Abstract
Macrophages from mouse strains with the naturally occurring mutation P451L in the purinergic receptor P2X7 have impaired responses to agonists (1). Because P2X7 receptors are expressed in bone cells and are implicated in bone physiology, we asked whether strains with the P451L mutation have a different bone phenotype. By sequencing the most common strains of inbred mice, we found that only a few strains (BALB, NOD, NZW, and 129) were harboring the wild allelic version of the mutation (P451) in the gene for the purinergic receptor P2X7. The strains were compared by means of dual energy X-ray absorptiometry (DXA), bone markers, and three-point bending. Cultured osteoclasts were used in the ATP-induced pore formation assay. We found that strains with the P451 allele (BALB/cJ and 129X1/SvJ) had stronger femurs and higher levels of the bone resorption marker C-telopeptide collagen (CTX) compared to C57Bl/6 (B6) and DBA/2J mice. In strains with the 451L allele, pore-formation activity in osteoclasts in vitro was lower after application of ATP. In conclusion, two strains with the 451L allele of the naturally occurring mutation P451L, have weaker bones and lower levels of CTX, suggesting lower resorption levels in these animals, which could be related to the decreased ATP-induced pore formation observed in vitro. The importance of these findings for the interpretation of the earlier reported effects of P2X7 in mice is discussed, along with strategies in developing a murine model for testing the therapeutic effects of P2X7 agonists and antagonists upon postmenopausal osteoporosis.
1. Introduction
In the past decade several reports have shown that P2-receptor signalling plays a central role in bone physiology [1–5]. Bone cells express several types of P2 receptors [6], allowing them to respond differently to nucleotides, depending on the types of nucleotides present, their concentration, and the duration of exposure [7, 8]. Prolonged exposure to high agonist concentrations initiates the formation of large pores in the membrane mediated by the P2 subtype P2X7, a feature often assessed by ATP-induced dye uptake [7, 9]. Activation of the P2X7 receptor results in changed cellular morphology and membrane blebbing [10], which initiates necrotic and apoptotic mechanisms in macrophages [11]. There are several studies directing a role to the P2X7 receptor in mediating ATP-induced apoptosis in other cell types [7] and accordingly increased osteoclast numbers have been found in mice with ablation of the P2X7 receptor (P2X7−/− mice) [3]. In humans the single nucleotide polymorphism Glu496Ala is associated with abolished pore formation activity of the P2X7 receptor [12] and with decreased ATP-induced apoptosis of osteoclasts in vitro [13]. The P2X7 receptors are expressed in both osteoclast precursors and resorbing osteoclasts [8, 14, 15], and therefore, in addition to activating the apoptotic pathway, the P2X7 receptor could play a role in osteoclast development [16–18] and activation [19].
In calvarial cells in vitro activation of P2X7 receptors increases expression of osteoblast markers, enhances mineralization, and induces membrane blebbing [20, 21]. The effects of P2X7 activation could also be mediated through the activation of the cytokine interleukin 1β (IL-1β) on bone cells or on cells adjacent to the bone compartment, resulting in a systemic effect on bone [22]. In macrophages from people carrying two C alleles of the Glu496Ala (A1513C) polymorphism, the processing of pro-IL-1β is impaired, leading to decreased levels of mature serum IL-1β.
The ablation of the P2X7 receptor generated by Solle (Pfizer P2X7−/−) in mice, led to reduced total bone mineral content (BMC), as a result of increased trabecular bone resorption, decreased periosteal circumference of the femur, reduced periosteal bone formation [3], and impaired response to mechanical loading [4]. Another murine model, with ablation of the P2X7 gene (GSK P2X7−/−), was generated by Chessell and his group [23] and has a dissimilar bone phenotype, showing increased cortical thickness in the tibial shaft, but no changes in total BMD [15]. The contradicting observations have been attributed to the dissimilar sample sizes, methods of the gene knockout, and different genetic backgrounds of the inbred strains used to generate the mice.
That the genetic background of the two P2X7−/− strains is important was shown in the study by Adriouch et al. [24] describing the presence of a naturally occurring mutation in the murine P2X7 gene. A thymine to cytosine change was found at nucleotide position 1352 (T1352C) in the B6 genome, but not in BALB/c and outbred mice. The mutation results in a change in the amino acid sequence (proline to leucine) at position 451 (P451L), in the cytoplasmic tail of the P2X7 receptor [24]. By transfecting HEK cells with constructs of both genotypes, they found that ATP-induced pore formation was reduced by approximately 50% in cells carrying the mutated 451L allele [24]. In murine thymocytes the P451L mutation affects apoptosis acting through the ATP-induced pore formation [25]. The reduced responsiveness of the P2X7 receptors to ATP in mice with 451L could have led to underestimation of the effects of the P2X7−/− upon bone and other parameters. Pfizer P2X7−/− mice were generated on 129/Ola × C57Bl/6(B6) × DBA/2 genetic backgrounds, and maintained on the B6 × DBA/2 background [3, 26]. The GSK P2X7−/− mice were maintained on B6 background, but originate from a B6/129 hybrid [15, 23].
Two papers have described the expression of a novel splice variant (called P2X7-k) for the rodent P2X7 [27, 28]. The splice variant was first found in lymphocytes of the GSK P2X7−/− mice by Taylor et al. [27]. Nicke et al. reported shortly after that P2X7-k shows higher agonist sensitivity and slower deactivation than the normal P2X7 receptor complexes [28]. The alternative exon of the P2X7-k splice variant includes the intracellular N-terminus and ~80% of the first transmembrane domain, and thereby escapes gene inactivation in the GSK P2X7−/− mice. However, the expression of the splice variant P2X7-k is tissue-specific, and not expressed on the plasma membrane of osteoclasts from BALB/cJ P2X7−/− [29].
None of the current P2X7−/− models are quite suitable for studying the therapeutic potential of the agonist acting through P2X7 in ovariectomized mice. First, the impaired ATP response in P451L mutated cells could mask the “true” effect of agonists and antagonists. Second, some of the founder strains included in the genetic background of these animals lacks response upon ovariectomy. The inbred mouse strains traditionally used for bone research represent different skeletal phenotypes during development and aging [30], which could be of importance in the interpretation of the precise role of P2X7 receptors in bone remodelling. In the present study, the major goal was to investigate the distribution of the P451L alleles in inbred strains of mice. Furthermore, we wanted to investigate the bone status of these animals in order to select a suitable strain with the preferred genetic background (i.e., carrying the P451 allele) for crossing the P2X7−/− genotype into.
