Abstract
Selective estrogen receptor modulators (SERMs), such as tamoxifen and raloxifene can act as estrogen receptor (ER) antagonists or agonists depending on the cell type. The antagonistic action of tamoxifen has been invaluable for treating breast cancer, whereas the agonist activity of SERMs also has important clinical applications as demonstrated by the use of raloxifene for osteoporosis. Whereas the mechanism whereby SERMs function as antagonists has been studied extensively very little is known about how SERMs produce agonist effects in different tissues with the two ER types; ERα and ERβ. We examined the regulation of 32 SERM-responsive regions with ERα and ERβ in transiently transfected MCF-7 breast, Ishikawa endometrial, HeLa cervical and WAR-5 prostate cancer cells. The regions were regulated by tamoxifen and raloxifene in some cell types, but not in all cell lines. Tamoxifen activated similar number of regions with ERα and ERβ in the cell lines, whereas raloxifene activated over twice as many regions with ERβ compared to ERα. In Ishikawa endometrial cancer cells, tamoxifen activated 17 regions with ERα, whereas raloxifene activated only 2 regions, which might explain their different effects on the endometrium. Microarray studies also found that raloxifene regulated fewer genes than tamoxifen in U2OS bone cancer cells expressing ERα, whereas tamoxifen was equally effective at regulating genes with ERα and ERβ. Our studies indicate that tamoxifen is a non-selective agonist, whereas raloxifene is a relative ERβ-selective agonist, and suggest that ERβ-selective SERMs might be safer for treating clinical conditions that are dependent on the agonist property of SERMs.
Keywords: Estrogen receptor, Selective estrogen receptor modulator, Gene regulation, Tamoxifen, Raloxifene, Estradiol, Microarray
1. Introduction
Two major classes of estrogenic drugs are used clinically to target the two estrogen receptor (ER) subtypes, ERα and ERβ (Dahlman-Wright et al., 2006; Heldring et al., 2007; Koehler et al., 2005). The first class of drugs that interact with ERs are a family of mammalian estrogens that include estradiol, estrone, and equilin, and synthetic estrogens such as ethinyl estradiol. The mammalian estrogens have been used extensively to treat menopausal symptoms and osteoporosis, whereas synthetic estrogens are commonly found in oral contraceptive pills. This class of estrogens function as agonists in tissues and regulate genes by recruiting coactivators to the activation function (AF)-2 surface of ERs (Smith and O’Malley, 2004). The second class of drugs used clinically to target the ERs is the selective ER modulators (SERMs). These compounds are distinguished from estrogens by their capacity to function as both agonists and antagonists in different cell types (Jordan, 2004). The main SERMs approved for clinical use are tamoxifen and raloxifene. The antagonistic action of tamoxifen on ER signaling in breast cells has been exploited for treating and preventing breast cancer (MacGregor and Jordan, 1998; O’Regan and Jordan, 2002). Raloxifene also acts as an antagonist in breast cells and decreases the risk of ER positive breast tumors (Cummings et al., 1999; Vogel et al., 2006). A major clinical difference in the antagonistic action of the two SERMs is that tamoxifen prevents both invasive and non-invasive breast tumors, whereas raloxifene only prevents invasive tumors (Grady et al., 2008). The molecular mechanism of the antagonistic action of SERMs has been studied extensively (MacGregor and Jordan, 1998). SERMs act as antagonists by at least three mechanisms. First, the SERMs bind to ERs with high affinity and competitively block the binding of estradiol. Second, the SERMs disrupt the movement of helix 12, which prevents the binding of coactivators to AF-2 in the ligand binding domain (Nettles and Greene, 2005; Shiau et al., 1998). Third, the SERMs induce the recruitment of corepressor proteins, such as N-CoR to ERs (Shang et al., 2000), which causes the repression of gene transcription by histone deacetylation and chromatin remodeling (Torchia et al., 1998).
The SERMs also have agonist activity in specific tissues. Some agonist actions of SERMs lead to beneficial effects, such as increasing bone mineral density (Love et al., 1988) and the prevention of fractures (Ettinger et al., 1999). This important property has led to the approval of raloxifene for the prevention and treatment of osteoporosis. However, the agonist properties can produce some adverse effects, including the stimulation of blood clots (Vogel et al., 2006). While much is known about the mechanism of the antagonist action of SERMs, little is known about how they produce agonist effects. It is also important to understand the mechanism whereby diverse SERMs produce different agonist effects from each other. For example, it is unclear why tamoxifen increases the risk of endometrial cancer, whereas raloxifene does not (Vogel et al., 2006). Studies indicate that the agonist effect is mediated by coactivators (Shang and Brown, 2002) that bind to and potentiate the constitutive activity of AF-1 in the A/B domain of ERs (Dutertre and Smith, 2003; Webb et al., 1998). This is in contrast to the agonist action of estrogens, which is mediated by the recruitment of coactivators to AF-2 in the ligand binding domain (Feng et al., 1998; Shiau et al., 1998). A more complete understanding of the molecular mechanism for the agonist actions of SERMs could lead to the development of safer and more selective SERMs.
One of the major problems that impede investigating the agonist action of SERMs at the genomic level is the lack of regulatory regions from native genes. Therefore, most studies have focused on the activation of an AP-1 site by the SERMs (Paech et al., 1997; Webb et al., 1995). While this site might be responsible for mediating the SERM’s effects on several genes, it is clearly not the only SERM-responsive regulatory region. To identify a broad range of regulatory regions from native target genes, we used a chromatin immunoprecipitation-cloning and sequencing strategy. We isolated multiple regions from different genes that were regulated by SERMs in U2OS osteosarcoma cells (Levy et al., 2008). Using some of these regulatory regions, the goal of this study was to determine if the SERM-responsive regions are activated by tamoxifen and raloxifene in ER subtype specific manner in different cell lines. Our findings suggest that tamoxifen is a non-selective ER subtype SERM, whereas raloxifene is a relative ERβ-selective SERM. These results provide a potential mechanism whereby tamoxifen and raloxifene produce different clinical effects.
2. Materials and methods
2.1. Materials
Estradiol, tamoxifen and raloxifene were obtained from Sigma–Aldrich. All other compounds were obtained as previously described (Levy et al., 2008).
2.2. Cell lines and cell culture
Tetracycline-inducible U2OS-ERα and U2OS-ERβ cells were characterized and maintained as previously described (Kian Tee et al., 2004). U2OS, MCF-7, HeLa, and Ishikawa cells were obtained from the UCSF cell culture facility and maintained as previously described (Kian Tee et al., 2004; Levy et al., 2008). WAR-5 prostate cancer cells were prepared as previously described (Ricke et al., 2006).
2.3. Plasmids, transfections, and luciferase assays
The 32 SERM-responsive regions were isolated by ChIP and cloned upstream of a minimal thymidine kinase luciferase as previously described (Levy et al., 2008). Transfections of plasmids into HeLa was carried out by electroporation (Mersereau et al., 2008), whereas Ishikawa, WAR-5 and MCF-7 cells were transfected using Lipofectamine 2000 according to manufacturer’s protocol (Invitrogen). Following transfections cells were then treated with 10 nM E2, 1 μM raloxifene or 1 μM tamoxifen for 24 h at 37 C. Cells were assayed for luciferase activity according to manufacture’s protocol (Promega).
