Highlights
► The role of DsbA and DsbD in cytochrome c maturation in E. coli was probed. ► DsbA is not essential for holocytochrome c production. ► DsbD is important but not essential for cytochrome c maturation. ► A model is proposed for cytochrome c biosynthesis in the periplasm of E. coli. ► Heme attachment to and oxidation of the apocytochrome are competing processes.
Keywords: Cytochrome c, DsbA, DsbD, Disulfide bond, Cysteine, Heme
Abstract
Heme attachment to c-type cytochromes in bacteria requires cysteine thiols in the CXXCH motif of the protein. The involvement of the periplasmic disulfide generation system in this process remains unclear. We undertake a systematic evaluation of the role of DsbA and DsbD in cytochrome c biogenesis in Escherichia coli and show unequivocally that DsbA is not essential for holocytochrome production under aerobic or anaerobic conditions. We also prove that DsbD is important but not essential for maturation of c-type cytochromes. We discuss the findings in the context of a model in which heme attachment to, and oxidation of, the apocytochrome are competing processes.
1. Introduction
c-Type cytochromes are proteins that contain covalently bound heme and are essential for the life of numerous organisms from all kingdoms of life. In Gram-negative bacteria the heme attachment reaction, typically to the two cysteines of a CXXCH protein motif, is generally performed in the oxidizing environment of the periplasm by the cytochrome c maturation (Ccm) system consisting of eight proteins (CcmA–H) [1,2]. Oxidation of the cysteine thiols to a disulfide might reasonably be expected to prevent the heme attachment reaction, creating a chemical paradox that needs to be resolved.
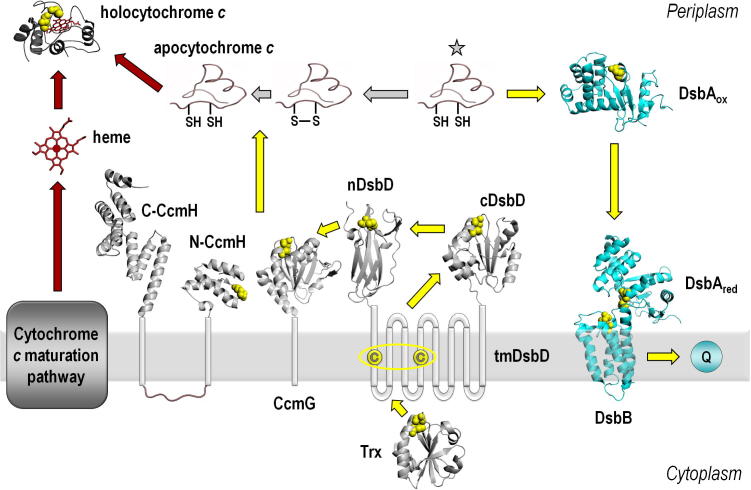
The periplasmic protein DsbA is crucial for the oxidative folding of extracytoplasmic and extracellular proteins that require disulfide bonds [3] and is linked to the virulence of many pathogens [4]. The characteristics of the modified thioredoxin-like structure of DsbA [5], along with other considerations [6,7], have given insight into its strongly oxidizing properties. DsbB reoxidizes DsbA [8] and transfers electrons to the respiratory chain [9]. The presence of DsbA creates a requirement for proteins with reducing functions in the periplasm to reverse the oxidation reaction and make the apocytochrome thiol groups available for heme attachment. The pathway for reductant provision to the Ccm system as it is currently understood in Escherichia coli, along with DsbA and DsbB, are shown in Fig. 1.
Fig. 1.
Scheme illustrating the proteins affecting the oxidation state of the CXXCH motif of the apocytochrome before covalent heme attachment in the bacterial periplasm. Apocytochrome enters the periplasm in a reduced state (both cysteines in a thiol form, indicated with a star) and can be oxidized by the protein DsbA which then transfers the acquired electrons to the membrane protein DsbB and itself is restored to its active oxidized state. DsbB passes the electrons to the respiratory chain through quinone (Q). The oxidized apocytochrome (disulfide bond between the cysteines of the CXXCH motif) is reduced by proteins CcmG and/or CcmH of the Ccm system. The necessary reducing power originates from cytoplasmic thioredoxin (Trx). Reductant is transferred sequentially to tmDsbD, cDsbD and nDsbD; the latter is the reductant provider for several periplasmic pathways. The reduced apocytochrome can then have heme attached by the heme-handling proteins CcmA–F. Yellow arrows indicate electron flow and red arrows indicate steps involving heme handling. Cysteines are depicted as yellow spheres. The PDB entries used are 2TRX, 1FVK, 2HI7, 2FWH, 1JPE, 2BLK, 2E2E, and 155C. Structures were rendered in Pymol (DeLano, W.L. The Pymol Molecular Graphics System (2002) http://www.pymol.org).
