Abstract
Background
Daily suppression of herpes simplex virus type 2 (HSV-2) reduces plasma HIV-1 concentrations and has been shown to delay HIV-1 disease progression modestly in one clinical trial. We investigated the impact of daily suppressive acyclovir on HIV-1 disease progression in Rakai, Uganda
Methods
In a single site trial, 440 HIV-1, HSV-2 dually infected consenting adults with CD4+ T-cell counts 300-400 cells/μL and not on antiretroviral therapy were randomized 1:1 to receive either acyclovir 400 mg orally twice daily or placebo; participants were followed for 24 months. The primary outcome was CD4 <250 or ART initiation for WHO stage IV disease. Intent-to-treat analysis used Cox proportional hazards (CPH) models, adjusting for baseline log10 viral load (VL), CD4 cell count, gender and age to assess the risk of disease progression. The impact of suppressive HSV-2 treatment by baseline VL was also investigated in a CPH model. This trial is registered with clinicaltrials.gov, number NCT00405821.
Findings
Overall, 110 participants in the placebo arm and 95 participants in the treatment arm reached the primary endpoint (Adj HR 0.75, 95% CI 0.58-0.99; p=0.040). In a sub-analysis stratified by baseline VL quintile, participants with a baseline VL >= 50,000 copies/ml experienced a 38% reduction in HIV disease progression in the treatment compared to placebo arm (Adj HR 0.62, 95% CI 0.43-0.96;p=0.03).
Interpretation
Acyclovir reduced the rate of disease progression by 25%, with the greatest impact occurring among individuals with high baseline VL. Suppressive acyclovir may be warranted among HSV-2/HIV-1 dually infected individuals with viral loads >= 50,000 copies/ml prior to antiretroviral treatment.
Keywords: HIV-1, herpes simplex virus, acyclovir, disease progression
Introduction
Interventions that slow HIV-1 disease progression could postpone the need for antiretroviral therapy (ART) and prolong life expectancy for HIV-infected persons, thus safe, effective, and low cost approaches to delay HIV disease progression are urgently needed. Despite the success achieved in scaling up ART, the majority of the 22.5 million HIV+ persons in low and middle-income countries are not yet on ART. With many programs facing funding constraints, cost-effective measures to delay ART initiation are needed
Herpes simplex virus type 2 (HSV-2) has been shown to up-regulate HIV-1 replication at the cellular level and to increase viral load.(1) HSV-2 is the most common cause of genital ulcer disease (GUD); seroprevalence among HIV-1 infected individuals ranges from 70% to over 90%.(2;3) GUD is prevalent in HIV-infected individuals and increases HIV transmission to uninfected partners.(4) Reactivation of HSV-2 is common among HIV-1 infected individuals and plasma and genital HIV-1 concentrations increase during reactivation.(3;5-7) HIV-1 viral load (VL) in plasma is the key determinant of HIV progression and transmission.(8;9) A recent meta-analysis of seven randomized trials of daily herpes suppressive therapy with acyclovir or valacyclovir for 8-12 weeks has shown a reduction in median plasma HIV-1 RNA concentration by 0.33 log10 copies/ml and one randomized controlled trial has shown a modest 16% reduced risk of disease progression with daily acyclovir.(10;11) We undertook a randomized, placebo-controlled, double blind trial of acyclovir 400mg twice daily to evaluate the impact on disease progression among dually HIV/HSV-2 infected individuals who were not yet eligible for ART in this Ugandan setting.
Methods
Participants
Four hundred and forty eligible HIV-1, HSV-2 co-infected clients 18 years and older from Rakai Health Sciences Program and affiliated HIV care programs in the area were recruited between May 2007 and November 2008. Inclusion criteria were seropositivity for both HIV-1 and HSV-2, and CD4 count between 300 and 400 cells/ul. We excluded individuals with AIDS defining illnesses, and individuals receiving ART. All participants provided written informed consent. The study was approved by the Uganda Virus Research Institute Science and Ethics Committee, the Uganda National Council for Science and Technology and the NIAID Intramural Institutional Review Board.
Study Design
The study was a double-blinded, individually randomized, placebo-controlled trial of 400mg acyclovir twice daily versus placebo. The study drug was manufactured by Carlsbad Laboratories (San Diego, CA, USA) with matched acyclovir 400mg and placebo tablets, packaged in monthly bottles of 60 tablets to provide a 30 day supply, and stored between 20°C – 25°C. Placebo tablets were identical in appearance, taste and weight to the acyclovir tablets. Participants were instructed to take one tablet in the morning and one in the evening. The sample size of 220 per arm was estimated based on time-to-event design and the log-rank test and the study was powered to detect a hazard ratio of 1.5-2.0 at 2 years with power of 80%, assuming that 40% of participants in the placebo arm will progress to CD4+ cell count < 250 cells/μL and a 10% loss to follow-up.
Randomization and masking
Participants were randomly assigned to the intervention or control groups after eligibility screening as follows. Treatment assignment was randomly generated using a fixed block randomization method of block size 4. Each of the 4 unique computer generated alphanumeric random numbers representing the two study arms were printed on a random assignment-card, and placed in an opaque envelope. Two teams of clinicians were assembled and each assigned 8 HIV-care clinics (hubs) provided with random assignment cards, without switching their assigned batches with the other team. For each enrolling team, a block of size 4 was exhausted first before picking another block of size 4; i.e. no replacement was done for each selected assignment card until whole block of size 4 was exhausted.
