Abstract
Background
Matrix metalloproteinase-9 (MMP-9) secreted by corneal epithelial cells has a role in the remodelling of extracellular matrix and migration of epithelial cells. Elevated levels of MMP-9 activity in the ocular surface may be involved in the pathogenesis of corneal diseases. N-acetylcysteine (NAC) has been used to treat corneal diseases, including recurrent epithelial erosions. In this study, its effects on the MMP-9 secretion and human corneal epithelial (HCE) cell migration were evaluated in vitro.
Methods
Confluent HCE cell cultures were treated with 0–20 mM NAC, and tested for MMP-9 secretion and epithelial cell migration by gelatin zymography and scratch wound assay, respectively. Comparisons between different treatment groups were made using analysis of variance, followed by multiple pairwise comparisons.
Results
Twenty mM NAC inhibited the secretion of MMP-9 significantly. Cell migration, assessed after 24 h of wounding, showed a highly significant dose-dependent inhibitory effect.
Conclusions
This study shows that NAC reduces MMP-9 production by HCE cells and inhibits cell migration in vitro. This information helps to elucidate the mechanisms by which NAC may be beneficial therapeutically and suggests that NAC may be useful for managing corneal erosions and related conditions.
Keywords: cornea, epithelium, healing, recurrent corneal erosions (RCE), N-acetylcysteine (NAC)
Introduction
Corneal injuries elicit inflammatory responses that eliminate the injurious agent and facilitate healing. Corneal inflammation occurs in various conditions and irrespective of the underlying cause, this often results in tissue destruction that leads to corneal ulceration, scarring, and even perforation. Pathological activity of proteolytic enzymes and active pro-inflammatory cytokines are implicated in the progression of tissue destruction.1, 2, 3 The early phases of healing that follow inflammation are characterised by deposition of extracellular matrix (ECM), and proliferation and migration of cells within this matrix, which acts as a scaffold. This is followed by a remodelling phase, in which the ECM is degraded by proteolytic enzymes.1 Matrix metalloproteinases (MMPs) are a group of proteases that have a role in ECM remodelling and the tissue inhibitors of metalloproteinases (TIMPs) interact with MMPs to modify their biological roles in tissues.1, 4, 5
MMPs are secreted by corneal epithelial cells and stromal keratocytes and have a role in the remodelling of ECM and migration of corneal epithelial cells on the underlying stroma. TIMPs are also secreted by corneal epithelial cells.5 The activity of MMP-9 is upregulated in ocular surface diseases, such as recurrent corneal erosion (RCE),3 peripheral ulcerative keratitis,2 ocular rosacea,6 and dry eye.4 Elevated MMP-9 activity in these conditions may be involved in the pathogenesis of the disease. MMPs participate at all stages of the ulcerative process, from initiating the epithelial defect to ulcer resolution and repair.1 Although collagenolytic and gelatinolytic processes are essential for tissue remodelling and wound healing, downregulation of their activity is essential to maintain tissue stability after initial wound healing.1 Therefore, prevention of ECM degradation through inhibition of MMP activity may be an effective therapeutic approach to treat corneal ulcers.
N-acetylcysteine (NAC), a mucolytic agent, has been used in ophthalmology to treat corneal diseases, including alkali-burned corneal ulcers,7 and filamentous keratitis.8 Although the effects of NAC on the corneal surface and wound healing have been described in several studies,9, 10, 11, 12, 13 the effects of NAC on the secretion of MMPs in human corneal epithelial (HCE) cells have not been investigated to date. We hypothesised that NAC modulates the MMP-9 secretion in the HCE cells and TIMPs may be involved in the regulation of MMP-9. The purpose of this study was to evaluate the effect of NAC on the secretion of MMP-9 and TIMPs and on corneal epithelial cell migration using cultured HCE cells.
Materials and methods
HCE cell cultures
Corneoscleral rims from the donor corneoscleral buttons remaining after corneal transplantation were used to culture HCE cells. These corneoscleral buttons were obtained from the Manchester Eye Bank. Ethical approval was obtained from Lothian NHS (Edinburgh) Research Ethics Committee. The HCE cells were isolated using 0.125% trypsin/EDTA and seeded onto mitomycin-C inactivated 3T3 fibroblasts (2.4 × 104 cells/cm2) in 12-well plates and incubated with culture media (Dulbecco's modified Eagle's medium and Ham's F12 mixture, supplemented with 10% FCS, 4 × 10−3 mol/l glutamine, 1.8 × 10−4 mol/l adenine, 5 μg/ml insulin, 2 × 10−7 mol/l triiodo-ℒ-thyronine, 0.4 μg/ml hydrocortisone, 10 ng/ml epidermal growth factor, 100 IU/ml penicillin-streptomycin ,and 0.5 μg/ml amphotericin B) at 37°C under 5% CO2. When the epithelial cells were confluent (almost all the 3T3 cells were removed), they were used for MMP-9, TIMPs, and migration assays.