2. Materials and Methods
2.1. Sequencing of P2X7
A single-coding mutation (T1352C) in the murine P2X7 gene resulting in a change from proline to leucine at position 451 (P451L) has earlier been reported in four major strains of inbred mice [24]. To examine the distribution of the two alleles of T1352C (P451L) among laboratory mice genomic DNA was analyzed by sequencing exon 13 in the murine P2X7 gene. DNA from 20 different inbred strains (C3H/HeJ, C3H/HeJCrl, NZB/B1NJ, NZW/J, SJL/J, AKR/J, BALB/cJ, BALB/cByJ, BALB/cAnNCrl, 129/J, 129X1/SvJ, SWR/J, C57L/J, C57BL/10J, DBA/1J, DBA/2J, SM/J, NOD, DDY/cJ, and CALB/Rk) and from 2 strains of outbred (Mus caroli/Ei and M. spretus) was obtained from The Jackson Laboratories (Bar Harbor, ME). The B6 originates from Charles River (Germany). The Danish Pest Infestation Laboratory (Lyngby, Denmark) donated ten wild Mus musculus domestica, from different locations. Earpieces and tail parts were used for DNA isolation with the QIAamp DNA Blood Mini Kit (Qiagen), which was used as template in PCR reactions with the following primers recognizing exon 13: F-ACT TGA GGG GTT GTC ATT GC and R-TCC AAG GGA AGC TGT ATT GTG giving an amplicon of 595 bp. For each PCR product two sequencing reactions were prepared with the specific sequencing primers (F-TGC TGA TGG GTC TGG AAA CT and R-CAT GAT GTG GCA GCC GTA) in the sequencing reaction with the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) as described by Ohlendorff et al. 2007 [13], and loaded on the ABI PRISM 3100 Genetic Analyzer. Duplicate amplification and sequencing reactions were run per strain to confirm the sequences.
2.2. Animals
Female mice of nine inbred strains (C3H/HeJ, NZB/B1NJ, SJL/J, AKR/J, BALB/cJ, 129X1/SvJ, SWR/J, C57L/J, and DBA/2J) were obtained at eight weeks of age from Jackson Laboratories (Bar Harbor, ME). The B6 originated from Charles River (Germany).
2.3. Study Protocol
The Danish Animal Welfare Council approved all animal procedures in advance (protocol: 2002/561–634). Female mice at the age of 120 days were starved overnight before euthanized by CO2. Blood was collected into 5 mL syringes by cardiac puncture. Serum was collected and stored at −80°C for later measurements of bone markers. The animals were scanned on a PIXImus (Lunar Corporation, Madison, WI) densitometer.
2.4. Bone Mineral Measurements and Body Composition
Bone mineral measurements and body composition of the animals were determined on the PIXImus densitometer. Animals were fixed in a standard position, and measurements were performed sequentially, with duplicate determinations. Intraassay CV was 0.47% and interassay CV 0.73%. Due to the large mineral content in the skull, it was excluded from the calculations.
2.5. Bone Formation and Resorption Markers
To investigate possible differences in bone formation markers between the genotypes, osteocalcin was measured in serum samples in duplicate using the Mouse Osteocalcin RIA Reagents from Biomedical Technologies, Inc. (Stoughton, MA), following the protocol supplied with the reagents. Interassay CV was 12% and intraassay CV was 6%.
Bone resorption as expressed by fragments of type I collagen in mouse serum (s-CTX) was measured in duplicate using the RatLaps Elisa Assay (C-telopeptide collagen type I fragment Assay) developed by Nordic Bioscience Diagnostics (Herlev, Denmark) and following the procedure supplied with the kit. Interassay CV was 14.8% and intraassay CV was 9.2%.
Alkaline phosphatase activity was measured in duplicate mouse serum using the Alkaline Phosphatase Reagent Kit (Sigma). Alkaline phosphatase activity was measured directly on the serum in multiwell plates, using a slight modification of the standard clinical chemistry procedure. Serum replicas were diluted with alkaline buffer solution and substrate solution was added to each well, and the plate was incubated at 37°C for 30 min. Finally 2.0N NaOH was added to each well to stop the reaction, before the absorbance was measured on a plate reader at 405 nm. Interassay CV was 5.9% and intraassay CV was 2.4%.
2.6. Bone Strength Measurements
On the day of sacrifice the mouse femurs were collected, cleaned for tissue, wrapped in saline gauze, and frozen at −20°C until biomechanical testing. The strength of the femur was measured by the 3-point-bending test on a Lloyd Instruments compression device (Lloyd Instruments, Fareham, UK), performed after rehydrating the femur in a saline solution at room temperature, as described earlier [31].
2.7. Bone Histomorphometry
To investigate histologic and morphometric changes in four different inbred strains histomorphometric analyses were performed, as described earlier [31]. In short, the total spines and tibias were collected and fixed in 70% ethanol at 5°C. The following indices were determined; bone volume in percentage of total volume (BV/TV%), cortical thickness (C.Th, μm), trabecular thickness (Tb.Th, μm), and eroded surface as percentage of bone surface (ES/BS%). Under fluorescent light the mineralizing surfaces were determined as percentage of bone surface (MS/BS%). Further, mineral appositional rate (MAR, μm/day) was calculated.
2.8. In Vitro Dye Uptake Assay
Osteoclasts were isolated from bone marrow from the long bones of 3-4 weeks old female mice of the strains; BALB/cJ, 129X1/SvJ, DBA, and B6 (10 mice per strain) as described by Wu et al. [32]. Bone marrow monocyte/macrophage lineage precursors were seeded in α-MEM (10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, 50 ng/mL M-CSF, and pH = 7.35–7.4) and plated with a density of 2 × 106 cells/cm3. More than 91% of both mononuclear and multinucleated cells in these cultures stained positively for TRAP (tartrate resistant acid phosphatase) using the leukocyte acid phosphatase kit (Sigma).
