Abstract
In the developing embryo, appropriate patterning of the endoderm fated to become pancreas requires the spatial and temporal coordination of soluble factors secreted by the surrounding tissues. Once pancreatic progenitor cells are specified in the developing gut tube epithelium, epithelial-mesenchymal interactions, as well as a cascade of transcription factors, subsequently delineate three distinct lineages, including endocrine, exocrine and ductal cells. Simultaneous morphological changes, including branching, vascularization, and proximal organ development, also influence the process of specification and differentiation. Decades of research using mouse genetics have uncovered many of the key factors involved in pancreatic cell fate decisions. When pancreas development or islet cell functions go awry, due to mutation in genes important for proper organogenesis and development, the result can lead to a common pancreatic affliction, diabetes mellitus. Current treatments for diabetes are adequate but not curative. Therefore researchers are utilizing the current understanding of normal embryonic pancreas development in vivo, to direct embryonic stem cells toward a pancreatic fate with the goal of transplanting these in vitro generated “islets” into patients. Mimicking development in vitro has proven difficult; however, significant progress has been made and the current differentiation protocols are becoming more efficient. The continued partnership between developmental biologists and stem cell researchers will guarantee that the in vitro generation of insulin-producing beta cells is a possible therapeutic option for the treatment of diabetes.
Introduction
The pancreas is made up of exocrine and endocrine compartments. Acinar cells, which form the exocrine tissue, produce digestive enzymes that are secreted through a ductal network and aid in digestion. The endocrine compartment is organized into structures known as the islets of Langerhans, which are composed of five types of hormone-producing cells. In mice, islet organization appears as a central core of insulin-producing beta cells surrounded by alpha (which express glucagon), epsilon (ghrelin), delta (somatostatin), and PP (pancreatic polypeptide) cells.
Disorders that afflict the pancreas can occur in both the exocrine and endocrine glands and research into the development of the pancreas has provided much insight into the aetiology of many of these diseases. The most common form of pancreatic cancer is adenocarcinoma, which is believed to arise from ductal and non-ductal origins (reviewed in 1, 2); cancers affecting islet cells are far more rare.3 Pancreatitis is an inflammation in the pancreas caused by the activation of digestive enzymes produced in the acini, which can secondarily affect endocrine pancreas survival and function. Diabetes mellitus is the disease most commonly associated with the endocrine pancreas, with onset attributable to genetic, metabolic, and/or physiological dysfunction. This review synthesizes our current understanding of pancreas development from studies in the embryo, with emphasis on those using mouse models, and discusses how this knowledge is assisting in the pursuit of therapeutics for the treatment of diabetes.
I. PANCREAS DEVELOPMENT
I.1 Patterning the endoderm
Between embryonic day (E) 8.5 and E9, the developing embryo rotates from a lordotic to a fetal position, which internalizes the endoderm layer to form a primitive gut tube. The gut tube epithelium is influenced by secreted factors and signalling pathways that induce regional transcriptional responses to create anterior-posterior patterning along the length of the tube. All endoderm-derived organs, including the oesophagus, lungs, thyroid, thymus, stomach, pancreas, liver and intestine, will eventually develop from this simple epithelial tube.4 Specifically, the dorsal pancreatic bud evaginates from the region of the dorsal foregut endoderm that lies between the putative stomach and intestine domains. Similarly, a region of the ventral foregut endoderm is patterned to become the ventral pancreatic bud, in proximity to the liver and biliary domain. Inductive and permissive cues from neighbouring regions influence ensuing organ specification. Figure 1 depicts stages during early embryonic development where signalling from adjacent tissues and cells influences the patterning and specification of the pancreatic endoderm. Many of the specific signals that influence early pancreatic patterning will be discussed.
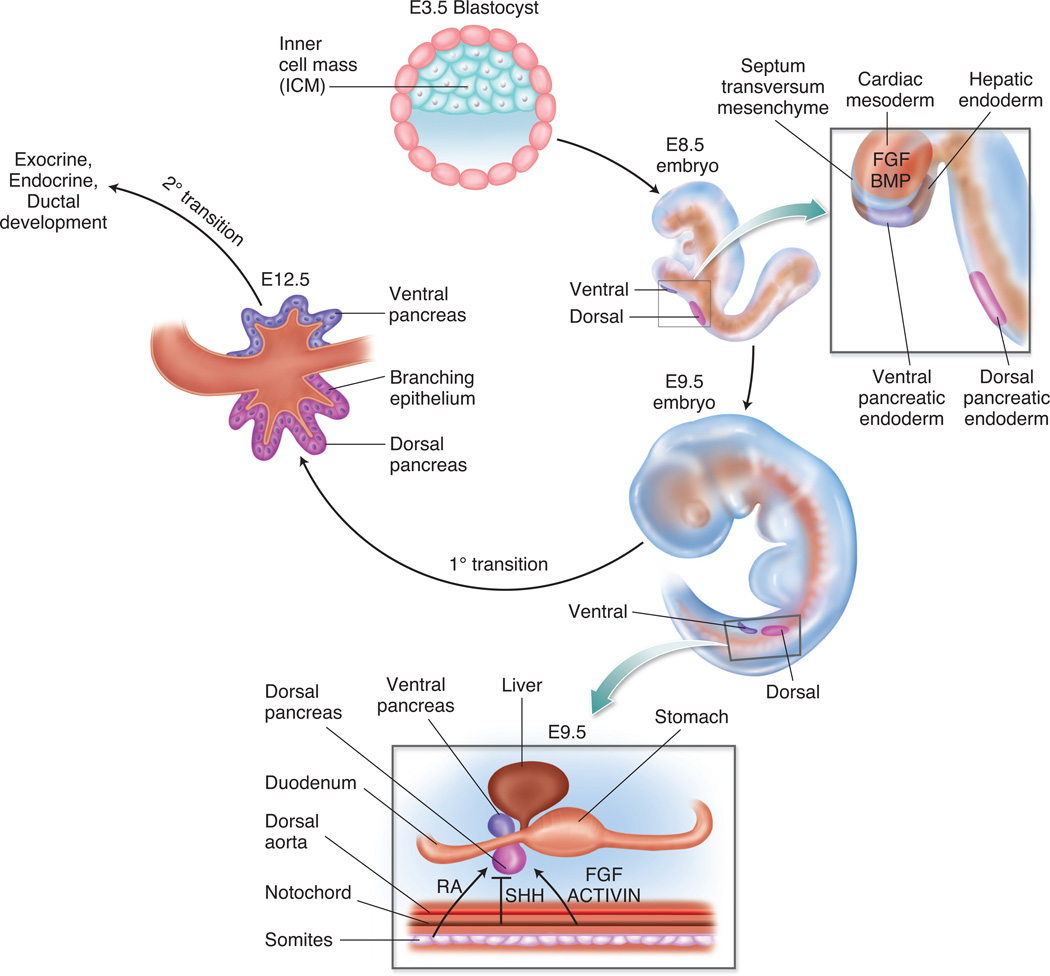
Figure 1. Pancreatic endoderm patterning and specification.
The portion of the endoderm fated to become dorsal and ventral pancreas is patterned and specified due to the influence of secreted molecules and signalling pathways from adjacent tissues and cells. The influence of FGF and BMP signalling from the cardiac mesoderm and septum transversum mesenchyme, respectively, affect the patterning of ventral pancreatic endoderm. Retinoic acid (RA), SHH, FGF and ACTIVIN signalling from the paraxial mesoderm and notochord allow for patterning of the dorsal pancreatic endoderm. The stage from E9.5 through E12.5 marks the primary transition. During this period, the pancreatic progenitor cells are specified, first wave endocrine cells are present, and epithelial branching morphogenesis begins. The secondary transition occurs after E12.5 and is marked by a major wave of endocrine cell specification and development, as well as further ductal morphogenesis and exocrine lineage differentiation.
Retinoic acid (RA) signalling from the paraxial mesoderm is required to define the endodermal anterior-posterior position of the pancreas as demonstrated by studies in Zebrafish and Xenopus.5, 6 In the mouse, RA has been found to have specific effects on the dorsal pancreatic domain. Aldh1a2 (formerly known as RALDH2), the gene that encodes the enzyme required to synthesize RA, is expressed in the dorsal pancreatic mesenchyme, and deletion of Aldh1a2 leads to dorsal pancreatic agenesis.7 Additionally, Pdx1 expression is lost in the dorsal, but not ventral, pancreatic domain7, which demonstrates the importance of RA to the development of the dorsal pancreatic bud. In addition to RA, FGF (fibroblast growth factor) signalling is important for early patterning of the endoderm.8 In particular, FGF4-mediated signalling directly patterns the gut tube endoderm to promote posterior fates and inhibit anterior fates.9
The notochord has been implicated as the source of signals that permit differentiation of the dorsal foregut endoderm fated to become pancreas; removal of the notochord was found to abolish the expression of pancreatic genes in the adjacent endodermal tissue.10 Further studies revealed the absence of Shh (sonic hedgehog) expression in the pancreatic endoderm, whereas adjacent regions of the gut tube rostral or caudal to the pancreatic domain express Shh.11 Moreover, in vitro studies determined that FGF2 (fibroblast growth factor 2) and activin, signalling factors expressed in the notochord, repress endodermal Shh and induce the pancreatic gene Pdx1 and pancreatic fates.12 Therefore the current model synthesized from studies in the mouse and chick, holds that in the dorsal foregut endoderm induction of the pancreatic program specifically requires FGF and activin to inhibit endodermal Shh, which permits mesenchymal PTCH1 (patched homolog 1) to initiate pancreatic specification.12
The above studies clearly identify factors originating from the notochord (FGF, activin) and paraxial mesoderm (RA, FGF) as necessary to affect dorsal pancreatic patterning. The factors influencing ventral foregut endoderm emanate from the cardiac mesoderm (source of FGFs) and the septum transversum mesenchyme (source of BMPs), which are positioned in proximity to the liver and ventral pancreatic domains. This was determined, in part, using ex vivo recombination studies from late gastrulation-stage embryos; the mesoderm and ectoderm were identified to differentially induce endodermal genes, providing evidence that the endoderm receives instructions from adjacent germ layers.8 In addition, soluble factors expressed in the mesoderm and primitive streak, create regional identity in areas of the endoderm, thus establishing organ domains.8 Without these initial instructions, ventral patterning does not proceed.
Subsequent work from Zaret and colleagues13 determined the importance of BMP and the timing of ventral specification. Using three to four somite (3-4S) stage embryos and half-embryo cultures it was determined that BMP signalling specifically influenced the expression of hepatic-specific genes. BMP signalling had the opposite effect on the pancreatic program, whereby Pdx1 expression was induced by noggin (NOG), an inhibitor of BMP signalling, and inhibited by BMP4; an effect that was reversed in the 5-6S stage embryos.13 In addition, conditional ablation of Smad4 at the 5-6S stage (using Foxa3-Cre) showed defective ventral pancreatic development13, whereas ablation at the 7-8S stage (using Pdx1-Cre) produced no pancreatic phenotype.14 Ultimately, it was determined that BMP signalling in the ventral foregut is necessary to induce Pdx1 expression and the pancreatic program. These studies demonstrate that ventral foregut patterning signals are progressive and dynamic, with opposing effects on the hepatic and pancreatic programs.