Study drug packaging and labeling
Each participant's monthly pills of 60 tablets for 24 months were packaged for the acyclovir and placebo, separately, by non-project staff completely independent of the study, but supervised by the two unblinded statisticians. Pill bottles were labeled with unique computer generated alphanumeric codes then arranged in numerical order of the random numbers and stored in the pharmacy prior to dispensing to the study participants. The randomization alphanumeric computer generated label on each pill bottle corresponded with the randomization number on the card used at the enrollment visit. The Pharmacy released pill bottles corresponding to packs of envelopes going to the field. All study staff, investigators and participants were blinded to the randomization code apart from two protocol statisticians (FM & NK).
Screening and follow-up
Study screening included: identification of HIV-1 and HSV-2 serostatus, willingness to adhere to study procedures and baseline laboratory evaluations (CD4, renal, hematology and liver function testing, HIV VL). Participants were seen monthly over 24 months follow-up for drug refill and adverse event review. Female participants provided a self administered vaginal swab and a urine sample for pregnancy testing. A symptom screen was performed and if deemed necessary a physical examination was done including a genital exam and swab of any visual ulcers for subsequent testing using a GUD multiplex assay (detecting HSV-1,2, H. ducreyi and T. pallidum). At every 6 months visit a more intensive history was taken including adverse event review, quality of life survey and a physical examination was performed. Blood was taken at these major 6 monthly visits for repeat CD4 and HIV VL testing.
To establish eligibility, HIV-1 serostatus was determined using two different enzyme immunoassays (Vironostika HIV-1, Organon Teknika, Charlotte, North Carolina, USA, and Cambridge Biotech, Worcester, Massachusetts, USA), with Western blot confirmation of all discordant EIAs (HIV-1 WB Bio-Merieux-Vitek, St Louis, Missouri, USA). HSV-2 serostatus was determined using Focus HerpeSelect-2 EIA (Focus Technologies, Cypress, CA, USA), with a cut-off of 3.4 to improve specificity.(12;13) CD4 testing was performed using a FACSCalibur (Becton Dickenson, Franklin Lakes, New Jersey, USA) and HIV VL testing was done using the Roche Monitor v1.5 assay (Roche Diagnostics, Indianapolis, USA)
The primary adherence measure was clinic-based pill count. For 25 (0.3%) visits where pill count was missing, we relied on self-report of subject. Percent adherence was defined as the number of pills taken during the previous month divided by the number of pills expected to be taken based on the time interval between visits. For missed dispensing visits, we attributed zero pills taken from the time of the scheduled visit for each subject. Pill counts were performed by study staff but no additional adherence counseling was done based on pill counts. For the intent-to-treat analysis, subjects who were permanently or temporarily discontinued from study drug (for example, for concomitant corticosteroid usage) were considered 0% adherent during the period of study drug discontinuation. Monthly data were aggregated quarterly and categorized as less than 90%, more than 90%, or missing; these categories were selected on the basis of adherence levels that had been achieved in previous efficacy trials.(14;15) Participants contributed to adherence data until reaching the primary endpoint, having started ART, being lost to follow-up or experiencing death.
Study endpoints
The primary outcome was HIV-1 disease progression defined as progression to a CD4 less than 250 or a WHO stage IV condition other than esophageal candidiasis (referred to as ART eligibility). A secondary composite endpoint analysis also included non-traumatic death and ART initiation for any reason apart from short course prevention of mother to child transmission (PMTCT). Similar composite measures have been used as outcomes in earlier studies of ART and have been proposed as outcomes for trials of preventive HIV-1 vaccines that might alter viral load and disease progression.(10;16-18)
Statistical analysis
The primary outcome, HIV-1 disease progression to ART eligibility (CD4<250 cells/ ul or WHO stage IV disease) was estimated assuming that this event occurred at the monthly visit in which it was detected. In both study arms, time from enrolment was cumulated up to the 24-month follow-up visit, end-point or censoring, whichever occurred first, and the incidence of ART eligibility was estimated per 100 person-years. Exploratory analyses assessed the comparability of the two study arms at enrolment; age, sex, baseline pregnancy status for women, viral load and CD4 count. We used an intention-to-treat approach for the primary efficacy analysis. Women who become pregnant during the study and were provided with short course prophylaxis PMTCT were analyzed in their randomization arm. Hazard ratios (HR) and 95% CI of ART eligibility in the acyclovir versus the placebo arm were estimated using Kaplan-Meier (KM) survival analysis and Cox proportional hazard regression for the adjusted analyses. Primary analyses adjusted for baseline viral load and CD4 counts as planned apriori(19) . A post-hoc analysis was also performed stratified by baseline HIV VL <50, 000 copies/ml or >= 50, 000 copies/ml. Baseline viral load was categorized into four groups; <10,000; 10,000-49,999; 50,000-99,999; and 100,000+ copies/mL while CD4 counts were categorized as 300-349 and 350+ cells/ul. The log-rank test was used to assess comparability of failure (ART eligibility) for the two arms. All analyses were deemed to be statistically significant at a two-sided α=0.05 level, and were conducted using Stata version 10.0 statistical analysis software.
For the formal statistical monitoring, we used the O'Brien-Fleming alpha spending-function at data accrual information fraction points of 50%, 75% and 100%, based on the Lan-DeMets group sequential approach with 2 interim and a final analysis. The 2-sided alpha cut off points for the first, second and last analyses were 0.00153, 0.00916, and 0.02200, respectively. The data cutoff date for the first interim analysis was March 31, 2009, when about 50% of projected person time had been accrued, and June 4, 2010 for the second interim analysis, when about 75% of person time had been accrued. None of the interim analyses showed a statistically significant difference in ART eligibility between the two study arms. WinLD was used for the alpha and Z-boundaries calculations at each look.