For MMP assay, the cells were washed with PBS and incubated with equal volumes of serum-free media containing NAC (Sigma, Poole, UK) at 0, 0.1, 1.0, 5.0, 10, and 20 mM. After 24 h, media were separated and stored at −20°C until the MMP assay. Four sets of experiments were conducted with four different donor samples. The viability of cells in each well after treatment was assessed in two sets of experiments by Trypan blue exclusion after trypsin digestion to exclude cytotoxicity. In addition, in two sets of experiments, cells were left with NAC for further 24 h to investigate the effect of longer exposure to NAC (48 h).
Gelatin zymography and reverse zymography
A volume of 3 μl of conditioned media from each sample was diluted 1 : 1 with deionised water, mixed with 6 μl of sample application buffer, and subjected to gelatin zymography as described by Ramaesh et al.14 For quantitative analysis, the gelatin zymograms were produced by loading further diluted conditioned media, containing only 1 μl of each sample. This was done to produce a clear non-overlapping band for each sample. The bands of gelatinolytic activity detected by zymography were analysed quantitatively by a GS-700 Imaging Densitometer (Bio-Rad, Hemel Hempstead, UK) and the density of the bands were expressed as uncalibrated optical density (uOD).14
The secretion of TIMPs was detected by reverse zymography as described in Riley et al.15 Briefly, samples of 7.5 μl conditioned media were separated according to molecular weight by PAGE (12% gels) containing gelatin (1 mg/ml) and a preparation of MMP-2 (conditioned medium from baby hamster kidney cells, BHK-21, which constitutively express MMP-2). Gels were washed, incubated in reverse zymography digestion buffer, and then stained with Coomassie blue. The inhibitory activity of TIMP with substrate degradation by MMP-2 appeared as dark bands against a lighter background.
Epithelial cell migration assay
HCE cells were grown to confluence in 12-well plates, and a 1-mm wide linear wound was made with a cell scraper. After being rinsed twice with PBS, the cells were allowed to migrate in serum-free media containing NAC at 0, 0.1, 1.0, 5.0, 10, and 20 mM at 37°C under 5% CO2 for 24 h. The wound width was measured at three different points for the linear wound in each well at 0, 6, and 24 h, and the migration distance was calculated for 6 and 24 h.
Immunostaining
Confluent HCE cells were fixed in 4% paraformaldehyde in PBS at 4°C for 10 min, washed in PBS, and then treated with 0.5% TritonX-100 in PBS at room temperature for 10 min. Cells were treated with normal serum (1% goat serum, 1% bovine serum albumin) for 30 min and incubated with primary antibodies, rabbit anti-human polyclonal cytokeratin 12 antibody (1 : 50, Santa Cruz, Wembley, UK), and rabbit anti-mouse polyclonal cytokeratin 19 (type 1) antibody (1 : 100, LifeSpan Biosciences, Newmarket, UK) at 4°C for overnight. The cells were incubated at room temperature for 1 h with goat anti-rabbit Alexa Fluor 488 (1 : 400, Invitrogen, Paisley, UK) finally counterstained with TOPRO-3 and mounted. Cells were washed with 0.2% TritonX-100 in PBS after incubation with antibodies. Control samples were incubated with normal serum, but otherwise treated in the same way. Samples were visualised by fluorescence microscopy.
Statistical analysis
MMP-9 secretion and cell migration of corneal epithelial cells were analysed by a single factor analysis of variance (ANOVA) for the optical densities and the migration distance, respectively, for different groups. Comparisons between different treatment groups were made using ANOVA, followed by multiple pairwise comparisons with Bonferroni/Dunn's post hoc tests and the results were considered statistically significant where P<0.0033, to allow for multiple tests.