I.2 Pancreatic Patterning and Specification
Induction of the ventral pancreatic endoderm occurs one day after that of the dorsal pancreatic endoderm; however in both cases, signals from the mesoderm lead to patterning of the epithelium and evagination of the pancreatic buds. The subsequent activation of a complex network of transcription factors (TFs) in the pancreatic progenitor cells is critical for downstream developmental events. Using mouse models, many TFs have been identified as both necessary and sufficient for pancreas development.
First and foremost, Pdx1 (pancreatic and duodenal homeobox 1; formerly known as Ipf1, Stf1) marks the pancreatic progenitor cells. Loss of Pdx1 results in pancreatic agenesis 15–17, and mutation leads to maturity onset diabetes of the young (MODY4)18 or permanent neonatal diabetes 19. Expression of Pdx1 is induced in foregut endoderm cells in a broad domain that encompasses the dorsal and ventral pancreas, the duodenum, and a portion of the stomach.20 Subsequent coexpression of Pdx1 and the basic helix-loop-helix TF Ptf1a (pancreas-specific transcription factor 1a; formerly known as p48) defines pancreatic progenitor cells and allows for the pancreatic program to proceed.21 As development progresses, Pdx1 expression becomes down-regulated in the cells that form the endocrine progenitor population, and by late gestation is selectively maintained at high levels in the beta cells and lower levels in the acinar cells.22, 23 Recent studies have demonstrated that these dynamic changes in Pdx1 expression levels are necessary for proper endocrine differentiation.24 Moreover, Wright and colleagues have recently demonstrated that altering the level of Pdx1 expression in the NEUROG3+ (neurogenin3; formerly known as Ngn3) progenitor cells can alter alpha and beta cell ratios both in the embryo and the adult pancreas.25 As with many TFs, timing, location and level of expression can result in different effects on differentiation and disease.
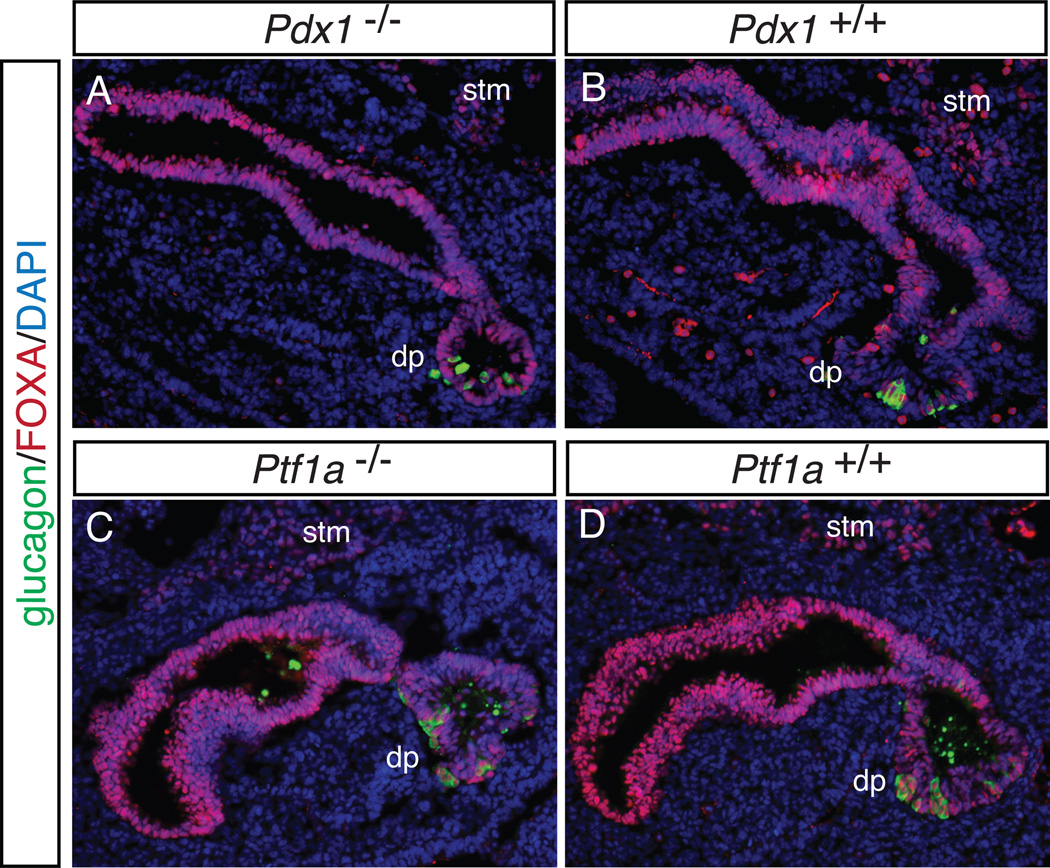
The expression domain of Ptf1a early in pancreas development is restricted to multipotent progenitor cells in the developing pancreatic epithelium 20, 26, which gives rise to the endocrine, exocrine and ductal lineages. Ptf1a was originally thought to be an exocrine-specific gene27; however, null mutations of Ptf1a results in pancreatic agenesis, with only a small remaining dorsal pancreatic rudiment.26, 28 Interestingly, descendants of Ptf1a-expressing cells were observed in the duodenum of the null and found to express pancreatic markers. Moreover, when Pdx1 is expressed under the control of Ptf1a regulatory sequences in Pdx1 null embryos, the pancreatic agenesis phenotype is rescued.26 Therefore both Pdx1 and Ptf1a are important for early pancreas development; however, specification of the pancreatic domain proceeds in the absence of either or both of these factors.26, 28 This is depicted in Figure 2, which shows glucagon-expressing cells in the dorsal pancreatic primordium that forms in the absence of either Pdx1 or Ptf1a.
Figure 2. Pancreatic specification is unaltered in embryos lacking either Pdx1 or Ptf1a.
Sagittal sections from E10.5 Pdx1 null (A) and wildtype littermate (B), as well as Ptf1a null (C) and wildtype littermate (D) embryos were stained by immunofluorescence with antibodies against the transcription factor FOXA (demarking endoderm) and the hormone glucagon (identifying the pancreatic domain). In both null embryos, the region fated to become the dorsal pancreas (dp) is present, contains glucagon-expressing cells, and appears similar in both the null and wildtype. stm, septum transversum mesenchyme. DAPI marks all nuclei. 20X
The FOXA genes are expressed in the dorsal and ventral endoderm. Similar to Pdx1 and Ptf1a, the pancreas-specific simultaneous deletion of Foxa1 (forkhead box A1) and Foxa2 (forkhead box A2) (in Foxa1flox/flox;Foxa2flox/flox;Pdx1-cre embryos) leads to pancreatic hypoplasia.29 Additionally, FOXA1 and FOXA2 regulate Pdx1 expression through occupancy of a distal Pdx1 enhancer, and this regulation of Pdx1 controls pancreatic anlagen expansion and differentiation.29
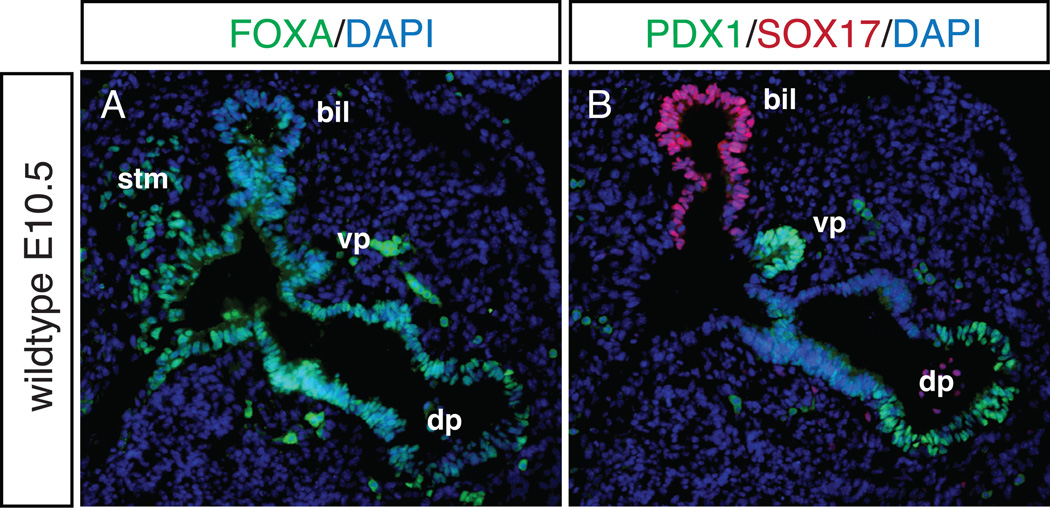
In addition to its broader role in general endoderm specification30, another gene identified as important in pancreatic specification is the SRY-related HMG-box TF, Sox17. The Wells group31 showed that the ventral pancreas and extrahepatobiliary system are derived from a common progenitor cell that coexpresses Pdx1 and Sox17 at E8.5. By E10.5 the Sox17+ biliary cells and the Pdx1+ ventral pancreatic cells occupy separate domains. When Sox17 is deleted from the ventral foregut at E8.5 (using Foxa3-cre), biliary structures are lost and ectopic pancreas tissue is found in the liver and common duct. Overexpression of Sox17 in the Pdx1+ domain resulted in the opposite phenotype, with loss of the ventral pancreas and ectopic biliary tissue in the pancreatic domain. As Hes1 is also expressed in the biliary domain 32, further analysis of Hes1 null embryos revealed an expanded Sox17 expression domain with scattered Sox17+ cells in the dorsal pancreas. The previously reported phenotype of gall bladder agenesis and ectopic pancreatic tissue in the common duct of Hes1 null 32 embryos supports observations by the Wells group that functionally links HES1 and SOX17 in controlling biliary development. Figure 3 depicts sagittal sections from an E10.5 wild-type embryo where the dorsal and ventral pancreatic domains marked by PDX1 are separate from the SOX17+ biliary domain.
Figure 3. Pancreatic and biliary domains in the wildtype E10.5 embryo.
