Abstract
Voluntary exercise is known to have an antidepressant effect. However, the underlying mechanism for this antidepressant action of exercise remains unclear, and little progress has been made in identifying genes that are directly involved. We have identified macrophage migration inhibitory factor (MIF) by analyzing existing mRNA microarray data and confirmed the augmented expression of selected genes under two experimental conditions: voluntary exercise and electroconvulsive seizure. A proinflammatory cytokine, MIF is expressed in the central nervous system and involved in innate and adaptive immune responses. A recent study reported that MIF is involved in antidepressant-induced hippocampal neurogenesis, but the mechanism remains elusive. In our data, tryptophan hydroxylase 2 (Tph2) and brain-derived neurotrophic factor (Bdnf) expression were induced after MIF treatment in vitro, as well as during both exercise and electroconvulsive seizure in vivo. This increment of Tph2 was accompanied by increases in the levels of total serotonin in vitro. Moreover, the MIF receptor CD74 and the ERK1/2 pathway mediate the MIF-induced Tph2 and Bdnf gene expression as well as serotonin content. Experiments in Mif−/− mice revealed depression-like behaviors and a blunted antidepressant effect of exercise, as reflected by changes in Tph2 and Bdnf expression in the forced swim test. In addition, administration of recombinant MIF protein produced antidepressant-like behavior in rats in the forced swim test. Taken together, these results suggest a role of MIF in mediating the antidepressant action of exercise, probably by enhancing serotonin neurotransmission and neurotrophic factor-induced neurogenesis in the brain.
Keywords: physical activity, major depressive disorder, glycosylation-inhibiting factor
Depression is one of the most common psychiatric disorders, affecting ∼121 million people worldwide and resulting in major social and economic consequences, yet it remains undertreated (1). Although numerous antidepressant drugs have been introduced, limits remain in both their degree of therapeutic efficacy and our understanding of their underlying mechanisms (2, 3). The effectiveness of selective serotonin reuptake inhibitors, which up-regulate synaptic serotonin content, suggests a crucial role of serotonin in the action of antidepressant drugs (4). However, the evidence regarding involvement of other systems, including the noradrenergic, dopaminergic, and glutamatergic systems, in antidepressant action suggests complex neurotransmission. In addition, although hippocampal neurogenesis by antidepressive treatment modalities, including electroconvulsive seizure (ECS), an animal model of electroconvulsive therapy, and various antidepressants, has been suggested to be responsible for the therapeutic effects (5–7), several recent studies have not supported the direct involvement of neurogenesis in antidepressant action (7, 8).
Regular physical activity is associated with diverse human health benefits, including prevention of cardiovascular dysfunction (9), metabolic diseases (10), and mood disorders. In particular, studies involving rodents (11) and humans (12) have suggested that long-term exercise reduces the symptoms and risk of depression. Although exercise therapy has received recent attention for its efficacy in the treatment of depression, the underlying mechanism linking exercise and depression remains unclear. Understanding the detailed mechanisms of the effect of exercise therapy on depression could lead to the identification of important targets for antidepressant drugs. In this study, we focused on the hippocampus, a component of the limbic system that is highly sensitive to environmental and stress hormones and that may play a crucial role in the development and treatment of depression (13).
Several previous studies have reported that exercise-modulated genes in the brain may be responsible for antidepressant-like effects (14, 15). To identify the candidate genes contributing to the antidepressant action of exercise, we compared previously reported mRNA microarray data on the rodent hippocampus after voluntary exercise with data on the rodent hippocampus after ECS, the most effective treatment for depression symptoms. One significantly up-regulated candidate gene was that encoding macrophage migration inhibitory factor (MIF), a proinflammatory cytokine that is highly expressed in immune cells as well as nonimmune cells, including those in the brain (16). The contribution of MIF to antidepressant-induced hippocampal neurogenesis was reported in a recent study (17); however, the underlying mechanism of MIF in hippocampal neurogenesis and its role in exercise-induced antidepressant therapy have not been reported. We subjected rats to 28 d of voluntary exercise or 10 d of repeated ECS treatment and then evaluated the expression of MIF as well as behavioral changes in the rats and mice during the forced swim test (FST). The results revealed a remarkably diminished antidepressant effect of exercise in Mif−/− mice and an enhanced antidepressant effect in MIF-injected rats, along with the induction of brain-derived neurotrophic factor (BDNF) and tryptophan hydroxylase 2 (TPH2) in MIF-infused rat brains and MIF-treated neuronal cell lines. Our results demonstrate an antidepressant-like effect of exercise in rodents as determined by measurements of MIF.
Results
Selection and Validation of Candidate Genes Related to the Antidepressant Action of Exercise.