DsbD is the sole provider of reducing power to the periplasm. Reductant is needed when incorrectly formed disulfides are inserted into proteins with more than two cysteines, and an isomerase is required, or presumably if c-type cytochrome apoproteins acquire disulfide bonds. DsbD is a three-domain protein comprising a transmembrane region with a soluble periplasmic domain at each terminus [10]. It interacts with thioredoxin in the cytoplasm and, via a disulfide cascade [11], transfers reductant to multiple periplasmic partners [12]. DsbC, a dimeric periplasmic disulfide isomerase [13] as well as TrbB, a disulfide bond thioredoxin-like isomerase involved in bacterial conjugation [14] interact with the N-terminal domain of DsbD (nDsbD). For transfer of reductant to the Ccm pathway, nDsbD interacts with the protein CcmG [15]. In the Ccm system CcmG and CcmH (Fig. 1) have been implicated in thiol-oxidoreductase functions. CcmG has a thioredoxin fold but it is not clear whether it interacts with apocytochromes directly, or via thiol–disulfide exchange with CcmH. CcmH has a pair of cysteine residues, in a three-helix bundle fold [16], though the cysteine residues are not essential for cytochrome c biogenesis under all growth conditions [17,18].
Several studies have investigated the requirement for DsbA and the involvement of DsbD in cytochrome c maturation, with different conclusions. Contrary to intuitive expectation, in E. coli strains lacking dsbA, the absence of endogenous cytochromes c under anaerobic conditions has been reported [19]. The absence of DsbA also resulted in failure to mature an exogenous mono-heme cytochrome c [20]. DsbB was found to be essential for cytochrome c biogenesis [21], consistent with its role as oxidant of DsbA. These observations were taken as an indication that the formation of disulfide bonds was an obligate step, rather than an undesirable diversion from the heme attachment reaction to apocytochromes. Also, it has been thought that the breakage of the S–S bond could provide a driving force for the formation of the thioether bonds of cytochromes to heme [22]. DsbD was shown to be absolutely essential for cytochrome c maturation in E. coli [23]. Lack of its analogue, CcdA, was also found to impair cytochrome c production in Rhodobacter capsulatus [24].
More recently, the failure of DsbA deletion strains to produce cytochromes in E. coli was found to vary according to the specific cytochrome involved. A cytochrome c from a hyperthermophile was produced at greater than wild-type levels in a ΔdsbA strain [25] and a c-type variant of cytochrome b562 was matured under aerobic conditions abundantly and correctly despite the absence of DsbA [26]. Experiments in R. capsulatus showed that deletion of dsbA did not result in loss of c-type cytochromes [27] but that lower cytochrome c levels are matured in ΔdsbA [24]. It was also shown that in R. capsulatus and Bacillus subtilis lack of the oxidative proteins (DsdA/DsbB and BdbC/BdbD, respectively) counteracts the cytochrome c deficiency in ΔccdA strains [24,27,28]. However, in E. coli, the paradigm for study of c-type cytochrome biogenesis, such comparative studies with isogenic strains have not been done and thus there are important unresolved contradictions between the original [19–21] and more recent publications.
In this work we have sought to clarify the involvement of DsbA and DsbD in cytochrome c biogenesis by examining several endogenous and exogenous cytochromes, with both mono- and multi-heme examples. By using a full set of gene deletion strains and large culture volumes (combined with extracted periplasms being concentrated in small volumes) to ensure that even low levels of cytochrome c are detected, we assess the contribution of DsbA and DsbD, both individually and in combination, under aerobic and anaerobic conditions.
2. Materials and methods
All bacterial strains and plasmids used in this study are listed in Table 1.
Table 1.
Bacterial strains and plasmids used in this work.
| Name | Description | Source |
|---|---|---|
| MC1000 | araD139, Δ(ara, leu)7697, ΔlacX74, galU, galK, strA | [45] |
| MC1000 (ΔdsbA) | MC1000 ΔdsbA::kan | Lab stock |
| MC1000 (ΔdsbD) | MC1000 ΔdsbD | [46] |
| MC1000 (ΔdsbA/ΔdsbD) | MC1000 ΔdsbA::kan, ΔdsbD | Lab stock |
| pEC86 | E. coli ccmABCDEFGH, CamR | [29] |
| pRZ001 | P. denitrificans cytochrome cd1, GentR | Lab stock |
| pKPD1 | P. denitrificans c550, AmpR | [47] |
2.1. Cell growth
Aerobic cell growth was conducted in 200 ml 2×TY medium (16 g l−1 peptone, 10 g l−1 yeast extract, 5 g l−1 NaCl) in 2.5 l flasks. Cultures were inoculated from single colonies and incubated at 37 °C for 15–18 h with shaking at 200 rpm. Fully aerobic growth conditions prevented expression of the endogenous E. coli Ccm system and the Ccm operon was constitutively expressed from plasmid pEC86 [29]. 1 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) was added to the cultures from inoculation. 100 μg ml−1 ampicillin, 20 μg ml−1 gentamicin and 34 μg ml−1 chloramphenicol were used when appropriate.
