Abstract
Graphene-related materials are in the forefront of nanomaterial research. One of the most common ways to prepare graphenes is to oxidize graphite (natural or synthetic) to graphite oxide and exfoliate it to graphene oxide with consequent chemical reduction to chemically reduced graphene. Here, we show that both natural and synthetic graphite contain a large amount of metallic impurities that persist in the samples of graphite oxide after the oxidative treatment, and chemically reduced graphene after the chemical reduction. We demonstrate that, despite a substantial elimination during the oxidative treatment of graphite samples, a significant amount of impurities associated to the chemically reduced graphene materials still remain and alter their electrochemical properties dramatically. We propose a method for the purification of graphenes based on thermal treatment at 1,000 °C in chlorine atmosphere to reduce the effect of such impurities on the electrochemical properties. Our findings have important implications on the whole field of graphene research.
Keywords: electrochemistry, synthesis
Graphene and graphene-derived materials have recently attracted enormous attention from the scientific community because of their extraordinary physical, chemical, and mechanical features (1, 2). Graphene materials can be used in several applications—including electronics (3), composite materials (4, 5), sensing (6), energy storage (7, 8), and medicine (9)—with expected or known advantages over conventional materials.
In general, there are two routes leading to the production of graphene: (i) a bottom-up approach, consisting of growing single/bilayered graphene onto a catalytic surface through chemical vapor deposition (CVD) technique (10, 11); and (ii) a top-down approach, starting from graphite to obtain single/few-layered graphene sheets by an exfoliation procedure (12, 13). Because exfoliation in the liquid phase is hardly achieved directly on graphitic materials because of the highly cohesive van der Waals forces between the graphene sheets (14), a chemical treatment is generally performed to oxidize graphite to graphite oxide (GO). The oxidation helps to increase the graphene interlayer distance for an easy exfoliation, which is then followed by the removal of the oxygen functionalities to give single/few-layered graphene (12). The second approach received particularly huge attention because it is suitable for large-scale production of graphene materials and is cost-effective, although the graphene produced presents significant structural defects and lower carrier mobility properties (12). Natural graphite is the preferred starting material for this method of preparation because it is available in great quantities and at a low cost. Alternatively, synthetic graphite is also widely adopted as a starting material. It is important to highlight the differences between these two graphitic materials with particular focus on the content of metallic impurities and possible sources of contamination.
Natural graphite is mined using standard hard or soft rock-mining techniques. If the ore is hard rock, it must be drilled, blasted, and then crushed in a mill. Soft rock ore can literally be dug and transported like sand directly to the floatation plant. The floatation process relies on the differences in surface chemistry between the soil rock and the graphite mineral to be extracted (15). In a water dispersion of the rock/graphite mixture, certain floatation agents that bind specifically to the graphitic material are added. The subsequent injection of air into the water mixture allows graphite to float on the liquid surface, whereas the rock particles sink to the bottom. After floatation, the graphite flakes are washed, dried, and finally packaged for shipment. Although the floatation process is able to remove most of the mineral grains attached “mechanically” to the surface of graphite flakes, it cannot change the purity of the discrete single graphite flake particle. The graphite particle still contains some mineral impurities, which are “intercalated” between groups or stacks of adjacent graphene layers. The intercalated impurities maintain an intimate association with graphite because graphite cannot be removed from the flake by mechanical methods. This type of impurity can only be removed by using chemical or thermal treatments. Graphite flakes in the purity range of 80–98 % have typically been purified using only froth floatation. Flakes above 98% purity require additional purification steps subsequent to floatation (16). Synthetic graphite powders are obtained from the graphitization of selected carbon precursors, like petroleum and coal tar-based cokes, by heat treatment at temperatures above 2,500 °C under an oxygen-free environment. During the heat-treatment process, the amorphous coke material is purified and converted into crystalline carbon. A traditional process exploits the Acheson furnace technology, in which the carbon raw material is positioned between two electrodes and covered by refractory powder material to protect it from oxidation. Electric current is then passed through the carbon bulk, which acts as an electric resistance between the electrodes while generating, by Joule effect, temperatures above 3,000 °C inside the furnace (16). By using high-purity cokes as starting material in the graphitization process, purity levels above 99.9 % can be achieved routinely. The final properties of the obtained synthetic graphite are therefore influenced by the characteristics of the precursor materials and the process parameters. It is, in fact, possible to have a level of contamination of up to 2%, even for synthetic graphite (17–19).
Regardless of the type of graphite, it is important to mention that a milling process is always performed prior to commercialization in order to obtain the desired particle size. This is because different applications are optimized to use only specific graphite particle sizes. Despite a relatively low value of hardness (0.5–1 on the Mohs scale), milling of graphite to fine particle sizes is very difficult because of its flaky shape and the strength of the covalent bonds within the graphene sheets. Such milling process may represent an important source of metallic contamination depending on the desired size of the final product. For sizes above 100 μm, conventional hammer mills and industrial sieving equipment can obtain the final product with low energy input and a low contamination level. On the other hand, when finer particle sizes (< 44 μm) are needed, the process becomes very energy-consuming and the level of metal contamination increases significantly (16).
It should be highlighted here that the graphene community does not anticipate metallic impurities to be present in graphenes. This is somewhat surprising given the fact that the presence of metallic impurities in graphite has been known for a long time (20, 21). The influence of metallic impurities has already been demonstrated for carbon nanotubes (CNTs) produced by CVD on metallic nanoparticle catalysts. It was clearly shown that residual metallic impurities present in CNT samples can alter and even dominate their electronic (22, 23), electrochemical (24–27), redox (28–30), adsorption (31) and toxicological properties (32, 33). However, research on the effects of impurities in graphene and graphene-related materials is still in its earliest stage despite the important implications of these impurities—implications including the possibility of altering the electrochemical and electronic behavior or toxicity of graphenes because these materials are being used as electrode surfaces (6) or tracers in cancer imaging (34).