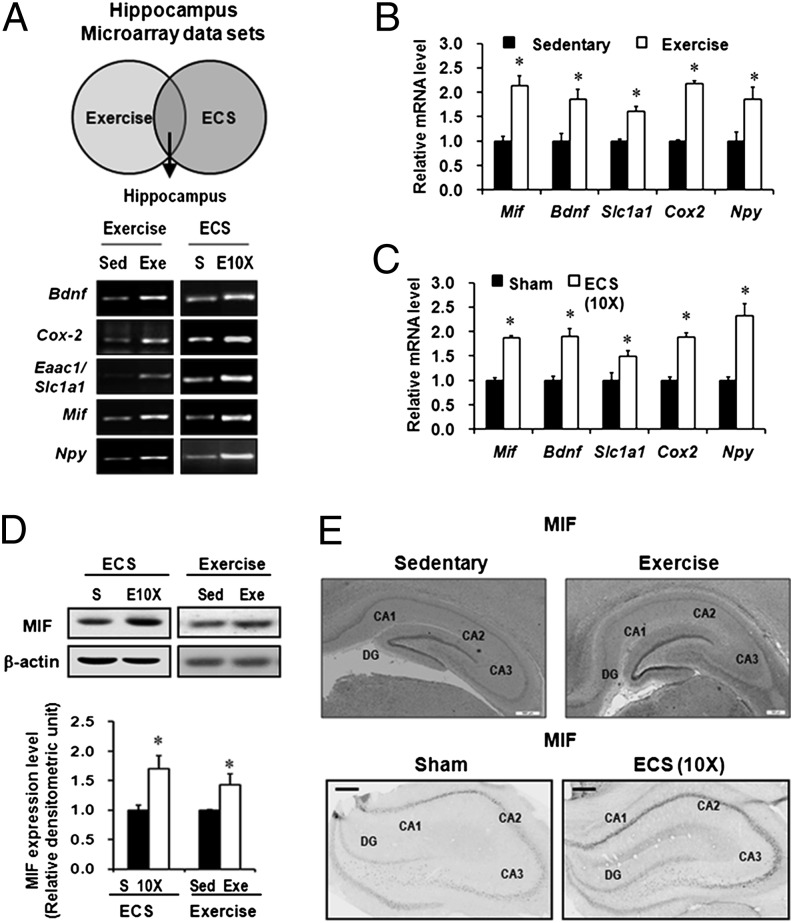
To identify candidate genes possibly contributing to the antidepressant action of voluntary exercise, we first compared previously reported mRNA microarray data on the rodents’ hippocampi after voluntary exercise with those data after ECS. Common differentially expressed candidate genes were selected in both the exercise and ECS experiments (Table S1) (14, 15, 18–22). To validate these genes, hippocampal tissues from rat brain were collected after 28 d of voluntary wheel running or after repeated ECS treatments for 10 d. The RT-PCR results showed that five genes were commonly induced by both long-term exercise and repeated ECS treatments (Fig. S1): Bdnf, cyclooxygenase-2 (Cox-2), glutamate transporter (Slc1a1), neuropeptide Y (Npy), and Mif (Fig. 1A and Fig. S1A). All of these genes were significantly induced by both exercise and ECS, as determined by real-time RT-PCR (P < 0.05 for all; Fig. 2 B and C). Consistent with previous reports (15, 19, 21, 23, 24), the expression of Bdnf, Cox-2, Slc1a1, and Npy increased after voluntary exercise and repeated ECS. In addition, expression of Mif also increased after both treatments. Furthermore, immunoblot analysis confirmed increased MIF protein levels in the rat hippocampi under both long-term exercise and repeated ECS compared with each control group (Fig. 1D). Immunohistochemistry analysis also indicated that the expression of MIF protein increased in the dentate gyrus subfields after long-term exercise (Fig. 1E and Fig. S2A) and repeated ECS (Fig. 1E). However, the protein MIF content in plasma did not differ between the exercised mice and the nonexercised mice (Fig. S2B). Taken together, our observations, the noticeable expression pattern in mossy fibers of the dentate gyrus, and the known biological role in antidepressant-induced hippocampal neurogenesis (17) indicate that MIF may be a feasible candidate protein contributing to exercise-mediated antidepressant activity (25).
Fig. 1.
MIF is induced by long-term voluntary exercise and repeated ECS treatments in rat hippocampus. (A) Scheme and RT-PCR results for selecting candidate genes regulated by both voluntary exercise and ECS. (B and C) Real-time RT-PCR was performed to measure the mRNA expression of five genes among candidate genes in rat hippocampi. mRNA expression was enhanced in long-term exercise-treated rats (28 d; n = 8) and after repeated ECS treatments (10 d; n = 5). (D) Quantitative immunoblotting showed that both exercise and ECS increased the level of MIF protein in the rat hippocampi (n = 4). (E) Immunohistochemistry showing that exercise (28 d) and ECS (10 d) increased MIF immunostaining in the rat hippocampus. Sed, sedentary; Exe, exercise; S, sham. Error bars show mean ± SE. Asterisks indicate statistically significant differences compared with the control groups (P < 0.05).
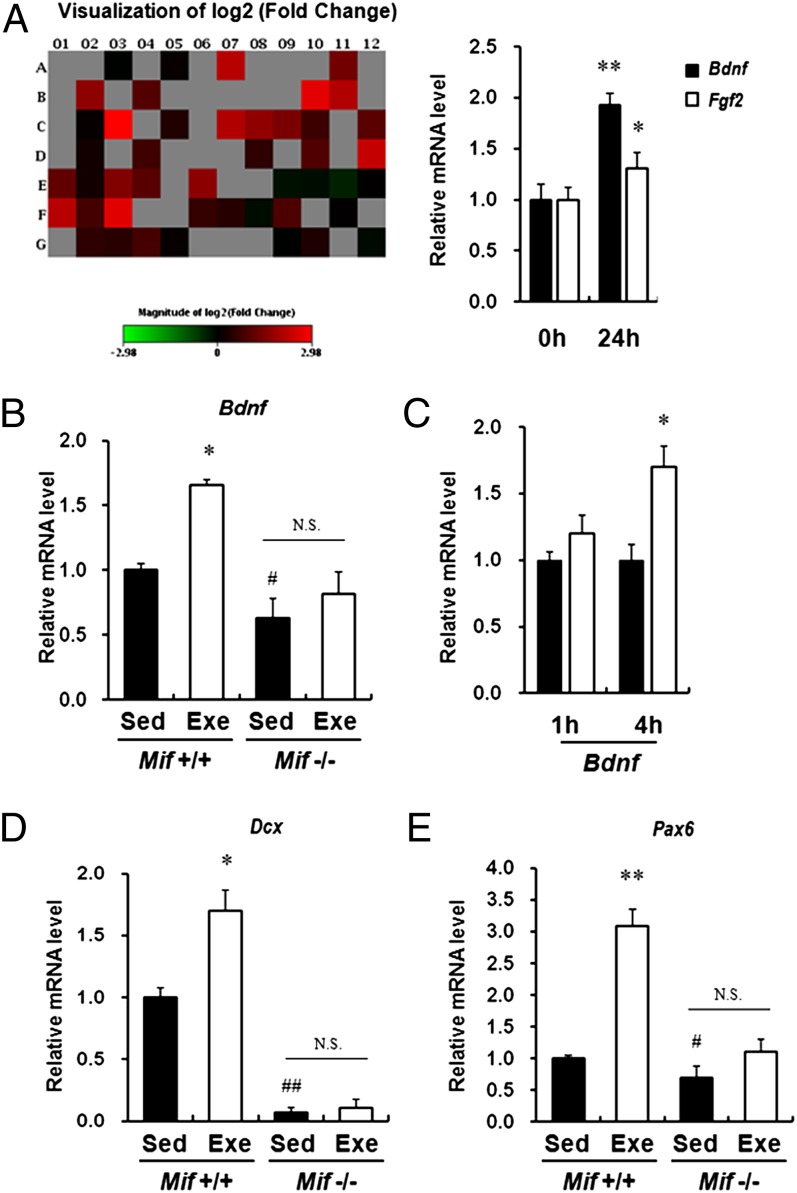
Fig. 2.