Cells were grown anaerobically, allowing the expression of the endogenous Ccm system, for 24 h in 1 l bottles filled to the top with growth media at 37 °C without shaking, inoculated from overnight starter cultures (also grown at 37 °C). Growth media were prepared as described previously [30]. 10 mM nitrate (or 5 mM nitrite in the case of expression of endogenous cytochromes) was used as terminal electron acceptor. 100 μg ml−1 ampicillin or 20 μg ml−1 gentamicin, were added when appropriate; for the expression of the endogenous E. coli cytochromes no antibiotics were used. Cytochrome c550 was induced with 1 mM IPTG from inoculation and cytochrome cd1 was induced by autoinduction [31]. When needed, l-cystine (Sigma) was added to the media from inoculation at a final concentration of 5 mM (l-cystine was dissolved in 1 M HCl at a concentration of 5% w/v before addition to the growth media).
2.2. Characterization of the cytochrome c content of periplasmic fractions
For extraction of the periplasmic fractions, cells were harvested and sphaeroplasted as described [32]. At least 6 replicates of each experiment were conducted. The production of cytochrome c550 was quantified by visible absorption spectroscopy on a Varian Cary 50 Bio spectrophotometer. Samples were normalized according to wet-cell pellet weights and were reduced by the addition of a few grains of disodium dithionite (Sigma). Absorbance values at 550 nm were used.
The production of cytochromes cd1, NapB and NrfA was quantified by SDS–PAGE analysis followed by densitometry on heme-staining bands. SDS–PAGE analysis was carried out on 12% Bis–Tris NuPAGE gels (Invitrogen) using MES–SDS or MOPS–SDS running buffer prepared according to Invitrogen specifications and loading prestained protein markers (Invitrogen, SeeBlue Plus 2). Gels were stained for covalently bound heme according to the method of Goodhew [33]. Gel loadings were normalized according to wet-cell pellet weights. Densitometry was used to quantify cytochrome c production using GeneSnap (SYNGENE). The linear relationship between the amount of mature cytochrome c present on the gel and the amount detected by densitometry was ensured by using subsaturated loading on gels [34].
Errors on the levels of cytochrome c production were calculated for datasets collected on different days. On average these values have a% error of 8%; details of the errors for each set of experiments can be found in Table 2.
Table 2.
Levels of cytochromes c produced under aerobic and anaerobic conditions in a set of four bacterial strains. The levels for cytochrome c550 were determined spectroscopically whereas for cytochromes cd1, NapB and NrfA SDS–PAGE analysis followed by densitometry on heme-stained gels was used. At least 6 replicates were done for each experiment and errors for each dataset are given in brackets. The amount of cytochrome produced by the wild-type strain is arbitrarily set to 100.
|
Aerobic conditions | |||
|---|---|---|---|
| Cytochrome c550 |
Cytochrome cd1 |
||
| Strain | Level of cytochrome c | Strain | Level of cytochrome c |
| MC1000 | 100 (±9) | MC1000 | 100 (±13) |
| ΔdsbA | 94 (±2) | ΔdsbA | 100 (±13) |
| ΔdsbD | 6 (±6) | ΔdsbD | 29 (±10) |
| ΔdsbA/ΔdsbD | 53 (±12) | ΔdsbA/ΔdsbD | 54 (±19) |
| Anaerobic conditions | |||
| Cytochrome c550 | Cytochrome cd1 | ||
| Strain | Level of cytochrome c | Strain | Level of cytochrome c |
| MC1000 | 100 (±8) | MC1000 | 100 (±8) |
| ΔdsbA | 30 (±0) | ΔdsbA | 39 (±8) |
| ΔdsbD | 10 (±5) | ΔdsbD | 23 (±4) |
| ΔdsbA/ΔdsbD | 21 (±19) | ΔdsbA/ΔdsbD | 31 (±10) |
| Cytochrome NapB | Cytochrome NrfA | ||
| Strain | Level of cytochrome c | Strain | Level of cytochrome c |
| MC1000 | 100 (±4) | MC1000 | 100 (±2) |
| ΔdsbA | 44 (±7) | ΔdsbA | 17 (±6) |
| ΔdsbD | 4 (±13) | ΔdsbD | 6 (±3) |
| ΔdsbA/ΔdsbD | 39 (±1) | ΔdsbA/ΔdsbD | 13 (±2) |
3. Results and discussion
3.1. Cytochrome c production under aerobic growth conditions
E. coli does not express its endogenous c-type cytochromes under aerobic conditions. We used two exogenous cytochromes c (cytochromes c550 and cd1 from Paracoccus denitrificans) as test substrates for the Ccm system in different genetic backgrounds (wild-type, ΔdsbA, ΔdsbD and ΔdsbA/ΔdsbD, see Table 1). Cytochrome cd1 is produced in its semi-apo form (i.e. without d1 heme and with heme attached to the c-type cytochrome domain) as the d1 heme cofactor is not produced by E. coli. The Ccm proteins were expressed constitutively from plasmid pEC86 [29].