Results
HCE cell cultures
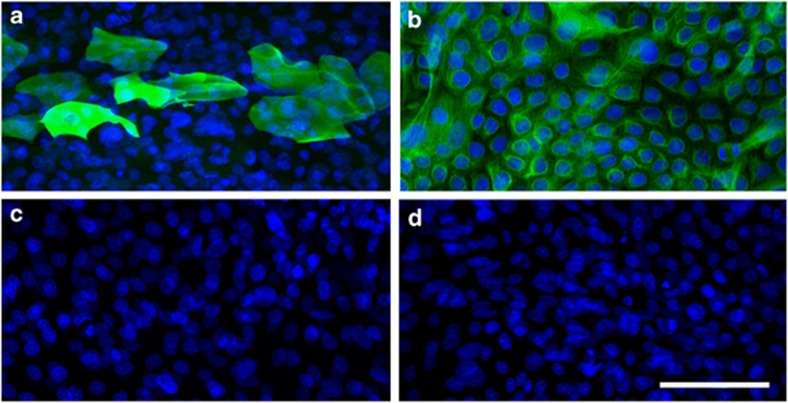
HCE cells cocultured with 3T3 cells became confluent around 14 days and by this time very few 3T3 cells remained in the cultures. The epithelial cell population consisted of colonies of large cells that were positive for K12 (Figure 1a) and small cells that were K19-positive (Figure 1b). The viability of cells, as assessed by Trypan blue after trypsin digestion to detach epithelial cells into cell suspension, was 87–95%.
Figure 1.
Immunocytochemistry images of cytokeratin 12 and 19 (K12 and K19) localisation in cultured HCE cells. (a) K12 immunostaining of differentiated HCE cells (green); (b) K19 immunostaining of undifferentiated cells (green); (c, d) negative controls for K12 and K19, respectively. Nuclei are stained blue with DAPI. Scale bar=100 μm.
Secreted MMP activity and TIMPs
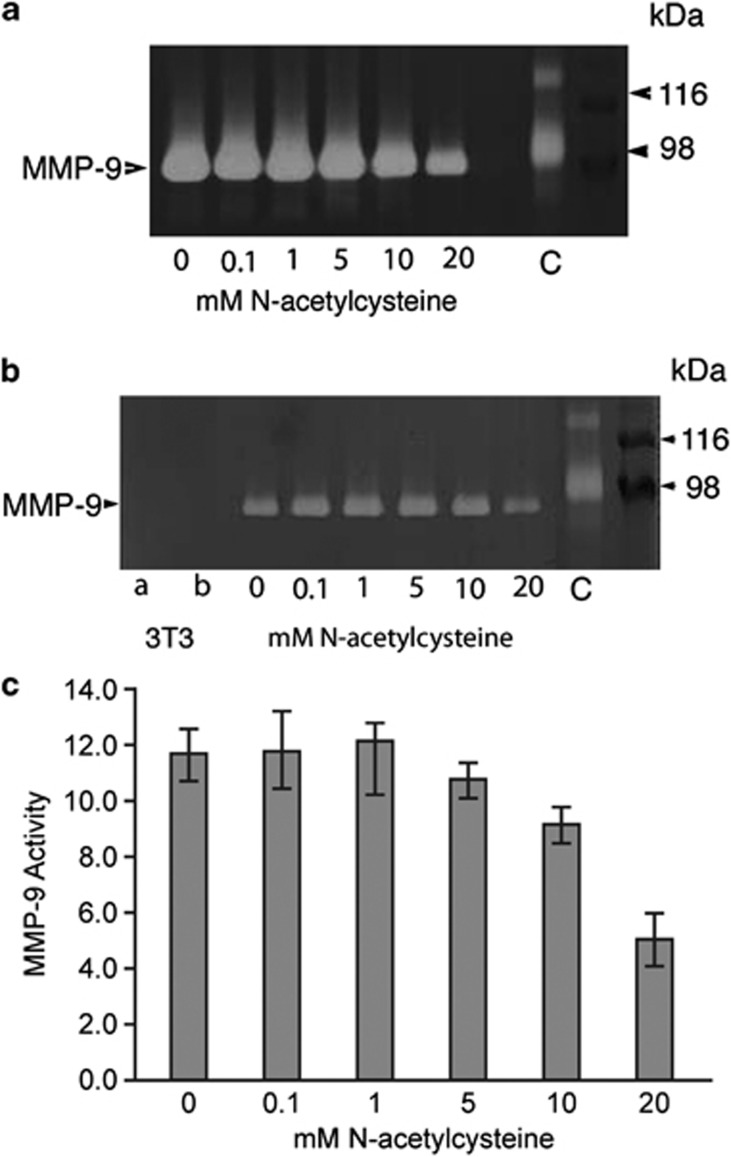
The conditioned media obtained from the cultures exhibited bands at 92 kDa corresponding to MMP-9 activity, when 3 μl of samples were loaded (Figure 2a). The zymograms showed a dose-dependent reduction of secreted MMP reflected in the activity assay for cells grown in different NAC concentrations. Gelatin zymograms produced with 1 μl volume samples showed non-overlapping bands (Figure 2b) and these zymograms were used for quantitative assays. Conditioned media obtained from the wells that contained only 3T3 cells did not produce MMP-9 bands (Figure 2b). A dose-related effect of NAC on MMP-9 activity is illustrated by the histogram (Figure 2c), which shows the mean optical density (uOD) values for the bands of MMP-9 activity. ANOVA showed a significant difference (P<0.0005) among treatments with different concentrations of NAC for MMP-9 activity (optical density of bands in the zymogram). Pairwise comparisons showed significant differences between 20 mM NAC and lower concentrations (Figure 2c).
