Abstract
Increasing evidence indicates that the CREB-dependent transcriptional activation of a number of genes requires the CREB co-activator, transducer of regulated CREB activity (TORC). Because of the central importance of CREB in many brain functions we examined the topographic distribution of TORC1, 2, and 3 mRNAs in specific regions of the rat forebrain. In situ hybridisation (ISH) analysis revealed that TORC1 is the most abundant isoform in most forebrain structures, followed by TORC2 and TORC3. All three TORC isoforms were found in a number of brain nuclei, the ventricular ependyma, and pia mater. While high levels of TORC1 were widely distributed in the forebrain, TORC2 was found in discrete nuclei and TORC3 mostly in the ependyma, and pia mater. The relative expression of TORC isoforms was confirmed by qRT-PCR analysis in the hippocampus and hypothalamus. In the paraventricular nucleus of the hypothalamus, TORC1 and 2 mRNAs were abundant in the parvicellular and magnocellular neuroendocrine compartments, while TORC3 expression was low. All three isoform mRNAs were found elsewhere in the hypothalamus, with the most prominent expression of TORC1 in the ventromedial nucleus, TORC2 in the dorsomedial and arcuate nuclei, TORCs 1 and 2 in the supraoptic, and TORC2 in the suprachiasmatic nuclei. These differential distribution patterns are consistent with complex roles for all three TORC isoforms in diverse brain structures, and provide a foundation for further studies on the mechanisms of CREB/TORC signalling on brain function.
Keywords: Gene regulation, Transcription factors, Neuropeptides, Hypothalamus, Telecephalon, Energy metabolism
INTRODUCTION
Cyclic AMP responsive element binding protein (CREB)-dependent transcription is critical for many functions in the brain, including neuronal development, synaptic plasticity, memory and the synthesis of neuropeptides and regulatory enzymes [1–4]. Although transcriptional activation clearly requires binding of CREB to a cyclic AMP responsive element (CRE) in the gene promoter, it is now evident that for several cyclic AMP-inducible genes, activation involves nuclear translocation of the CREB co-activator, transducer of regulated CREB activity (TORC) also called CREB regulated transcription co-activator (CRTC) [5,6]. Three TORC isoforms—1, 2, and 3—have been identified in mammals [8]. The amino-terminal regions of these isoforms are highly conserved and are responsible for the interaction with CREB [5,7]. In basal conditions TORC is retained in the cytoplasm in an inactive state through phosphorylation at Ser 171 and binding to the scaffolding protein 14-3-3 [7]. The two known isoforms of salt inducible kinase (SIK)—a Ser/Thr kinase belonging to the AMP dependent kinase family—are responsible for phosphorylating and thereby inactivating TORC. Subsequent activation and nuclear translocation of TORC involves dephosphorylation driven by cyclic AMP/PKA-dependent inhibition of SIK, and calcineurin-dependent dephosphorylation of TORC [8]. Dephosphorylation dissociates TORC from its scaffolding protein thereby permitting its translocation to the nucleus. Most studies examining the physiological significance of TORC have focused on its role in regulation of gluconeogenic enzymes in the liver and on glucose homeostasis, functions in which TORC2 appear to be the most important [5,9]. TORC1 is abundant in the brain and there is increasing evidence for a role of this subtype in the brain. TORC1 is required for late-phase long-term potentiation of hippocampal neurones [10,11], for dendritic outgrowth in cortical neurones in vitro [12]. TORC3 has been less studied but it is known to be present in the liver where it regulates mitochondrial biogenesis [13] and to attenuate beta-adrenergic signaling in adipocytes [14].
Recent studies using a hypothalamic neuronal cell line and primary cultures of hypothalamic neurones show that phospho-CREB is not sufficient to drive corticotrophin releasing hormone (CRH) gene transcription, but that its activation requires the nuclear translocation of TORC [15]. Silencing RNA knock-down experiments suggest that all 3 TORC subtypes are involved, but TORC2 appears to be the most important. Immunohistochemical studies have shown co-localization of TORC2 in CRH neurones in the paraventricular nucleus of the hypothalamus (PVH) [16]. However, the presence of TORC1 and 3 in CRH neurones remains unknown because of the current lack of suitable antibodies.
Although TORC is clearly important for several aspects of neuronal function, little is known about the relative importance, functional differences, or the specific locations in brain of the three TORC isoforms. The purpose of the present study is to use in situ hybridisation (ISH) to examine the topographic distribution of TORC1, 2 and 3 mRNAs in the rat forebrain, especially in the hypothalamus.
MATERIALS AND METHODS
Animals and Tissue Preparation
Adult male Sprague-Dawley rats (~300g BW; for USC, obtained from Harlan, Placentia CA, USA; for NICHD, obtained from Harlan Sprague Dawley, Frederick, MD) were housed in a temperature- and humidity-controlled vivarium with a 12h light-12h dark cycle (lights on 06.00h) and unrestricted access to food and water. The Animal Care and Use Committees of the University of Southern California and NICHD approved all animal experimental procedures.
For ISH, three rats were anaesthetised with isoflurane and decapitated between 09.00h and 10.00h. Brains were rapidly removed from the skull, fixed by immersion in chilled borate buffered (pH9.5) 4% paraformaldehyde for 48h, and then post-fixed and cryoprotected by overnight immersion in the same fixative containing 12% w/v sucrose. Brains were frozen in hexane supercooled over powdered dry ice. Eight 1-in-8 20 µm coronal sections were cut through the forebrain from approximately levels 22 to 30 of the Swanson rat brain atlas [17] using a sliding microtome. One series was stained with thionin; the remaining series were prepared for ISH as previously described [18].
In Situ Hybridisation Histochemistry
Plasmids harboring rat TORC1, 2, and 3 cDNA fragments were created by cloning TORC PCR fragments into pGEM-T Easy vector (Promega). The sequences of the PCR primers used for each TORC isoform are shown in Table 1. The resulting plasmids were linearised with appropriate restriction enzymes and then used to synthesise sense and antisense 35S-UTP-labelled complementary RNA (cRNA) probes using the Promega Gemini kit (Promega Inc., Madison WI) and appropriate RNA polymerases. The plasmids generated riboprobes of approximately 310bp, 600 bp and 700 bp for TORC1, TORC2 and TORC3, respectively.
TABLE 1.
Primer sequences used to generate the sense and antisense cRNA probes for in situ hybridisation of the three TORC isoform mRNAs, and for the q-RT-PCR analysis of the three TORC isoform mRNAs.
| Primer Sequences for In Situ Hybridisation Probes |
| TORC1 |
| 5’-TATGGCACCGTGTACCTCTCG-3’ (forward) |
| 5’-AAGTGGATGGTGCTGAGGTCAG-3’ (reverse) |
| TORC2 |
| 5’-AAGTCTAGTTATACCCAGCCATCC-3’ (forward) |
| 5’-TCACTGTAGCCGATCACTACGGAATG-3’ (reverse) |
| TORC3 |
| 5’-ACACCCTGACGGTAGTCACAGCATAG-3’ (forward) |
| 5’-AAGATGGAGGGCATTGTGTGCAGG-3’ (reverse) |
| Primer Sequences for q-RT-PCR |
| TORC1 |
| CACCAGAGCACAATGACACC (forward) |
| GCCTTCTTTGAGTCCCATGA (reverse) |
| TORC2 |
| CCCACCCCAAAGTCTCTACA (forward) |
| CCCCAGGCTGAAGTCATTTA (reverse) |
| TORC3 |
| AAGCCAGGTACCCTCCAACT (forward) |
| GCACATACAGGAAAGCAGCA (reverse) |
ISH was performed as previously described [18,19]. Adjacent sections were hybridised with a 35S-UTP-labelled complementary RNA (cRNA) probes transcribed from each of the three TORC isoform cDNAs. One series from each brain was also stained with thionin and used as reference to anatomical regions using the scheme and nomenclature of Swanson [17]. Sections hybridised with the radiolabelled sense and antisense riboprobes were exposed to Mamoray HDR-C™ X-ray film (Agfa Corp., Goose Creek SC) for 12 and 21 days. Sense strand cRNA probes did not yield detectable hybridisation for any of TORC isoforms (Fig 1A2–C2).