Table 2 shows the quantitation of the levels of production of the two cytochromes as determined spectroscopically (for c550) and densitometrically (for semi-apo cd1). The amount of holocytochrome in the wild-type (WT) strain MC1000 is arbitrarily set to 100. For both substrates the heme attachment levels in ΔdsbA were very close to those observed in the parental strain. Around 50% levels were detected in the double deletion strain ΔdsbA/ΔdsbD in both cases. In the situation where both the oxidizing protein (DsbA) and reducing protein (DsbD) are absent it might be expected that cytochrome levels would approach those seen in the WT strain. However, in the heavily aerated cultures used here, we expect that oxygen would make a significant contribution to spontaneous disulfide bond formation in the periplasm. DsbD would therefore be needed to reduce the proportion of cytochrome that became oxidized via that route. In ΔdsbD we found detectable levels of holocytochrome c550 and significant amounts of holocytochrome cd1. These observations contrast with previous reports that DsbD is absolutely essential for cytochrome c maturation in E. coli [35] although the latter study was performed under anaerobic conditions. It has, however, been shown that DsbD is not needed for production of cytochromes c in Pseudomonas aeruginosa [36] grown aerobically. The absence of DsbD would be expected to attenuate cytochrome levels, as we have observed, because no reductant is being provided to reverse the oxidizing effects of both DsbA and oxygen.
The difference in the relative maturation levels between the two substrate cytochromes is possibly due to their different properties. The accessibility of the pair of cysteine thiols on an apocytochrome delivered to the periplasm will depend on the folding rate of the apoprotein once it is transported by the Sec system. Also, the reduction potential of the thiol–disulfide couple, and the pKa values of the cysteine pair of the CXXCH motif, will determine their tendency for oxidation. The potentials could vary dramatically; for example in thioredoxins, which also have a CXXC motif, the character of the XX values, the effect of the helix dipole, and other factors have significant effects on the reduction potentials [37].
3.2. Cytochrome c production under anaerobic growth conditions
A comparable set of growths to those described above were performed in anaerobic cultures. E. coli grown under these conditions produces its endogenous cytochromes c [38]; two of these are soluble periplasmic proteins that we have quantitated here, along with the exogenous cytochromes c550 and cd1. The endogenous NapB is a di-heme cytochrome c involved in nitrate reduction [39] and NrfA is a pentaheme nitrite reductase that has one of its hemes attached to an unusual CXXCK motif [40]. Results are presented in Table 2. There are variations in the heme attachment levels seen for the different cytochromes in the various strains but the trends are the same in all cases. There is a significant decrease in holocytochrome level in ΔdsbA compared to WT, a similar level when comparing ΔdsbA with ΔdsbA/ΔdsbD, and a reduction in ΔdsbD, but not to undetectable levels in any case.
In ΔdsbA the significant decrease in holocytochrome level compared to the aerobic cultures highlights the effect of spontaneous disulfide bond formation under aerobic but not under anaerobic conditions. The presence of an oxidant appears to increase holocytochrome levels (as long as a source of reductant (DsbD) is also present) possibly because the disulfide stabilizes the apocytochrome; apocytochromes are readily degraded in the periplasm and are usually not detectable when heme has not been attached to them [41]. It could also be that the oxidized apocytochrome is protected from degradation while interacting with the reducing components of the Ccm system (CcmG or CcmH) and that the most productive holocytochrome biogenesis pathway is one in which the apocytochrome is oxidized, then reduced by the Ccm proteins before heme attachment. To provide evidence for this hypothesis, we measured holocytochrome c550 levels in anaerobic cultures of ΔdsbA supplemented with an oxidant in the growth medium (5 mM l-cystine) and found that it fully complements for the lack of DsbA. For a given set of cultures the amount of holo-c550 produced in ΔdsbA is 39% of what is produced in the parental strain whereas the same strain grown in the presence of 5 mM l-cystine yields 106% holo-c550. It seems therefore, that DsbA can be replaced not only by oxygen, but by chemical oxidants, in increasing holocytochrome c production.
Under anaerobic conditions, similar levels of holocytochrome in ΔdsbA and ΔdsbA/ΔdsbD are expected. In both cases reduced apocytochrome enters the periplasm and encounters no oxidant (DsbA or oxygen); the presence of DsbD is therefore irrelevant. Hence, the Ccm system attaches heme to the same fraction of apocytochrome in each of the two strains, and the remainder becomes degraded. Again the different properties of the individual cytochromes appear to affect their levels of maturation in the strains examined. These effects are most obvious in the ΔdsbD strain (where the level of competition between the Ccm system and DsbA depends on the intrinsic properties of each cytochrome) and the ΔdsbA/ΔdsbD strain (where any source of reductant and oxidant, except for spontaneous reoxidation, are removed). More cytochrome cd1 is produced in ΔdsbD (compared to the other cytochromes), both aerobically and anaerobically. Its large size, along with the fact that the protein forms a dimer [42], could protect the CXXCH motif of each monomer from immediate oxidation by DsbA. A larger variation can be seen for the amounts of holocytochromes produced in the ΔdsbA/ΔdsbD strain, varying from ∼15% for NrfA to ∼40% for NapB. With any source of oxidant (DsbA, oxygen or chemical) absent, the tendency of the CXXCH motif to find itself in the dead-end situation of disulfide bond formation (since DsbD is absent) could depend on the microenvironment around each motif, which varies for the four substrates (differences in folding rate, number of CXXCH motifs, pKa values, and reduction potentials of the cysteine pair being contributing factors).