To investigate pore formation in osteoclasts from the four strains, as described for osteoblasts by Ke et al. [3], the medium was changed after 7 days in culture to a HEPES/glucose (20/10 mM) divalent cation-free buffer and the osteoclasts ability to take up the dye YO-PRO (Molecular Probes) upon ATP (200 nM) stimulation was assessed. After 9 minutes Hoechst 33342 (5 μg/mL, BioWhittaker) was added to the culture for another minute to reveal unstained nuclei in the osteoclasts. Only multinucleated osteoclasts (with 3 or more nuclei) were counted on a Leica BZ:03 Microscope and the number of YO-PRO containing cells was compared to the number of total osteoclasts in ten randomly chosen fields of sight. Control cultures were incubated with YO-PRO and Hoechst, but not ATP. Interassay CV was 9.8% and intraassay CV was 5.4%.
2.9. Resorption Assay
Osteoclasts were isolated and cultured as described above for the dyeuptake assay, but seeded in 96-well plates on bovine bone slices (Nordic Bioscience, Herlev Denmark) or glass-cover slips. After 10 days of culture the osteoclasts on glass-cover slips were stained for TRAP and counted by visible inspection of ten randomly chosen fields of sight. Only TRAP positive cells with more than 3 nuclei were quantified as multinucleated osteoclasts. The cells were removed from the bone slices and the resorption pits were stained with hematoxylin, prior to quantification with the C.A.S.T. (Computer Assisted Sterological Toolbbox) Grid system on Olympus Bx51. The results were calculated as area resorbed in percentage of total area. Interassay CV was 0.67% and intraassay CV was 0.45%.
2.10. Statistics
Statistical analyses were performed using the SPSS software, v.11.5. Standard parametric and nonparametric tests were used. For the comparison of results from the different strains the one-way ANOVA was used, with multiple comparisons and post hoc Bonferroni corrections. Differences were considered statistically insignificant when P ≤ 0.05. Simple descriptives of data were presented as means ± standard error of the mean (SEM).
3. Results
3.1. Distribution of the P451L Mutation in the Inbred Strains
The distribution of the two alleles of the P451L mutation among common strains of laboratory mouse strains was determined in 21 strains and sublines by sequencing genomic DNA. Only mice from wild populations, outbred strains and a few of the inbred strains, which included the four major strains, BALB, NOD, NZW, and 129, had the P451 allele, hereafter called the original genotype of the P451L mutation. The rest of the examined strains and sublines had the mutated 451L allele (Table 1).
Table 1.
The distribution of the strains in two groups with different allelic versions of the P451L mutation. Strains written in italic are outbred or mice from wild populations. ∗Confirming the data also shown by Adriouch et al. [24].
| Strains with P451 | Strains with 451L |
|---|---|
| Mus spretus ∗ | C57BL/6J (B6)∗ |
| Mus musculus ∗ | C57BL/10J∗ |
| Mus caroli ∗ | C57BL/6NCrl |
| BALB/cByJ∗ | DBA/1J∗ |
| BALB/cAnNCrl∗ | DBA/2J∗ |
| BALB/cJ | AKR/J |
| NOD∗ | C3H/HeJ |
| NZW∗ | C57L/J |
| 129/J∗ | NZB/BINJ |
| 129X1/SvJ | SJL/J |
| SWR/J | |
| DDY/J | |
| SM/J | |
| CALB/RkJ |
3.2. Bone Phenotypes in Relation to P451L Mutation
Ten inbred strains frequently used in bone related studies, were examined by DXA, three-point bending and serum concentration of bone markers to reveal the bone phenotype. As summarized in Table 2 there were significant differences in all measured parameters between groups, but only few associated to the different allelic versions of the P2X7 P451L mutation. With the genetic background of the two existing P2X7−/− mice [23, 26] in mind, the inbred strains of 129X1/SvJ and BALB/cJ (P451) were compared with B6 and DBA/2 (451L) with respect to bone status and these background strains were further examined by histomorphometric analysis, resorption activity, and in vitro dyeuptake assay.
Table 2.
Bone parameters and concentration of in vivo bone markers presented as means (±SD). Number of animals in each strain is indicated at the top of the table (n). BMD, BMC, and bone area, in whole body or femoral region was determined by DXA scanning. An electronic digital caliper measured femoral length, before bone strength of the femur was determined by a 3-point bending test. In vivo bone markers were measured on serum using commercial available kits. One-way ANOVA was performed to test differences between groups and post hoc Bonferroni corrections, using 0.05 as the significance level. Simple descriptive of data is presented as means and standard deviations (SD). Significant difference between the 129X1/SvJ, BALB/cJ, B6, and DBA/2 animals at the P < 0.05 level is extracted from Bonferroni multiple comparison analysis and indicated with asterisks when different from the three other strains, with A when different from 129X1/SvJ, with B when different from B6, with C when different from BALB/cJ, and with D when different from DBA/2J.