Figure 1.
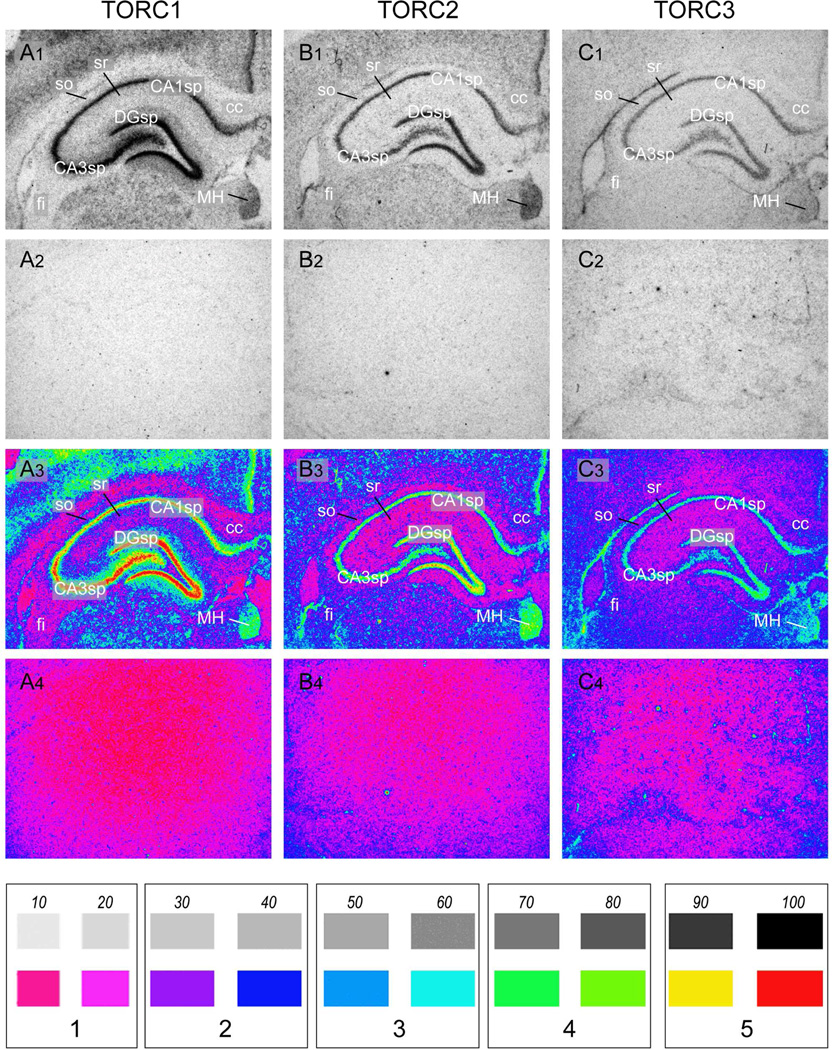
Representative photomicrographs of serial coronal sections through the dorsal hippocampus from one of 3 rats showing in situ hybridisation for TORC1 mRNA (A1); TORC2 mRNA (B1); TORC3 mRNA (C1). Note that although each TORC isoform is expressed in the same regions, levels of TORC1 mRNA were significantly higher than TORC2 mRNA, and that all three isoforms were expressed at low levels in white matter fibre tracts, with TORCs 2 and 3 being at a somewhat higher level that TORC1. Panels A2 – C2 show corresponding sections hybridised with control sense-strand cRNA probes for each isoform mRNA. Panels A3 – C3 and A4–C4 show pseudocolored images of panels A1 – C2 and A2 – C2, as described in the Methods. The scale at the bottom of the Figure shows a 10 – 100% gray scale with the correspondingly assigned pseudocolor. Each pair of gray scale values was assigned a value of 1 – 5, which were used for the results in Table 1.
Abbreviations: CA1sp, field CA1 Ammon’s horn, pyramidal layer; CA3sp, field CA3 Ammon’s horn, pyramidal layer; cc, corpus callosum; DGsp, dentate gyrus, granule cell layer; fi, fimbria; MH, medial habenula; so, stratum oriens ; sr, stratum radiatum.
Greyscale images from the Mamoray HDR-C film were obtained using IPLab Spectrum software for the Mac (v3.9; Signal Analytics) controlling an uncooled QImaging Micropublisher™ CCD Digital Camera (QImaging, Surrey, British Columbia, Canada). All the sections used to generate the illustrated greyscale images were obtained from a single animal with the same exposure time for all 3 TORC probes (21 days). Sections from the remaining animals were used to confirm the results. Pseudocolour images were used for the semi-quantitation reported in Table 2. These were obtained from the same sections used for the greyscale images, but were exposed to the Mamoray HDR-C film for only 12 days to minimize signal saturation. Pseudocolour transformation of these images was performed using the IPLab Spectrum software (see Fig. 1A3 – C4 for examples). A linear 0 – 100 greyscale (in 10% increments) was generated in Adobe Photoshop CS3™ (Adobe Systems, Inc., San Jose, CA, USA) and pseudocoloured in the same manner (Fig. 1), and the resulting values (0 – 5, in 20% increments) assigned to the expression levels in regions anatomically identified using adjacent thionin-stained sections.
TABLE 2.
The relative expression levels of TORC1 – 3 mRNA in selected regions of the rat forebrain. Results were determined by comparing the signal levels from pseudocoloured images of in situ hybridisation for each TORC isoform mRNA with a pseudocoloured 0 (white) −100% (black) greyscale. This grayscale was then divided into 5 in 20% greyscale increments (1 – 5). The expression level of each of the three TORC isoform mRNAs in the various forebrain regions was assigned a value of 1 – 5 based on pseudocolored hybridisation images. See Methods and Figure 1 for further details.