We analysed the annual rate of change in log10 VL using multi-level linear mixed effects models (random intercept and random slope) with an unstructured covariance structure. The annual rate of change in log10 viral load was estimated for each study arm and between study arms using a time-study arm interaction term. Participants were censored from trajectory analyses when they reached a study endpoint (immunological or clinical), started ART, were lost to follow up (died, refused), or reached administrative censoring point. Linear regression modeling was also used to assess the difference in mean viral load (log10 VL) between treatment and placebo, adjusting for baseline viral load.
Role of the funding source
SJR, TQ are employees of NIAID/NIH and involved in the study design, data collection, data analysis, data interpretation and writing of the report. SJR, FM, KN, NK, MJW, RHG, DS & TCQ contributed to design of the study and writing the protocol, undertook all analyses, wrote the manuscript, and had final responsibility for the decision to submit for publication. FM & NK were the protocol statisticians and had access to the raw data. PS, GM, IB contributed to study conduct, laboratory support and writing of the manuscript.
Results
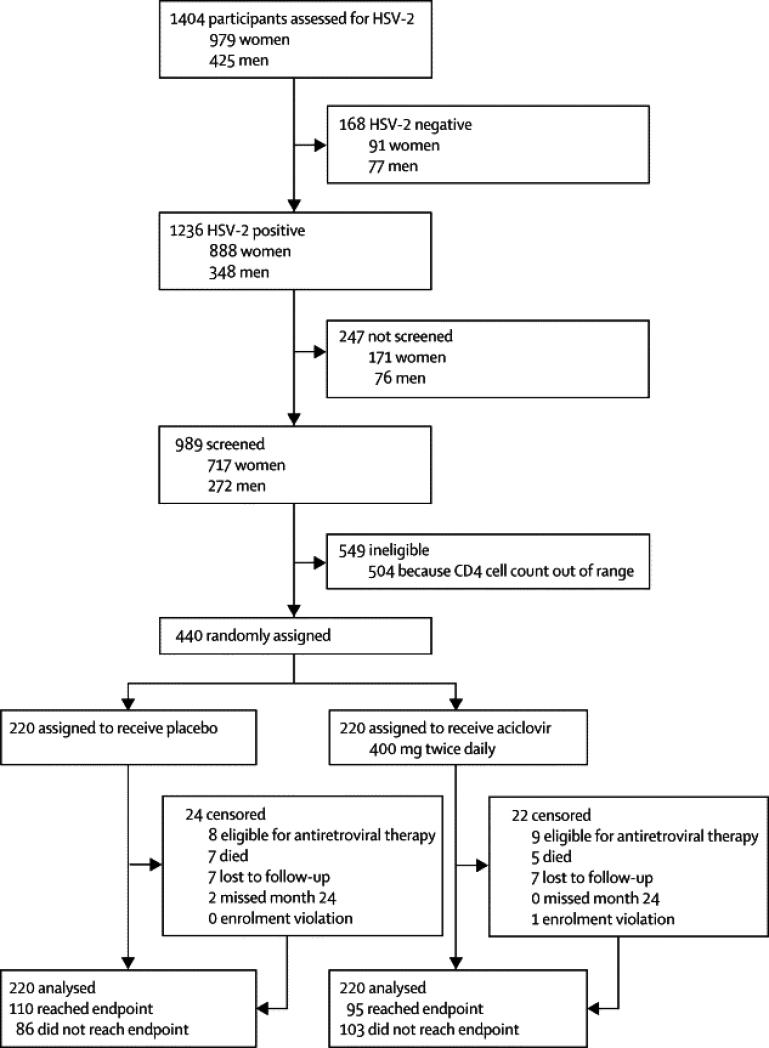
The CONSORT diagram is given in Figure 1. A total of 1,404 potential participants were assessed for HSV-2 seropositivity of which 1,236 were HSV-2 seropositive and 989 (80.0%) were screened sequentially for enrollment up to the time of full enrolment. The major screening criteria excluding enrollment was CD4 out of the eligibility range. Among the 504 ineligible subjects due to CD4, the high (n=290) and low (n=214) median CD4 cells/ul values (IQR) were 475 (434-549) and 248 (219-276) respectively. 440 eligible subjects were randomized equally across both treatment arms. Table 1 shows baseline demographic and laboratory parameters by study arm. There were no statistically significant baseline differences between study arms by sex, age, CD4 and viral load. Overall the majority (71%) of participants were women, 42% were aged 20-39 years and about 61% had viral load<50,000copies/ml. The median baseline CD4 was 350 (IQR 323-375) and baseline HIV-1 plasma RNA was 4.4 log10 copies per ml (IQR 3.8-5.1).
Figure 1.
Overview of Recruitment and Retention
ART: antiretroviral therapy, LTF: lost to follow-up
Table 1.