Adjacent sagittal sections from E10.5 wildtype embryo, stained by immunofluorescence with antibodies against FOXA (A), and PDX1, SOX17 (B). The domains of the dorsal pancreas (dp), ventral pancreas (vp), biliary domain (bil) and septum transversum mesenchyme (stm) are noted. While all domains express FOXA (A), PDX1+ cells are found only in the pancreatic domains, and are distinct from the SOX17+ cells in the biliary domain (B). DAPI marks all nuclei. 20X
Sox9 (SRY-box containing gene 9) expression overlaps with Pdx1 in the early pancreatic buds and is also a marker of pancreatic progenitor cells. By E15.5, Sox9 becomes restricted to a subset of Pdx1+ cells in the epithelial cords that are also Hes1+ and mitotically active.33 Depletion of the progenitor cell pool in the pancreas-specific deletion of Sox9 (in Sox9flox/flox;Pdx1-cre embryos) leads to pancreatic hypoplasia.33 Subsequent lineage tracing revealed that Sox9+ cells located in the epithelial cords give rise to both endocrine and exocrine cells.34 Furthermore, mice hypomorphic for Sox9 show reduced endocrine cell mass, but overall organ size remains unchanged, which suggests that SOX9 is involved in endocrine cell fate determination.34 In two studies that lineage trace Sox9-expressing cells in the wild-type and injured states, it was determined that, while Sox9 was expressed continuously throughout the pancreatic and biliary ductal epithelium and Sox9+ cells have the capacity to give rise to all cell types in the embryonic pancreas35, Sox9+ cells do not give rise to endocrine cells in normal or injured postnatal/adult pancreas.36
As evidenced by the studies outlined in this section, signals originating from adjacent tissues must intersect on the primitive gut tube in order for pancreatic organ formation to begin. Subsequently, a large number of TFs are activated to influence the patterning and specification of this early pancreatic domain, as well as the later decision of cells to maintain the pancreatic program (studies summarized in Table 1). Importantly, it has become increasingly clear that a unique combination of endoderm-specific and pancreatic-specific TFs must interact to specify the different pancreatic cell fates and to direct islet cell lineage decisions.
Table 1.
Summary of knockout mouse models with severe pancreatic phenotypes
| Gene(s) | Genotype studied | Gross Pancreatic Phenotype |
Reference |
|---|---|---|---|
| Pdx1 | Pdx1−/− | pancreatic agenesis | Jonsson J et al., Nature 1994 |
| Ptf1a | Ptf1a−/− | pancreatic agenesis | Krapp A et al., Genes Dev 1998; Kawaguchi Y et al., Nat Genet 2002 |
| Foxa1, Foxa2 | FoxA1flox/flox;FoxA2 flox/flox;Pdx1-cre | pancreatic hypoplasia | Gao N et al., Genes Dev 2008 |
| Sox9 | Sox9 flox/flox;Pdx1-cre | pancreatic hypoplasia | Seymour PA et al., PNAS 2007 |
| Fgf10 | Fgf10−/− | pancreatic hypoplasia | Bhushan A et al., Dev 2001 |
| Cdh2 | Cdh2−/− | dorsal pancreatic agenesis | Esni F et al., Dev Biol 2001 |
| Hlxb9 | Hlxb9−/− | dorsal pancreatic agenesis | Harrison KA et al., Nat Genet 1999; Li H et al., Nat Genet 1999 |
| Raldh2 | Raldh2−/− | dorsal pancreatic agenesis | Martin M et al., Dev Biol 2005 |
| Isl1 | Isl1−/− | Lack of dorsal mesenchyme formation and exocrine differentiation; islet cells absent | Ahlgren U et al., Nature 1997 |
| Sox17 | Sox17flox/flox;FoxA3-cre | Absent biliary structures; ectopic pancreas tissue in the liver and common duct | Spence JR et al., Dev Cell 2009 |
| Hes1 | Hes1−/− | Gall bladder agenesis; ectopic pancreas in the common duct | Sumazaki R et al., Nat Genet 2004; Fukuda A et al., J Clin Invest 2006 |
I.3 Morphological development and cellular interactions in pancreas development
Morphological changes in the pancreas and the presence of neighbouring non-pancreatic cells have a profound affect on development and differentiation. In particular, many genes expressed in the pancreatic mesenchyme have been identified to influence proper pancreas formation. Fgf10 (fibroblast growth factor 10) is expressed in the mesenchyme surrounding the early pancreatic buds. Pancreatic specification is normal in Fgf10 null mice; however, subsequent branching morphogenesis, pancreatic growth, and differentiation do not occur resulting in severe pancreatic hypoplasia.37 While the pancreatic buds form and Pdx1+ progenitor cells were identified in the epithelium at E9.5, maintenance of Pdx1 expression was lost by E10.5 in the dorsal pancreas and reduced in the ventral pancreas; without FGF10 signalling in the mesenchyme Pdx1+ progenitor cells were not maintained in the epithelium.
Isl1 (ISL1 transcription factor, LIM/homeodomain; formerly known as islet1) is expressed in the mesenchymal cells that surround the dorsal pancreatic bud. During development and in the adult pancreas, Isl1 is also expressed in postmitotic islet cells. Deletion of Isl1 leads to an arrest in embryonic development around E9.5, following the initial pancreatic budding.38 Interestingly, in this mutant the mesenchyme surrounding the dorsal bud does not form. Using explant culture of embryos null for Isl1, it was demonstrated that all differentiated islet cells are lost, and exocrine differentiation is absent in the dorsal but not ventral bud.38 Therefore ISL1 is required for two distinct stages of pancreas development including the development of the mesenchyme, which influences pancreas specification, as well as differentiation of both endocrine and exocrine cells.
Signals from the vasculature can also affect pancreas organogenesis. Yoshitomi and Zaret 39 describe how emergence of the dorsal bud and expression of Pdx1 require endothelial cell interactions or the presence of the aorta. Ptf1a expression is also induced in the dorsal pancreatic endoderm by aortic endothelial cells; however later in development, endogenous pancreatic endothelial cells localize to the trunk epithelium where they function to limit acinar cell differentiation.40 Endothelial signalling does not appear to be sufficient for inducing endocrine differentiation but can enhance insulin expression and beta cell proliferation in response to external stimuli (reviewed in 41).
A study by Villasenor et al. 42 highlights branching morphogenesis as another determinant in pancreatic development and differentiation. The authors performed an extensive anatomical characterization of branching and epithelial cell dynamics in the embryonic pancreas. Subsequently, it was demonstrated that EPH (eph-related receptor tyrosine kinase) signalling is required to support proper branching morphogenesis. Ephb2/Ephb3 compound mutants display reduced pancreas size and thickness, and many wildtype branching features were not observed; later stage embryos have reduced exocrine mass, and defects in branch length and pancreas size were observed in the adult. Concomitantly, mesenchymal gene expression was altered; however, most striking was the change in epithelial dynamics. Defective EPH signalling lead to disorganized rosette structures, early lumen collapse, and an overall delay in tubulogenesis and remodelling.42 Given that the ductal epithelium houses the endocrine progenitor cells, the effect of altered branching may indirectly affect the differentiation of other pancreatic cell lineages.
Late in development, islet cell aggregation is another morphological feature that, when defective, can influence proper pancreatic function. The overexpression of dominant negative E-cadherin (cadherin1, Cdh1) in beta cells resulted in perturbed cell clustering at E13.5, and abrogation of the coalescing of endocrine cells into islets at E17.5.43 Further studies identified that RAC1 (RAS-related C3 botulinum substrate 1) signalling can modulate CDH1 mediated cell adhesion thereby affecting islet cell migration.44 Clearly, the expression of factors within and surrounding the progenitor cells has a critical affect on pancreatic patterning as evidenced by the multiple models of pancreatic agenesis and hypoplasia; however, changes to the anatomical morphology of the organ and the influence of intercalated endothelial cells also impacts organ development. Additional reviews of signalling pathways as well as cellular interactions that affect pancreas development can be found in the “Further Reading/Resources” section.
I.4 Endocrine Differentiation
The endocrine versus exocrine lineage decision can only occur early in development when the progenitors are multipotent.45 Notch signalling participates in this lineage decision, although it also plays complex roles in other aspects of pancreas development and function (see additional review 46). Notch signalling in pancreatic progenitor cells activates the Notch target, HES1, which directly represses Neurog3 expression to promote the exocrine cell lineage.47 Consistently, deletion of Dll1 (delta-like gene 1) or Rbpj (recombination signal binding protein for immunoglobulin kappa J region), or overexpression of Neurog3, results in accelerated endocrine differentiation.48 However, the misexpression of activated Notch in pancreatic progenitor (Pdx1+) cells blocks the differentiation of both the exocrine and endocrine lineages, and instead promotes the maintenance of progenitor cells.49 This suggests there are additional complexities to Notch-regulated lineage decisions. Clearly the development and differentiation of the exocrine lineage is intimately related to that of the endocrine lineage; however, the process of exocrine differentiation is a complex area of study, in its own right, which has been recently documented in a comprehensive review and will not be addressed further here.50
The specification and differentiation of the five endocrine cell types begins with the onset of pancreas development. A subset of hormone-expressing cells can be found as early as E9.5; however, these early “first wave” endocrine cells have not been well characterized due to the lack of definitive markers. Most endocrine cells are specified during the secondary transition, the stage of pancreas development between E12.5 and E15.5.51 During this stage, key TFs necessary for endocrine specification and maturation are upregulated, resulting in the appearance of hormone-expressing cells that differentiate from the endocrine progenitors and delaminate from the ductal epithelial cords.52
Cells expressing NEUROG3 represent the endocrine progenitor population. Gradwohl et al.53 established this fact with the generation of the Neurog3 null mouse, which lacks all hormone-expressing endocrine cells; further confirmation was provided by lineage tracing experiments.54, 55 Interestingly, using a transgenic mouse model, temporal induction of Neurog3 influenced the proportional distribution of differentiated endocrine cell populations.56 Additionally, the level of expression of Neurog3 was demonstrated to be important for the endocrine versus exocrine decision in pancreatic progenitor cells57, as well as for the delamination of endocrine cells from the epithelium.58 These studies demonstrate that modulating temporal, spatial, and level of expression of a TF can affect specification and differentiation. In this section, we will highlight additional TFs important in the fate determination of each endocrine cell lineage.
I.4a The insulin-expressing beta cell
The most highly studied endocrine cell is the insulin-producing beta cell largely because of the implications for the treatment of diabetes. Interestingly, a number of TFs expressed in early pancreatic progenitor cells are also found to function in the beta cell. In particular, mice deficient for Pdx1 (heterozygous deletion) are glucose intolerant.59 Moreover, deletion of Pdx1 specifically in the beta cell results in beta cell dysfunction.60 While expression studies clearly identify PDX1 as an early pancreas progenitor factor, these studies discern additional functions for PDX1 in the proper functioning of mature beta cells. Furthermore, transcriptional activation of several beta cell genes, including insulin (Ins1) 61 and islet amyloid polypeptide (Iapp) 62, occurs as a result of direct regulation by PDX1.