Figure 2.
Effects of NAC on MMP-9 secretion. (a, b) Gelatin zymography of conditioned media from cultured HCE cells treated with different concentrations of NAC. (a) Conditioned media exhibited strong bands at 92 kDa, corresponding to MMP-9 activity. (b) The same sample as in (a), loaded at a lower volume (1 μl). In the first two lanes, conditioned media of 3T3 cells was loaded at (a) 1 μl and (b) 7.5 μl, and they did not exhibit bands for MMP-9. C, control sample, human amniotic fluid, which contains MMP-9. Right: protein markers and the corresponding molecular weights. (c) Histogram showing the MMP-9 activity assay, expressed as optical densities of the bands (uOD). Each bar represents the mean±SEM of four samples. Pairwise comparisons showed significant differences between 0 vs 20 mM (P=0.0001), 0.1 vs 20 mM (P<0.0001), 1.0 vs 20 mM (P<0.0001), and 5 vs 20 mM (P=0.0005) NAC treatment groups.
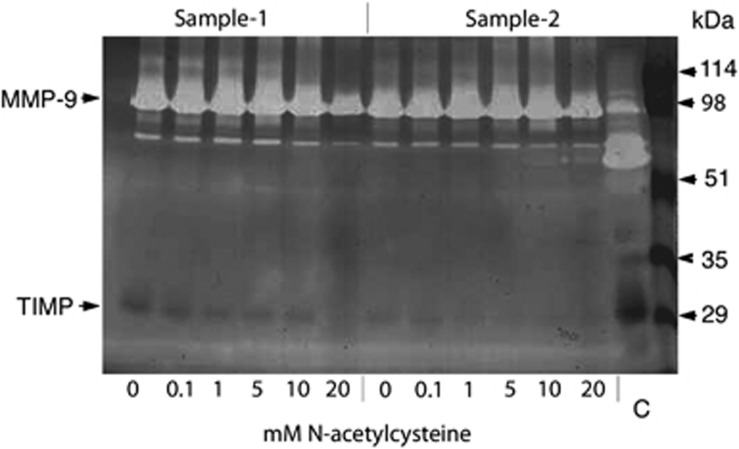
Reverse zymograms of conditioned media showed bands at 28 kDa corresponding to TIMP-1 for some samples (Figure 3), although the results were not consistent.
Figure 3.
Effects of NAC on TIMPs secretion. Reverse zymography of conditioned media from cultured human corneal epithelial cells treated with different concentrations of NAC-acetylcysteine for two samples. Bands at 28 kDa, corresponding to TIMP-1, were detected for some of the NAC treatments, but the results were not consistent for all samples (eg, sample 2).