MIF increases expression of Bdnf in vitro and in vivo. (A) (Left) Heat maps representing the relative expression levels for all genes (involved in neurogenesis and neuronal stem cells) induced by treatment with MIF (300 ng/mL) for 24 h on the real-time PCR arrays. Data for each gene were normalized to a panel of housekeeping transcripts and are expressed as fold change compared with the vehicle-treated group. (Right) Real-time RT-PCR was performed to measure mRNA expression of the selected MIF-target genes from RT-PCR and the PubMed search, Bdnf and Fgf2. Treatment with recombinant MIF protein (300 ng/mL) for 24 h increased the expression level of Bdnf and Fgf2. Error bars show mean ± SD. *P < 0.05; **P < 0.01 vs. 0 h. (B) Bdnf mRNA expression was reduced in the hippocampi of Mif−/−mice compared with WT littermates. Furthermore, Bdnf mRNA expression level was not significantly changed by long-term exercise in hippocampi of Mif−/−mice compared with sedentary Mif−/−mice (n = 6–8 for each group). Error bars show mean ± SE. #P < 0.05; *P < 0.05. (C) ICV injection of MIF protein increased Bdnf gene expression at 4 h after injection (n = 4–6 for each group). (D and E) Dcx and Pax6 mRNA expression was reduced in the hippocampi of Mif−/− mice compared with WT littermates. In addition, the Dcx and Pax6 mRNA expression level was not changed significantly by long-term exercise in the hippocampus of Mif−/− mice compared with sedentary Mif−/− mice (n = 6–8 for each group). Error bars show mean ± SE. NS, not significant. *P < 0.05; #P < 0.05; ##P < 0.01.
BDNF Levels Are Induced by MIF.
The role of MIF in antidepressant-stimulated adult hippocampal neurogenesis (17) and increased expression after long-term exercise and ECS treatment (26, 27) led us to investigate the molecular mechanism of MIF in neurogenesis. We first measured the effective concentration of recombinant MIF on the neuronal cell response by immunoblot analysis. We then analyzed the expression pattern of genes involved in neurogenesis and neuronal stem cells using PCR array (SABiosciences), and found that 14 genes were elevated by MIF (300 ng/mL) for 24 h after treatment in Neuro-2A cells (Fig. 2A and Fig. S3A). After screening out those selected molecules by searching for words with each gene name and “depression” in PubMed, we identified Bdnf and Fgf2 as the targets of the antidepressant action of MIF.
We further validated and assessed the changes in Bdnf and Fgf2 expression by real-time RT-PCR. Cells were treated with MIF for various durations, and gene expression was evaluated. Bdnf expression increased significantly from 3 h to 24 h after treatment, whereas Fgf2 expression was elevated only at 24 h compared with vehicle treatment (Fig. 2A and Fig. S3B). In addition, we measured BDNF protein levels after MIF treatment using immunoblot analysis (Fig. S3C). To validate the in vitro findings in the in vivo brain samples, we measured the expression levels of Bdnf in hippocampi of Mif−/− mice and MIF-injected rats. Basal expression of Bdnf in hippocampi of Mif−/− mice was reduced compared with that in WT littermates. Furthermore, mRNA expression of Bdnf was also induced by long-term exercise in the hippocampus of WT littermates. However, compared with sedentary Mif−/− mice, long-term exercise did not significantly induce Bdnf mRNA expression in Mif−/− mice (Fig. 2B). Furthermore, mRNA expression of Bdnf increased significantly by 4 h after injection (Fig. 2C). In addition, the mRNA levels of a marker for newborn neurons, Dcx, and a key transcription factor that controls neurogenesis, Pax6, were decreased in Mif−/− mice. Long-term exercise had a marginal effect on the expression of these genes in the hippocampi of Mif−/− mice (Fig. 2D).
These results led us to expand our observations on the effect of MIF on candidate genes induced by both long-term exercise and ECS treatment. We also noted that the expression levels of other selected candidate genes were dynamically regulated by MIF treatment in the neuroblastoma cell line Neuro-2A (Fig. S3B). These findings provide a biological basis for a possible mechanism through which exercise-induced MIF mediates hippocampal neurogenesis.
TPH2 and Brain Serotonin Levels Are Induced by MIF.