| TORC 1 | TORC 2 | TORC 3 | ||||
|---|---|---|---|---|---|---|
| Cortical Plate and Cortical Subplate (Cortical Amygdalar Nuclei) | ||||||
| Cortical Plate | ||||||
| Sensory/Motor cortex | 1 | 2 | 2 | 1 | ||
| 2 | 5 | 3 | 1–2 | |||
| 3 | 5 | 3 | 1–2 | |||
| 4 | 4 | 3 | 1–2 | |||
| 5 | 5 | 3 | 1–2 | |||
| 6 | 4 | 3 | 1–2 | |||
| Retrospenial Area | 5 | 3–4 | 2 | |||
| Pyriform cortex (PIR) | 1 | 4 | 1–2 | 2–3 | ||
| 2 | 5 | 3–5 | 1–2 | |||
| 3 | 4–5 | 4 | 1 | |||
| Cortical Subplate | ||||||
| Basolateral nucleus of the amygdala (BLA) | 4 | 3 | 1 | |||
| Basomedial nucleus of the amygdala | 4 | 3 | 1 | |||
| Lateral nucleus of the amygdala | 4 | 3 | 1 | |||
| Posterior nucleus of the amygdala | 5 | 4 | 2 | |||
| Dorsal Hippocampal formation | ||||||
| Ammon's Horn field CA1 (CA1) | 5 | 4–5 | 3 | |||
| Ammon's Horn field CA2 (CA2) | 5 | 4–5 | 3 | |||
| Ammon's Horn field CA3 (CA3) | 5 | 4–5 | 3 | |||
| Dentate gyrus (DG) | 5 | 5 | 3 | |||
| Pallidum | ||||||
| Globus Pallidus (GP) | 4–5 | 3 | 1 | |||
| Bed nuclei of the stria terminalis | ||||||
| posterior part (BSTp) | 5 | 3–4 | 1–2 | |||
| Striatum | ||||||
| Caudate putamen (CP) | 2 | 2 | 1–2 | |||
| Striatal Amygdalar Nuclei | ||||||
| Central nucleus (CEA) | 4 | 3 | 1 | |||
| Medial nucleus (MEA) | 5 | 3 | 2 | |||
| Intercalated nuclei (IA) | 5 | 3 | 2 | |||
| Thalamus | ||||||
| Reticular nucleus (RT) | 2 | 3 | 1 | |||
| Lateral habenula | 2 | 2 | 2 | |||
| Medial habenula (MH) | 4–5 | 4–5 | 2–3 | |||
| Zona incerta | 4 | 3 | 2 | |||
| Other thalamic nuclei | 3–4 | 2–3 | 1 | |||
| Hypothalamus | ||||||
| Periventricular Zone | ||||||
| Medial Preoptic Area | 4 | 3 | 1–2 | |||
| Suprachiasmatic nucleus (SCH) | 4 | 4–5 | 2–3 | |||
| Supraoptic nucleus (SO) | 4 | 4–5 | 2 | |||
| Paraventricular nucleus | ||||||
| neuroendoc parvicellular | 4 | 4 | 2–3 | |||
| neuroendoc magnocellular | 4 | 4–5 | 3 | |||
| lateral parvicellular | 4 | 4 | 1–2 | |||
| Arcuate nucleus (ARH) | 4–5 | 4 | 2–3 | |||
| Median Eminence | 3 | 3 | 3 | |||
| Medial Zone | ||||||
| Anterior hypothalamic area | 4 | 4 | 1–2 | |||
| Dorsomedial nucleus (DMH) | ||||||
| posterior part (DMHp) | 4 | 4 | 1–2 | |||
| ventral part (DMHv) | 3–4 | 3–4 | 1–2 | |||
| Ventromedial nucleus (VMH) | ||||||
| dorsomedial part | 5 | 3–4 | 2 | |||
| central part | 4 | 2–3 | 2 | |||
| ventrolateral part | 5 | 3–4 | 2 | |||
| Lateral Zone | ||||||
| Lateral hypothalamic area | 3–4 | 2–3 | 1 | |||
| Other Structures | ||||||
| ventricular ependyma (ve) | 3 | 3 | 3–4 | |||
| myelinated fiber tracts | 1–2 | 1–2 | 1–2 | |||
| pia mater (pia m.) | 2 | 3 | 3 | |||
Quantitative Real Time PCR for the TORC Isoform mRNAs
Brains from 3 rats were rapidly removed after decapitation and placed in an ice chilled stainless steel brain slicer. A coronal section containing the hypothalamus was obtained by blade cuts at the levels of the optic chiasm and the mammilary bodies and placed flat on a chilled rubber cork and cut at the top and 2mm lateral at each side of the 3rd ventricle with a scalpel blade. The hippocampus was dissected out by removing the cortex and thalamic regions with the help of two forceps. Microdissected hypothalamic and hippocampal tissue from individual rats was immediately frozen in 1.5 ml microfuge tubes on dry ice and stored at −80 C. Total RNA was prepared using TRIzol reagent (Invitrogen, Hopkinton, MA, USA), followed by purification using RNeasy mini kit reagents and column DNase digestion (Qiagen, Valencia, CA, USA) to remove genomic DNA contamination. Complementary DNA was reverse transcribed from 1µg of total RNA as previously described [20]. Levels of TORC1, 2 and 3 mRNAs were measured by quantitative real time PCR (qRT-PCR) using the sets of primers shown in Table 1.
Power SYBR green PCR mix (ABI) was used for the amplification mixture with each primer at a final concentration of 200nM and 1µl of 1:10 dilution cDNA for a total reaction volume of 12.5µl. PCR reactions were performed on spectrofluorometric thermal cycler (ABI 7900HT Fast Real-Time System, Applied Biosystems, Foster City, CA), as previously described [20]. Samples were amplified by an initial denaturation at 50°C for 2min, 95°C for 10min and then cycled (40) using 95°C for 15s, and 60°C for 1 min. Standard curves were generated to measure absolute TORC1, 2, and 3 mRNA content per total RNA unit using the TORC fragments cloned into pGEM-T Easy vector described above as a template.
RESULTS
General Distribution of TORC Isoform mRNAs in the Forebrain
Although all three TORC isoforms are widely expressed in the forebrain, TORC1 mRNA is substantially higher than those for the other two isoforms in almost all regions (Figs. 1–6, Table 2). This is particularly evident in the entire cerebral cortex including the dorsal part of the hippocampal formation (Fig. 1). In contrast to the high levels of TORC1 mRNA, TORC2 and 3 mRNAs levels were much lower and more evenly distributed across the forebrain with the exception of some discrete areas, and particularly the hippocampal formation, PVH, supraoptic (SO), suprachiasmatic (SCH), ventromedial (VMH) nuclei, and the piriform cortex. In these regions TORC2 and 3 mRNAs were detected above the rather diffuse distribution evident elsewhere in the forebrain. The distribution pattern of all three TORC mRNAs is consistent with the notion that they are not confined to neurons or their cell bodies, For example, TORC3 mRNA was clearly evident in the ventricular ependyma, while the diffuse TORC2 and 3 mRNA hybridisation in the neuropil and myelinated fibre tracts would be consistent with expression in dendrites and/or glia (Fig. 1). However, further studies using double-labelling techniques will be needed to determine categorically whether each TORC isoform is present in these locations.
Figure 6.
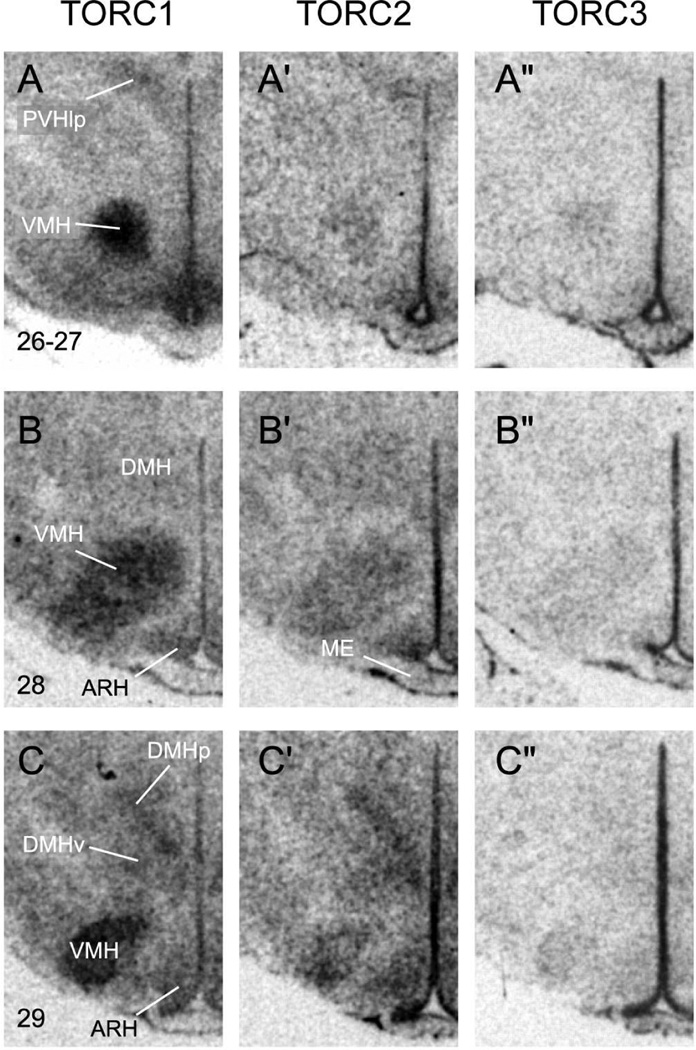
Representative photomicrographs of serial coronal sections through the region of ventomedial (VMH) and dorsomedial (DMH) nuclei of the hypothalmus from one of 3 rats showing in situ hybridisation for TORC1 mRNA (A – C); TORC2 mRNA (A’ – C’); TORC3 mRNA (A” – C”). Numbers in the bottom left of panels A–C, refer to the levels in the Swanson rat atlas [15]. Other abbreviations: ARH, arcuate nucleus; DMHp, posterior part of the DMH; DMHv, ventral part of the DMH; ME, median eminence; PVHlp, lateral parvicellular part of the paraventricular nucleus of the hypothalamus.