It was shown by theoretical modeling that the interaction of transition metal impurities with graphene may alter its electronic properties dramatically (35, 36). We recently demonstrated that metallic impurities present in synthetic graphite still remain and significantly interfere with the electrochemistry of related graphene materials produced by means of thermal reduction/exfoliation of graphite oxide (37). Here, we investigate the metallic-impurities content of both natural and synthetic graphite used as precursors for the preparation of chemically reduced graphene materials. We follow the variation of such contamination during the synthetic procedures consisting of (i) oxidation of graphite to graphite oxide, and (ii) chemical reduction of graphite oxide to chemically reduced graphene (CRG) by means of hydrazine. We show that a significant amount of metallic impurities still remains at the end of the process and that these impurities could dramatically influence the electrochemical properties of the graphene materials. In particular, we demonstrate that the most abundant impurities, Fe and Ni, display prominent catalytic effects with consequently possible important implications in toxicological events.
Results and Discussion
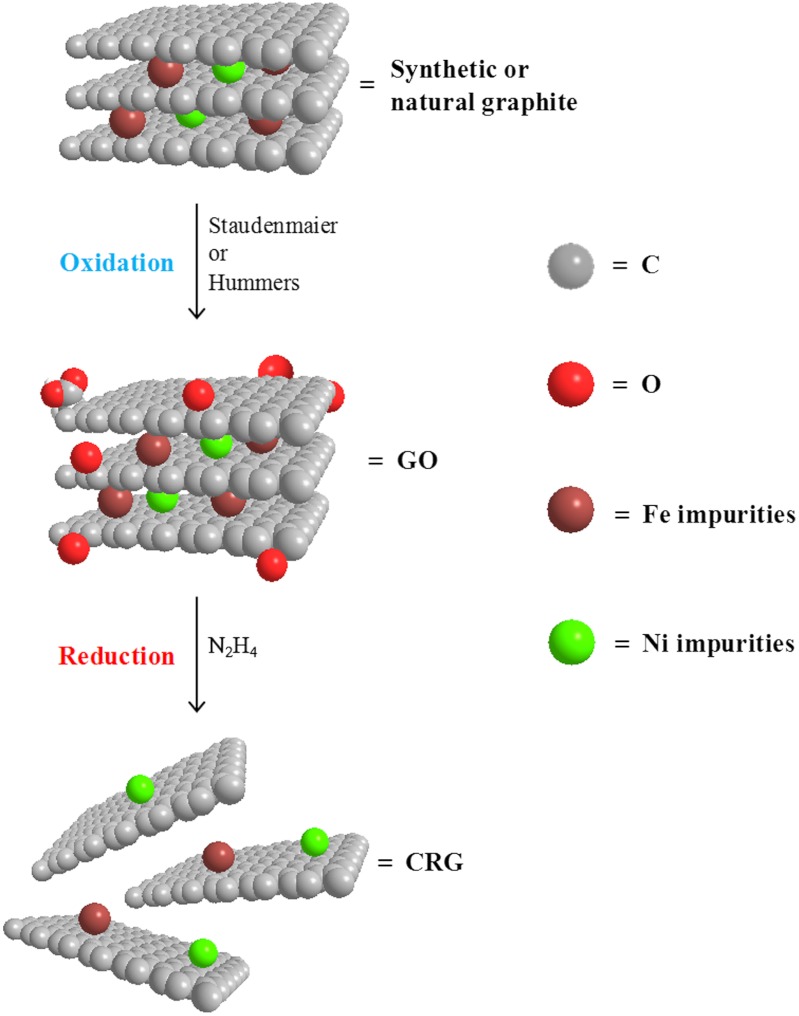
The solution-based production of graphene materials follows the steps illustrated in Fig. 1. Graphite starting material (synthetic or natural) is oxidized to graphite oxide in order to introduce oxygen functionalities, which facilitate the exfoliation of the graphene sheets. Such oxidative treatment is typically carried out using the Hummers or Staudenmaier methods (see Materials and Methods), which differ on the oxidation agent used. After the exfoliation of graphite oxide, a chemical reduction is performed to eliminate the oxygen functionalities and to obtain single/few-layered graphene sheets.
Fig. 1.
Schematic for the preparation of chemically reduced graphene. Synthetic or natural graphite are preliminarily oxidized to GO using the modified Hummers or Staudenmaier methods. CRG is obtained by the chemical reduction of GO using hydrazine. Metallic impurities (Ni, Fe) present in graphite still remain after the chemical treatments.
