Abstract
Toll-like receptors (TLRs) are critical components of innate immunity and function as rapid pathogen sensors. TLR4 is expressed on CD4+ T cells as well, the functional significance of which is unclear. In this study, we analyzed the function of TLR4 in T cells but did not find a role in promoting T helper (Th) cell polarization. Instead, TLR4 ligation enhanced both CD4+ T-cell proliferation and survival in vitro. Using the experimental autoimmune encephalomyelitis (EAE) model, we found that the loss of TLR4 solely in CD4+ T cells almost completely abrogated disease symptoms, mainly through blunted Th17 and, to a lesser degree, Th1 responses. Moreover, Tlr4−/− γδ T cells were defective in IL-17 and IFN-γ production following EAE induction. This study supports an important role of this innate receptor in the direct regulation of T-cell activation and survival during autoimmune inflammation.
Keywords: innate T cell activation, multiple sclerosis
Toll-like receptors (TLRs) detect conserved microbial patterns and are largely thought to function in cells of the innate response where activation leads to antigen-presenting cell (APC) maturation and inflammatory cytokine production (1). Recent work has demonstrated a role of TLR-signaling pathways within cells of the adaptive immune response as well (2). For example, TLR2 provided a costimulatory signal for both CD4+ and CD8+ T cells (3, 4) and was found to regulate IL-17 production by γδ T cells (5) and CD4+ T helper 17 (Th17) cells (6).
Naïve CD4+ T cells differentiate into various effector lineages depending on the environment at the time of activation (7). Th1 cells are characterized by a requirement for IL-12 as well as the production of IFN-γ. The recently identified Th17 lineage is characterized by IL-17 expression (8, 9). Both Th17 and Th1 cells are involved in promoting autoimmune inflammation, especially during multiple sclerosis and EAE (8, 10, 11); however, Th17 cells may be the major inflammatory subset involved in promoting EAE (12), and our group has shown that the Th17 subset can regulate Th1 cells during EAE pathogenesis (13).
The expression of TLR4 has been linked to central nervous system (CNS) inflammation although studies have been largely focused on innate cells. Two reagents commonly used for EAE induction, Mycobacterium tuberculosis and pertussis toxin, have been shown to signal through the TLR4 pathway (14–16). Consequently, EAE was less severe when induced in TLR4-deficent animals (14). Another group, conversely, found that complete TLR4 deficiency resulted in the exacerbation of EAE symptoms (17). Thus, the role of TLR4 in EAE remains controversial; however, other work supports the idea that TLR4 may promote CNS inflammation. For example, LPS administration was found to promote EAE in a CD4+ T-cell transgenic model (18), and TLR4 expression in microglial cells was also found to promote brain injury in a model of cerebral ischemia (19).
Recent studies have provided insights on the function of TLR4 in CD4+ T cells. TLR4 signaling was found to ameliorate inflammation in a T-cell transfer model of colitis. In this setting, TLR4 activation led to ERK 1/2 inhibition and the down-regulation of Th1 responses (20). Human CD4+ T cells were found to have enhanced adherence to fibronectin following LPS stimulation, which was a result of PKC and p38 pathway activation (21). Here we demonstrate that TLR4 activation in CD4+ T cells enhances proliferation and survival of CD4+ T cells. Most importantly, we found that TLR4 expression by T cells is essential for the development of EAE. Overall, we provide evidence of an innate mechanism that can directly regulate adaptive immune responses during inflammatory disease.
Results
TLR4 Signaling Augments CD4+ T-Cell Activation in Vitro and in Vivo.
We previously found that TLR4 was expressed on CD4+ T cells and that Th17 cells express high TLR4 mRNA (6). Thus, we examined whether TLR4 activation could influence CD4+ T-cell polarization by first differentiating naïve CD4+ T cells under Th conditions in the absence or presence of LPS (Fig. 1A). Although no discernable differences in cytokine production were found, we did, however, observe a tendency for naïve cells to proliferate more extensively when LPS was added to the cultures. Indeed, we found that treatment of naïve CD4+ T cells with LPS alone induced proliferation compared with untreated cells (Fig. 1B). At suboptimal doses of αCD3, LPS enhanced proliferation, but at larger doses this effect was minimal. In addition, we examined the effect of TLR4 in the survival of CD4+ T cells cultured in the absence of TcR signals. Roughly 40% of CD4+ T cells left untreated for 3 d exhibited signs of cellular death and apoptosis as measured by 7-aminoactinomycin D (7-AAD) and annexin-V staining (Fig. 1C). However, when LPS was added to the culture, CD4+ T cells exhibited little to no signs of apoptosis and cellular death. Thus, TLR4 signaling in CD4+ T cells acts to enhance activation in the absence of TcR stimulation and promote survival.