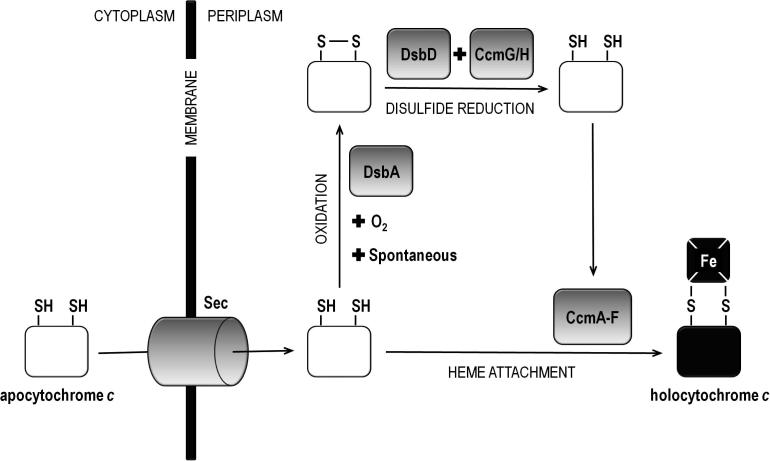
We propose a model for E. coli (shown in Fig. 2) in which the apocytochrome can undergo the competing processes of either heme attachment to, or oxidation of, its cysteine thiols. A proportion of the apoprotein interacts with the Ccm heme attachment proteins directly, involving no thiol–disulfide reactions (for this fraction of apocytochrome DsbD and CcmG/H are irrelevant). Another fraction of the apoprotein instead is oxidized by DsbA (or oxygen when present); the apoform of a CXXCH-containing variant of cytochrome b562 with a disulfide in its CXXCH motif has been show to be formed [26]. This fraction then is dependent on the presence of the reducing proteins for covalent heme attachment. The ratios of the apocytochrome that will enter each of these pathways will depend on the properties of the individual proteins as illustrated by the varied relative levels of the different substrates in the different strains examined here. It should be taken into account that for multi-heme cytochromes (like NapB and NrfA) it is likely that CXXCH motifs on the same polypeptide chain have different properties. Also attachment of heme to one of the motifs might affect the subsequent heme attachment to the remaining ones. A previous study indicated that a heme binding motif incorporated into a thioredoxin-like fold did not result in high levels of heme attachment [43] demonstrating that the efficiency of heme attachment to CXXCH can be very low depending on the properties of the individual substrate.
Fig. 2.
Model illustrating the involvement of DsbA, DsbD and the Ccm system in the formation of holocytochrome c in E. coli. Upon entering into the periplasm, through the Sec system, the reduced and unfolded apocytochrome can undergo the competing processes of either heme attachment to, or oxidation of, its cysteine thiols. A fraction of the apoprotein interacts with the Ccm heme attachment proteins (CcmA–F) directly. Another fraction instead is oxidized by with DsbA (or oxygen when present). Then it is dependent on the presence of the reducing proteins DsbD and CcmG/H, which eventually deliver it in the reduced state to CcmA–F, for heme attachment. The final product, holocytochrome c, can be produced via both pathways.
3.3. Concluding remarks
DsbA is a powerful oxidase that functions outside the cytoplasmic membranes of many bacteria and has a necessarily broad substrate specificity. c-Type cytochromes, with their characteristic CXXCH heme-binding motifs, are delivered to the periplasm by the Sec system in their unfolded form [44] and therefore easily become targets for DsbA-catalyzed disulfide-bond formation. The original studies, although a complementation with some small molecule disulfides was seen [20], erroneously concluded that DsbA is essential for holocytochrome c production [19–21]. We suspect that the experimental conditions used [19–21] fortuitously led to this conclusion. The present work establishes that the absence of DsbA does have consequences for c-type cytochrome formation in E. coli for a variety of reasons as explored here which may also relate to comparable observations made in R. capsulatus. The Ccm proteins are able to reverse the oxidation event allowing heme attachment to occur; DsbD functions as a house-keeping protein that corrects the unwanted oxidation of apocytochromes. This, along with the potential stabilization of the oxidized apocytochrome, seems to be the most efficient way for these two essential but opposing pathways to coexist in the periplasm but the key point is that mechanistically it is now unambiguous that a disulfide bond is not a prerequisite for heme attachment to a CXXCH motif catalyzed by the Ccm system.