HCE cell migration
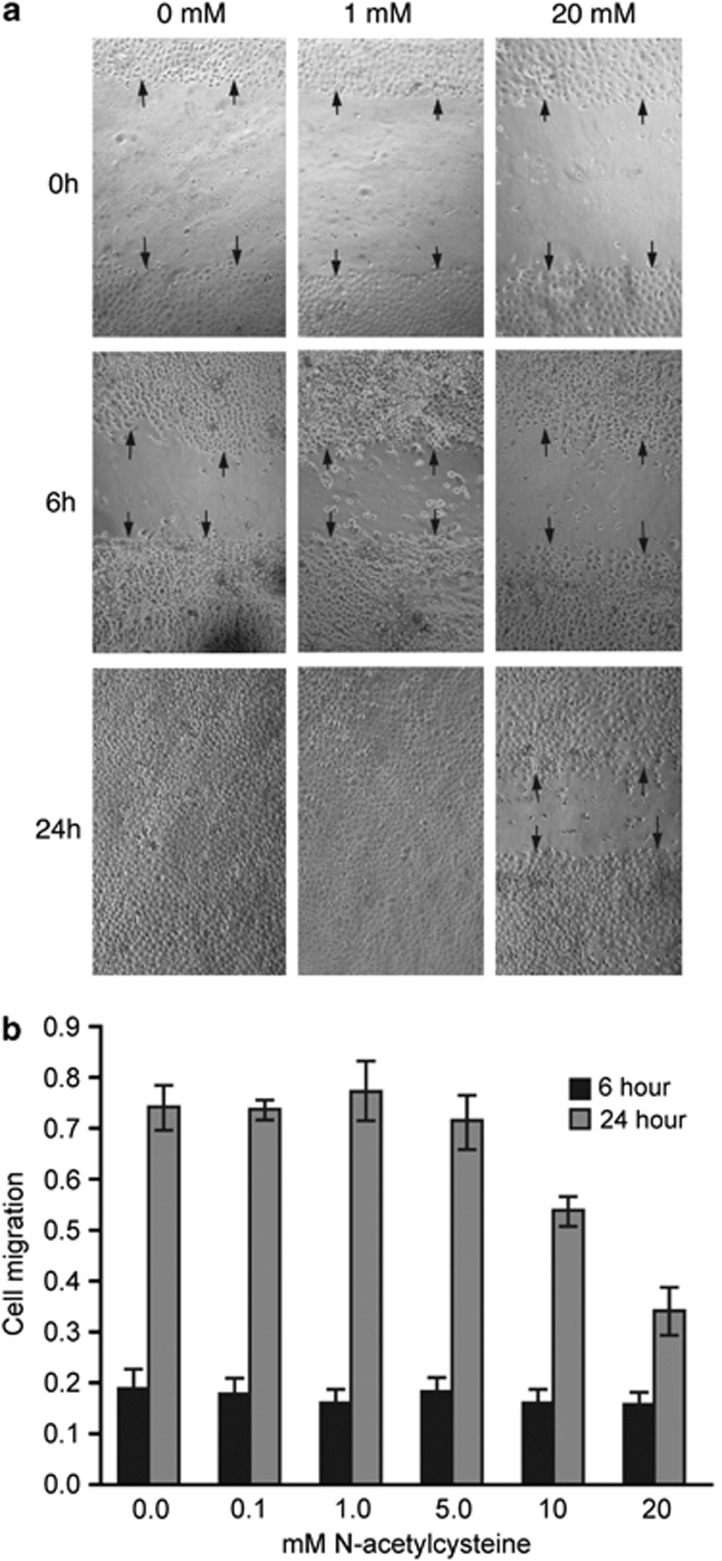
Reduction in wound size was observed in all treatment groups after 6 h. Representative images of some of the treatment groups are shown in Figure 4a. Average cell migration distance was calculated for each linear wound after 6 and 24 h, and the mean values are shown in Figure 4b. There was no significant difference between groups for cell migration distance after 6 h. However, after 24 h a dose-dependent reduction in cell migration was observed with the wounds completely closed in most of the wells treated with 0, 0.1, 1.0, 5.0, and 10 mM NAC and only partially closed in most of the wells treated with 20 mM NAC. There was a significant difference (P<0.0001) among treatments for cell migration distance after 24 h by ANOVA. Pairwise comparisons showed significant differences between higher and lower concentrations (Figure 4).
Figure 4.
Effects of NAC on corneal epithelial cell migration. (a) Representative phase contrast images of in vitro human corneal epithelial wound closure for three different concentrations (0, 1.0, and 20 mM) of NAC. Wound closure was photographed immediately (0 h), 6 h, and 24 h after wounding. Arrows indicate the edge of the wounds. (b) Histogram showing the migration of epithelial cells after 6 and 24 h for different concentrations of NAC. Each bar represents the mean±SEM of five samples. Multiple pairwise comparisons showed significant differences between 0 vs 10 mM (P=0.0029), 0 vs 20 mM (P<0.0001), 0.1 vs 20 mM (P<0.0001), 1.0 vs 10 mM (P=0.0008), 1.0 vs 20 mM (P<0.0001), and 5 vs 20 mM (P<0.0001) NAC treatment groups.