2.4. Microarrays
U2OS-ERα and U2OS-ERβ cells were plated in 6-well plates. When the cells reached 80% confluence they were treated with 1 μg/ml doxycline for 12 h to induce ERα or ERβ. The cells were then treated with 10 nM E2, 1 μM raloxifene, 1 μM tamoxifen for 6 h at 37 C. Total cellular RNA was isolated with the Aurum RNA isolation kit, Bio-Rad (Hercules, CA) according to manufacturer’s protocol. Total RNA was first quantified by standard spectrophotometry, and then qualitatively evaluated by capillary electrophoresis employing the Bio-Rad Experion system (Hercules, CA). Biotin-labeled cRNA samples were prepared with 750 ng of total RNA template. Following synthesis and purification, the biotin-labeled samples were evaluated by both 260/280 absorbance spectrophotometry and capillary electrophoresis. The final labeled cRNA samples were hybridized overnight against Human genome HG U133A-2.0 GeneChip arrays containing more than 22,200 probe sets (Affymetrix, Santa Clara, CA). The array hybridizations, washing, staining, and scanning were performed at the J.D. Gladstone Genomics Core (San Francisco, CA).
2.5. Data analysis
The microarrays were preprocessed with a procedure similar to GCRMA (Wu and Irizarry, 2005), except that the background adjustment step is modified. Instead of using the probe sequence to predict non-specific binding (as in GCRMA), the non-specific binding for each probe is estimated from a database composed of hybridization data on the same platform of microarrays used in a variety of experiments. The new procedure is therefore dubbed dbRMA. Background parameters were estimated for each probe separately in dbRMA and avoided borrowing information across probes sharing similar but not identical sequences. Assessment on calibration data (Affymetrix Latin Square experiment) showed better accuracy of background parameters compared to those predicted by sequence. The normalization and summarization steps in the preprocessing procedures remain the same as GCRMA. The details of dbRMA procedure will be presented in a separate manuscript. After the preprocessing step, probesets were selected for further analysis if the fold change was greater than 2 and multiple testing adjusted p-value using Benjamini and Hochberg procedure (BH-adjusted p-value) was less than 0.05 (Dudoit et al., 2003). The heatmaps of log intensities of genes across different experiments were produced using Cluster and TreeView software (Eisen et al., 1998). Cluster software was used to perform the hierarchical clustering based on Pearson correlation coefficients to find clusters of genes with similar expression patterns. TreeView was then used to visualize the clusters and produce the figures.
2.6. Functional enrichment analysis of target genes
To elucidate the biological processes of target genes, we searched for enriched GO annotations using GOstat software (Beissbarth and Speed, 2004). For each annotated GO term, GOstat counted the number of overlapping genes from the input gene list, and compared it with the one expected from a reference list (GO annotation human (http://www.ebi.ac.uk/GOA/human_release.html). Fisher’s exact test was performed to compute a p-value for each GO category and BH-adjusted p-values were calculated. Results for GO “biological process” annotation are reported.
3. Results
3.1. Cell type-specific regulation of SERM regulatory regions
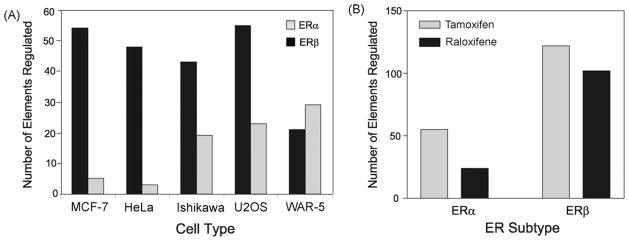
Using a chromatin immunoprecipitation-cloning and sequencing strategy, we previously isolated 173 regulatory regions from ERα target genes in U2OS cells (Levy et al., 2008). Most of these regions were regulated by the SERMs, tamoxifen and raloxifene in U2OS cells. In the current study, we examined whether regulatory regions are regulated by SERMs in a cell type-specific manner with ERα and ERβ. Of the 173 original regulatory regions, 32 were studied. These regions were selected because they were all regulated in U2OS cells (Levy et al., 2008) and contained different and uncharacterized ER binding elements. Of the 32 regulatory regions, 4 had an ERE, 15 had an AP-1 site, 6 had a Sp1 site, 13 had a FOXA1, 8 had a NFκB site and 24 had a GATA2 site (Supplemental Table 1). Each cell line was co-transfected with the 32 ChIP-derived regions in the presence of an expression vector for ERα or ERβ. Cell lines representing known ER target tissues were selected, which included the Ishikawa endometrial, MCF-7 breast, WAR-5 prostate and HeLa cervical cancer cell lines. We also compared the results from these four cell lines with our previous findings with the U2OS osteosarcoma cells (Levy et al., 2008). Following transfections the cells were treated with E2, tamoxifen or raloxifene and luciferase activity was measured. We considered regulation to be an activation of 2-fold or greater or repression that is 0.5 or greater from the untreated control and statistically significant (p < 0.05). The vitellogenin estrogen response element, which was used as a positive control was activated by E2 with both ERα and ERβ in all cell types (Table 1, bottom line). All 32 regions were activated by tamoxifen and/or raloxifene in at least one cell type, whereas only 9 regions were activated by E2 (Table 1). These results demonstrate that all of the ChIP fragments can function as SERM regulatory regions. There was a remarkable difference in response to the SERMs in the various cell types as some regions were regulated in some cell lines, but not others. Except for the WAR-5 cells the regions also displayed ER subtype regulation as more regions were regulated by the SERMs in MCF-7, HeLa, Ishikawa and U2OS cells with ERβ compared to ERα (Fig. 1A). In the 5 cell lines transfected with ERβ there was a combined activation of 122 regions by tamoxifen and 102 regions by raloxifene, indicating that both SERMs were equally effective at activating the regulatory regions (Fig. 1B). However, in the presence of ERα there was a difference in response to the SERMs. The activation of regulatory regions by tamoxifen occurred 55 times in the five cell lines, whereas with raloxifene only 24 regions were activated. Our findings demonstrate that the SERMs are more effective at activating regulatory regions with ERβ and that raloxifene acts as an ERβ-selective SERM relative to tamoxifen. Our data also show that many regions displayed opposite responses to E2 and SERMs with ERβ in the U2OS and MCF-7 cells. In these cell types most of the regions were repressed by E2 and activated by the SERMs. None of the regions were regulated in opposite direction by SERMs with ERα.
Table 1.