Using inductively coupled plasma mass spectrometry (ICP-MS) technique, we assessed the starting metallic-impurities content of synthetic (S) and natural (N) graphite samples as well as the variation of the impurities after the preliminary oxidation process to graphite oxide and, finally, after the chemical reduction to graphene. Table 1 summarizes the results for the analyses. In order to obtain a complete set of information, we decided to perform the oxidation procedure using both the Hummers and Staudenmaier methods. For the final reduction, hydrazine was employed for both materials following an identical procedure. It can be seen in Table 1 that synthetic graphite, as expected, contains 10 to 100 times lower concentrations of impurities compared to natural graphite, with the presence of Fe and Ni as the most abundant elements in both cases. With regard to the oxidative procedures, it can be noted that, by using the Staudenmaier method on synthetic graphite, only slight changes in the metallic contents resulted, with all elements remaining at the same order of magnitude as the precursor graphite. However, substantial decreases in the metallic contents were observed with the Hummers method on natural graphite. The element concentrations were, in fact, approximately 10 times lower than the parental graphite. Interestingly, both the graphite-oxide materials showed very similar metal contents for all elements with the exception of Fe, which was approximately 10 times higher for GO produced from natural graphite. Proceeding with the chemical reduction of graphite oxide, we noticed increments of the metal contents. In order to seek possible sources of contamination, we carefully analyzed all the reagents involved in the oxidation as well as the reduction procedure by ICP-MS (Table S1). We found that none of the chemicals used in the procedures had significant amounts of metal impurities (< detection limit) or metal contents in the order of parts per billion. The increase in the amount of metallic impurities after the chemical reduction can only be explained by the fact that some of the impurities present in both the synthetic and natural graphite are strongly intercalated within the graphitic structure and cannot be released by the microwave acid-based digestion performed prior to the ICP-MS analyses. We suggest that the ultrasonication treatments performed to exfoliate GO before the chemical reduction could have possibly exposed those trapped impurities. In order to verify such hypothesis, we repeated the ICP-MS analysis of the GO samples (synthetic and natural) with inclusion of a 2-h ultrasonication treatment prior to the digestion steps. Using this method, the metallic-impurities content of the GO samples almost matched (or were in the same order of magnitude) that of the CRG samples, thus confirming that a significant portion of impurities were trapped within the graphitic structure (Table S2).
Table 1.
Metallic-impurities content (ppm) in synthetic and natural graphite, GO, and CRG materials as determined by ICP-MS analysis
| Fe | Co | Cu | Mo | Ni | |
| Graphite synthetic | 55.2 ± 1.3 | 0.03 ± 0.01 | 1.2 ± 0.2 | 0.45 ± 0.01 | 2.9 ± 0.6 |
| GO (Staudenmaier) | 39.0 ± 3.7 | 0.05 ± 0.01 | 3.0 ± 0.6 | 1.5 ± 0.2 | 3.17 ± 0.02 |
| S-CRG | 108.9 ± 16.7 | 0.07 ± 0.01 | 16.7 ± 0.3 | < DL | 20.2 ± 1.0 |
| Graphite natural | 4224 ± 250 | 3.30 ± 0.02 | 15.1 ± 0.7 | 18.1 ± 0.3 | 33.7 ± 2.8 |
| GO (Hummers) | 529.6 ± 32.4 | 0.09 ± 0.01 | 3.7 ± 0.8 | 1.5 ± 0.2 | 5.2 ± 0.6 |
| N-CRG | 927.6 ± 159.9 | 0.27 ± 0.01 | 16.6 ± 1.0 | < DL | 18.0 ± 1.7 |
DL, detection limit; ±, standard deviation.
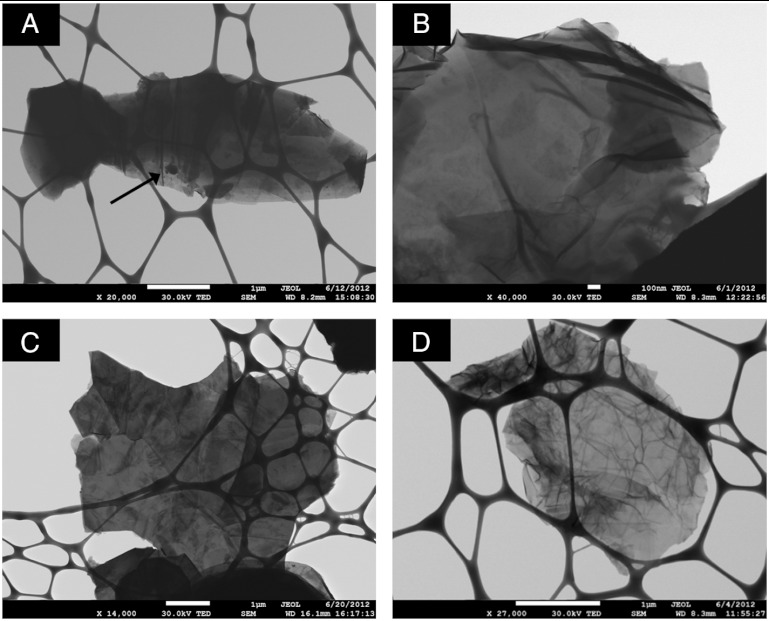
We adopted scanning transmission electron microscopy with energy-dispersive X-ray spectroscopy (STEM/EDS) to image the studied materials. It can be seen in Fig. 2 that the natural and synthetic graphite (Fig. 2 A and C, respectively) showed multilayered structures with mostly smooth basal planes and sharp edges. The chemically reduced materials N-CRG and S-CRG (Fig. 2 B and D, respectively) presented exfoliated morphologies with single/few-layered sheet morphology and several wrinkles on the surfaces, as typically observed on graphene materials derived from graphite oxide. An iron-based metallic impurity is also visible in Fig. 2A (natural graphite), as indicated by the arrow. EDS analysis confirmed the presence of Fe (Fig. S1).
Fig. 2.
STEM images of (A) natural graphite, (B) chemically reduced graphene produced from natural graphite, (C) synthetic graphite, and (D) chemically reduced graphene produced from synthetic graphite. (A) The dark spot indicated by the arrow represents Fe-based metallic impurity. Scale bars, 1 μm (A, C, and D) and 100 nm (B).