| Sample size (n) | 129X1/SvJ | BALB/cJ | B6 | DBA/2J | SWR/J | C57L/J | AKR/J | SJL/J | NZB/BlNJ | C3H/HeJ | ANOVA one way |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 14 | 16 | 14 | 14 | 15 | 15 | 15 | 15 | 15 | ||
| P451L genotype | P | P | L | L | L | L | L | L | L | L | |
| Bone Parameters | |||||||||||
| BMD (g/cm2) | 0.0562∗ | 0.0513A, D | 0.0502A, D | 0.0468∗ | 0.0477 | 0.0579 | 0.0576 | 0.0548 | 0.0525 | 0.0563 | <0.001 |
| (±0.0020) | (±0.0018) | (±0.0020) | (±0.0022) | (±0.0013) | (±0.0030) | (±0.0021) | (±0.0015) | (±0.0041) | (±0.0018) | ||
| BMC (g) | 0.535∗ | 0.471A, D | 0.458A, D | 0.358∗ | 0.394 | 0.548 | 0.593 | 0.459 | 0.465 | 0.558 | <0.001 |
| (±0.045) | (±0.028) | (±0.031) | (±0.029) | (±0.035) | (±0.042) | (±0.046) | (±0.025) | (±0.064) | (±0.036) | ||
| Area (cm2) | 9.53D | 9.19D | 9.13D | 7.65∗ | 8.26 | 9.46 | 10.29 | 8.38 | 8.83 | 9.90 | <0.001 |
| (±0.53) | (±0.30) | (±0.40) | (±0.41) | (±0.54) | (±0.35) | (±0.60) | (±0.40) | (±0.60) | (±0.50) | ||
| BMD in femur region (g/cm2) | 0.0798B, D | 0.0764B, D | 0.0655A, C | 0.0639A, C | 0.0650 | 0.0786 | 0.0782 | 0.0774 | 0.0740 | 0.0826 | <0.001 |
| (±0.0039) | (±0.0021) | (±0.0033) | (±0.0028) | (±0.0024) | (±0.0049) | (±0.0036) | (±0.0032) | (±0.0052) | (±0.0037) | ||
| BMC in femur region (g) | 0.0439B,D | 0.0402D | 0.0364A, D | 0.0281∗ | 0.0307 | 0.0464 | 0.0466 | 0.0391 | 0.0378 | 0.0439 | <0.001 |
| (±0.0044) | (±0.0028) | (±0.0026) | (±0.0026) | (±0.0021) | (±0.0044) | (±0.0029) | (±0.0030) | (±0.0060) | (±0.0036) | ||
| Area of femur region (cm2) | 0.552D | 0.529D | 0.558D | 0.443∗ | 0.476 | 0.595 | 0.599 | 0.507 | 0.511 | 0.533 | <0.001 |
| (±0.035) | (±0.031) | (±0.027) | (±0.035) | (±0.031) | (±0.046) | (±0.026) | (±0.032) | (±0.053) | (±0.028) | ||
| Femur length (mm) | 15.25D | 15.06D | 15.53D | 14.34∗ | 14.29 | 15.79 | 16.00 | 14.08 | 15.90 | 15.61 | <0.001 |
| (±0.36) | (±0.64) | (±0.23) | (±0.45) | (±0.40) | (±0.33) | (±0.69) | (±0.28) | (±0.45) | (±0.64) | ||
| Strength/Max. load femurs (N) | 31.5∗ | 24.6∗ | 16.3A, C | 18.7A, C | 16.7 | 28.0 | 30.5 | 20.0 | 23.3 | 33.9 | <0.001 |
| (±3.2) | (±1.7) | (±1.1) | (±1.5) | (±0.8) | (±2.4) | (±2.8) | (±0.8) | (±3.0) | (±2.3) | ||
|
| |||||||||||
| In vivo bone markers | |||||||||||
| Osteocalcin (ng/mL) | 40.0C, D | 55.91∗ | 38.42C, D | 71.72∗ | 38.44 | 36.49 | 72.37 | 51.09 | 48.49 | 63.22 | <0.001 |
| (±9.48) | (±11.78) | (±11.11) | (±10.89) | (±8.97) | (±8.75) | (±11.25) | (±10.20) | (±8.06) | (±11.38) | ||
| ALP (nmol/mL) | 266.8 | 314.2 | 260.2 | 317.6 | 272.5 | 179.7 | 190.9 | 213.7 | 299.4 | 270.6 | <0.001 |
| (±56.1) | (±41.2) | (±63.8) | (±60.5) | (±39.9) | (±48.3) | (±62.5) | (±59.6) | (±36.3) | (±56.3) | ||
| RatLaps-Telopeptide collagen (ng/mL) | 14.28B,C | 19.09∗ | 9.81A, C | 12.13C | 10.75 | 9.37 | 7.07 | 7.15 | 8.83 | 8.76 | <0.001 |
| (±3.45) | (±4.40) | (±1.86) | (±1.88) | (±1.62) | (±2.53) | (±2.62) | (±1.83) | (±1.95) | (±4.02) | ||
3.3. Bone Status of the Background Strains
When focusing on BMD/BMC 129X1/SvJ had significantly (P < 0.001) higher whole body bone mineral (BMD and BMC) compared to the other three strains (Figure 1(a)). In the load-bearing region, femoral BMD and BMC were higher in the strains 129X1/SvJ and BALB/cJ than B6 and DBA/2 (P < 0.001. Figure 1(b)).
Figure 1.
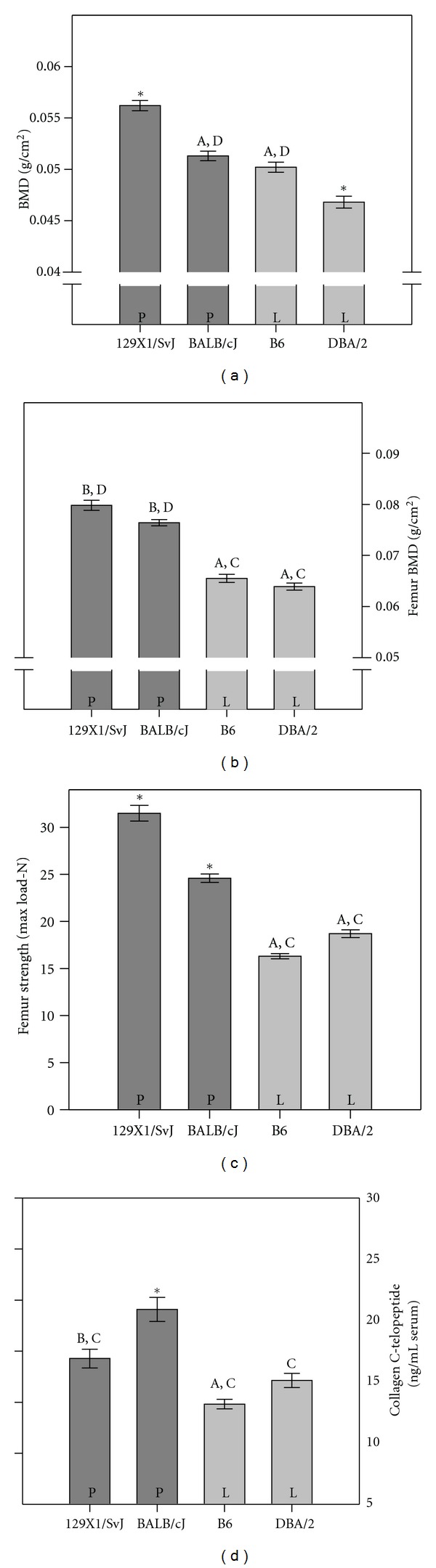
Bone parameters in the 129X1/SvJ, BALB/c, B6, and DBA/2 inbred strains. Significant difference between the strains at the P < 0.05 level is indicated with asterisks when different from the three other strains, with A when different from 129X1/SvJ, with B when different from B6, with C when different from BALB/cJ, and with D when different from DBA/2. P451L genotype indicated as P or L at each strain. (a) In BALB/c and B6 whole body BMDs were not significantly different from each other, but significantly lower than 129X1/SvJ and BALB/cJ. BALB/cJ had higher BMD than DBA. (b) The femoral BMD in 129X1/SvJ and BALB/cJ were significantly higher than B6 and DBA/2. The latter were not significantly different from each other. (c) The femoral strength assessed by a three-point bending test showed significantly higher strength in 129X1/SvJ femurs. BALB/c had stronger femurs than the DBA and B6 strains had. The latter two were not significantly different from each other. (d) The serum concentration of the resorption marker s-CTX-I, showed significantly higher resorption in the BALB/cJ strain and lowest in the B6 strain.