Cell type specific regulation of regulatory regions by E2 and SERMs. MCF-7, HeLa, Ishikawa, U2OS or WAR-5 cells were transfected with an estrogen response element or 32 regulatory regions from a ChIP library (Levy et al., 2008), located upstream of minimal TK promoter, and an ERα or ERβ expression vector. Cells were treated with 10 nM E2, 1 μM tamoxifen or 1 μM raloxifene for 18 h and luciferase assays were performed. The values in lighter shades of gray are activated by 2.0-fold or greater and those values in the darker shade of gray were repressed by 0.5 or greater. Each number represents the mean of triplicate samples. The S.E.M. was less than 10%.
| Element’s Origin | MCF-7
|
HeLa
|
Ishikawa
|
U2OS
|
WAR-5
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERα | ERβ | ERα | ERβ | ERα | ERβ | ERα | ERβ | ERα | ERβ | |||||||||||||||||||||
| E2 | T | R | E2 | T | R | E2 | T | R | E2 | T | R | E2 | T | R | E2 | T | R | E2 | T | R | E2 | T | R | E2 | T | R | E2 | T | R | |
| Progesterone receptor | 0.3 | 0.6 | 1.0 | 0.5 | 1.2 | 2.1 | 0.6 | 0.8 | 1.2 | 0.3 | 4.1 | 8.0 | 0.8 | 1.2 | 1.3 | 0.9 | 1.5 | 3.2 | 1.1 | 1.0 | 1.3 | 0.8 | 2 | 6.5 | 2.2 | 4.8 | 2.2 | 0.9 | 1.7 | 2.0 |
| tRNA serine 1 | 0.3 | 1.1 | 1.5 | 0.5 | 4.4 | 4.7 | 0.8 | 1.1 | 1.2 | 0.3 | 5.3 | 13.1 | 0.9 | 1.0 | 1.0 | 0.7 | 1.0 | 1.1 | 1.1 | 1.3 | 1.1 | 0.8 | 1.8 | 8 | 1.8 | 3.8 | 2.4 | 0.9 | 1.4 | 1.7 |
| Olfactory receptor, family 7 | 0.5 | 1.2 | 1.6 | 0.5 | 3.0 | 4.0 | 0.8 | 1.0 | 1.1 | 0.4 | 4.8 | 9.8 | 1.2 | 1.2 | 1.5 | 1.0 | 1.6 | 2.6 | 3.6 | 2.8 | 3.9 | 0.9 | 2.5 | 5.5 | 2.5 | 4.7 | 2.3 | 1.1 | 1.5 | 1.8 |
| Cytoplasmic polyadenylation element bp 4 | 0.4 | 0.8 | 1.5 | 0.3 | 22.4 | 4.2 | 0.8 | 1.0 | 1.2 | 0.3 | 4.2 | 8.3 | 1.1 | 1.4 | 1.8 | 1.0 | 1.3 | 2.0 | 0.4 | 0.4 | 0.5 | 0.3 | 3.3 | 4.4 | 1.6 | 2.6 | 1.7 | 1.0 | 1.4 | 1.9 |
| Similar to Ig kappa chain V region | 0.4 | 1.0 | 1.4 | 0.5 | 3.3 | 4.0 | 0.9 | 1.2 | 1.2 | 0.9 | 6.1 | 13.0 | 0.9 | 1.9 | 1.4 | 0.8 | 2.6 | 5.2 | 0.6 | 1.0 | 0.9 | 0.5 | 3.1 | 4.5 | 1.7 | 2.8 | 1.9 | 1.1 | 1.6 | 1.8 |
| hypothetical LOC401038 | 0.3 | 1.0 | 15 | 0.4 | 2.6 | 3.2 | 1.3 | 1.1 | 1.1 | 1.0 | 0.4 | 0.6 | 1.1 | 3.8 | 1.7 | 0.4 | 1.2 | 1.3 | 0.5 | 1.5 | 1.3 | 0.3 | 6.4 | 9.0 | 2.1 | 3.9 | 2.4 | 1.0 | 1.8 | 2.9 |
| Phosphodiesterase 4D interacting protein | 0.3 | 0.8 | 1.2 | 0.7 | 3.0 | 2.9 | 1.1 | 0.9 | 1.0 | 0.9 | 1.3 | 1.7 | 1.2 | 4.1 | 1.2 | 0.9 | 4.0 | 3.3 | 0.9 | 0.9 | 1.0 | 0.6 | 4.5 | 6.2 | 1.1 | 2.2 | 1.9 | 0.8 | 2.1 | 2.4 |
| Solute carrier family 37 member 2 | 0.4 | 0.7 | 1.5 | 0.5 | 3.3 | 5.0 | 1.0 | 1.2 | 1.2 | 0.9 | 1.5 | 1.8 | 1.1 | 2.1 | 1.7 | 0.5 | 2.8 | 3.6 | 0.9 | 1.6 | 2.0 | 0.9 | 8.4 | 9.7 | 1.6 | 3.2 | 1.4 | 1.2 | 2.0 | 2.0 |
| Chemokine orphan receptor 1 | 0.3 | 0.7 | 1.3 | 0.4 | 3.9 | 5.2 | 0.8 | 0.9 | 1.2 | 0.7 | 1.6 | 2.3 | 0.8 | 1.2 | 0.9 | 1.2 | 2.2 | 1.4 | 1.1 | 7.4 | 2.9 | 0.9 | 4.3 | 7.2 | 1.2 | 2.2 | 1.4 | 0.9 | 1.7 | 1.8 |
| Similar to RIKEN cDNA 5730421E18 gene | 0.2 | 0.7 | 1.2 | 0.3 | 3.0 | 4.9 | 1.3 | 1.7 | 1.9 | 0.5 | 7.6 | 17.1 | 2.3 | 5.2 | 1.9 | 0.5 | 1.4 | 1.9 | 1.0 | 0.9 | 0.8 | 1.3 | 3.4 | 4.9 | 2.2 | 3.6 | 1.1 | 1.0 | 1.7 | 1.9 |
| Cat eye syndrome chromosome region 6 | 4.9 | 0.7 | 0.6 | 2.8 | 1.4 | 1.9 | 5.4 | 1.1 | 0.6 | 3.0 | 1.7 | 1.8 | 8.6 | 3.1 | 1.7 | 1.7 | 1.0 | 1.4 | 70.2 | 18.9 | 8.1 | 15.8 | 1.4 | 2.4 | 2.2 | 1.8 | 1.4 | 7.1 | 0.6 | 0.5 |
| Ryanodine receptor 3 | 0.2 | 0.7 | 1.4 | 0.1 | 1.3 | 1.8 | 1.3 | 1.4 | 1.1 | 0.8 | 2.4 | 5.3 | 3.1 | 5.0 | 4.1 | 0.9 | 3.0 | 3.4 | 1.2 | 2.3 | 3.1 | 0.6 | 3.0 | 4.5 | 1.1 | 1.7 | 1.1 | 1.0 | 1.8 | 2.1 |
| LOC441580 | 0.5 | 0.8 | 1.7 | 0.3 | 1.0 | 2.4 | 1.5 | 2.6 | 1.4 | 1.1 | 4.1 | 6.1 | 0.8 | 2.3 | 1.3 | 0.8 | 3.3 | 4.0 | 1.0 | 2.6 | 2.0 | 0.4 | 2.4 | 3.9 | 1.1 | 1.1 | 1.0 | 1.0 | 1.2 | 1.0 |