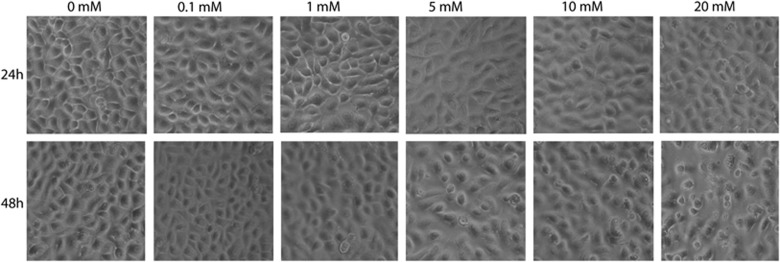
Although after 24 h there was no difference in the cell morphology and viability of cells treated with different concentration of NAC, cell morphology was affected after 48 h in cultures treated with higher concentrations of NAC (5, 10, and 20 mM, Figure 5). The epithelial cells tended to detach from the surface and the nuclei were fragmented in some cells implying cytotoxicity in cultures with continuous treatment of NAC.
Figure 5.
Morphology of human corneal epithelial cells treated with different concentrations of NAC after 24 and 48 h. Cells with different treatments appeared similar after 24 h (top row), although after 48h cells treated with 5.0, 10, and 20 mM NAC tended to round up and detach from the culture wells.
Discussion
These results are the first to demonstrate that that NAC is effective at reducing MMP-9 secretion in HCE cell cultures in a concentration-dependent manner (significant for 20 mM and a non-significant trend for 10 mM). Although TIMPs were produced by HCE cells in cultures treated with NAC, they were not detected in all samples. This study also shows that NAC decreased the rate of HCE cell migration in vitro (significant for both 10 and 20 mM).
NAC is a derivative of cysteine, which inhibits collagenase irreversibly by reducing disulphide bonds and by chelating calcium or zinc. It also inhibits MMP-9, potentially by similar mechanisms, although it is less potent and therefore requires a higher concentration.16 NAC also inhibits MMP-9 produced by bladder cancer cells17 and fetal membranes.18 Further, NAC has been shown to inhibit inflammatory cytokine responses in amniotic fluid and placental tissue in rats.19 The present finding of inhibitory effect of NAC on MMP-9 secretion and the reports on the inflammatory responses suggest that NAC may be useful clinically to treat destructive corneal conditions in which MMP-9 activity and inflammatory cytokines are upregulated.
Although the exact mechanism of inhibition of MMP-9 secretion by NAC is currently unknown, the inhibitory properties of NAC on inflammation has been shown to act through nuclear factor-κB (NF-κB), which has a pivotal role in inducing the expression of multiple genes in immune and inflammatory responses.18, 20 Inactive NF-κB is cytoplasmic and in response to activation by oxidative stress and action of reactive oxygen species it undergoes phosphorylation and translocates to the nucleus to initiate transcription by high affinity binding to regulatory κB motifs.21 These κB motifs have been identified in the promoter region of a number of genes involved in the phospholipid metabolic pathway, pro-inflammatory cytokines, MMP-9, and urokinase-type plasminogen activator.21 NAC has been shown to inhibit upstream events that lead to NF-κB activation. As well as inhibiting MMP-9 via its action on NF-κB, NAC may also have a direct inhibitory effect on MMP-9 by reducing disulphide bonds, as noted above.
In our in vitro system, the corneal epithelial wound healing was inhibited in the presence of 10 and 20 mM NAC after 24 h, although there was no difference in healing after 6 h. Further, we have found that continuous treatment of NAC for 48 h showed toxic effects on HCE cells. In most in vitro studies, NAC is measured in terms of molar concentration (molarity), whereas clinical studies usually quote a percentage concentration. Our maximum dose of 20 mM NAC is equivalent to a 0.33% solution, which is much lower than the solutions used clinically. Although, in clinical studies, topical applications of 5 and 20% NAC have been shown to be effective in the treatment of Sjogren syndrome22 and long-standing corneal epithelial defects,23 respectively, without toxic effects, the reports on animal experimental studies are contradictory. In all, 3% NAC has been shown to reduce the healing time in dog and rabbit corneas12, 13 whereas 10 and 20% concentrations had no effect in dogs.12 Non-toxic effects of NAC have been reported in rabbit cornea with topical application of 20% NAC 9, 10 but frequent application of 0.1 M NAC for 2 h and 20% NAC over 15 min were reported to have toxic effects.11 Further, 1% NAC was shown to reduce the cell survival and gelatinase activity of primary rabbit corneal epithelial cells.24 A recent study reported that 40 mM NAC inhibited the corneal epithelial wound healing in a pig eye organ culture system and constant presence of NAC in the medium caused extensive HCE cell death within 8 h.25 These reports and the finding of the present study suggest that prolonged treatment of NAC has adverse effects in the cornea. However, 20 mM or lower doses may not be a problem clinically because NAC eye drops are applied topically and NAC will become diluted in the tear film immediately after application, contrary to these cell cultures where dilution will not occur.