Similar to Pdx1, the homeobox TF gene Nkx6-1 (NK6 homeobox 1) is expressed throughout the epithelium early in pancreas development63; however, after the secondary transition it becomes restricted to beta cells. Deletion of Nkx6-1 results in the loss of beta cell precursors.64 Beta cells were restored to the Nkx6-1 null pancreas by transgenic expression of either Nkx6-1 or Nkx6-2 from the Pdx1 promoter; however, beta cells were not rescued with transgenic expression of Nkx6-1 from the Neurog3 promoter, which places Nkx6-1 upstream of Neurog3 in beta cell differentiation.65 Interestingly, although the related family member, Nkx6-2 (NK6 homeobox 2), is also expressed in a similar domain to Pdx1 in early pancreatic development63, the phenotype of the Nkx6-2 null mouse showed that this TF is dispensable for normal endocrine development.66 However, simultaneous deletion of Nkx6-1 and Nkx6-2 demonstrated functional redundancy between the two family members in both alpha and beta cell development, as the number of alpha and beta cells was reduced compared with the single knockout of Nkx6-1.66
Just as the function of a TF can change over the course of pancreatic development, many TFs have been identified to be necessary for the development and differentiation of the beta cell lineage while not expressed exclusively therein. Mice with a deletion of Nkx2-2 (NK2 transcription factor related, locus 2) die from severe hyperglycemia due to the complete absence of beta cells.67 The pancreas of this null mouse is also deficient in alpha cells and a subset of PP cells, with a concomitant increase in ghrelin-expressing cells.68
Another factor expressed in, but not exclusive to, the beta cell is the basic helix-loop-helix (bHLH) TF neurogenic differentiation 1 (NEUROD1, formerly known as BETA2). During embryonic development, Neurod1 expression can be detected in all endocrine cell types, except for delta cells.69 Mice homozygous for the deletion of Neurod1 also die perinatally from diabetes, due to beta cell apoptosis.70 More recently, Lee and colleagues discovered an additional function for Neurod1 in the postnatal maturation of beta cells.71 The deletion of Neurod1 in all insulin-expressing cells (using both RIP-cre and Pdx1-creER) resulted in the arrest of postnatal beta cell maturation, characterized by the expression of increased glycolytic genes and Ldha (lactate dehydrogenase A), and lead to severe glucose intolerance and impaired insulin secretion.71
Additional TFs important in beta cell development include the PAX and MAF families of genes. The absence of Pax4 (paired box gene 4) leads to loss of beta cells 72, whereas deletion of the related family member, Pax6 (paired box gene 6), affects predominately the alpha and beta cell lineage, in addition to other endocrine cell types.73, 74 Interestingly, the Pax4/Pax6 double mutant lacks all mature endocrine cells.75 The MAF family of basic leucine zipper TFs behaves similarly in their individual and overlapping affects on alpha and beta cells. Mafb (v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B) null mice die at birth, and the pancreatic phenotype shows reduced alpha and beta cells despite an unchanged endocrine cell number.76 Mafa (v-maf musculoaponeurotic fibrosarcoma oncogene family, protein A) null mice are viable but develop glucose intolerance and diabetes due to beta cell dysfunction.77 Moreover, the deletion of Mafa lead to reduced expression of the beta cell genes Ins1, Ins2, Neurod1, and Glut2. Zhao and colleagues 78 also demonstrated that MAFA controls the level of insulin gene expression, together with Pdx1 and Neurod1. Interestingly, in the developing and postnatal mouse pancreas Mafa expression is exclusive to the beta cell and can be used as a marker of the mature insulin-producing cells.79, 80
Finally, it is interesting to note that when the Ins1 and Ins2 genes were directly disrupted, beta cell differentiation was not affected, rather these double mutant mice showed insulin deficiency and subsequent perinatal death from diabetes 81. Upon further investigation, enlarged islets were observed in the Ins1/Ins2 mutant pancreata, pointing to insulin as a negative regulator of growth.82
I.4b The glucagon-expressing alpha cell
Similar to TFs important for beta cell development, a number of TFs have been described as markers of the glucagon-expressing cells because of their involvement in alpha cell development, despite their lack of exclusive expression.
Mice carrying a deletion of Arx (aristaless-related homeobox) display perinatal hypoglycemia and die shortly after birth.83 The pancreas of the Arx null mouse is deficient for alpha cells after E12.5, thereby defining Arx as necessary for alpha cell development. However, the Arx mutant also shows an increase in insulin- and somatostatin-expressing cells.83 Further study of the function of Arx in the pancreas uncovered that when misexpressed in Pdx1- or Pax6-expressing cells dramatic postnatal hyperglycemia and death resulted, due to a loss of beta and delta cells, and an increase in alpha and PP cells.84 The misexpression of Arx in the beta cell also induced an increase in alpha and PP cell numbers, which when combined with lineage tracing determined that beta cells were being converted to alpha or PP cells.84 Compound mutants have also identified the importance of Arx function in the developing pancreas. Whereas the loss of both Arx and Pax4 resulted in loss of alpha and beta cells, with a concomitant increase in somatostatin-expressing cells 85, the combined loss of Arx and Nkx2-2 most significantly restored the PP cell population lost in the Nkx2-2 single mutant mouse 86.
POU3F4 (POU domain, class 3, transcription factor 4; formally known as Brn4) is a TF expressed in glucagon-expressing cells as early as E9.5.20 BRN4 facilitates the transcriptional activation of the glucagon gene; however, deletion of Brn4 does not disrupt alpha cell development.87, 88 While not exclusive, Irx1 (iroquois related homeobox 1) and Irx2 (iroquois related homeobox 2) are also expressed in alpha cells.89 Interestingly, mutant mice with a deletion of Irx2 are phenotypically normal90; Irx1 null pancreata have not yet been investigated. In contrast, Pax6 is expressed in the early pancreatic epithelium and in differentiated endocrine cells not exclusively alpha cells 20; however, deletion of Pax6 results in a loss of predominantly alpha cells, in addition to other endocrine cell types.74
While under appreciated given its importance to beta cell differentiation, Neurod1 is also expressed in the glucagon-expressing cells.69, 70 In addition to the beta cell phenotype described in the Neurod1 null, alpha cell deficiency was also observed late in development.70 Moreover, an epistatic relationship was identified between Neurod1 and Nkx2-2, whereby simultaneous loss of both TFs restores the alpha cells lost in the Nkx2-2 null.91 Clearly, these studies highlight the complexity of the TF network in endocrine cell fate decisions.
Similar to the deletion of the insulin genes, Hayashi et al.,92 deleted the glucagon (Gcg) gene to determine the effect on alpha cells and general pancreatic function. These mice display alpha cell hyperplasia, but are viable and normoglycemic, demonstrating that glucagon is dispensable for survival.
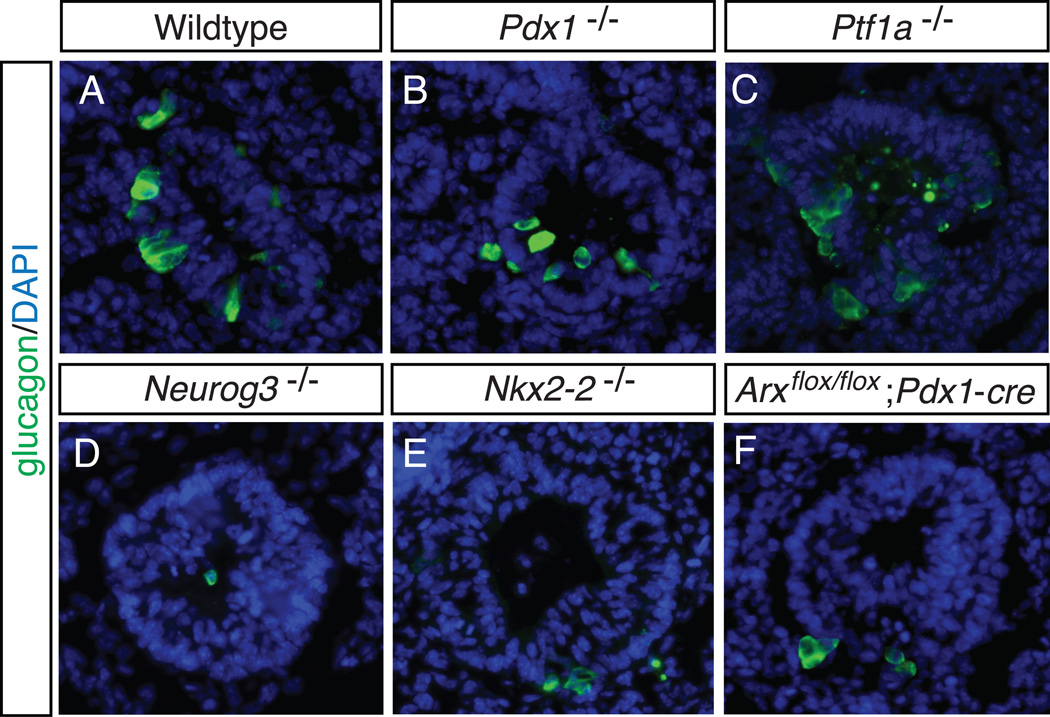
Interestingly, in nearly all models where pancreatic development was severely compromised, a population of glucagon-expressing alpha cells remained. In addition, the mutant mouse models for specific genes identified as important in alpha cell development, including Nkx2-2 and Arx, also do not display a complete loss of this endocrine cell population. These “early” endocrine cells have also been termed “first wave” endocrine cells, and in many cases exclusively express the hormone glucagon. The origin of these cells is as of yet unknown; however, as depicted by the images in Figure 4, first wave glucagon-expressing cells exist despite the deletion of factors necessary for pancreas and/or endocrine cell development, including Pdx1, Ptf1a and Neurog3. While research has uncovered many of the genes and signals necessary and sufficient for pancreatic specification and endocrine cell differentiation, unexplained observations like the “early” glucagon-expressing cell population remind us that there is still much to uncover.
Figure 4. Early “first wave” glucagon-expressing alpha cells.