| Similar to salivary proline-rich protein | 0.0 | 1.3 | 0.5 | 0.5 | 2.9 | 4.5 | 0.9 | 1.4 | 1.1 | 0.9 | 4.5 | 7.3 | 1.1 | 2.1 | 1.6 | 1.1 | 1.4 | 1.8 | 1.1 | 2.9 | 3.1 | 0.3 | 1.7 | 2.7 | 1.2 | 1.4 | 1.1 | 0.8 | 1.0 | 1.0 |
| Killer cell lectin-like receptor subfamily C 2 | 0.3 | 0.8 | 1.2 | 0.4 | 3.5 | 2.9 | 0.8 | 0.8 | 0.9 | 1.1 | 3.1 | 6.6 | 1.8 | 3.2 | 1.5 | 1.0 | 2.4 | 3.8 | 1.1 | 1.2 | 1.1 | 0.6 | 3.9 | 5.5 | 1.3 | 1.9 | 1.4 | 0.9 | 1.4 | 1.4 |
| Similar to CG7467-PA | 0.2 | 0.8 | 1.6 | 0.3 | 4.0 | 5.8 | 1.2 | 1.6 | 1.1 | 1.0 | 5.3 | 7.9 | 1.7 | 5.3 | 2.2 | 0.8 | 2.0 | 1.3 | 1.0 | 2.5 | 2.1 | 0.4 | 2.6 | 3.6 | 1.5 | 1.5 | 1.6 | 1.0 | 1.3 | 1.6 |
| Ankyrin repeat and SOCX box-containing 17 | 1.5 | 1.2 | 1.1 | 1.8 | 0.8 | 1.3 | 2.2 | 1.6 | 1.0 | 1.0 | 1.3 | 1.2 | 1.6 | 1.5 | 1.2 | 1.3 | 3.0 | 3.5 | 24.6 | 2.5 | 1.5 | 0.3 | 4.1 | 5.1 | 1.8 | 1.2 | 1.2 | 4.5 | 0.5 | 0.5 |
| Hypothetical protein MGC26143 | 0.1 | 0.4 | 0.9 | 0.2 | 3.6 | 7.3 | 0.8 | 0.8 | 0.9 | 1.0 | 1.7 | 0.8 | 0.6 | 1.5 | 1.2 | 0.7 | 7.0 | 7.7 | 0.5 | 0.8 | 1.1 | 0.6 | 3.5 | 5.1 | 1.1 | 2.0 | 2.0 | 0.8 | 1.9 | 2.8 |
| Lipase hepatic | 0.1 | 0.3 | 0.6 | 0.3 | 4.6 | 10.7 | 0.7 | 1.0 | 1.0 | 1.0 | 1.5 | 2.3 | 0.7 | 2.3 | 1.2 | 0.6 | 7.9 | 10.7 | 0.5 | 1.3 | 1.7 | 0.4 | 5.6 | 11.6 | 1.4 | 2.8 | 1.5 | 1.0 | 1.8 | 2.5 |
| Fibroblast growth factor 12 | 0.2 | 0.5 | 1.0 | 0.2 | 2.4 | 3.8 | 1.2 | 1.2 | 1.5 | 0.7 | 5.9 | 15.6 | 0.9 | 1.0 | 1.0 | 0.9 | 1.5 | 1.6 | 0.6 | 1.2 | 1.3 | 0.4 | 1.8 | 2.5 | 1.5 | 2.2 | 1.5 | 1.0 | 1.3 | 1.4 |
| FLJ45721 protein | 0.1 | 0.4 | 1.0 | 0.3 | 3.0 | 6.6 | 0.8 | 0.7 | 0.9 | 0.6 | 4.6 | 11.0 | 0.7 | 2.2 | 0.9 | 1.5 | 3.9 | 5.9 | 0.3 | 0.7 | 0.7 | 0.6 | 3.4 | 4.0 | 1.2 | 2.0 | 1.6 | 0.8 | 1.7 | 2.5 |
| Cytidine monophosphate | 0.4 | 1.0 | 1.7 | 0.4 | 2.3 | 2.9 | 1.0 | 1.1 | 0.9 | 0.9 | 8.1 | 12.0 | 1.2 | 3.8 | 1.3 | 0.3 | 5.9 | 5.2 | 0.8 | 1.1 | 0.9 | 0.5 | 3.0 | 3.5 | 1.4 | 2.4 | 1.4 | 0.8 | 1.8 | 1.9 |
| Similar to DEAD box polypeptide 10 | 0.4 | 1.0 | 1.4 | 0.2 | 1.5 | 1.9 | 0.8 | 1.1 | 1.6 | 0.2 | 4.8 | 8.8 | 1.0 | 1.5 | 1.2 | 0.9 | 1.4 | 1.5 | 1.5 | 0.9 | 1.1 | 0.7 | 2.4 | 6.3 | 1.0 | 1.4 | 1.7 | 0.7 | 1.9 | 2.0 |
| Hypothetical gene supported by BC031617 | 0.4 | 1.1 | 1.5 | 1.4 | 2.6 | 2.6 | 1.1 | 1.6 | 1.6 | 1.7 | 16.4 | 24.1 | 0.6 | 2.6 | 1.2 | 0.5 | 5.6 | 4.5 | 0.8 | 2.0 | 1.4 | 0.4 | 3.3 | 4.4 | 1.4 | 2.6 | 2.0 | 0.9 | 2.6 | 2.8 |
| Hypothetical gene supported by BC040860 | 0.3 | 0.9 | 1.3 | 0.5 | 3.0 | 3.8 | 0.9 | 0.9 | 1.0 | 0.3 | 1.2 | 2.5 | 0.6 | 1.3 | 0.7 | 1.2 | 3.5 | 3.7 | 0.4 | 1.1 | 1.1 | 0.8 | 4.8 | 6.5 | 1.0 | 0.9 | 0.7 | 1.0 | 1.5 | 1.9 |
| Similar to galectin-related inter-fiber protein | 0.4 | 1.0 | 1.2 | 0.5 | 3.7 | 5.8 | 1.1 | 1.0 | 0.8 | 1.1 | 1.5 | 1.7 | 0.7 | 1.7 | 1.2 | 0.5 | 3.9 | 9.4 | 0.7 | 2.0 | 1.7 | 0.4 | 3.4 | 6.9 | 1.5 | 3.2 | 2.2 | 1.2 | 2.1 | 2.9 |
| Similar to TFIIH basal transcription factor | 0.5 | 0.9 | 1.3 | 0.5 | 2.7 | 3.3 | 2.7 | 1.4 | 1.0 | 2.8 | 5.3 | 10.5 | 1.6 | 3.6 | 1.5 | 1.4 | 2.6 | 3.8 | 7.1 | 2.2 | 2.0 | 2.9 | 1.5 | 2.5 | 1.3 | 1.7 | 1.4 | 1.8 | 1.4 | 1.4 |
| Similar to hypothetical protein DKFZp586O | 0.4 | 0.7 | 1.4 | 0.6 | 2.2 | 2.5 | 0.9 | 0.8 | 0.9 | 1.6 | 5.7 | 14.6 | 1.3 | 1.2 | 1.3 | 0.8 | 4.4 | 6.2 | 0.6 | 1.4 | 1.4 | 0.3 | 2.4 | 3.0 | 1.4 | 3.8 | 2.4 | 0.9 | 1.3 | 2.4 |
| Roundabout, axon guidance receptor | 0.6 | 0.8 | 1.2 | 0.4 | 2.2 | 4.3 | 1.3 | 1.7 | 1.6 | 0.9 | 3.6 | 8.2 | 0.8 | 1.9 | 1.4 | 1.4 | 1.3 | 1.7 | 0.9 | 1.9 | 2.2 | 0.4 | 2.9 | 2.8 | 1.2 | 1.9 | 1.6 | 0.7 | 1.6 | 2.0 |
| KIAA1944 protein | 0.5 | 1.2 | 1.7 | 0.5 | 3.4 | 4.3 | 1.7 | 0.4 | 1.2 | 0.7 | 3.8 | 9.7 | 1.2 | 2.0 | 1.7 | 1.0 | 2.0 | 4.1 | 0.9 | 1.3 | 1.4 | 1.7 | 0.4 | 1.2 | 1.0 | 2.0 | 1.8 | 0.9 | 1.5 | 1.6 |