Baseline characteristics by trial arm
| Enrolment characteristics | Placebo | Treatment | P-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Total | 220 | 100.0 | 219 | 100.0 | |
| Sex | |||||
| Female | 161 | 73.2 | 150 | 68.5 | |
| Male | 59 | 26.8 | 69 | 31.5 | 0.295 |
| Age (years) | |||||
| 20-29 | 44 | 20.0 | 46 | 21.0 | |
| 30-39 | 93 | 42.3 | 93 | 42.5 | 0.956 |
| 40-49 | 53 | 24.1 | 54 | 24.7 | |
| 50+ | 30 | 13.6 | 26 | 11.9 | |
| CD4 | |||||
| 300-349 | 105 | 47.7 | 106 | 48.4 | |
| 350-399 | 115 | 52.3 | 115 | 51.6 | 0.924 |
| Viral load | |||||
| <50000 | 137 | 62.3 | 133 | 60.7 | 0.769 |
| 50000+ | 83 | 37.7 | 86 | 39.3 | |
| Further Viral load categories | |||||
| <10 000 | 65 | 29.6 | 67 | 30.6 | |
| 10 000-49 999 | 72 | 32.7 | 66 | 30.1 | 0.901 |
| 50 000-99 999 | 23 | 10.5 | 21 | 9.6 | |
| 100 000+ | 60 | 27.3 | 65 | 29.7 | |
| Log10 Viral load | |||||
| Mean (SD) | 4.40 | (0.87) | 4.40 | (0.86) | 0.964 |
| Median (IQR) | 4.44 | (3.80,5.05 ) | 4.43 | (3.85,5.07) | 0.962 |
SD: standard deviation, IQR (interquartile range)
Four hundred three (92%) subjects completed the study intervention phase up to month 24. Study retention was high at all time points with 98% (430) retained at month 6, 95% (420) retained at month 12 and 94% (413) retained at month 18. A total of 235 subjects were censored in the primary analysis by month 24. Fourteen (3.2%) participants were lost to follow-up, 7 in the intervention and 7 in the control arm. Twelve subjects were censored for death, 5 in the intervention arm and 7 in the control arm. Seventeen subjects were censored for having initiated ART, of which 9 were in the treatment arm and 8 in the control arm. One subject in the treatment arm was an enrolment violation, and contributed no follow-up time to the primary outcomes analysis. Eighty-six subjects in the control arm (39.1%) and 103 subjects in the intervention arm (46.8%) reached month 24 without meeting the primary endpoint or censoring for any reason. There were 2 subjects in the control arm who missed the scheduled month 24 visit and were thus censored at time of last study visit at month 23. Adherence rates to study drug versus placebo were similar between arms (table 2); the proportion of subjects achieving > 90% drug coverage during each quarterly period ranged from 80.9% to 91.0% in the intervention arm, and from 81.7% to 95.0% in the control arm. There were 24/150 (16%) incident pregnancies in the treatment arm and 29/160 (18%) in the placebo arm; relative risk 0.88, 95% CI 0.54-1.45; p=0.619). Among the incident pregnancies not censored for ART initiation, 19/150 (13%) in the treatment arm and 28/160 (17%) in the placebo arm were provided short course ART (zidovudine plus lamivudine)from 28 weeks gestation for prevention of mother to child transmission (PMTCT) of HIV; relative risk 0.73, 95% CI 0.42-1.24; p=0.2689). Apart from the twelve deaths resulting in censoring, there were 8 additional deaths among participants after they had reached the primary endpoint but prior to month 24. Of the 20 deaths during the study, 19 were HIV-associated (2 were meningitis, 2 febrile illness, 1 gastroenteritis and 14 thought to be HIV related but of unknown cause) and 1 death was due to spousal assault. The median (IQR) CD4 cell count among the HIV-associated deaths was 349 cells/ul (248-390 cells/ul). One subject in the acyclovir and two subjects in the placebo group died after starting antiretroviral therapy.
Table 2.
Quarterly Study Drug Compliance Summary
| M1-M3 | M4-M6 | M6-M9 | M9-M12 | M12-M15 | M15-M18 | M18-M21 | M21-M24 | |
|---|---|---|---|---|---|---|---|---|
| Acyclovir | 93.8% | 92.4% | 94.0% | 94.8% | 94.2% | 93.9% | 95.4% | 94.6% |
| Placebo | 95.2% | 92.0% | 93.3% | 94.6% | 97.4% | 96.4% | 97.2% | 96.9% |
| Completed visits | 1299 | 1276 | 1088 | 1073 | 935 | 924 | 758 | 739 |
| Missed Dispensing | 4/1299 (0%) | 39/1276 (3%) | 30/1088 (3%) | 23/1073 (2%) | 14/935 (1%) | 17/924 (2%) | 8/758 (1%) | 10/739 (1%) |
* This table includes scheduled monthly study drug dispensing visits for uncensored subjects and subjects not yet meeting an endpoint for the primary analysis.
Table 3 shows the number of enrollees reaching the endpoint and the rate of ART eligibility by study arm. During follow-up, 205 participants (46.7 %) reached the primary composite endpoint, 110 in the placebo arm (50.0%) and 95 in the treatment arm (43.2%); one participant reached a clinical endpoint and 204 subjects reached an immunologic endpoint.
Table 3.