Early “first wave” alpha cells are present in mouse models despite the deletion of factors necessary for pancreas development and endocrine specification. Sagittal sections of E10.5 embryos from a representative wildtype littermate (A), Pdx1 null (B), Ptf1a null (C), Neurog3 null (D), Nkx2-2 null (E), and pancreas-specific deletion of Arx (F), were stained by immunofluorescence for the hormone glucagon (A–F). In all images glucagon-expressing cells are present. All images are of the dorsal pancreas. DAPI marks all nuclei. 40X
I.4c The ghrelin-expressing epsilon cell
The ghrelin-expressing endocrine cell lineage was first identified in human pancreatic islets by Sundler and colleagues.93 While rare compared with other endocrine populations, ghrelin+ cells were described as most abundant in embryonic islets, present in the neonate, but greatly reduced in the adult pancreas.93 Moreover, ghrelin+ cells were identified in islets, exocrine tissue, and ductal epithelium.93 Further analysis in the rat identified that ghrelin+ cells occasionally coexpressed glucagon or pancreatic polypeptide; these double positive cells were detectable from late gestation through early postnatal time points, and from this it was suggested that ghrelin+, glucagon+ and pancreatic polypeptide+ cells are of the same lineage.94
In the mouse, Sussel and colleagues 68 observed an increase in ghrelin+ cells (subsequently named “epsilon” cells) in place of the deficient beta, alpha and PP cells in the pancreas of the Nkx2-2 null mouse. Similar to previous reports in the rat and human, epsilon cells were described as the fifth endocrine lineage and identified in small number in the wildtype islet. Additional TF mutants, including Pax6 null embryos, were also discovered to have an increase in ghrelin+ cells.68,95 Subsequent studies identified that, similar to the rat, ghrelin-expressing cells and glucagon/ghrelin coexpressing cells can be found in the embryonic pancreas, and these cells derive from a Neurog3+ endocrine progenitor.95 Interestingly, the glucagon/ghrelin coexpressing cells are lost with deletion of either Nkx2-2 68 or Arx 95, but increased with deletion of Pax4.95
In light of these studies, there is ongoing investigation to understand the epsilon cell lineage, as well as the significance of the presence of ghrelin in the pancreas. Not surprisingly the hormone ghrelin, like insulin and glucagon, was deleted (Ghrl null mouse) and found to be dispensable for endocrine development and differentiation.96
I.4d The pancreatic polypeptide-expressing PP cell
In recent years, pancreatic polypeptide (PPY)-expressing PP cells have not been studied to the same extent as alpha and beta cells. However, a number of early studies investigating the connection between all endocrine lineages uncovered a controversial relationship between PPY-expressing cells and the endocrine progenitor population. Vassalli and colleagues52 examined the pattern of hormone expression throughout pancreas development and identified glucagon- and PPY-expressing cells at E10.5, insulin cells at E11.5 and somatostatin cells at E13.5; using RT-PCR the early appearance of Ppy expression was confirmed and these early PPY+ cells were believed to coexpress other hormones.52 In the following years, hormone expression in the pancreas was re-examined by Hanahan and colleagues97 and insulin and glucagon were found to coexpress in the E9.5 pancreas, and the presence of PPY expression was not identified until postnatal day 1. These opposing results were explained with the observation that antibodies against PPY cross reacts with a related family member, neuropeptide Y (NPY), which is expressed in early hormone+ cells.97 Subsequent studies using in vivo endocrine cell ablation, determined that PP cells are indispensable for beta and delta cell differentiation because the ablation of PPY-expressing cells resulted in embryonic pancreata devoid of insulin- and somatostatin-expressing cells.98 However, further studies by Herrera99 utilized a lineage tracing technique and demonstrated definitively that islet beta cells do not produce PPY.
TFs exclusive to PP cells have yet to be identified; however, a number of mouse models previously discussed also describe alteration to the PP cell population. Interestingly, the deletion of Pyy (peptide YY), another NPY family member, results in the loss of PP cells.100 More recently, in a Neurog3 null background, Neurog3 expression was temporally induced to determine if progenitor cells change their competence throughout development, and it was demonstrated that pancreatic progenitors acquire the competence to make PP cells between E10.5 and E12.5; however induction of Neurog3 after E14.5 induced a greater number of PP cells late in development.56
I.4e The somatostatin-expressing delta cell
Although somatostatin-producing cells play an important role in the paracrine regulation of islet function101, delta cell markers remain scarce, with few somatostatin cell-specific TFs identified. Inactivation of Pax4 causes a loss of delta cells, in addition to beta cells, leading to the hypothesis that delta cells and beta cells arise from a common lineage.72 However, a common beta/delta progenitor has yet to be identified. Alternatively, a handful of studies have described phenotypes that potentially link the alpha and delta cell lineages. Gannon et al.,60 deleted Pdx1 specifically in the beta cell and found that the absence of Pdx1 results in beta cell dysfunction and an increase in alpha and delta cell numbers. In addition, the Arx null pancreas displays an increase in delta cells, concomitant with a decrease in alpha cells.85 In general, the development of the delta cell is quite under-studied and, similar to other hormone mutants, the deletion of somatostatin (Sst) has no reported pancreatic phenotype.102
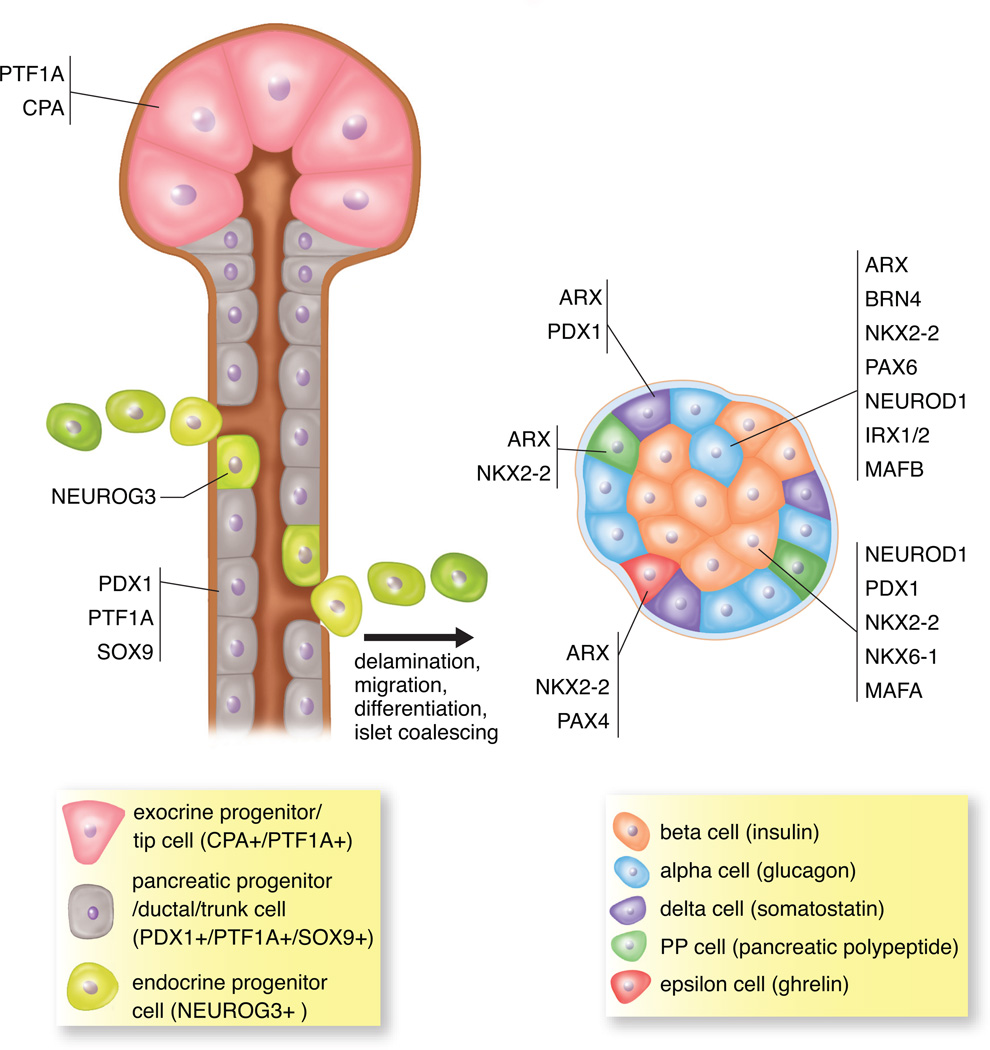
Table 2 summarizes some of the key TFs noted to have defects in endocrine differentiation; Figure 5 illustrates the genesis of the hormone-producing cell lineages from the delaminating endocrine progenitors, which differentiate from the multipotent pancreatic progenitor cells that reside in the ductal epithelium of the developing pancreas.
Table 2.
Summary of knockout mouse models that affect endocrine differentiation
| Gene | Genotype studied |
Viability | Pancreatic Phenotype (expression changes vs. to WT) | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| alpha | beta | delta | epsilon | PP | ||||
| Neurog3 | Neurog3−/− | perinatal death | absent* | absent | absent | absent | absent | Gradwohl G et al., PNAS 2000 |
| Pdx1 | Pdx1flox/flox;RIP-cre | viable/diabetic | increase | decrease | increase | Gannon M et al., Dev Biol 2008 | ||
| Nkx6-1 | Nkx6-1−/− | perinatal death | decrease | Sander M et al., Dev 2000 | ||||
| Nkx6-2 | Nkx6-2−/− | viable | Henseleit KD et al., Dev 2005 | |||||
| Nkx6-1/Nkx6-2 | Nkx6-1−/−; Nkx6-2−/− | perinatal death | decrease | decrease | Henseleit KD et al., Dev 2005 | |||
| Nkx2-2 | Nkx2-2−/− | perinatal death | decrease* | absent | increase | decrease | Sussel et al., Dev 1998 | |
| Neurod1 | Neurod1−/− | perinatal death | decrease | absent | Naya FJ et al., Genes Dev 1997 | |||
| Nkx2-2/Neurod1 | Nkx2.2−/−; Neurod1−/− | perinatal death | absent | increase | Chao CS et al., Dev Biol 2007 | |||
| Pax4 | Pax4−/− | perinatal death | increase | absent | absent | Sosa-Pineda B et al., Nature 1997 | ||
| Pax6 | Pax6−/− | perinatal death | decrease | decrease | decrease | increase | decrease | Sander M et al., Genes Dev 1997 |
| Pax4/Pax6 | Pax4−/−; Pax6−/− | perinatal death | absent | absent | absent | absent | St-Onge L et al., Nature 1997 | |
| MafA | MafA−/− | viable/diabetic | dysfunction | Zhang C et al., MCB 2005 | ||||
| MafB | MafB−/− | perinatal death | decrease | decrease | Artner I et al., PNAS 2007 | |||
| Arx | Arx−/− | perinatal death | absent* | increase | increase | Collombat P et al., Genes Dev 2003 | ||
| Foxa2 | Foxa2flox/flox;Foxa3-cre | perinatal death | decrease | Lee CS et al., Dev Biol 2005 | ||||
first wave alpha cells present
Figure 5. Islet cell specification in the developing pancreas.
Multipotent pancreatic progenitor cells (PDX1+/PTF1A+/SOX9+) are present in the ductal epithelium in the developing pancreas. In addition, multipotent CPA+/PTF1A+ cells at the tips in early development are multipotent progenitor cells but later in development are restricted to differentiating into only the exocrine lineage. All hormone-producing endocrine cell lineages are derived from the endocrine progenitor cells (NEUROG3+), which delaminate from the ductal epithelium. Endocrine progenitors can differentiate into all five hormone-expressing cell types including insulin-producing beta cells, glucagon-producing alpha cells, somatostatin-producing delta cells, pancreatic polypeptide-producing PP cells and ghrelin-producing epsilon cells. As development proceeds, these differentiated hormone+ cells will coalesce and form the islets of Langerhans. The influence of various transcription factors determines the specific endocrine cell produced.
II. DIABETES
II.1 Diabetes
Diabetes mellitus (“diabetes”) represents a family of metabolic disease characterized primarily by pancreatic dysfunction. Under the umbrella of diabetes, there are multiple forms of the disease with different etiology but the same resultant endpoint. Type 1 diabetes (T1D) is caused by the autoimmune destruction of beta cells and can affect normal weight children and adults; however, childhood onset is most prevalent (reviewed in 103). This form of diabetes is fatal if not treated with exogenous insulin to compensate for the lack of production of this hormone by the body. Although autoimmunity is the primary effecter of T1D, it is still not known which combinations of genetic and environmental stimuli trigger the immune response and why the pancreatic beta cells are specifically targeted for destruction. These are ongoing areas of research.
