global measure of adequate relief predicts clinically important difference in pain and is independent of baseline pain severity in IBS. Gastroenterology. 2007;132:A–140. [Google Scholar]
- 29.Ford AC, Moayyedi P. Meta-analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther. 2010;32:144–158. doi: 10.1111/j.1365-2036.2010.04328.x. [DOI] [PubMed] [Google Scholar]