Acknowledgements
This work was supported by the Wellcome Trust (Grant Number 092532 and a Value-in-People Award) and the Biotechnology and Biological Sciences Research Council (Grant BB/H017887/1). D.A.I.M. acknowledges funding from the Alexander S. Onassis Public Benefit Foundation and the E.P.A. Cephalosporin Fund. We are grateful to Prof. Linda Thöny-Meyer for providing us with strains MC1000ΔdsbA and MC1000ΔdsbA/ΔdsbD.
Contributor Information
Stuart J. Ferguson, Email: stuart.ferguson@bioch.ox.ac.uk.
Julie M. Stevens, Email: julie.stevens@bioch.ox.ac.uk.
References
- 1.Kranz R.G., Richard-Fogal C., Taylor J.S., Frawley E.R. Cytochrome c biogenesis: mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol. Mol. Biol. Rev. 2009;73:510–528. doi: 10.1128/MMBR.00001-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens J.M., Mavridou D.A., Hamer R., Kritsiligkou P., Goddard A.D., Ferguson S.J. Cytochrome c biogenesis System I. FEBS J. 2011;278:4170–4178. doi: 10.1111/j.1742-4658.2011.08376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shouldice S.R., Heras B., Walden P.M., Totsika M., Schembri M.A., Martin J.L. Structure and function of DsbA, a key bacterial oxidative folding catalyst. Antioxid. Redox. Signal. 2011;14:1729–1760. doi: 10.1089/ars.2010.3344. [DOI] [PubMed] [Google Scholar]
- 4.Peek J.A., Taylor R.K. Characterization of a periplasmic thiol:disulfide interchange protein required for the functional maturation of secreted virulence factors of Vibrio cholerae. Proc. Natl. Acad. Sci. U S A. 1992;89:6210–6214. doi: 10.1073/pnas.89.13.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin J.L., Bardwell J.C., Kuriyan J. Crystal structure of the DsbA protein required for disulphide bond formation in vivo. Nature. 1993;365:464–468. doi: 10.1038/365464a0. [DOI] [PubMed] [Google Scholar]
- 6.Grauschopf U., Winther J.R., Korber P., Zander T., Dallinger P., Bardwell J.C. Why is DsbA such an oxidizing disulfide catalyst? Cell. 1995;83:947–955. doi: 10.1016/0092-8674(95)90210-4. [DOI] [PubMed] [Google Scholar]
- 7.Zapun A., Bardwell J.C., Creighton T.E. The reactive and destabilizing disulfide bond of DsbA, a protein required for protein disulfide bond formation in vivo. Biochemistry. 1993;32:5083–5092. doi: 10.1021/bi00070a016. [DOI] [PubMed] [Google Scholar]
- 8.Inaba K., Murakami S., Nakagawa A., Iida H., Kinjo M., Ito K., Suzuki M. Dynamic nature of disulphide bond formation catalysts revealed by crystal structures of DsbB. EMBO J. 2009;28:779–791. doi: 10.1038/emboj.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inaba K., Ito K. Structure and mechanisms of the DsbB–DsbA disulfide bond generation machine. Biochim. Biophys. Acta. 2008;1783:520–529. doi: 10.1016/j.bbamcr.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Chung J., Chen T., Missiakas D. Transfer of electrons across the cytoplasmic membrane by DsbD, a membrane protein involved in thiol–disulphide exchange and protein folding in the bacterial periplasm. Mol. Microbiol. 2000;35:1099–1109. doi: 10.1046/j.1365-2958.2000.01778.x. [DOI] [PubMed] [Google Scholar]
- 11.Katzen F., Beckwith J. Transmembrane electron transfer by the membrane protein DsbD occurs via a disulfide bond cascade. Cell. 2000;103:769–779. doi: 10.1016/s0092-8674(00)00180-x. [DOI] [PubMed] [Google Scholar]
- 12.Stirnimann C.U., Grutter M.G., Glockshuber R., Capitani G. NDsbD: a redox interaction hub in the Escherichia coli periplasm. Cell. Mol. Life Sci. 2006;63:1642–1648. doi: 10.1007/s00018-006-6055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haebel P.W., Goldstone D., Katzen F., Beckwith J., Metcalf P. The disulfide bond isomerase DsbC is activated by an immunoglobulin-fold thiol oxidoreductase: crystal structure of the DsbC–DsbDalpha complex. EMBO J. 2002;21:4774–4784. doi: 10.1093/emboj/cdf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemmis C.W., Berkmen M., Eser M., Schildbach J.F. TrbB from conjugative plasmid F is a structurally distinct disulfide isomerase that requires DsbD for redox state maintenance. J. Bacteriol. 2011;193:4588–4597. doi: 10.1128/JB.00351-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stirnimann C.U., Rozhkova A., Grauschopf U., Grutter M.G., Glockshuber R., Capitani G. Structural basis and kinetics of DsbD-dependent cytochrome c maturation. Structure. 2005;13:985–993. doi: 10.1016/j.str.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Di Matteo A., Gianni S., Schinina M.E., Giorgi A., Altieri F., Calosci N., Brunori M., Travaglini-Allocatelli C. A strategic protein in cytochrome c maturation: three-dimensional structure of CcmH and binding to apocytochrome c. J. Biol. Chem. 2007;282:27012–27019. doi: 10.1074/jbc.M702702200. [DOI] [PubMed] [Google Scholar]