Fig. 1.
TLR4 signaling enhances CD4+ T-cell activation. (A) Sorted naïve CD4+ T cells were differentiated under various Th conditions for 4 d followed by intracellular cytokine staining. (B) Naïve CD4+ T cells from WT or Tlr4−/− mice were treated 3 d with media, 1 μg/mL αCD28, or αCD28 + αCD3 in the absence or presence of LPS. Data are presented as mean + SD for triplicate determinations; *P < 0.05; Student's t test. (C) WT or Tlr4−/− CD4+ T cells were left untreated for 3 d in the absence or presence of LPS before analysis by 7-AAD and Annexin-V staining. Data are representative of three independent experiments.
We next examined whether TLR4 signaling could regulate CD4+ T-cell activation in vivo. We transferred WT or Tlr4−/− CD4+ T cells into Rag1−/− hosts, immunized with keyhole limpet hemocyanin (KLH), and then analyzed T-cell responses in the draining lymph nodes (DLN) and spleen. We observed substantial reductions in antigen-specific IL-17 and IFN-γ responses in hosts receiving Tlr4−/− CD4+ T cells (Fig. 2 A and B). The overall CD4+ T-cell numbers remained unchanged between groups, indicating that TLR4 did not influence homeostatic proliferation (Fig. 2B). The 3-d KLH recall responses revealed a similar trend where WT CD4+ T cells proliferated more extensively and produced higher amounts of Th17 and Th1 cytokines following KLH restimulation (Fig. 2 C and D). In contrast, within the DLN, only Th17 cytokines were significantly higher in WT CD4+ T cells (Fig. 2D). Taken together, our results indicate that signaling through the TLR4 pathway can enhance the activation and persistence of Th17 and, to a lesser degree, Th1 responses.
Fig. 2.
Tlr4−/− CD4+ T cells exhibit blunted effector cytokine responses in vivo. (A) Representative staining of CD4+ T cells derived from the DLN of WT- (Left) or Tlr4−/−-transferred (Right) Rag1−/− mice immunized 7 d with KLH. Cells were restimulated overnight with KLH before intracellular cytokine staining. (B) Summary of CD4+ T-cell cytokine production in the DLN following KLH immunization. (C) Proliferation analysis of WT and Tlr4−/− CD4+-transferred DLN following 7 d KLH immunization. (D) Cytokine production analysis of splenic (Top) and DLN (Bottom) CD4+ T cells following 3 d KLH restimulation. For all experiments, n = 5 per group; *P < 0.05; Student's t test. Data are representative of three independent experiments.
Tlr4−/− CD4+ T Cells Are Inadequate in Promoting EAE.