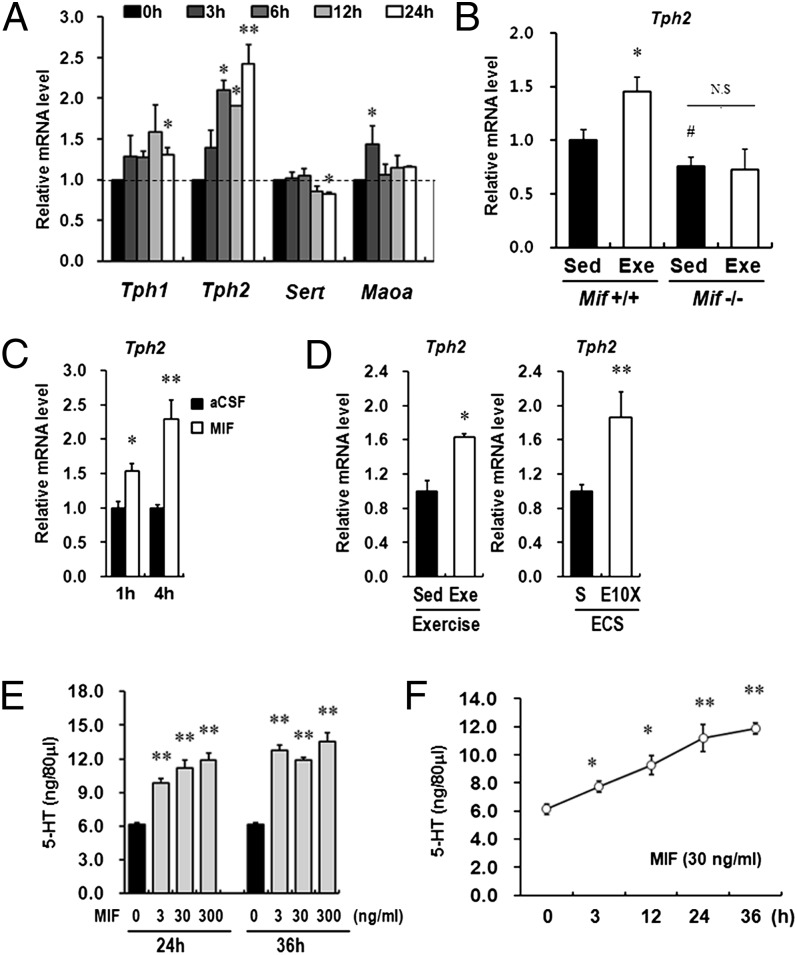
Exercise and ECS treatment have been established to increase brain serotonin (5-HT) through the activation of neurotransmission systems (28, 29). To evaluate whether MIF contributes to 5-HT neurotransmission, we analyzed several genes associated with neurotransmission in antidepressant action, including molecular markers involved in synthesis or transport of 5-HT (4, 30), by real-time RT-PCR. Strikingly, expression of Tph2 increased significantly from 6 h to 24 h after treatment in Neuro-2A cells (Fig. 3A). We also measured TPH2 protein levels via immunoblot analysis after MIF treatment (Fig. S4A). We then measured the expression levels of Tph2 in the hippocampi of Mif−/− mice and MIF-injected rats. Similar to bdnf mRNA expression, basal expression of Tph2 was significantly lower in the hippocampi of Mif−/− mice compared with their WT littermates. Furthermore, mRNA expression of Tph2 was induced by long-term exercise in the hippocampi of WT littermates, but not of Mif−/− mice (Fig. 3B). The expression of Tph2 was increased at 1 h and 4 h after injection (Fig. 3C). In addition, repeated ECS treatments and long-term exercise increased Tph2 expression in rat hippocampi (Fig. 3D).
Fig. 3.
MIF activates the 5-HT system. (A) Treatment with recombinant MIF protein (300 ng/mL) increased the expression level of Tph2 in a time-dependent manner among neurotransmission-related genes. Error bars show mean ± SD. *P < 0.05 vs. 0 h. (B) Tph2 mRNA expression was reduced in the hippocampus of Mif−/− mice compared with WT littermates. In addition, the Tph2 mRNA expression level was not changed significantly by long-term exercise in the hippocampi of Mif−/− mice compared with sedentary Mif−/− mice (n = 6–8 for each group). Error bars show mean ± SE. *P < 0.05; #P < 0.05. (C) ICV injection of MIF protein increases Tph2 gene expression at 1 h and 4 h after injection (n = 4–6 for each group). (D) Both repeated ECS treatments and voluntary exercise increased Tph2 gene expression in the rat hippocampus (n = 4–6 for each group). (E) Treatment with MIF induced a dose-dependent (3, 30, and 300 ng/mL) increase in 5-HT levels in RBL2H3 cells at 24 h and 36 h after treatment (n = 3). (F) MIF (300 ng/mL) treatment induced a time-dependent (3, 12, 24, and 36 h) increase in 5-HT levels in RBL2H3 cells (n = 3). NS, not significant. Error bars show mean ± SD. *P < 0.05; **P < 0.01 vs. 0 h.
Because TPH2 is a critical rate-limiting enzyme in the biosynthesis of 5-HT in the brain, we further characterized the effect of MIF on 5-HT levels (31). In this experiment, we used RBL2H3, a basophilic leukemia cell line, which is the optimal in vitro model system for investigating the serotonin synthesis pathway (32). Before 5-HT measurement, we confirmed that Tph2 expression also increased in a time-dependent manner after MIF treatment in RBL2H3 cells (Fig. S4B). The intracellular 5-HT concentration increased after 24 h of MIF treatment in a dose-dependent manner (Fig. 3D) and tended to be saturated at 36 h. In accordance with the enhanced Tph2 gene expression, the total 5-HT concentration increased on treatment with MIF (300 ng/mL) in a time-dependent manner (Fig. 3E). These results indicate that activation of the 5-HT system produced by MIF may be involved in neuronal functions induced by exercise.