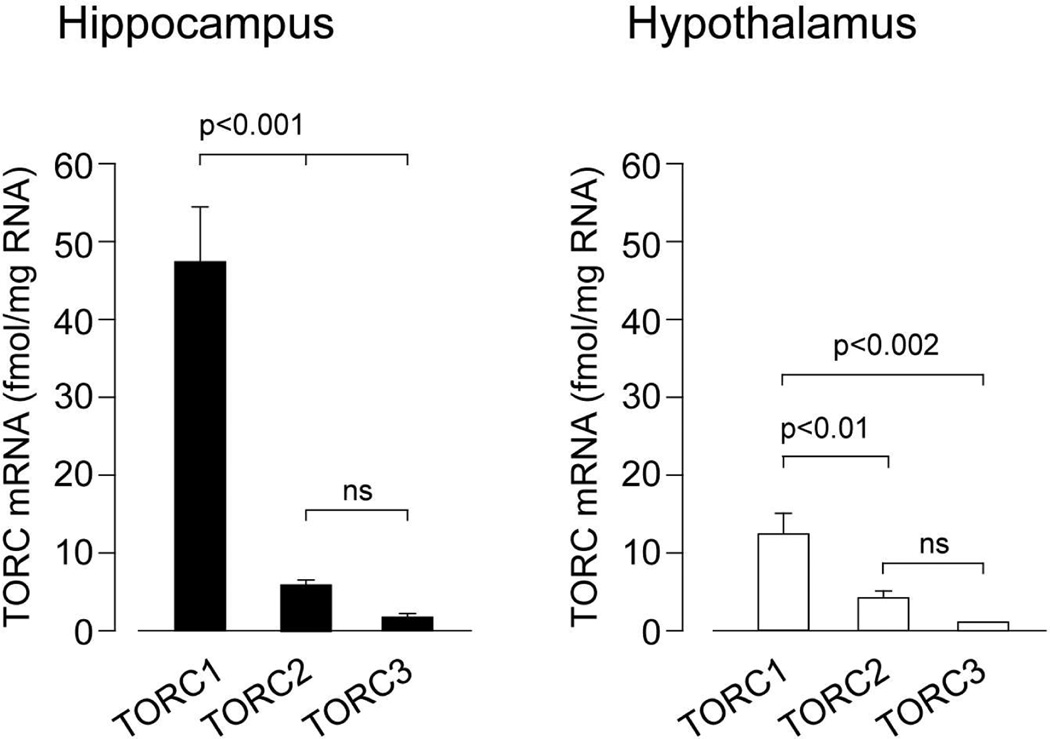
A semi-quantitative analysis of the relative expression of the three TORC subtypes mRNAs in different forebrain regions is shown in Table 2. The relative levels of the mRNA obtained from the ISH analyses (Table 2) were confirmed by quantitative RT-PCR measurements of the three isoform mRNAs in dissected hippocampal and hypothalamic tissue (Fig. 2).
Figure 2.
TORC isoform mRNAs in the hippocampus and hypothalamus as determined by quantitative RT-PCR. Bars show the mean and SEM of the values obtained in measurements in 3 individual rats.
Cortical Plate and Cortical Subplate (Cortical Amygdalar Nuclei)
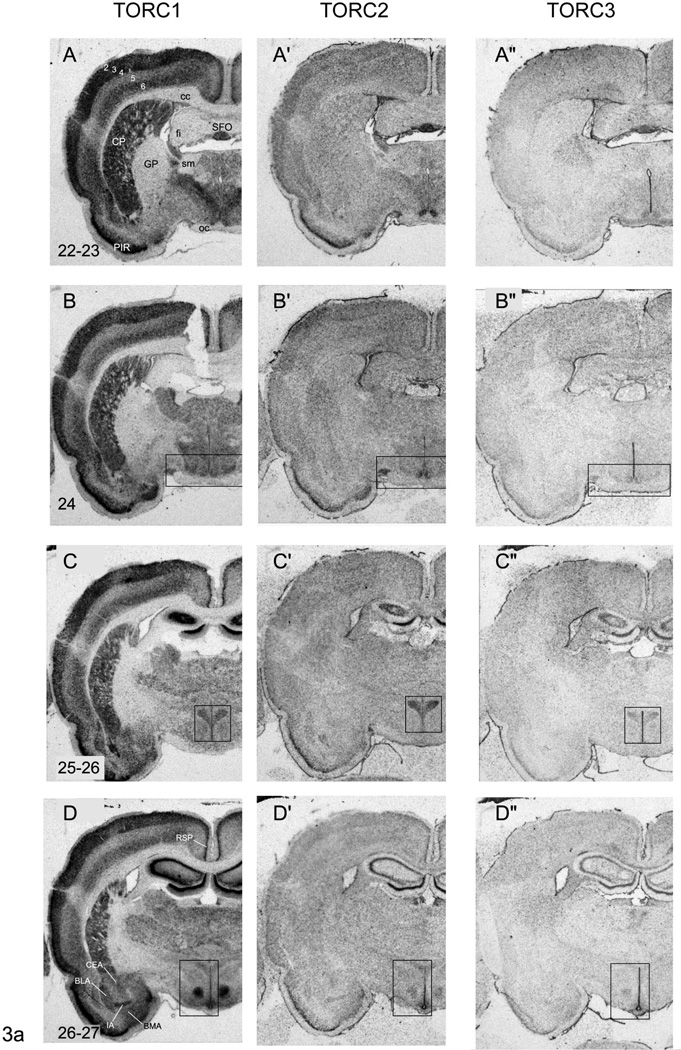
TORC1 mRNA is heavily expressed in the cortical plate, but is particularly abundant in layers 2, 3, and 5 of the primary and secondary motor and sensory areas (Fig. 3A–G), and in layer 2 of the pyriform cortex (Fig. 3A–G) and retrosplenial area (Fig. 3D–G). Although TORC2 mRNA shows a similar distribution pattern to TORC1 mRNA, its levels are significantly lower except for layer 2 of the pyriform cortex and retrosplenial area, where it is significantly higher than the rest of the cortical plate (Fig. 3A’–G’). Very low levels of TORC3 mRNA are detectable in the cortical plate (Fig. 3A”–G”), and no laminar distribution pattern is discernable, except for layer 2 of the pyriform cortex and retrosplenial area (Fig. 3A”–G”).
Figure 3.