The ultimate way of determining bone quality is to test the ability of bone to resist mechanical forces. Determined by a three-point bending test, the femoral bone strength was different among the strains of mice (Table 2). Of the four background strains 129X1/SvJ and BALB/cJ were more resistant to mechanical forces at the femur than both DBA/2 and B6 strains (P < 0.001, Table 2, Figure 1(c)).
In vivo bone markers are summarized in Table 2. The serum concentration of the bone formation markers, alkaline phosphatase and osteocalcin, were not associated with the P451L mutation in the P2X7 gene. The BALB/cJ strain had significantly (P < 0.001) higher s-CTX than all the other strains (Figure 1(d)). 129X1/SvJ mice had significantly higher s-CTX than the B6 mice, but not the DBA/2 (Table 2).
3.4. Histomorphometric Analysis in Relation to the P451L Mutation
Bone volume (%) in the vertebrae was higher in 129X1/SvJ than in the BALB/cJ and the B6 (P < 0.001), whereas BALB/cJ and B6 did not differ significantly (Figure 2(a)). No significant difference was found in the Tb.Th between the different strains. The cortical thickness (C.Th) for BALB/cJ (102.1 μm) was not significantly different from B6 (93.0 μm) and DBA/2 (104,4 μm). The mean C.Th was 121.5 μm for the 129X1/SvJ strain, was significantly higher than in the three other strains. Neither MAR nor MS/BS showed any significant differences between the four strains. However ES/BS% showed that the 129X1/SvJ strain had a significantly higher ES/BS% than B6, and BALB/cJ, B6, and DBA/2 (Figure 2(b)).
Figure 2.
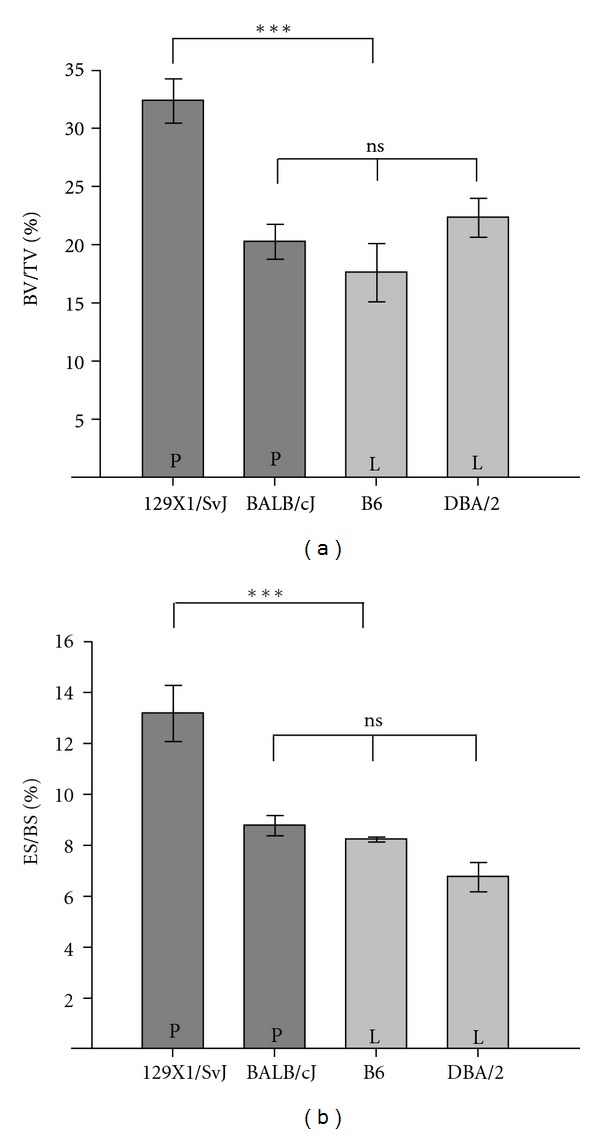
Histomorphometric analysis of the vertebrae in the strains 129X1/SvJ, BALB/cJ, B6, and DBA/2 displayed as means ± SEM. P and L refers to P451 and 451L, respectively. (a) The BV/TV% of 129X1/SvJ was between 32% and 46% lower than the BV/TV% in the other strains. (b) The percentage of eroded surfaces (ES/BS%) was nearly 50% higher in the 129X1/SvJ strain compared to the other strains.
3.5. Ex Vivo Bone Resorption Assay
The area of resorption pits per bone slice was calculated as percentage of total area in 10 mice from each strain. BALB/cJ and 129X1/SvJ osteoclasts showed 19.1% and 18.7% area resorbed in comparison to 17.4% and 16.3% for B6 and DBA/2, with no significant difference (P = 0.478 between BALB/cJ and DBA/2). The number of TRAP-stained osteoclasts did not differ significantly. BALB/cJ and 129X1/SvJ had 79.5 and 83.7 TRAP-positive osteoclasts in comparison to 93.6 and 97.4 for B6 and DBA/2, with no significant difference (P = 0.108 between BALB/cJ and DBA/2).
3.6. In Vitro Dye Uptake Assay
To elucidate the cellular bases of these changes, we examined the response to ATP stimulation in a dye uptake assay, comparing the pore formation in 4 cultures from 10 mice in each of the following strains; B6, BALB/cJ, DBA/2, and 129X1/SvJ. Half of the cell cultures were incubated with dye, but without ATP stimulation. These cultures were used as controls to determine the spontaneous background staining, compared to the total number of cells in the assay. A fraction of the osteoclasts in the un-stimulated control cultures showed spontaneous dye uptake, with no significant difference between the strains. Interestingly, the percentage of osteoclasts taking up dye upon ATP-stimulation was significantly higher when isolated from strains carrying the P451 allele (BALB/cJ and 129X1/SvJ) as compared to strains with the 451L-allele (DBA/2 and B6) (P < 0.05) (Figure 3). When corrected for background dye uptake, the number of dye uptaking osteoclasts from P2X7 451L strains was reduced by approximately 30% compared to osteoclasts from the P451 strains, suggesting deleterious effects of the mutation on P2X7-mediated pore formation.