- 17.Robertson I.B., Stevens J.M., Ferguson S.J. Dispensable residues in the active site of the cytochrome c biogenesis protein CcmH. FEBS Lett. 2008;582:3067–3072. doi: 10.1016/j.febslet.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 18.Fabianek R.A., Hofer T., Thöny-Meyer L. Characterization of the Escherichia coli CcmH protein reveals new insights into the redox pathway required for cytochrome c maturation. Arch. Microbiol. 1999;171:92–100. doi: 10.1007/s002030050683. [DOI] [PubMed] [Google Scholar]
- 19.Metheringham R., Griffiths L., Crooke H., Forsythe S., Cole J. An essential role for DsbA in cytochrome c synthesis and formate-dependent nitrite reduction by Escherichia coli K-12. Arch. Microbiol. 1995;164:301–307. doi: 10.1007/BF02529965. [DOI] [PubMed] [Google Scholar]
- 20.Sambongi Y., Ferguson S.J. Mutants of Escherichia coli lacking disulphide oxidoreductases DsbA and DsbB cannot synthesise an exogenous monohaem c-type cytochrome except in the presence of disulphide compounds. FEBS Lett. 1996;398:265–268. doi: 10.1016/s0014-5793(96)01256-2. [DOI] [PubMed] [Google Scholar]
- 21.Metheringham R., Tyson K.L., Crooke H., Missiakas D., Raina S., Cole J.A. Effects of mutations in genes for proteins involved in disulphide bond formation in the periplasm on the activities of anaerobically induced electron transfer chains in Escherichia coli K12. Mol. Gen. Genet. 1996;253:95–102. doi: 10.1007/pl00013815. [DOI] [PubMed] [Google Scholar]
- 22.Moore G.R., Pettigrew G.W. Springer-Verlag; New York: 1990. Cytochrome c: evolutionary, structural and physicochemical aspects. [Google Scholar]
- 23.Sambongi Y., Ferguson S.J. Specific thiol compounds complement deficiency in c-type cytochrome biogenesis in Escherichia coli carrying a mutation in a membrane-bound disulphide isomerase-like protein. FEBS Lett. 1994;353:235–238. doi: 10.1016/0014-5793(94)01053-6. [DOI] [PubMed] [Google Scholar]
- 24.Turkarslan S., Sanders C., Ekici S., Daldal F. Compensatory thio-redox interactions between DsbA, CcdA and CcmG unveil the apocytochrome c holdase role of CcmG during cytochrome c maturation. Mol. Microbiol. 2008;70:652–666. doi: 10.1111/j.1365-2958.2008.06441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojima N., Yamanaka M., Ichiki S., Sambongi Y. Unexpected elevated production of Aquifex aeolicus cytochrome c555 in Escherichia coli cells lacking disulfide oxidoreductases. Biosci. Biotechnol. Biochem. 2005;69:1418–1421. doi: 10.1271/bbb.69.1418. [DOI] [PubMed] [Google Scholar]
- 26.Allen J.W., Barker P.D., Ferguson S.J. A cytochrome b562 variant with a c-type cytochrome CXXCH heme-binding motif as a probe of the Escherichia coli cytochrome c maturation system. J. Biol. Chem. 2003;278:52075–52083. doi: 10.1074/jbc.M307196200. [DOI] [PubMed] [Google Scholar]
- 27.Deshmukh M., Turkarslan S., Astor D., Valkova-Valchanova M., Daldal F. The dithiol:disulfide oxidoreductases DsbA and DsbB of Rhodobacter capsulatus are not directly involved in cytochrome c biogenesis, but their inactivation restores the cytochrome c biogenesis defect of CcdA-null mutants. J. Bacteriol. 2003;185:3361–3372. doi: 10.1128/JB.185.11.3361-3372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erlendsson L.S., Hederstedt L. Mutations in the thiol-disulfide oxidoreductases BdbC and BdbD can suppress cytochrome c deficiency of CcdA-defective Bacillus subtilis cells. J. Bacteriol. 2002;184:1423–1429. doi: 10.1128/JB.184.5.1423-1429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arslan E., Schulz H., Zufferey R., Kunzler P., Thöny-Meyer L. Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem. Biophys. Res. Commun. 1998;251:744–747. doi: 10.1006/bbrc.1998.9549. [DOI] [PubMed] [Google Scholar]