Based on the ICP-MS results, it is clear that the metallic impurities, in particular Fe and Ni, are still present after the procedures followed to fabricate graphene materials. Such metal contaminations can dramatically influence the electrochemical behavior of the materials. It has already been demonstrated, in fact, how metallic impurities influence the electrochemical properties of carbon nanotubes (24–28), even at ppm levels (38). We recently demonstrated an altered electrochemical behavior of thermally exfoliated graphene by comparing the electrochemistry of such material with that of edge-plane–pyrolitic graphite electrode (EPPG) (37). It is well-known that the electrochemistry of graphene and graphene-based materials resembles that of the EPPG electrode (39, 40) because of the high density of defects and edge-plane sites available in such materials. The heterogeneous electron transfer (HET) at edge sites of carbon materials is a factor 107 faster than that at the basal-plane sites; therefore, the EPPG electrode, with only exposed edge-plane sites of the graphitic carbon material, represents the perfect reference system for comparison (39). In this way, any possible deviations from the electrochemistry of the EPPG electrode can only be caused by the presence of metallic impurities that are extremely electrochemically active.
In the following text, we will demonstrate the effect of residual Fe impurities on the electrochemistry of chemically reduced graphene materials using cumene hydroperoxide (CHP) as a molecular probe. Hydrogen peroxide (25) and organic peroxides (41) are known to be very sensitive to the presence of Fe impurities, which catalyze their reduction.
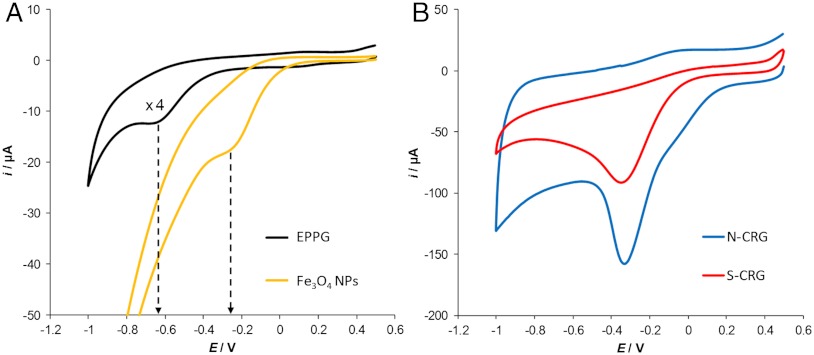
Fig. 3A shows the cyclic voltammetric measurements of 10 mM CHP with EPPG electrode and Fe3O4 nanoparticle-modified glassy carbon (GC) electrode, both acquired after purging the solution with nitrogen to remove any dissolved atmospheric oxygen.
Fig. 3.
Cyclic voltammograms recorded in the presence of 10 mM CHP. (A) Fe3O4 nanoparticle-modified GC electrode and EPPG electrode; (B) electrode modified with chemically reduced graphene obtained from N-CRG and S-CRG. Supporting electrolyte, 50 mM phosphate buffered solution at pH 7.2. Scan rate, 0.1 V/s. Reference electrode, Ag/AgCl.
It can be seen that the reduction peak of CHP occurs at the EPPG electrode at about -0.62 V (versus Ag/AgCl). However, with the Fe3O4-modified GC electrode, the reduction signal is recorded at a more positive potential (-0.25 V), indicating that the Fe3O4 nanoparticles exert a catalytic effect on the reduction of CHP. Such potential shift of almost 400 mV on the reduction of CHP is clear evidence of the catalytic effect of Fe3O4 nanoparticles, which, in other words, facilitate the reduction of CHP to the corresponding alcohol. No catalytic effects resulted from using Co-, Co3O4-, Cu2O-, Ni, NiO-, MoO2-, or MoO3-nanoparticle–modified electrodes (Fig. S2). Subsequently, we proceeded with the cyclic voltammetric measurements of CHP using GC electrodes modified with chemically reduced graphene produced from natural and synthetic graphite. It can be seen in Fig. 3B that the reduction peak for CHP appeared at about -0.32 V and -0.37 V for N-CRG and S-CRG, respectively. It is again evident that a catalytic effect is taking place in such a redox reaction. The reduction peaks from both materials were recorded, in fact, at potentials very close to those obtained with Fe3O4 nanoparticles (Fig. 3A), indicating that a similar catalytic behavior is occurring. Because ICP-MS analysis confirmed the presence of Fe-based impurities within the graphene materials, we can conclude that such impurities are responsible for the altered electrochemical behavior.
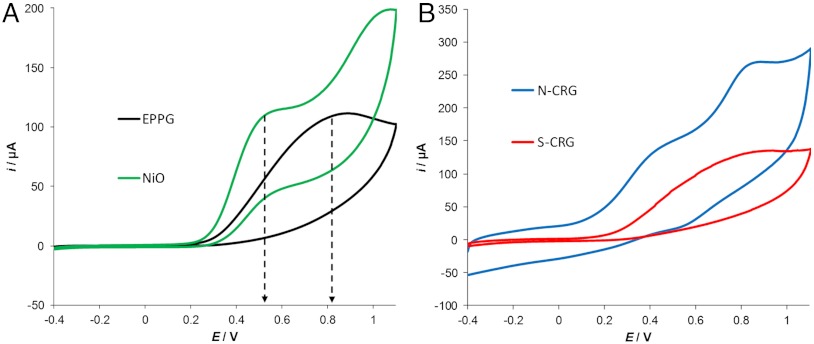
We also tested the effect of metallic impurities towards the oxidation of hydrazine, which is known to be sensitive to metallic impurities that can result in the lowering of its oxidation potential (24, 42). Fig. 4A shows representative cyclic voltammetric measurements in the presence of 5 mM hydrazine using an EPPG electrode and a NiO nanoparticle-modified GC electrode. It can be seen that NiO nanoparticles have a catalytic effect on the oxidation of hydrazine, which resulted in an oxidation potential about 300 mV lower than the signal recorded using the EPPG electrode. By measuring the electrochemical behavior of hydrazine with a GC electrode modified with N-CRG, it could be noted that the oxidative wave presented two features (Fig. 4B). The first signal, occurring at about 0.41 V, can be attributed to the oxidation of hydrazine catalyzed by the presence of metallic impurities within N-CRG. The second signal, with maximum at about 0.8 V, can be attributed to the oxidation of hydrazine over the underlying GC electrode surface. Such a phenomenon is a typical characteristic of heterogeneous electrode surfaces. It is clear that, when using the N-CRG–modified electrode, the oxidation of hydrazine occurs at a lower potential than the EPPG electrode (0.81 V) and at a similar potential compared to the NiO nanoparticle-modified electrode (0.46 V). This is again clear evidence of the presence of metallic impurities within the N-CRG material, which is capable of altering the electrochemical properties.