In conclusion, these in vitro results show that NAC inhibits the MMP-9 secretion in HCE cells, which is a novel finding that may have potential application in the management of conditions such as RCE and non-healing corneal ulcers, where raised MMP-9 activity has been implicated in the pathogenesis. Specifically, we suggest NAC may have a potential therapeutic role for treatment of RCEs.
Acknowledgments
We would like to thank Rosemary Leask for her technical assistance with MMP assays, and Prof Dylan Edwards for reagents for the reverse zymography assay. This work was supported by a grant from the Ross Foundation for the Prevention of Blindness, Scotland (to KR, BD, and JDW) and an Ophthalmology Research Grant from the Royal College of Surgeons of Edinburgh (to KR, JDW, and BD).
The authors declare no conflict of interest.
References
- Wong TT, Sethi C, Daniels JT, Limb GA, Murphy G, Khaw PT. Matrix metalloproteinases in disease and repair processes in the anterior segment. Surv Ophthalmol. 2002;47 (3:239–256. doi: 10.1016/s0039-6257(02)00287-4. [DOI] [PubMed] [Google Scholar]
- Coleman CM, Hannush S, Covello SP, Smith FJ, Uitto J, McLean WH. A novel mutation in the helix termination motif of keratin K12 in a US family with Meesmann corneal dystrophy. Am J Ophthalmol. 1999;128 (6:687–691. doi: 10.1016/s0002-9394(99)00317-7. [DOI] [PubMed] [Google Scholar]
- Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Res. 2002;21 (1:1–14. doi: 10.1016/s1350-9462(01)00015-5. [DOI] [PubMed] [Google Scholar]
- Garrana RM, Zieske JD, Assouline M, Gipson IK. Matrix metalloproteinases in epithelia from human recurrent corneal erosion. Invest Ophthalmol Vis Sci. 1999;40 (6:1266–1270. [PubMed] [Google Scholar]
- Sobrin L, Liu Z, Monroy DC, Solomon A, Selzer MG, Lokeshwar BL, et al. Regulation of MMP-9 activity in human tear fluid and corneal epithelial culture supernatant. Invest Ophthalmol Vis Sci. 2000;41 (7:1703–1709. [PubMed] [Google Scholar]
- Afonso AA, Sobrin L, Monroy DC, Selzer M, Lokeshwar B, Pflugfelder SC. Tear fluid gelatinase B activity correlates with IL-1alpha concentration and fluorescein clearance in ocular rosacea. Invest Ophthalmol Vis Sci. 1999;40 (11:2506–2512. [PubMed] [Google Scholar]
- Brown SI, Tragakis MP, Pearce DB. Treatment of the alkali-burned cornea. Am J Ophthalmol. 1972;74 (2:316–320. doi: 10.1016/0002-9394(72)90552-1. [DOI] [PubMed] [Google Scholar]
- Haut J, Labrune P, Ullern M, Chermet M. New trial treatment of dry eye with acetylcysteine ophthalmic solution. Bull Soc Ophtalmol Fr. 1977;77 (2:165–167. [PubMed] [Google Scholar]
- Petroutsos G, Guimaraes R, Giraud JP, Renard G, Pouliquen Y. Effect of acetylcysteine (Mucomyst) on epithelial wound healing. Ophthalmic Res. 1982;14 (4:241–248. doi: 10.1159/000265198. [DOI] [PubMed] [Google Scholar]
- Sugar A, Waltman SR. Corneal toxicity of collagenase inhibitors. Invest Ophthalmol. 1973;12 (10:779–782. [PubMed] [Google Scholar]
- Thermes F, Molon-Noblot S, Grove J. Effects of acetylcysteine on rabbit conjunctival and corneal surfaces. A scanning electron microscopy study. Invest Ophthalmol Vis Sci. 1991;32 (11:2958–2963. [PubMed] [Google Scholar]