- 30.Mavridou D.A., Saridakis E., Kritsiligkou P., Goddard A.D., Stevens J.M., Ferguson S.J., Redfield C. Oxidation state-dependent protein-protein interactions in disulfide cascades. J. Biol. Chem. 2011;286:24943–24956. doi: 10.1074/jbc.M111.236141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Studier F.W. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Allen J.W., Tomlinson E.J., Hong L., Ferguson S.J. The Escherichia coli cytochrome c maturation (Ccm) system does not detectably attach heme to single cysteine variants of an apocytochrome c. J. Biol. Chem. 2002;277:33559–33563. doi: 10.1074/jbc.M204963200. [DOI] [PubMed] [Google Scholar]
- 33.Goodhew C.F., Brown K.R., Pettigrew G.W. Haem staining in gels, a useful tool in the study of bacterial c-type cytochromes. Biochim. Biophys. Acta. 1986;852:288–294. [Google Scholar]
- 34.Goddard A.D., Stevens J.M., Rao F., Mavridou D.A., Chan W., Richardson D.J., Allen J.W., Ferguson S.J. C-Type cytochrome biogenesis can occur via a natural Ccm system lacking CcmH, CcmG, and the heme-binding histidine of CcmE. J. Biol. Chem. 2010;285:22882–22889. doi: 10.1074/jbc.M110.133421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crooke H., Cole J. The biogenesis of c-type cytochromes in Escherichia coli requires a membrane-bound protein, DipZ, with a protein disulphide isomerase-like domain. Mol. Microbiol. 1995;15:1139–1150. doi: 10.1111/j.1365-2958.1995.tb02287.x. [DOI] [PubMed] [Google Scholar]
- 36.Page M.D., Saunders N.F., Ferguson S.J. Disruption of the Pseudomonas aeruginosa dipZ gene, encoding a putative protein-disulfide reductase, leads to partial pleiotropic deficiency in c-type cytochrome biogenesis. Microbiology. 1997;143(Pt 10):3111–3122. doi: 10.1099/00221287-143-10-3111. [DOI] [PubMed] [Google Scholar]
- 37.Chivers P.T., Prehoda K.E., Raines R.T. The CXXC motif: a rheostat in the active site. Biochemistry. 1997;36:4061–4066. doi: 10.1021/bi9628580. [DOI] [PubMed] [Google Scholar]
- 38.Iobbi-Nivol C., Crooke H., Griffiths L., Grove J., Hussain H., Pommier J., Mejean V., Cole J.A. A reassessment of the range of c-type cytochromes synthesized by Escherichia coli K-12. FEMS Microbiol. Lett. 1994;119:89–94. doi: 10.1111/j.1574-6968.1994.tb06872.x. [DOI] [PubMed] [Google Scholar]
- 39.Berks B.C., Richardson D.J., Reilly A., Willis A.C., Ferguson S.J. The napEDABC gene cluster encoding the periplasmic nitrate reductase system of Thiosphaera pantotropha. Biochem. J. 1995;309(Pt 3):983–992. doi: 10.1042/bj3090983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Einsle O. Structure and function of formate-dependent cytochrome c nitrite reductase. NrfA. Methods Enzymol. 2011;496:399–422. doi: 10.1016/B978-0-12-386489-5.00016-6. [DOI] [PubMed] [Google Scholar]
- 41.Gao T., O’Brian M.R. Control of DegP-dependent degradation of c-type cytochromes by heme and the cytochrome c maturation system in Escherichia coli. J. Bacteriol. 2007;189:6253–6259. doi: 10.1128/JB.00656-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koppenhofer A., Turner K.L., Allen J.W., Chapman S.K., Ferguson S.J. Cytochrome cd1 from Paracoccus pantotrophus exhibits kinetically gated, conformationally dependent, highly cooperative two-electron redox behavior. Biochemistry. 2000;39:4243–4249. doi: 10.1021/bi000192a. [DOI] [PubMed] [Google Scholar]
- 43.Mavridou D.A., Braun M., Thony-Meyer L., Stevens J.M., Ferguson S.J. Avoidance of the cytochrome c biogenesis system by periplasmic CXXCH motifs. Biochem. Soc. Trans. 2008;36:1124–1128. doi: 10.1042/BST0361124. [DOI] [PubMed] [Google Scholar]
- 44.Thöny-Meyer L., Kunzler P. Translocation to the periplasm and signal sequence cleavage of preapocytochrome c depend on sec and lep, but not on the ccm gene products. Eur. J. Biochem. 1997;246:794–799. doi: 10.1111/j.1432-1033.1997.t01-1-00794.x. [DOI] [PubMed] [Google Scholar]
- 45.Casadaban M.J., Cohen S.N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 1980;138:179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- 46.Stewart E.J., Katzen F., Beckwith J. Six conserved cysteines of the membrane protein DsbD are required for the transfer of electrons from the cytoplasm to the periplasm of Escherichia coli. EMBO J. 1999;18:5963–5971. doi: 10.1093/emboj/18.21.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambongi Y., Ferguson S.J. Synthesis of holo Paracoccus denitrificans cytochrome c550 requires targeting to the periplasm whereas that of holo Hydrogenobacter thermophilus cytochrome c552 does not. Implications for c-type cytochrome biogenesis. FEBS Lett. 1994;340:65–70. doi: 10.1016/0014-5793(94)80174-6. [DOI] [PubMed] [Google Scholar]