Fig. 4.
Cyclic voltammograms recorded in the presence of 5 mM hydrazine. (A) NiO nanoparticle-modified GC electrode and EPPG electrode; (B) electrode modified with chemically reduced graphene obtained from N-CRG and S-CRG graphite. Supporting electrolyte, 50 mM phosphate buffered solution at pH 7.2. Scan rate, 0.1 V/s. Reference electrode, Ag/AgCl.
For an S-CRG–modified electrode, the catalytic effect is less evident because a broader oxidation signal was obtained. This could be caused by a lower content of Fe impurities compared to the N-CRG.
An efficient procedure to purify the graphene materials would represent an important contribution to the field. Reducing the amount of metallic impurities to levels at which they have no electrocatalytic effect or trigger no toxicological event while preserving the electronic, electrochemical, and physical properties of the graphene materials is of great interest and importance. To this aim, we selected and investigated the efficiency of three possible purification methods: (i) soaking and refluxing in a concentrated hydrochloric (HCl) and nitric acid (HNO3) mixture; (ii) sonication in a mixture of hydrogen peroxide (H2O2) and HCl; and (iii) thermal treatment in a Cl2 atmosphere. The first two methods have been proposed in the past for the purification of carbon nanotubes (43–45), whereas the thermal treatment is commonly adopted for the purification of graphite (46–48) or other carbonaceous materials (49). We monitored the metallic content, with particular focus on Fe impurities, by means of ICP-MS. We observed that only the thermal treatment at 1,000 °C in Cl2 atmosphere was able to slightly reduce the amount of Fe, from 930 ppm to a value of about 840 ppm, whereas the other two methods resulted in unaltered levels of Fe. Such results confirm that the reduction of metallic content to levels below 100 ppm (0.01 wt%, where they have no catalytic effects; see ref. 38) is an extremely challenging task. To the best of our knowledge, only methods adopting extreme conditions (2,000–3,000 °C in halogen atmosphere) may be able to lower those levels (46–48); however, such methods also cause structural damages with detrimental consequences to the material properties. The liquid-phase purifications in HCl-HNO3 or H2O2-HCl mixtures have been documented to reach levels of purity in the order of 1–5 wt% (10,000–50,000 ppm), two orders of magnitude higher than the content measured in our graphene materials (43–45).
As mentioned above, the metallic-impurities content in our graphene materials decreased by about 90 ppm upon the Cl2 (at 1,000 °C) treatment and was practically unaltered when using the wet chemistry purification methods. We tested whether the metallic impurities in the treated materials were still electrocatalytic towards the reduction of CHP. The materials treated in aqueous-phase purifications (methods 1 and 2) presented an electrochemical behavior almost identical to that of the unpurified materials. However, the graphenes purified by the thermal treatment in Cl2 atmosphere (method 3) showed a significantly reduced electrocatalytic behavior (Fig. S3). The thermally treated materials showed, in fact, a reduction peak for CHP about 200 mV lower than the untreated ones and very close to that of the EPPG electrode. This is likely caused by the removal of surface-accessible metallic impurities while the remaining trace metal impurities are still sheathed by the graphene sheets (i.e., between the graphene layers) and thus electrochemically inactive.
To summarize, we demonstrated that metallic impurities present in both synthetic and natural graphite still remain after their oxidation to graphite oxide by the most common oxidation methods, such as Hummers and Staudenmaier. These impurities persist even after the chemical reduction of graphite oxide, which is employed to fabricate graphene materials. Such metallic impurities are extremely active as catalysts, as we have demonstrated here by means of electrochemical methods. We propose a thermal treatment of graphene in halogen atmosphere as means of purification from the electrochemically active impurities. Because metallic impurities dramatically alter the electronic, electrochemical, redox, catalytic, and toxicological properties of the carbon materials, and because the graphene-researching community seldom takes into account the existence of metallic impurities present in chemically reduced graphenes, our findings could have important implications for the whole graphene field.
Materials and Methods
Natural graphite (flake size, 45 μm) was kindly donated by Asbury Carbons (Asbury, NJ). Synthetic graphite (particle size, < 20 μm), sulfuric acid (95–98%), potassium chlorate (98%), hydrochloric acid (37%), N,N-dimethylformamide (DMF), potassium phosphate dibasic, sodium phosphate monobasic, potassium chloride, nickel oxide (II) nanoparticles, and iron oxide (II, III) nanoparticles (diameter, < 50 nm) were purchased from Sigma-Aldrich. Cumene hydroperoxide (80%) and hydrazine were purchased from Alfa Aesar. Fuming nitric acid (> 90%) and ultrapure nitric acid (for digestions for ICP analysis) were obtained from J.T. Baker. GC and EPPG electrodes with a diameter of 3 mm were obtained from Metrohm Autolab.
Elemental analyses were performed using an Agilent model 7700x ICP-MS, and microwave digestions with concentrated nitric acid were performed on a Mars CEM system. All voltammetric experiments were performed on a μAutolab type-III electrochemical analyzer (Eco Chemie) connected to a personal computer and controlled by General Purpose Electrochemical Systems Version 4.9 software (Eco Chemie). Electrochemical experiments were performed in a 5-mL voltammetric cell at room temperature by using a three-electrode configuration. A platinum electrode (Autolab) served as an auxiliary electrode, whereas an Ag/AgCl electrode (CH Instruments) served as a reference electrode. All electrochemical potentials in this paper are stated versus Ag/AgCl reference electrode.