| Hypothetical protein FLJ10305 | 0.4 | 0.7 | 1.1 | 0.5 | 2.2 | 2.4 | 1.0 | 0.7 | 0.4 | 0.8 | 2.8 | 4.2 | 0.8 | 2.7 | 1.5 | 0.8 | 7.4 | 10.4 | 0.9 | 1.7 | 1.4 | 0.4 | 3.5 | 5.8 | 2.5 | 3.7 | 3.4 | 0.9 | 1.5 | 2.4 |
| Hypotetical protein FLJ35827 | 0.4 | 1.0 | 1.7 | 0.5 | 2.8 | 3.1 | 1.2 | 1.9 | 1.8 | 0.7 | 3.1 | 6.1 | 1.6 | 1.2 | 1.0 | 1.1 | 3.6 | 4.5 | 1.0 | 1.5 | 1.2 | 0.6 | 2.6 | 4.1 | 1.2 | 1.5 | 1.8 | 0.9 | 1.3 | 1.8 |
| Estrogen Response Element (ERE) | 14.0 | 1.6 | 0.8 | 2.8 | 0.6 | 1.1 | 109 | 6.9 | 3.5 | 2.7 | 0.8 | 1.1 | 15.6 | 1.4 | 1.9 | 2.3 | 1.2 | 1.2 | 136 | 3.2 | 1.4 | 10.1 | 0.6 | 1.0 | 10.0 | 1.2 | 0.3 | 2.2 | 0.9 | 1.1 |
Fig. 1.
Cell and ER subtype regulation by tamoxifen and raloxifene. (A) The regions regulated by the SERMs in Table 1 are shown as the % of the 32 regions that were regulated in MCF-7, HeLa, Ishikawa, U2OS or WAR-5 cell lines. (B) The sum of the total number of regions regulated by tamoxifen or raloxifene in the presence of ERα or ERβ in all five cell lines.
3.2. ER subtype-specific regulation of genes by tamoxifen and raloxifene
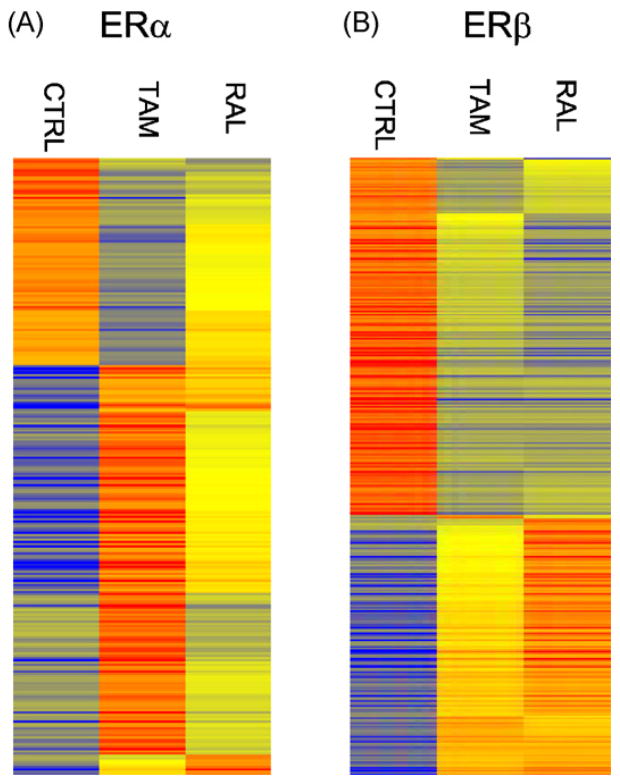
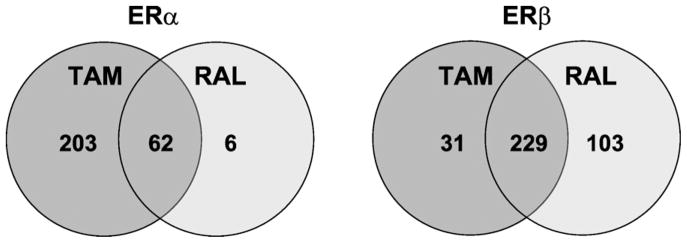
As mentioned our transfection studies suggested that raloxifene is a relative ERβ-selective SERM. We next used microarrays to determine if a similar pattern of regulation occurs with tamoxifen and raloxifene on endogenous genes in U2OS cells stably expressing ERα and ERβ. We previously showed that these cells produce comparable levels of ERs by binding assays and that raloxifene and tamoxifen regulated different genes after treatment for 18 h (Kian Tee et al., 2004). Because of the long treatment time this study identified both primary and secondary target genes. It is conceivable that the genes differentially regulated by raloxifene and tamoxifen were secondary genes rather than primary genes. To determine if raloxifene and tamoxifen regulated different primary target genes, we treated U2OS-ERα and U2OS-ERβ with raloxifene and tamoxifen for only 6 h. The heatmaps show that the genes regulated by tamoxifen and raloxifene in the U2OS-ERα cells (Fig. 2A) were distinct from those regulated in the U2OS-ERβ cells (Fig. 2B). A gene was considered to be activated or repressed if the magnitude was 2-fold or greater and statistically significant (p < 0.05). A list of genes regulated by each drug and the magnitude of regulation are shown in Supplemental Table 2. Venn diagrams show that in the U2OS-ERα cells tamoxifen regulated 265 genes compared to 68 genes with raloxifene (Fig. 3). There were 203 genes specifically regulated by tamoxifen and 6 genes regulated only by raloxifene. 62 genes were commonly regulated by both tamoxifen and raloxifene. An opposite pattern was observed in the U2OS-ERβ cells, where raloxifene regulated more specific genes than tamoxifen (103 vs. 31 genes). 229 genes were commonly regulated (Fig. 3).
Fig. 2.
Heatmaps of log-intensities of the regulated genes by tamoxifen and raloxifene in U2OS-ERα or U2OS-ERβ cells. Genes regulated by at least one of the compounds in U2OS-ERα (A) or U2OS-ERβ cells (B) cells are shown in rows. U2OS-ERα or U2OS-ERβ cells were treated for 6 h with 1 μM tamoxifen (Tam) or raloxifene (Ral). For each gene (row), the average log intensities are colored yellow, relatively higher expression are colored with reds of increasing intensity, and relatively lower expression are colored with blues of increasing intensity. The controls and treated samples for microarrays were done in triplicate. Each activated gene was 2-fold or greater and each repressed gene was 0.5-fold or greater with p-values < 0.05.