CD74-GTPase RhoA-ERK1/2 Pathway Is Involved in MIF-Induced Expression of TPH2, BDNF, and the Amount of 5-HT.
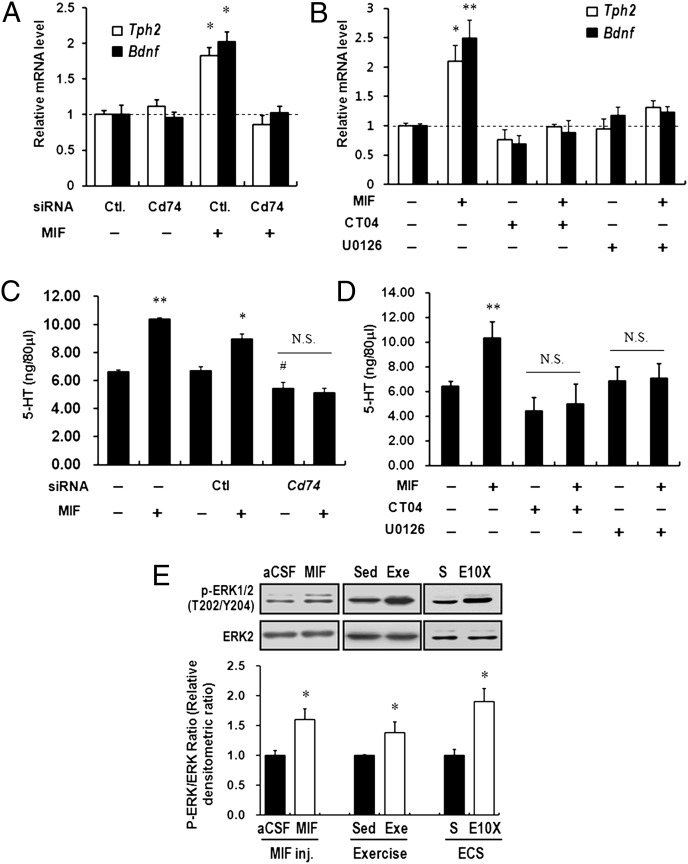
MIF has been reported to stimulate the ERK1/2 pathway, both transiently and in a sustained manner (33) via CD74-GTPase RhoA (34). In addition, activated ERK1/2 is involved in TPH (35) and BDNF (36) expression in various types of neuronal cells. We found robust expression of Cd74 in rat hippocampi as well as in Neuro-2A cells (Fig. S5A). Inhibition of Cd74 expression by siRNAs reduced the expression of Tph2 and Bdnf induced by MIF treatment in Neuro-2A cells (Fig. 4A and Fig. S5B). To evaluate the role of the GTPase RhoA–ERK pathway in Tph2 and Bdnf induction by MIF, we used the GTPase RhoA inhibitor CT04 (Cytoskeleton) and MEK inhibitor U0126 (Cell Signaling Technology). Pretreatment of CT04 (5 μg/mL) and U0126 (10 μM) for 60 min significantly decreased Tph2 and Bdnf mRNA levels induced by MIF treatment for 24 h (Fig. 4B). Furthermore, inhibition of CD74, GTPase RhoA, and the ERK1/2 pathway by Cd74 siRNAs, CT04, and U0126, respectively, reduced the amount of 5-HT induced by MIF treatment (Fig. 5 C and D). In addition, intracerebroventricular (ICV) injection of MIF increased ERK1/2 phosphorylation in rat hippocampi at 1 h after administration, as did long-term voluntary exercise and repeated ECS treatments (Fig. 4E). These results suggest that the CD74–ERK1/2 pathway seems to be required for mediating the action of MIF.
Fig. 4.
MIF increases the amount of 5-HT via the CD74-ERK1/2 pathway. (A) Down-regulation of CD74 prevented MIF action on the expression of Tph2 and Bdnf. *P < 0.05 vs. control siRNA (or control siRNA with MIF treatment). (B) Pretreatment with CT04 (5 μg/mL), a GTPase RhoA inhibitor, or U0126 (10 μM), a selective MEK inhibitor, diminished the MIF (300 ng/mL)-induced expression of Tph2 and Bdnf in Neuro-2A cells. *P < 0.05; **P < 0.01 vs. the vehicle treatment (or vehicle with MIF treatment). (C and D) Down-regulation of Cd74 reduced the basal amount of 5-HT. Cd74 siRNA transfection and pretreatment with CT04 (5 μg/mL) or U0126 (10 μM) prevented the amount of 5-HT induction at 24 h after MIF treatment (300 ng/mL). NS, not significant. Error bars show mean ± SD. *P < 0.05; #P < 0.05 vs. control siRNA (or control siRNA with MIF treatment). **P < 0.01 vs. the vehicle treatment (or vehicle with MIF treatment). (E) ICV administration of MIF, chronic exercise, and repeated ECS treatments increased ERK1/2 phosphorylation in the rat hippocampi.
Fig. 5.
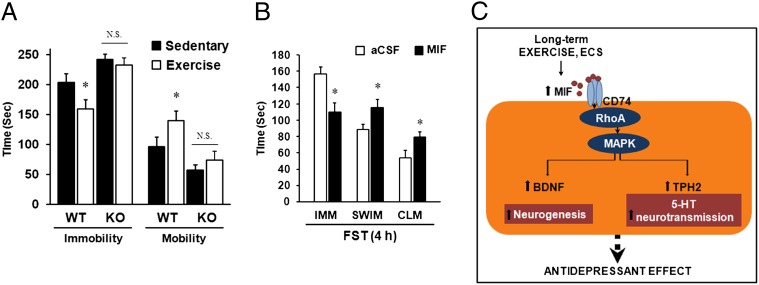
Antidepressant action of MIF as evidenced by the behavioral test. (A) Mif−/− (KO) mice had longer immobility times compared with WT littermates, and no antidepressant action of exercise was evident in Mif−/− mice on the FST (n = 6–8 for each group). (B) ICV injection of recombinant MIF protein (5 μL of a 300-μg/mL solution) into rats significantly reduced immobility time on the FST at 4 h after injection (n = 8–10 for each group). IMM, immobility; SWM, swimming; CLM, climbing. Error bars show mean ± SE. *P < 0.05. (C) Schematic model showing the mechanism of MIF on the antidepressant effect induced by exercise.