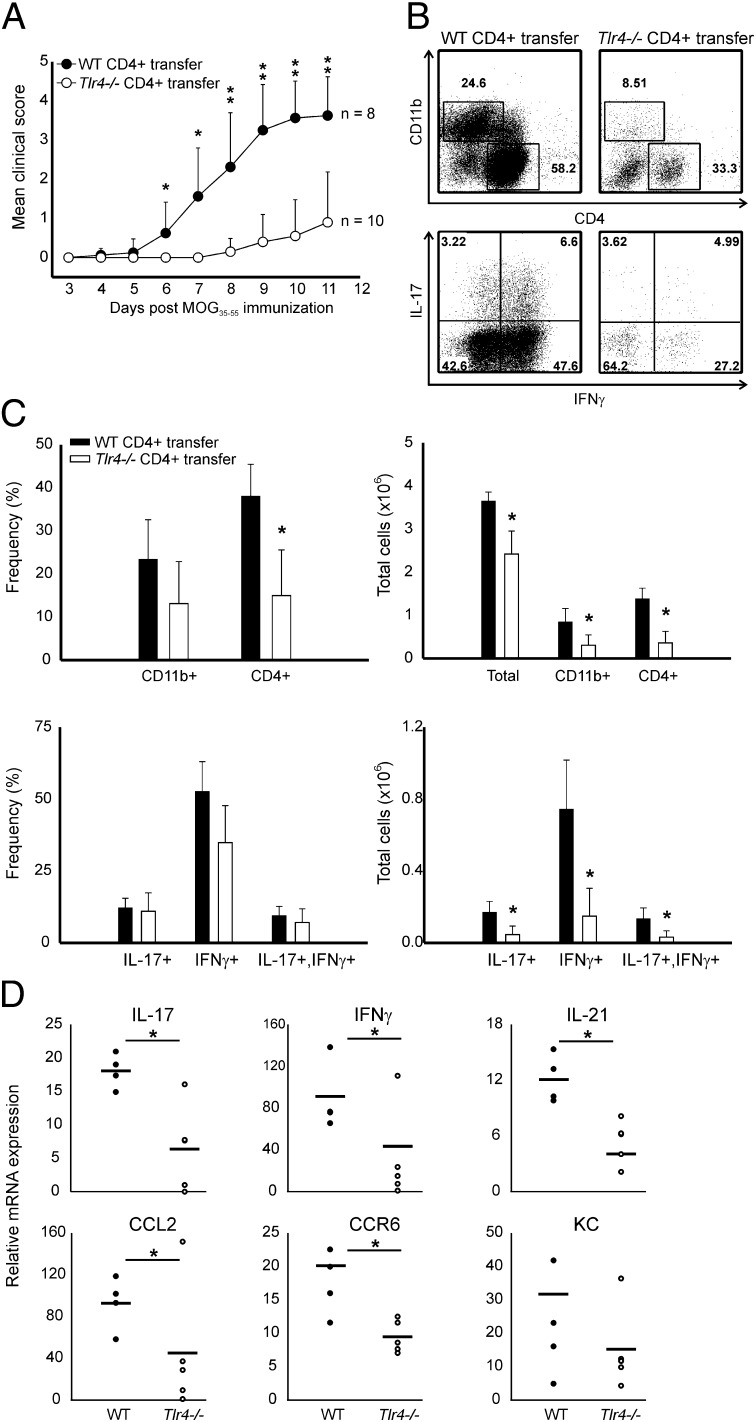
Th17 cells are the primary regulators of CNS inflammation in the EAE model (12), and Th1 cells are thought to be important as well (11, 22). Due to the reduction we observed in Tlr4−/− Th1 and Th17 responses following single immunization, we next analyzed whether TLR4 signaling directly in CD4+ T cells could promote EAE. WT or Tlr4−/− CD4+ T cells were transferred into Rag1−/− hosts and EAE was induced. All of the mice receiving WT CD4+ T cells developed EAE within 7 d (mean clinical score ∼3.5; Fig. 3A). In sharp contrast, only 50% of Rag1−/− mice receiving Tlr4−/− CD4+ T cells developed EAE, and the animals that did develop clinical symptoms exhibited delayed onset (9 d) and significantly reduced EAE severity (mean score ∼1.2). In our model we observed a much stronger EAE phenotype when TLR4 is deficient only in CD4+ T cells compared with the full knockouts (17). The reason for this observation is not currently known but could reflect the ubiquitous nature of TLR4 signaling in that various cell types use TLR4 in different ways.
Fig. 3.
Tlr4−/− CD4+ T cells are defective in promoting EAE. (A) Representative clinical scores of Rag1−/− mice reconstituted with WT or Tlr4−/− CD4+ T cells. n = 8 for WT CD4+ T-cell transfer and n = 10 for TLR4-deficient CD4+ T-cell transfer. (B) Representative CNS staining of mice presented in A. CD4+ and CD11b+ cells were analyzed by surface staining (Upper). CD4+ T cells were analyzed for the production of IL-17 and IFN-γ following 6 h phorbol myristate acetate (PMA) restimulation (Lower). (C) Summary of CNS infiltration and cytokine production of the mice presented in A. (D) mRNA analysis of EAE brain and spinal cord tissues of Rag1−/− mice reconstituted with WT or Tlr4−/− CD4+ T cells. n = 5 mice per group. Data are representative of four independent experiments; *P < 0.05, **P < 0.001; Student's t test.