Type 2 diabetes (T2D) is characterized by insulin resistance, whereby there is a reduced ability to respond to insulin in the pancreatic beta cells and peripheral tissues. Ultimately, the disease is associated with the disruption of pancreatic beta cell function and the loss of beta cell mass. This form of diabetes is most prevalent in adults; however, more recently T2D has been described in an increasing number of younger individuals.104 T2D is a polygenic disease influenced by many environmental factors, which has complicated the identification of a general underlying genetic cause as well as universal therapeutic treatments.
A third form of diabetes that is often mistaken for T1D due to its early onset, is maturity onset diabetes of the young (MODY). MODY is characterized by beta cell dysfunction due to single gene mutations. This monogenic disease has autosomal-dominant inheritance and early onset, usually in childhood or adolescence before 25 years of age.104 The genes implicated in the pathogenesis of MODY are expressed in the beta cell, and a heterozygous mutant state has a range of resultant defects including abnormal insulin secretion, glucose sensing, and beta cell development and function. While MODY genes encode TFs important in the pancreas, a number of these genes are also expressed in other tissues, namely the liver (HNF4A, GCK, HNF1A, HNF1B, KLF11, BLK) and kidney (HNF1B) (Table 3). Dysfunction in these organs can also accompany the disease in individuals that harbour the associated mutation.
Table 3.
Genes implicated in Maturity Onset Diabetes of the Young (MODY)
| MODY | Gene | Gene Name | Former gene names |
Resulting Defect | Reference |
|---|---|---|---|---|---|
| MODY 1 | HNF4A | hepatocyte nuclear factor-4-alpha | HNF4, TCF14 | insulin secretion and beta cell mass | Bell GI et al., PNAS 1991; Stoffel M et al., PNAS 1996 |
| MODY 2 | GCK | glucokinase | GK, GLK, HK4, LGLK | glucose sensing by beta cells; hepatic glucose storage | Froguel P et al., Nature 1992 |
| MODY 3 | HNF1A | hepatocyte nuclear factor-1 alpha | TCF1, HNF1 | insulin secretion and beta cell mass | Vaxillaire M et al Nat Gen 1995; Menzel S. et al., Diabetes 1995 |
| MODY 4 | PDX1 | pancreas/duodenum homeobox protein-1 | IPF1, STF1, IDX1 | transcriptional regulation of beta cell development and function | Stoffers DA et al., Nat Gen 1997 |
| MODY 5 | HNF1B | hepatic nuclear factor-1 beta | TCF2, HNF2 | insulin secretion and beta cell mass; 'renal cysts and diabetes syndrome' | Horikawa Y et al., Nat Gen 1997 |
| MODY 6 | NEUROD1 | neurogenic differentiation 1 | BETA2 | transcription/regulation of beta cell development and function | Malecki MT et al., Nat Gen 1999 |
| MODY 7 | KLF11 | kruppel-like factor-11 | TIEG2 | impaired insulin promoter activation leading to decreased insulin expression | Neve B et al., PNAS 2005 |
| MODY 8 | CEL | carboxyl-ester lipase | BSDL, BSSL | lipase function in pancreatic acinar cells; 'exocrine pancreatic dysfunction' | Raeder H et al., Nat Gen 2006 |
| MODY 9 | PAX4 | paired box gene 4 | repressed activity of the insulin and glucagon promoters | Plengvidhya N et al., J Clin Endocr Metab. 2007 | |
| MODY 10 | INS | insulin | proinsulin | insulin gene processing | Edghill EL et al., Diabetes 2008; Molven A et al., Diabetes 2008 |
| MODY 11 | BLK | tyrosine kinase, B-lymphocyte specific | beta cell function (insulin synthesis and secretion) | Kim SH et al., Diabetes 2004; Borowiec M et al., PNAS 2009 |
Finally, rare forms of neonatal diabetes manifest with other syndromes, and are the result of single gene alterations. One example is a rare syndrome characterized by neonatal diabetes and congenital hypothyroidism (NDH), which has been linked to a genetic alteration in GLIS3.105 In addition, mutation in the gene RFX6 leads to Mitchell-Riley syndrome, typified by hypoplastic pancreas, neonatal diabetes, intestinal atresia and small/absent gall bladder.106 Additional genes implicated in rare forms of neonatal diabetes include PTF1A107, EIF2AK3 (Wolcott-Rallison syndrome108), INSULIN109, FOXP3 (Immunodysregulation, Polyendocrinopathy, and Enteropathy, X-linked syndrome)110, and KCNJ11111.
II.2 Current therapies
The use of exogenous insulin for the treatment of diabetes stemmed from the seminal work of Frederick Banting, Charles Best, James Collip and J.J.R. MacLeod, researchers at the University of Toronto (Canada) in 1921.112 While the first successful extraction of insulin (at the time termed pancrein) can be traced to Nicolae Paulescu in 1921 (published in 1922)113, the work by Banting and colleagues, who injected purified extract from fetal calf pancreas into patients affected with diabetes, revolutionized the treatment of this fatal disease. At present, the insulin prescribed to diabetic patients is biosynthetic recombinant human insulin and provides effective treatment. The creation of continuous subcutaneous insulin infusion therapy, i.e. the insulin pump, further assists patients in that the device provides consistent insulin injections.114 Additionally, in patients where the disease has not progressed, many drugs have been designed to assist the beta cell in its proper function.
Exogenous insulin and pharmaceuticals, while gold standards of care, burden the patient with constant treatment and monitoring of their disease. Alternate therapies are being pursued with the goal of alleviating the requirement for exogenous therapeutics. The question has become, is it possible to identify a therapeutic strategy that restores islet cells, thereby returning normal pancreatic function to patients with diabetes, effectively “curing” the disease?
One such breakthrough came in 1999 at the University of Alberta (Canada), with the first human pancreatic islet transplantation.115 The Edmonton Protocol, as it has been termed, isolates pancreatic islets from up to three donated human cadaveric pancreata and transplants these islets into the portal vein of the recipient.115 As with any organ transplant, recipients are also administered immunosuppressive drug therapy to prevent rejection. One-year follow-up data describes that more than half of the transplant recipients do not require exogenous insulin; however, these numbers decrease with increasing post-operative time.116 While the Edmonton protocol provides a window of curative hope, this revolutionary therapeutic option has limitations, first and foremost, the availability of donor islets. This limitation could be resolved with the in vitro generation of large quantities of functional “islets”.
II.3 The future
Many of the developmental studies outlined in Section I have been instrumental in guiding the creation of protocols to engineer functional insulin-producing beta cells in vitro for therapeutic use in the treatment of diabetes. Ultimately, it has been demonstrated that to successfully produce pancreatic islet cells in vitro, the in vivo developmental stages must be recapitulated. These stages include the induction of definitive endoderm, the subsequent patterning and specification of the pancreatic progenitor cells, followed by the differentiation into hormone-producing endocrine cells, specifically insulin-responsive beta cells. Mimicking development is not trivial, but work in the mouse is assisting in translating the known signals required for specification and differentiation into the in vitro setting.
The relative ease of procurement and manipulation of mouse embryonic stem (mES) cells, as well as mouse embryonic or adult pancreatic tissue, makes these platforms ideal for the creation and testing of in vitro differentiation protocols. Various techniques have been used to manipulate these cells with the goal of understanding their capacity to produce insulin-expressing cells. The direct differentiation of mES cells toward endoderm using soluble factors was found to be successful, as this recapitulated the known developmental signals that induce the specification of pancreatic endoderm.117 Building on this work, small molecules were identified that mimicked these soluble factors and produced endoderm more efficiently.118 However, in both cases the starting cellular platform was the mES cell. From a therapeutic standpoint, the production of insulin-producing cells from adult tissue would mean that cells from a patient could be used to create customized beta cells for transplantation, circumventing the need for immunosuppressive drugs.
To that end, the creation of induced pluripotent stem (iPS) cells 119, which are adult somatic cells reprogrammed to an ES cell state using Oct4, Klf4, cMyc, Sox2, has created a new platform for testing in vitro protocols and the differentiation of many cell lineages (reviewed in 120). Ultimately, the use of human iPS or approved human embryonic stem (hES) cell lines to produce differentiation protocols is more translatable to the clinical realm. To date, research in many organ systems has been successful in the in vitro differentiation of hES cells into cardiac (reviewed in 121), haematopoetic122, and neural123 cells, thus providing great therapeutic promise for the differentiation of hES cells into pancreatic islet cells.
The manipulation of mES and iPS cells has provided adequate proof of principle that in vitro differentiation can be successful, in general. However, understanding the organ-specific requirements, and specifically the plasticity of cells in the pancreas, has provided further insight into the ability to direct or redirect cells toward a pancreatic fate. The capacity of adult mouse pancreatic tissue to transdifferentiate into insulin-producing cells was demonstrated; Zhou and colleagues infected adult mouse exocrine tissue with virus expressing the TF genes Pdx1, Neurog3 and MafA, and determined that insulin-expressing cells could be produced.124 More recently, using either the overexpression of certain TF genes or by extreme pancreatic injury, transdifferentiation between alpha and beta cells has been demonstrated in both the adult and embryonic pancreas.84, 125
The coalescing of decades of pancreatic development research with years of in vitro technology pursuits produced a successful template for the in vitro differentiation of hES cells toward the pancreatic lineage, first reported by ViaCyte (formerly Novocell).126 In this study, the authors outline the manipulation of hES cells toward a hormone-producing fate in vitro, using soluble factors. While successful in the differentiation of definitive endoderm127, and pancreatic and endocrine progenitors126, the ability to complete the differentiation process in vitro was not demonstrated; only after transplantation of endocrine precursors into the mouse were insulin-responsive cells produced.128
Building on these studies, the Keller group recently outlined a protocol with an increased differentiation potential.129 Similar to the in vivo developmental program, this protocol first induces hES cells toward endoderm using FGF, followed by the addition of RA, noggin (BMP inhibitor) and cyclopamine (Hedgehog inhibitor) to generate Pdx1-expressing cells. Ultimately the resulting differentiated endocrine cells were in greater proportion to previous protocols, with an increased number of insulin+ cells produced. While much work still remains to be done, this in vitro differentiation protocol as well as those discussed throughout this section, provides great promise for the creation of insulin-producing cells for therapeutic use. The continued application of developmental biology research to in vitro differentiation technology may ultimately produce the long awaited therapeutic cure for diabetes.