Fig. 3.
Venn diagrams of genes regulated by tamoxifen or raloxifene. The number of genes regulated specifically by 1 μM tamoxifen (Tam) or raloxifene (Ral) in U2OS-ERα (left) or U2OS-ERβ (right) cells. The numbers in the middle represent the genes commonly regulated by tamoxifen and raloxifene.
3.3. Raloxifene regulates fewer classes of genes than tamoxifen in U2OS-ERα cells
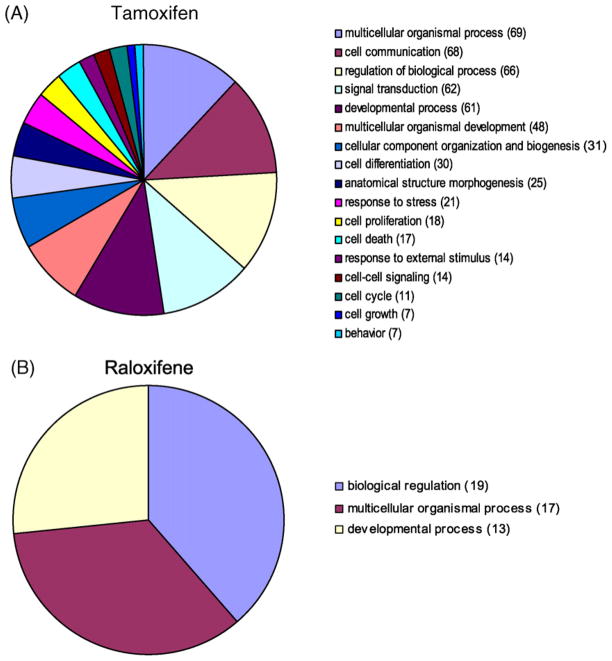
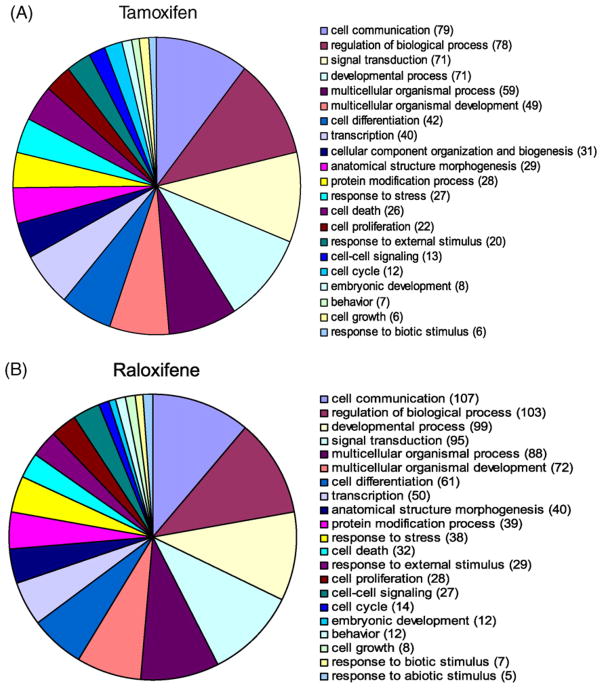
Gene ontologies show that raloxifene regulated only three major classes of genes in U2OS-ERα cells (Fig. 4B), compared to 17 major classes of genes for tamoxifen, including a class of genes involved in cell growth and proliferation (Fig. 4A). The major classes of genes regulated by tamoxifen (Fig. 5A) were similar to those regulated by raloxifene in U2OS-ERβ cells (Fig. 5B). However, with GO terms corresponding very small groups, there is some difference between genes regulated by tamoxifen and genes regulated by raloxifene (data not shown), which is consistent with the differences observed in genes regulated by the SERMs (Supplemental Table 2).
Fig. 4.
Pie charts comparing significantly enriched GO terms for genes regulated by tamoxifen or raloxifene in U2OS-ERα cells. Gene ontology (GO) terms significantly enriched in various biological processes for genes regulated by tamoxifen (A) and raloxifene (B) in U2OS-ERα cells. Threshold 0.05 was used for selecting GO terms using BH-adjusted p-values. Significant GO slim terms are presented in the pie chart. The number of genes regulated in the each biological class is shown in parentheses.
Fig. 5.
Pie charts comparing significantly enriched GO terms for genes regulated by tamoxifen or raloxifene in U2OS-ERβ cells. Gene ontology (GO) terms significantly enriched in various biological processes for genes regulated by tamoxifen (A) and raloxifene (B) in U2OS-ERβ cells. Threshold 0.05 was used for selecting GO terms using BH-adjusted p-values. Significant GO slim terms are presented in the pie chart. The number of genes regulated in the each biological class is shown in parentheses.
3.4. ERβ regulates genes in opposite direction in response to E2 and the SERMs
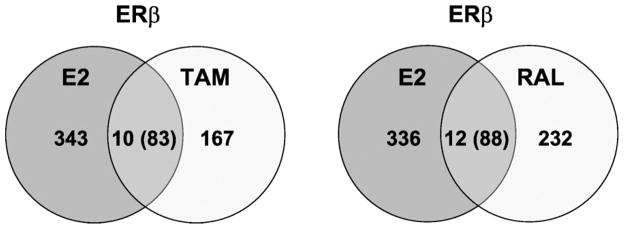
Our transfection studies also showed that some regions responded to E2 and the SERMs in an opposite direction in the cells transfected with ERβ, but not the ERα. To determine if a similar pattern occurred with endogenous genes, we compared the genes regulated by SERMs with those genes regulated by E2. Consistent with the transfection studies there were no genes regulated in an opposite direction with E2 and the SERMs in the U2OS-ERα cells. In the U2OS-ERβ cells, there were a total of 93 genes that were commonly regulated by E2 and tamoxifen, of which 83 were regulated in an opposite direction (Fig. 6). Raloxifene and E2 regulated 100 genes in common and 88 of those genes were regulated in an opposite direction (Supplemental Table 3). These findings indicate that ERβ can regulate genes in opposite direction in response to E2 and the SERMs.
Fig. 6.
Venn diagrams of genes regulated by E2, tamoxifen or raloxifene. The number of genes regulated by 10 nM estradiol (E2 ), 1 μM tamoxifen (Tam) or raloxifene (Ral) in U2OS-ERβ cells. The numbers in the middle represent the genes commonly regulated by E2 and tamoxifen (left) or E2 and raloxifene (right). The numbers in parentheses represent the genes that were regulated in an opposite direction.