Rate and Hazard Ratio for ART eligibility
| N | Events/pyr | Rate/100pyr | Crude HR (95%CI) | Adjusted HR (95%CI) | |
|---|---|---|---|---|---|
| Overall | 438 | 205/ 631.98 | 32.4 | ||
| Study arm | |||||
| Placebo | 219 | 110/303.8 | 36.21 | 1.0 | 1.0 |
| Treatment | 219 | 95/328.1 | 28.95 | 0.79(0.60, 1.04) | 0.75 (0.57,0.99) |
| Sex | |||||
| Female | 310 | 143/440.4 | 32.5 | 1.0 | 1.0 |
| Male | 128 | 62/191.6 | 32.4 | 0.98 (0.73,1.33) | 0.79 (0.57,1.08) |
| Age-group | |||||
| 20-29 | 90 | 49/126.2 | 38.8 | 1.0 | 1.0 |
| 30-39 | 185 | 82/267.1 | 30.7 | 0.80 (0.56,1.14) | 0.87 (0.60,1.24) |
| 40-49 | 107 | 49/162.3 | 30.2 | 0.78 (0.53,1.16) | 0.95 (0.63,1.44) |
| 50+ | 56 | 25/76.3 | 32.7 | 0.90 (0.56,1.46) | 1.01 (0.62,1.67) |
| Baseline CD4 | |||||
| 300-349 | 211 | 117/282.3 | 25.2 | 1.0 | 1.0 |
| 350-399 | 227 | 88/349.7 | 41.4 | 0.57 (0.43,0.75) | 0.58 (0.44,0.77) |
| Baseline Viral load | |||||
| <10 000 | 132 | 37/218.8 | 16.9 | 1.0 | |
| 10 000-49 999 | 138 | 73/202.1 | 36.1 | 2.25 (1.51,3.34) | 2.20 (1.47,3.29) |
| 50 000-99 999 | 43 | 27/61.1 | 44.2 | 2.92 (1.77,4.79) | 2.82 (1.70,4.68) |
| 100 000+ | 125 | 68/149.97 | 45.3 | 3.01 (2.02,4.50) | 3.17 (2.10,4.78) |
| Baseline Viral load | |||||
| <50 000 | 270 | 110/420.9 | 26.1 | 1.0 | |
| 50 000+ | 168 | 95/211.1 | 45.0 | 1.88 (1.43,2.48) |
ART: antiretroviral therapy, pyr: person years, HR: hazard ratio
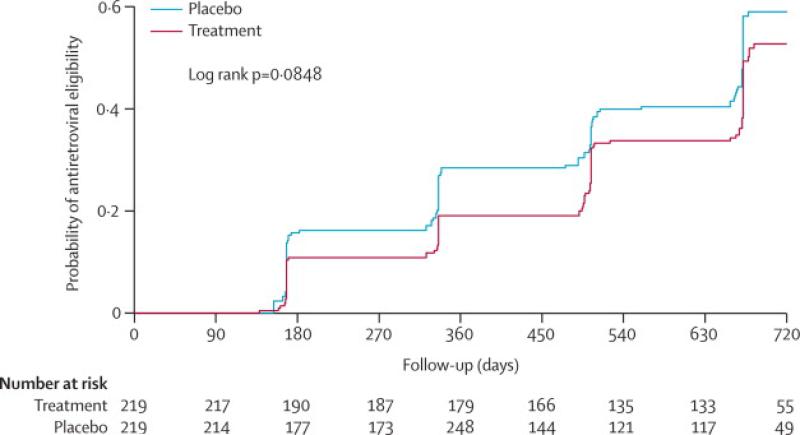
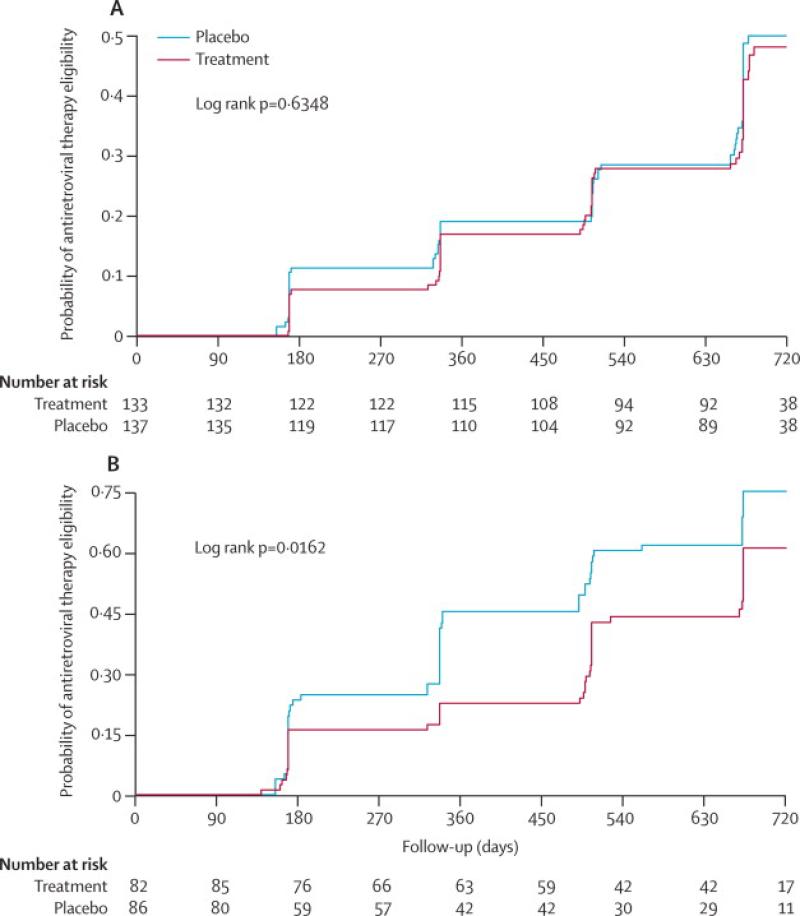
In the unadjusted time to event analysis, the unadjusted hazard ratio (HR) of disease progression was lower in the treatment arm compared to placebo but not statistically significant (HR 0.79; 95% CI 0.60-1.04; p=0.093) (table 3; figure 2). In the protocol-specified analysis that adjusted for baseline viral load and CD4, the treatment effect was more pronounced (Adj HR 0.75; 95% CI 0.58-0.99; p=0.040). In a further analysis stratified by baseline HIV VL, participants with baseline VL <50 000 copies/ml, had a modest and non-statistically significant 10% reduction in disease progression (Adj HR 0.90; 95% CI 0.54-1.5, p=0.688), whereas among participants with baseline VL ≥ 50 000 copies/ml, a 38% reduction in HIV disease progression was observed in the treatment compared to the placebo arm (Adj HR 0.62; 95% CI 0.43-0.96, p=0.03)(table 3; figure 3a & 3b). Among participants who reached the primary endpoint, the median time was 336 days (IQR 168-560) among placebo and 497 days (IQR 322-664) among treatment participants, a difference of 161 days (p= 0.111). Among those with baseline VLs ≥50,000 cps/mL, the difference in median time to the primary endpoint was 168 days, whereas for those with VLs <50,000 cps/mL difference in median time was only one day.