Mif KO Mice Demonstrate Diminished Antidepressant Effect of Long-Term Exercise.
We examined the effects of Mif deletion on the depression-like behavior and antidepressive effects of chronic voluntary exercise in Mif−/− mice. We observed a significant decrease in the mobility of Mif−/− mice compared with their WT littermates (P < 0.05 for both) (Fig. 5A), consistent with findings reported by Conboy et al. (17). In addition, in the WT littermates, exercise for 28 d significantly decreased the immobility time during FST (Fig. 5A), consistent with previous reports (37, 38). However, compared with the WT littermates, Mif−/− mice did not exhibit significantly decreased immobility time after long-term exercise, indicating that Mif−/− mice are incapable of fully reproducing the antidepressant effects of long-term exercise (Fig. 5A).
We also subjected the mice to the novelty-suppressed feeding test, which assesses stress-induced anxiety by measuring the latency of an animal to approach and eat a familiar food in an aversive environment. Latency was significantly longer in the Mif−/− mice compared with WT littermates (Fig. S6A). Conversely, locomotor activity did not differ between Mif−/− mice and WT littermates (Fig. S6B). These results suggest that MIF is a required element in the association between exercise and antidepressive action.
Effect of Injection of Recombinant MIF Protein in Rats on Antidepressant-Like Behavioral Changes.
To directly examine whether MIF exerts antidepressant activity, we investigated the influence of intrabrain injections of MIF on FST, the most reliable behavioral test of depression (39, 40). We generated recombinant MIF protein as described in SI Materials and Methods. Before the ICV injection experiments, we confirmed that 100 ng/mL of MIF achieved half the effective concentration for ERK phosphorylation, a well-known target of MIF (Fig. S7 A and B). Purified MIF protein or artificial cerebrospinal fluid was infused into the lateral ventricles of cannulated rats, and their behavior in the FST was examined. MIF infusion (5 μL of a 300-μg/mL solution) led to significant increases in mobility at 1 h and 4 h after treatment (P < 0.05 for both) compared with infusion of artificial cerebrospinal fluid (Fig. 5B and Fig. S6C). A significant increase in swimming at 1 h and 4 h after injection (P < 0.05 for both) and climbing events at 4 h after injection was observed compared with each control group (Fig. 5B and Fig. S6C). These findings indicate that an increasing amount of MIF protein in the brain is sufficient to induce antidepressive-like behavior.
Discussion
In this paper, we provide evidence that MIF is regulated by long-term exercise and that it mediates induction of 5-HT and neurotrophic factors, resulting in the amelioration of depressive behaviors in a rodent model. MIF was up-regulated during both long-term voluntary exercise and repeated ECS treatments. We propose a unique mechanism by which MIF exerts its antidepressant-like action by demonstrating ERK1/2 dependent up-regulation of TPH2 and BDNF expression in neuronal cell lines. We found that MIF also induced Tph2 and Bdnf expression in rat brain along with ERK1/2 activation. Consistent with these findings, 5-HT levels were increased by MIF. Furthermore, Mif−/− mice also displayed depression-like behavior. Notably, compared with the exercised WT group, the exercised Mif−/− mice demonstrated limited improvement in antidepressive-like behavior. In addition, direct intrabrain administration of MIF induced an antidepressant-like response in the FST. Taken together, these results demonstrate that MIF induces antidepressive-like behavior and mediates the effect of exercise on mood improvement.
We found that the cytokine MIF was induced by exercise as well as by ECS. MIF is a proinflammatory cytokine that plays a role in the innate and adaptive immune responses. MIF is presented in most cells, including pituitary cells, T cells, macrophages/monocytes, and several neural cells, such as those found in the hippocampus, and is released in response to infection and stress (25, 41). A recent study reported that MIF plays an important role in antidepressant-induced hippocampal neurogenesis (17). Moreover, MIF dynamically regulates the expression of other selected candidate genes, suggesting that it may modulate the antidepressant activity of exercise.
Our analysis of changes in the expression levels of neurogenesis and neurotransmission-related genes revealed significantly increased expression of Tph2 and Bdnf in response to MIF treatment. MIF−/− mice showed reduced expression levels, and exercise did not lead to recovery of expression of mRNA levels of Tph2 and Bdnf. ICV administration of MIF also up-regulated the mRNA levels of Tph2 and Bdnf in rat brains. A rate-limiting enzyme of the 5-HT synthesis pathway, TPH2 is specifically expressed in the brain (31). The neurotransmitter serotonin is believed to be involved in various brain functions, including control of mood, aggression, anxiety, pain, cognition, appetite, and sexual behavior, which points to a central role of 5-HT in the treatment of depression (42, 43). We also observed that MIF treatment increased the amount of 5-HT in the RBL2H3 cell line, along with induction of Tph2. The widespread occurrence of 5-HT–positive nerve ends throughout the brain and the density of serotonergic fibers appear to exhibit considerable local differences in the hippocampus (44). In the present study, MIF increased Tph2 expression, 5-HT levels, and exercise- and ECS-induced Tph2 expression. These findings suggest that MIF may enhance serotonin neurotransmission by inducing TPH2, which could contribute to the antidepressant action of MIF.