Figure 3.
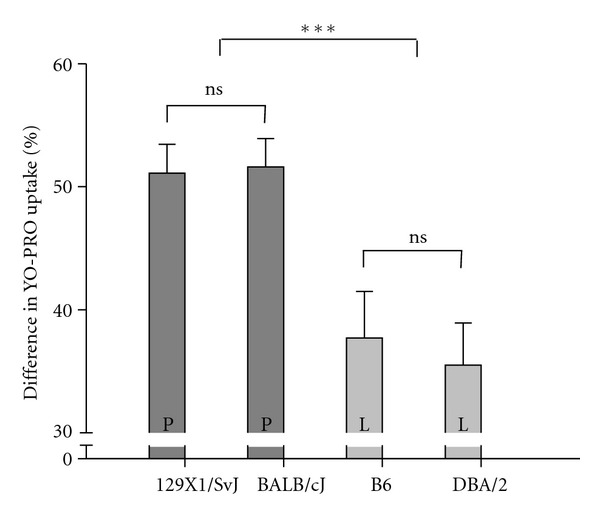
Dye uptake in the four major strains 129X1/SvJ, BALB/cJ, DBA/2, and B6, displayed as mean of differences between the dye uptake in the control cultures and the dye uptake in the ATP, stimulated cell cultures (±SEM). P and L refer to P451 and 451L, respectively.
4. Discussion
The P451 allele of the P451L mutation in P2X7 was present in different wild mice populations, in the outbred strains Mus caroli/Ei and M. spretus and the four inbred strains, BALB, NOD, NZW, and 129 (Table 1). In the bone phenotypic study, two of the strains we characterized, had the natural version (P451) of the mutation, the other eight strains had the 451L version. We found great diversity in all the examined bone phenotypic parameters, but only a few were associated to the P451L mutation. The strain 129X1/SvJ had higher BMD, stronger femurs and higher bone resorption in bone histomorphometric analysis, compared to BALB/cJ, DBA/2 and B6. BALB/cJ had stronger femurs with higher BMD and higher levels of the bone resorption marker s-CTX than DBA/2 and B6.
We found strong femurs with high BMD in the P451 mice. Mechanical stress of load-bearing parts of the body could induce release of ATP in the bone compartment. Mechanical stimulation of bone formation has been shown to be partly mediated through the P2X7 receptor [4]. However, we did not detect any significant differences in bone formation markers associated to the P451L alleles. Perhaps the local effect of ATP stimulation in the femurs cannot be detected on the systemic bone markers in the serum samples. The histomorphometric analysis was only of the vertebrae, where higher BV/TV% was found in the P451 mice.
In the bone phenotypic part of the study we found that strains with the naturally occurring mutation 451L in the gene for P2X7 had lower resorption than strains with the wild type allele (P451). Agonists activating P2X7 in low concentrations can initiate osteoclast formation [33, 34]. Significant differences in the number of osteoclasts after 10 days of culture were not detected, but bear in mind that osteoclast culture was done without agonist stimulation. The absence of an effect of the P451L mutation on osteoclast development in vitro could also be due to the lack of systemic hormones, mechanical stimulation, and lack of accessory cells in the culture system.
The next step was to investigate if there were any differences in resorption activity in vitro though we did not detect a significant difference in resorbed area there appeared to be a tendency to higher resorption in cultures with osteoclasts derived from P451 strains. The duration of resorption was not investigated in this study, but keeping in mind that the P2X7 receptor is involved in apoptosis of osteoclasts [13], there could be differences in the length of the osteoclasts' resorption phase related to the P451L allelic versions.
In humans, the polymorphism called Glu496Ala abolishes the pore formation activity of the P2X7 receptor [12] and has been associated with decreased ATP-induced apoptosis of osteoclasts in vitro [13]. A tendency towards more TRAP-positive osteoclasts was seen in the 451L osteoclast cultures. Apoptosis was not directly addressed in this study, but in vitro osteoclasts derived from the examined strains showed differences in the ATP-induced dye uptake assay. The examined mutation P451L is in the C-terminal part of P2X7, which is thought to be involved in the initiation of the pore formation, by activating pannexin-1 [35, 36]. In this manner the 451L mutated P2X7 receptors could blunt the pannexin-1 dependent dye uptake, and explain the reduced dye uptake we found in B6 and DBA/2 osteoclasts.
The physiological importance of the pore formation remains to be elucidated, but besides having a role in apoptosis it could be related to IL-1β processing and its release through pannexin-1 [37, 38]. Interleukin-1β has been reported to affect both bone resorption and bone formation dependent on the duration of the administration [39]. Interleukin-1β has also been shown to induce osteoclast precursor proliferation and differentiation, and increases bone resorption [40]. This suggests that IL-1β induces bone resorption initially, but that the long-term effect increased bone turnover, which corresponds to our data.
In macrophages from people carrying two C alleles of the Glu496Ala (A1513C) polymorphism, the processing of pro-IL-1β is impaired, leading to decreased levels of mature serum IL-1β. Following the theory that the reduced activity of the cytoplasmic tail of P2X7 (measured by pore formation) has significant importance in determining the bone cells' response to ATP, the mechanical stimulation of bone cells in strains with the mutation should be impaired, and thereby the release of mature IL-1β reduced. Then the level of IL-1β induced resorption would be decreased in strains with the mutation and bone remodelling would favour bone formation, and that correlates with the decreased levels of resorption measured in vivo in B6 and DBA/2.
Background strains of both the previously described P2X7−/− mice harbor the 451L allele of the examined mutation in P2X7. The observed differences in the two P2X7−/− murine models cannot be addressed to the P451L mutation in P2X7 found in the background strains, since both models are maintained on genetic background harboring the 451L allele. However, the different bone status of DBA/2 and B6 may affect the direction of the effect of the ablation of P2X7. The observed lack of mechanical stimulation upon bone formation in the Pfizer P2X7−/−, would probably not have been found in a pure B6 background since the DBA/2 shows increased remodeling compared to the B6 mice. The best reason for the different observations will be the escape of gene knockout due to the splice variant P2X7-k [28] in the GSK P2X7−/− model [23]. Even though no expression of the splice variant P2X7-k has been found in osteoclasts [29], and GSK P2X7−/− osteoblasts shows no dye uptake, the splice variant P2X7-k could still be found in other cells in the bone environment.