We next examined the CNS and found that Rag1−/− mice receiving Tlr4−/− T cells displayed a reduction of infiltrating CD4+ and CD11b+ cells (Fig. 3 B and C). This result was surprising in that similar mice immunized with KLH had comparable T-cell counts in the DLN (Fig. 2). It is likely that Tlr4−/− CD4+ T cells are not being fully activated and therefore fail to migrate and be reactivated in the CNS during EAE. Of the CD4+ T cells present in the CNS, those lacking TLR4 showed a reduction in both the Th1 and Th17 compartments (Fig. 3 B and C). Total CNS mRNA analysis revealed similar reductions in IL-17, IFN-γ, CCR6, as well as CCL2 in mice receiving Tlr4−/− CD4+ T cells (Fig. 3D).
Recent work has shown that IL-23-driven production of GM-CSF by Th17 cells is critical for the development of EAE (23, 24). Indeed, in our model, GM-CSF expression was also sharply reduced in CD4+ T cells lacking Tlr4−/− expression (Fig. S1). Moreover, FoxP3+ Treg cells are important suppressors of autoimmune-associated inflammation (reviewed in ref. 25). We found that FoxP3+ cells were increased in the CNS tissues of Rag1−/− mice receiving WT T cells (Fig. S2), indicating that Treg suppression is not the reason for blunted EAE development caused by TLR4 deletion. Thus, the persistence of inflammatory Th cells is dependent on TLR4 expression by CD4+ T cells. Others have demonstrated the opposite in regard to Th1 cells (20). The reasons for this discrepancy are unclear but the substantial Th17 and Th1 reductions we observed suggest that TLR4 is critical in promoting autoimmune CNS inflammation.
In further support of our observations, we generated chimeras by reconstituting Rag1−/− mice with a 1:1 ratio of B6SJL and Tlr4−/− bone marrow and induced EAE. In the CNS and spleen we found that the frequency of CD45.1 and CD45.2 CD4+ T cells was virtually identical (Fig. 4A), demonstrating effective reconstitution and homeostatic proliferation by Tlr4−/− T cells. A dramatic decrease of IL-17+ T cells, however, was found in the CNS CD45.2 population (Fig. 4 B and C). IFN-γ production, although reduced in Tlr4−/− CD4+ T cells, did not reach statistical significance in comparison with CD45.1 cells. Upon reactivation of splenic T cells, the Th1 compartment was identical where a modest, but significant, decrease of IL-17 production was observed for Tlr4−/− CD4+ T cells (Fig. 4 D–F), suggesting that TLR4 is more important for Th17 and Th1 function at the inflamed tissue rather than the periphery.
Fig. 4.
TLR4-deficient CD4+ T cells lack encephalitogenic cytokine production. (A) Representative CD4 and CD45.1 staining in the CNS (Left) and spleen (Right) of B6SJL:Tlr4−/− chimeric mice following EAE induction. (B) Representative cytokine staining of WT (CD45.1) and Tlr4−/− (CD45.2) CD4+ T cells in the CNS following EAE induction and PMA restimulation. (C) Summary of CD4+ T-cell frequencies in the CNS of mixed bone marrow–reconstituted mice following EAE induction. n = 5 mice per group. (D) Intracellular cytokine staining of splenocytes from the mice in A following overnight MOG35–55 restimulation. Each column (Top and Bottom) is representative of the CD45.1 and CD45.2 CD4+ T cells derived from one individual mouse. (E) Compilation of the data presented in D. (F) Cytokine production analysis of splenic CD45.1 or CD45.2 CD4+ T cells that were cultured 3 d with APCs in the absence or presence of MOG35–55 peptide. Data are representative of three independent experiments. *P < 0.05; Student's t test.
Our previous data suggested that Tlr4−/− T cells are able to activate and migrate to the CNS when in the same inflammatory environment as WT CD4+ T cells. Once in the CNS Tlr4−/− T cells are either dying or failing to expand, which may be mediated through endogenous TLR4 agonists released by dying cells (26). Indeed, when myelin oligodendrocyte glycoprotein (MOG)-specific WT or Tlr4−/− T cells were expanded with IL-23 in vitro, we found equivalent IL-17 and IFN-γ (Fig. S3A). However, once transferred, only WT CD4+ T cells were able to induce EAE (Fig. S3B). Tlr4−/− CD4+ T cells were present in the CNS but did not produce encephalitogenic cytokines (Fig. S3C). Splenic cytokine production was similar between WT and Tlr4−/− CD4+ T cells (Fig. S3D), suggesting that TLR4-mediated reactivation in the CNS is needed for EAE pathogenesis.