Samples for ICP analysis were prepared by accurately weighing into a clean Easy Prep vessel followed by addition of 1.5 mL ultrapure HNO3.
Digestion procedure consisted of microwave treatment at 1,600 W (100%), ramping the temperature from 25 °C to 120 °C with 20 min ramping time and maintaining the final temperature for 15 min. Final temperatures of 180 °C and 200 °C were also adopted to examine the influence of temperature and ramping time on elements determination.
The calculated results showed good agreement between the different methods and their replicates.
Natural graphite and synthetic graphite were tested as received with no further treatment.
Graphite oxide from synthetic graphite (S-GO) was prepared according to the Staudenmaier method (50). Thus, 17.5 mL of sulphuric acid (95–98%) and 9 mL of nitric acid (fuming) were added to a reaction flask (round bottom) containing a magnetic stir bar and cooled by immersion in an ice bath for 15 min. Next, 1 g of graphite was added to the mixture under vigorous stirring to obtain a homogeneous dispersion. While keeping the reaction flask in the ice bath, 11 g of potassium chlorate were added to the mixture over 15 min in order to avoid any sudden increment in temperature and formation of explosive chlorine dioxide gas. After the complete dissolution of potassium chlorate, the reaction flask was loosely capped to allow evolution of gas and the mixture was stirred vigorously for 96 h at room temperature. On completion of the reaction, the mixture was poured into 1 L of deionized water and filtered. GO was then redispersed and washed repeatedly in HCl (5%) solutions to remove sulphate ions, and finally washed with deionized water until neutral pH of the filtrate was obtained. Resulting GO powder was then dried in a vacuum oven at 60 °C for 48 h before usage.
Graphite oxide from natural graphite (N-GO) was prepared according to the modified Hummers method: 0.5 g of graphite, 0.5 g of NaNO3, and 23 mL of H2SO4 were stirred together in an ice bath. Next, 3 g of KMnO4 was added slowly to the mixture at 0 °C. Once mixed, the solution was heated to 35 °C and stirred for about 1 h, forming a thick paste. Next, 40 mL of water were added, and the solution was stirred for 30 min while the temperature was raised to 90 °C. The temperature was maintained at 90 °C for 15 min, and then 100 mL of water was added followed by the slow addition of 3 mL of H2O2 (30%), turning the color of the solution from dark brown to yellow, until gas evolution stopped. The warm solution was then filtered and washed with about 100 mL of warm water. The filter cake created was redispersed in water by mechanical agitation. Centrifugation was done at about 6,600 × g for 15 min in water until a neutral pH of the supernatant was obtained. GO powder was finally dried in oven at 60 °C for 2 d before usage.
Chemically reduced graphene was obtained from the natural GO (N-CRG) and synthetic GO (S-CRG) using hydrazine as reducing agent. Dry graphite oxide powder (49.8 mg) was dispersed in ultrapure water to give a 1.0 mg mL-1 colloidal solution and ultrasonicated (150 W) for 3 h. Hydrazine hydrate (2 mL, 32.1 mmol) was added dropwise at 47 °C and the reaction mixture was heated up to 100 °C for 24 h. After cooling to room temperature, the mixture was washed repeatedly with methanol and ultrapure water using centrifugation (8,000 rpm, 10 min). The sample pellet was dried at 60 °C for 2 d prior to usage.
The EPPG and GC electrode surfaces were renewed by polishing with 0.05-mm alumina particles on a cloth and ultrasonicated in ultrapure water for 10 min. Immobilization of the carbon materials onto the working electrode (GC) was performed firstly by preparing a suspension of the desired material with a concentration of 5 mg mL-1 in DMF with a 10-min sonication, followed by a deposition of 3-μL aliquot of the appropriate suspension onto the electrode surface. The solvent was then allowed to evaporate at room temperature. Nanoparticle-modified GC electrodes were prepared in a similar fashion by dispersing nanoparticles in DMF (5 mg mL-1) and subsequent deposition of 3 μL of the dispersed NPs on the GC surface. Cyclic voltammetry experiments were performed at a scan rate of 100 mV s-1 using 50 mM phosphate buffer solution (pH 7.2) as supporting electrolyte and 10 mM CHP or 5 mM hydrazine as molecular probes.
In procedure 1 of purification method using aqua regia, 0.2 mg of N-CRG was dispersed in the mixture of concentrated hydrochloric acid and nitric acid (3∶1 by volume). The mixture was heated under reflux for 1 h. The strong evolution of nitrous gases occurred during the heating. The mixture was allowed to cool at room temperature, and after 4 h the graphene was separated by suction filtration, repeatedly washed with deionized water, and dried under vacuum at 60 °C.
Procedure 2 of purification in hydrochloric acid-hydrogen peroxide mixture consisted of the following steps. About 10 mg of the N-CRG sample was mixed directly with 5 mL of 1-N hydrochloric acid and 5 mL of 30 % H2O2 in a 15-mL glass tube. The suspension was sonicated for 2 h, leaving the sonicator bath temperature to rise up to 50 °C. The N-CRG material was then separated from the mixture by filtration and washed with about 500 mL of ultrapure water. The sample was finally dried at 60 °C for 3 d in a vacuum oven before use.