4. Discussion
The SERMs are a unique class of drugs that bind to ERs, and are distinguished from estrogens by their capacity to act as an antagonist or agonist in various tissues (MacGregor and Jordan, 1998). While the antagonist property of SERMs have been exploited for breast cancer treatment for decades the value of the agonist property of SERMs has only recently been realized in clinical practice by use of raloxifene for osteoporosis prevention and treatment (Ettinger et al., 1999). However, the agonist property of current SERMs is associated with some serious side-effects such as an increased risk of endometrial cancer with tamoxifen (Fisher et al., 1994; Shang, 2006) and blood clots with both tamoxifen and raloxifene (Vogel et al., 2006). A better understanding of the molecular mechanisms whereby SERMs produce agonist effects could lead to the development of SERMs that produce less side-effects. This might be achieved by developing SERMs that have greater tissue selectivity or ER-subtype specificity.
The agonist effect of SERMs has been studied using a simple AP-1 element (Kushner et al., 2000; Webb et al., 1995). Our discovery of new and diverse SERM-responsive regions from native genes (Levy et al., 2008) provided an opportunity to compare the cell type and ER subtype responses to tamoxifen and raloxifene with more complex regulatory regions from endogenous genes. We showed that 32 regulatory regions responded to tamoxifen or raloxifene in five different cell lines, further demonstrating the authenticity of the SERM-responsive regions. Whereas AP-1 is the most common known transcription factor that mediates SERM activation of genes only 15 of the 32 regulatory regions had an AP-1 site (Supplemental Table 1). Furthermore, there was not a different pattern of regulation between regions containing an AP-1 element and those that did not. These results suggest that other elements besides AP-1 can mediate the agonist action of SERMs. It is unlikely that the ERE or Sp1 was responsible for SERM activation, because only 4 and 6 regulatory regions contained these elements, respectively. GATA2 was the most common element observed as 24 regulatory regions contained this site, but its role in mediating the effects of SERMs is not known.
The SERM-responsive regions were regulated in a cell type specific manner as some regions were regulated in some cell types, but not others. This property demonstrates a feature about the agonist activity of SERMs that is different from the agonist property of estrogens, because E2 activated a classical estrogen response element in all five cell lines. Another difference between E2 and the SERMs was the finding that E2 was much more effective at repressing the regions in the five cell lines compared to the SERMs. We were unable to identify a motif by bioinformatics that was associated with repression in the regulatory regions, most likely because there were an insufficient number of regions tested. It is also conceivable that multiple, distinct elements rather than a single motif are responsible for repression by E2. We also found that there is little correlation between the regulation of the endogenous genes by PCR and transfection assays with the regulatory regions. We speculate that this is likely due to mapping of the regulatory region to the nearest gene. However, it is likely that some regulatory regions regulate distant genes rather than the nearest gene.
The mechanism of the cell type specific action of the SERMs is not clear. The regulation of genes by estrogenic ligands requires ERs, coregulators and transcription factors (Carroll et al., 2005; Laganiere et al., 2005; Smith and O’Malley, 2004). The differential expression of each of these factors could contribute to the cell specific effects. One major determinant of cell type selectivity is the expression levels of coregulators in different tissues (Smith and O’Malley, 2004). This was demonstrated by the observation that the agonist effect of tamoxifen in endometrial cells was due to the high expression of SRC-1 (Shang and Brown, 2002). We analyzed the microarray data to determine the relative expression of SRC-1, SRC-2 and SRC-3 in the 5 cell lines. With the exception of higher expression of SRC-3 in MCF-7 cells there was similar expression levels of these three classes of coactivators in all five cell lines (data not shown). These findings indicate that other coactivators or factors are responsible for the tissue selective effects of the SERMs. In addition to coactivators, genome-wide screens with tiling arrays have found that the regulation of genes by ERs require transcription factors, such as FOXA1 (Carroll et al., 2005; Laganiere et al., 2005), which was present in 13 out of the 32 gene regulatory regions. We also showed that the activation of the NKG2E gene by estrogens requires a cluster of transcription factors that include c-jun, HSF-2 and C/EBPα (Levy et al., 2007). These findings raise the possibility that the different responses to the SERMs in the five cell lines could be due to a differential expression of transcription factors.
We also found that tamoxifen and raloxifene had different effects on the regulatory regions in the presence of ERα and ERβ. Tamoxifen regulated nearly equal number of regions with ERα and ERβ, whereas raloxifene regulated about twice as many regions with ERβ in the five cell lines. These findings indicate that tamoxifen is a non-selective SERM, whereas raloxifene is a relative ERβ selective SERM. We also found a similar pattern with endogenous genes in U2OS cells. Microarrays showed that tamoxifen regulated about four times as many genes compared to raloxifene in U2OS-ERα cells, whereas the number of genes regulated by the tamoxifen and raloxifene were similar in U2OS-ERβ cells. The Study of Tamoxifen and Raloxifene (STAR) trial compared the effectiveness of raloxifene to tamoxifen at reducing the risk of invasive breast cancer in premenopausal and postmenopausal women (Vogel et al., 2006). While both drugs were equally effective at reducing the risk of invasive breast cancer the side-effect profiles in STAR demonstrated that tamoxifen and raloxifene have different clinical effects, as subjects treated with raloxifene had fewer cataracts, blood clots and uterine cancers than those treated with tamoxifen (Vogel et al., 2006). Based on our findings it is conceivable that tamoxifen might cause more adverse effects than raloxifene by its greater action on ERα. We found that tamoxifen activated 17 regions with ERα in Ishikawa cells compared to only 2 regions with raloxifene. The lack of a pronounced effect of raloxifene with ERα in the Ishikawa endometrial cancer cell line might also explain why it does not increase the risk of endometrial cancer (Cummings et al., 1999; Martinez Lage et al., 2000) like tamoxifen. The GO analysis found that tamoxifen, but not raloxifene regulated cell proliferation genes in the ERα cells. Taken together, these findings suggest that the lack of effect of raloxifene on endometrial cancer might be due to its weak action on ERα, which is responsible for regulating genes involved in cell proliferation in endometrial cells (Shang, 2006).
The agonist property of SERMs is beginning to be translated to clinical practice for osteoporosis and more clinical indications for SERMs are on the horizon, such as vaginal dryness and mastalgia (Shelly et al., 2008). While SERMs are more selective than estrogens it is conceivable that more selective SERMs can be developed that will have a safer profile. Our results indicate that SERMs that are targeted selectively to ERα or ERβ should have different clinical effects. Surprisingly, we found that the SERMs regulated the regions and endogenous genes in an opposite direction to E2 with ERβ, but not ERα. This finding suggests that ERβ-selective SERMs might produce effects that are opposite to E2, which increases the risk of breast and endometrial cancer. In addition, our studies showing that raloxifene is a relative ERβ selective SERM and the clinical findings that it is associated with less side-effects (Vogel et al., 2006) indicate that ERβ-selective SERMs might be safer for treating conditions, such as osteoporosis that depend on the agonist activity.
Supplementary Material
Acknowledgments
We thank Pierre Chambon and Jan-Åke Gustafsson for providing plasmids. We also thank Zhijin Wu and Yunxia Sui for providing us the dbRMA package for microarray analysis. This work was supported by a grant from the American Cancer Society to D.C.L.
Abbreviations
- E2
estradiol
- ER
estrogen receptor
- SRC
steroid receptor coactivator
- ChIP
chromatin immunoprecipitation
- PCR
polymerase chain reaction
- BH
Benjamini and Hochberg
- GO
gene ontology
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.mce.2008.10.050.