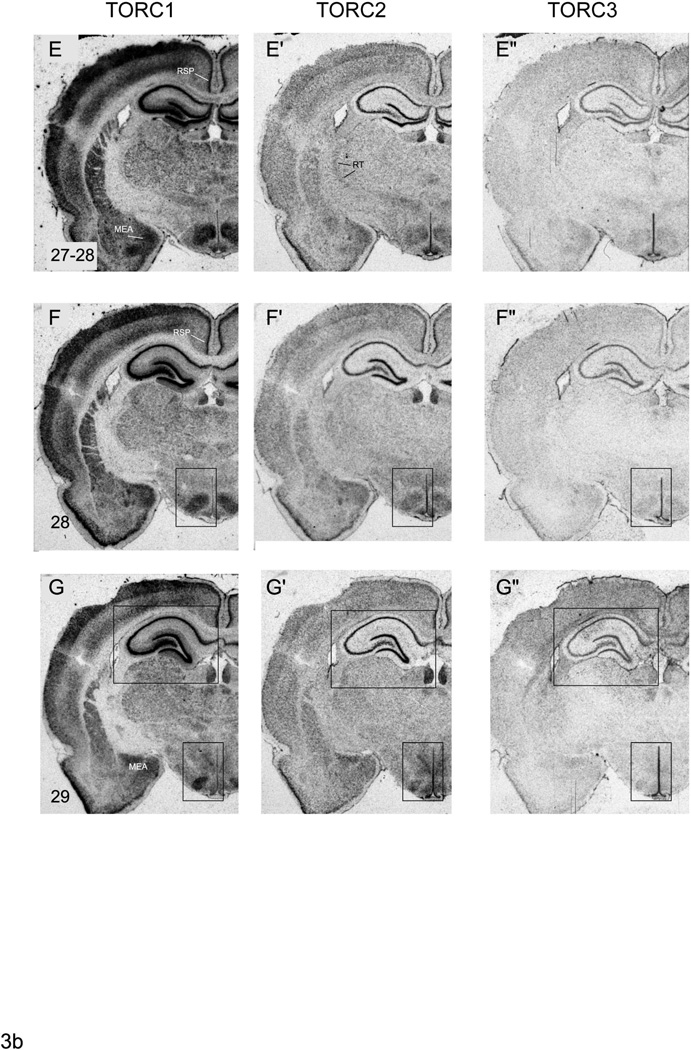
Representative photomicrographs of serial coronal sections through the forebrain of one of 3 rats showing in situ hybridisation for TORC1 mRNA (A – G), TORC2 mRNA (A’ – G’), TORC3 mRNA (A” – G”). Numbers in the bottom right of panel A – G, refer to the levels in the Swanson rat atlas [17]. Abbreviations are as in Figure 1 except: CEA, central nucleus of the amygdala; CP; caudate putamen; GP, globus pallidus; MEA, medial nucleus of the amygdala; BLA, basolateral nucleus of the amgydala; PIR, piriform cortex; RT, reticular nucleus of the thalamus; SFO, sub-fornical organ; sm, stria medullaris. Numbers 2 – 6 in panel A refer to cortical layers. Rectangles in panels B – B”, C – C”, D – D”, F – F” and G – G” indicate regions that are shown at higher magnification in Figures 1, 4 – 6.
The relative levels of all three TORC isoform mRNAs in the basolateral, lateral, and basomedial amygdalar nuclei are similar to those seen in layer 6 of the cortical plate, with TORC1 mRNA being the most abundant and TORC3 mRNA barely detectable (Fig. 3D–D”).
Dorsal Hippocampal Formation
All three isoforms, but particularly TORC1 mRNA, are heavily expressed in the pyramidal cell layers of CA1, CA2 and CA3, and in the granule cell layer of the dentate gyrus (Fig. 1A–C). Indeed, these parts of the hippocampal formation along with the ventricular ependyma and pial membrane have the highest levels of TORC3 mRNA in the forebrain. In contrast to neuronal patterns of the expression, TORC2 and particularly TORC3 mRNAs are clearly evident in the fimbria at levels that are noticeably higher than those seen in the stratum oriens and stratum radiatum (Fig. 1A–C), consistent with widespread extra-neuronal expression. For comparison, Figs 1A2–C2 shows the background hybridisation signal in the same regions from sense-strand control probes.
Striatum and Pallidum
The pattern of TORC1 mRNA expression in striatal and pallidal regions is quite distinct, with very abundant levels in the caudoputamen, but a virtually absence in the globus pallidus (Fig. 3A–G). It is also evident that TORC1 mRNA is absent from the myelinated fascicles that pass through the caudoputamen (Fig. 3A–G). In contrast to TORC1, levels of TORC2 mRNA were very low in the caudoputamen and the globus pallidus (Fig. 3A’–G’). Hybridisation signal for TORC3 mRNA is detectable in the caudoputamen and globus pallidus. But apart from a noticeably higher level of TORC3 mRNA in the most dorsal part of the globus pallidus (Fig. 3A”), levels are barely above background with no clearly discernible distribution pattern (Fig. 3A”–G”).
The expression levels of all three TORC mRNAs in the striatal parts of the amygdala parallel those seen in the cortical plate and caudoputamen (Fig. 3C–E). Thus, TORC1 mRNA is the most abundant and TORC3 mRNA the least. However, the medial and intercalated amygdalar nuclei have significantly higher levels of all three TORC mRNA compared to the more laterally situated central amygdalar nucleus (Fig. 3D–G).
Thalamus
All thalamic regions contain TORC mRNAs with TORC1 and TORC2 mRNAs being significantly more abundant than TORC3 (Fig. 3A–G). Apart from the medial habenula, which shows quite high levels of TORC1 and 2 mRNAs (Fig. 1) and the reticular nucleus of the thalamus with somewhat higher levels of TORC2 mRNA (Fig. 3E’), there is little apparent regional variation in the expression levels across thalamic nuclei. Again, like other parts of the forebrain the hybridisation signal for TORC3 mRNA is just above background, and substantially lower than those for the other two isoforms.
Hypothalamus
Suprachiasmatic Nucleus
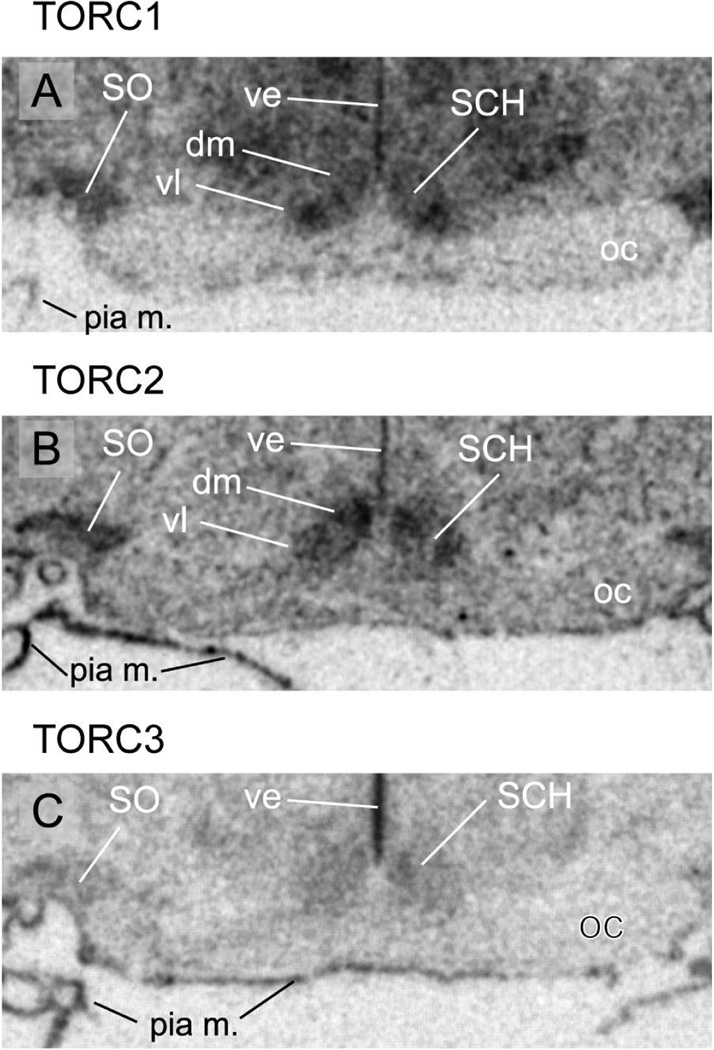
Both TORC1 and 2 mRNAs are strongly expressed throughout the SCH, but each shows a distinct pattern. TORC1 mRNA is more heavily expressed in the ventral and ventrolateral parts of the nucleus where many VIP neurones are found (Fig. 4A), whereas TORC2 mRNA is abundant throughout the nucleus with more concentration in the dorsomedial part (Fig. 4B). TORC3 mRNA in the SCH is found at levels higher than peri-SCH regions, but the regional differences seen with TORC1 and 2 are not apparent (Fig. 4C).