Procedure 3, based on thermal treatment of graphene, was performed in quartz-glass reactor equipped with magnetic manipulator at the temperature of 1,000 °C∶0.2 g of graphene was placed in a porous quartz-glass capsule connected to magnetic manipulator inside vacuum-tight tube furnace with controlled atmosphere. The reactor was flushed with nitrogen by repeated evacuation of tube furnace to remove any traces of oxygen and quickly inserted by magnetic manipulator to preheated furnace. At first stage the graphene sample was heated in hydrogen-nitrogen atmosphere for 30 min to remove remaining oxygen functionalities after chemical reduction of graphene and to reduce remaining metallic impurities and increase their reactivity toward chlorine. Subsequently, the reactor was repeatedly evacuated and flushed with nitrogen to remove hydrogen from the reactor to avoid danger of explosive reaction of chlorine with hydrogen. The graphene was then treated in chlorine-nitrogen atmosphere for 60 min. The flow of hydrogen and nitrogen during the first step of purification procedure was 500 mL min-1 of each gas (ratio 1∶1), and the flow of chlorine and nitrogen was 1,000 mL min-1 of each gas (ratio 1∶1) during the second step of purification procedure. The purity of hydrogen and nitrogen used in purification procedure was 99.9999%, whereas the purity of chlorine was 99.8% (the residual 0.2% is water vapor).
Supplementary Material
ACKNOWLEDGMENTS.
The authors thank Drs. S. Terrinoni and S. Mazziotti Tagliani for helpful discussions about geology and graphite formation. M.P. thanks the Nanyang Assistant Professorship Start Up Grant (Nanyang Technological University) and Japan Society for the Promotion of Science/Nanyang Technological University fund. Z.S. were supported by the Ministry of Education of the Czech Republic (research projects no. MSM6046137302) and by Specific University Research grant (MSMT No 21/2012).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205388109/-/DCSupplemental.
References
- 1.Novoselov KS, et al. Electric field effect in atomically thin carbon films. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 2.Geim AK, Novoselov KS. The rise of graphene. Nat Mater. 2007;6:183–191. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 3.Rogers JA. Electronic materials: Making graphene for macroelectronics. Nat Nanotechnol. 2008;3:254–255. doi: 10.1038/nnano.2008.115. [DOI] [PubMed] [Google Scholar]
- 4.Stankovich S, et al. Graphene-based composite materials. Nature. 2006;442:282–286. doi: 10.1038/nature04969. [DOI] [PubMed] [Google Scholar]
- 5.Ramanathan T, et al. Functionalized graphene sheets for polymer nanocomposites. Nat Nanotechnol. 2008;3:327–331. doi: 10.1038/nnano.2008.96. [DOI] [PubMed] [Google Scholar]
- 6.Pumera M. Graphene-based nanomaterials and their electrochemistry. Chem Soc Rev. 2010;39:4146–4157. doi: 10.1039/c002690p. [DOI] [PubMed] [Google Scholar]
- 7.Pumera M. Graphene-based nanomaterials for energy storage. Energy Environ Sci. 2011;4:668–674. [Google Scholar]
- 8.Stoller MD, Park SJ, Zhu YW, An JH, Ruoff RS. Graphene-based ultracapacitors. Nano Lett. 2008;8:3498–3502. doi: 10.1021/nl802558y. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008;130:10876–10877. doi: 10.1021/ja803688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science. 2009;324:1312–1314. doi: 10.1126/science.1171245. [DOI] [PubMed] [Google Scholar]
- 11.Reina A, et al. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2008;9:30–35. doi: 10.1021/nl801827v. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Ruoff RS. Chemical methods for the production of graphenes. Nat Nanotechnol. 2009;4:217–224. doi: 10.1038/nnano.2009.58. [DOI] [PubMed] [Google Scholar]
- 13.Zhu YW, et al. Graphene and graphene oxide: Synthesis, properties, and applications. Adv Mater. 2010;22:3906–3924. doi: 10.1002/adma.201001068. [DOI] [PubMed] [Google Scholar]
- 14.Zacharia R, Ulbricht H, Hertel T. Interlayer cohesive energy of graphite from thermal desorption of polyaromatic hydrocarbons. Phys Rev B. 2004;69:155406. [Google Scholar]
- 15.Pierson HO. Handbook of Carbon, Graphite, Diamond, and Fullerenes: Properties, Processing, and Applications. Park Ridge, NJ: Noyes Publications; 1993. [Google Scholar]
- 16.Marsh H, Heintz EA, Rodríguez-Reinoso F. Introduction to Carbon Technologies. Alicante: Universidad de Alicante; 1997. p. 572. [Google Scholar]
- 17.Superior Graphite. MetalPURE (powder metal additive) Online Catalog. 2012. Available at http://graphites-carbons.superiorgraphite.com/item/all-categories/metalpure-powder-metal-additive-/item-1016.
- 18.Cocan Graphite. SupeRecarb: Regular synthetic. Online Catalog. 2012. Available at http://www.cocangraphite.com/EN/Product/SupeRecarb/
- 19.Famous Minerals and Chemicals Private Limited. High purity synthetic graphite. Online Catalog. 2012. Available at http://www.famousminerals.net/high-purity-synthetic-graphite.html.