- Aldavood SJ, Behyar R, Sarchahi AA, Rad MA, Noroozian I, Ghamsari SM, et al. Effect of acetylcysteine on experimental corneal wounds in dogs. Ophthalmic Res. 2003;35 (6:319–323. doi: 10.1159/000074070. [DOI] [PubMed] [Google Scholar]
- Sarchahi AA, Maimandi A, Tafti AK, Amani M. Effects of acetylcysteine and dexamethasone on experimental corneal wounds in rabbits. Ophthalmic Res. 2008;40 (1:41–48. doi: 10.1159/000111158. [DOI] [PubMed] [Google Scholar]
- Ramaesh T, Ramaesh K, Leask R, Springbett A, Riley SC, Dhillon B, et al. Increased apoptosis and abnormal wound-healing responses in the heterozygous pax6+/− mouse cornea. Invest Ophthalmol Vis Sci. 2006;47 (5:1911–1917. doi: 10.1167/iovs.05-1028. [DOI] [PubMed] [Google Scholar]
- Riley SC, Leask R, Denison FC, Wisely K, Calder AA, Howe DC. Secretion of tissue inhibitors of matrix metalloproteinases by human fetal membranes, decidua and placenta at parturition. J Endocrinol. 1999;162 (3:351–359. doi: 10.1677/joe.0.1620351. [DOI] [PubMed] [Google Scholar]
- Hook CW, Brown SI, Iwanij W, Nakanishi I. Characterization and inhibition of corneal collagenase. Invest Ophthalmol. 1971;10 (7:496–503. [PubMed] [Google Scholar]
- Kawakami S, Kageyama Y, Fujii Y, Kihara K, Oshima H. Inhibitory effect of N-acetylcysteine on invasion and MMP-9 production of T24 human bladder cancer cells. Anticancer Res. 2001;21 (1A:213–219. [PubMed] [Google Scholar]
- Lappas M, Permezel M, Rice GE. N-Acetyl-cysteine inhibits phospholipid metabolism, proinflammatory cytokine release, protease activity, and nuclear factor-kappaB deoxyribonucleic acid-binding activity in human fetal membranes in vitro. J Clin Endocrinol Metab. 2003;88 (4:1723–1729. doi: 10.1210/jc.2002-021677. [DOI] [PubMed] [Google Scholar]
- Beloosesky R, Gayle DA, Amidi F, Nunez SE, Babu J, Desai M, et al. N-acetyl-cysteine suppresses amniotic fluid and placenta inflammatory cytokine responses to lipopolysaccharide in rats. Am J Obstet Gynecol. 2006;194 (1:268–273. doi: 10.1016/j.ajog.2005.06.082. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Li X, Stark GR. NF kappaB-dependent signaling pathways. Exp Hematol. 2002;30 (4:285–296. doi: 10.1016/s0301-472x(02)00777-4. [DOI] [PubMed] [Google Scholar]
- Williamson J, Doig WM, Forrester JV, Tham MH, Wilson T, Whaley K, et al. Management of the dry eye in Sjogren's syndrome. Br J Ophthalmol. 1974;58 (9:798–805. doi: 10.1136/bjo.58.9.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MB. Collagenase inhibitors: rationale for their use in treating corneal ulceration. Int Ophthalmol Clin. 1975;15 (4:49–66. doi: 10.1097/00004397-197501540-00006. [DOI] [PubMed] [Google Scholar]
- Fitton JH, Ziegelaar BW, Hicks CR, Clayton AB, Crawford GJ, Constable IJ, et al. Assessment of anticollagenase treatments after insertion of a keratoprosthetic material in the rabbit cornea. Cornea. 1998;17 (1:108–114. doi: 10.1097/00003226-199801000-00016. [DOI] [PubMed] [Google Scholar]
- Huo Y, Qiu WY, Pan Q, Yao YF, Xing K, Lou MF. Reactive oxygen species (ROS) are essential mediators in epidermal growth factor (EGF)-stimulated corneal epithelial cell proliferation, adhesion, migration, and wound healing. Exp Eye Res. 2009;89 (6:876–886. doi: 10.1016/j.exer.2009.07.012. [DOI] [PubMed] [Google Scholar]