Figure 2.
Cumulative probability of ART eligibility
ART: antiretroviral therapy, AHR: adjusted hazard ratio
Figure 3a.
Cumulative probability of ART eligibility
ART: antiretroviral therapy
Figure 3b: Cumulative probability of ART eligibility
ART: antiretroviral therapy
We performed a secondary analysis to investigate the impact of acyclovir on progression to the composite endpoint of death, AIDS, CD4<250 cells/ul or initiation of ART for any indication other than short course PMTCT. In this analysis the reduction in disease progression was similar to that observed in the primary analysis (Adj HR 0.76; 95% CI 0.58-0.98, p=0.034).
HIV viral load during study follow-up was also included as a secondary endpoint in the study. In the acyclovir arm, HIV VL declined at an annual rate of –0.061 log10 copies/ml (95% CI -0.250,-0.129) and in the placebo arm HIV VL increased at an annual rate of 0.402 log10 copies/ml (95% CI 0.212-0.592). The difference in the annual rate of change of VL between the 2 study arms was -0.463 log copies/ml (95% CI -0.731,-0.194, p=0.001). Relative to placebo arm, the mean viral load over 24 months was -0.16 log copies/ml lower in the treatment arm (95% CI; -0.24, -0.08, p<0.001).
There were 27 GUD episodes (for 20 of 219 subjects, 9.1%) in the acyclovir arm, and 81 GUD episodes (for 47 of 220 subjects, 21.4%) in the placebo arm. The incidence of GUD episodes was 8.2/100 py (27/328.1 py) in the treatment and 26.7/100 py (81/303.8py) in the control arm (IRR=0.31, 95%CI 0.19-0.48, p<0.0001).
Discussion
Acyclovir 400mg twice daily for suppression of HSV-2 in HIV-1 co-infected individuals reduced the risk of HIV-1 disease progression by 25%, ours is the second study examining a disease progression endpoint and our results are similar although stronger than the 16% reduction in the Partners in Prevention HSV/HIV Transmission study.(10) This difference in efficacy may be due to differences in enrollment criteria; CD4 counts between 300-400 cells/ul in the present study, whereas the Partners study enrolled individuals with any CD4 count above 250 cells/ul (median enrollment CD4 462 cells/ul, log10VL 4.1 copies/ml). A novel finding in our study was the greater impact observed on disease progression among participants with baseline viral loads ≥50,000 copies/mL. The more advanced HIV disease stage of our population with slightly higher baseline viral load (log10 VL 4.4 copies/ml) may explain some of the differences in the results of the two studies. Our results provide further evidence that specific anti-herpes therapy in the absence of ART can delay HIV-1 disease progression by approximately 161 days.
Several studies have shown that acyclovir and valacyclovir reduce plasma HIV VL with a median reduction of 0.33 log10copies/ml from a meta-analysis of seven randomized controlled trials(11;20), consistent with the 0.463 log10copies/ml reduction in this trial. We postulate that the reduction in HIV-1 concentration observed in our study mediated the reduction in HIV-1 disease progression. Our results are consistent with a recent review of observational data which estimated a 25% reduction in disease progression for every 0.3 log10copies reduction of plasma HIV-1 RNA.(21) A recent trial from Kenya using high dose (valacyclovir 1.5g twice daily) reported a more dramatic reduction in HIV-1 VL with a 1.23 log reduction in HIV-1 VL.(22) The greater bioavailability of valacyclovir and high dose in this latter study may provide a greater impact on reduction in HIV disease progression.
The mechanism of action of acyclovir on HIV-1 VL and disease progression remains a subject of debate. Acyclovir is a highly specific chain terminator of herpes simplex virus, preferentially incorporated by the herpes virus DNA polymerase.(23) The reduction in HSV-2 reactivation and symptomatic GUD through suppressive therapy has been proposed as one of the probable mechanisms for acyclovir's effect in reducing HIV-1 concentrations.(10) We observed a 69% reduction in symptomatic genital ulcer disease incidence in the treatment arm which supports this hypothesis.
In vitro studies have also shown that acyclovir can directly inhibit HIV-1 replication, possibly in a similar fashion to the reverse-transcriptase inhibitor class of drugs.(24) These studies raised some concern because the in-vitro the reverse transcriptase inhibitor mutation, V75I, was found to emerge after HIV-1 exposure to high dose acyclovir.(25) However, the 0.463 log10 reduction in HIV-1 viral load trajectories in our study persisted over 24 months follow-up without HIV-1 viral rebound, consistent with earlier studies suggesting that this mutation had not developed with HSV-2 suppressive therapy at standard doses.(26)
The finding in our secondary analysis that the impact of acyclovir was greater among individuals with HIV-1 VL ≥50,000 copies/ml) may reflect the fact that individuals with higher baseline VLs are more rapid progressors (19) and therefore more likely to reach an endpoint over 24 months follow-up.