- 20.McKee DW. Effect of metallic impurities on gasification of graphite in water-vapor and hydrogen. Carbon. 1974;12:453–464. [Google Scholar]
- 21.Heintz EA, Parker WE. Catalytic effect of major impurities on graphite oxidation. Carbon. 1966;4:473–478. [Google Scholar]
- 22.Azevedo S, Chesman C, Kaschny JR. Stability and electronic properties of carbon nanotubes doped with transition metal impurities. Eur Phys J B. 2010;74:123–128. [Google Scholar]
- 23.Odom TW. Electronic properties of single-walled carbon nanotubes. Aust J Chem. 2001;54:601–604. [Google Scholar]
- 24.Banks CE, Crossley A, Salter C, Wilkins SJ, Compton RG. Carbon nanotubes contain metal impurities which are responsible for the “electrocatalysis” seen at some nanotube-modified electrodes. Angew Chem Int Ed. 2006;45:2533–2537. doi: 10.1002/anie.200600033. [DOI] [PubMed] [Google Scholar]
- 25.Sljukic B, Banks CE, Compton RG. Iron oxide particles are the active sites for hydrogen peroxide sensing at multiwalled carbon nanotube modified electrodes. Nano Lett. 2006;6:1556–1558. doi: 10.1021/nl060366v. [DOI] [PubMed] [Google Scholar]
- 26.Batchelor-McAuley C, Wildgoose GG, Compton RG, Shao LD, Green MLH. Copper oxide nanoparticle impurities are responsible for the electroanalytical detection of glucose seen using multiwalled carbon nanotubes. Sensor Actuator B Chem. 2008;132:356–360. [Google Scholar]
- 27.Dai X, Wildgoose GG, Compton RG. Apparent “electrocatalytic” activity of multiwalled carbon nanotubes in the detection of the anaesthetic halothane: Occluded copper nanoparticles. Analyst. 2006;131:901–906. doi: 10.1039/b606197d. [DOI] [PubMed] [Google Scholar]
- 28.Ambrosi A, Pumera M. Regulatory peptides are susceptible to oxidation by metallic impurities within carbon nanotubes. Chem Eur J. 2010;16:1786–1792. doi: 10.1002/chem.200902534. [DOI] [PubMed] [Google Scholar]
- 29.Guo L, et al. Iron bioavailability and redox activity in diverse carbon nanotube samples. Chem Mater. 2007;19:3472–3478. [Google Scholar]
- 30.Liu XY, et al. Bioavailability of nickel in single-wall carbon nanotubes. Adv Mater. 2007;19:2790–2793. [Google Scholar]
- 31.Tian X, et al. Metal impurities dominate the sorption of a commercially available carbon nanotube for pb(II) from water. Env Sci Technol. 2010;44:8144–8149. doi: 10.1021/es102156u. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Guo L, Morris D, Kane AB, Hurt RH. Targeted removal of bioavailable metal as a detoxification strategy for carbon nanotubes. Carbon. 2008;46:489–500. doi: 10.1016/j.carbon.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koyama S, et al. In vivo immunological toxicity in mice of carbon nanotubes with impurities. Carbon. 2009;47:1365–1372. [Google Scholar]
- 34.Hong H, et al. In vivo targeting and imaging of tumor vasculature with radiolabeled, antibody-conjugated nanographene. ACS Nano. 2012;6:2361–2370. doi: 10.1021/nn204625e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu FM, Ma TX, Lin HQ, Gubernatis JE. Magnetic impurities in graphene. Phys Rev B. 2011;84:075414. [Google Scholar]
- 36.Krasheninnikov AV, Nieminen RM. Attractive interaction between transition-metal atom impurities and vacancies in graphene: A first-principles study. Theor Chem Acc. 2011;129:625–630. [Google Scholar]
- 37.Ambrosi A, et al. Metallic impurities in graphenes prepared from graphite can dramatically influence their properties. Angew Chem Int Ed. 2012;51:500–503. doi: 10.1002/anie.201106917. [DOI] [PubMed] [Google Scholar]
- 38.Pumera M, Miyahara Y. What amount of metallic impurities in carbon nanotubes is small enough not to dominate their redox properties? Nanoscale. 2009;1:260–265. doi: 10.1039/b9nr00071b. [DOI] [PubMed] [Google Scholar]
- 39.Davies TJ, Hyde ME, Compton RG. Nanotrench arrays reveal insight into graphite electrochemistry. Angew Chem Int Ed. 2005;44:5121–5126. doi: 10.1002/anie.200462750. [DOI] [PubMed] [Google Scholar]
- 40.Ambrosi A, Bonanni A, Sofer Z, Cross JS, Pumera M. Electrochemistry at chemically modified graphenes. Chem Eur J. 2011;17:10763–10770. doi: 10.1002/chem.201101117. [DOI] [PubMed] [Google Scholar]
- 41.Stuart EJE, Pumera M. Electrochemistry of a whole group of compounds affected by metallic impurities within carbon nanotubes. J Phys Chem C. 2010;114:21296–21298. [Google Scholar]
- 42.Pumera M, Iwai H. Multicomponent metallic impurities and their influence upon the electrochemistry of carbon nanotubes. J Phys Chem C. 2009;113:4401–4405. [Google Scholar]
- 43.Dujardin E, Ebbesen TW, Krishnan A, Treacy MMJ. Purification of single-shell nanotubes. Adv Mater. 1998;10:611–613. [Google Scholar]
- 44.Rinzler AG, et al. Large-scale purification of single-wall carbon nanotubes: Process, product, and characterization. Appl Phys A. 1998;67:29–37. [Google Scholar]
- 45.Wang YH, et al. A highly selective, one-pot purification method for single-walled carbon nanotubes. J Phys Chem B. 2007;111:1249–1252. doi: 10.1021/jp068229+. [DOI] [PubMed] [Google Scholar]
- 46.Ulrich H. 2,914,383. US Patent. 1959
- 47.Graphite Concept Products. Graphitization. Online Catalog. 2012. Available at http://www.graphiteconcept.com/content/view/33/27/
- 48.Büchel KH, Moretto HH, Woditsch P. Industrial Inorganic Chemistry. New York: Wiley; 2008. p. 511. [Google Scholar]
- 49.Im CJ, Durney T, Matuszak ML. Halogen treatment of char for the removal of sulfur and mineral matter. Fuel Proc Technol. 1993;33:49–60. [Google Scholar]
- 50.Staudenmaier L. Verfahren zur darstellung der graphitsäure. Ber Dtsch Chem Ges. 1898;31:1481–1487. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.