Footnotes
Disclosure
N.L., C.G. and W.A.R. have nothing to declare. X.Z., M.T., and I.C., are employees of Bionovo, Inc. T.P.S., G.L.F. and D.C.L. are on the Scientific Advisory Board of Bionovo, Inc. D.C.L. has received financial support for research from Bionovo, Inc.
References
- Beissbarth T, Speed TP. GOstat: find statistically overrepresented gene ontologies within a group of genes. Bioinformatics. 2004;20:1464–1465. doi: 10.1093/bioinformatics/bth088. [DOI] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Cummings S, Eckert S, Krueger K, Grady D, Powles T, Cauley J, Norton LTN, Bjarnason N, Morrow M, MEL, Black D, Glusman J, Costa A, Jordan V. The effect of raloxifene on risk of breast cancer in postmenopausal women. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson JA. International union of pharmacology. LXIV Estrogen receptors. Pharmacol Rev. 2006;58:773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- Dudoit S, Shaffer JP, Boldrick JC. Multiple hypothesis testing in microarray experiments. Stat Sci. 2003;18:71–103. [Google Scholar]
- Dutertre M, Smith CL. Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor-alpha: regulation by phosphorylation sites in the A/B region depends on other receptor domains. Mol Endocrinol. 2003;17:1296–1314. doi: 10.1210/me.2001-0316. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger B, Black D, Mitlak B, Knickerbocker R, Nickelsen T, Genant H, Christiansen C, Delmas P. Reduction of vertebral fracture risk in post-menopausal women with osteoprosis treated with raloxifene. Results from a 3-year randomized clinical trial. JAMA. 1999;282:637–644. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- Feng W, Ribeiro RC, Wagner RL, Nguyen H, Apriletti JW, Fletterick RJ, Baxter JD, Kushner PJ, West BL. Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science. 1998;280:1747–1749. doi: 10.1126/science.280.5370.1747. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino J, Redmond C, Fisher E, Wickerham L, Cronin W. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86:527–537. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- Grady D, Cauley JA, Geiger MJ, Kornitzer M, Mosca L, Collins P, Wenger NK, Song J, Mershon J, Barrett-Connor E. Reduced incidence of invasive breast cancer with raloxifene among women at increased coronary risk. J Natl Cancer Inst. 2008;100:854–861. doi: 10.1093/jnci/djn153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell. 2004;5:207–213. doi: 10.1016/s1535-6108(04)00059-5. [DOI] [PubMed] [Google Scholar]
- Kian Tee M, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors {alpha} and {beta} Mol Biol Cell. 2004;15:1262–1272. doi: 10.1091/mbc.E03-06-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev. 2005;26:465–478. doi: 10.1210/er.2004-0027. [DOI] [PubMed] [Google Scholar]
- Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- Laganiere J, Deblois G, Lefebvre C, Bataille AR, Robert F, Giguere V. From the cover: location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc Natl Acad Sci USA. 2005;102:11651–11656. doi: 10.1073/pnas.0505575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N, Tatomer D, Herber CB, Zhao X, Tang H, Sargeant T, Ball LJ, Summers J, Speed TP, Leitman DC. Differential regulation of native estrogen receptor-regulatory elements by estradiol, tamoxifen, and raloxifene. Mol Endocrinol. 2008;22:287–303. doi: 10.1210/me.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N, Zhao X, Tang H, Jaffe RB, Speed TP, Leitman DC. Multiple transcription factor elements collaborate with estrogen receptor {alpha} to activate an inducible estrogen response element in the NKG2E gene. Endocrinology. 2007;148:3449–3458. doi: 10.1210/en.2006-1632. [DOI] [PubMed] [Google Scholar]
- Love RR, Mazess RB, Tormey DC, Barden HS, Newcomb PA, Jordan VC. Bone mineral density in women with breast cancer treated with adjuvant tamoxifen for at least two years. Breast Cancer Res Treat. 1988;12:297–302. doi: 10.1007/BF01811242. [DOI] [PubMed] [Google Scholar]
- MacGregor JI, Jordan VC. Basic guide to the mechanisms of antiestrogen action. Pharmacol Rev. 1998;50:151–196. [PubMed] [Google Scholar]
- Martinez Lage JM, Oliveros-Cid A, Martinez-Lage P. Estrogens and Alzheimer’s disease: rationale, promises, and facts. Med Clin (Barc) 2000;114:747–755. [PubMed] [Google Scholar]
- Mersereau JE, Levy N, Staub RE, Baggett S, Zogric T, Chow S, Ricke WA, Tagliaferri M, Cohen I, Bjeldanes LF, Leitman DC. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol Cell Endocrinol. 2008;283:49–57. doi: 10.1016/j.mce.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettles KW, Greene GL. Ligand control of coregulator recruitment to nuclear receptors. Annu Rev Physiol. 2005;67:309–333. doi: 10.1146/annurev.physiol.66.032802.154710. [DOI] [PubMed] [Google Scholar]
- O’Regan RM, Jordan VC. The evolution of tamoxifen therapy in breast cancer: selective oestrogen-receptor modulators and downregulators. Lancet Oncol. 2002;3:207–214. doi: 10.1016/s1470-2045(02)00711-8. [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper G, Nilsson S, gustafsson JA, Kushner P, Scanlan T. Differential ligand activation of estrogen receptors ERa and ERb at API sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Ricke WA, Ishii K, Ricke EA, Simko J, Wang Y, Hayward SW, Cunha GR. Steroid hormones stimulate human prostate cancer progression and metastasis. Int J Cancer. 2006;118:2123–2131. doi: 10.1002/ijc.21614. [DOI] [PubMed] [Google Scholar]
- Shang Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat Rev Cancer. 2006;6:360–368. doi: 10.1038/nrc1879. [DOI] [PubMed] [Google Scholar]
- Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103:843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- Shelly W, Draper MW, Krishnan V, Wong M, Jaffe RB. Selective estrogen receptor modulators: an update on recent clinical findings. Obstet Gynecol Surv. 2008;63:163–181. doi: 10.1097/OGX.0b013e31816400d7. [DOI] [PubMed] [Google Scholar]
- Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- Torchia J, Glass C, Rosenfeld MG. Co-activators and co-repressors in the integration of transcriptional responses. Curr Opin Cell Biol. 1998;10:373–383. doi: 10.1016/s0955-0674(98)80014-8. [DOI] [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Jr, Wade JL, 3rd, Robidoux A, Margolese RG, James J, Lippman SM, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP study of tamoxifen and raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- Webb P, Lopez GN, Uht RM, Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- Webb P, Nguyen P, Shinsako J, Anderson C, Feng W, Nguyen MP, Chen D, Huang SM, Subramanian S, McKinerney E, Katzenellenbogen BS, Stallcup MR, Kushner PJ. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol Endocrinol. 1998;12:1605–1618. doi: 10.1210/mend.12.10.0185. [DOI] [PubMed] [Google Scholar]
- Wu Z, Irizarry RA. Stochastic models inspired by hybridization theory for short oligonucleotide arrays. J Comput Biol. 2005;12:882–893. doi: 10.1089/cmb.2005.12.882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.