The underlying goal was to find a candidate strain of mice that could be used as new genetic background for the P2X7−/− mice, in the search for the therapeutic potential of P2X7 agonists and antagonists in a murine model for postmenopausal osteoporosis. Four strains were determined to carry the P451 allele by sequencing, however the NOD mice develop type I diabetes (during which P2X7 expression change [41]) and the NZB mice get autoimmune anaemia. Only two strains with the P451 allele of the mutation were left, namely, 129X1/SvJ and BALB/cJ. In this study we found that DBA/2 and B6 had low BMD (totally and regionally in the femur) and had a low bone strength, which could make it difficult to detect changes in the parameters. Besides carrying the P451 allele, high baseline BMD, relatively strong bones, and high trabecular bone volume should characterize the selected strain. All that fits well with the bone phenotype of 129X1/SvJ, but mice from this strain show no cancellous bone loss upon oestrogen depletion, as has been reported in postmenopausal women and oestrogen depleted rats [42, 43]. In contrast, BALB/cJ has relatively strong bones and high BMD in the femur, high Tb.Th and number, and respond as predicted upon ovariectomy [44].
In conclusion, we find that the P451 strains have stronger femurs and higher BMD, but the cellular basis could not be established in this study. The major finding in this study is that the genetic background could be of significant importance when determining the effect of the P2X7 ablation and that an optimal strain for studying the bone-specific effect of the P2X7 ablation could be the BALB/cJ mice.
Acknowledgments
The authors would like to acknowledge the Bartholin Institute, Copenhagen Municipal Hospital for guidance and for housing the animals. The technical assistance of Stine Ohlendorff and Zanne Henriksen was greatly appreciated. The Danish Pest Infestation Laboratory was kindly providing mus musculus from different locations. The work was kindly supported by the European Commission under the 7th Framework Programme (proposal no. 202231) performed as a collaborative project among the members of the ATPBone Consortium (Copenhagen University Hospital Glostrup, University College London, University of Maastricht, University of Ferrara, University of Liverpool, University of Sheffield, and Université Libre de Bruxelles), and is a substudy under the main study “Fighting osteoporosis by blocking nucleotides: purinergic signalling in bone formation and homeostasis.” Furthermore, this work was funded by the Research Foundation on Hvidovre Hospital H:S, Denmark in 2003 and 2006.
References
- 1.Dixon SJ, Sims SM. P2 purinergic receptors on osteoblasts and osteoclasts: potential targets for drug development. Drug Development Research. 2000;49(3):187–200. [Google Scholar]
- 2.Grol MW, Panupinthu N, Korcok J, Sims SM, Dixon SJ. Expression, signaling, and function of P2X7 receptors in bone. Purinergic Signalling. 2009;5(2):205–221. doi: 10.1007/s11302-009-9139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ke HZ, Qi H, Weidema AF, et al. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Molecular Endocrinology. 2003;17(7):1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. Journal of Biological Chemistry. 2005;280(52):42952–42959. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- 5.Gartland A, Buckley KA, Hipskind RA, et al. Multinucleated osteoclast formation in vivo and in vitro by P2X 7 receptor-deficient mice. Critical Reviews in Eukaryotic Gene Expression. 2003;13(2-4):243–253. doi: 10.1615/critreveukaryotgeneexpr.v13.i24.150. [DOI] [PubMed] [Google Scholar]
- 6.Bowler WB, Buckley KA, Gartland A, Hipskind RA, Bilbe G, Gallagher JA. Extracellular nucleotide signaling: a mechanism for integrating local and systemic responses in the activation of bone remodeling. Bone. 2001;28(5):507–512. doi: 10.1016/s8756-3282(01)00430-6. [DOI] [PubMed] [Google Scholar]
- 7.North RA. Molecular physiology of P2X receptors. Physiological Reviews. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 8.Naemsch LN, Dixon SJ, Sims SM. Activity-dependent Development of P2X7 Current and Ca2+ Entry in Rabbit Osteoclasts. Journal of Biological Chemistry. 2001;276(42):39107–39114. doi: 10.1074/jbc.M105881200. [DOI] [PubMed] [Google Scholar]
- 9.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272(5262):735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari D, Chiozzi P, Falzoni S, et al. Extracellular ATP triggers IL-1β release by activating the purinergic P2Z receptor of human macrophages. Journal of Immunology. 1997;159(3):1451–1458. [PubMed] [Google Scholar]
- 11.Ferrari D, Los M, Bauer MKA, Vandenabeele P, Wesselborg S, Schulze-Osthoff K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Letters. 1999;447(1):71–75. doi: 10.1016/s0014-5793(99)00270-7. [DOI] [PubMed] [Google Scholar]
- 12.Gu BJ, Zhang W, Worthington RA, et al. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. Journal of Biological Chemistry. 2001;276(14):11135–11142. doi: 10.1074/jbc.M010353200. [DOI] [PubMed] [Google Scholar]
- 13.Ohlendorff SD, Tofteng CL, Jensen J-EB, et al. Single nucleotide polymorphisms in the P2X7 gene are associated to fracture risk and to effect of estrogen treatment. Pharmacogenetics and Genomics. 2007;17(7):555–567. doi: 10.1097/FPC.0b013e3280951625. [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen NR, Henriksen Z, Sørensen OH, Eriksen EF, Civitelli R, Steinberg TH. Intercellular calcium signaling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2X7 receptors. Journal of Biological Chemistry. 2002;277(9):7574–7580. doi: 10.1074/jbc.M104608200. [DOI] [PubMed] [Google Scholar]
- 15.Gartland A, Buckley KA, Hipskind RA, et al. Multinucleated osteoclast formation in vivo and in vitro by P2X7 receptor-deficient mice. Critical Reviews in Eukaryotic Gene Expression. 2003;13(2-4):243–253. doi: 10.1615/critreveukaryotgeneexpr.v13.i24.150. [DOI] [PubMed] [Google Scholar]
- 16.Denlinger LC, Sommer JA, Parker K, et al. Mutation of a dibasic amino acid motif within the C terminus of the P2X7 nucleotide receptor results in trafficking defects and impaired function. Journal of Immunology. 2003;171(3):1304–1311. doi: 10.4049/jimmunol.171.3.1304. [DOI] [PubMed] [Google Scholar]