TLR4 Expression by γδ T Cells Contributes to Autoimmune Inflammation.
γδ T cells also produce IL-17 (27) and TLR4 promoted IL-17 production by γδ T cells during E. coli infection (28). This effect, however, was not mediated by TLR4 signaling in γδ T cells. Additionally, TLR4 was not observed in γδ T cells derived from the spleen or peritoneal cavity (5). Conversely, TLR4 was increased in γδ T cells following burn injury (29) and human γδ T cells expressed TLR4 following lipid A stimulation (30). More recently, we found TLR4 mRNA expression in a population of splenic and lymph node–derived γδ T cells (6). Thus, to further test TLR4 relevance, we analyzed a heterogeneous pool of γδ T cells derived from lymphoid organs and lungs of healthy IL-17F–RFP reporter mice (10) that were stimulated in vitro with IL-23 for 2 d. RFP+, and to a lesser extent RFP−, γδ T cells were found to express TLR4 mRNA (Fig. 5A); whereas, IL-23 was also found to up-regulate TLR4 expression (Fig. 5B). To examine whether TLR4 could promote IL-17 production, we examined γδ T cells stimulated in vitro for 5 h with LPS, IL-23, or a combination of both. IL-17 mRNA was produced upon IL-23 stimulation, even more so with the addition of LPS (Fig. 5C). At the protein level, γδ T cells stimulated under similar conditions produced higher IL-17 and IL-17F when LPS was present (Fig. 5D). We also observed that γδ T cells proliferated extensively in the presence of LPS, similar to what was observed for CD4+ T cells (Fig. 1). Indeed, TCR-independent LPS stimulation enhanced γδ T-cell proliferation, where IL-23 was dispensable (Fig. 5E). Thus, TLR4 activation promotes expansion and IL-17 production by γδ T cells in vitro.
Fig. 5.
TLR4 signaling in γδ T cells promotes expansion and IL-17 production. (A) γδ T cells were sorted from healthy IL-17F–RFP mice, stimulated for 2 d with IL-23, and then analyzed for TLR4 mRNA expression. Dendritic cells and sorted WT and Tlr4−/− γδ T cells derived from the spleen were used as expression controls. (B) Sorted γδ T cells from healthy WT animals were stimulated with IL-23 0–16 h before the analysis of TLR4 mRNA. (C) Sorted splenic, lymph node, and respiratory γδ T cells derived from healthy WT and Tlr4−/− mice were stimulated for 5 h with LPS and/or IL-23 before IL-17 and IL-17F mRNA analysis. (D) Cytokine production was examined from the supernatants of sorted γδ T cells stimulated 2 d with LPS and/or IL-23. (E) carboxyfluorescein succinimidyl ester (CFSE) dilution of sorted γδ T cells stimulated for 2 d with αCD3, LPS, or IL-23 was assessed by flow cytometry. Data are representative of at least three independent experiments; *P < 0.05; Student's t test.
We next wanted to investigate whether TLR4 signaling could promote IL-17 production by γδ T cells in a competitive EAE model as γδ T cells have been shown to promote CNS inflammation (31). Low γδ T-cell numbers and cytokines were observed in the CNS of healthy mice (Fig. 6A). Upon EAE induction, we observed a substantial influx of γδ T cells into the CNS but no difference was observed in the frequency of CD45.1 versus CD45.2 γδ T cells (Fig. 6B). However, WT γδ T cells produced both IL-17 and IFN-γ and Tlr4−/− γδ T cells exhibited ∼50% reduction in both cytokines. We also found that the majority of IL-17- and IFN-γ-producing cells were γδ TCRlo (Fig. S4). Thus, TLR4 signaling in γδ T cells promotes effector cytokine production during autoimmunity similar to what was observed for CD4+ T cells.
Fig. 6.