Subsequent to the initiation and conduct of our study, the World Health Organization (WHO) revised their recommendations to start antiretroviral therapy at CD4 counts less than 350 cells/ul.(27) Our enrollment criteria precluded examination of the impact of acyclovir on disease progression during earlier stages of HIV disease. Retention in HIV care pre-ART has emerged as an important implementation challenge and suppressive therapy for HSV-2 may offer an opportunity to improve retention among those not eligible for ART. Earlier combination ART will almost certainly have a more profound impact on disease progression than acyclovir. However, funding constraints has limited the ability to achieve early initiation of ART and acyclovir provides a cheaper alternative strategy allowing delay in initiation of ART for approximately 161 days. (28)
Although a formal cost-effectiveness study was not planned for our study, we calculated the number needed to treat to prevent initiation of ART from our overall disease progression delay (25%) and among those with high baseline HIV-1 VL (>= 50,000 copies/ml) which corresponds to 15 and 7 persons needed to treat, respectively. At an estimated yearly cost estimate of ART of $240, excluding monitoring, treating 15 persons with acyclovir 400mg twice daily at $24/year would be more expensive at $360 based purely on drug cost alone but this excludes ART delivery costs which would need to be examined in a formal costing analysis. Among those with high baseline HIV-1 VL, we would only need to treat 7 individuals resulting in a yearly cost of $168 plus the cost of an HIV-1 VL (estimated at $50) resulting in a total yearly cost of $218. The availability of HIV-1 VL testing in resource-constrained settings remains limited which would complicate implementation of a strategy based on HIV-1 VL screening but as treatment programs continue to expand and wider availability of lower cost HIV-1 VL technologies enter the market the situation could change. More data is needed to assess the optimal dose of HSV-2 anti-viral therapy including cost, tolerability of higher dosing and the impact of higher dose therapy on HIV-1 VL and disease progression.
We have shown the acyclovir suppressive therapy in HIV-1/HSV-2 co-infected individuals delays HIV-1 disease progression and the need for antiretroviral therapy by 25% over two years. Further investigation is needed to assess the role of other antiviral agents such as valacyclovir among populations with earlier HIV stage disease to establish if suppression of herpes virus could play a role in routine care of HIV-1/HSV-2 co-infected individuals not eligible for ART. Future work investigating the impact of acyclovir on inflammatory cytokines could also help further elucidate the mechanism of action on HIV disease progression.
Panel: Research in context
Systemic review
Seven randomized trials published to date have investigated the impact of acyclovir or valacyclovir on HIV-1 plasma viral load among HIV-1/HSV-2 co-infected adults.(11) Although these studies had different lengths of follow-up and used different methods to compare changes in HIV-1 viral load, all showed a consistent reduction in mean plasma viral load among individuals treated with acyclovir or valacyclovir.
Interpretation
This study is the second to investigate the role of suppressive HSV-2 therapy with acyclovir 400mg twice daily among HIV-1/HSV-2 co-infected individuals and the impact on HIV-1 disease progression. Consistent with the earlier multi-center study reported by the Partners in Prevention group, we also showed a delay in disease progression although ours was more pronounced (25% versus 16% in the Partners in Prevention study).(10) A novel finding from our study was the observation that HIV-1 disease progression was greatest among those with high (>=50,000 copies/ml) baseline HIV-1 VL with a 38% reduction in disease progression observed. Although more research is needed on costing, optimal dosage and tolerability of higher dose suppressive therapy, this study provides important evidence for the role of HSV-2 suppressive therapy among HIV-1/HSV-2 co-infected individuals not eligible for antiretroviral therapy.
Acknowledgements
We would like to thanks the trial participants; the staff of the Rakai Health Sciences Program and Larry Corey for his design input and support. We also thank the NIH Data Safety and Monitoring Board and the Institutional Review Boards (UVRI Science and Ethics Committee and the NIAID IRB) for their technical advice and oversight of this study.
Source of funding:
This research was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E, and in part through program project grant PO1 AI-30731-19. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
We declare that we have no conflicts of interest
Reference List
- 1.Moriuchi M, Moriuchi H, Williams R, Straus SE. Herpes simplex virus infection induces replication of human immunodeficiency virus type 1. Virology. 2000 Dec 20;278(2):534–40. doi: 10.1006/viro.2000.0667. [DOI] [PubMed] [Google Scholar]
- 2.Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008 Oct;86(10):805–12. doi: 10.2471/BLT.07.046128. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mbopi-Keou FX, Gresenguet G, Mayaud P, Weiss HA, Gopal R, Matta M, et al. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J Infect Dis. 2000 Oct;182(4):1090–6. doi: 10.1086/315836. [DOI] [PubMed] [Google Scholar]
- 4.Serwadda D, Gray RH, Sewankambo NK, Wabwire-Mangen F, Chen MZ, Quinn TC, et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect Dis. 2003 Nov 15;188(10):1492–7. doi: 10.1086/379333. [DOI] [PubMed] [Google Scholar]
- 5.McClelland RS, Wang CC, Overbaugh J, Richardson BA, Corey L, Ashley RL, et al. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS. 2002 Dec 6;16(18):2425–30. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 6.Mole L, Ripich S, Margolis D, Holodniy M. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis. 1997 Sep;176(3):766–70. doi: 10.1086/517297. [DOI] [PubMed] [Google Scholar]