Figure 4.
Representative photomicrographs of serial coronal sections through the suprachiasmatic (SCH) and supraoptic nuclei (SO) from one of 3 rats showing in situ hybridisation for TORC1 mRNA (A); TORC2 mRNA (B); TORC3 mRNA (C). Other abbreviations: dm, dorsomedial part of the SCH; oc, optic chiasm; pia m., pia mater; ve, ventricular ependyma; vl, ventrolateral part of the SCH.
Paraventricular Nucleus of the Hypothalamus
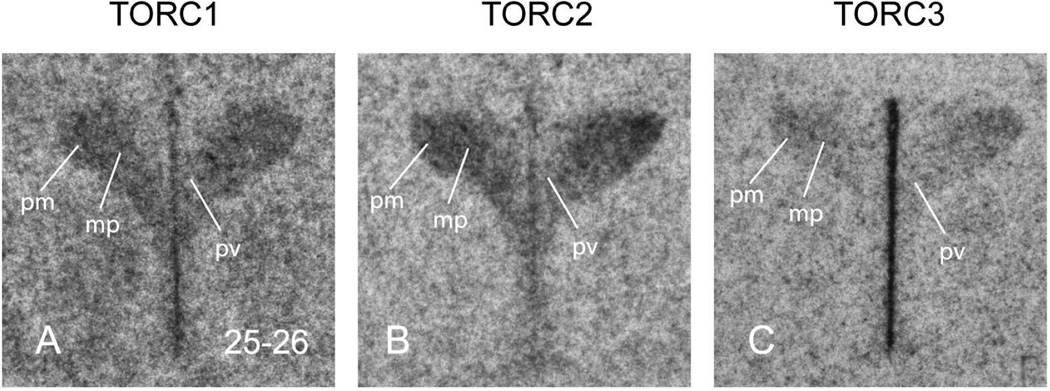
All three isoforms are clearly expressed in each of the main functional compartments of the PVH: magnocellular, parvicellular neuroendocrine, and parvicellular pre-autonomic (Fig. 5, 6A). However, TORC2 mRNA is the most abundant of the three isoforms, closely followed by TORC1 mRNA. TORC3 mRNA is much lower than the other two isoforms, but still clearly evident, particularly in the magnocellular regions (Fig. 5C).
Figure 5.
Representative photomicrographs of serial coronal sections through the paraventricular nucleus of the hypothalamus (PVH) from one of 3 rats showing in situ hybridisation for TORC1 mRNA (A); TORC2 mRNA (B); TORC3 mRNA (C). Numbers in the bottom right of panel A, refer to the levels in the Swanson rat atlas [17]. Other abbreviations: mp, medial parvicellular part of the PVH; pm, posterior magnocellular part of the PVH.
Ventromedial Nucleus of the Hypothalamus
Like the PVH, the mRNAs for all three isoforms are seen throughout the entire rostrocaudal extent of the VMH (Fig. 6). But unlike the PVH, the levels of TORC1 mRNA are much greater than TORC2, which in turn is greater than TORC3. Within the VMH the mRNAs for all TORCs are seen in all three subdivisions: dorsomedial, central, and ventrolateral, with the levels of expression reflecting the respective density of neurones within each subdivision (Fig. 6).
Dorsomedial Nucleus of the Hypothalamus (DMH)
The relative abundance of the three TORC mRNAs in the DMH is more similar to that seen in the PVH than the VMH, with TORC1 and 2 mRNAs showing more or less equal expression, with much lower levels of TORC3 mRNA (Fig. 6B,C). Particular enrichment of TORCs 1 and 2 is seen in the posterior part of the DMH (Fig. 6C). But this may simply be a consequence of the greater cell density in this part of the DMH than in its anterior and posterior parts [17].
Arcuate Nucleus (ARH)
Like other hypothalamic nuclei all three TORC isoform mRNAs are found throughout the rostrocaudal extent of the ARH. Their relative abundance matches that seen in the VMH and DMH (Fig. 6).
Non-Neuronal Tissues
The expression of the three isoforms is not limited to neurones. Although low-level expression of all isoform mRNAs is seen in myelinated fibre tracts, the expression of TORC2 is generally greater than TORC3, while TORC1 mRNA is barely above control hybridisation (Fig. 1). Similarly, TORC3 mRNA is particularly abundant in the choroids plexus, ventricular ependyma (Fig. 4–6), and the pial membrane (Figs. 3C’, 3C”, & 4). However, the hybridisation signals for TORC1 and particularly TORC2 mRNAs is also evident in these structures, with TORC1 mRNA being found at a reduced levels.
DISCUSSION
Increasingly convergent sets of evidence demonstrate that TORC is an important regulator of CREB-dependent transcription for many genes [7,16,21]. CREB has a prominent role as a transcriptional regulator in the brain [1–4, 22–24], and the high levels of TORC isoform expression we observe in the forebrain suggest that CREB-dependent transcription in the brain at least partially requires TORC as a co-activator. Using ISH analysis we show a markedly divergent distribution of the three TORC isoforms in the rat forebrain, not only between different regions, but also between different cell types. While all 3 isoforms are widely distributed in the brain, TORC1 is the most abundant isoform mRNA and displays high expression levels in most forebrain structures. Although TORC2 and 3 mRNAs are also widely distributed their levels are generally much lower, with TORC3 being the least abundant. However, high levels of TORC2 and 3 mRNA were found at discrete areas in the brain suggesting functional specificity of the different TORC isoforms. TORC3 mRNA was particularly prominent in the ventricular ependyma and pia mater. The distribution pattern of the hybridisation signal shows that all three TORC isoforms are present in regions with a predominance of non-neuronal cell types, including myelinated fibre tracts, ventricular ependyma, and brain membranes. This wide distribution of TORC indicates that expression is not restricted to neurones and suggests that the co-activator is involved in a multiplicity of functions in the brain.
The ISH results show that TORC1 mRNA is clearly more abundant in the forebrain than TORC2 and 3. From a technical standpoint, it is worth noting that the relative abundance of the different TORC isoforms we observed using ISH does not reflect different probe efficiencies. The use of a standard curve for the qRT-PCR analyses in RNA from dissected hippocampal and hypothalamic tissue made it possible to measure absolute values of the different isoform mRNAs, precluding the influence of primer efficiency, and clearly confirmed that the ISH results represent true differential expression of each TORC isoform mRNA.
CREB is involved in a wide variety of neural functions including memory acquisition and consolidation in the hippocampus [25–27]; protection against ischemia-related cell death [28]; the regulation of neuropeptide gene expression [29–32]; dendrite outgrowth in cortical neurones [12]; and the sensitization of striatal mechanisms to cocaine [33]. The wide distribution of TORC mRNAs in the brain especially in regions related to CREB-regulated transcription suggests the involvement of the co-activator in a variety of brain functions with recognised CREB-dependence. CREB is ubiquitously expressed and is regulated by phosphorylation at several different serine sites by a variety of signalling pathways and kinases, including protein kinase A, Ca2+/calmodulin kinase IV, and several kinases in the mitogen-activated protein-kinase cascade. Phospho-CREB dimers bind to cAMP-response elements in the regulatory regions of target genes. Since CREB represents a site of convergence for diverse signalling pathways and the stimuli that activate these pathways, it is likely that differential requirement of co-activators such as TORC determine the specificity of the responses. The high levels of TORC1 mRNA that we find in the hippocampus agree with the results of Zhou et al. [11]. This same study also showed that TORC1 translocates to the nucleus in response to elevated intracellular cyclic AMP and calcium concentrations, and that TORC translocation is required for cyclic AMP/CRE dependent transcription together with the induction and maintenance of long term potentiation [11]. We now show the presence of significant amounts of TORC2 and 3 mRNAs in the hippocampus, which suggests that these two isoforms are also involved in hippocampal function. Similarly, the high expression of TORC1 mRNA we see in the cortical plate is consistent with the finding that TORC1 is required for dendritic outgrowth in cortical neurones in culture [12], and the more recent observation that SIK2 acts to protect cortical neurones against ischemic damage by way of a TORC1-dependent process [34].