BDNF, one of the molecules induced by MIF, is a critical modulator of synaptic plasticity in the central nervous system, supporting the survival of existing neurons and encouraging the growth and differentiation of new neurons. Several studies have reported an association between the BDNF gene and depressive disorders and decreased levels of BDNF in patients with depression (45, 46). Various antidepressants have been reported to increase BDNF expression, which can contribute to neurogenesis (7). In the present study, exercise and ECS increased the BDNF level with neurogenesis markers in the hippocampus. A recent study identified MIF as a potent molecular target for neurogenesis (17). In the present study, MIF induced expression of the Bdnf gene in vitro and in vivo, suggesting the possible involvement of another mechanism in the antidepressant effect of MIF besides induction of 5-HT synthesis through TPH2 expression. MIF-induced BDNF expression may be associated with the effect on neurogenesis, but this cause-and-effect relationship requires further investigation.
CD74 mediates MIF induction of ERK1/2 activation (33, 34), which plays a key role in the transduction of extracellular signals to cellular responses, related mainly to cell growth. In the present study, we observed the activation of ERK1/2 in the brain after MIF treatment in vitro and in vivo. Inhibition of the CD74, GTPase RhoA, and ERK1/2 pathways diminished the effect of MIF on the expression of Tph2 and Bdnf, as well as on the amount of 5-HT. These results suggest that the CD74–MAPK pathway is involved in Tph2 and Bdnf induction after MIF treatment.
Finally, 28 d of voluntary exercise did not recover the depressive-like behavior of Mif−/− mice, but did induce antidepression-like behavioral changes in their WT littermates. The Mif−/− mice displayed increased despair phenotypes compared with littermate controls, which is consistent with recently reported data showing that genetic deletion of MIF resulted in increased depression-like behavior (17). In addition, analysis of MIF administration indicated that MIF has antidepressant-like behavioral effects. Intrabrain injection of MIF reduced depressive-like behavior in the FST. These results suggest that MIF itself induces an antidepressive effect and mediates the antidepressant action of long-term exercise.
Disturbances in the immunologic system have long been considered possible pathogenetic mechanisms of depression (47). Initially, MIF was identified as a proinflammatory cytokine involved in innate and adaptive immune responses. In the present study we have identified a different role for MIF, as an antidepressant. In particular, we found that MIF mediates the antidepressant action of long-term voluntary exercise. Moreover, MIF induces TPH2 through the activation of serotonin neurotransmission and BDNF with a possible neurogenesis action, which could mediate the antidepressant action of MIF. Note that MIF may exert its effects in the brain through the activation of two different mechanisms that are the most critical for the therapeutic interventions of depression. Taken together, our findings identify an important player in the field of antidepressants and indicate that MIF may be a significant antidepressive mediator in response to exercise.
Materials and Methods
Additional details on all methods are provided in SI Materials and Methods. For statistical analysis, all data are expressed as mean ± SE or ± SD, and are representative of at least three different experiments. Data were analyzed using the two-tailed Student t test or one- or two-way ANOVA, followed by the least significant differences post hoc test. For all cases, a P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 17.0.
Supplementary Material
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean government [2011-0000878 and Fusion Pioneer Project (PGB013) and KRF-2007-341-C00027]. We thank Dr. Kim Wan-Uk (Catholic University of Korea) and Dr. Nam Jeong-Seok and Dr. Choi Cheol Soo (Gil Medical Center, Gachon University of Medicine and Science) for their help with the animal experiments.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205535109/-/DCSupplemental.
References
- 1.Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 2.Cassano P, Fava M. Tolerability issues during long-term treatment with antidepressants. Ann Clin Psychiatry. 2004;16:15–25. doi: 10.1080/10401230490281618. [DOI] [PubMed] [Google Scholar]
- 3.Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- 4.Eley TC, et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- 5.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 6.Madsen TM, et al. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 7.Kempermann G, Kronenberg G. Depressed new neurons—adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry. 2003;54:499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- 8.David DJ, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Souza SB, et al. Role of exercise training in cardiovascular autonomic dysfunction and mortality in diabetic ovariectomized rats. Hypertension. 2007;50:786–791. doi: 10.1161/HYPERTENSIONAHA.107.095000. [DOI] [PubMed] [Google Scholar]
- 10.Morris RT, et al. Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J Appl Physiol. 2008;104:708–715. doi: 10.1152/japplphysiol.01034.2007. [DOI] [PubMed] [Google Scholar]
- 11.Zheng H, et al. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res. 2006;168:47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: Efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Alfonso J, Frasch AC, Flugge G. Chronic stress, depression and antidepressants: Effects on gene transcription in the hippocampus. Rev Neurosci. 2005;16:43–56. doi: 10.1515/revneuro.2005.16.1.43. [DOI] [PubMed] [Google Scholar]
- 14.Hunsberger JG, et al. Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007;13:1476–1482. doi: 10.1038/nm1669. [DOI] [PubMed] [Google Scholar]
- 15.Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001;8:1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- 16.Fingerle-Rowson GR, Bucala R. Neuroendocrine properties of macrophage migration inhibitory factor (MIF) Immunol Cell Biol. 2001;79:368–375. doi: 10.1046/j.1440-1711.2001.01024.x. [DOI] [PubMed] [Google Scholar]