- 7.Schacker T, Ryncarz AJ, Goddard J, Diem K, Shaughnessy M, Corey L. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA. 1998 Jul 1;280(1):61–6. doi: 10.1001/jama.280.1.61. [DOI] [PubMed] [Google Scholar]
- 8.Mellors JW, Rinaldo CR, Jr., Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996 May 24;272(5265):1167–70. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 9.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000 Mar 30;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 10.Lingappa JR, Baeten JM, Wald A, Hughes JP, Thomas KK, Mujugira A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010 Mar 6;375(9717):824–33. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludema C, Cole SR, Poole C, Chu H, Eron JJ. Meta-analysis of randomized trials on the association of prophylactic acyclovir and HIV-1 viral load in individuals coinfected with herpes simplex virus-2. AIDS. 2011 Jun 19;25(10):1265–9. doi: 10.1097/QAD.0b013e328347fa37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golden MR, Ashley-Morrow R, Swenson P, Hogrefe WR, Handsfield HH, Wald A. Herpes simplex virus type 2 (HSV-2) Western blot confirmatory testing among men testing positive for HSV-2 using the focus enzyme-linked immunosorbent assay in a sexually transmitted disease clinic. Sex Transm Dis. 2005 Dec;32(12):771–7. doi: 10.1097/01.olq.0000175377.88358.f3. [DOI] [PubMed] [Google Scholar]
- 13.Laeyendecker O, Henson C, Gray RH, Nguyen RH, Horne BJ, Wawer MJ, et al. Performance of a commercial, type-specific enzyme-linked immunosorbent assay for detection of herpes simplex virus type 2-specific antibodies in Ugandans. J Clin Microbiol. 2004 Apr;42(4):1794–6. doi: 10.1128/JCM.42.4.1794-1796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delany-Moretlwe S, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008 Jun 21;371(9630):2109–19. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mertz GJ, Jones CC, Mills J, Fife KH, Lemon SM, Stapleton JT, et al. Long-term acyclovir suppression of frequently recurring genital herpes simplex virus infection. A multicenter double-blind trial. JAMA. 1988 Jul 8;260(2):201–6. [PubMed] [Google Scholar]
- 16.Gilbert PB, Sun Y. Failure time analysis of HIV vaccine effects on viral load and antiretroviral therapy initiation. Biostatistics. 2005 Jul;6(3):374–94. doi: 10.1093/biostatistics/kxi014. [DOI] [PubMed] [Google Scholar]
- 17.Hammer SM, Katzenstein DA, Hughes MD, Gundacker H, Schooley RT, Haubrich RH, et al. A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. AIDS Clinical Trials Group Study 175 Study Team. N Engl J Med. 1996 Oct 10;335(15):1081–90. doi: 10.1056/NEJM199610103351501. [DOI] [PubMed] [Google Scholar]
- 18.MacArthur RD, Novak RM, Peng G, Chen L, Xiang Y, Hullsiek KH, et al. A comparison of three highly active antiretroviral treatment strategies consisting of non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or both in the presence of nucleoside reverse transcriptase inhibitors as initial therapy (CPCRA 058 FIRST Study): a long-term randomised trial. Lancet. 2006 Dec 16;368(9553):2125–35. doi: 10.1016/S0140-6736(06)69861-9. [DOI] [PubMed] [Google Scholar]
- 19.Jurriaans S, Van GB, Weverling GJ, Van SD, Nara P, Coutinho R, et al. The natural history of HIV-1 infection: virus load and virus phenotype independent determinants of clinical course? Virology. 1994 Oct;204(1):223–33. doi: 10.1006/viro.1994.1526. [DOI] [PubMed] [Google Scholar]
- 20.Barnabas RV, Wasserheit JN, Huang Y, Janes H, Morrow R, Fuchs J, et al. Impact of herpes simplex virus type 2 on HIV-1 acquisition and progression in an HIV vaccine trial (the Step study). J Acquir Immune Defic Syndr. 2011 Jul 1;57(3):238–44. doi: 10.1097/QAI.0b013e31821acb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008 Oct 18;22(16):2179–85. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mugwanya K, Baeten JM, Mugo NR, Irungu E, Ngure K, Celum C. High-dose Valacyclovir HSV-2 Suppression Results in Greater Reduction in Plasma HIV-1 Levels Compared With Standard Dose Acyclovir Among HIV-1/HSV-2 Coinfected Persons: A Randomized, Crossover Trial. J Infect Dis. 2011 Dec;204(12):1912–7. doi: 10.1093/infdis/jir649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elion GB. The biochemistry and mechanism of action of acyclovir. J Antimicrob Chemother. 1983 Sep;12(Suppl B):9–17. doi: 10.1093/jac/12.suppl_b.9. [DOI] [PubMed] [Google Scholar]
- 24.Lisco A, Vanpouille C, Tchesnokov EP, Grivel JC, Biancotto A, Brichacek B, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008 Sep 11;4(3):260–70. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tchesnokov EP, Obikhod A, Massud I, Lisco A, Vanpouille C, Brichacek B, et al. Mechanisms associated with HIV-1 resistance to acyclovir by the V75I mutation in reverse transcriptase. J Biol Chem. 2009 Aug 7;284(32):21496–504. doi: 10.1074/jbc.M109.024026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baeten JM, Lingappa J, Beck I, Frenkel LM, Pepper G, Celum C, et al. Herpes simplex virus type 2 suppressive therapy with acyclovir or valacyclovir does not select for specific HIV-1 resistance in HIV-1/HSV-2 dually infected persons. J Infect Dis. 2011 Jan 1;203(1):117–21. doi: 10.1093/infdis/jiq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammer SM, Eron JJ, Jr., Reiss P, Schooley RT, Thompson MA, Walmsley S, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008 Aug 6;300(5):555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 28.Geng EH, Bwana MB, Kabakyenga J, Muyindike W, Emenyonu NI, Musinguzi N, et al. Diminishing availability of publicly funded slots for antiretroviral initiation among HIV-infected ART-eligible patients in Uganda. PLoS One. 2010;5(11):e14098. doi: 10.1371/journal.pone.0014098. [DOI] [PMC free article] [PubMed] [Google Scholar]