TLR4-deficient γδ T cells have reduced IL-17 and IFN-γ responses during EAE. Intracellular cytokine staining of γδ+ T cells derived from healthy (A) or EAE (B) CNS tissues of chimeric mice (clinical score = 1). Lymphocytes were pooled from five individual mice and γδ T cells were restimulated 5 h with PMA. Intracellular cytokine staining was assessed in both WT (CD45.1) and TLR4−/− (CD45.2) fractions. Data are representative of two individual experiments.
Discussion
Toll-like receptors are critical innate mechanisms for host defense against invading pathogens. Specifically, TLR4 is involved in the recognition of LPS present on Gram-negative bacteria (1) but has also been shown to respond to other ligands such as tuberculosis and pertussis toxin (14–16). Previously we found that TLR2 signaling could promote the expansion and polarization of naïve cells to the Th17 lineage (6). Although we found that Th17 cells had higher TLR4 expression compared with other Th lineages, LPS activation had no effect in promoting lineage commitment. However, naïve T cells stimulated through the TLR4 pathway did proliferate more extensively and exhibited enhanced survival (Fig. 1). LPS stimulation of naïve CD4+ T and γδ T cells in the absence of TcR signal still resulted in measurable proliferation above background levels, suggesting a conserved innate-signaling mechanism that promotes survival of these cells in the presence of danger signals. Consequently, TLR4 represents a mechanism for the regulation of inflammation by T cells. The mechanisms governing proliferation and survival following TLR4 stimulation remain unclear and will be the subject of future investigation.
We found that Tlr4−/− CD4+ T cells had a dramatic reduction in Th1 and Th17 cytokines (Figs. 2–4) following the induction of inflammation, which was surprising because LPS had no effect on polarization in vitro. The positive effect of TLR4 activation on the survival of CD4+ T cells in vitro (Fig. 1), however, can instead explain this observation. Following initial activation and lineage commitment, CD4+ T cells also require signals for survival and reactivation. Thus, TLR4 signals (provided by exogenous stimuli or endogenous inflammatory byproducts) regulate the persistence of Th lineages. Furthermore, this pathway is robust enough to maintain and promote Th effector function, which is severely lacking in CD4+ T cells lacking TLR4 expression. We conclude then that in the absence of TLR4 signaling, T cells are unable to survive or reactivate, leading to a reduction in autoimmune-associated inflammation. Our studies involving bone marrow chimeras (Fig. 4) and passive transfer EAE (Fig. S3) strongly argue for a role of TLR4 in regulating activation and survival in vivo rather than Th17 or Th1 development.
We found that TLR4 activation specifically in CD4+ T cells was critical for EAE development (Figs. 3 and 4); however, there are conflicting reports about the necessity of TLR4 in this model when full Tlr4−/− animals were used (14, 17). Our data also demonstrates that TLR4 deficiency solely in CD4+ T cells leads to a phenotype that is considerably stronger compared with that observed when using full Tlr4−/− animals. The reason for this observation is unclear but could reflect differential TLR4-signaling depending on cell type. For example, innate cells produce pro- and anti-inflammatory mediators, such as IL-12 and IL-10, following TLR4 activation that could moderately suppress Th17 and EAE development. In this scenario, TLR4 expression would not be as important as WT and Tlr4−/− CD4+ T cells are receiving different signals from the environment; the lack of inhibitory signals provided through TLR4 activation in other cell types could allow CD4+ T cells to promote inflammation in a similar manner compared with WT animals. Thus, in the presence of other TLR4-responding cells, this activation effect is amplified as both WT and KO CD4+ T cells are receiving the same environmental signals.
A recent publication demonstrated that CD4+ T cells deficient in both IL-10 and TLR4 exacerbated colitis through high Th1 activity compared with controls (20). In the EAE model, however, we found that the presence of TLR4 had the opposite effect in promoting CNS inflammation. One possible explanation for this discrepancy is that the same mechanism exists in our model but the outcome is quite different. Th1 responses antagonize Th17 generation and function (32), thus a reduction in Th1 generation in the absence of TLR4 could potentially led to the enhancement of EAE-promoting Th17 responses. The fact that we observed a reduction in both Th17 and Th1 compartments could then be explained by a recent report from our group that Th17 cells have a role in regulating Th1 responses during EAE (13). However, we did not observe a direct effect of TLR4 in Th polarization, suggesting that this pathway is not selective for Th1 or Th17 cells. Finally, TLR4 signaling could lead to different outcomes in CD4+ T cells when comparing the pathogen-rich environment of the gut to the sterile environment of the CNS.