Conclusion
Developmental biology research has, and will continue to, decipher the mystery of organogenesis. With respect to endoderm patterning, pancreatic specification and endocrine differentiation, the utilization of mouse models has been successful in deciphering many of the key factors involved in this process. Factors identified in tissue surrounding the prospective pancreatic endoderm, including SHH, activin, FGF, BMP, and RA, are necessary for the proper induction of patterning in the epithelial gut tube. Following patterning, transcription factors, most importantly PDX1, PTF1A, FOXA and SOX9, specify the pancreatic progenitor cells from which the endocrine, exocrine and ductal lineages are derived. The subsequent development and differentiation of all hormone-producing endocrine cells relies on the influence of mesenchyme and vasculature, as well as branching morphogenesis, ductal delamination and islet cell coalescing. Taken together, understanding the specific cues that allow for proper development has aided in determining the plasticity of cells in the pancreas. Researchers are now translating these key findings into in vitro differentiation protocols, whereby adult or embryonic stem cells can be directed toward a pancreatic fate with the goal of generating insulin-responsive beta cells for the purpose of transplantation into patients affected with diabetes. These in vitro protocols outline the sequential addition of soluble factors, small molecules, or drugs to mimic the in vivo developmental process, thereby creating the developmental milieu in a dish to direct cells to become insulin-producing beta cells. With continued support from developmental biology research, the promise of a curative therapeutic option for the treatment of diabetes is on the horizon.
Acknowledgements
We would like to thank Dr. Jeffrey Raum, Dr. Shouhong Xuan, and Payman Samavarchi-Tehrani for critical reading of this manuscript. TLM is supported by a JDRF Postdoctoral Fellowship (3-2010-791). LS is supported by funding from the NIH (R01 DK082590, R01 DK087711, U01 DK089523 (Beta Cell Biology Consortium)), the Foundation for Diabetes Research, and the Naomi Berrie Diabetes Center.
Footnotes
- Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn 2011, 240(3): 530-65.
- Kim W, Shin YK, Kim BJ, Egan JM. Notch signaling in pancreatic endocrine cell and diabetes. Biochem Biophys Res Commun 2010, 392(3):247–251.
- Macdonald RJ, Swift GH, Real FX. Transcriptional control of acinar development and homeostasis. Prog Mol Biol Transl Sci 2010, 97:1–40.
- Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009, 326(1):4–35.
- Jorgensen MC, Ahnfelt-Ronne J, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev 2007, 28(6): 685–705.
Contributor Information
Teresa L. Mastracci, Email: tm2377@columbia.edu, Department of Genetics and Development, Columbia University.
Lori Sussel, Email: lgs2@columbia.edu, Department of Genetics and Development, Columbia University.
References
- 1.Murtaugh LC, Leach SD. A case of mistaken identity? Nonductal origins of pancreatic "ductal" cancers. Cancer Cell. 2007;11:211–213. doi: 10.1016/j.ccr.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Pour PM, Pandey KK, Batra SK. What is the origin of pancreatic adenocarcinoma? Mol Cancer. 2003;2:13. doi: 10.1186/1476-4598-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oberg K. Pancreatic endocrine tumors. Semin Oncol. 2010;37:594–618. doi: 10.1053/j.seminoncol.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Pan FC, Brandes N, Afelik S, Solter M, Pieler T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev Biol. 2004;271:144–160. doi: 10.1016/j.ydbio.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 6.Stafford D, Prince VE. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol. 2002;12:1215–1220. doi: 10.1016/s0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- 7.Martin M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, Dolle P, Gradwohl G. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev Biol. 2005;284:399–411. doi: 10.1016/j.ydbio.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- 9.Dessimoz J, Opoka R, Kordich JJ, Grapin-Botton A, Wells JM. FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech Dev. 2006;123:42–55. doi: 10.1016/j.mod.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Kim SK, Hebrok M, Melton DA. Notochord to endoderm signaling is required for pancreas development. Development. 1997;124:4243–4252. doi: 10.1242/dev.124.21.4243. [DOI] [PubMed] [Google Scholar]
- 11.Kim SK, Hebrok M, Melton DA. Pancreas development in the chick embryo. Cold Spring Harb Symp Quant Biol. 1997;62:377–383. [PubMed] [Google Scholar]
- 12.Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12:1705–1713. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science. 2009;324:1707–1710. doi: 10.1126/science.1174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 16.Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- 17.Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 18.Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17:138–139. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- 19.Nicolino M, Claiborn KC, Senee V, Boland A, Stoffers DA, Julier C. A novel hypomorphic PDX1 mutation responsible for permanent neonatal diabetes with subclinical exocrine deficiency. Diabetes. 2010;59:733–740. doi: 10.2337/db09-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorgensen MC, Ahnfelt-Ronne J, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev. 2007;28:685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- 21.Burlison JS, Long Q, Fujitani Y, Wright CV, Magnuson MA. Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev Biol. 2008;316:74–86. doi: 10.1016/j.ydbio.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CV, Teitelman G. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- 23.Wu KL, Gannon M, Peshavaria M, Offield MF, Henderson E, Ray M, Marks A, Gamer LW, Wright CV, Stein R. Hepatocyte nuclear factor 3beta is involved in pancreatic beta-cell-specific transcription of the pdx-1 gene. Mol Cell Biol. 1997;17:6002–6013. doi: 10.1128/mcb.17.10.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujitani Y, Fujitani S, Boyer DF, Gannon M, Kawaguchi Y, Ray M, Shiota M, Stein RW, Magnuson MA, Wright CV. Targeted deletion of a cis-regulatory region reveals differential gene dosage requirements for Pdx1 in foregut organ differentiation and pancreas formation. Genes Dev. 2006;20:253–266. doi: 10.1101/gad.1360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang YP, Thorel F, Boyer DF, Herrera PL, Wright CV. Context-specific alpha -to-beta cell reprogramming by forced Pdx1 expression. Genes Dev. 2011:25. doi: 10.1101/gad.16875711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 27.Krapp A, Knofler M, Frutiger S, Hughes GJ, Hagenbuchle O, Wellauer PK. The p48 DNA-binding subunit of transcription factor PTF1 is a new exocrine pancreas-specific basic helix-loop-helix protein. EMBO J. 1996;15:4317–4329. [PMC free article] [PubMed] [Google Scholar]
- 28.Krapp A, Knofler M, Ledermann B, Burki K, Berney C, Zoerkler N, Hagenbuchle O, Wellauer PK. The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev. 1998;12:3752–3763. doi: 10.1101/gad.12.23.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis SL, Tam PP. Definitive endoderm of the mouse embryo: formation, cell fates, and morphogenetic function. Dev Dyn. 2006;235:2315–2329. doi: 10.1002/dvdy.20846. [DOI] [PubMed] [Google Scholar]
- 31.Spence JR, Lange AW, Lin SC, Kaestner KH, Lowy AM, Kim I, Whitsett JA, Wells JM. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev Cell. 2009;17:62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36:83–87. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- 33.Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seymour PA, Freude KK, Dubois CL, Shih HP, Patel NA, Sander M. A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation. Dev Biol. 2008;323:19–30. doi: 10.1016/j.ydbio.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 36.Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–5117. doi: 10.1242/dev.128.24.5109. [DOI] [PubMed] [Google Scholar]
- 38.Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- 39.Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131:807–817. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
- 40.Pierreux CE, Cordi S, Hick AC, Achouri Y, Ruiz de Almodovar C, Prevot PP, Courtoy PJ, Carmeliet P, Lemaigre FP. Epithelial: Endothelial cross-talk regulates exocrine differentiation in developing pancreas. Dev Biol. 2010;347:216–227. doi: 10.1016/j.ydbio.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 41.Eberhard D, Kragl M, Lammert E. 'Giving and taking': endothelial and beta-cells in the islets of Langerhans. Trends Endocrinol Metab. 2010;21:457–463. doi: 10.1016/j.tem.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Villasenor A, Chong DC, Henkemeyer M, Cleaver O. Epithelial dynamics of pancreatic branching morphogenesis. Development. 2011;137:4295–4305. doi: 10.1242/dev.052993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahl U, Sjodin A, Semb H. Cadherins regulate aggregation of pancreatic beta-cells in vivo. Development. 1996;122:2895–2902. doi: 10.1242/dev.122.9.2895. [DOI] [PubMed] [Google Scholar]
- 44.Greiner TU, Kesavan G, Stahlberg A, Semb H. Rac1 regulates pancreatic islet morphogenesis. BMC Dev Biol. 2009;9:2. doi: 10.1186/1471-213X-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaffer AE, Freude KK, Nelson SB, Sander M. Nkx6 transcription factors and Ptf1a function as antagonistic lineage determinants in multipotent pancreatic progenitors. Dev Cell. 2010;18:1022–1029. doi: 10.1016/j.devcel.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim W, Shin YK, Kim BJ, Egan JM. Notch signaling in pancreatic endocrine cell and diabetes. Biochem Biophys Res Commun. 2010;392:247–251. doi: 10.1016/j.bbrc.2009.12.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 48.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 49.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macdonald RJ, Swift GH, Real FX. Transcriptional control of acinar development and homeostasis. Prog Mol Biol Transl Sci. 2010;97:1–40. doi: 10.1016/B978-0-12-385233-5.00001-5. [DOI] [PubMed] [Google Scholar]
- 51.Pictet R, Rutter WJ. Development of the embryonic endocrine pancreas. Vol. 1. Washington, DC: Williams and Wilkins; 1972. [Google Scholar]
- 52.Herrera PL, Huarte J, Sanvito F, Meda P, Orci L, Vassalli JD. Embryogenesis of the murine endocrine pancreas; early expression of pancreatic polypeptide gene. Development. 1991;113:1257–1265. doi: 10.1242/dev.113.4.1257. [DOI] [PubMed] [Google Scholar]
- 53.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu G, Brown JR, Melton DA. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev. 2003;120:35–43. doi: 10.1016/s0925-4773(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 55.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 56.Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell. 2007;12:457–465. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 57.Wang S, Yan J, Anderson DA, Xu Y, Kanal MC, Cao Z, Wright CV, Gu G. Neurog3 gene dosage regulates allocation of endocrine and exocrine cell fates in the developing mouse pancreas. Dev Biol. 2010;339:26–37. doi: 10.1016/j.ydbio.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gouzi M, Kim YH, Katsumoto K, Johansson K, Grapin-Botton A. Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development. Dev Dyn. 2011;240:589–604. doi: 10.1002/dvdy.22544. [DOI] [PubMed] [Google Scholar]
- 59.Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CV, Powers AC. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem. 2002;277:11225–11232. doi: 10.1074/jbc.M111272200. [DOI] [PubMed] [Google Scholar]
