Hepatocellular carcinoma (HCC) is a common and highly lethal tumor that is currently the third-leading cause of cancer-related deaths (1). Hepatitis B virus (HBV) is responsible for more than 50% of HCC cases worldwide, making it the second most important known carcinogen for all types of cancer. Although prevention of HBV infection by implementation of universal infant vaccination strategies is starting to have an impact on the subsequent incidence of HCC (1), there remains a huge burden of disease in store for the 400 million people estimated to be already infected with this virus. There is therefore a pressing need to develop a better understanding of how HBV infection triggers the development of HCC, so that earlier intervention can prevent this complication of persistent infection. Remarkable findings by Sitia et al. in PNAS (2) shed new light on the pathogenesis of HBV-related liver cancer, revealing a surprising effect of antiplatelet drugs in preventing its development.
HBV is a hepatotropic DNA virus that is not directly cytopathic; the resultant liver disease is instead mediated by the immune responses it triggers (3). HBV infects only humans and chimpanzees, making it challenging to decipher the immune correlates of viral control and disease pathogenesis. Many mechanistic insights have come from the application of mouse models such as the one used by Sitia et al. (2), in which an HBV transgene is expressed at high level in the liver, and the resultant tolerance of the immune system is overcome by transfer of HBV-primed T cells from a nontransgenic syngeneic strain (4). Taken together, studies in these different hosts have concluded that the HBV-specific CD8 T-cell response is a key player in triggering viral control and liver disease. A strong, functionally efficient CD8 T-cell response is a pivotal component of the coordinated immune response able to control acute HBV infection (5). By contrast, an inadequate virus-specific T-cell response in the setting of persistent infection can trigger recurrent hepatocyte damage, amplified by the influx of a large non–antigen-specific inflammatory infiltrate (6, 7). Hepatocyte death initiates the laying down of scar tissue, resulting in progressive hepatic fibrosis and eventually cirrhosis, whereas hepatocyte regeneration in an inflammatory milieu promotes the development of procarcinogenic mutations and ultimately HCC.
So where do platelets come into this scenario? Previous groundbreaking work from Iannacone et al. (8) revealed an unprecedented role for activated platelets in mediating CTL-induced liver damage in mouse models of acute viral hepatitis. In the study of Sitia et al. (2), from the same laboratory, the authors go on to show that platelet activation is a critical driver of the fatal sequelae of chronic HBV infection. To do this, Sitia et al. (2) take advantage of a mouse model that has previously been shown to allow persistent, high-level expression of the HBsAg transgene in all hepatocytes, and to result in the development of high rates of HCC in a CD8 T cell-dependent manner (9). In this elegant study, in 540 mice carefully monitored for their lifetime, Sitia et al. (2) demonstrate that the administration of drugs able to block platelet activation [aspirin and clopidogrel (Asp/Clo)] potently reduces the development of HCC, thereby markedly enhancing survival. The development of cirrhosis cannot be assessed in this model, but, importantly, the authors also observe significant amelioration in the progression of liver fibrosis. Reductions in these outcomes are accompanied by a decrease in the number of HBV-specific CD8 and nonspecific inflammatory cells accumulating in the livers of the transgenic mice expressing high levels of the HBV surface protein (i.e., HBsAg) (Fig. 1) (2). Although platelets could have procarcinogenic effects independent of T cells, Sitia et al. (2) show that antiplatelet drugs are unable to block carcinogenesis in a model of liver cancer that is not dependent on an inflammatory infiltrate (2). They therefore conclude that the capacity of Asp/Clo to block the intrahepatic accumulation and/or expansion of CTL is responsible for the striking reductions in HCC.
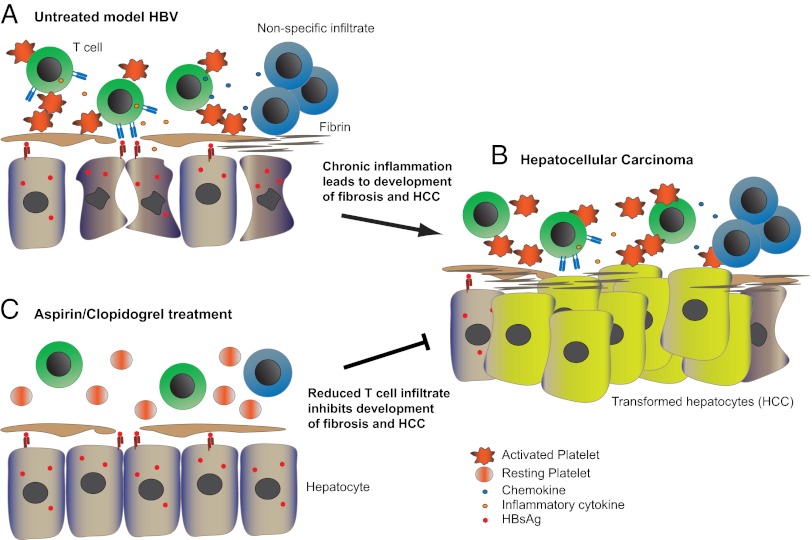
Fig. 1.
Inhibiting platelet activation can ameliorate the development of HBV-induced immune-mediated HCC. (A) Activated platelets promote the accumulation of HBsAg-specific CD8 T cells in the liver. Upon recognition of hepatocytes expressing HBV antigen, specific T cells produce inflammatory cytokines, inducing chemokines that recruit additional antigen-nonspecific immune cells. (B) Repetitive cycles of hepatocyte death and regeneration in the milieu of chronic liver inflammation lead to the development of fibrosis and HCC. Transformed hepatocytes lose the expression of viral antigens and can no longer be recognized by CD8 T cells. (C) Suppressing platelet activation by Asp/Clo treatment significantly decreases the number of HBV-specific CD8 T cells and secondary nonspecific infiltrate, ameliorating liver injury and fibrosis and reducing the development of HCC.