Our previous work has demonstrated that TLR2 signaling in T cells also promotes EAE development (6). Consequently, if both TLR pathways are critical for CD4+ T cells to promote EAE, one might find it puzzling that there is a lack of redundancy between these pathways. However, we believe the explanation lies in the distinct mechanisms each TLR uses to promote inflammation, where deletion of either pathway is sufficient to confer protection against EAE. In the case of TLR2, we found that ligation could directly promote Th17 generation as well as expansion. During the development of autoimmune inflammation, TLR2 directly promotes the generation of encephalitogenic Th17 responses. In the case of TLR4, we did not observe a role in polarization; instead we found that ligation enhanced proliferation and survival. During EAE, Th17 and Th1 cells are generated regardless of TLR4 expression, but cells expressing TLR4 are able to persist and reactivate in the CNS. Another reason for lack of redundancy between pathways is likely the presence of endogenously generated TLR2 or TLR4 ligands in the CNS that are critical for the amplification of T-cell responses. We have shown that mice immunized in the absence of exogenous TLR2 signals still exhibited reduced EAE as a result of TLR2 deficiency (6). The same may be true for TLR4, as endogenous ligands generated as a result of inflammation have been shown to signal through this pathway (26). We are currently conducting studies to address this point, but the argument could be made that endogenous ligands specific for TLR2 promote Th17, where endogenous ligands specific for TLR4 promote survival and general activation.
Overall, our results demonstrate the crucial and innate role of TLR4 in promoting the activation of CD4+ and γδ T cells, which contributes to the initiation of autoimmune inflammation. In combination with our previous studies showing that TLR2 expression on CD4+ T cells also promotes the development of EAE, our results suggest that targeting TLR pathways directly on T lymphocytes may prove to be effective in treating autoimmune-inflammatory disorders such as multiple sclerosis.
Materials and Methods
Mice.
C57BL/6, Tlr4−/−, B6SJL, and Rag1−/− mice were purchased from The Jackson Laboratory. Mice were housed under specific pathogen-free conditionals at the M.D. Anderson Cancer Center. All experiments were performed with 6–10-wk-old animals under strict adherence to the protocols approved by the MD Anderson Cancer Center Institutional Animal Care and Use Committee.
In Vitro T-Cell Assays.
Naïve CD4+ T cells were sorted from the spleen and lymph nodes of C57BL/6 or Tlr4−/− mice and the details for each assay are described in SI Materials and Methods.
CD4+ T-Cell Transfer.
CD4+ T cells from the spleen and lymph nodes of healthy C57BL/6 and TLR4−/− mice were purified using the AutoMACS system (Miltenyi). Cell were washed 3× in PBS and 5 × 106 cells were injected i.v. into the tail veins of Rag1−/− animals. KLH and EAE immunizations occurred the day following CD4+ transfer.
KLH Immunization.
CD4+-reconstituted Rag1−/− animals were s.c. immunized with KLH (0.5 mg/mL; Sigma) emulsified in complete Freund's adjuvant (CFA) (0.5 mg/mL) at the base of the tail. Seven days following immunization, DLN and spleen were harvested for analysis detailed in SI Materials and Methods.
Induction of EAE.
EAE was induced using 150 μg MOG35–55 peptide (RS Synthesis) emulsified in CFA (5 μg/mL) as described in SI Materials and Methods.
Bone Marrow Reconstitution.
Tibias and femurs from B6SJL and Tlr4−/− mice were flushed with PBS. Bone marrow was then filtered, depleted of erythrocytes, and washed 3× in PBS. Bone marrow from each strain was pooled in a 1:1 ratio and 10 × 106 cells were injected i.v. into the tail veins of sublethally irradiated Rag1−/− hosts.
mRNA Analysis.
CNS and γ/δ T cell mRNA was purified and analyzed as described in SI Materials and Methods.
Statistical Analysis.
Student's t tests were performed to analyze statistical significance. P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank the entire C.D. laboratory and the flow-cytometry core at the MD Anderson Cancer Center for their help. J.M.R. was supported by National Cancer Institute training Grant T32CA009598. This work is supported by research grants from the National Institutes of Health (to C.D.). C.D. is a Leukemia and Lymphoma Society Scholar.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120585109/-/DCSupplemental.