- 60.Gannon M, Ables ET, Crawford L, Lowe D, Offield MF, Magnuson MA, Wright CV. pdx-1 function is specifically required in embryonic beta cells to generate appropriate numbers of endocrine cell types and maintain glucose homeostasis. Dev Biol. 2008;314:406–417. doi: 10.1016/j.ydbio.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watada H, Kajimoto Y, Kaneto H, Matsuoka T, Fujitani Y, Miyazaki J, Yamasaki Y. Involvement of the homeodomain-containing transcription factor PDX-1 in islet amyloid polypeptide gene transcription. Biochem Biophys Res Commun. 1996;229:746–751. doi: 10.1006/bbrc.1996.1875. [DOI] [PubMed] [Google Scholar]
- 63.Pedersen JK, Nelson SB, Jorgensen MC, Henseleit KD, Fujitani Y, Wright CV, Sander M, Serup P. Endodermal expression of Nkx6 genes depends differentially on Pdx1. Dev Biol. 2005;288:487–501. doi: 10.1016/j.ydbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- 65.Nelson SB, Schaffer AE, Sander M. The transcription factors Nkx6.1 and Nkx6.2 possess equivalent activities in promoting beta-cell fate specification in Pdx1+ pancreatic progenitor cells. Development. 2007;134:2491–2500. doi: 10.1242/dev.002691. [DOI] [PubMed] [Google Scholar]
- 66.Henseleit KD, Nelson SB, Kuhlbrodt K, Hennings JC, Ericson J, Sander M. NKX6 transcription factor activity is required for alpha- and beta-cell development in the pancreas. Development. 2005;132:3139–3149. doi: 10.1242/dev.01875. [DOI] [PubMed] [Google Scholar]
- 67.Sussel L, Kalamaras J, Hartigan-O'Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- 68.Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci U S A. 2004;101:2924–2929. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson KR, Torres CA, Solomon K, Becker TC, Newgard CB, Wright CV, Hagman J, Sussel L. Cooperative transcriptional regulation of the essential pancreatic islet gene NeuroD1 (beta2) by Nkx2.2 and neurogenin 3. J Biol Chem. 2009;284:31236–31248. doi: 10.1074/jbc.M109.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997;11:2323–2334. doi: 10.1101/gad.11.18.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gu C, Stein GH, Pan N, Goebbels S, Hornberg H, Nave KA, Herrera P, White P, Kaestner KH, Sussel L, et al. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab. 2011;11:298–310. doi: 10.1016/j.cmet.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 73.Raum JC, Hunter CS, Artner I, Henderson E, Guo M, Elghazi L, Sosa-Pineda B, Ogihara T, Mirmira RG, Sussel L, et al. Islet beta-cell-specific MafA transcription requires the 5'-flanking conserved region 3 control domain. Mol Cell Biol. 2011;30:4234–4244. doi: 10.1128/MCB.01396-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sander M, Neubuser A, Kalamaras J, Ee HC, Martin GR, German MS. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997;11:1662–1673. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- 75.St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- 76.Artner I, Blanchi B, Raum JC, Guo M, Kaneko T, Cordes S, Sieweke M, Stein R. MafB is required for islet beta cell maturation. Proc Natl Acad Sci U S A. 2007;104:3853–3858. doi: 10.1073/pnas.0700013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao L, Guo M, Matsuoka TA, Hagman DK, Parazzoli SD, Poitout V, Stein R. The islet beta cell-enriched MafA activator is a key regulator of insulin gene transcription. J Biol Chem. 2005;280:11887–11894. doi: 10.1074/jbc.M409475200. [DOI] [PubMed] [Google Scholar]
- 79.Artner I, Hang Y, Mazur M, Yamamoto T, Guo M, Lindner J, Magnuson MA, Stein R. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2011;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsuoka TA, Artner I, Henderson E, Means A, Sander M, Stein R. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc Natl Acad Sci U S A. 2004;101:2930–2933. doi: 10.1073/pnas.0306233101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duvillie B, Cordonnier N, Deltour L, Dandoy-Dron F, Itier JM, Monthioux E, Jami J, Joshi RL, Bucchini D. Phenotypic alterations in insulin-deficient mutant mice. Proc Natl Acad Sci U S A. 1997;94:5137–5140. doi: 10.1073/pnas.94.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Duvillie B, Currie C, Chrones T, Bucchini D, Jami J, Joshi RL, Hill DJ. Increased islet cell proliferation, decreased apoptosis, and greater vascularization leading to beta-cell hyperplasia in mutant mice lacking insulin. Endocrinology. 2002;143:1530–1537. doi: 10.1210/endo.143.4.8753. [DOI] [PubMed] [Google Scholar]
- 83.Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17:2591–2603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collombat P, Hecksher-Sorensen J, Krull J, Berger J, Riedel D, Herrera PL, Serup P, Mansouri A. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J Clin Invest. 2007;117:961–970. doi: 10.1172/JCI29115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Collombat P, Hecksher-Sorensen J, Broccoli V, Krull J, Ponte I, Mundiger T, Smith J, Gruss P, Serup P, Mansouri A. The simultaneous loss of Arx and Pax4 genes promotes a somatostatin-producing cell fate specification at the expense of the alpha- and beta-cell lineages in the mouse endocrine pancreas. Development. 2005;132:2969–2980. doi: 10.1242/dev.01870. [DOI] [PubMed] [Google Scholar]
- 86.Mastracci TL, Wilcox C, Panea C, Golden JA, May CL, Sussel L. Nkx2.2 and Arx genetically interact to regulate pancreatic endocrine cell development and endocrine hormone expression. Dev Biol. 2011 doi: 10.1016/j.ydbio.2011.08.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hussain MA, Lee J, Miller CP, Habener JF. POU domain transcription factor brain 4 confers pancreatic alpha-cell-specific expression of the proglucagon gene through interaction with a novel proximal promoter G1 element. Mol Cell Biol. 1997;17:7186–7194. doi: 10.1128/mcb.17.12.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hussain MA, Miller CP, Habener JF. Brn-4 transcription factor expression targeted to the early developing mouse pancreas induces ectopic glucagon gene expression in insulin-producing beta cells. J Biol Chem. 2002;277:16028–16032. doi: 10.1074/jbc.M107124200. [DOI] [PubMed] [Google Scholar]
- 89.Petri A, Ahnfelt-Ronne J, Frederiksen KS, Edwards DG, Madsen D, Serup P, Fleckner J, Heller RS. The effect of neurogenin3 deficiency on pancreatic gene expression in embryonic mice. J Mol Endocrinol. 2006;37:301–316. doi: 10.1677/jme.1.02096. [DOI] [PubMed] [Google Scholar]
- 90.Lebel M, Agarwal P, Cheng CW, Kabir MG, Chan TY, Thanabalasingham V, Zhang X, Cohen DR, Husain M, Cheng SH, et al. The Iroquois homeobox gene Irx2 is not essential for normal development of the heart and midbrain-hindbrain boundary in mice. Mol Cell Biol. 2003;23:8216–8225. doi: 10.1128/MCB.23.22.8216-8225.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chao CS, Loomis ZL, Lee JE, Sussel L. Genetic identification of a novel NeuroD1 function in the early differentiation of islet alpha, PP and epsilon cells. Dev Biol. 2007;312:523–532. doi: 10.1016/j.ydbio.2007.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hayashi Y, Yamamoto M, Mizoguchi H, Watanabe C, Ito R, Yamamoto S, Sun XY, Murata Y. Mice deficient for glucagon gene-derived peptides display normoglycemia and hyperplasia of islet {alpha}-cells but not of intestinal L-cells. Mol Endocrinol. 2009;23:1990–1999. doi: 10.1210/me.2009-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept. 2002;107:63–69. doi: 10.1016/s0167-0115(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 94.Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem. 2004;52:301–310. doi: 10.1177/002215540405200301. [DOI] [PubMed] [Google Scholar]
- 95.Heller RS, Jenny M, Collombat P, Mansouri A, Tomasetto C, Madsen OD, Mellitzer G, Gradwohl G, Serup P. Genetic determinants of pancreatic epsilon-cell development. Dev Biol. 2005;286:217–224. doi: 10.1016/j.ydbio.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 96.Hill JT, Mastracci TL, Vinton C, Doyle ML, Anderson KR, Loomis ZL, Schrunk JM, Minic AD, Prabakar KR, Pugliese A, et al. Ghrelin is dispensable for embryonic pancreatic islet development and differentiation. Regul Pept. 2009;157:51–56. doi: 10.1016/j.regpep.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development. 1993;118:1031–1039. doi: 10.1242/dev.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 98.Herrera PL, Huarte J, Zufferey R, Nichols A, Mermillod B, Philippe J, Muniesa P, Sanvito F, Orci L, Vassalli JD. Ablation of islet endocrine cells by targeted expression of hormone- promoter-driven toxigenes. Proc Natl Acad Sci U S A. 1994;91:12999–13003. doi: 10.1073/pnas.91.26.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 100.Schonhoff S, Baggio L, Ratineau C, Ray SK, Lindner J, Magnuson MA, Drucker DJ, Leiter AB. Energy homeostasis and gastrointestinal endocrine differentiation do not require the anorectic hormone peptide YY. Mol Cell Biol. 2005;25:4189–4199. doi: 10.1128/MCB.25.10.4189-4199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson IC, Low MJ, Christie MR, Persaud SJ, Jones PM. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes. 2009;58:403–411. doi: 10.2337/db08-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Low MJ, Otero-Corchon V, Parlow AF, Ramirez JL, Kumar U, Patel YC, Rubinstein M. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest. 2001;107:1571–1580. doi: 10.1172/JCI11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 104.Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med. 2001;345:971–980. doi: 10.1056/NEJMra002168. [DOI] [PubMed] [Google Scholar]
- 105.Senee V, Chelala C, Duchatelet S, Feng D, Blanc H, Cossec JC, Charon C, Nicolino M, Boileau P, Cavener DR, et al. Mutations in GLIS3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat Genet. 2006;38:682–687. doi: 10.1038/ng1802. [DOI] [PubMed] [Google Scholar]
- 106.Pearl EJ, Jarikji Z, Horb ME. Functional analysis of Rfx6 and mutant variants associated with neonatal diabetes. Dev Biol. 2011;351:135–145. doi: 10.1016/j.ydbio.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sellick GS, Barker KT, Stolte-Dijkstra I, Fleischmann C, Coleman RJ, Garrett C, Gloyn AL, Edghill EL, Hattersley AT, Wellauer PK, et al. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet. 2004;36:1301–1305. doi: 10.1038/ng1475. [DOI] [PubMed] [Google Scholar]
- 108.Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 109.Stoy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104:15040–15044. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 111.Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 112.Bliss M. Resurrections in Toronto: the emergence of insulin. Horm Res. 2005;6498(Suppl 2) doi: 10.1159/000087765. [DOI] [PubMed] [Google Scholar]
- 113.Murray I. Paulesco and the isolation of insulin. J Hist Med Allied Sci. 1971;26:150–157. doi: 10.1093/jhmas/xxvi.2.150. [DOI] [PubMed] [Google Scholar]
- 114.Schaepelynck P, Renard E, Jeandidier N, Hanaire H, Fermon C, Rudoni S, Catargi B, Riveline JP, Guerci B, Millot L, et al. A Recent Survey Confirms the Efficacy and the Safety of Implanted Insulin Pumps During Long-Term Use in Poorly Controlled Type 1 Diabetes Patients. Diabetes Technol Ther. 2011 doi: 10.1089/dia.2010.0209. [DOI] [PubMed] [Google Scholar]
- 115.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 116.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 117.Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 118.Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL, Melton DA. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 120.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 121.Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res. 2002;91:189–201. doi: 10.1161/01.res.0000027865.61704.32. [DOI] [PubMed] [Google Scholar]
- 122.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 124.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 127.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 128.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 129.Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X, Micallef SJ, Park IH, Basford C, Wheeler MB, et al. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]