- 17.Conboy L, et al. Macrophage migration inhibitory factor is critically involved in basal and fluoxetine-stimulated adult hippocampal cell proliferation and in anxiety, depression, and memory-related behaviors. Mol Psychiatry. 2011;16:533–547. doi: 10.1038/mp.2010.15. [DOI] [PubMed] [Google Scholar]
- 18.Conti B, et al. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Mol Psychiatry. 2007;12:167–189. doi: 10.1038/sj.mp.4001897. [DOI] [PubMed] [Google Scholar]
- 19.Newton SS, et al. Gene profile of electroconvulsive seizures: Induction of neurotrophic and angiogenic factors. J Neurosci. 2003;23:10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molteni R, Ying Z, Gómez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- 21.Ploski JE, Newton SS, Duman RS. Electroconvulsive seizure-induced gene expression profile of the hippocampus dentate gyrus granule cell layer. J Neurochem. 2006;99:1122–1132. doi: 10.1111/j.1471-4159.2006.04156.x. [DOI] [PubMed] [Google Scholar]
- 22.Gurok U, et al. Laser capture microdissection and microarray analysis of dividing neural progenitor cells from the adult rat hippocampus. Eur J Neurosci. 2007;26:1079–1090. doi: 10.1111/j.1460-9568.2007.05734.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen JX, Zhao X, Yue GX, Wang ZF. Influence of acute and chronic treadmill exercise on rat plasma lactate and brain NPY, L-ENK, DYN A1-13. Cell Mol Neurobiol. 2007;27:1–10. doi: 10.1007/s10571-006-9110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikkelsen JD, Woldbye D, Kragh J, Larsen PJ, Bolwig TG. Electroconvulsive shocks increase the expression of neuropeptide Y (NPY) mRNA in the piriform cortex and the dentate gyrus. Brain Res Mol Brain Res. 1994;23:317–322. doi: 10.1016/0169-328x(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 25.Bacher M, et al. MIF expression in the rat brain: Implications for neuronal function. Mol Med. 1998;4:217–230. [PMC free article] [PubMed] [Google Scholar]
- 26.Scott BW, Wojtowicz JM, Burnham WM. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol. 2000;165:231–236. doi: 10.1006/exnr.2000.7458. [DOI] [PubMed] [Google Scholar]
- 27.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Béquet F, Gomez-Merino D, Berthelot M, Guezennec CY. Exercise-induced changes in brain glucose and serotonin revealed by microdialysis in rat hippocampus: Effect of glucose supplementation. Acta Physiol Scand. 2001;173:223–230. doi: 10.1046/j.1365-201X.2001.00859.x. [DOI] [PubMed] [Google Scholar]
- 29.Garattini S, Kato R, Lamesta L, Valzelli L. Electroshock, brain serotonin, and barbiturate narcosis. Experientia. 1960;16:156–157. doi: 10.1007/BF02157732. [DOI] [PubMed] [Google Scholar]
- 30.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutknecht L, et al. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J Neural Transm. 2008;115:1127–1132. doi: 10.1007/s00702-008-0096-6. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa H, Kojima M, Iida Y, Oguro K, Nakanishi N. Stimulation of tryptophan hydroxylase production in a serotonin-producing cell line (RBL2H3) by intracellular calcium mobilizing reagents. FEBS Lett. 1996;392:289–292. doi: 10.1016/0014-5793(96)00834-4. [DOI] [PubMed] [Google Scholar]
- 33.Lue H, et al. Rapid and transient activation of the ERK MAPK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on JAB1/CSN5 and Src kinase activity. Cell Signal. 2006;18:688–703. doi: 10.1016/j.cellsig.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Leng L, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood JL, Russo AF. Autoregulation of cell-specific MAP kinase control of the tryptophan hydroxylase promoter. J Biol Chem. 2001;276:21262–21271. doi: 10.1074/jbc.M007520200. [DOI] [PubMed] [Google Scholar]
- 36.Obrietan K, Gao XB, Van Den Pol AN. Excitatory actions of GABA increase BDNF expression via a MAPK-CREB-dependent mechanism—a positive feedback circuit in developing neurons. J Neurophysiol. 2002;88:1005–1015. doi: 10.1152/jn.2002.88.2.1005. [DOI] [PubMed] [Google Scholar]
- 37.Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Droste SK, Schweizer MC, Ulbricht S, Reul JM. Long-term voluntary exercise and the mouse hypothalamic-pituitary-adrenocortical axis: Impact of concurrent treatment with the antidepressant drug tianeptine. J Neuroendocrinol. 2006;18:915–925. doi: 10.1111/j.1365-2826.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 39.Bianchi M, Moser C, Lazzarini C, Vecchiato E, Crespi F. Forced swimming test and fluoxetine treatment: In vivo evidence that peripheral 5-HT in rat platelet-rich plasma mirrors cerebral extracellular 5-HT levels, whilst 5-HT in isolated platelets mirrors neuronal 5-HT changes. Exp Brain Res. 2002;143:191–197. doi: 10.1007/s00221-001-0979-3. [DOI] [PubMed] [Google Scholar]
- 40.Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- 41.Bucala R. MIF rediscovered: Cytokine, pituitary hormone, and glucocorticoid-induced regulator of the immune response. FASEB J. 1996;10:1607–1613. doi: 10.1096/fasebj.10.14.9002552. [DOI] [PubMed] [Google Scholar]
- 42.Caspi A, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 43.Levinson DF. The genetics of depression: A review. Biol Psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 44.Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 45.Duncan LE, Hutchison KE, Carey G, Craighead WE. Variation in brain-derived neurotrophic factor (BDNF) gene is associated with symptoms of depression. J Affect Disord. 2009;115:215–219. doi: 10.1016/j.jad.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen L, et al. Genetic association study of BDNF in depression: Finding from two cohort studies and a meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:814–821. doi: 10.1002/ajmg.b.30686. [DOI] [PubMed] [Google Scholar]
- 47.Catena-Dell’Osso M, et al. Inflammatory and neurodegenerative pathways in depression: A new avenue for antidepressant development? Curr Med Chem. 2011;18:245–255. doi: 10.2174/092986711794088353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.