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.MacLeod H, Wetzler LM. T cell activation by TLRs: A role for TLRs in the adaptive immune response. Sci STKE. 2007;2007:pe48. doi: 10.1126/stke.4022007pe48. [DOI] [PubMed] [Google Scholar]
- 3.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci USA. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercier BC, Cottalorda A, Coupet CA, Marvel J, Bonnefoy-Bérard N. TLR2 engagement on CD8 T cells enables generation of functional memory cells in response to a suboptimal TCR signal. J Immunol. 2009;182:1860–1867. doi: 10.4049/jimmunol.0801167. [DOI] [PubMed] [Google Scholar]
- 5.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds JM, et al. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 2010;32:692–702. doi: 10.1016/j.immuni.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong C, Flavell RA. Cell fate decision: T-helper 1 and 2 subsets in immune responses. Arthritis Res. 2000;2:179–188. doi: 10.1186/ar85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 10.Yang XO, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lees JR, Golumbek PT, Sim J, Dorsey D, Russell JH. Regional CNS responses to IFN-gamma determine lesion localization patterns during EAE pathogenesis. J Exp Med. 2008;205:2633–2642. doi: 10.1084/jem.20080155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong C. Regulation and pro-inflammatory function of interleukin-17 family cytokines. Immunol Rev. 2008;226:80–86. doi: 10.1111/j.1600-065X.2008.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamazaki T, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerfoot SM, et al. TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. J Immunol. 2004;173:7070–7077. doi: 10.4049/jimmunol.173.11.7070. [DOI] [PubMed] [Google Scholar]
- 15.Heldwein KA, et al. TLR2 and TLR4 serve distinct roles in the host immune response against Mycobacterium bovis BCG. J Leukoc Biol. 2003;74:277–286. doi: 10.1189/jlb.0103026. [DOI] [PubMed] [Google Scholar]
- 16.van de Veerdonk FL, et al. Mycobacterium tuberculosis induces IL-17A responses through TLR4 and dectin-1 and is critically dependent on endogenous IL-1. J Leukoc Biol. 2010;88:227–232. doi: 10.1189/jlb.0809550. [DOI] [PubMed] [Google Scholar]
- 17.Marta M, Andersson A, Isaksson M, Kämpe O, Lobell A. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur J Immunol. 2008;38:565–575. doi: 10.1002/eji.200737187. [DOI] [PubMed] [Google Scholar]
- 18.Waldner H, Collins M, Kuchroo VK. Activation of antigen-presenting cells by microbial products breaks self tolerance and induces autoimmune disease. J Clin Invest. 2004;113:990–997. doi: 10.1172/JCI19388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyakkoku K, et al. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 2010;171:258–267. doi: 10.1016/j.neuroscience.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 20.González-Navajas JM, et al. TLR4 signaling in effector CD4+ T cells regulates TCR activation and experimental colitis in mice. J Clin Invest. 2010;120:570–581. doi: 10.1172/JCI40055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanin-Zhorov A, et al. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J Immunol. 2007;179:41–44. doi: 10.4049/jimmunol.179.1.41. [DOI] [PubMed] [Google Scholar]
- 22.Hirota K, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Codarri L, et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 24.El-Behi M, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zozulya AL, Wiendl H. The role of regulatory T cells in multiple sclerosis. Nat Clin Pract Neurol. 2008;4:384–398. doi: 10.1038/ncpneuro0832. [DOI] [PubMed] [Google Scholar]
- 26.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds JM, Angkasekwinai P, Dong C. IL-17 family member cytokines: Regulation and function in innate immunity. Cytokine Growth Factor Rev. 2010;21:413–423. doi: 10.1016/j.cytogfr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 29.Schwacha MG, Daniel T. Up-regulation of cell surface toll-like receptors on circulating gammadelta T-cells following burn injury. Cytokine. 2008;44:328–334. doi: 10.1016/j.cyto.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui Y, Kang L, Cui L, He W. Human gammadelta T cell recognition of lipid A is predominately presented by CD1b or CD1c on dendritic cells. Biol Direct. 2009;4:47. doi: 10.1186/1745-6150-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Stumhofer JS, Silver J, Hunter CA. Negative regulation of Th17 responses. Semin Immunol. 2007;19:394–399. doi: 10.1016/j.smim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.