- 17.Hiken JF, Steinberg TH. ATP downregulates P2X7 and inhibits osteoclast formation in RAW cells. American Journal of Physiology, Cell Physiology. 2004;287(2):C403–C412. doi: 10.1152/ajpcell.00361.2003. [DOI] [PubMed] [Google Scholar]
- 18.Hoebertz A, Arnett TR, Burnstock G. Regulation of bone resorption and formation by purines and pyrimidines. Trends in Pharmacological Sciences. 2003;24(6):290–297. doi: 10.1016/S0165-6147(03)00123-8. [DOI] [PubMed] [Google Scholar]
- 19.Morrison MS, Turin L, King BF, Burnstock G, Arnett TR. ATP is a potent stimulator of the activation and formation of rodent osteoclasts. Journal of Physiology. 1998;511(2):495–500. doi: 10.1111/j.1469-7793.1998.495bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panupinthu N, Rogers JT, Zhao L, et al. P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. Journal of Cell Biology. 2008;181(5):859–871. doi: 10.1083/jcb.200708037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panupinthu N, Zhao L, Possmayer F, Ke HZ, Sims SM, Dixon SJ. P2X7 nucleotide receptors mediate blebbing in osteoblasts through a pathway involving lysophosphatidic acid. Journal of Biological Chemistry. 2007;282(5):3403–3412. doi: 10.1074/jbc.M605620200. [DOI] [PubMed] [Google Scholar]
- 22.Sluyter R, Shemon AN, Wiley JS. Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1β release from human monocytes. Journal of Immunology. 2004;172(6):3399–3405. doi: 10.4049/jimmunol.172.6.3399. [DOI] [PubMed] [Google Scholar]
- 23.Chessell IP, Hatcher JP, Bountra C, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114(3):386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. Journal of Immunology. 2002;169(8):4108–4112. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- 25.Le Stunff H, Auger R, Kanellopoulos J, Raymond MN. The Pro-451 to leu polymorphism within the C-terminal tail of P2X7 receptor impairs cell death but not phospholipase D activation in murine thymocytes. Journal of Biological Chemistry. 2004;279(17):16918–16926. doi: 10.1074/jbc.M313064200. [DOI] [PubMed] [Google Scholar]
- 26.Solle M, Labasi J, Perregaux DG, et al. Altered cytokine production in mice lacking P2X7 receptors. Journal of Biological Chemistry. 2001;276(1):125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 27.Taylor SRJ, Gonzalez-Begne M, Sojka DK, et al. Lymphocytes from P2X7-deficient mice exhibit enhanced P2X7 responses. Journal of Leukocyte Biology. 2009;85(6):978–986. doi: 10.1189/jlb.0408251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicke A, Kuan YH, Masin M, et al. A functional P2X7 splice variant with an alternative transmembrane domain 1 escapes gene inactivation in P2X7 knock-out mice. Journal of Biological Chemistry. 2009;284(38):25813–25822. doi: 10.1074/jbc.M109.033134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen RR, Nielsen CK, Nasser A, et al. P2X7 receptor-deficient mice are susceptible to bone cancer pain. Pain. 2011;152(8):1766–1776. doi: 10.1016/j.pain.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18(5):397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 31.Furlan F, Galbiati C, Jorgensen NR, et al. Urokinase plasminogen activator receptor affects bone homeostasis by regulating osteoblast and osteoclast function. Journal of Bone and Mineral Research. 2007;22(9):1387–1396. doi: 10.1359/jbmr.070516. [DOI] [PubMed] [Google Scholar]
- 32.Wu X, McKenna MA, Feng XU, Nagy TR, McDonald JM. Osteoclast apoptosis: the role of Fas in vivo and in vivo. Endocrinology. 2003;144(12):5545–5555. doi: 10.1210/en.2003-0296. [DOI] [PubMed] [Google Scholar]
- 33.Gartland A, Buckley KA, Bowler WB, Gallagher JA. Blockade of the pore-forming P2X7 receptor inhibits formation of multinucleated human osteoclasts in vitro. Calcified Tissue International. 2003;73(4):361–369. doi: 10.1007/s00223-002-2098-y. [DOI] [PubMed] [Google Scholar]
- 34.Korcok J, Raimundo LN, Ke HZ, Sims SM, Dixon SJ. Extracellular nucleotides act through P2X7 receptors to activate NF-κB in osteoclasts. Journal of Bone and Mineral Research. 2004;19(4):642–651. doi: 10.1359/JBMR.040108. [DOI] [PubMed] [Google Scholar]
- 35.Iglesias R, Locovei S, Roque A, et al. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. American Journal of Physiology, Cell Physiology. 2008;295(3):C752–C760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Letters. 2007;581(3):483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brough D, Pelegrin P, Rothwell NJ. Pannexin-1-dependent caspase-1 activation and secretion of IL-1β is regulated by zinc. European Journal of Immunology. 2009;39(2):352–358. doi: 10.1002/eji.200838843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO Journal. 2006;25(21):5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tatakis DN. Interleukin-1 and bone metabolism: a review. Journal of Periodontology. 1993;64(5, supplement):416–431. [PubMed] [Google Scholar]
- 40.Uy HL, Dallas M, Calland JW, Boyce BF, Mundy GR, Roodman GD. Use of an in vivo model to determine the effects of interleukin-1 on cells at different stages in the osteoclast lineage. Journal of Bone and Mineral Research. 1995;10(2):295–301. doi: 10.1002/jbmr.5650100217. [DOI] [PubMed] [Google Scholar]
- 41.Coutinho-Silva R, Robson T, Beales P, Burnstock G. Changes in expression of P2X7 receptors in NOD mouse pancreas during the development of diabetes. Autoimmunity. 2007;40(2):108–116. doi: 10.1080/08916930601118841. [DOI] [PubMed] [Google Scholar]
- 42.Riggs BL, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocrine Reviews. 2002;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 43.Iwaniec UT, Yuan D, Power RA, Wronski TJ. Strain-dependent variations in the response of cancellous bone to ovariectomy in mice. Journal of Bone and Mineral Research. 2006;21(7):1068–1074. doi: 10.1359/jbmr.060402. [DOI] [PubMed] [Google Scholar]
- 44.Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG. Ovariectomy-induced bone loss varies among inbred strains of mice. Journal of Bone and Mineral Research. 2005;20(7):1085–1092. doi: 10.1359/JBMR.050307. [DOI] [PubMed] [Google Scholar]