In the hypothalamus, all three TORC mRNAs are found in a number of functionally distinct nuclei, with TORC1 and 2 mRNAs generally being expressed at significantly higher levels than TORC3 mRNA. This expression pattern suggests that TORC can regulate CREB-dependent gene expression in neurones concerned with a range of behavioral, autonomic, and neuroendocrine functions. A remarkable finding was the differential expression of the three isoform mRNAs between hypothalamic nuclei, which suggests distinct functional roles for the different isoforms in neuroendocrine activity. While TORC1 was generally the most abundant isoform mRNA in the forebrain, certain hypothalamic nuclei expressed equally high levels of TORC1 and 2 mRNAs. Levels of TORC2 were particularly prominent in the SO, posterior magnocellular part of the PVH and the SCH, with levels being as high as those of TORC1 anywhere else in the forebrain. The presence of TORC mRNAs in the PVH is consistent with recent reports demonstrating the involvement of TORC in the regulation of CRH transcription [15] and the presence of immunoreactive TORC2 in the PVH [16]. Immunoreactive TORC2 is found in CRH neurones where it undergoes translocation to the nucleus and binding to the CRH promoter during stress [16]. Although studies using overexpression and siRNA blockade of TORC in the hypothalamic cell line 4B and primary cultures of hypothalamic neurones demonstrated that all 3 TORC isoforms influence CRH gene transcription, TORC2 appeared to be the most important [15]. The present demonstration of high TORC2 mRNA expression in the medial parvicellular PVH is consistent with these findings. It is noteworthy that mRNAs for all 3 TORC isoforms were also found in the posterior magnocellular part of the PVH and in the SO. At least for TORC2, the present finding is in keeping with the previous demonstration of high levels of immunoreactive TORC2 in magnocellular neurones of the PVH and SO [16], and supports a role for TORC in the regulation of magnocellular neuroendocrine function.
It should be emphasized that in the SCH there were notable differences of expression between the three isoform mRNAs within the nucleus. TORC2 mRNA was more prominent in the dorsomedial part where the majority of neurones express vasopressin [35,36], whereas TORC1 mRNA was more heavily expressed in the ventrolateral region that contains more VIP neurones [37,38]. On the other hand, TORC3 mRNA is evenly distributed throughout the SCH but at much lower levels than the other two isoform mRNAs. CREB is involved in SCH function and CREB-dependent transcription shows daily variations [39]. The prominence of TORC in the SCH and differential topographic distribution of the mRNA isoforms within the nucleus suggest region-specific roles for the different isoforms in its function.
TORC was first characterised as a key regulator of CREB-dependent transcription of gluconeogenic enzymes in the liver [40,41]. Furthermore, studies in Drosophila have shown that starvation triggers TORC activation, which is critical for maintaining energy balance through induction of CREB target genes in the brain [9]. In this context, it is striking that some of the most prominent expression of TORC1 and 2 mRNA in the hypothalamus is in the PVH, VMH, DMH, and ARH, which are nuclei heavily implicated in the hypothalamic control of food intake and energy metabolism [42–44]. These findings are consistent with a role for TORC in the central mechanisms controlling energy homeostasis in the rat.
The generally similar distribution of all three TORC isoform mRNAs in many regions of the forebrain (eg. field CA1 and the DG of the dorsal hippocampal formation) raises the possibility that at least some neurones or glia cells express all three isoforms. However, the functional implications of multiple TORC isoforms in the same cell are currently unknown. In this regard, it is worth noting that in addition to its role as CREB co-activator, recent studies have associated TORC with other transcription factors such as the homeobox protein MEIS1A in the transcriptional regulation of Hoxb2 and Meis1 independently of CREB [45]. Thus it is possible that at some locations TORC may have a role other than as CREB co-activator.
In summary, we show that the mRNAs encoding all 3 recognised isoforms of the CREB co-activator TORC are broadly expressed in the rat forebrain, but that each shows very different levels of expression within particular regions. Not only are all three mRNAs found in neurones, but they are all detectable in other cell types in the brain. These findings are consistent with the notion that the various TORC isoforms play important modulatory roles on a wide variety of different functions in the central nervous system. They provide the anatomical basis for further studies on the role that TORC isoforms play in regulating CREB-dependent gene transcription in the brain.
ACKNOWLEDGEMENTS
This project was supported by NINDS NS029728 (AGW) and NICHD intramural funds (GA).
REFERENCES
- 1.Kaang BK, Kandel ER, Grant SG. Activation of cAMP-responsive genes by stimuli that produce long-term facilitation in Aplysia sensory neurons. Neuron. 1993;10:427–435. doi: 10.1016/0896-6273(93)90331-k. [DOI] [PubMed] [Google Scholar]
- 2.Frank DA, Greenberg ME. CREB: a mediator of long-term memory from mollusks to mammals. Cell. 1994;79:5–8. doi: 10.1016/0092-8674(94)90394-8. [DOI] [PubMed] [Google Scholar]
- 3.Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- 4.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 5.Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB, Montminy M. TORCs: transducers of regulated CREB activity. Mol Cell. 2003;12:413–423. doi: 10.1016/j.molcel.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Iourgenko V, Zhang W, Mickanin C, Daly I, Jiang C, Hexham JM, Orth AP, Miraglia L, Meltzer J, Garza D, Chirn GW, McWhinnie E, Cohen D, Skelton J, Terry R, Yu Y, Bodian D, Buxton FP, Zhu J, Song C, Labow MA. Identification of a family of cAMP response element-binding protein coactivators by genome-scale functional analysis in mammalian cells. Proc Natl Acad Sci U S A. 2003;100:12147–12152. doi: 10.1073/pnas.1932773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takemori H, Kajimura J, Okamoto M. TORC-SIK cascade regulates CREB activity through the basic leucine zipper domain. FEBS J. 2007;274:3202–3209. doi: 10.1111/j.1742-4658.2007.05889.x. [DOI] [PubMed] [Google Scholar]
- 8.Bittinger MA, McWhinnie E, Meltzer J, Iourgenko V, Latario B, Liu X, Chen CH, Song C, Garza D, Labow M. Activation of cAMP response element-mediated gene expression by regulated nuclear transport of TORC proteins. Curr Biol. 2004;14:2156–2161. doi: 10.1016/j.cub.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Wang B, Goode J, Best J, Meltzer J, Schilman PE, Chen J, Garza D, Thomas JB, Montminy M. The insulin-regulated CREB coactivator TORC promotes stress resistance in Drosophila. Cell Metab. 2008;7:434–444. doi: 10.1016/j.cmet.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovács KA, Steullet P, Steinmann M, Do KQ, Magistretti PJ, Halfon O, Cardinaux JR. TORC1 is a calcium- and cAMP-sensitive coincidence detector involved in hippocampal long-term synaptic plasticity. Proc Natl Acad Sci U S A. 2007;104:4700–4705. doi: 10.1073/pnas.0607524104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Wu H, Li S, Chen Q, Cheng XW, Zheng J, Takemori H, Xiong ZQ. Requirement of TORC1 for late-phase long-term potentiation in the hippocampus. PLoS One. 2006 Dec 20;1:e16. doi: 10.1371/journal.pone.0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Zhang C, Takemori H, Zhou Y, Xiong ZQ. TORC1 regulates activity-dependent CREB-target gene transcription and dendritic growth of developing cortical neurons. J Neurosci. 