This work makes an exciting addition to the growing body of literature revealing an unappreciated role for platelets within the immune system. Platelets are tiny (2 μm) anucleate cells, but their simple structure is deceptive; they express mRNA and can synthesize nascent proteins as well as storing those they have taken up (10). Platelets have a large number of intracellular granules containing immunomodulatory ligands, cytokines, and chemokines; they can secrete more than 300 different proteins, many of which are not involved in their primary function of hemostasis (11). Because they are so numerous and have a low threshold for activation, they have been proposed to perform a sentinel function within the immune system, acting as pivotal mediators of cellular communication. As they do not leave the circulation, the main opportunity for platelets to interact with immune cells is thought to arise in the liver and spleen, where they may become activated in response to damaged endothelium. The mechanism by which platelets can interact with T cells to enhance their local accumulation and promote pathologic processes in the setting of HBV remains to be elucidated. One plausible candidate is CD154 (CD40 ligand), which is abundantly expressed by activated platelets, in amounts shown to be sufficient to augment lymphocyte function (12) in addition to triggering an inflammatory reaction in endothelial cells (13). Interestingly, platelets are also estimated to be the major repository for TGF-β in the body (11), raising the possibility that antiplatelet therapy could diminish the release of this profibrogenic cytokine. Thus, in addition to promoting the clearance of a number of pathogens (11), platelets are emerging as potent drivers of immune-mediated tissue damage.
A caveat of the study by Sitia et al. (2) is the fact that the transgenic mouse model that had to be used to allow the development of HCC expressed only HBsAg, precluding assessment of the impact of the full virion. A number of viral factors, such as host cell genomic integration and transactivation of oncogenes by other viral proteins have been implicated in the development of HCC (3, 4). The mouse model used by Sitia et al. (2, 9) suggests that the immune response to HBV is necessary and sufficient to trigger liver cancer. However, viral factors may play an important secondary role, promoted by the chronic inflammatory environment. This model also does not allow any assessment of the negative impact that antiplatelet drugs, and the consequent reduction in numbers of HBV-specific T cells, might have on viremia and infectivity. Similarly, the possibility that Asp/Clo will reduce T cells able to provide tumor surveillance needs to be considered; it is possible that the lack of HBsAg expression by HCC cells noted in this study (2) is the end result of T cell-driven cancer immunoediting.
An important aspect of the study by Sitia et al. (2) is that treatment was started only after inflammation was established, making it applicable to the clinical situation, in which many patients with HBV are under surveillance before the onset of significant immunopathologic conditions requires the commencement of treatment. The decrease in viral load achieved with existing nucleos(t)ide inhibitors reduces all complications of HBV-induced liver disease but certainly cannot eliminate the risk of HCC (1); in addition, long-term suppressive therapy with these agents is currently too expensive for many of the countries in which HBV is most prevalent. Could antiplatelet drugs constitute a simple, well-tolerated, and cheap additional approach to the prevention of HCC? The use of drugs that antagonize platelet aggregation seems counterintuitive in patients with liver disease, who are already considered to be at increased risk of bleeding. However, recent work suggests that liver disease may conversely increase thrombotic tendencies, perhaps justifying trials of antiplatelet drugs in these patients (14). Blocking platelet activation also carries the risk of interfering with their emerging protective roles against various infections, as exemplified by the ability of aspirin to block platelet-mediated killing of malaria parasites (15). Weighed against this, antiplatelet drugs are widely used to prevent cardiovascular disease, and, in such studies, have additionally been found to reduce the rates of overall cancer deaths (16). Prospective studies to specifically address the effects of Asp/Clo on the development of HCC in patients with chronic HBV infection will require extended follow-up, but viral load, and the progression of fibrosis, will be easier outcomes to measure. Initial studies could use the woodchuck hepatitis virus model (closely related to HBV) to assess the impact of antiplatelet drugs on viremia, liver inflammation, and HCC.
HCC arises in the context of chronic necroinflammatory liver disease caused by a number of agents in addition to HBV, including HCV and alcohol. The striking effects of antiplatelet drugs demonstrated by Sitia et al. (2) should prompt investigation of their effects in these related pathological situations and inspire further research into the underlying mechanisms of interactions between platelets and T cells.
Acknowledgments
Work in the authors' lab is supported by Medical Research Council, United Kingdom.
Footnotes
References
- 1.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nature reviews. Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sitia G, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci USA. 2012;109:E2165–E2172. doi: 10.1073/pnas.1209182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dandri M, Locarnini S. New insight in the pathobiology of hepatitis B virus infection. Gut. 2012;61:6–17. doi: 10.1136/gutjnl-2012-302056. [DOI] [PubMed] [Google Scholar]
- 4.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 5.Thimme R, et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kakimi K, et al. Blocking chemokine responsive to gamma-2/interferon (IFN)-gamma inducible protein and monokine induced by IFN-gamma activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J Exp Med. 2001;194:1755–1766. doi: 10.1084/jem.194.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maini MK, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iannacone M, et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11:1167–1169. doi: 10.1038/nm1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler P, Chisari FV. Immune pathogenesis of hepatocellular carcinoma. J Exp Med. 1998;188:341–350. doi: 10.1084/jem.188.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denis MM, et al. Escaping the nuclear confines: Signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 12.Elzey BD, et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 13.Henn V, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 14.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–156. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 15.McMorran BJ, et al. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 2009;323:797–800. doi: 10.1126/science.1166296. [DOI] [PubMed] [Google Scholar]
- 16.Rothwell PM, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: Analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]