2009;29:2334–2343. doi: 10.1523/JNEUROSCI.2296-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Than T, Lou H, Ji C, Win S, Kaplowitz N. Role of CREB regulated transcription coactivator 3 (CRTC3) in the initiation of mitochondrial biogenesis and stress response in liver cells. J Biol Chem. 2011 doi: 10.1074/jbc.M111.240481. (May 2-ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song Y, Altarejos J, Goodarzi MO, Inoue H, Guo X, Berdeaux R, Kim JH, Goode J, Igata M, Paz JC, Hogan MF, Singh PK, Goebel N, Vera L, Miller N, Cui J, Jones MR, Chen YD, Taylor KD, Hsueh WA, Rotter JI, Montminy M CHARGE Consortium; GIANT Consortium. CRTC3 links catecholamine signalling to energy balance. Nature. 2010;468:933–939. doi: 10.1038/nature09564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Coello AG, Grinevich V, Aguilera G. Involvement of transducer of regulated cAMP response element-binding protein activity on corticotropin releasing hormone transcription. Endocrinology. 2010;151:1109–1118. doi: 10.1210/en.2009-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Knobloch HS, Grinevich V, Aguilera G. Stress Induces Parallel Changes in Corticotrophin-Releasing Hormone (CRH) Transcription and Nuclear Translocation of Transducer of Regulated cAMP Response Element-Binding Activity 2 in Hypothalamic CRH Neurones. J Neuroendocrinol. 2011;23:216–223. doi: 10.1111/j.1365-2826.2010.02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swanson LW. Brain Maps: Structure of the Rat Brain. Third Edition. San Diego: Academic Press; 2004. [Google Scholar]
- 18.Watts AG, Tanimura SM, Sanchez-Watts G. Crh and Avp gene transcription in the hypothalamic paraventricular nucleus of unstressed rats: daily rhythms and their corticosterone dependence. Endocrinology. 2004;145:529–540. doi: 10.1210/en.2003-0394. [DOI] [PubMed] [Google Scholar]
- 19.Watts AG, Sanchez-Watts G. Physiological regulation of peptide messenger RNA colocalization in rat hypothalamic paraventricular medial parvicellular neurons. J Comp Neurol. 1995;352:501–514. doi: 10.1002/cne.903520403. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Kamitakahara A, Kim AJ, Aguilera G. Cyclic adenosine 3',5'-monophosphate responsive element binding protein phosphorylation is required but not sufficient for activation of corticotropin-releasing hormone transcription. Endocrinology. 2008;149:3512–3520. doi: 10.1210/en.2008-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Cao JL, Vialou VF, Lobo MK, Robison AJ, Neve RL, Cooper DC, Nestler EJ, Han MH. Essential role of the cAMP-cAMP response-element binding protein pathway in opiate-induced homeostatic adaptations of locus coeruleus neurons. Proc Natl Acad Sci U S A. 2010;107:17011–17016. doi: 10.1073/pnas.1010077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem. 2011;116:1–9. doi: 10.1111/j.1471-4159.2010.07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barco A, Pittenger C, Kandel ER. CREB, memory enhancement and the treatment of memory disorders: promises, pitfalls and prospects. Expert Opin Ther Targets. 2003;7:101–114. doi: 10.1517/14728222.7.1.101. [DOI] [PubMed] [Google Scholar]
- 26.Izquierdo LA, Barros DM, Vianna MR, Coitinho A, deDavid e Silva T, Choi H, Moletta B, Medina JH, Izquierdo I. Molecular pharmacological dissection of short- and long-term memory. Cell Mol Neurobiol. 2002;22:269–287. doi: 10.1023/A:1020715800956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens CF. CREB and memory consolidation. Neuron. 1994;13:769–770. doi: 10.1016/0896-6273(94)90244-5. [DOI] [PubMed] [Google Scholar]
- 28.Mabuchi T, Kitagawa K, Kuwabara K, Takasawa K, Ohtsuki T, Xia Z, Storm D, Yanagihara T, Hori M, Matsumoto M. Phosphorylation of cAMP response element-binding protein in hippocampal neurons as a protective response after exposure to glutamate in vitro and ischemia in vivo. J Neurosci. 2001;21:9204–9213. doi: 10.1523/JNEUROSCI.21-23-09204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfl S, Martinez C, Majzoub JA. Inducible binding of cyclic adenosine 3',5'-monophosphate (cAMP)-responsive element binding protein (CREB) to a cAMP-responsive promoter in vivo. Mol Endocrinol. 1999;13:659–669. doi: 10.1210/mend.13.5.0282. [DOI] [PubMed] [Google Scholar]
- 30.Nikodemova M, Kasckow J, Liu H, Manganiello V, Aguilera G. Cyclic adenosine 3',5'-monophosphate regulation of corticotropin-releasing hormone promoter activity in AtT-20 cells and in a transformed hypothalamic cell line. Endocrinology. 2003;144:1292–1300. doi: 10.1210/en.2002-220990. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto K, Zanger K, Hollenberg AN, Cohen LE, Radovick S, Wondisford FE. cAMP response element-binding protein-binding protein mediates thyrotropin-releasing hormone signaling on thyrotropin subunit genes. J Biol Chem. 2000;275:33365–33372. doi: 10.1074/jbc.M006819200. [DOI] [PubMed] [Google Scholar]
- 32.Boutillier AL, Gaiddon C, Lorang D, Roberts JL, Loeffler JP. Transcriptional activation of the proopiomelanocortin gene by cyclic AMP-responsive element binding protein. Pituitary. 1998;1:33–43. doi: 10.1023/a:1009966808106. [DOI] [PubMed] [Google Scholar]
- 33.Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C, Conkright MD, Kenny PJ. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466:197–202. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki T, Takemori H, Yagita Y, Terasaki Y, Uebi T, Horike N, Takagi H, Susumu T, Teraoka H, Kusano K, Hatano O, Oyama N, Sugiyama Y, Sakoda S, Kitagawa K. SIK2 is a key regulator for neuronal survival after ischemia via TORC1-CREB. Neuron. 2011;69:106–119. doi: 10.1016/j.neuron.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Vandesande F, Dierick K, De Mey J. Identification of the vasopressin-neurophysin producing neurons of the rat suprachiasmatic nuclei. Cell Tiss Res. 1975;156:377–380. doi: 10.1007/BF00225365. [DOI] [PubMed] [Google Scholar]
- 36.van den Pol AN, Tsujimoto KL. Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience. 1985;15:1049–1086. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]
- 37.Card JP, Brecha N, Karten HJ, Moore RY. Immunocytochemical localization of vasoactive intestinal polypeptide-containing cells and processes in the suprachiasmatic nucleus of the rat: light and electron microscopic analysis. J Neurosci. 1981;1:1289–1303. doi: 10.1523/JNEUROSCI.01-11-01289.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J Comp Neurol. 1987;258:230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- 39.Obrietan K, Impey S, Smith D, Athos J, Storm DR. Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. Biol Chem. 1999;274:17748–17756. doi: 10.1074/jbc.274.25.17748. [DOI] [PubMed] [Google Scholar]
- 40.Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR, 3rd, Takemori H, Okamoto M, Montminy M. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 42.Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294:E827–E832. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 44.Watts AG, Donovan CD. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol. 2010;31:32–43. doi: 10.1016/j.yfrne.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goh S, Looi Y, Shen H, Fang J, Bodner C, Houle M, Ng A-H, Screaton RA, Featherstone M. Transcriptional activation by MEIS1A in response to protein kinase A signaling requires the transducers of regulated CREB family of CREB co-activators. J Biol Chem. 2009;284:18904–18912. doi: 10.1074/jbc.M109.005090. [DOI] [PMC free article] [PubMed] [Google Scholar]